Abstract
Introduction
A critical unmet need exists for precision therapies for chronic kidney disease. GFB-887 is a podocyte-targeting, small molecule inhibitor of transient receptor potential canonical-5 (TRPC5) designed specifically to treat patients with glomerular kidney diseases characterized by an overactivation of the TRPC5-Rac1 pathway. In a first-in-human study, GFB-887 was found to be safe and well tolerated, had a pharmacokinetic (PK) profile allowing once-daily dosing, and dose dependently decreased urinary Rac1 in healthy adults.
Methods
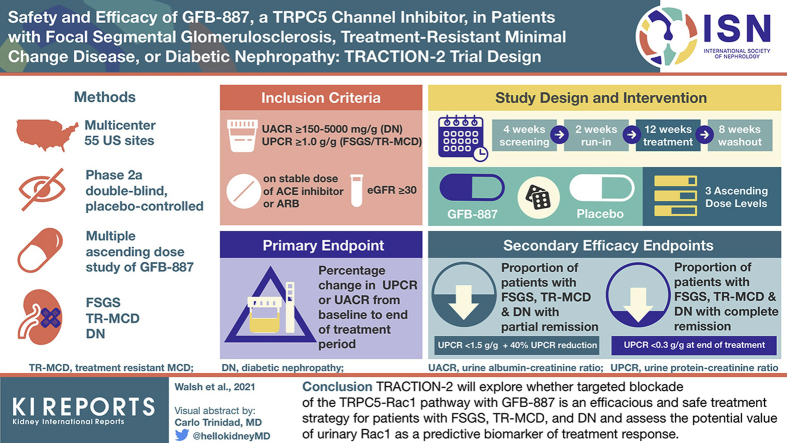
TRACTION-2 is a phase 2a, double-blind, placebo-controlled, multiple−ascending dose study of GFB-887 in patients with focal segmental glomerulosclerosis (FSGS), treatment-resistant minimal change disease (TR-MCD), or diabetic nephropathy (DN) (NCT04387448). Adult patients on stable renin−angiotensin system blockade and/or immunosuppression with persistent proteinuria will be randomized and dosed in 3 ascending dose levels to GFB-887 or placebo for 12 weeks. Cohorts may be expanded or biomarker-enriched depending upon results of an adaptive interim analysis.
Results
The primary objective is to evaluate the effect of increasing doses of GFB-887 on proteinuria. Safety and tolerability, quality of life, pharmacokinetic/pharmacodynamic profiles, and the potential association of urinary Rac1 with efficacy will also be evaluated. The projected sample size has 80% power to detect a treatment difference in proteinuria of 54% (FSGS/TR-MCD) or 44% (DN) compared to placebo.
Conclusion
TRACTION-2 will explore whether targeted blockade of the TRPC5-Rac1 pathway with GFB-887 is an efficacious and safe treatment strategy for patients with FSGS, TR-MCD, and DN and the potential value of urinary Rac1 as a predictive biomarker of treatment response.
Keywords: FSGS, proteinuria, TRPC5, Rac1, biomarker, precision medicine
Graphical abstract
Focal segmental glomerulosclerosis (FSGS), treatment-resistant minimal change disease (TR-MCD), and diabetic nephropathy (DN) are chronic kidney diseases (CKDs) that are characterized by podocyte dysfunction leading to proteinuria and are associated with a high risk of end-stage kidney failure.1, 2, 3, 4, 5, 6 Although current therapies are effective at reducing proteinuria in some patients, disease progression marked by persistent proteinuria and decline in eGFR remains a significant unmet medical need, requiring development of both novel and targeted therapies in this area.7, 8, 9 Because underlying mechanisms of podocyte injury and loss are being elucidated,10 the development of targeted therapies that are tailored to the specific underlying molecular pathways of disease progression and characteristics of individual patients is increasingly feasible.11,12 Using this precision medicine approach has the potential to improve outcomes while minimizing toxicity.13,14
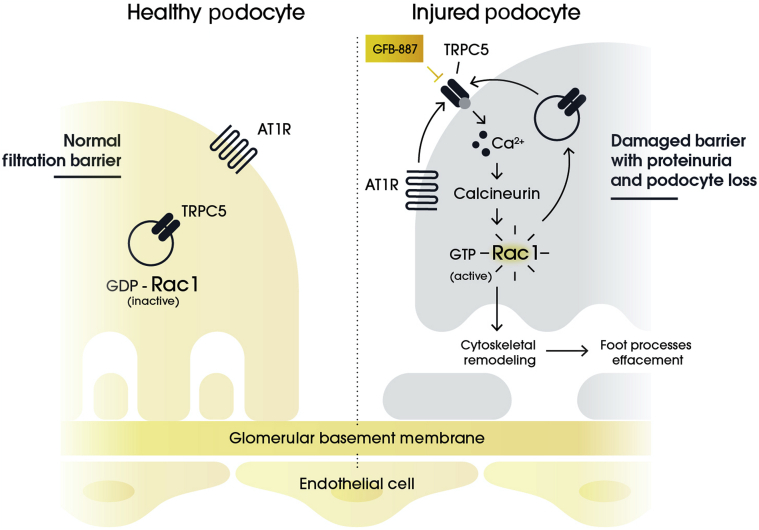
The TRPC5-Rac1 (transient receptor potential canonical-5-Ras-related C3 botulinum toxin substrate 1) pathway is central to the pathogenesis of podocyte injury (Figure 1).15,16 Rac1 promotes cytoskeletal remodeling and podocyte death through elevation of reactive oxygen species. In addition, Rac1 activation leads to the translocation of TRPC5, a Ca2+-permeable nonselective cationic channel, to the podocyte cell membrane. The activation of TRPC5 leads to an elevation in cytoplasmic calcium, which further drives Rac1 activity in a feed-forward loop.17,18
Figure 1.
TRPC5-Rac1 pathway activation and podocyte injury.
The importance of maintaining homeostasis of the TRPC5-Rac1 pathway is highlighted by rare forms of FSGS characterized by excess Rac1 activation driven by mutations in its regulators (e.g., ARHGAP24 and ARHGDIA).19, 20, 21, 22 In addition, significant increases in Rac1 have been observed in podocytes of immunostained kidney biopsy samples from patients with FSGS and MCD compared to healthy kidneys, and recent data indicate that urinary Rac1 levels are significantly elevated in patients with FSGS and DN compared with healthy controls.16,23 Activation of Rac1 in kidney diseases, such as FSGS, is thought to occur specifically in podocytes allowing the targeting of the TRPC5-Rac1 pathway in a cell-specific manner. Other activators of Rac1 also include hyperglycemia, angiotensin II type 1 receptor, and epidermal growth factor receptor, which would feed into the TRPC5-Rac1 feed-forward loop.21,24 Thus, reducing Rac1 levels by inhibiting TRPC5 has potential as a targeted treatment for podocytopathies. In fact, inhibition of TRPC5 has been shown to ameliorate proteinuria and to prevent podocyte loss in animal models of FSGS without off-target toxicities.15,25 The safety of inhibiting TRPC5 is further supported by the lack of a significant phenotype in TRPC5 knock-out mice.26,27
GFB-887 is a podocyte-targeting, small molecule TRPC5 inhibitor designed specifically to treat patients with kidney diseases characterized by an overactivation of the TRPC5-Rac1 pathway. GFB-887 has been shown, in both in vitro and in vivo models of kidney disease, to prevent podocyte damage mediated by activation of Rac1 signaling (Figure 1). Thus, GFB-887−reduced albuminuria in a deoxycorticosterone acetate (DOCA) salt-induced hypertensive rat model of FSGS, a puromycin aminonucleoside injury rat model of MCD, and a Zucker diabetic Sprague−Dawley (ZDSD) rat model of DN.28 GFB-887 also reduced pathogenic podocyte migration in an in vitro migration assay and protected human induced pluripotent stem cell−derived podocytes and kidney organoids from cytoskeletal disruption after co-incubation with protamine sulfate (PS), a known indirect activator of TRPC5.29,30 Although TRPC5 is also expressed outside the kidney, including in the brain, there were no adverse findings observed during an extensive preclinical evaluation of GFB-887, both in pharmacological and in toxicological evaluations.
We recently completed a first-in-human single−ascending dose study of GFB-887 (5−900 mg) dosed orally to 70 healthy participants (Study GFB-887-101; Clinicaltrials.gov Identifier NCT03970122).31 GFB-887 exhibited a favorable PK profile, and only mild treatment-emergent adverse events (e.g., headache, nausea) were reported. No dose-limiting toxicities or clinically significant abnormalities in laboratory values, vital signs, or electrocardiographic parameters were observed. GFB-887 exhibited dose-dependent reductions in urinary Rac1, providing evidence that GFB-887 engages the low basal levels of TRPC5 in healthy podocytes, inhibiting the TRPC5-Rac1 pathway. This finding further suggests that urinary Rac1 could be used as a response and/or pharmacodynamic biomarker to guide treatment or dosing decisions.
Collectively, these findings provided the rationale for initiating TRACTION-2, the first-in-patient clinical trial of GFB-887. Herein, we describe the study design and methodology of this trial, including some study design features that are novel for nephrology studies (Table 1). TRACTION-2 will explore whether targeted blockade of TRPC5-Rac1 pathway with GFB-887 is an efficacious and safe treatment strategy that is well tolerated by patients with FSGS, TR-MCD, and DN. Given the mechanism of action of GFB-887 and data supporting upregulation of the TRPC5-Rac1 pathway in patients with FSGS and DN, this study will also evaluate whether baseline urinary Rac1 levels predict treatment response. Furthermore, we summarize the use of an adaptive study design, which allows for prospectively planned modifications to the protocol on the basis of accumulating data from study participants.32
Table 1.
TRACTION-2 novel study design features
| Study design feature | Description |
|---|---|
| Adaptive design | Depending upon the results of a preplanned interim analysis, sample size can be increased and/or enriched for populations more likely to respond to GFB-887 treatment on the basis of baseline urinary Rac1 levels |
| Dose for Dose Levels 2 and 3 will be determined based upon the pharmacokinetic determinations performed on an ongoing basis | |
| “Basket” design | Enrollment of patient cohorts across various chronic kidney disease etiologies (DN, FSGS/TR-MCD) to characterize the effect of a single-targeted therapy (GFB-887) in multiple disorders |
| Patient-focused | Remote study conduct: Assessments at the patient’s home using a home healthcare nurse or using telemedicine options |
| Robust evaluation of quality of life scores, symptomatology, and edema |
DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; TR-MCD, treatment-resistant minimal change disease.
Materials and Methods
Study Participants and Sites
Patients of either sex, any race, and 18 to 75 years of age with FSGS, TR-MCD, and DN are eligible to participate in this trial. Inclusion and exclusion criteria for all patients and those specific to diagnoses are shown in Tables 2 and 3. Briefly, participants are required to have an estimated glomerular filtration rate (eGFR) ≥30 ml/min per 1.73 m2 and to have albuminuria/proteinuria (urine albumin-to-creatinine ratio [UACR] ≥150 to 5000 mg/g for DN patients; urine protein-to-creatinine ratio [UPCR] ≥1.0 g/g for FSGS or TR-MCD patients) while receiving a stable dose of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), or, as indicated, steroids or other anti-proteinuric agents (e.g., sodium glucose co-transporter−2 [SGLT2]) inhibitors or blood pressure−reducing agents for at least 4 weeks before screening. Patients not receiving ACE inhibitors or ARBs because of allergy or intolerance to ACE inhibitors or ARBs are still eligible. Persistent proteinuria (>1.0 g/24-h urine or >1.0 g/g UPCR) following a standard course of corticosteroids or another immunosuppressive therapy is required for participants to be considered to have TR-MCD. A biopsy or genetic testing is required to confirm a diagnosis of FSGS or TR-MCD. In addition, participants on calcineurin inhibitor (CNI) treatment or who have a history of CNI resistance are ineligible, given the shared pathway of CNIs with TPRC5 inhibitors.33 Resistance is defined as having a suboptimal antiproteinuric response to CNIs (<40% proteinuria reduction) after at least an 8-week course at therapeutic dose levels. Up to 125 patients will be enrolled at an estimated 55 sites in the United States (see Supplementary Material).
Table 2.
TRACTION-2 key inclusion criteria
| Diagnosis | Inclusion criteria |
|---|---|
| All Patients |
Men or women (18 to 75 years of age, of any race) |
| eGFR ≥30 mL/min/1.73 m2 at screening | |
| Currently receiving an ACE inhibitor or ARB for at least the last 3 months before screening, with a stable dose for at least 4 weeks before screening (patients not receiving ACE inhibitors or ARBs because of allergy or intolerance to ACE inhibitors or ARBs are also eligible) | |
| DN | Diagnosis of type 2 diabetes with HbA1c level ≤11% at screening |
| Average UACR ≥150 to 5000 mg/g during screening and run-in visits | |
| FSGS or TR-MCD | Diagnosis based on either biopsy, as documented by pathology report, or FSGS based on genetic testing |
| TR-MCD defined as incomplete resolution of proteinuria, defined as persistent proteinuria (>1.0 g/24-h urine or >1.0 g/g UPCR) following at least 8 weeks of corticosteroids or another immunosuppressive therapy | |
| Average UPCR ≥1.0 g/g during screening and run-in visits | |
| Patients currently receiving corticosteroids or mycophenolate mofetil must have been receiving the medication for at least the last 3 months before screening, with a stable dose for at least 4 weeks before screening |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; TR-MCD, treatment-resistant minimal change disease; UACR, urinary albumin-to-creatinine ratio; UPCR, urinary protein-to-creatinine ratio.
Table 3.
TRACTION-2 key exclusion criteria
| Diagnosis | Exclusion criteria |
|---|---|
| All Patients |
Evidence of another kidney disease clinically or on biopsy |
| Uncontrolled BP (systolic BP >160 mm Hg or diastolic BP >90 mm Hg) | |
| BMI >45 kg/m2 for DN patients; >40 kg/m2 for FSGS/TR-MCD patients | |
| ALT and/or AST >2 × ULN at screening or a known history of severe or chronic hepatobiliary disease | |
| Women who are pregnant or are breastfeeding | |
| Positive HIV test result or hepatitis B or C infection | |
| Clinically significant cardiovascular disease | |
| History of kidney transplantation | |
| DN | Current or past renal disease that requires immunosuppressive therapy |
| FSGS or TR-MCD | Currently receiving CNI therapy or history of CNI resistance |
| Systemic immunosuppressive or corticosteroid therapy for non−kidney disease indications | |
| Received rituximab or cyclophosphamide within 120 days of screening | |
| Received plasmapheresis within 84 days of screening | |
| Biopsy consistent with collapsing FSGS |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CNI, calcineurin inhibitor; DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; HIV, human immunodeficiency virus; TR-MCD, treatment-resistant minimal change disease; ULN, upper limit of normal.
This study is being conducted in accordance with consensus ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation (ICH) Good Clinical Practice (GCP) Guidelines, and applicable laws and regulations. An Institutional Review Board/Ethics Committee reviewed and approved the protocol and other relevant documents before study initiation. All participants will be required to provide written informed consent after the details of the study are fully explained to them. The study is sponsored by Goldfinch Bio, Inc.
Study Design and Treatment
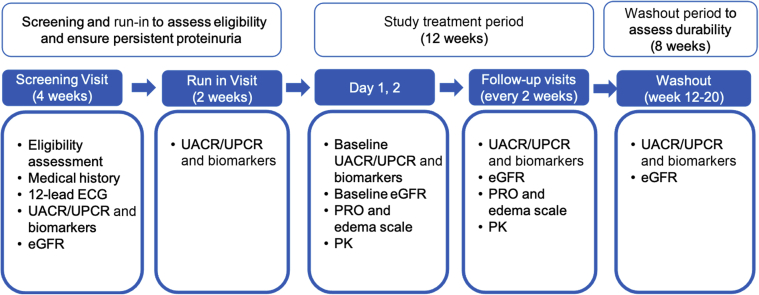
This is a multicenter, Phase 2a, randomized, double-blind, placebo-controlled clinical trial to evaluate the safety, tolerability, and PK and PD profiles of multiple ascending doses of GFB-887 in patients with FSGS, TR-MCD, and DN (Clinicaltrials.gov Identifier NCT04387448). The total study duration is planned to be up to 26 weeks and will consist of the following study periods: a 4-week screening period after informed consent is obtained; a 2-week run-in period before study drug administration, to confirm that UACR and UPCR levels continue to meet eligibility criteria; a 12-week period in which patients will receive either GFB-887 or placebo; and an 8-week washout period to evaluate durability in treatment response after the end of study treatment (Figure 2).
Figure 2.
TRACTION-2 study design and schedule of key assessments. ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; PK, pharmacokinetics; PRO, patient-reported outcome; UACR, urine albumin-to-creatinine ratio; UPCR, urine protein-to-creatinine ratio.
Before any patient was enrolled in the study, several measures were put into place to mitigate potential disruptions in study activities due to the ongoing COVID-19 global pandemic. These measures included the following: allowing patients to electronically sign the informed consent form and to receive study treatment supply at home; alternative methods to conduct study visits, including assessments at the patient’s home using a home healthcare nurse, via telephone, or using other telemedicine options; and use of a symptom-directed physical examination for visits conducted via home nursing or telemedicine. Each study site completed a sponsor-prepared COVID-19 checklist to assess their readiness for implementation of these measures.
Dose Escalation
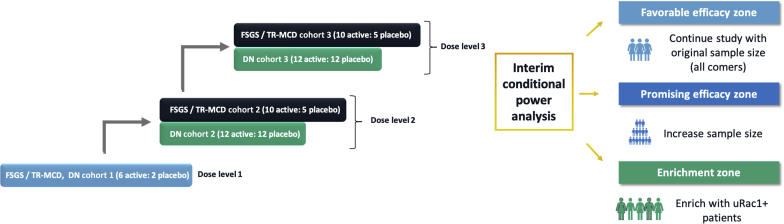
During the double-blind treatment period, patients will be randomized and dosed in 3 ascending dose levels to either GFB-887 or placebo (Figure 3). In Dose Level 1, a mixed population of 8 patients with FSGS/TR-MCD or DN will be randomized to receive GFB-887 or placebo in a 6:2 ratio in a single cohort. Dose Levels 2 and 3 will each enroll up to 15 FSGS/TR-MCD patients (randomized 2 [GFB-887]:1 [placebo]) and 24 DN patients (randomized 1 [GFB-887]:1 [placebo]) in separate cohorts. In Dose Levels 2 and 3, randomization for FSGS/TR-MCD will be covariate-balanced for baseline UPCR (<3 g/g, ≥3 g/g), prior use of CNI (yes/no), and presence of 2 apolipoprotein L1 gene (APOL1) risk variants. Randomization for DN patients will be covariate-balanced for baseline UACR (<300 mg/g, ≥300 mg/g) and concurrent use of an SGLT2 inhibitor.
Figure 3.
Multiple ascending doses and adaptive study features of TRACTION-2. Up to 125 patients with DN, FSGS, or TR-MCD will be randomized in 3 ascending dose levels to either GFB-887 or placebo (6:2 [active: placebo] for Dose Level 1; and 2:1 for FSGS/TR-MCD and 1:1 for DN for Dose Levels 2 and 3). Dose Level 1 will include 8 patients with any of the 3 diseases under study. Dose Levels 2 and 3 will include 24 DN patients and 15 FSGS/TR-MCD patients. An adaptive interim efficacy analysis will allow for enrollment of additional patients depending on which “zones” the results fall into: “favorable efficacy zone,” the trial will be completed as initially planned with no sample size increase or population enrichment; “promising efficacy zone,” the total sample size may be increased; “enrichment zone,” the population may be enriched by restricting the future enrollment to the subpopulation identified by urinary Rac1. If the results of the adaptive interim analysis do not fall into any of these zones, then the trial will be completed as initially planned, with no sample size increase or population enrichment. DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; PK, pharmacokinetics; TR-MCD, treatment-resistant minimal change disease; uRac1, urinary Ras-related C3 botulinum toxin substrate 1.
Endpoints and Assessments
Efficacy endpoints are presented in Table 4, and the schedule of assessments is shown in Figure 2. The primary endpoint is the percentage change from baseline to the end of the treatment period in UPCR in patients with FSGS/TR-MCD or UACR in patients with DN. Key secondary efficacy endpoints include the following: (i) proportions of FSGS/TR-MCD patients achieving a modified partial remission, defined as a UPCR <1.5 g/g and a 40% reduction from baseline at the end of treatment; and (ii) proportions of FSGS/TR-MCD patients achieving a complete remission, defined as UPCR <0.3 g/g at the end of treatment.34 The association between efficacy and baseline urinary Rac1 measurements, as well as on-treatment changes in urinary Rac1 levels, will be investigated as exploratory endpoints.
Table 4.
TRACTION-2 efficacy endpoints
| Primary efficacy endpoint | Percentage change from baseline in UACR or UPCR in patients with DN and FSGS/TR-MCD, respectively |
| Secondary efficacy endpoints | Proportions of FSGS/TR-MCD patients achieving a modified partial remission, defined as a UPCR <1.5 g/g and a 40% reduction from baseline at the end of treatment |
| Proportions of FSGS/TR-MCD patients achieving a complete remission, defined as UPCR <0.3 g/g at the end of treatment | |
| Percentage change from baseline in 24-hour urine protein/albumin excretion | |
| Proportion of responders, defined as patients with at least 30%, 40%, and 50% reductions in UACR/UPCR from baseline | |
| Proportion of FSGS/TR-MCD patients achieving modified partial remission, complete remission, or a 40% reduction in UPCR from baseline | |
| Exploratory efficacy endpoints | Change from baseline in HRQoL scores |
| Change from baseline in Nephrotic Syndrome Edema−Clinician Rating Scale |
DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; HRQoL, health-related quality of life; TR-MCD, treatment-resistant minimal change disease; UACR, urinary albumin-to-creatinine ratio; UPCR, urinary protein-to-creatinine ratio.
To better evaluate the impact of GFB-887 on quality of life, patients and clinicians will be asked to complete health-related quality of life (HRQoL) questionnaires during the study. The FSGS/MCD PRO will be used for measurement of patient-reported outcomes (PROs) for patients with FSGS and TR-MCD.35 The Quality of Life Disease Impact Scale–7 item scale (QDIS-7),36 Quality of Life General Survey (QGEN),37 and Patient Global Impressions of Change and Severity (PGI-C, PGI-S)38 will be used for measurement of CKD-specific and generic PROs for all patients. The clinician-reported outcome (ClinRO) Nephrotic Syndrome Edema−Clinician Rating Scale assessment will be used for standardized measurement of edema in patients with FSGS/TR-MCD.39
TRACTION-2 will also characterize the PK profile of GFB-887 at all dose levels. Full plasma PK profiles will be determined after the first and last study doses, with additional collections at various times over the course of the study. Urine collections will also be performed to evaluate GFB-887 excretion. These plasma and urine samples may also be used for future biomarker analyses to study the mechanism of action of GFB-887. A molecular genetics report including the results of a Clinical Laboratory Improvement Amendment (CLIA) certified laboratory analysis of APOL1 and other genes associated with FSGS will be returned to the investigator. Genotyping will also be performed as an exploratory endpoint to further characterize the genetic signature of the patient population and the response to GFB-887, which will not be returned to the investigator.
The safety and tolerability of GFB-887 will be assessed via the incidence and severity of AEs, and the incidence of clinically significant changes in 12-lead electrocardiographic parameters, vital signs, physical examinations, and laboratory parameters. Adverse events will be coded using the Medical Dictionary for Regulatory Activities (MedDRA). The safety of GFB-887 will be assessed at regular intervals by both a blinded and an unblinded data review team (DRT). The unblinded team will be independent from the operational study team and include external nephrology experts and supported by an independent statistical team.
Statistical Analysis
Sample Size and Analysis of the Primary Efficacy Endpoint
The sample size calculation is based on the primary endpoint, the percentage change from baseline in UPCR and UACR to the end of the treatment period in patients with FSGS/TR-MCD and DN, respectively, in cohorts at Dose Level 2 and higher. The sample size is based on the predicted efficacy of GFB-887 using cyclosporine as an analogue. Although not completely characterized, cyclosporine exerts a significant antiproteinuric effect of at least 50%.40,41 The sample size is considered sufficient to establish proof-of-concept for GFB-887 given the early phase of development. On the basis of previous studies with comparable populations, the SD of the change from baseline in the log-transformed primary efficacy scales for both treatment groups is assumed to be 0.8.42, 43, 44 As such, under these assumptions, 30 FSGS/TR-MCD patients (with 20 active and 10 placebo) and 48 DN patients (with 24 active and 24 placebo) will provide 80% power to detect a treatment difference of 54% and 44% versus placebo on the change from baseline to the end of treatment measured on the log-transformed primary efficacy scale, respectively, at a 1-sided significance level of 0.05. If, however, the predicted efficacy is observed to be lower at an interim analysis, the adaptive nature of the study allows for an increase in the sample size based on the conditional power (see Adaptive Interim Analysis below).
The change from baseline to endpoint in the log-transformed UPCR (FSGS/TR-MCD) and UACR (DN) will be analyzed using an analysis of covariance model, which includes treatment groups (GFB-887 vs. placebo) as a fixed factor and baseline log-transformed UPCR/UACR as a covariate. Anti−log-transformed values of the least squares (LS) means and 90% confidence intervals (CIs) will be calculated. In addition to the above, a Bayesian analytic approach may be used to derive posterior probabilities of statistically and clinically relevant differences between GFB-887 and placebo.
Analysis of Secondary Efficacy Endpoints
Secondary efficacy endpoints of percentage of patients achieving response or remission based on UACR and UPCR will be analyzed using a χ2 test or Cochran−Mantel−Haenszel χ2 test stratified by the prespecified stratification factors as appropriate. Change in 24-hour urine protein/albumin excretion compared with baseline will be analyzed using the same statistical method as for the primary endpoint.
Pharmacokinetic Analysis
Individual and summary plasma concentration versus time profiles will be plotted using linear and semi-logarithmic scales for each treatment. The PK parameters for GFB-887 will be calculated using noncompartmental methods and presented using descriptive statistics (mean, SD, minimum, median, maximum, coefficient of variation, and geometric mean) where applicable. Dose proportionality of GFB-887 area under the plasma concentration−time curve (AUC) and observed Cmin and Cmax will be evaluated using descriptive statistics and visual inspection of the scatter plots of these parameters versus treatment.
Interim PK analyses will be conducted to assist in defining the doses for Dose Levels 2 and 3. Available PK data will be fitted to a model, and the model will be used to predict the dose needed to attain target concentrations for the 2 groups. Those doses will be recommended to a blinded data review team for implementation prior to the start of enrollment of those groups.
Exploratory Analysis of Rac1
Pharmacodynamic (PD) measurements will be summarized by treatment group and collection time. Urinary Rac1 measures from predosing to each postdosing time point will be analyzed using a mixed-effects repeated-measures model. In addition, the area under the urinary Rac1 concentration−time curve will be compared between treatment groups and placebo graphically or using statistical tests, such as analysis of covariance or multivariate regression.
Adaptive Interim Analysis
For each disease type (FSGS/TR-MCD and DN), the trial has 1 prespecified adaptive interim analysis that will be performed by the unblinded data review team to optionally increase the sample size and/or to enrich the study population by restricting subsequent enrollment to a subpopulation identified as more likely to respond to treatment (Figure 3). The decision to adaptively increase the sample size or to enrich for a certain threshold of baseline urinary Rac1 patients will be based on the evaluation of the conditional power for demonstrating the significant treatment effect at the planned number of patients using a 1-sided significance level of α = 0.05. In general, conditional power is defined as the probability that, conditional on the current value of the test statistic and given a true effect size, the trial will achieve a statistically significant result at the final analysis. Pre-defined “zones” for this interim analysis are defined as shown in Figure 3. If the results of the adaptive interim analysis fall into the “favorable efficacy zone,” then they will be considered favorable for the full population, and the trial will be completed as initially planned with no sample size increase or population enrichment. If the results fall into the “promising efficacy zone,” then they will be considered promising for the full population, and the total sample size may be increased. In this case, the sample size will be increased to ensure a pre-defined conditional power that can be achieved in the second stage of the trial. The actual decision rules regarding the boundaries that define the promising zone and the target conditional power are kept blinded until the end of the study. As a hypothetical example, if, in the FSGS population, an interim placebo-adjusted effect size of 35% is observed on UPCR, and if this corresponds to a conditional power of 60% (assumed to be in the promising zone), additional patients will be enrolled to boost the power for the remainder of the study. This increases the likelihood of observing a statistically significant result in the presence of a clinically meaningful effect that may be lower than initially planned. If the results fall into the “enrichment zone,” then the population may be enriched by restricting the future enrollment to the subpopulation identified by urinary Rac1 and a possible sample size increase in this enriched population to achieve a certain conditional power, similar to the hypothetical example above. If the results do not fall into any of these zones, then the trial will be completed as initially planned, with no sample size increase or population enrichment. For all decisions related to adaptive sample size re-estimation/enrichment, the results of the safety analyses to date may additionally be used to influence subsequent allocation of patients.
Discussion
Currently, there are no therapies approved by the United States Food and Drug Administration (FDA) for FSGS and TR-MCD, nor are there approved therapies for proteinuric kidney diseases that specifically target the podocyte, a primary component of the glomerular filtration barrier. Novel precision medicine−based strategies that protect the podocytes are therefore needed to advance therapeutic development for these progressive kidney diseases. Toward this aim, GFB-887, a subtype-selective and potent small molecule inhibitor of the TRPC5 ion channel, is being investigated as a targeted disease-modifying therapy for patients with podocytopathies. In this report, we describe the design of TRACTION-2, a first-in-patient, randomized, double-blind, placebo-controlled, dose-escalating clinical trial of GFB-887. TRACTION-2 was designed to achieve proof of concept as efficiently and safely as possible to develop a targeted therapy in this area of high unmet clinical need. Our primary objectives are to explore whether targeted blockade of the TRPC5-Rac1 pathway with GFB-887 is an efficacious and safe treatment strategy for patients with podocytopathies and whether proteinuria response rates are enhanced in patients with elevated Rac1 levels. This may inform the design of future GFB-887 studies by identifying a population most likely to be responsive to TRPC5 inhibition.
Activation of either calcineurin or TRPC5 in podocytes is sufficient to cause synaptopodin degradation, podocyte dysfunction, and proteinuria. In fact, calcineurin inhibition with cyclosporin or tacrolimus serves as an analogue to the potential efficacy of GFB-887 given their activity on the TRPC5-Rac1 pathway.33 The use of CNIs has been shown to achieve significant reductions in proteinuria in patients with steroid-resistant FSGS, which is independent of their immunosuppressive effects.40,41,45 However, the off-target nephrotoxicity of CNIs limits their long-term use.46 The safety of TRPC5 inhibition, and of GFB-887 in particular, has been established in preclinical models and does not recapitulate the toxicities associated with calcineurin inhibition. Given that TRPC5 and calcineurin are components of the TRPC5-Rac1 pathway, patients who have demonstrated CNI resistance or are currently being treated with CNI are not eligible for TRACTION-2 but will be studied in future trials. Resistance to CNIs may indicate the activation of an alternative pathological pathway, highlighting the need for alternative treatments for patients with FSGS.
TRACTION-2 includes several notable design features, including the selection of an at-risk patient population, the use of disease-specific patient reported outcome tools, and novel adaptive elements. The study population includes patients who remain at high risk for disease progression given that patients have residual albuminuria/proteinuria despite background therapy of stable RAS blockade. In addition, the TRACTION-2 study will include separate cohorts of patients with FSGS, TR-MCD, and DN, thus using a “basket” trial design approach to efficiently characterize the effect of GFB-887 across multiple CKD etiologies. Approximately half of those patients with untreated primary FSGS will develop kidney failure over 5 to 8 years.47 In addition, MCD is considered a related podocytopathy to FSGS, and, although the majority of patients with MCD respond to an initial course of corticosteroids, many adults with MCD are resistant to treatment. Although not fully elucidated, the prognosis of patients with TR-MCD is similar to that of patients with FSGS, and their categorization likely reflects limitations in establishing a diagnosis of FSGS on the basis of a kidney biopsy rather than underlying biological differences.48,49 Finally, DN accounts for nearly half of all patients with CKD, and the prevalence is rising globally as a consequence of the diabetes and obesity epidemic.5
TRACTION-2 is designed to provide early human data on the short-term effect of increasing doses of GFB-887 on UPCR/UACR levels. These objective surrogate endpoints are known to predict long-term kidney function and survival.50 In addition to the biochemical evaluation of efficacy, the study will evaluate HRQoL during treatment with GFB-887 compared with placebo using several different patient-reported and clinician-reported outcomes. First, the FSGS/MCD PRO is a measure designed to support a comprehensive assessment of symptoms and symptom impact in adults with FSGS or TR-MCD.35 Second, the QDIS is a 1-minute HRQoL impact measure with the breadth of widely used functional health and well-being surveys.36 Third, the QGEN survey will measure the following 8 functional health and well-being domains: physical, role (physical or emotional or social) functioning, pain, vitality, general health, and emotional well-being.37 Fourth, the PGI assessment will be self-administered to evaluate severity (PGI-S) of current kidney disease and change (PGI-C) from baseline in severity of kidney disease.38 Finally, the edema ClinRO will be used to assess edema, a debilitating symptom in patients with FSGS/TR-MCD.39 This tool was developed based upon clinician input to enable standardized assessment of edema in clinical and research contexts.
In line with recent progress made in the design of kidney disease trials to ensure that the right patients are selected for treatment,51, 52, 53 the TRACTION-2 study will also use an adaptive study design to ensure that the sample size and study population are guided by emerging efficacy, pharmacodynamics, and safety data. TRACTION-2 will adaptively enroll additional patients if the results of an interim conditional power analysis indicate promising efficacy, or will enrich the study population with Rac1-positive patients, as they may be more likely to respond favorably to treatment. This approach is supported by the FDA for the design of clinical trials intended to support the effectiveness and safety of drugs.32
Some potential limitations of this study must also be taken into consideration. First, although the sample sizes selected for each dose cohort are considered sufficient to evaluate the objectives of the study, larger prospective clinical trials of longer duration will be needed to evaluate the impact of GFB-887 on eGFR and other clinically relevant outcomes. Second, even though Rac1 is considered a key driver of podocyte injury in FSGS/TR-MCD and DN, the heterogeneity of these diseases may limit the ability to detect a treatment effect. Finally, although an adaptive study design is thought to bring forward efficiencies, it can be more challenging to construct, conduct, and analyze.32
The inhibition of TRPC5 is a novel therapeutic approach for proteinuric kidney diseases, supported by promising preclinical data. GFB-887 is the first TRPC5 inhibitor for kidney disease in clinical trials and is the first agent in general to target the podocyte, with the potential to provide long-term kidney protection. TRACTION-2 will explore whether targeted blockade of the TRPC5-Rac1 pathway with GFB-887 may be an efficacious and safe treatment strategy for patients with FSGS, TR-MCD, and DN, and will assess whether baseline urinary Rac1 levels are a useful biomarker to predict response. The information obtained from this phase 2 trial will inform subsequent clinical trial development: namely, whether enrichment of future studies with patients who are most likely to be responsive to GFB-887 is feasible and is likely to improve outcomes. This innovative trial design will be applicable for the evaluation of other precision medicines for kidney diseases.
Disclosure
This study was supported by Goldfinch Bio, Inc., Cambridge, Massachusetts, USA. LW, JFR, CC, GAG, LJ, YMKF, XP, and FSC are employees of Goldfinch Bio, Inc. KP and JRW are paid consultants to Goldfinch Bio, Inc. HT has served as a consultant to and/or a member of a data monitoring committee for Goldfinch Bio, Inc., Travere Therapeutics, Otsuka, and Chemocentryx, and received a research grant from Natera. DSG, HJLH, KRT are consultants to Goldfinch Bio, Inc.
Acknowledgments
This study is funded by Goldfinch Bio, Inc., Cambridge, Massachusetts, USA. The authors wish to thank Agnes Costello of Goldfinch Bio, Inc. for providing significant support to the authors in the coordination and development of this manuscript and critically reviewing this manuscript, John Lawler of Goldfinch Bio, Inc. for leading the clinical operations team for this study, Tingting Ge, formerly of Goldfinch Bio, Inc. for her contributions to this study, and Barbara Gillespie of Covance for her guidance on study design and clinical operations. Cindy C. Taylor of Synterex, Inc. provided medical writing support. Portions of this manuscript were presented at the American Society of Nephrology—Kidney Week, 2020 (virtual): Walsh L, Reilly JF, Cornwall C, et al. Safety and efficacy evaluation of GFB-887, a TRPC5 channel inhibitor, in patients with diabetic nephropathy, focal segmental glomerulosclerosis, or treatment-resistance minimal change disease: phase 2 study design (TRACTION-2) (INFO22). Clinicaltrials.gov Identifier: NCT04387448.
Author Contributions
LW and JFR conceived the study, contributed to its design, and authored substantial parts of the manuscript. GAG and YMKF serve as medical monitors for the trial (eg study conduct, amendments), contributed to study design, and authored parts of the manuscript related to study operations. CC is responsible for day-to-day conduct of the study and study amendments, and provided input during the preparation of the manuscript. DSG, HJLH, LJ, HT, KRT, KP, JRW, XP, and FSC contributed to study design and reviewed and edited the manuscript. All authors reviewed, contributed to, and approved the final version of this manuscript.
Footnotes
Listing of TRACTION-2 Study Sites (United States) as of April 7, 2021
Supplementary Material
Listing of TRACTION-2 Study Sites (United States) as of April 7, 2021
References
- 1.Kitiyakara C., Kopp J.B., Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:172–182. doi: 10.1053/snep.2003.50025. [DOI] [PubMed] [Google Scholar]
- 2.McGrogan A., Franssen C.F.M., de Vries C.S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 3.Hogan J., Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol. 2013;24:702–711. doi: 10.1681/ASN.2012070734. [DOI] [PubMed] [Google Scholar]
- 4.Mason P.D., Hoyer P.F. In: Comprehensive Clinical Nephrology. Floege J., Johnson R.J., Feehally J., editors. Elsevier Saunders; St. Louis, Missouri: 2010. Minimal change nephrotic syndrome; pp. 218–227. [Google Scholar]
- 5.Alicic R.Z., Rooney M.T., Tuttle K.R. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2019. 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States.https://www.usrds.org/annual-data-report/ Available at: [Google Scholar]
- 7.Gipson D.S., Gibson K., Gipson P.E. Therapeutic approach to FSGS in children. Pediatr Nephrol. 2007;22:28–36. doi: 10.1007/s00467-006-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int. 2011;79:678–685. doi: 10.1038/ki.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaudreuil S., Lorenzo H.K., Elias M. Optimal management of primary focal segmental glomerulosclerosis in adults. Int J Nephrol Renovasc Dis. 2017;10:97–107. doi: 10.2147/IJNRD.S126844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C.C., Wang G.H., Lu J. Role of podocyte injury in glomerulosclerosis. Adv Exp Med Biol. 2019;1165:195–232. doi: 10.1007/978-981-13-8871-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown K.D., Campbell C., Roberts G.V. Precision medicine in kidney disease: the patient’s view. Nat Rev Nephrol. 2020;16:625–627. doi: 10.1038/s41581-020-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyatt C.M., Schlondorff D. Precision medicine comes of age in nephrology: identification of novel biomarkers and therapeutic targets for chronic kidney disease. Kidney Int. 2016;89:734–737. doi: 10.1016/j.kint.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Heerspink H.J.L., List J., Perkovic V. New clinical trial designs for establishing drug efficacy and safety in a precision medicine era. Diabetes Obes Metab. 2018;20(suppl 3):14–18. doi: 10.1111/dom.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heerspink H.J.L., Oberbauer R., Perco P. Drugs meeting the molecular basis of diabetic kidney disease: bridging from molecular mechanism to personalized medicine. Nephrol Dial Transplant. 2015;30(suppl 4):iv105–iv112. doi: 10.1093/ndt/gfv210. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Castonguay P., Sidhom E.H. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science. 2017;358:1332–1336. doi: 10.1126/science.aal4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robins R., Baldwin C., Aoudjit L. Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int. 2017;92:349–364. doi: 10.1016/j.kint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Bezzerides V.J., Ramsey I.S., Kotecha S. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 18.Tian D., Jacobo S.M.P., Billing D. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen Y., Shah S., Campbell K.N. Molecular mechanisms of proteinuria in focal segmental glomerulosclerosis. Front Med (Lausanne) 2018;5:98. doi: 10.3389/fmed.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akilesh S., Suleiman H., Yu H. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011;121:4127–4137. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greka A., Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol. 2011;22:1969–1980. doi: 10.1681/ASN.2011040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee H.Y., Saisawat P., Ashraf S. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagon Y., Raghu H., Ge T. Urinary Rac1, a novel predictive biomarker, is elevated in FSGS and diabetic nephropathy patients and reduced by TRPC5 inhibition with GFB-887 in a rat FSGS model (PO1965) J Am Soc Nephrol. 2020;31:607. [Google Scholar]
- 24.Lv Z., Hu M., Fan M. Podocyte-specific Rac1 deficiency ameliorates podocyte damage and proteinuria in STZ-induced diabetic nephropathy in mice. Cell Death Dis. 2018;9:342. doi: 10.1038/s41419-018-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M., Ledeboer M.W., Daniels M. Discovery of a potent and selective TRPC5 inhibitor, efficacious in a focal segmental glomerulosclerosis model. ACS Med Chem Lett. 2019;10:1579–1585. doi: 10.1021/acsmedchemlett.9b00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaldecker T., Kim S., Tarabanis C. Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest. 2013;123:5298–5309. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riccio A., Li Y., Moon J. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly J.F., Coeffet-LeGal M.F., Pan-Zhou X. GFB-887, a small molecule inhibitor of TRPC5, attenuates proteinuria in animal models of FSGS, minimal change disease and diabetic nephropathy (TH-PO1063) J Am Soc Nephrol. 2019;30:398. [Google Scholar]
- 29.Westerling-Bui A., Soare T.W., Venkatachalan S. A translational kidney organoid system bolsters human relevance of clinical development candidate. bioRxiv. 2019;12:30.891440. [Google Scholar]
- 30.Westerling-Bui A.D., Beconi M., Hoang H.G. Small molecule inhibition of TRPC5 protects against podocyte injury and proteinuria in FSGS (SA-PO317) J Am Soc Nephrol. 2018;29:817. [Google Scholar]
- 31.Walsh L., Lynam C., Johnson L. GFB-887, a TRPC5 inhibitor, is safe and well tolerated and engages the TRPC5 target leading to reductions in urinary Rac1 in healthy subjects (PO1886) J Am Soc Nephrol. 2020;31:586. [Google Scholar]
- 32.US Food and Drug Administration . 2019. Adaptive designs for clinical trials of drugs and biologics guidance for industry.https://www.regulations.gov/docket/FDA-2018-D-3124 Available at: [Google Scholar]
- 33.Faul C., Donnelly M., Merscher-Gomez S. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troost J.P., Trachtman H., Nachman P.H. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13:414–421. doi: 10.2215/CJN.04780517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlozzi N.E., Trachtman H., Walsh L. FSGS minimal change disease patient-reported outcome (PRO) measure development (TH-PO1000) J Am Soc Nephrol. 2019;30:380. [Google Scholar]
- 36.Ware J.E., Jr., Gandek B., Guyer R. Standardizing disease-specific quality of life measures across multiple chronic conditions: development and initial evaluation of the QOL Disease Impact Scale (QDIS®) Health Qual Life Outcomes. 2016;14:84. doi: 10.1186/s12955-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware J.E., Jr., Gandek B. Improving single-item generic health survey measures. Qual Life Res. 2017;26:43–44. [Google Scholar]
- 38.Guy W., ECDEU Assessment manual for psychopharmacology . U.S. Dept. of Health, Education, and Welfare; Rockville, MD: 1976. U.S. Dept. of Health, Education, and Welfare, publication no. 76-338. [Google Scholar]
- 39.Carlozzi N.E., Trachtman H., Walsh L. Development of a clinician-reported outcome measure for edema assessment (TH-PO1047) J Am Soc Nephrol. 2019;30:393. [Google Scholar]
- 40.Cattran D.C., Appel G.B., Hebert L.A. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 41.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koomen J.V., Stevens J., Mostafa N.M. Determining the optimal dose of atrasentan by evaluating the exposure-response relationships of albuminuria and bodyweight. Diabetes Obes Metab. 2018;20:2019–2022. doi: 10.1111/dom.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson F., Lewis J.B., Lewis E.J. Impact of baseline renal function on the efficacy and safety of aliskiren added to losartan in patients with type 2 diabetes and nephropathy. Diabetes Care. 2010;33:2304–2309. doi: 10.2337/dc10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coyne D.W., Andress D.L., Amdahl M.J. Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant. 2013;28:2260–2268. doi: 10.1093/ndt/gft227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberman K.V., Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996;7:56–63. doi: 10.1681/ASN.V7156. [DOI] [PubMed] [Google Scholar]
- 46.Lusco M.A., Fogo A.B., Najafian B. AJKD atlas of renal pathology: calcineurin inhibitor nephrotoxicity. Am J Kidney Dis. 2017;69:e21–e22. doi: 10.1053/j.ajkd.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Korbet S.M. The treatment of primary focal segmental glomerulosclerosis. Ren Fail. 2000;22:685–696. doi: 10.1081/jdi-100101956. [DOI] [PubMed] [Google Scholar]
- 48.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 49.Waldman M., Crew R.J., Valeri A. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–453. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 50.Mathias S.D., Vallow S., Gipson D.S. Development of focal segmental glomerulosclerosis patient-reported outcome measures: symptom diary and symptom impact questionnaire. Am J Kidney Dis. 2017;70:532–540. doi: 10.1053/j.ajkd.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 51.Heerspink H.J.L., Andress D.L., Bakris G. Rationale and protocol of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018;20:1369–1376. doi: 10.1111/dom.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Library of Medicine (U.S.) ClinicalTrials.gov. Tumor necrosis factor inhibition in focal segmental glomerulosclerosis and treatment resistant minimal change disease. Identifier: NCT04009668. 2021. https://clinicaltrials.gov/ct2/show/NCT04009668 Available at:
- 53.National Library of Medicine (U.S.) ClinicalTrials.gov. Phase 2a study of VX-147 in adults with APOL1-mediated focal segmental glomerulosclerosis. Identifier: NCT04340362. 2021. https://clinicaltrials.gov/ct2/show/NCT04340362 Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.