Abstract
Objective
Flattening in the anteroposterior direction (AP flattening) of the terminal ileum (TI) or sigmoid colon (SC) lying across the psoas muscle, on magnetic resonance enterography (MRE), might mimic bowel inflammation in the coronal view. This study investigated the prevalence of AP flattening and the factors associated with its development.
Materials and Methods
A total of 364 surgery-naïve patients with Crohn's disease (CD) who had undergone MRE were retrospectively reviewed. AP flattening was defined as a luminal collapse in the anteroposterior direction, with a bowel width in the axial plane < 1/4 of the normal diameter without reduction of bowel width in coronal images. The prevalence of AP flattening of the TI and SC on MRE in patients with bowel segments lying across the psoas muscle was determined. We further compared the rate of AP flattening between MRE and computed tomography enterography (CTE) in a subcohort of patients with prior CTE. The factors associated with AP flattening were analyzed using multivariable logistic regression in a subcohort of patients with endoscopic findings of TI.
Results
Three hundred and twenty-two and 363 patients, respectively, had TI and SC lying across the psoas muscle. The prevalence of AP flattening on MRE was 7.5% (24/322) in TI and 5.2% (19/363) in SC. The prevalences were significantly higher on MRE than on CTE in both the TI (7.3% [12/164] vs. 0.6% [1/164]; p = 0.003) and SC (5.8% [11/190] vs. 1.6% [3/190]; p = 0.039). AP flattening of the TI was independently and strongly associated with the absence of CD inflammation on endoscopy, with an adjusted odds ratio of 0.066 (p = 0.003) for the presence versus the absence (reference) of inflammation.
Conclusion
AP flattening of the TI or SC lying across the psoas muscle was uncommon and predominantly observed on MRE of the bowel without CD inflammation.
Keywords: Crohn disease, Inflammatory bowel diseases, Magnetic resonance imaging, Diagnostic imaging
INTRODUCTION
Magnetic resonance enterography (MRE) and computed tomography enterography (CTE) are the primary imaging modalities used to evaluate patients with Crohn's disease (CD) [1,2,3]. Owing to the absence of radiation exposure, MRE is particularly useful for patients with CD, who tend to be young and require lifelong repetitive examinations [4,5]. The cardinal findings of bowel inflammation in CD on enterography include bowel wall thickening and hyperenhancement [6,7]. In clinical practice, flattening in the anteroposterior direction (AP flattening) of the terminal ileum or sigmoid colon lying across the psoas muscle is occasionally observed on MRE (Fig. 1). AP flattening creates an appearance resembling mural thickening and hyperenhancement of the bowel in the coronal view, which is the primary imaging plane of MRE [1,8], as coronal cross-sectional planes cut through the sheet-like flattened bowel wall. Therefore, AP flattening mimics bowel inflammation in CD. Unlike the usual concentric collapse of the bowel, it is difficult to recognize AP flattening unless the reader is familiar with the finding or makes a close correlation with axial images because the bowel width appears slightly enlarged or normal instead of shrunken in the coronal view. Therefore, we noted that cases of AP flattening were sometimes misinterpreted as bowel inflammation in our practice.
Fig. 1. AP flattening of the terminal ileum and sigmoid colon lying across the psoas muscle.
A. A schematic drawing showing AP flattening of the terminal ileum (white arrow) and sigmoid colon (black arrow) lying across the psoas muscle (asterisks). AP flattening was empirically defined as a luminal collapse in the anteroposterior direction, with a bowel width in axial images of < 1/4 of the diameter of the well-distended adjacent bowel segments. B, C. AP flattening of the terminal ileum (arrowheads) mimics mural thickening and hyperenhancement in the coronal view (B). The pseudo-abnormality created by bowel collapse (arrowheads) in the anteroposterior direction is recognizable in axial images (C). The bowel width in the segment showing AP flattening (arrowheads) is 4.1 mm in the axial view, which is < 1/4 of the 1.9-cm diameter of the adjacent well-distended bowel segment. The bowel segment showing AP flattening (arrowheads) appears slightly stretched craniocaudally (2.2 cm in width) in the coronal view compared to the adjacent well-distended bowel segment. D, E. AP flattening of the sigmoid colon (arrowheads) mimics mural thickening and hyperenhancement in the coronal view (D). The pseudo-abnormality created by bowel collapse (arrowheads) is recognizable in correlation with axial images (E). The bowel width in the segment showing AP flattening (arrowheads) is 4.5 mm in the axial view, which is < 1/4 of the 1.9-cm diameter of the adjacent well-distended bowel segment. The width of the segment showing AP flattening (arrowheads) is maintained in the coronal view (1.9 cm) compared to the adjacent well-distended bowel segment. AP flattening = flattening in the anteroposterior direction
To our knowledge, this unique pattern of bowel collapse as a potential interpretive pitfall has not been described in the literature, although the challenges in interpreting MRE posed by the usual concentric bowel collapse are well known [9]. Furthermore, considering that the terminal ileum and sigmoid colon are the bowel regions frequently affected by CD [10], further knowledge of the phenomenon of AP flattening would be particularly beneficial for a more accurate interpretation of MRE in patients with CD. Therefore, we investigated the prevalence of AP flattening of the terminal ileum or sigmoid colon on MRE in patients with CD, as well as the factors associated with the development of AP flattening.
MATERIALS AND METHODS
The Institutional Review Board of Asan Medical Center approved this retrospective study (IRB No. 2020-0813) and waived the requirement for written informed consent.
Study Participants
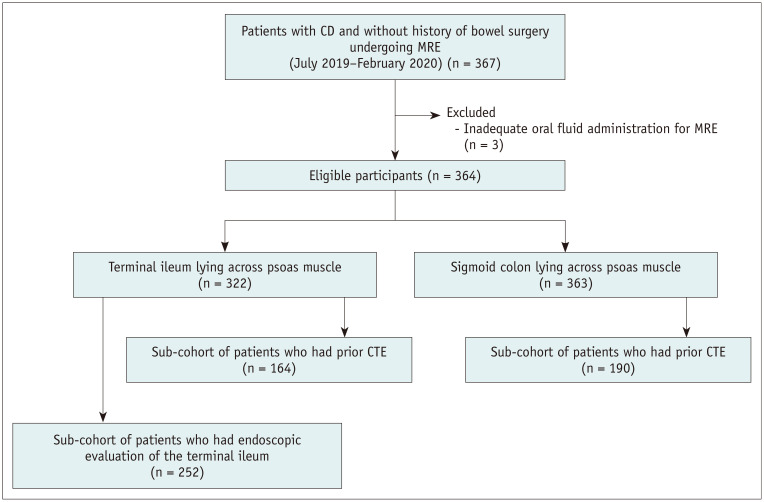
Figure 2 summarizes the patient selection process. A total of 367 consecutive patients with CD with no history of bowel surgery and who had undergone MRE between July 2019 and February 2020 at Asan Medical Center, a tertiary referral center, were initially recruited. The indications for MRE in CD at our institution were consistent with those recommended [2,11] for the baseline or follow-up assessment for medical therapy. After reviewing the technical notes taken at the time of MRE examination, to avoid confounding effects on bowel distention, we excluded three patients who did not receive the complete oral fluid administration required for MRE, leaving a study cohort comprising 364 patients (269 male and 95 female; mean age ± standard deviation, 31.2 ± 9.4 years). We further identified subcohorts of patients who had undergone CTE as the prior enterography examination and whose endoscopic findings of the terminal ileum were available (see Clinical Data Collection and Statistical Analysis sections).
Fig. 2. Flow diagram of the study participants.
CD = Crohn's disease, CTE = computed tomography enterography, MRE = magnetic resonance enterography
MRE and CTE Acquisition
MRE was performed immediately after the oral administration of 1200 mL polyethylene glycol (approximately 30–40 minutes; 150 mL every 5 minutes). Scanning was performed with the patient in the supine position using a 3T magnetic resonance imaging scanner (Ingenia; Philips Healthcare) and included the following sequences: coronal T2-weighted half-Fourier sequences with and without fat suppression, axial T2-weighted half-Fourier sequence with fat suppression, coronal diffusion-weighted imaging (DWI) with b-factors of 0 and 900 s/mm2 and apparent diffusion coefficient map; coronal pre- and post-contrast (enteric and portal phases) T1-weighted spoiled gradient-echo sequences with fat suppression; and axial delayed post-contrast T1-weighted spoiled gradient-echo sequence with fat suppression. For contrast enhancement, 0.2 mL/kg of gadoterate meglumine (Dotarem; Guerbet) was intravenously administered at a rate of 2 mL/s, followed by a saline flush. To avoid bowel peristalsis, two 10-mg doses of scopolamine-N-butyl bromide (Buscopan; Boehringer Ingelheim) were administered intravenously, once at the start of the examination and the other before T1-weighted imaging. The total patient time on the scanner was approximately 30 minutes. The detailed technical parameters are provided in Supplementary Table 1.
CTE was performed after administering oral fluid in the same manner as for MRE. However, no spasmolytic agents were administered. Enteric-phase images were obtained in the supine position after the administration of an intravenous bolus of non-ionic iodinated contrast material (100–150 mL of 320 mgI/mL) at 3 mL/s using 64- or 128-detector row scanners (SOMATOM series; Siemens). The scan parameters were as follows: beam pitch, 1; gantry rotation time, 0.5 seconds; field of view to fit, 100 or 120 kVp; and automated tube current modulation (CARE Dose 4D) with quality reference mAs set at 200. Both axial and coronal images were obtained at 3-mm thickness and 3-mm intervals.
MRE and CTE Analysis
The images were reviewed by a board-certified gastrointestinal radiologist with a 2-year experience in interpreting MRE and CTE after confirmation of a near-complete agreement with a senior radiologist with approximately 8 years of experience in MRE (further details are provided in Supplementary Fig. 1). Coronal and axial T2- and T1-weighted images were analyzed. Axial DWI scans were absent and DWI did not provide as many anatomical details as T2- or T1-weighted images. Therefore, DWI scans were not analyzed. The reader first determined whether the terminal ileum or sigmoid colon was lying across the psoas muscle. When such a finding was noted, the reader then determined the presence of AP flattening in the bowel segments. AP flattening was empirically defined as a luminal collapse in the anteroposterior direction with a bowel width in axial images of < 1/4 of the diameter of the well-distended adjacent bowel segments (Fig. 1) with no change or slight stretching of the bowel width craniocaudally in coronal images, unlike the usual circumferential concentric bowel collapse in all directions. AP flattening was assessed separately for the T2- and T1-weighted images. In the subcohort of patients with prior CTE (Fig. 2), the CTE images were analyzed similarly to the MRE images.
In the subgroup of patients in which endoscopic findings of the terminal ileum were available (Fig. 2), an additional anthropometric analysis was performed for the terminal ileum. We recorded the level of the terminal ileum lying across the psoas muscle using the ipsilateral iliac vessels as landmarks (at the level of the external iliac vessels vs. at or above the level of the common iliac vessels). In axial images, we measured the thickness of the anterior abdominal wall over the terminal ileum lying across the psoas muscle (abdominal wall thickness) and the anteroposterior distance between the inner margin of the anterior abdominal wall and the anterior margin of the psoas muscle (peritoneal space width) (Fig. 3). These measurements were obtained from T1-weighted axial images, as the skin-side boundary of the abdominal wall is obscure in fat-suppressed T2-weighted axial images.
Fig. 3. Measurement of the abdominal wall thickness (solid double-headed arrow) and peritoneal space width (dashed double-headed arrow) at the level of the terminal ileum (asterisk) lying across the psoas muscle in an axial magnetic resonance enterography image.
Clinical Data Collection
Demographic and CD-related patient characteristics at the time of MRE were collected through a review of the electronic medical records (Table 1). Patients who had undergone endoscopic evaluation of the terminal ileum within 3 months before or after MRE were identified, and their endoscopic findings of the terminal ileum were collected retrospectively from captured images and clinical endoscopic reports. The 3-month limit was chosen because it is considered an acceptable time interval for comparing endoscopic findings with other data in studies of CD based on the chronic nature of the disease [12,13]. Board-certified gastroenterologists specializing in inflammatory bowel disease performed the endoscopic examinations. In institutional routine clinical practice, endoscopic findings of the terminal ileum were described as the presence (ranging from aphthous ileitis to deep ulcerations) or absence of active inflammation, and corresponding images were captured. As the examiners did not routinely use formal severity scores, such as the Crohn's Disease Endoscopic Index of Severity score [14] or Simple Endoscopic Score for Crohn's Disease [15], such scores were not available for this study.
Table 1. Patient Characteristics.
| Characteristics | All Patients (n = 364) |
Patients with the Terminal Ileum Lying Across the Psoas Muscle | Patients with the Sigmoid Colon Lying Across the Psoas Muscle | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Patients in the Group (n = 322) |
Subcohort of Patients with Prior CTE (n = 164) |
P * | Subcohort of Patients with Endoscopic Results (n = 252) |
P * | All Patients in the Group (n = 363) |
Subcohort of Patients with Prior CTE (n = 190) |
P † | |||
| Age, year | 31.2 ± 9.4 | 30.9 ± 9.5 | 32.4 ± 9.7 | 0.115 | 30.7 ± 9.4 | 0.776 | 31.2 ± 9.4 | 32.7 ± 9.5 | 0.079 | |
| Sex | 0.456 | 0.767 | 0.408 | |||||||
| Male | 269 (73.9) | 238 (73.9) | 116 (70.7) | 189 (75.0) | 268 (73.8) | 134 (70.5) | ||||
| Female | 95 (26.1) | 84 (26.1) | 48 (29.3) | 63 (25.0) | 95 (26.2) | 56 (29.5) | ||||
| Height, cm | 169.5 ± 7.5 | 169.6 ± 7.5 | 168.6 ± 7.1 | 0.165 | 169.7 ± 7.5 | 0.850 | 169.5 ± 7.6 | 168.5 ± 7.2 | 0.116 | |
| Weight, kg | 65.6 ± 13.3 | 65.7 ± 13.3 | 64.8 ± 12.3 | 0.445 | 66.5 ± 13.6 | 0.509 | 65.6 ± 13.3 | 64.6 ± 12.5 | 0.392 | |
| BMI, kg/m2 | 22.7 ± 3.7 | 22.7 ± 3.7 | 22.7 ± 3.4 | 0.865 | 23.0 ± 3.8 | 0.484 | 22.7 ± 3.7 | 22.6 ± 3.5 | 0.844 | |
| CDAI score | 0.461 | 0.850 | 0.674 | |||||||
| < 150 | 319 (87.6) | 283 (87.9) | 146 (89.0) | 223 (88.5) | 318 (87.6) | 169 (88.9) | ||||
| 150–219 | 19 (5.2) | 15 (4.7) | 10 (6.1) | 13 (5.2) | 19 (5.2) | 11 (5.8) | ||||
| 220–450 | 26 (7.1) | 24 (7.5) | 8 (4.9) | 16 (6.3) | 26 (7.2) | 10 (5.3) | ||||
| > 450 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
Data are expressed as mean ± standard deviation for continuous variables and as number (percentage) for categorical variables. Patient characteristics were compared using the independent t test or chi-square test, as appropriate, according to the data type. *For comparison with all 322 patients in the group, †For comparison with all 363 patients in the group. BMI = body mass index, CTE = computed tomography enterography, CDAI = Crohn's Disease Activity Index
The clinical radiology reports following MRE were reviewed in patients showing AP flattening to assess how AP flattening affected the ultimate interpretation of MRE. We investigated whether the bowel segments were interpreted in the clinical reports as having inflammation. We used data from the terminal ileum alone because our MRE reporting of the sigmoid colon was often inconclusive. This reporting style was based on the fact that the sigmoid colon is not the main area of interest for MRE and is examined primarily by endoscopy as the technical condition for magnetic resonance imaging is suboptimal at times in the colorectum, unlike the small bowel.
Statistical Analysis
The primary study outcome was the prevalence of AP flattening in the terminal ileum and sigmoid colon. The prevalence of AP flattening on MRE was compared to that on CTE in a subcohort of patients who had undergone prior CTE (Fig. 2) using McNemar's test.
Additionally, factors associated with the occurrence of AP flattening on MRE were analyzed in a subcohort of patients who underwent terminal ileum endoscopy within ± 3 months of MRE (Fig. 2). We performed the analysis for the terminal ileum but not for the sigmoid colon for the following reasons. The inflammatory status of the bowel should be examined as a potential factor for which endoscopic findings are necessary. Unlike the terminal ileum, retrospective matching of the bowel location between endoscopy and MRE for the sigmoid colon lying across the psoas muscle was deemed unreliable and inaccurate because the sigmoid colon is typically much longer and convoluted compared to the terminal ileum. We performed multivariable logistic regression analysis, including the various demographic characteristics, level of the terminal ileum, abdominal wall thickness, peritoneal space width, and endoscopic findings as independent variables. Firth's correction was used for the logistic regression analysis to address complete separation in our data; that is, no incidents of AP flattening in patients with terminal ileal inflammation on endoscopy [16]. Variables with p < 0.25 in univariable analysis were included in the multivariable analysis.
R software version 4.0.2 (R Foundation for Statistical Computing) was used to perform the statistical analysis. p < 0.05 was considered statistically significant.
RESULTS
Study Participants
The constitution of the study patients and baseline patient characteristics at the time of MRE are summarized in Figure 2 and Table 1. Of the 364 patients, 322 and 363 had the terminal ileum and sigmoid colon lying across the psoas muscle, respectively. Of them, 164 and 190 patients, respectively, had undergone CTE for enterography examination before MRE (median, 24 months; interquartile range, 15–30 months). The characteristics of the subcohort of patients who had undergone prior CTE did not differ significantly from those of the entire corresponding set of patients (Table 1). Of the 322 patients with the terminal ileum lying across the psoas muscle, 252 had available endoscopic findings of the terminal ileum obtained within ± 3 months (median, 5 days; interquartile range, 2–8 days) of MRE. The characteristics of the 252 patients did not differ significantly from those of the corresponding set of patients (Table 1).
Prevalence of AP Flattening
AP flattening in T2- or T1-weighted images was observed in the terminal ileum in 7.5% (24/322) of patients and in the sigmoid colon in 5.2% (19/363) of patients (Table 2). When classified based on the MRE sequence, AP flattening was noted in T2-weighted images, T1-weighted images, and both sequences in the terminal ileum in 5.9% (19/322), 6.8% (22/322), and 5.3% (17/322) of patients, respectively, and in the sigmoid colon in 4.1% (15/363), 5.0% (18/363), and 3.9% (14/363) of patients, respectively.
Table 2. Prevalence of AP Flattening.
| Patients with the Terminal Ileum Lying Across the Psoas Muscle | Patients with the Sigmoid Colon Lying Across the Psoas Muscle | ||||||
|---|---|---|---|---|---|---|---|
| All Patients (n = 322) |
Subcohort of Patients with Prior CTE (n = 164) |
All Patients (n = 363) |
Subcohort of Patients with Prior CTE (n = 190) |
||||
| MRE | MRE | CTE | P | MRE | MRE | CTE | P |
| 7.5% (24/322) | 7.3% (12/164) | 0.6% (1/164) | 0.003 | 5.2% (19/363) | 5.8% (11/190) | 1.6% (3/190) | 0.039 |
Numbers in parentheses represent the corresponding numbers of patients. AP flattening = flattening in the anteroposterior direction, CTE = computed tomography enterography, MRE = magnetic resonance enterography
According to our clinical reports following MRE, 45.8% (11/24) of the terminal ileal segments showing AP flattening were interpreted as having inflammation. Nine of the 11 cases occurred during the first half of the study period. Endoscopic confirmation was available for 22 of the 24 cases of AP flattening, including the 11 cases. None of the patients had inflammation in the terminal ileum on endoscopy.
Comparison with CTE
For the subcohort of patients who had undergone prior CTE, the results of the comparison of the rates of AP flattening between MRE and CTE are summarized in Table 2. The prevalence of AP flattening in the terminal ileum was significantly higher on MRE than on CTE (7.3% [12/164 patients] vs. 0.6% [1/164 patients]; p = 0.003; Fig. 4). The prevalence of AP flattening in the sigmoid colon was also significantly higher on MRE than on CTE (5.8% [11/190 patients] vs. 1.6% [3/190 patients]; p = 0.039).
Fig. 4. A patient showing AP flattening of the terminal ileum on MRE (arrowheads on the left; A, B) but not on CTE (arrowheads on the right; A, B).
AP flattening = flattening in the anteroposterior direction, CTE = computed tomography enterography, MRE = magnetic resonance enterography
Factors associated with AP Flattening
In the subgroup of 252 patients with available endoscopic findings for the terminal ileum, multivariable logistic regression analysis (Table 3) revealed that the absence of inflammation in the terminal ileum (reference category), as compared to active inflammation on endoscopy, was a significant independent factor associated with AP flattening of the terminal ileum, with an adjusted odds ratio of 0.066 (95% confidence interval, 0.001–0.498; p = 0.003). The likelihood of AP flattening of the terminal ileum was approximately 15 times greater when there was no inflammation than when there was active inflammation. While 63 of 252 patients had active inflammation in the terminal ileum on endoscopy, none showed AP flattening on MRE. None of the 22 patients who showed AP flattening on MRE had inflammation in the terminal ileum on endoscopy. None of the other variables were significantly associated with AP flattening.
Table 3. Factors associated with AP Flattening of the Terminal Ileum.
| Variables | Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Adjusted Odds Ratio | 95% CI | P | |||
| Demographic findings | ||||||||
| Age, year | 0.963 | 0.907–1.013 | 0.153 | 0.964 | 0.905–1.017 | 0.192 | ||
| Sex | ||||||||
| Male | Reference category | Reference category | ||||||
| Female | 0.506 | 0.130–1.467 | 0.225 | 1.032 | 0.209–4.365 | 0.967 | ||
| Height, cm | 1.052 | 0.991–1.119 | 0.096 | 1.062 | 0.979–1.152 | 0.146 | ||
| Weight, kg | 1.011 | 0.979–1.042 | 0.495 | |||||
| BMI, kg/m2 | 1.001 | 0.888–1.116 | 0.983 | |||||
| MRE findings | ||||||||
| Level of the traversing terminal ileum | ||||||||
| External iliac vessels | Reference category | Reference category | ||||||
| Common iliac vessels or higher | 2.346 | 0.875–5.838 | 0.088 | 2.174 | 0.767–5.868 | 0.139 | ||
| Abdominal wall thickness, mm | 1.002 | 0.959–1.043 | 0.933 | |||||
| Peritoneal space width, mm | 0.999 | 0.965–1.030 | 0.961 | |||||
| Endoscopic findings | ||||||||
| Inflammation in the terminal ileum | ||||||||
| Absent | Reference category | Reference category | ||||||
| Present | 0.059 | 0.000–0.434 | 0.001 | 0.066 | 0.001–0.498 | 0.003 | ||
Firth's correction was applied to the logistic regression analysis to deal with the complete separation of the data. Factors with p < 0.25, in the univariable analysis, were included in the multivariable analysis. An odds ratio > 1 indicates a greater likelihood of AP flattening compared to the reference. AP flattening = flattening in the anteroposterior direction, BMI = body mass index, CI = confidence interval, MRE = magnetic resonance enterography
DISCUSSION
The results of the present study indicated that AP flattening occurring in the terminal ileum or sigmoid colon lying across the psoas muscle, which may mimic bowel inflammation of CD in the coronal view, is an uncommon phenomenon predominantly seen on MRE. However, since the terminal ileum and sigmoid colon are bowel regions frequently affected by CD [10], findings that can mimic inflammation in these locations should be particularly noteworthy to avoid false interpretation of bowel inflammation.
The observation of AP flattening in both T2- and T1-weighted images in most patients with AP flattening (17/24 in the terminal ileum and 14/19 in the sigmoid colon) requires further attention regarding the potential interpretive pitfall as persistent flattening across sequences may prevent the recognition of pseudo-abnormalities by presenting the well-distended bowel on either sequence. Both coronal and axial images are recommended to be acquired and reviewed in MRE examinations, although the coronal plane is the primary imaging plane for enterographic evaluation of the bowel [1,8]. Therefore, when inflammation is suspected in the terminal ileum or sigmoid colon lying across the psoas muscle in the coronal view during MRE interpretation, a careful correlation with axial images is needed to confirm whether the segments are flattened to avoid interpretive pitfalls.
The exact mechanism of AP flattening can only be speculated. The fact that the phenomenon was noted nearly exclusively by MRE and not by CTE provides some plausible explanations, which may involve several factors. First, unlike CTE, MRE was performed after the intravenous administration of spasmolytics, which decrease bowel tone [17,18], as recommended to avoid artifacts due to peristaltic motion [1,2,6,8]. Second, patients spend longer times on the scanner for MRE compared to CTE [19]. Third, the terminal ileum or sigmoid colon lying over the psoas muscle, which protrudes somewhat anteriorly, is at a more non-dependent location in the supine position than the adjacent bowel parts. Combined, these factors may result in a relative tendency for the luminal fluid in the segment over the psoas muscle to passively shift to regions in more dependent locations because of gravity in MRE compared to CTE. The same phenomenon is less likely to occur during CTE as a greater bowel tone and occasional peristalsis might resist the passive fluid shift; moreover, the patient time on the scanner is also much shorter [19].
The results of this study also revealed that the absence of inflammation in the bowel was independently and strongly associated with AP flattening. None of the 63 patients with endoscopically proven active inflammation in the terminal ileum showed AP flattening on MRE. This result seems logical because inflammation causes tissue induration, which would likely resist flattening of the bowel owing to edema and, in the case of CD, because of fibrosis, which often coexists with bowel inflammation [20,21,22,23]. In some sense, once the reader becomes familiar with the finding of AP flattening, it may be used as a sign to help exclude bowel inflammation instead of merely being an interpretive pitfall.
This study had the following limitations. First, in this retrospective study, not all patients had undergone prior CTE or endoscopy for comparison with MRE. As MRE and CTE were compared and the analysis of factors associated with AP flattening was performed in select patients, our study had an inherent risk of selection bias. This risk cannot be ignored despite the similarity in baseline patient characteristics between the subcohorts and the entire corresponding set of patients. Second, regarding the analysis of factors associated with AP flattening, a shorter interval between MRE and endoscopy and (semi-) quantitative scoring of the inflammatory severity on endoscopy, as often adopted in prospective studies, would have allowed more precise analysis. We chose a 3-month interval for our study as this duration is considered an acceptable, if not ideal, time window for comparing endoscopic findings to other data in retrospective studies of CD considering the chronic nature of the disease [12,13]. Previous studies have adopted a broader interval of 6 months [24].
In conclusion, AP flattening of the terminal ileum or sigmoid colon lying across the psoas muscle was an uncommon finding predominantly seen on MRE. This may represent a potential interpretive pitfall that mimics bowel inflammation in CD. Nonetheless, once the reader recognizes the finding, it may be used as a sign to help exclude bowel inflammation.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Seong Ho Park.
- Formal analysis: Dong Wook Kim, Seong Ho Park.
- Investigation: Dong Wook Kim.
- Methodology: Dong Wook Kim, Seong Ho Park.
- Project administration: Seong Ho Park.
- Resources: Jong Seok Lee, Hyun Jin Kim, Ah Young Kim, Byong Duk Ye, Suk-Kyun Yang.
- Supervision: Seong Ho Park, Byong Duk Ye, Suk-Kyun Yang.
- Visualization: Dong Wook Kim.
- Writing—original draft: Dong Wook Kim.
- Writing—review & editing: Seong Ho Park, Jong Seok Lee, Hyun Jin Kim, Ah Young Kim, Byong Duk Ye, Suk-Kyun Yang.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2020.1420.
Technical Parameters of Magnetic Resonance Enterography
Inter-reader agreement for assessing AP flattening.
References
- 1.Grand DJ, Guglielmo FF, Al-Hawary MM. MR enterography in Crohn's disease: current consensus on optimal imaging technique and future advances from the SAR Crohn's disease-focused panel. Abdom Imaging. 2015;40:953–964. doi: 10.1007/s00261-015-0361-8. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Chang KJ, Fowler KJ, Cash BD, Garcia EM, Kambadakone AR, et al. ACR appropriateness criteria® Crohn disease. J Am Coll Radiol. 2020;17:S81–S99. doi: 10.1016/j.jacr.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Park SH, Ye BD, Lee TY, Fletcher JG. Computed tomography and magnetic resonance small bowel enterography: current status and future trends focusing on Crohn's disease. Gastroenterol Clin North Am. 2018;47:475–499. doi: 10.1016/j.gtc.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Desmond AN, O'Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, et al. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524–1529. doi: 10.1136/gut.2008.151415. [DOI] [PubMed] [Google Scholar]
- 5.Levi Z, Fraser E, Krongrad R, Hazazi R, benjaminov O, meyerovitch J, et al. Factors associated with radiation exposure in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30:1128–1136. doi: 10.1111/j.1365-2036.2009.04140.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruining DH, Zimmermann EM, Loftus EV, Jr, Sandborn WJ, Sauer CG, Strong SA Society of Abdominal Radiology Crohn's Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn's disease. Radiology. 2018;286:776–799. doi: 10.1148/radiol.2018171737. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmo FF, Anupindi SA, Fletcher JG, Al-Hawary MM, Dillman JR, Grand DJ, et al. Small bowel Crohn disease at CT and MR enterography: imaging atlas and glossary of terms. Radiographics. 2020;40:354–375. doi: 10.1148/rg.2020190091. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SA, Avni F, Cronin CG, Hoeffel C, Kim SH, Laghi A, et al. The first joint ESGAR/ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol. 2017;27:2570–2582. doi: 10.1007/s00330-016-4615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha R, Verma R, Verma S, Rajesh A. MR enterography of Crohn disease: part 1, rationale, technique, and pitfalls. AJR Am J Roentgenol. 2011;197:76–79. doi: 10.2214/AJR.10.7253. [DOI] [PubMed] [Google Scholar]
- 10.Timmer A, Breuer-Katschinski B, Goebell H. Time trends in the incidence and disease location of Crohn's disease 1980–1995: a prospective analysis in an urban population in Germany. Inflamm Bowel Dis. 1999;5:79–84. doi: 10.1097/00054725-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Grand DJ, Beland M, Harris A. Magnetic resonance enterography. Radiol Clin North Am. 2013;51:99–112. doi: 10.1016/j.rcl.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Koulaouzidis A, Sipponen T, Nemeth A, Makins R, Kopylov U, Nadler M, et al. Association between fecal calprotectin levels and small-bowel inflammation score in capsule endoscopy: a multicenter retrospective study. Dig Dis Sci. 2016;61:2033–2040. doi: 10.1007/s10620-016-4104-7. [DOI] [PubMed] [Google Scholar]
- 13.Noh SM, Oh EH, Park SH, Lee JB, Kim JY, Park JC, et al. Association of faecal calprotectin level and combined endoscopic and radiological healing in patients with Crohn's disease receiving anti-tumour necrosis factor Therapy. J Crohns Colitis. 2020;14:1231–1240. doi: 10.1093/ecco-jcc/jjaa042. [DOI] [PubMed] [Google Scholar]
- 14.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 16.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21:2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 17.Cronin CG, Dowd G, Mhuircheartaigh JN, DeLappe E, Allen RH, Roche C, et al. Hypotonic MR duodenography with water ingestion alone: feasibility and technique. Eur Radiol. 2009;19:1731–1735. doi: 10.1007/s00330-009-1346-1. [DOI] [PubMed] [Google Scholar]
- 18.Menys A, Taylor SA, Emmanuel A, Ahmed A, Plumb AA, Odille F, et al. Global small bowel motility: assessment with dynamic MR imaging. Radiology. 2013;269:443–450. doi: 10.1148/radiology.13130151. [DOI] [PubMed] [Google Scholar]
- 19.Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 20.Adler J, Punglia DR, Dillman JR, Polydorides AD, Dave M, Al-Hawary MM, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn's disease. Inflamm Bowel Dis. 2012;18:849–856. doi: 10.1002/ibd.21801. [DOI] [PubMed] [Google Scholar]
- 21.Barkmeier DT, Dillman JR, Al-Hawary M, Heider A, Davenport MS, Smith EA, et al. MR enterography-histology comparison in resected pediatric small bowel Crohn disease strictures: can imaging predict fibrosis? Pediatr Radiol. 2016;46:498–507. doi: 10.1007/s00247-015-3506-6. [DOI] [PubMed] [Google Scholar]
- 22.Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn's disease. Am J Gastroenterol. 2007;102:2541–2550. doi: 10.1111/j.1572-0241.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 23.Zappa M, Stefanescu C, Cazals-Hatem D, Bretagnol F, Deschamps L, Attar A, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17:984–993. doi: 10.1002/ibd.21414. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes SR, Rodrigues RV, Bernardo S, Cortez-Pinto J, Rosa I, da Silva JP, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn's disease. Inflamm Bowel Dis. 2017;23:1403–1409. doi: 10.1097/MIB.0000000000001143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical Parameters of Magnetic Resonance Enterography
Inter-reader agreement for assessing AP flattening.