The foot processes of podocytes in glomeruli are essential filtration barriers in the kidneys. Podocytopathy results in proteinuria and nephrotic syndrome.1 Many genetic diseases related to congenital nephrotic syndrome are caused by podocytopathy.1 Triglyceride deposit cardiomyovasculopathy (TGCV) is an emerging orphan disorder (ORPHA code: 565612)2,3 that we first reported in patients with severe heart failure (HF) requiring cardiac transplantation. A known cause of TGCV is genetic mutations in PNPLA2, which encodes adipose triglyceride lipase (ATGL).2 ATGL is a rate-limiting enzyme in the hydrolysis of intracellular triglycerides (TGs) that releases long-chain fatty acids for energy production in the mitochondria. Mutations in PNPLA2 can also cause neutral lipid storage disease, with myopathy as another phenotype.4 Patients with TGCV exhibit cardiomyocyte steatosis and severe adult onset HF with reduced ejection fraction (HFrEF).2,5 These symptoms may be accompanied by proteinuria; however, the underlying mechanisms are unknown because severe heart failure usually prevents further investigation of kidney lesions. Here, we report a novel type of podocytopathy in patients with TGCV with genetic ATGL deficiency.
Cases
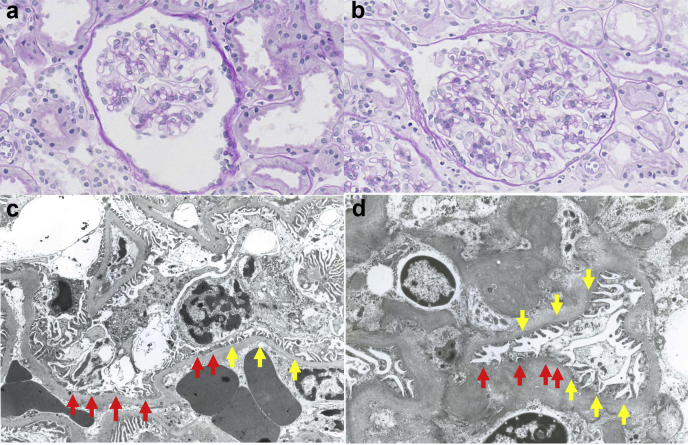
Two patients from 1 family with proteinuria preceding HFrEF were examined. Their parents were not consanguineous, but they came from the same area in the countryside of Japan, and both had been healthy. Proteinuria levels of 0.8 to 1.0 g/day were detected during school health checkups in 2 brothers aged 9 and 14 years. Their creatinine levels were normal (0.5 and 0.6 mg/dl, respectively). High levels of creatine kinase were observed in both patients from the beginning of observations, but neurological findings were normal, and they did not suffer from any muscle pain. The serological findings did not support autoimmune disease (Supplementary Table 1). Primary glomerulonephritis, including immunoglobulin (Ig)A nephropathy, which is the most common type at these ages, was suspected. Therefore, kidney biopsies were performed in both patients at the age of 20. The results of periodic acid-Schiff staining were almost normal except for a slight increase in the mesangial matrix (Figure 1a and b) in both patients. Immunofluorescence staining revealed no immune complex deposition, which ruled out IgA nephropathy and other primary glomerulonephritis with immune deposition in glomeruli. Minimal change nephrotic syndrome and focal segmental glomerulosclerosis were ruled out by lack of foot process effacement on electron microscopy. Almost all known genetic podocytopathy diseases are accompanied by nephrotic levels of proteinuria from birth,1 which were not observed with these cases. Electron microscopy revealed peculiar podocytopathy. In both brothers, the foot processes were flattened and agglutinated disproportionately, whereas a major part of the foot processes appeared normal; this has not been reported in any disease (Figure 1c and d). At that time, the cause of proteinuria remained undetermined because the genetic backgrounds were unknown.
Figure 1.
(a,b) Periodic acid-Schiff staining. (c,d) Electron microscopy findings. (a,c) Older brother. (b,d) Younger brother. Periodic acid-Schiff stained glomeruli in the brothers were almost normal except for a slight increase in the mesangial matrix (a,b). There were no abnormalities in the interstitial area, including the proximal tubules (a,b). In both brothers, some foot processes were disproportionately flattened and agglutinated, whereas the majority of the foot processes appeared normal (c,d). Abnormal foot processes are shown by red arrows, and normal foot processes are shown by yellow arrows.
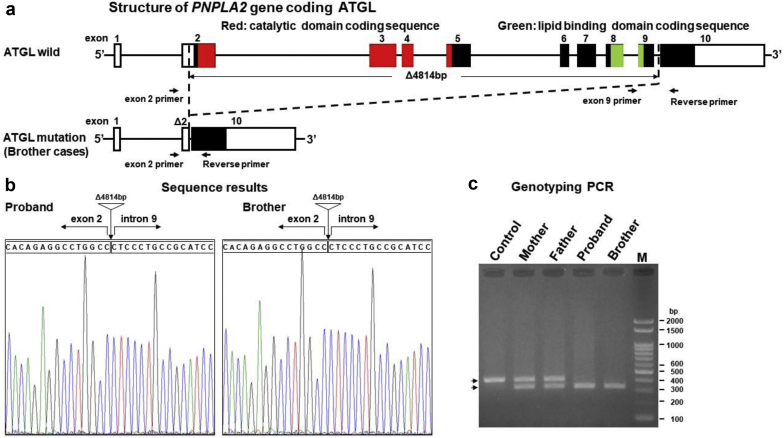
Proteinuria persisted until the onset of HFrEF in their 30s; the brothers had no hematuria during the observation periods, and their serum albumin levels remained normal. Both patients started standard therapy for HFrEF, including beta-blockers. The elder brother had decompensated intractable HFrEF and was referred to the hospital for cardiac transplantation. TGCV diagnosis was made based on a severely reduced left ventricular ejection fraction, cardiomyocyte steatosis with negative staining of ATGL in endomyocardial biopsy specimens, and Jordan’s anomaly in peripheral leukocytes.2,5 Considering these facts, the sequences of the PNPLA2 gene, which encodes ATGL, were analyzed in both patients. Briefly, polymerase chain reaction products specific for PNPLA2 gene loci were amplified from cDNA pools prepared from peripheral white blood cells and directly sequenced to detect mutations. Consequently, genetic analyses revealed that both patients were homozygous for a novel large deletion spanning exon 2 to intron 9 in PNPLA2 (Figure 2; gene ID LC508023). This deletion is the largest within the PNPLA2 gene among those reported thus far. Because the putative ATGL protein is a short polypeptide completely lacking the catalytic and lipid-binding domains essential for lipase activities, even if it is properly translated, this genetic deficiency of ATGL may cause the observed podocytopathy. Notably, a high level of creatine kinase is a common feature in patients with PNPLA2 mutations 4. In addition, genetic analyses revealed that both parents, who were healthy, were heterozygous for the large deletion, indicating possible autosomal recessive inheritance.
Figure 2.
Genetic mutations in adipose triglyceride lipase (ATGL). (a) Schematic representation of the large deletion in the ATGL gene. A 4814-bp deletion in the patient removed the majority of the protein-coding exons, spanning from exon 2 to an internal region of intron 9. Protein-coding sequences are shown in black. Arrows indicate the forward and reverse primers used for the genotyping polymerase chain reaction (PCR). (b) Sequence analysis of the large deletion areas from the 2 patients. Vertical arrows and triangles indicate the points of deletion. (c) Genotyping PCR test for the large deletion. Genotyping PCR was conducted using 3 primers: an exon 2 primer, an exon 9 primer, and a reverse primer. Lane 1: healthy control. Lane 2: mother. Lane 3: father. Lane 4: older brother. Lane 5: younger brother. Lane M: ladder marker. A single 350-bp fragment was observed in both patients who were homozygous for this large deletion. A single 430 bp band represents the wildtype allele.
Discussion
The novel podocytopathy described here is characterized by childhood-onset proteinuria, disproportionately flattened and agglutinated foot processes in podocytes, and association with ATGL deficiency.
In ATGL knockout mice, 2 distinctive pathological lesions in kidneys have been reported. One is disproportionately flattened and agglutinated foot processes in podocytes related to cellular oxidative stress and apoptosis.6 The other is vacuolization in the proximal tubules.7 The former pathology in the mouse podocytes was similar to that observed in the 2 brothers. The latter pathology was not observed in the renal biopsy specimens obtained in the earlier stages of disease but was similar to that observed in the autopsy specimens from other patients with TGCV who died of advanced HFrEF.8 Therefore, it is likely that the primary lesions induced by a lack of ATGL in podocytopathy are followed by secondary lesions in the proximal tubules and the development of chronic kidney disease (CKD). A disturbance of TG metabolism induced by a lack of ATGL might prevent enough energy to maintain foot processes in glomeruli.6 In ATGL-deficient mice, F-actin rearrangement was observed in podocytes, and stress fiber reorganization from the perinucleus toward the periphery of the cells was also observed, resulting in disproportionate foot process effacement. ATGL deficiency destroys the integrity of the glomerular filtration barrier via this foot process abnormality.6 The pressure of arterioles in glomeruli decreases according to the length of the afferent arteriole. The maintenance of the foot process of podocytes requires more energy under high than under low arteriole pressure. These tiny differences in the condition of the foot processes of podocytes might cause disproportionately flattened and agglutinated foot processes in podocytes.
Podocyte injury usually causes massive proteinuria because foot processes are important barriers against proteins. Low levels of protein are considered to be induced by endothelial dysfunction or abnormalities of the basal membrane in glomeruli. However, the disproportionate flattening and agglutination of foot processes in podocytes can also explain the low level of proteinuria. Approximately 20% of diabetic nephropathy dialysis patients are considered to have a high risk of TGCV.9 Recently, we reported a higher prevalence of TGCV in hemodialysis patients and the severe clinical outcomes of these patients, irrespective of the presence or absence of diabetes mellitus.10 Patients with CKD have abnormal TG metabolism and suffer from cardiovascular diseases. Injuries of glomerular endothelial cells play a role in the early stages of CKD. Our observations indicate that disproportionate podocytopathy might be another key player involved in the initiation of common CKD and related cardiovascular diseases.
Conclusion
Several key points from these cases should be mentioned (Table 1). One is that disproportionate flattening and agglutination of foot processes in podocytes can be accompanied by TGCV with a low level of proteinuria. Second, kidney biopsy can provide clues even after a long disease duration because new insights sometimes reveal the true clinical diagnosis. Third, this newly described type of podocyte injury might be more common than previously believed, although further study is required.
Table 1.
Teaching points regarding TGCV with proteinuria
| Triglyceride deposit cardiomyovasculopathy (TGCV) might manifest with proteinuria prior to heart failure symptoms. |
| Disproportionate flattening and agglutination of foot processes in podocytes might indicate TGCV. |
| Kidney biopsy can provide clues even after a long disease duration because new insights sometimes reveal the true clinical diagnosis. |
Author Contributions
YN, TK, YH, and KIH designed this study. TO, TK, TM, YI and KIH contributed to the diagnosis of TGCV in both patients. YN and MH contributed to the diagnosis of renal pathology in both patients. YH and KIH contributed to the determination of the mutation in both patients. YN, TO YH and KIH wrote the manuscript, which all authors read and approved.
Disclosure
The authors declare that they have no relevant financial interest.
Patient Consent
Written informed consent from patients was obtained using the format of the American Journal of Kidney Disease.
Acknowledgments
We thank Professor Hidekazu Shigematsu (deceased), Division of Pathology, Shinsyu University, Matsumoto, Japan, for useful comments on renal pathological findings. We thank Dr. Yasuchika Kato, Department of Cardiology, Fujita Health University, Toyoake, Japan, and Dr. Hideo Oishi, Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan, for their extensive efforts in the clinical management of these patients. We thank Dr. Akinori Sawamura, Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan, and Dr. Masahisa Katsuno, Department of Neurology, Nagoya University Graduate School of Medicine, Nagoya, Japan, for their contributions to the clinical diagnosis of TGCV. This work was supported by a research grant for rare and intractable disease from the Ministry of Health, Labour, and Welfare of Japan (20FC1008). This work was supported by a research grant for rare and intractable disease from the Ministry of Health, Labour, and Welfare of Japan (20FC1008). This work was also supported by JSPS KAKENHI grant nos. JP17K09721, JP19K10098, JP20K10225, and JP21K08242 and AMED grant nos. JP20ek0109479 and JP21ek0109479.
Footnotes
Table S1. Patient profiles at the time of renal biopsy.
Supplementary Material
Table S1. Patient profiles at the time of renal biopsy.
References
- 1.Kopp J.B., Anders H.J., Susztak K. Podocytopathies. Nat Rev Dis Primers. 2020;6:68. doi: 10.1038/s41572-020-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirano K., Ikeda Y., Zaima N. Triglyceride deposit cardiomyovasculopathy. N Engl J Med. 2008;359:2396–2398. doi: 10.1056/NEJMc0805305. [DOI] [PubMed] [Google Scholar]
- 3.Li M., Hirano K.I., Ikeda Y. Triglyceride deposit cardiomyovasculopathy: a rare cardiovascular disorder. Orphanet J Rare Dis. 2019;14:134. doi: 10.1186/s13023-019-1087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer J., Lefevre C., Morava E. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K., Sakata Y., Miyauchi H. 2020 Diagnostic criteria for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol. 2020 doi: 10.17996/anc.20-00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W., Jiang Y., Han J. Atgl deficiency induces podocyte apoptosis and leads to glomerular filtration barrier damage. FEBS J. 2017;284:1070–1081. doi: 10.1111/febs.14038. [DOI] [PubMed] [Google Scholar]
- 7.Chen W., Zhang Q., Cheng S. Atgl gene deletion predisposes to proximal tubule damage by impairing the fatty acid metabolism. Biochem Biophys Res Commun. 2017;487:160–166. doi: 10.1016/j.bbrc.2017.03.170. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M., Ikeda Y., Li M. A historical case of primary triglyceride deposit cardiomyovasculopathy. Pathol Int. 2020;70:58–61. doi: 10.1111/pin.12884. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda Y., Zaima N., Hirano K. Coronary triglyceride deposition in contemporary advanced diabetics. Pathol Int. 2014;64:325–335. doi: 10.1111/pin.12177. [DOI] [PubMed] [Google Scholar]
- 10.Onishi T., Nakano Y., Hirano K.I. Prevalence and clinical outcomes of triglyceride deposit cardiomyovasculopathy among haemodialysis patients. Heart. 2020 doi: 10.1136/heartjnl-2020-317672. https://heart.bmj.com/content/107/2/127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.