Abstract
Introduction
Familial hyperkalemic hypertension is a rare inherited form of arterial hypertension. Four genes are responsible for this disease, the variants of these genes cause disruption in the regulation of ion transport in the distal renal tubule. Whether the genotype explains the large phenotypic heterogeneity has not been fully explored.
Methods
We retrospectively analyzed clinical and genetic data of 153 cases (84 probands, 69 relatives) with familial hyperkalemic hypertension.
Results
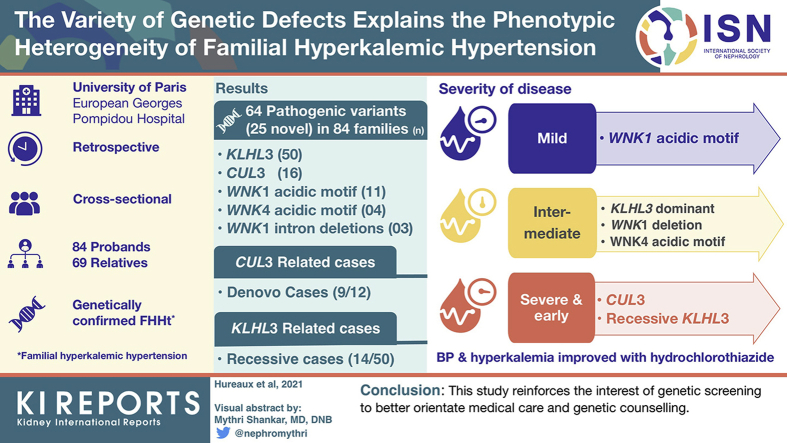
Pathogenic variants (25 novel variants) were identified as follows: KLHL3 (n = 50), CUL3 (n = 16), WNK1 acidic motif (n = 11), WNK4 acidic motif (n = 4) and WNK1 intron 1 deletions (n = 3). De novo cases were mainly observed in the CUL3-related cases (9 of 12) and recessive cases were only observed in KLHL3-related cases (14 of 50). More severe forms were observed in recessive KLHL3 and CUL3 cases that were also associated with growth retardation. Patients with WNK1 acidic motif variants had a typical biological phenotype and lower frequency of hypertension conversely to WNK4 variants affecting the same motif. Patients with heterozygous KLHL3 and WNK1 deletions had milder forms. Familial screening in 178 relatives allowed detection and care for 69 positive cases. Blood pressure and hyperkalemia were improved by hydrochlorothiazide in all groups.
Conclusions
This study confirms the phenotypic variability ranging from the severe and early forms associated with CUL3 and recessive KLHL3 genotypes through intermediate forms associated with KLHL3 dominant, WNK4 and WNK1 deletion to mild form associated with WNK1 acidic motif genotype and reinforces the interest of genetic screening to better orientate medical care and genetic counseling.
Keywords: aldosterone, arterial hypertension, distal nephron, genetics, potassium
Graphical abstract
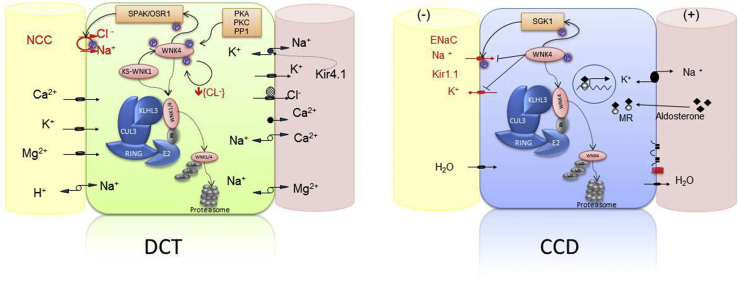
Familial hyperkalemic hypertension (FHHt), also known as Gordon syndrome or pseudohypoaldosteronism type 2, is a rare form of inherited arterial hypertension associated with hyperkalemia, hyperchloremic metabolic acidosis, and high sensitivity to thiazide diuretics. Clinically described from the 1960s,1,2,3 linkage studies first identified WNK1 and WNK44 as the genetic basis of FHHt. The with-no-lysine kinases (WNKs) were found to be instrumental in fine tuning potassium, sodium, and chloride metabolism in the aldosterone sensitive nephron.5 Ten years later, Boyden et al.6 and Louis-Dit-Picard et al.7 identified genes KLHL3 and CUL3 as responsible of the remaining forms of the disease. These genes encode proteins participating in the E3 ligase ubiquitination system. Kelch-like 3 is a member of substrate adaptators for proteins degraded in the proteasome through interaction with cullin-3–based ubiquitin-ligase complexes.8 Kelch-like 3 is exquisitely expressed in the renal distal tubule and has been identified as a substrate adaptator for WNK1 and WNK4.9 FHHt-related KLHL3 variants alter this interaction, thus decreasing WNKs degradation by the proteasome, leading to increased activity of the Ste20p-related Proline Alanine-rich Kinase which activates the sodium-chloride cotransport in distal convoluted tubule and secondary effects on renal outer medullary potassium channel and epithelial sodium channel in cortical collecting duct.8 Cullin-3 is an ubiquitously expressed protein and a key component of cullin RING-ligase complexes, implicated in the ubiquitination of other proteins. An animal model bearing a mutation similar to that observed in CUL3-related FHHt also suggests a vascular effect.10, 11, 12, 13 The alteration of any member of this pathway (WNK1, WNK4, KLHL3, and CUL3) could result in a common phenotype, which severity and specificity depend upon the specific expression of the altered protein and its possible interaction with others.8,14,15 Figure 1 summarizes the interaction and effects of these proteins in the distal convoluted tubule and cortical collecting duct cells.
Figure 1.
Schematic representations of a cell of the distal convoluted tubule (DCT), and a principal cell of the cortical collecting duct (CCD). Inside each cell, the interaction of proteins implicated in the pathophysiology of the familial hyperkalemic hypertension is shown. In the DCT, the activity of the sodium chloride cotransporter (NCC) is maintained via a phosphorylation cascade implicating Ste20p-related Proline Alanine-rich Kinase (SPAK)/OSR/WNKs. WNK4 is active in its phosphorylated form; it can be phosphorylated by several kinases (PKC, PKA, PP1, and KS-WNK1) and is also autophosphorylated when the intracellular chloride concentration is low. Activated WNK4 phosphorylates SPAK/OSR, which, in turn, phosphorylates NCC increasing the apical NaCl reabsorption. WNK1/4 protein levels are regulated by the complex CUL3-KLHL3-E3, which targets WNK1/4 for degradation by promoting their ubiquitylation. KLHL3 binds to the acidic motif of WNK kinases through its propeller domain. Mutations in the acidic motif of WNK1/4 disrupt its interaction with KLHL3 and prevent WNK4 degradation. Mutations in KLHL3 impair either KLHL3 binding to WNK1/4 or KLHL3 binding to CUL3. Finally, mutations in CUL3 seem to modify the interaction with the COP9 signalosome enzyme, resulting in hyperneddylation and increasing ubiquitin ligase activity directed toward the substrate adaptor KLHL3. The final result is the accumulation of WNK4 with the subsequent NCC phosphorylation and increase of NaCl reabsorption. In the principal cells of the CCD, WNK4 has been implicated as a negative regulator of Na reabsorption and K secretion by epithelial sodium channel (ENaC) and renal outer medullary potassium channel (ROMK) channels, respectively.
This study aimed to (1) compare FHHt patients’ phenotypes according to the implicated gene in dominant and recessive forms, and establish a genotype-phenotype correlation based on the analysis of clinical and biological data from a cohort of 153 FHHt patients; (2) present new cases of FHHt caused by CUL3 pathogenic variants; and (3) discuss the presence of gene dosage effect of KLHL3 pathogenic variants.
Methods
Patients
In this cross-sectional study, we retrospectively analyzed 153 patients (84 probands, 69 relatives) with genetic confirmation of FHHt in our center. Analysis was performed after obtaining consent in accordance with French bioethics law of 2004.
Genetic Analysis
Genetic analyses were performed by Sanger sequencing, except for 20 families whose cases were received after 2015 and who were analyzed using next-generation sequencing. For Sanger sequencing, exons and flanking intron sequences were amplified by polymerase chain reaction, sequenced with BigDye Terminator v3.1 cycle sequencing kits, and run on ABI Prism 3730XL DNA Analyzer (Perkin Elmer Applied Biosystems, Foster City, CA); DNA variants were identified with Sequencher software, by comparison with the reference sequences (NM_018979.2 for WNK1, NM_032387.4 for WNK4, NM_017415.2 for KLHL3, and NM_003590.4 for CUL3). For next-generation sequencing, library preparation, massive parallel sequencing, and bioinformatics analysis were performed using a panel of genes as previously described.16,17 Identified variants were confirmed in both cases on a second sample by Sanger sequencing, and classified according to the American College of Medical Genetics (ACMG).18 In silico analysis of variants was performed using Alamut V.2.5.1 software (Interactive Biosoftware, Rouen, France). To exclude intron 1 deletion for samples analyzed by Sanger, we performed a quantitative polymerase chain reaction of WNK1 exon and intron 1, using forward primer (5’-tagctgggactacagg-3’) and reverse primer (5’-cctgtagtcccagcta-3’), on a Quant studio 7 flex system (Real Time PCR, Thermo Fisher Scientific, Waltham, Massachusetts, USA). Results were by analyzed by CopyCaller Software v2.0 software (Thermo Fisher Scientific), using the ΔΔCt methods.
Clinical Analysis
Clinical and biological data were analyzed based on questionnaires filled out by the prescribing physicians describing the basal phenotype and the phenotype under hydrochlorothiazide (HCTZ) treatment. To evaluate growth parameters, WHO charts were adjusted to age and sex; growth failure was defined by a Z-score of height and/or of weight inferior or equal to -2 SDs. As our population included infants, children, and adults, classification of the values of systolic blood pressure and diastolic blood pressure into blood pressure (BP) stages adapted to age was used following the European recommendations.19,20 As the designations of the hypertension stages were different in children and adults, five categories were designated (Supplementary Table S1); categories 3, 4, and 5 correspond in children to hypertension, stage 1 hypertension, and stage 2 hypertension, respectively, and in adults to grade 1, grade 2, and grade 3 hypertension, respectively.
Statistical Analysis
Results are reported as medians and interquartile ranges (IQRs) for continuous variables and frequency for categorical variables; quantitative values were compared by Kruskal-Wallis test and post hoc Dunn’s multiple comparison tests using Prism V6 (GraphPad Software, Inc., San Diego, CA, USA); P values were adjusted to account for multiple comparisons. When more than 50% of the data was missing in one group for a given variable, this group was excluded from the analysis. The association between delta of K+ and the basal plasma potassium level was studied by linear regression. Because for some genotypes there were no patients in one or more categories, categories 1 and 2 were grouped as “normal” BP and 3 to 5 were grouped as hypertensive in order to compare using chi square or Fishers exact tests.
Results
Genetic Analysis: The KLHL3 Gene is the Most Frequently Affected Gene
Clinical and genetic characteristics were analyzed in a cohort of 84 families harboring pathogenic mutations in one of the four known genes responsible for FHHt. This cohort contained 153 affected patients, including 84 index cases and 69 affected relatives.
We identified 64 pathogenic (class 5) or probably pathogenic (class 4) variants, (Supplementary Table S2). Among them, 25 mutations (39%) were not previously described. In silico prediction of the new missense and splicing variants, and segregation analysis in the families harboring these variants are shown in Supplementary Tables S3a and S3b.
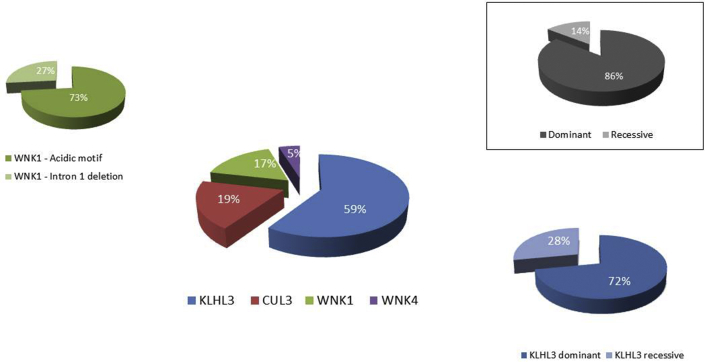
Among the 84 families, the most commonly implicated gene was KLHL3 (n = 50, 60%) followed by CUL3 (n = 16, 19%), WNK1 (n = 14, 17%), and WNK4 (n = 4, 5%). Whereas dominant inheritance was observed for the CUL3, WNK1, and WNK4 genes, KLHL3 variants were associated with dominant (n = 36, 72%) and recessive inheritance (n = 14, 28%) (Figure 2). Genotype distribution in adult and pediatric populations is shown in Supplementary Figure S1. Globally, dominant inheritance was associated with all four genes and represented 83% of cases (Figure 2).
Figure 2.
Genetics of familial hyperkalemic hypertension in pie chart representation according to the involved gene and inheritance mode.
Variants at the KLHL3 Gene
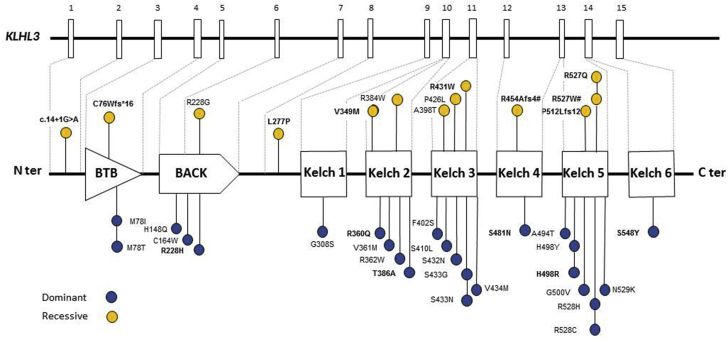
A total of 38 likely pathogenic or pathogenic variants were found located along the gene and affecting all domains (KELCH, BACK, and BTB) (Figure 3, Supplementary Table S2). Twenty-five different missense variants were detected in 50 families with dominant inheritance (KLHL3 autosomal dominant [AD]) and 13 variants (9 missense, 3 frameshift, and 1 splicing) in 14 families with recessive inheritance (KLHL3 autosomal recessive [AR]). Segregation studies were performed in 11 families with the dominant form; in four of them the mutation appeared de novo. Genetic screening of relatives allowed the identification of six additional heterozygous patients. Among the 14 KLHL3 AR families, 13 were homozygous and one compound heterozygous. Familial history was available for 10 of 13 homozygous patients and consanguinity was present in eight families. Segregation studies were performed in nine of 14 families including the one compound heterozygous case. In all, parents were heterozygous for one or the familial variant. These studies identified two additional homozygous affected relatives.
Figure 3.
Schematic representation of the KLHL3 gene and protein with the several domains BTB, BACK and the KELCH domains, and pathogenic variants according the recessive (yellow circles) or dominant (blue circles) mode of transmission. Thirty-eight pathogenic variants were found in KLHL3 gene, including 25 in the dominant form and 13 in the recessive form. Dominant and recessive variants are mostly missense variants, distributed all along the gene, on the different domains BTB, BACK and KELCH domain. #Variants detected in the compound heterozygous patient. Variants not previously described are highlighted in bold.
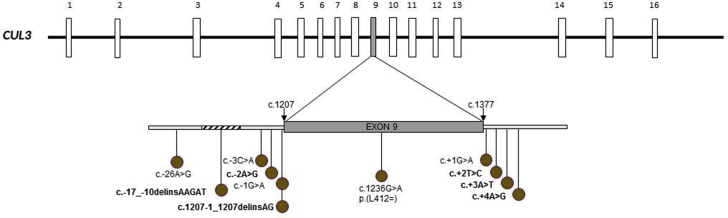
Exon 9 Splice Variants of CUL3 Gene
Eleven different pathogenic or likely pathogenic variants were found located in the exon 9 or in the two adjacent introns of the CUL3 gene. All variants detected were splice mutations causing deletion of CUL3 exon 9 encoding a 57 amino acid sequence (Figure 4, Supplementary Table S2). Five variants were not previously described. Of all the variants, four were found in two or three unrelated families; nine were substitution mutations; and two were deletion-insertion variants. We identified heterozygous de novo variants in nine of 12 families (75%) in whom segregation studies could be performed; variants were inherited from the mother in two families and from the father in one family. Parent DNA was not available for the remaining four families. Familial studies allowed the identification of three affected relatives.
Figure 4.
Schematic representation of the CUL3 gene and its 20 exons. Eleven different pathogenic variants were found in 13 families. All CUL3 variants are located in regions affecting splicing of exon 9 and causing deletion of CUL3 exon 9 encoding a 57–amino acid sequence. Variants not previously described are highlighted in bold.
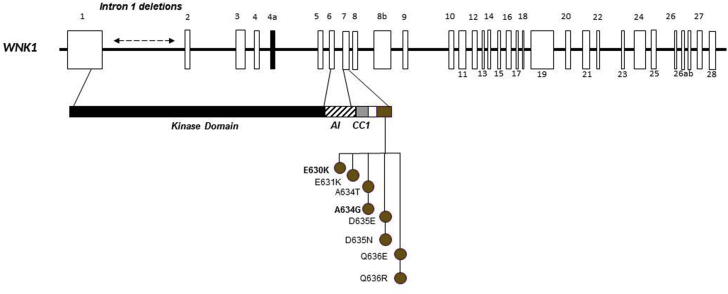
Two Types of WNK1 Variants
Eleven likely pathogenic or pathogenic variants were found in 14 families; eight of which were at exon 7, in the acidic motif. This motif corresponds to 10 amino acids negatively charged "EPEEPEADQH" downstream of a coiled-coil domain. Most missense variants and families were recently reported21; here, we describe two novel variants. The three other variants correspond to large intron 1 deletion of different sizes (21 kb, 23 kb, and 41 kb) (Figure 5), located in the kinase domain of WNK1. The first family was described by Archard et al.,22 and the corresponding deletion was described in the seminal paper identifying WNK1 and WNK4 as causal genes from Wilson et al.4 The two other intron 1 deletions and corresponding families were not previously described. Segregation studies were performed for eight families, identifying 18 affected relatives with variants in the acidic motif and 20 affected relatives harboring intron 1 deletion.
Figure 5.
Schematic representation of the WNK1 gene and its 28 exons and location of pathogenic variants. Twelve pathogenic variants were found in 13 families. There are nine missense variants located in acidic motif and three large intronic 1 deletions of 23 kb, 21 kb, and 41 kb. Variants not previously described are highlighted in bold.
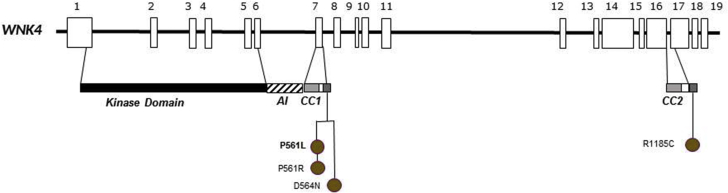
Missense WNK4 Variants
Four different missense variants, one novel, were detected in the WNK4 gene in four families; three variants are located at exon 7 in the acidic motif of this gene (Figure 6). The fourth missense variant, also changing-charge residue, was located downstream at the putative second coiled-coil domain. Segregations studies performed in three families identified six affected relatives.
Figure 6.
Schematic representation of the WNK4 gene and its 19 exons and location of pathogenic variants. Four pathogenic variants were found in four families. All variants are missense variants located in acidic motif. Variants not previously described are highlighted in bold.
The More Severe Phenotypes Are Occurring in CUL3 or Recessive KLHL3 Cases
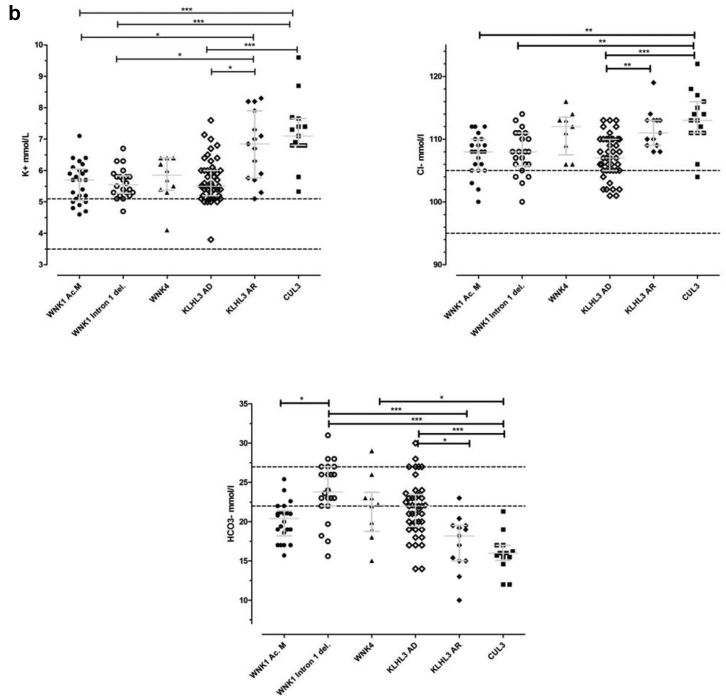
As expected, severe forms of FHHt were observed in CUL3 de novo or dominant cases. Nineteen patients were identified with the CUL3 variant genotype, including 16 probands and three affected relatives, with an equal occurrence of male (n = 9) and female (n = 10) subjects. Phenotype characteristics showed normal values for sodium, estimated glomerular filtration rate, total calcium, magnesium, low renin values, and normal or low aldosterone values (CUL3 Table 1 and Supplementary Table S4). Probands had a median age at onset of symptoms (failure to thrive, malaise, or paralysis) of 5.5 (IQR: 2.0 to 14.3) years. Hyperchloremia (Cl- >107 mmol/l) was present in 86.6% (n = 13 of 15; 4 missing values), with median of 113 (IQR: 111 to 116) mmol/l, hyperkalemia (K+ >5 mmol/l) in all patients with median of 7.1 (IQR: 6.8 to 7.6) mmol/l, and high BP stage 3, 4, or 5 in 93% of cases. Growth failure phenotype was observed in 71% of the patients (n = 10 of 14 patients, 5 missing values), concerning predominantly height, the median Z-score for height and weight was -2 SD (IQR: -2.25 to -0.75) and -1.25 SD (IQR: -2.5 to -0.75), respectively (Supplementary Table S4). Individual values of all parameters in CUL3 group are detailed in Supplementary Tables S4a and S4b.
Table 1.
Biological characteristics of the 153 familial hyperkalemic hypertension patients according to their genotype
| All patients (N = 153) | Normal values (when applicable) |
CUL3 n = 19 |
KLHL3 AR n = 16 |
KLHL3 AD n = 56 |
WNK4 n = 10 |
WNK1 intron 1 del. n = 23 |
WNK1 acidic motif n = 29 |
P (Kruskal-Wallis test) |
|---|---|---|---|---|---|---|---|---|
| Sex M/F | 9/10 | 13/3 | 23/33 | 5/5 | 9/14 | 13/16 | ||
| age at work-up, years | 7 [2-16]Hν | 9 [4-26] | 33 [19-52] ν | 38 [13-43] | 36 [17-49] H | 27 [6-45] | <0.0001 | |
| age at discovery∗ | 5.5 [2.0-14.3]N | 5.5 [2.5-31.7] | 24.0 [11.0-44.0]N | 19.0 [15.8 -27.5] | 36.0 [8.0-37.0] | 16.5 [4.8-24.25] | 0.0308 | |
| Na+, mmol/l | 136-145 | 138 [136-140]7 | 138 [136-139]3 | 139 [137-140]7 | 139 [137-141] | 139 [139-140]1 | 140 [139-141]7 | 0.04 |
| K+, mmol/l | 3.5-5.1 | 7.1 [6.8-7.6]4€Hν | 6.9 [5.8-8.0]1Δθμ | 5.5 [5.2-6.0]9μν | 5.9 [5.4-6.4] | 5.5 [5.3-5.8]1θH | 5.7 [5.1-6.1]4Δ€ | <0.0001 |
| Cl-, mmol/l | 95-105 | 113 [111-116]4Eην | 111 [109-113]3M | 107 [105-110]11Mν | 112 [108-114]1 | 108 [106-111]1η | 108 [106-110]7E | <0.0001 |
| HCO3-, mmol/l | 22.0-27.0 | 16.0 [15.1-17.0]6Hνλ | 18.2 [15.0-19.5]3Θμ | 22.0 [19.4-23.5]13μν | 22.1 [18.8-23.8]λ | 23.8 [22.0-27.0]1ΘHα | 19.7 [18.2-21.1]7α | <0.0001 |
| eGFR, ml/min per 1.73 m2∗∗ | >90 | 117 [100-131]10 | 108 [104-193]8 | 94 [82-108]21 | 96 [84-138]1 | 88 [58-139]4 | 94 [73-124]11 | nd |
| Protides, g/l | 63-78 | 70 [60-73]12 | 72 [66-83]12 | 70 [65-73]11 | 69 [67-70] | 69 [65-71]2 | 66 [64-73]10 | nd |
| Uric acid, μmol/l | 137-393 | 225 [170-369]13 | 291 [291-316]12 | 279 [166-320]47 | 222 [177-311]5 | 182 [182-182]22 | 178 [160-193]14 | nd |
| Total calcium, mmol/l | 2.09-2.52 | 2.38 [2.30-2.50]13 | 2.31 [2.20-2.40]11 | 2.32 [2.21-2.44]34 | 2.31 [2.27-2.40] | 2.48 [2.48-2.48]22 | 2.32 [2.21-2.40]8 | nd |
| Magnesium, mmol/l | 0.64-0.90 | 0.86 [0.84-0.89]17 | 0.73 [0.70-0.90]12 | 0.82 [0.75-0.86]49 | 0.86 [0.86-0.86]9 | 0.69 [0.66-0.79]19 | 0.79 [0.76-0.81]22 | nd |
| Renin lying, pg/ml ∗∗∗ | 1.5-17.0 | 2.5 [0.6-2.9]12 | 2.7 [1.5-4.4]9 | 3.3 [1.4-5.0]35 | 6.1 [3.4-10.0]3 | 5 [2.8-9.0]13 | 2.3 [0.8-5.1]16 | nd |
| Aldosterone lying, pg/ml (RIA)∗∗∗ | 40-200 | 102 [28-514]9 | 50 [21-148]7 | 56 [37-127]30 | 190 [41-258]2 | 105 [60-207]12 | 188 [69-242]12 | nd |
AD, autosomal dominant; AR, autosomal recessive; del., deletion; eGFR, estimated glomerular filtration rate; F, female; M, male; nd, not done; RIA, radioimmunoassay.
Values are given in Median [interquartile range]. The number of missing values is after the brackets.
KLHL3 AD vs CUL3 with ∗: N, with ∗∗∗: ν ;
WNK1 acidic motif vs KLHL3 AR with ∗:Δ;
WNK1 acidic motif vs CUL3 with ∗∗: Ε and with ∗∗∗:€;
WNK1 intron 1 deletion vs KLHL3 AR with ∗: θ and with ∗∗∗: Θ;
WNK1 intron 1 deletion vs CUL3 with ∗∗: η and with ∗∗∗:Η;
KLHL3 AD vs KLHL3 AR with ∗: μ; with ∗∗:M
WNK1 acidic motif vs WNK1 intron 1 deletion with ∗: α;
WNK4 vs CUL3 with ∗: λ.
age at discovery for index patients only.
eGFR for estimated Glomerular Filtration Rate, using Modification of Diet in Renal Disease to estimate the clearance of creatinine for patients >18 years old; using Schwartz formula for patients < 18 years old.
Renin and Aldosterone lying excepted newborns. P values correspond to Kruskal-Wallis test. Statistical significance is given by superscript after square brackets as follow: ∗is P < 0.1; ∗∗is P < 0.01; ∗∗∗is P< 0.001 (P values were adjusted to account for multiple comparisons).
The 14 KLHL3 recessive homozygous or compound heterozygous cases had a marked FHHt phenotype associating a strong hyperkalemia (6.9 [IQR: 5.8 to 8.0] mmol/l) and a marked hyperchloremic acidosis (HCO3- = 18.2 [IQR: 15.0 to 19.5] mmol/l and Cl- = 111 [IQR: 109 to 113] mmol/l) accompanied by a normal-low renin level (2.7 [IQR: 1.5 to 4.7] mU/ml). Clinically, patients were young (age at discovery 5.5 [IQR: 2.5 to 31.7] years), with high BP (stage 3, 4, or 5 in 67% of cases). Growth failure was observed in half of patients from whom we had relevant information (n = 3 of 6).
Comparison Between Patients With KLHL3 Dominant Forms and Their Corresponding Wild-Type Relatives With Patients With Recessive Forms and Their Corresponding Heterozygous Relatives
KLHL3 Dominant Heterozygous Cases
Fifty-six patients were identified with KLHL3 dominant variant, including 36 index cases and 20 affected relatives, with a predominance of women (23 males and 33 women). As for all genotypes, plasma sodium, calcium, magnesium, estimated glomerular filtration rate, proteins, and uric acid were normal, except mild hyperkalemia (5.5 [IQR:5.2 to 6.0] mmol/l) and hyperchloremic acidosis (HCO3- = 22.0 [IQR: 19.4 to 23.5] mmol/l; Cl- = 107[IQR: 105 to 110] mmol/l) accompanied by a normal-low plasma renin level (3.3 [IQR: 1.4 to 5.0] mU/ml). Probands had a median age at discovery of symptoms at 24 (IQR: 11 to 44) years. Patients with normal to normal-high BP (stage 1 or 2) represented 46%. Growth failure concerned 34% of patients.
Clinical experience from our center, combined with published results6,23 suggested the absence or a very moderate FHHt phenotype in heterozygous relatives in families with KLHL3 AR. Expression variability was studied by comparing clinical and biological phenotypes of the following four groups: (1) relatives with wild-type of KLHL3 AD; (2) relatives heterozygous for KLHL3 AR; (3) patients with KLHL3 AD; and (4) patients (homozygous or compound heterozygous) with KLHL3 AR. Statistical significant differences were observed between these four groups for the following parameters: age at workup, plasma potassium, chlorides, and bicarbonates, with, in order of severity: patients with the KLHL3 AR form > patients with KLHL3 AD > relatives heterozygous for KLHL3 AR > relatives with wild-type KLHL3 AD (Table 2, Figure 7a and b).
Table 2.
Phenotype of the four groups of patients
| Clinical data | Patients KLHL3 AR (n = 16) | Patients KLHL3 AD (n = 56) | Relatives heterozygous KLHL3 AR (n = 13 with available data) | Relatives wild-type of KLHL3 AD (n = 11) | P (Kruskal-Wallis test) |
|---|---|---|---|---|---|
| Sex M/F | 13/3 | 23/33 | 5/8 | 5/6 | |
| Age at workup, years | 6 [4-26] α1 | 33 [19-52] α 1 | 16 [4-31] | 40 [12-60] | 0.0165 |
| n | 13 | 54 | 13 | 11 | |
| K+, mmol/l | 6.9 [5.8-7.9] β 3γ 3 | 5.5 [5.2-6.0] δ 3 ε 3 | 4.6 [4.3-5.0] β 3 δ 3 | 4.2 [3.7-4.5] γ 3 ε 3 | <0.0001 |
| n | 15 | 47 | 13 | 11 | |
| Cl-, mmol/l | 111 [109-113] α 1 β 3γ 3 | 107 [105-110] α 1δ 2 | 103 [100-104.5] β 3 δ 2 | 101 [100-105] γ 3 | <0.0001 |
| n | 13 | 43 | 13 | 8 | |
| HCO3-, mmol/l | 18 [15-20] α 2γ 3 | 22 [19-23] α 2 | 22 [19-25] | 27 [26-29] γ 3 | 0.0001 |
| N | 13 | 42 | 12 | 8 | |
| SBP >15 years old | 154 [130-163] | 142 [121-157] | 124 [116-135] | 117 [96-133] | ns |
| n | 7 | 37 | 5 | 4 | |
| DBP >15 years old | 85 [83-113] | 82 [76-95] γ 1 | 67 [62-90] | 64 [54-80] γ 1 | 0.0104 |
| n | 7 | 36 | 5 | 4 | |
| Z-score weight | 0.0 [0.0-0.2] | 1.3 [0.0-1.7] | 0.0 [-2.0-0.3] | 0.0 [0.0-2.0] | ns |
| 6 | 33 | 3 | 3 | ||
| Z-score height | -1.5 [-2.5 to 0.4] | -1.5 [-2.0 to -1.0] | -1 [-1.5 to -0.5] | 0.0 [-1 to 3.0] | ns |
| n | 6 | 32 | 3 | 3 |
AD, autosomal dominant; AR, autosomal recessive; DBP, diastolic blood pressure; F, female; M, male; ns, not significant; SBP, systolic blood pressure.
Values are given in Median [interquartile range], n correspond to the number of values. Statistical significance is given by superscript after square brackets as follow: Patients KLHL3 AR vs Patients KLHL3 AD :α, Patients KLHL3 AR vs Relatives heterozygous of KLHL3 AR:β; Patients KLHL3 AR vs Relatives Wild-type KLHL3:γ; Patients KLHL3 AD vs Relatives heterozygous of KLHL3 AR:δ; Patients KLHL3 AD vs Relatives Wild-type of KLHL3:ε; Relatives heterozygous of KLHL3 AR vs Relatives Wild-type of KLHL3 AD: f.∗ is 1, ∗∗ is 2, ∗∗∗ is 3 and ∗ is P < 0.1; ∗∗ is P < 0.01; ∗∗∗ is P < 0.001.
Figure 7.
Graphic representation of phenotypic difference between patients who are KLHL3 autosomal recessive (AR), patients who are KLHL3 autosomal dominant (AD), heterozygous relatives of KLHL3 AR, and wild-type relatives KLHL3 AD. (a) Age at workup and Z-scores for weight and height. (b) Values of systolic and diastolic blood pressure. Values are expressed as median and interquartile range in grey bars, and normal values in dotted lines. P values: ∗ is P < 0.1; ∗∗ is P < 0.01; ∗∗∗ is P < 0.001.
Variable Phenotype Caused by Genetic Defects in the WNK1 and WNK4 Genes
The WNK1 group encompassed 29 patients including 11 index cases with WNK1 acidic motif variants and 23 patients including three index cases with WNK1 intron 1 deletion. Both intron 1 deletion and acidic motif showed a mild and similar phenotype. However, in patients with the WNK1 acidic motif variants, acidosis was more pronounced (bicarbonates 20.4 [IQR: 18.2 to 21.4] versus 23.8 [IQR: 22.0 to 27.0] mmol/l), and age at first symptoms was younger for WNK1 acidic motif (16.5 [IQR: 4.8 to 24.3] years versus 36.0 [IQR: 8.0 to 37.0] years). Patients with high BP stage 3, 4, or 5 represented 39% in WNK1 acidic motif, and 43% in WNK1 intron 1 deletion. Growth failure concerned 28% for acidic motif and 11% for intron 1 deletion.
The WNK4 group involved 10 patients including four index cases. They had typical FHHt phenotype, with hyperkalemia (5.9 [IQR: 5.4 to 6.4] mmol/l), hyperchloremic acidosis (HCO3-= 22.1 [IQR: 18.8 to 23.8] mmol/l, Cl- = 112 [IQR: 108 to 114] mmol/l) and high BP (67% had stage 3, 4, or 5). For the six patients with available growth data during infancy, none had growth failure.
Overall Comparison Between Groups Classified by Genes
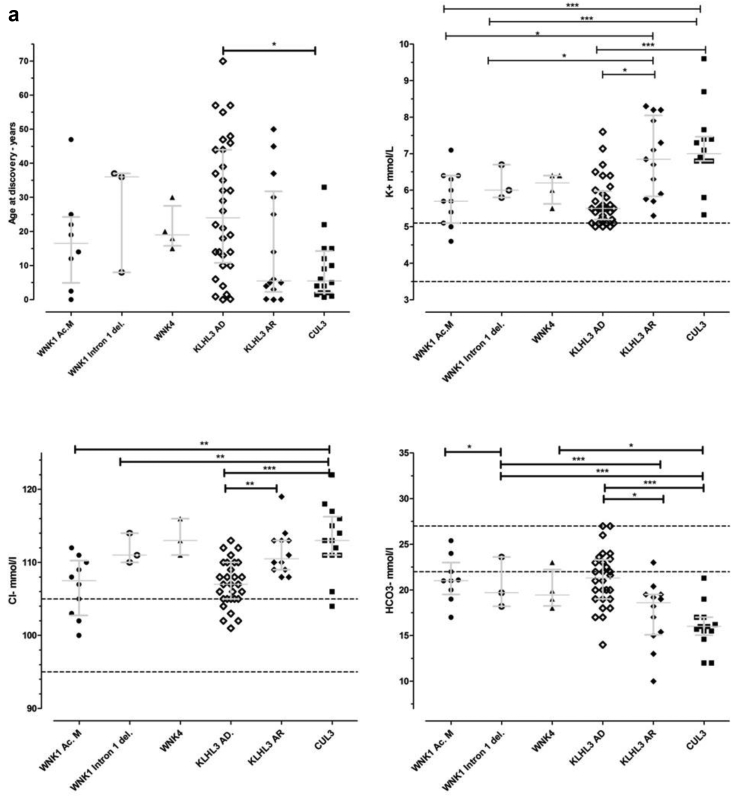
Statistically significant differences were observed between groups for the following variables: age at onset, potassium, chloride, and bicarbonates plasma concentrations (Table 1, Figure 8a and 8b). The most contrasted were the CUL3 and KLHL3 AD. A significant difference was also observed between CUL3 and WNK1 intron 1 deletion for K+, Cl-, and HCO3-. CUL3 versus WNK1 acidic motif showed significant difference for K+ and Cl- and CUL3 versus WNK4 only for HCO3-. The most severe phenotype was associated to CUL3 genotype closely followed by KLHL3 AR form in comparison to the four other genotypes, in which the severity hierarchy differed depending on the parameter.
Figure 8.
(a) Graphic representation of age at discovery and biochemical basal values of the six groups of probands with familial hyperkalemic hypertension. (b) Graphic representation of age at discovery and biochemical basal values of the six groups of probands and affected relatives with familial hyperkalemic hypertension. Median and interquartile range are shown as grey bars, and normal values as dotted lines. P values: ∗ is P < 0.1; ∗∗ is P < 0.01; ∗∗∗ is P < 0.001. Groups: ● WNK1 acidic motif;  WNK1 intron 1 deletion; ▲ WNK4;
WNK1 intron 1 deletion; ▲ WNK4;  KLHL3 autosomal dominant; ○ KLHL3 autosomal recessive; and ▪ CUL3.
KLHL3 autosomal dominant; ○ KLHL3 autosomal recessive; and ▪ CUL3.
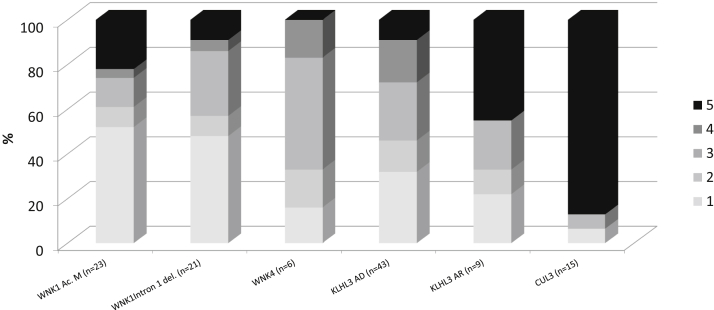
We compared the proportion of each stage of BP between the different genotypes. The KLHL3 AR group and CUL3 group presented a high percentage of grade 5 hypertension (45% and 87%, respectively) compared to others groups (Figure 9). When BP categories were classed in normal (categories 1 and 2) and hypertension (categories 3 to 5), the comparison between genotypes showed a statistically significant difference (P = 0.02). The P value was lower (P = 0.0024) when BP was compared gathering patients in two groups: severe phenotype (CUL3 and KLHL3 AR) and mild phenotype (WNK1, WNK4, and KLHL3 AD) (Supplementary Figure S2a and S2b).
Figure 9.
Percentage of blood pressure (BP) stages according to the familial hyperkalemic hypertension genotype. BP was adjusted to age following recommendations from the European Society of Cardiology and the European Society of Hypertension (see Supplementary Table S1).
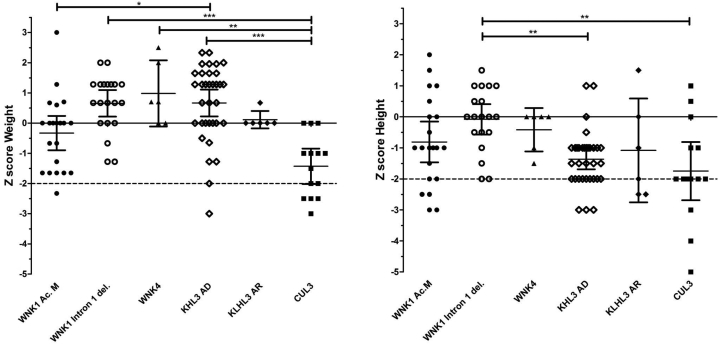
For analysis of the available data from Z-score for height and weight, we used the WHO charts (Figure 10, Supplementary Table S5). A significantly lower weight was observed in CUL3 patients compared to WNK1 intron 1 deletion and KLHL3 AD patients (P < 0.001), and to WNK4 (P < 0.001). A significantly smaller height was observed in CUL3 patients compared to WNK1 intron 1 del. (P < 0.001). Growth failure was present in all groups except WNK4, reaching the higher percentages in KLHL3 AR and CUL3 patients (50% and 71%, respectively).
Figure 10.
Graphic representation of Z-score of weight and height according to the genotype using the WHO charts. Growth retardation is defined by a Z-score less than -2 standard deviations.
Finally, the sensitivity of each group to HCTZ was assessed. For this purpose and when available, we assessed the differences between basal and under HCTZ of clinical and biochemical parameters (named the “delta analyses”). The HCTZ doses ranged from 0.1 mg/kg per day to 2.6 mg/kg per day. The delta of K+ analyses showed a significant difference (P = 0.015) between genotypes with higher response to treatment in CUL3 patients and a significant post-test difference between KLHL3 AD and CUL3 (Supplementary Table S6, Supplementary Figure S3). As this higher sensitivity could be explained by the higher initial values in this group, we correlated the delta of K+ with the basal kalemia in these four genotypes. The slopes were not significantly different (Supplementary Figure S4a) and a global significant association (P < 0.0001) existed between the basal plasma K+ values and the decrease observed in HCTZ (Supplementary Figure S4b).
Discussion
This study describes a large cohort of 153 individuals belonging to 84 families with FHHt. We have shown a genotype-phenotype correlation, where the most severe phenotype was associated with CUL3 variants. We described novel molecular variants in each gene, enriching the genetic database of FHHt with 25 novel variants, including five new CUL3 variants.
CUL3 is Associated With the Most Severe Form of FHHt
The discovery of a unique type of variant leading to exon 9 skip in the CUL3 gene by Boyden et al.6 is confirmed in our cohort, as well as the association with an earlier and more severe phenotype. CUL3 variants were presented in 19% of FHHt cases in our cohort and 14 of 16 were pediatric cases. As in the cohort in the study by Boyden et al.6, we observe a high rate of de novo mutation (75%). Boyden et al.6 revealed that patients with a CUL3 mutation were diagnosed younger (9 ± 6 years) compared to patients with WNK1 mutations (36 ± 20 years), WNK4 (28 ± 18 years), and dominant KLHL3 mutations (24 ± 18 years). Our study observed similar results concerning age of discovery and growth failure which were also confirmed in other publications.6,24,25 Although growth failure was observed in all groups except WNK4, it was significantly higher in CUL3 patients (71%). A lower body weight and body length was observed in cul3 mice deleted for exon 912 (and unpublished data from our lab). In contrast, no significant difference in weight or height was observed in the animal models after invalidation of other genes.26, 27, 28 Further studies are necessary to evaluate growth including data at birth and growth charts to confirm and evaluate the nature and progression of this failure. There was no correlation between Z-scores for weight and height and the degree of hypertension. This suggests that other pathways linked to the effect of the mutated protein could explain the growth failure, such as a possible role of CUL3 in fibroblast migration and cell mobility.29 The high rate of de novo variants correlated with these observations is consistent with the severity of the disease. This finding could be partly explained by the vascular phenotype associated to Cullin-3.10,11
Variants in the WNK1 acidic motif phenotype are associated with a marked metabolic acidosis compared to WNK1 intron 1 deletion and WNK4 acidic motif. We recently described missense mutations in the acidic motif of WNK1 in patients with early onset of a hyperkalemic, hyperchloremic acidosis and normal BP in most of cases.21 This motif, similar to that of WNK4, is responsible for binding to the substrate adapter KLHL3.9,12,30 While the acidic motif variants increase abundance of the kidney-specific isoform KS-WNK1,7 delta-intron 1 variants increase the L-WNK1,31 indicating a different pathophysiology. Based on this observation, we would expect a difference in the phenotype severity according to WNK1 variant type; however, no significant difference was observed except for bicarbonates.
The WNK4-related phenotype appears to be typical of the classic FHHt initially described1, 2, 3 and intermediate between WNK1/KLHL3 AD phenotypes and KLHL3 AR/CUL3 phenotypes; a higher number of patients with this genotype should help to confirm this trend.
Considering the BP, the percentages of patients with normal BP (categories 1 and 2) reflect the large spectrum of severity from less to more severe forms as follows: 61% WNK1 acidic motif, 57% WNK1 intron 1 deletion, 46% KLHL3 AD, 33% WNK4 and KLHL3 AR, and 6.5 % CUL3 patients.
The Different Phenotype Observed in the KLHL3 Genotype is due to a Gene Dosage Effect Rather Than a Dominant Negative Effect
Boyden et al.6 showed that subjects with dominant KLHL3 mutations had significantly higher serum kalemia levels than heterozygotes relatives of patients with recessive inheritance. They first inferred that mutations in dominant kindreds were likely dominant negative because they phenocopied the recessive disease.
When comparing the four subgroups (wild-type KLHL3, recessive KLHL3, dominant KLHL3, and heterozygous relatives of KLHL3 AR), we found an increasing severity of the disease suggesting a gene dosage effect. The difference observed between heterozygous relatives and dominant relatives should be considered with the difference in the age at workup: heterozygous relatives of KLHL3 AR form with almost normal phenotype have a lower age at workup than heterozygous patients of the KLHL3 AD form. It would be necessary to characterize heterozygous relatives of KLHL3 AR form as adults to evaluate whether their phenotype might approach the phenotype of patients with the dominant form.
The dominant variations observed in the cohort from the study by Boyden et al.6 are found in the three domains BTB, BACK, and KELCH. These three domains corresponds to bric-a-brac, tramtrack, broad complex domain for BTB domain, involved in oligomerization of the protein; BACK domain for BTB-And C-terminal kelch domain and KELCH domain for its “cup” shape (KELCH in German) formed by six kelch repeats folded in a six-bladed β-propeller. The BTB and BACK domains form a platform that interacts with the N-terminal region of Cul3, and the β-propeller structure of KELCH domain serves as a cup for substrate binding.32 We observed similar distribution of dominant KLHL3 variants (Figure 3). Among all described cases of KLHL3-related FHHt in literature, there are some discordant results compared to ours. First, the variant p.Gly500Val was first described in a patient with a recessive form without clinical data,33 whereas in our cohort and that of Boyden et al.,6 this variant was detected in patients with a dominant form. Second, the variant p.Ser410Leu was described in a patient with recessive form by Boyden et al.,6 whereas we observed a dominant form (Figure 3). We have also observed that different amino acid changes affecting the same residue can have a different inheritance pattern. For example, the change p.Arg384Trp was observed in recessive form whereas the change p.Arg384Gln was observed in a dominant one. These observations also argue for a gene dosage effect biased by the mode of detection of the patients.
Our study has the limitations of a retrospective analysis of patients affected by a rare disease. Patients with molecular anomalies in the WNK4 gene were rare in our population. Conclusions regarding the response to treatment by HCTZ are limited by the number of patients with available data with treatment and the variability of doses and duration of treatment.
In conclusion, the analysis of this unique cohort has shown that FHHt patients have variability in phenotypes ranging from the severe and early forms associated with CUL3 and KLHL3 AR genotypes through intermediate forms associated with KLHL3 AD, WNK4, and WNK1 intron 1 deletion genotypes to mild form associated with WNK1 acidic motif genotype. The identification of the causal mutation in a patient with a moderate form of FHHt allows the screening and therapeutic adaptation in other hypertensive relatives. Further studies on genetically screened patients and prospective follow-up of patients are necessary to better appreciate this variability as well as long-term prognosis and response to therapy.
Disclosure
Funding for this study was kindly provided by the European Union, FP7 (grant agreement 2012-305608 “European Consortium for High-Throughput Research in Rare Kidney Diseases [EURenOmics]”). The authors declared no competing interests. The authors declare no conflict of interest.
Acknowledgments
The authors thank patients and their families, referent clinicians, and all the technical and medical staff who contributed to the realization of this work.
Written consent for publication of clinical information and genetic analyses was obtained from all individuals, in accordance with French bioethics law of 2004, with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and with local ethical committee.
The datasets analyzed during the present study are available from the corresponding authors on reasonable request.
Footnotes
Table S1. European recommendations for blood pressure classification stages adapted to age.
Table S2. List of the 64 pathogenic variants detected in the 4 genes of patients with familial hyperkalemic hypertension.
Table S3a. Criteria of pathogenicity of new missense variants.
Table S3b. Criteria of pathogenicity of new splice-site variants.
Table S4a. Basic clinical and biochemical characteristics of the 19 CUL3 patients (index cases and affected relatives).
Table S4b. Growth clinical and biological data of CUL3 patients (index cases and affected relatives).
Table S5. Growth parameters for all familial hyperkalemic hypertension genotypes.
Table S6. Response to hydrochlorothiazide.
Figure S1. Genotype distribution of pediatric and adult populations with familial hyperkalemic hypertension.
Figure S2. Blood pressure and familial hyperkalemic hypertension genotypes.
Figure S3. Response to hydrochlorothiazide.
Figure S4. Delta of K+ under hydrochlorothiazide treatment versus basal kalemia.
STROBE checklist.
Contributor Information
Rosa Vargas-Poussou, Email: rosa.vargas@aphp.fr.
Xavier Jeunemaitre, Email: xavier.jeunemaitre@aphp.fr.
Supplementary Material
Table S1. European recommendations for blood pressure classification stages adapted to age.
Table S2. List of the 64 pathogenic variants detected in the 4 genes of patients with familial hyperkalemic hypertension.
Table S3a. Criteria of pathogenicity of new missense variants.
Table S3b. Criteria of pathogenicity of new splice-site variants.
Table S4a. Basic clinical and biochemical characteristics of the 19 CUL3 patients (index cases and affected relatives).
Table S4b. Growth clinical and biological data of CUL3 patients (index cases and affected relatives).
Table S5. Growth parameters for all familial hyperkalemic hypertension genotypes.
Table S6. Response to hydrochlorothiazide.
Figure S1. Genotype distribution of pediatric and adult populations with familial hyperkalemic hypertension.
Figure S2. Blood pressure and familial hyperkalemic hypertension genotypes.
Figure S3. Response to hydrochlorothiazide.
Figure S4. Delta of K+ under hydrochlorothiazide treatment versus basal kalemia.
STROBE checklist.
References
- 1.Paver W.K., Pauline G.J. Hypertension and hyperpotassaemia without renal disease in a young male. Med J Aust. 1964;2:305–306. doi: 10.5694/j.1326-5377.1964.tb115766.x. [DOI] [PubMed] [Google Scholar]
- 2.Arnold J.E., Healy J.K. Hyperkalemia, hypertension and systemic acidosis without renal failure associated with a tubular defect in potassium excretion. Am J Med. 1969;47:461–472. doi: 10.1016/0002-9343(69)90230-7. [DOI] [PubMed] [Google Scholar]
- 3.Gordon R.D. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension. 1986;8:93–102. doi: 10.1161/01.hyp.8.2.93. [DOI] [PubMed] [Google Scholar]
- 4.Wilson F.H., Disse-Nicodème S., Choate K.A. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 5.Faure S., Delaloy C., Leprivey V. WNK kinases, distal tubular ion handling and hypertension. Nephrol Dial Transplant. 2003;18:2463–2467. doi: 10.1093/ndt/gfg426. [DOI] [PubMed] [Google Scholar]
- 6.Boyden L.M., Choi M., Choate K.A. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis-Dit-Picard H., Barc J., Trujillano D. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron [published correction appears in Nat Genet. 2012;44(5):609] Nat Genet. 2012;4 doi: 10.1038/ng.2218. 456-S3. [DOI] [PubMed] [Google Scholar]
- 8.Hadchouel J., Ellison D.H., Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 9.Ohta A., Schumacher F.R., Mehellou Y. The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–122. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelham C.J., Ketsawatsomkron P., Groh S. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel Khalek W., Rafael C., Loisel-Ferreira I. Severe Arterial Hypertension from Cullin 3 Mutations Is Caused by Both Renal and Vascular Effects. J Am Soc Nephrol. 2019;30(5):811–823. doi: 10.1681/ASN.2017121307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumacher F.R., Siew K., Zhang J. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med. 2015;7:1285–1306. doi: 10.15252/emmm.201505444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agbor L.N., Ibeawuchi S.C., Hu C. Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight. 2016;1:e91015. doi: 10.1172/jci.insight.91015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu A., Wolley M., Stowasser M. The interplay of renal potassium and sodium handling in blood pressure regulation: critical role of the WNK-SPAK-NCC pathway. J Hum Hypertens. 2019;33:508–523. doi: 10.1038/s41371-019-0170-6. [DOI] [PubMed] [Google Scholar]
- 15.Cornelius R.J., Yang C.L., Ellison D.H. Hypertension-causing cullin 3 mutations disrupt COP9 signalosome binding. Am J Physiol Renal Physiol. 2020;318:F204–F208. doi: 10.1152/ajprenal.00497.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashton E.J., Legrand A., Benoit V. Simultaneous sequencing of 37 genes identified causative mutations in the majority of children with renal tubulopathies. Kidney Int. 2018;93:961–967. doi: 10.1016/j.kint.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Hureaux M., Ashton E., Dahan K. High-throughput sequencing contributes to the diagnosis of tubulopathies and familial hypercalcemia hypocalciuria in adults. Kidney Int. 2019;96:1408–1416. doi: 10.1016/j.kint.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lurbe E., Agabiti-Rosei E., Cruickshank J.K. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887–1920. doi: 10.1097/HJH.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 20.Williams B., Mancia G., Spiering W. 2018 ESC/ESH Guidelines for the management of arterial hypertension [published correction appears in Eur Heart J. 2019 Feb 1;40(5):475] Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 21.Louis-Dit-Picard H., Kouranti I., Rafael C. Mutation affecting the conserved acidic WNK1 motif causes inherited hyperkalemic hyperchloremic acidosis. J Clin Invest. 2020;130:6379–6394. doi: 10.1172/JCI94171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achard J.M., Warnock D.G., Disse-Nicodème S. Familial hyperkalemic hypertension: phenotypic analysis in a large family with the WNK1 deletion mutation. Am J Med. 2003;114:495–498. doi: 10.1016/s0002-9343(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 23.Kliuk-Ben Bassat O., Carmon V., Hanukoglu A. Familial hyperkalemia and hypertension (FHHt) and KLHL3: description of a family with a new recessive mutation (S553L) compared to a family with a dominant mutation, Q309R, with analysis of urinary sodium chloride cotransporter. Nephron. 2017;137:77–84. doi: 10.1159/000475825. [DOI] [PubMed] [Google Scholar]
- 24.Osawa M., Ogura Y., Isobe K., Uchida S., Nonoyama S., Kawaguchi H. CUL3 gene analysis enables early intervention for pediatric pseudohypoaldosteronism type II in infancy. Pediatr Nephrol. 2013;28:1881–1884. doi: 10.1007/s00467-013-2496-6. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji S., Yamashita M., Unishi G. A young child with pseudohypoaldosteronism type II by a mutation of Cullin 3. BMC Nephrol. 2013;14:166. doi: 10.1186/1471-2369-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadchouel J., Soukaseum C., Büsst C. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci U S A. 2010;107:18109–18114. doi: 10.1073/pnas.1006128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu P.Y., Cheng C.J., Wu Y.C. SPAK deficiency corrects pseudohypoaldosteronism II caused by WNK4 mutation. PLoS One. 2013;8:e72969. doi: 10.1371/journal.pone.0072969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susa K., Sohara E., Rai T. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23:5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 29.Guo D.F., Rahmouni K. The Bardet-Biedl syndrome protein complex regulates cell migration and tissue repair through a Cullin-3/RhoA pathway. Am J Physiol Cell Physiol. 2019;317:C457–C465. doi: 10.1152/ajpcell.00498.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakabayashi M., Mori T., Isobe K. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013;3:858–868. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Petiot E., Elvira-Matelot E., Mutig K. WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci U S A. 2013;110:14366–14371. doi: 10.1073/pnas.1304230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji A.X., Privé G.G. Crystal structure of KLHL3 in complex with Cullin3. PLoS One. 2013;8:e60445. doi: 10.1371/journal.pone.0060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glover M., Ware J.S., Henry A. Detection of mutations in KLHL3 and CUL3 in families with FHHt (familial hyperkalaemic hypertension or Gordon's syndrome) Clin Sci (Lond) 2014;126:721–726. doi: 10.1042/CS20130326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.