Abstract
Introduction
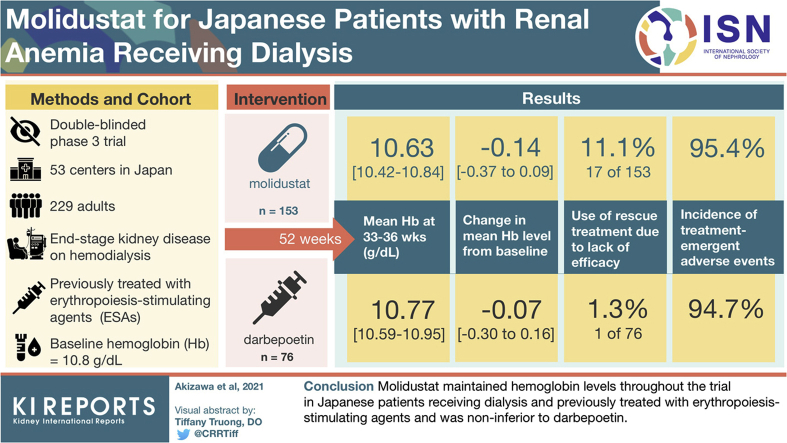
Molidustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor for renal anemia treatment, was evaluated in 5 phase 3 studies (MIYABI program). We report the results of the MIYABI hemodialysis-maintenance study.
Methods
This 52-week, randomized, double-blinded, double-dummy study compared the efficacy and safety of molidustat and darbepoetin in Japanese patients receiving hemodialysis and erythropoiesis-stimulating agents. Molidustat (starting dose: 75 mg/day) and darbepoetin were titrated to maintain hemoglobin (Hb) levels in the target range (≥10.0 and <12.0 g/dl). Primary outcomes were mean Hb level during the evaluation period (weeks 33–36) and its change from baseline. Safety outcomes included adverse events.
Results
Overall, 229 patients were randomized (molidustat, n = 153; darbepoetin, n = 76). Baseline characteristics were well balanced. Mean baseline Hb level was 10.8 g/dl. Mean (95% confidence interval [CI]) for mean Hb levels during the evaluation period were within the target range in both groups (molidustat: 10.63 [10.42–10.84] g/dl; darbepoetin: 10.77 [10.59–10.95] g/dl). Least-squares mean (95% CI) change in mean Hb level during the evaluation period from baseline was –0.14 (–0.37 to 0.09) g/dl for molidustat and –0.07 (–0.30 to 0.16) g/dl for darbepoetin; molidustat was noninferior to darbepoetin (least-squares mean difference [95% CI] [molidustat–darbepoetin]: –0.13 [–0.46 to 0.19] g/dl), based on a noninferiority margin of 1.0 g/dl. In line with published literature, and as expected in this patient population, most participants had ≥1 treatment-emergent adverse event.
Conclusion
Molidustat maintained Hb levels throughout the trial in patients receiving dialysis and previously treated with erythropoiesis-stimulating agents, and was noninferior to darbepoetin.
Keywords: darbepoetin alfa, dialysis, erythropoiesis-stimulating agent, molidustat, hypoxia-inducible factor prolyl hydroxylase inhibitor, renal anemia
Graphical abstract
Chronic kidney disease (CKD) can reduce the ability of the kidneys to produce erythropoietin, thereby lowering red blood cell production and leading to anemia,1,2 which has been reported to occur twice as often in patients with CKD than in the general population.3 In patients with CKD, the prevalence of anemia increases with decreasing renal function and tends to be higher in patients receiving hemodialysis than in those not receiving dialysis.1 Furthermore, low hemoglobin (Hb) levels (<8 g/dl) are associated with significantly increased risks of hospitalization and death compared with Hb within recommended levels (reference category, 11.00–11.99 g/dl) in patients receiving hemodialysis.4,5 Established treatment options for anemia in patients with CKD include iron supplementation, erythropoiesis-stimulating agents (ESAs), and red blood cell transfusion.6 ESAs are the current standard of care for renal anemia, but are ineffective at raising Hb to prespecified levels in 10% to 20% of patients, irrespective of dialysis status, and have been associated with several adverse events (AEs).7
Differences in the characteristics of patients receiving hemodialysis and in the management of renal anemia have been observed across Japan and other regions—namely, Europe and the United States (e.g., less intensive treatment in Japan than in Europe and the United States, with lower target Hb levels and lower doses of ESA).1,8,9 The Japanese Society for Dialysis Therapy guidelines for the treatment of renal anemia recommend that Hb levels are maintained between 10 and 12 g/dl in adults receiving hemodialysis and that ESA dose reduction or discontinuation should be considered at Hb levels >12 g/dl.9
Molidustat is a novel inhibitor of hypoxia-inducible factor prolyl hydroxylase (HIF-PH) that is under investigation as an alternative to ESAs for the treatment of renal anemia.7,10, 11, 12 In preclinical studies, molidustat stimulated endogenous erythropoietin production within its normal physiological range.10 On the basis of positive findings from preclinical and clinical studies, the molidustat once daily improves renal anemia by inducing erythropoietin (MIYABI) program of 5 phase 3 trials was initiated to evaluate oral molidustat for the treatment of renal anemia in Japan.7,11,12 Here, we present findings from the MIYABI Hemodialysis-Maintenance (MIYABI HD-M) study, which build on results from a previous open-label, phase 2b trial (DIALOGUE 4)13 and its extension study (DIALOGUE 5).14 MIYABI HD-M was conducted to compare the efficacy and safety of molidustat and an ESA (darbepoetin alfa [darbepoetin]) for the maintenance treatment of renal anemia in Japanese patients receiving dialysis and who were previously treated with ESAs.7 It was hypothesized that the mean Hb level in the molidustat group would be within the target range during the evaluation period (weeks 33–36) and that molidustat would be noninferior to darbepoetin for change in Hb level from baseline.
Methods
Study Design
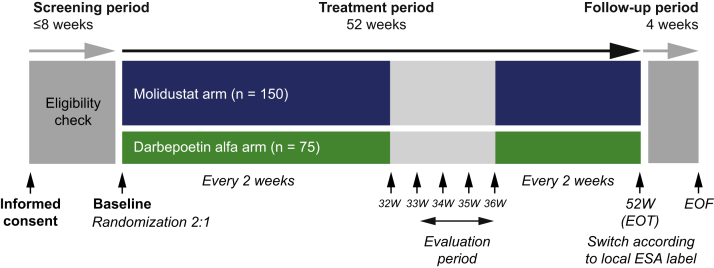
The design of the MIYABI HD-M study (ClinicalTrials.gov: NCT03543657) has been described previously.7 Briefly, this was a randomized, double-blinded, double-dummy, parallel-group, phase 3 trial comparing molidustat with darbepoetin in Japanese patients with CKD receiving dialysis and ESAs. The trial was conducted at 53 centers in Japan, and the treatment duration was 52 weeks. There was a screening period of up to 8 weeks before randomization and patients were scheduled to visit the study site at least once every 2 weeks until week 52, with weekly visits between week 32 and week 36. After the 52-week treatment period, there was a 4-week follow-up period (Figure 1).
Figure 1.
Study design. EOF, end of follow-up; EOT, end of treatment; ESA, erythropoiesis-stimulating agent; W, week.
The trial protocol was approved by the relevant independent ethics committee or institutional review board for each study center. The trial was conducted in accordance with the ethical principles detailed in the Declaration of Helsinki and Good Clinical Practice guidance. Informed consent was obtained from each participant before any study-specific examinations or procedures were conducted.
Selection Criteria
MIYABI HD-M included adults (aged ≥20 years) with end-stage kidney disease who received dialysis at least weekly for ≥12 weeks before randomization and were treated with the same ESA for ≥8 weeks before the screening period. Dialysis procedures could include hemodiafiltration, hemofiltration, hemodialysis, and other modalities except for peritoneal dialysis. ESA treatment could include an administration of darbepoetin weekly or every 2 weeks, administration of epoetin beta pegol monthly or every 2 weeks, or administration of epoetin alfa/beta weekly, every 2 weeks, twice per week, or 3 times per week, with ≤1 dose change within 8 weeks before randomization. Eligible patients had mean Hb levels of ≥9.5 and <12.0 g/dl (with a difference between the lowest and highest measured level of <1.2 g/dl), and either ferritin levels ≥100 ng/ml or transferrin saturation ≥20% at screening. Key exclusion criteria included: New York Heart Association class III or IV congestive heart failure; history of cardiovascular or cerebrovascular events within 6 months before randomization; sustained, poorly controlled arterial hypertension or hypotension at randomization; and proliferative choroidal or retinal disease at screening. A complete list of selection criteria has been published previously.7
Treatments
Eligible patients were randomized 2:1 to receive molidustat or darbepoetin, stratified by previous ESA dose (low or high) and history of thromboembolic events (yes or no; myocardial infarction, pulmonary thromboembolism, stroke [excluding hemorrhagic stroke], or acute limb ischemia). Treatment allocation was conducted via an interactive voice/web response system (IxRS), and the computer-prepared randomization list was provided to the IxRS supplier by the sponsor.7 Investigators and patients were blinded to treatment allocation. In this double-dummy trial, patients in the molidustat group were treated with both molidustat and darbepoetin placebo, and patients in the darbepoetin group were treated with both darbepoetin and molidustat placebo. Molidustat and molidustat placebo were administrated orally once daily, using a starting dose of 75 mg/day. Doses of molidustat and molidustat placebo were available in multiple doses of 5-, 12.5-, 25-, and 50-mg tablets. Darbepoetin and darbepoetin placebo were administrated weekly or once every 2 weeks by intravenous injection. For these trial products, the starting dose and dose interval were provided to the study investigator by an IxRS and according to the previous ESA dose and dose interval at randomization, as previously described.7 The maintenance dose of molidustat/molidustat placebo (≤200 mg) or darbepoetin/darbepoetin placebo (≤180 μg) was also provided by the IxRS and adjusted to achieve Hb levels within the target range of ≥10.0 and <12.0 g/dl. Patients with iron, folate, or vitamin B12 deficiency were to be treated before study enrollment and during the study. Supplements were administered at the investigator’s discretion, in line with current guidelines. During the treatment period, iron supplements were administered intravenously to achieve a serum ferritin level of ≥100 ng/ml or transferrin saturation ≥20%, in line with Japanese guidelines.9 Rescue treatment, which was prespecified in the protocol and defined as red blood cell transfusion due to renal anemia or any ESA treatment started due to lack of efficacy as judged by the investigator, was administered at the investigator’s discretion, for example, in patients with a Hb level <8.0 g/dl. Red blood cell transfusions administered due to study procedures or to treat acute bleeding, were recorded but not considered as rescue treatment.
Outcomes
The primary outcomes were mean Hb level during the evaluation period (weeks 33–36) and its change from baseline. Secondary efficacy outcomes included Hb level and change from baseline in Hb level at each visit, proportion of patients whose Hb level was in, above, or below the target range at each visit, and proportion of patients whose rise in Hb level from the previous scheduled visit was >0.5 g/dl/week. Use of rescue treatment was also evaluated.
Safety assessments included study drug exposure, AEs, and vital signs. A treatment-emergent AE (TEAE) was defined as any event that occurred between the first administration of study drug and the end-of-treatment/premature discontinuation visit plus 3 days, inclusive. If the patient had no end-of-treatment/premature discontinuation visit, the last date of drug administration was used. AEs of special interest included AEs with an outcome of death and myocardial infarction, unstable angina pectoris, stroke or transient ischemic attack, pulmonary thromboembolism, and acute limb ischemia. The incidence of major adverse cardiovascular events (MACE), adjudicated by an independent committee, was also assessed. MACE were defined as cardiovascular or undetermined death, myocardial infarction, unstable angina pectoris, ischemic stroke (ischemic stroke or ischemic stroke with hemorrhagic transformation), pulmonary thromboembolism, and acute limb ischemia. Ophthalmologic- and neoplasm (benign, malignant, and unspecified)-related TEAEs were also reported.
Statistical Analysis
The target sample size was 225 patients: 150 patients in the molidustat group and 75 patients in the darbepoetin group.7 Randomizing 150 patients to the molidustat group would provide ≥98% power to establish whether mean Hb levels and its 2-sided 95% confidence interval (CI) during the evaluation period were within the target range, assuming mean Hb levels of 10.5 to 11.5 g/dl and an SD of 1.3–1.5 g/dl based on previous phase 2b studies.7 The sample size calculated had >90% power to reject the null hypothesis that molidustat is inferior to darbepoetin, with a noninferiority margin of 1 g/dl and at a 1-sided 2.5% significance level.7 A between-group difference of 0 g/dl was assumed, with a common SD of 1.3 to 1.5 g/dl.7 The sample size calculated was expected to provide sufficient data to assess the safety of molidustat therapy over the study period, assuming a discontinuation rate of approximately 30%.7 Efficacy and safety analyses were performed using the full analysis set and safety analysis set, respectively. The full analysis set included all randomized patients who had ≥1 baseline Hb measurement before the first dose of study drug, with patients included according to their randomized group. The safety analysis set included all randomized patients who received ≥1 dose of study drug, with patients included according to their treatment. All variables were summarized using descriptive statistics. In the primary analyses, the mean Hb level during the evaluation period was calculated, together with its 2-sided 95% CI. The latter was estimated using 1-sample t-statistics and compared with the target Hb range.7 If the 2-sided 95% CI was within the target range, noninferiority of molidustat to darbepoetin was evaluated.7 The between-group difference in the response variable (i.e., least-squares [LS] mean for change from baseline in mean Hb level during the evaluation period) with 2-sided 95% CI was estimated using an analysis of covariance model with treatment group, previous ESA dose group (low/high), and previous thromboembolic events (yes/no) as fixed effects and baseline Hb level as a covariate to adjust for the impact of fixed effects and covariates (i.e., previous ESA dose, previous thromboembolic events and baseline Hb levels).7 Noninferiority of molidustat to darbepoetin was to be established if the lower limit of the 2-sided 95% CI for the difference (molidustat–darbepoetin) was above –1.0 g/dl, with a noninferiority margin of 1.0 g/dl.7 Data were analyzed for subgroups defined by characteristics such as age, sex, baseline weight, previous thromboembolic events, main cause of CKD, previous ESA dose, and rescue treatment use during the trial.
Results
Patient Disposition and Baseline Characteristics
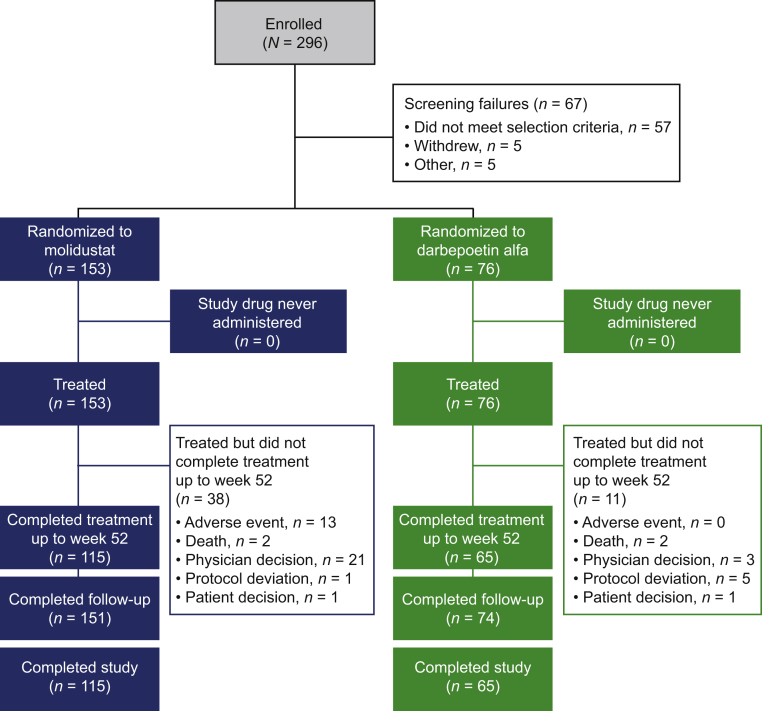
Overall, 296 patients were enrolled in the trial (Figure 2). Of 229 patients randomized to receive molidustat (n = 153) or darbepoetin (n = 76), 180 completed treatment up to week 52 and follow-up (n = 115 and n = 65 with molidustat and darbepoetin, respectively) (Figure 2). Of the 21 patients who discontinued molidustat on the advice of the treating physician, 17 received rescue treatment for lack of efficacy. In the darbepoetin group, 1 patient out of the 3 who discontinued treatment based on physician decision received rescue treatment. All randomized patients were eligible for inclusion in the full analysis set and safety analysis set. All patients were Japanese, and 61.1% of patients were male. In the overall population, mean (SD) age was 65.7 (10.4) years, mean (SD) body mass index was 22.5 (3.3) kg/m2, and mean (SD) baseline central Hb level was 10.8 (0.6) g/dl. Mean (SD) duration of dialysis was 7.9 (7.5) years. In general, demographics and baseline characteristics were well balanced between the treatment groups (Table 1). At baseline, patients in the molidustat group had a numerically longer mean duration of CKD and higher mean folate and serum C-reactive protein levels than patients in the darbepoetin group.
Figure 2.
Patient disposition. Screening failure: terminated the study before randomization (for any reason). Completed study: completed both treatments up to week 52 and follow-up.
Table 1.
Patient demographics and baseline characteristics
| Parameter | Molidustat (n = 153) | Darbepoetin alfa (n = 76) |
|---|---|---|
| Sex, n (%) | ||
| Male | 91 (59.5) | 49 (64.5) |
| Female | 62 (40.5) | 27 (35.5) |
| Age, years | 66.2 (10.3) | 64.8 (10.6) |
| Weight, kg | 58.52 (10.25) | 58.62 (12.38) |
| Height, cm | 160.86 (8.47) | 161.76 (8.44) |
| BMI, kg/m2 | 22.61 (3.34) | 22.25 (3.12) |
| Smoking history, n (%) | ||
| Never | 75 (49.0) | 33 (43.4) |
| Former | 60 (39.2) | 30 (39.5) |
| Current | 18 (11.8) | 13 (17.1) |
| Central Hb level, g/dl | 10.77 (0.64) | 10.84 (0.65) |
| Mean central Hb level during screening, g/dl | 10.79 (0.65) | 10.87 (0.64) |
| Previous ESA dose group,an (%) | ||
| Low | 152 (99.3) | 76 (100.0) |
| High | 1 (0.7) | 0 |
| Previous thromboembolic event,bn (%) | ||
| No | 141 (92.2) | 69 (90.8) |
| Yes | 12 (7.8) | 7 (9.2) |
| Main cause of CKD, n (%) | ||
| Diabetic nephropathy | 48 (31.4) | 24 (31.6) |
| Other | 93 (60.8) | 51 (67.1) |
| Chronic glomerulonephritis | 47 (30.7) | 25 (32.9) |
| Nephrosclerosis | 25 (16.3) | 15 (19.7) |
| Polycystic kidney disease | 13 (8.5) | 2 (2.6) |
| IgA nephropathy | 1 (0.7) | 3 (3.9) |
| Unknown | 12 (7.8) | 1 (1.3) |
| Duration of CKD, years | ||
| Mean (SD) | 12.067 (9.390) | 10.842 (8.841) |
| Median (range) | 9.593 (1.03–39.75) | 7.936 (1.10–36.79) |
| Duration of dialysis, years | ||
| Mean (SD) | 8.065 (7.588) | 7.706 (7.445) |
| Median (range) | 5.350 (0.31–34.39) | 5.677 (0.24–32.59) |
| Pulse rate, bpm | 72.2 (10.8) | 71.9 (11.4) |
| Systolic blood pressure, mmHg | 149.8 (17.2) | 148.1 (18.5) |
| Diastolic blood pressure, mmHg | 78.7 (11.2) | 79.7 (10.3) |
| Total iron binding capacity, μmol/l | 42.1 (6.6) | 42.1 (7.0) |
| Transferrin saturation, % | 29.2 (11.1) | 29.8 (10.0) |
| Hepcidin, ng/ml | 48.0 (40.1) | 48.9 (41.5) |
| Total iron, μg/dl | 67.6 (24.1) | 70.1 (27.1) |
| Ferritin, ng/ml | 118.7 (115.5) | 115.4 (119.1) |
| Vitamin B12, pmol/l | 336.6 (184.0) | 324.9 (172.7) |
| Folate, nmol/l | 224.8 (634.4) | 166.1 (596.5) |
| Serum C-reactive protein, mg/dl | 0.256 (0.623) | 0.217 (0.629) |
| Serum erythropoietin, IU/l | 11.378 (9.362) | 12.190 (13.462) |
BMI, body mass index; CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; IgA, immunoglobulin A.
Data presented are mean (SD) unless otherwise stated and are for the full analysis set.
Low previous ESA dose: darbepoetin alfa or epoetin beta pegol ≤50 μg/week and all doses of epoetin alfa or beta; high previous ESA dose: darbepoetin alfa or epoetin beta pegol >50 μg/week.
Previous thromboembolic event: previous myocardial infarction, pulmonary thromboembolism, stroke (excluding hemorrhagic stroke), or acute limb ischemia.
Efficacy
Mean (95% CI) for mean Hb levels during the evaluation period were within the target range in both treatment groups (10.63 [10.42–10.84] g/dl with molidustat and 10.77 [10.59–10.95] g/dl with darbepoetin). The LS mean (95% CI) change in mean Hb level during the evaluation period from baseline was –0.14 (–0.37 to 0.09) g/dl for molidustat and –0.07 (–0.30 to 0.16) g/dl for darbepoetin. Noninferiority of molidustat to darbepoetin for the change in mean Hb level from baseline to weeks 33–36 was established (LS mean difference [95% CI] for molidustat vs darbepoetin: −0.13 [−0.46 to 0.19] g/dl).
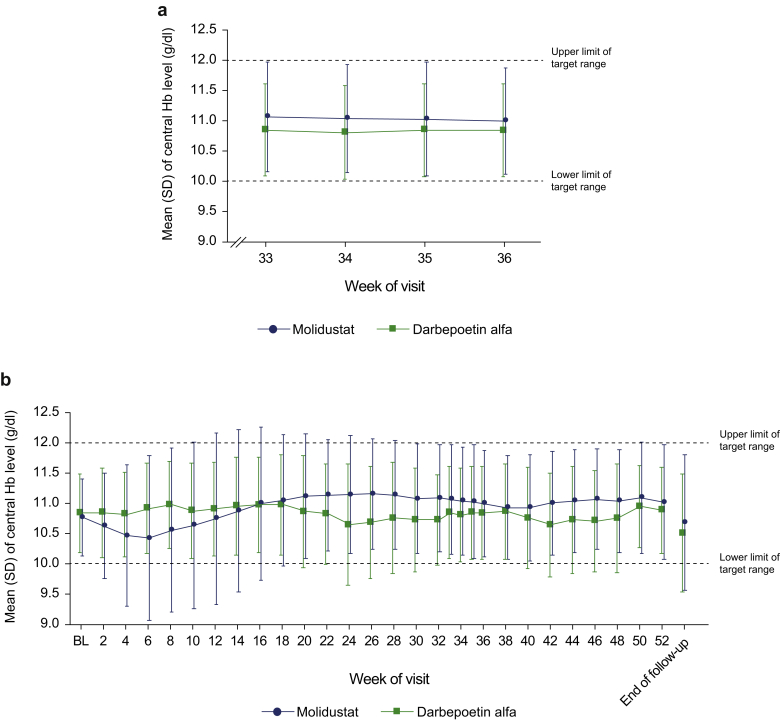
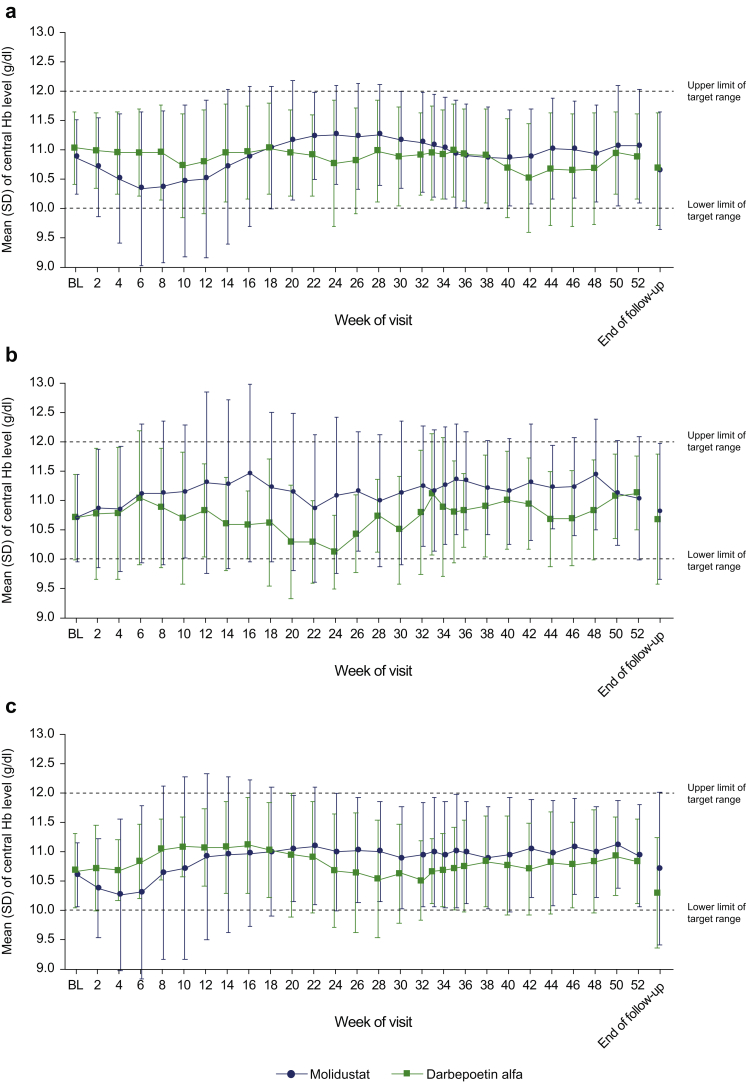
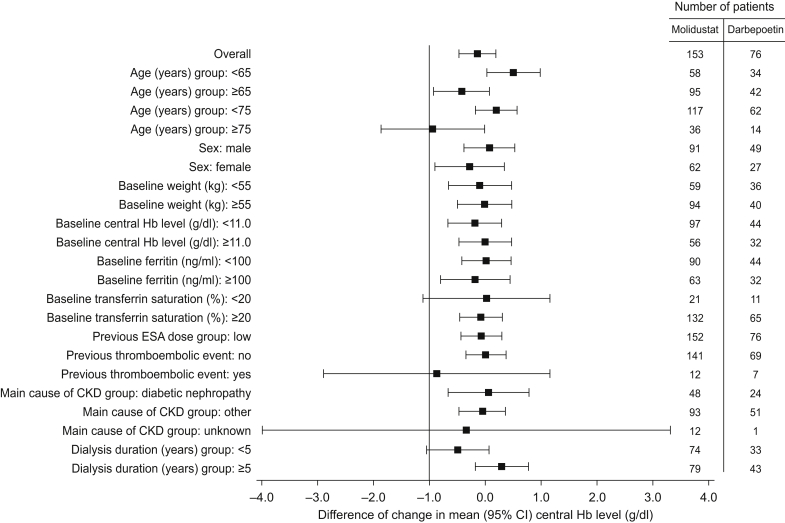
Mean Hb levels at each visit are displayed in Figure 3. In the molidustat group, the mean central Hb level decreased up to week 6, but remained within the target range and subsequently recovered (Figure 3). The proportion of patients with Hb level within the target range also initially decreased, but remained between 61.2% and 77.8% from week 18 to week 52 (Supplementary Figure S1A). In the darbepoetin group, the mean central Hb level remained within the target range throughout the study (Figure 3) and 68.7% to 88.7% of patients remained within the target range from week 2 to week 52 (Supplementary Figure S1B). Mean Hb levels at each visit by previous ESA treatment are displayed in Figure 4 and Supplementary Figure S2. Changes in mean Hb level in the subgroups of patients treated with molidustat and previously treated with darbepoetin or epoetin alfa or beta were not significantly different compared with the overall study population. However, the mean Hb level in the subgroup of patients receiving molidustat and previously treated with epoetin beta pegol did not tend to decrease slightly from baseline as observed in the overall study population from week 6 (Figure 4). In the subgroups of patients receiving molidustat and previously treated with darbepoetin or epoetin beta pegol, there was no significant difference between the low-dose and high-dose populations. However, the Hb levels in the subgroup of patients receiving molidustat and previously treated with high doses of epoetin alfa or beta were lower than in those previously treated with low doses of epoetin alfa or beta from week 2 to week 18, and were similar from week 20 to end of treatment (Supplementary Figure S2). The proportion of patients who experienced a rise in central Hb level from the previous scheduled visit of >0.5 g/dl/week from week 0 to week 52 was 49.0% in the molidustat group and 47.3% in the darbepoetin group (Supplementary Table S1). In all the subgroup analyses according to baseline characteristics, the LS mean difference between treatments in the change in mean Hb level during the evaluation period was above −1.0 g/dl (Figure 5).
Figure 3.
Mean central Hb levels by visit (full analysis set) during the evaluation period (a) and during the overall treatment period (b). BL, baseline; Hb, hemoglobin.
Figure 4.
Mean central Hb levels by visit stratified by previous ESA treatment: (a) darbepoetin alfa, (b) epoetin beta pegol, or (c) epoetin alfa or beta. BL, baseline; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin. BL Hb level is defined as the mean of all Hb levels during the screening period and Hb level at week 0. The number of patients treated with epoetin beta pegol was smaller than that receiving other ESAs, which might have resulted accidentally in bias. (a) At baseline: n = 86 for molidustat and n = 35 for darbepoetin. (b) At baseline: n = 19 for molidustat and n = 9 for darbepoetin. (c) At baseline: n = 48 for molidustat and n = 32 for darbepoetin.
Figure 5.
Subgroup analysis of the difference (molidustat – darbepoetin) of changes in mean (95% CI) central Hb level (g/dl) during the evaluation period from baseline (full analysis set). ANCOVA, analysis of covariance; CI, confidence interval; CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; IxRS, interactive voice/web response system. For the overall population, least-squares mean difference (95% CI) was estimated using ANCOVA. The between-group difference in the response variable (i.e. change from baseline in mean Hb level during the evaluation period) with 2-sided 95% CI was estimated using ANCOVA with treatment group, previous thromboembolic events recorded in IxRS, previous ESA dose group (low/high) recorded in IxRS as fixed effect and baseline central Hb level as covariate. For each subgroup, the 2-sided 95% CI was estimated using t statistics.
Overall, the proportion of patients who received rescue treatment due to lack of efficacy was 11.1% (17/153 patients including 4 patients who received rescue treatment after the treatment period had completed) in the molidustat group and 1.3% (1/76 patients) in the darbepoetin group. Among those 17 patients in the molidustat group, 15 received an ESA treatment and 2 received a red blood cell transfusion. In a subgroup analysis by rescue treatment, there was no apparent difference in demographics and baseline characteristics including Hb level, ferritin, transferrin saturation, duration of dialysis, last ESA dose before the start of study drug and erythropoietin resistance index between patients who received rescue treatment and those who did not in the molidustat group (data not shown).
Safety
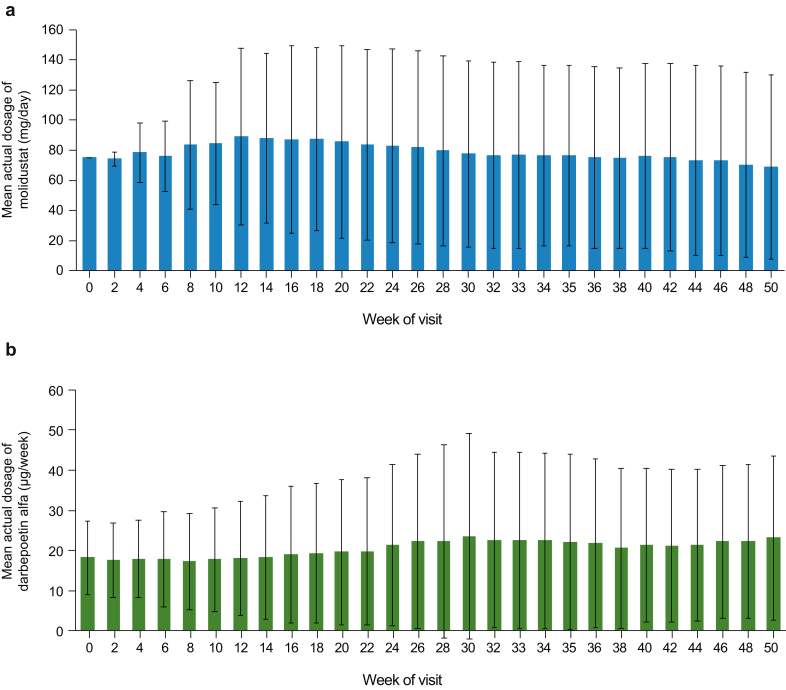
The mean (SD) treatment duration was 296.2 (117.2) days in the molidustat group and 315.9 (106.8) days in the darbepoetin group (median, 364 days in both groups). Most patients (69.3% in the molidustat group and 78.9% in the darbepoetin group) had a treatment duration of ≥336 days (≥48 weeks). Figure 6 shows the mean (SD) dosages of molidustat and darbepoetin at each study visit. The mean (SD) dosage was 79.02 (42.65) mg/day in the molidustat group and 20.16 (14.70) μg/week in the darbepoetin group. In the molidustat group, the most common maximum dosage was 75 mg/day (47.7%), followed by 200 mg/day (20.9%) and 100 mg/day (17.6%). In the darbepoetin group, the most common maximum dosage was 30 μg/week (19.7%), followed by 15 μg/week (18.4%) and 20 μg/week (18.4%). The proportions of patients requiring dose adjustments up to week 52 were similar in the 2 groups: 93.3% in the molidustat group and 93.2% in the darbepoetin group.
Figure 6.
Mean (SD) dosage of (a) molidustat and (b) darbepoetin alfa at each visit (full analysis set).
There were no notable differences between groups in the incidence of TEAEs (molidustat, 95.4%; darbepoetin, 94.7%), TEAEs with an outcome of death (1.3%; 2.6%) (Table 2), or AEs of special interest (4.6%; 3.9%). Table 2 and Supplementary Table S2 display TEAEs that occurred in ≥5% of patients in any treatment group. TEAEs occurring in ≥10% of patients in either treatment group included nasopharyngitis, diarrhea, contusion, vomiting, shunt stenosis, and nausea (Supplementary Table S2). Most TEAEs were mild or moderate in intensity (Table 2). Severe TEAEs that occurred in >1 patient in any treatment group included shunt occlusion (1.3% in each treatment group) and aortic dissection (1.3% in the molidustat group). Serious TEAEs were reported in 24.2% of patients in the molidustat group and 18.4% of patients in the darbepoetin group (Table 2). MACE that occurred after the start of study drug treatment were reported in 3.3% of patients in the molidustat group and 2.6% of patients in the darbepoetin group (Table 3). TEAEs of neoplasms benign, malignant, and unspecified (including cysts and polyps) by primary system organ class were reported in 9.8% of patients (15/153) in the molidustat group and in 5.3% of patients (4/76) in the darbepoetin group (Table 2 and Supplementary Table S3). Ocular TEAEs were reported in 30.1% of patients (46/153) in the molidustat group and 18.4% (14/76) in the darbepoetin group (Table 4). Some differences in the incidences of TEAEs resulting in discontinuation (molidustat, 8.5%; darbepoetin, 0%) (Table 5), ocular AEs (30.1%; 18.4%), influenza (7.8%; 2.6%), and nausea (3.9%; 14.5%) were observed. The TEAEs resulting in discontinuation of molidustat included 6 neoplasm events (benign, malignant, or unspecified); all were considered as unrelated to study drug by the investigators. Serum VEGF concentrations at baseline, week 36, week 52 and end of follow-up are presented in Supplementary Table S4.
Table 2.
Summary of TEAEs up to week 52 (safety analysis set)
| Molidustat (n = 153) | Darbepoetin (n = 76) | Total (N = 229) | |
|---|---|---|---|
| Any TEAE, n (%) | 146 (95.4) | 72 (94.7) | 218 (95.2) |
| Mild | 93 (60.8) | 54 (71.1) | 147 (64.2) |
| Moderate | 40 (26.1) | 13 (17.1) | 53 (23.1) |
| Severe | 13 (8.5) | 5 (6.6) | 18 (7.9) |
| Any serious TEAE, n (%) | 37 (24.2) | 14 (18.4) | 51 (22.3) |
| TEAE leading to death, n (%) | 2 (1.3) | 2 (2.6) | 4 (1.7) |
| TEAEs by primary system organ class and preferred terma | |||
| GI disorders, n (%) | 79 (51.6) | 59 (77.6) | 138 (60.3) |
| General disorders and administration site conditions, n (%) | 1 (0.7) | 6 (7.9) | 7 (3.1) |
| Infections and infestations, n (%) | 88 (57.5) | 48 (63.2) | 136 (59.4) |
| Injury, poisoning, and procedural complications, n (%) | 49 (32.0) | 25 (32.9) | 74 (32.3) |
| Metabolism and nutrition disorders, n (%) | 3 (2.0) | 4 (5.3) | 7 (3.1) |
| Musculoskeletal and connective tissue disorders, n (%) | 37 (24.2) | 16 (21.1) | 53 (23.1) |
| Nervous system disorders, n (%) | 12 (7.8) | 10 (13.2) | 22 (9.6) |
| Skin and subcutaneous disorders, n (%) | 12 (7.8) | 15 (19.7) | 27 (11.8) |
| Specific TEAEs, n (%) | |||
| Any neoplasms, benign, malignant, and unspecifiedb | 15 (9.8) | 4 (5.3) | 19 (8.3) |
| Malignant tumorsc | 6 (3.9) | 1 (1.4) | 7 (3.1) |
| Nonmalignant tumors | 9 (5.9) | 3 (3.9) | 12 (5.2) |
GI, gastrointestinal; TEAE, treatment-emergent adverse event.
Adverse events are presented by primary system organ class and preferred term. A patient is counted only once within each preferred term or any primary system organ class.
The number of adverse events are the sum of individual TEAEs which were reported in ≥5% of patients in either treatment group. Details are provided in Table S2.
Details are provided in Supplementary Table S3.
Categorized using the standardized MedDRA Queries (SMQ).
Table 3.
MACE including undetermined death evaluated by a specialist after the start of the study drug and up to week 52 (safety analysis set)
| Category and event by adjudicatora | Molidustat (n = 153) | Darbepoetin (n = 76) | Total (N = 229) |
|---|---|---|---|
| Patients with ≥1 MACE,bn (%) | 5 (3.3) | 2 (2.6) | 7 (3.1) |
| Cardiovascular death, n (%) | 2 (1.3) | 1 (1.3) | 3 (1.3) |
| Myocardial infarction, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Ischemic stroke, n (%) | 2 (1.3) | 1 (1.3) | 3 (1.3) |
MACE, major adverse cardiovascular events.
Primary system organ class term from Medical Dictionary for Regulatory Activities, version 22.0.
MACE including cardiovascular or undetermined death, myocardial infarction, unstable angina pectoris, ischemic stroke, pulmonary thromboembolism, and acute limb ischemia.
Table 4.
Ocular treatment-emergent adverse events (safety analysis set)
| Molidustat (n = 153) | Darbepoetin (n = 76) | Total (N = 229) | |
|---|---|---|---|
| Any ocular adverse events, n (%) | 46 (30.1) | 14 (18.4) | 60 (26.2) |
| Eye disorders, n (%) | 41 (26.8) | 12 (15.8) | 53 (23.1) |
| Angle closure glaucoma | 1 (0.7) | 0 | 1 (0.4) |
| Blepharitis | 1 (0.7) | 0 | 1 (0.4) |
| Borderline glaucoma | 0 | 1 (1.3) | 1 (0.4) |
| Cataract | 5 (3.3) | 0 | 5 (2.2) |
| Chalazion | 1 (0.7) | 0 | 1 (0.4) |
| Conjunctival deposit | 1 (0.7) | 0 | 1 (0.4) |
| Conjunctival hemorrhagea | 4 (2.6) | 2 (2.6) | 6 (2.6) |
| Conjunctivitis allergic | 3 (2.0) | 0 | 3 (1.3) |
| Corneal erosion | 1 (0.7) | 0 | 1 (0.4) |
| Corneal opacity | 1 (0.7) | 0 | 1 (0.4) |
| Diabetic retinal edemaa | 0 | 1 (1.3) | 1 (0.4) |
| Diabetic retinopathya | 4 (2.6) | 1 (1.3) | 5 (2.2) |
| Dry eye | 5 (3.3) | 1 (1.3) | 6 (2.6) |
| Entropion | 1 (0.7) | 0 | 1 (0.4) |
| Eye allergy | 1 (0.7) | 0 | 1 (0.4) |
| Eye pain | 0 | 1 (1.3) | 1 (0.4) |
| Eyelid edema | 1 (0.7) | 0 | 1 (0.4) |
| Eyelid rash | 1 (0.7) | 0 | 1 (0.4) |
| Foreign body sensation in eyes | 0 | 1 (1.3) | 1 (1.4) |
| Glaucoma | 2 (1.3) | 1 (1.3) | 3 (1.3) |
| Keratitis | 1 (0.7) | 0 | 1 (0.4) |
| Lenticular opacities | 1 (0.7) | 0 | 1 (0.4) |
| Ocular hyperemia | 3 (2.0) | 2 (2.6) | 5 (2.2) |
| Periorbital inflammation | 1 (0.7) | 0 | 1 (0.4) |
| Posterior capsule opacification | 0 | 1 (1.3) | 1 (0.4) |
| Retinal artery stenosisa | 1 (0.7) | 0 | 1 (0.4) |
| Retinal detachment | 1 (0.7) | 0 | 1 (0.4) |
| Retinal exudatesa | 1 (0.7) | 0 | 1 (0.4) |
| Retinal hemorrhagea | 3 (2.0) | 0 | 3 (1.3) |
| Retinal tear | 0 | 1 (1.3) | 1 (0.4) |
| Retinopathy hypertensivea | 2 (1.3) | 0 | 2 (0.9) |
| Rhegmatogenous retinal detachment | 0 | 1 (1.3) | 1 (0.4) |
| Trichiasis | 1 (0.7) | 0 | 1 (0.4) |
| Visual acuity reduced | 2 (1.3) | 0 | 2 (0.9) |
| Vitreous floaters | 0 | 1 (1.3) | 1 (0.4) |
| Immune system disorders, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Drug hypersensitivity | 1 (0.7) | 0 | 1 (0.4) |
| Infections and infestations, n (%) | 6 (3.9) | 1 (1.3) | 7 (3.1) |
| Conjunctivitis | 5 (3.3) | 0 | 5 (2.2) |
| Hordeolum | 1 (0.7) | 1 (1.3) | 2 (0.9) |
| Injury, poisoning and procedural complications, n (%) | 2 (1.3) | 1 (1.3) | 3 (1.3) |
| Eye contusion | 1 (0.7) | 0 | 1 (0.4) |
| Eye injury | 0 | 1 (1.3) | 1 (0.4) |
| Injury corneal | 1 (0.7) | 0 | 1 (0.4) |
| Product issues, n (%) | 0 | 1 (1.3) | 1 (0.4) |
| Device dislocation | 0 | 1 (1.3) | 1 (0.4) |
| Surgical and medical procedures, n (%) | 1 (0.7) | 1 (1.3) | 2 (0.9) |
| Cataract operation | 1 (0.7) | 1 (1.3) | 2 (0.9) |
A patient is counted only once within each preferred term or any primary system organ class.
Vascular-related events.
Table 5.
TEAEs resulting in discontinuation of study drug by primary system organ class and preferred term (full analysis set)
| Primary system organ class and preferred term | Molidustat (n = 153) | Darbepoetin (n = 76) | Total (N = 229) |
|---|---|---|---|
| Patients with ≥1 TEAE leading to discontinuation, n (%) | 13 (8.5) | 0 | 13 (5.7) |
| Cardiac disorders, n (%) | 2 (1.3) | 0 | 2 (0.9) |
| Angina pectoris | 1 (0.7) | 0 | 1 (0.4) |
| Angina unstable | 1 (0.7) | 0 | 1 (0.4) |
| Aortic valve stenosis | 1 (0.7) | 0 | 1 (0.4) |
| Arteriosclerosis coronary artery | 1 (0.7) | 0 | 1 (0.4) |
| Infections and infestations, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Septic shock | 1 (0.7) | 0 | 1 (0.4) |
| Injury, poisoning, and procedural complications, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Shunt occlusion | 1 (0.7) | 0 | 1 (0.4) |
| Neoplasms benign, malignant, and unspecified, n (%) | 6 (3.9) | 0 | 6 (2.6) |
| Benign cardiac neoplasm | 1 (0.7) | 0 | 1 (0.4) |
| Breast cancer | 1 (0.7) | 0 | 1 (0.4) |
| Gastric cancer | 1 (0.7) | 0 | 1 (0.4) |
| Prostate cancer | 1 (0.7) | 0 | 1 (0.4) |
| Renal cancer | 1 (0.7) | 0 | 1 (0.4) |
| Tumor inflammation | 1 (0.7) | 0 | 1 (0.4) |
| Nervous system disorders, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Cerebral infarction | 1 (0.7) | 0 | 1 (0.4) |
| Skin and subcutaneous disorders, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Drug eruption | 1 (0.7) | 0 | 1 (0.4) |
| Vascular disorders, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Aortic dissection | 1 (0.7) | 0 | 1 (0.4) |
TEAE, treatment-emergent adverse event.
Adverse events are presented by primary system organ class and preferred term. A patient is counted only once within each preferred term or any primary system organ class.
Iron Treatment
Over the 52 weeks of treatment, oral iron treatment (excluding iron treatment, which was not intended to supply iron) was administered to 19 of 153 patients in the molidustat group and 3 of 76 patients in the darbepoetin group. Intravenous iron treatment was administered to 95 patients in the molidustat group and to 48 patients in the darbepoetin group. The mean (SD) for the mean dosage of oral iron treatment during the 52-week treatment period was 29.18 (29.64) mg/day in the molidustat group and 42.99 (49.38) mg/day in the darbepoetin group. The mean (SD) for the mean dosage of intravenous iron treatment during the 52-week treatment period was 18.16 (11.81) mg/week in the molidustat group and 15.20 (9.14) mg/week in the darbepoetin group (Supplementary Table S5). Iron concentration, total iron binding capacity, unsaturated iron binding capacity, transferrin saturation and levels of hepcidin 25 and ferritin are presented in Supplementary Figure S3.
Discussion
This randomized, phase 3 trial compared the efficacy and safety of molidustat with darbepoetin for the maintenance treatment of renal anemia (mean screening Hb ≥9.5 and <12.0 g/dl) in Japanese patients receiving dialysis and ESAs. Throughout the 52-week treatment period, molidustat and darbepoetin maintained mean Hb levels within the target range of ≥10.0 and <12.0 g/dl. Molidustat was noninferior to darbepoetin for the change in mean Hb level during the evaluation period from baseline. Therefore, both hypotheses tested were supported. Overall, molidustat and darbepoetin were considered to have comparable safety and tolerability profiles.
These findings are consistent with previous studies that demonstrated that Hb levels were maintained within the target range after switching from an ESA (darbepoetin or epoetin) to molidustat in patients with renal anemia.13,14 Similar Hb levels were also previously reported in Japanese patients with renal anemia who were undergoing hemodialysis and who received roxadustat or daprodustat.15,16 Taken together, the current study and previous evidence suggest that HIF-PH inhibitors will likely become important tools for the management of renal anemia, although further safety studies are warranted.17
Molidustat was generally well tolerated in these previous phase 2 studies of up to 36 months’ duration.13,14 In the present study, the high incidences of TEAEs observed in both treatment groups were not surprising in this patient population and were in line with published data.16,18 There were numeric imbalances in the incidences of TEAEs resulting in discontinuation (including neoplasms considered by the investigator to be unrelated to study drug), of ocular AEs, of moderate and severe TEAEs, and in the number of patients who received rescue treatment that favored darbepoetin. Interestingly, in patients not receiving dialysis, the incidences of neoplasms and ocular AEs were similar between molidustat (3.7% and 19.5%, respectively) and darbepoetin (4.9% and 20.7%, respectively).19 It is also worth noting that, in the present study, none of the malignant tumors were considered to be related to molidustat by the investigators. In addition, the study was not designed to evaluate the outcome of tumors because patients with cancer at baseline could not be excluded from the study with certainty and because no protocol for tumor detection was prespecified. Finally, some emerging evidence from preclinical studies indicate that HIF-PH inhibitors might have anticancer effects.20,21 However, understanding the relationship between molidustat and the incidence of malignant tumors will require long-term evaluation (postmarketing surveillance, other real-world studies) and additional investigations, including basic mechanistic studies. The incidence of malignancy will be evaluated further alongside results from other phase 3 studies of molidustat.
The clinical impact of the increase in serum VEGF observed in the molidustat group remains unclear because there is no defined magnitude of increase in VEGF serum levels that is recognized as clinically significant. Furthermore, previous data in healthy Japanese individuals have indicated that molidustat did not have a dose-dependent effect on serum VEGF concentration during the treatment period.22
Strengths of this study include its multicenter, randomized, double-blinded, double-dummy, active comparator-controlled design, and its power to assess noninferiority of molidustat to darbepoetin. Although randomization generally balanced the treatment groups for demographics and baseline characteristics, there were numeric differences in some of these characteristics that could represent potential sources of bias. The exclusion of patients with a history of strokes or heart disease is another acknowledged limitation of this study. Moreover, the findings from this trial may not be generalizable to other countries or to patients who are younger than 20 years old, not Asian, or to patients previously untreated with ESAs. Results from a single-arm, phase 3 study of molidustat for anemia correction in Japanese patients receiving hemodialysis and untreated with ESAs (MIYABI Hemodialysis-Correction)7 has been published separately.12 Patients receiving peritoneal dialysis were not eligible for inclusion in MIYABI HD-M; effects of molidustat in these patients have been evaluated in another phase 3 study.7 Further studies are required to establish the long-term effects of molidustat treatment.
In conclusion, in Japanese patients receiving dialysis and previously treated with ESAs, molidustat maintained Hb levels during 52 weeks of treatment and was non-inferior to darbepoetin for change in mean Hb level; however, further investigation will be required to fully evaluate the safety and tolerability of molidustat in this patient population.
Disclosure
This study was funded by Bayer Yakuhin, Ltd. TA received consulting and lecture fees from Bayer Yakuhin, Ltd., during the conduct of the study. He also received consulting, lecture, or manuscript fees outside the submitted work from Astellas, Chugai Pharmaceutical, FUSO Pharmaceutical Industries, GlaxoSmithKline, Japan Tobacco Pharmaceuticals, KISSEI, Kyowa Kirin, NIPRO, Ono Pharmaceutical, Otsuka Pharmaceutical, Sanwa Chemical, and Torii Pharmaceutical. TY, KN, YM, YH, and TH are employees of Bayer Yakuhin, Ltd. HY received consulting and lecture fees from Bayer Yakuhin, Ltd. during the conduct of the study.
Acknowledgments
The authors thank Eriko Ogura for leading the protocol development and management of the trial and Takuto Yamashita for his contribution to the manuscript. Medical writing support was provided by Nicolas Bertheleme, PhD, of Oxford PharmaGenesis, Oxford, UK, with funding from Bayer Yakuhin, Ltd.
Author Contributions
HY, KN, TA,TY, and YM participated in the study concept and design. All authors were involved in the acquisition, analysis, and interpretation of data. All authors participated in preparing the manuscript.
DATA SHARING STATEMENT
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations/Pharmaceutical Research and Manufacturers of America Principles for Responsible Clinical Trial Data Sharing. This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing, on request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the European Union and United States, as necessary, for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after January 1, 2014.
Footnotes
Table S1. Proportion of patients whose rise in hemoglobin (Hb) level from the previous scheduled visit was >0.5 g/dl/week.
Table S2. Common treatment-emergent adverse events up to week 52 (safety analysis set).
Table S3. Treatment-emergent adverse events of neoplasms benign, malignant, and unspecified (including cysts and polyps).
Table S4. Serum vascular endothelial growth factor concentrations
Table S5. Iron treatment dose during the treatment period (safety analysis set).
Table S6. Coordinating investigator, central adjudication specialists, and data-monitoring committee members.
Table S7. Investigators and participating centers.
Figure S1. Proportion of patients with hemoglobin (Hb) levels in, above, or below the target range (full analysis set) following treatment with molidustat (a) and darbepoetin alfa (b).
Figure S2. Mean Hb levels by visit stratified by previous erythropoiesis-stimulating agent doses (below or above the median): (a) darbepoetin alfa, (b) epoetin beta pegol, or (c) epoetin alfa or beta.
Figure S3. Mean (SD) (a) serum iron concentration, (b) total iron binding capacity, (c) unsaturated iron binding capacity, (d) serum hepcidin 25 concentration, (e) serum ferritin concentration, and (f) serum transferrin saturation.
Supplementary Material
Table S1. Proportion of patients whose rise in hemoglobin (Hb) level from the previous scheduled visit was >0.5 g/dl/week.
Table S2. Common treatment-emergent adverse events up to week 52 (safety analysis set).
Table S3. Treatment-emergent adverse events of neoplasms benign, malignant, and unspecified (including cysts and polyps).
Table S4. Serum vascular endothelial growth factor concentrations
Table S5. Iron treatment dose during the treatment period (safety analysis set).
Table S6. Coordinating investigator, central adjudication specialists, and data-monitoring committee members.
Table S7. Investigators and participating centers.
Figure S1. Proportion of patients with hemoglobin (Hb) levels in, above, or below the target range (full analysis set) following treatment with molidustat (a) and darbepoetin alfa (b).
Figure S2. Mean Hb levels by visit stratified by previous erythropoiesis-stimulating agent doses (below or above the median): (a) darbepoetin alfa, (b) epoetin beta pegol, or (c) epoetin alfa or beta.
Figure S3. Mean (SD) (a) serum iron concentration, (b) total iron binding capacity, (c) unsaturated iron binding capacity, (d) serum hepcidin 25 concentration, (e) serum ferritin concentration, and (f) serum transferrin saturation.
References
- 1.Akizawa T., Okumura H., Alexandre A.F. Burden of anemia in chronic kidney disease patients in Japan: a Literature Review. Ther Apher Dial. 2018;22:444–456. doi: 10.1111/1744-9987.12712. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases Anemia in chronic kidney disease. https://www.niddk.nih.gov/health-information/kidney-disease/anemia
- 3.Stauffer M.E., Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9:e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisoni R.L., Bragg-Gresham J.L., Young E.W. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:94–111. doi: 10.1053/j.ajkd.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F., Pisoni R.L., Akizawa T. Anemia management for hemodialysis patients: Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines and Dialysis Outcomes and Practice Patterns Study (DOPPS) findings. Am J Kidney Dis. 2004;44:27–33. doi: 10.1053/j.ajkd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 7.Akizawa T., Taguchi M., Matsuda Y. Molidustat for the treatment of renal anaemia in patients with dialysis-dependent chronic kidney disease: design and rationale of three phase III studies. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Schaubel D.E., Kalbfleisch J.D. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int. 2012;81:1108–1115. doi: 10.1038/ki.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto H., Nishi S., Tomo T. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Renal Replace Ther. 2017;3:36. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 10.Flamme I., Oehme F., Ellinghaus P. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H., Taguchi M., Matsuda Y. Molidustat for the treatment of renal anaemia in patients with non-dialysis-dependent chronic kidney disease: design and rationale of two phase III studies. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akizawa T, Nobori K, Matsuda Y, et al. Molidustat for anemia correction in Japanese patients undergoing hemodialysis: a single-arm, phase 3 study. Ther Apher Dial. Posted online January 21, 2021. https://doi.org/10.1111/1744-9987.13627. [DOI] [PMC free article] [PubMed]
- 13.Macdougall I.C., Akizawa T., Berns J.S. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akizawa T., Macdougall I.C., Berns J.S. Long-term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE extension studies. Am J Nephrol. 2019;49:271–280. doi: 10.1159/000499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akizawa T., Iwasaki M., Yamaguchi Y. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akizawa T., Nangaku M., Yonekawa T. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol. 2020;15:1155–1165. doi: 10.2215/CJN.16011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta N., Wish J.B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69:815–826. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Nangaku M, Kondo K, Ueta K, et al. Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemic patients on hemodialysis: a phase 3, multicenter, randomized, double-blind study. Preprint. Posted online 21 February 2021. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab055. [DOI] [PMC free article] [PubMed]
- 19.Yamamoto H., Nobori K., Matsuda Y. Molidustat for renal anemia in non dialysis patients previously treated with erythropoiesis-stimulating agents: a randomized, open-label, phase 3 study. Am J Nephrol. 2021;9:e026704. doi: 10.1136/bmjopen-2018-026704. [DOI] [PubMed] [Google Scholar]
- 20.Nishide S., Uchida J., Matsunaga S. Prolyl-hydroxylase inhibitors reconstitute tumor blood vessels in mice. J Pharmacol Sci. 2020;143:122–126. doi: 10.1016/j.jphs.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Kachamakova-Trojanowska N., Podkalicka P., Bogacz T. HIF-1 stabilization exerts anticancer effects in breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2020;175:113922. doi: 10.1016/j.bcp.2020.113922. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa K, Uemura M, Matsuno K, et al. Safety, pharmacodynamics and pharmacokinetics of the oral selective hypoxia-inducible factor prolyl hydroxylase inhibitor molidustat in Japanese healthy subjects. In Proceedings of the 18th World Congress of Basic and Clinical Pharmacology. The Japanese Pharmacological Society, The Japanese Society of Clinical Pharmacology, 2018. https://doi.org/10.1254/jpssuppl.WCP2018.0_PO1-11-4
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.