Abstract
Objective
Arrhythmogenic mitral valve prolapse (MVP) is an important cause of sudden cardiac death characterized by fibrosis of the papillary muscles or left ventricle (LV) wall, and an association between late gadolinium enhancement (LGE) of the LV papillary muscles and ventricular arrhythmia in MVP has been reported. However, LGE of the papillary muscles may be observed in other causes of mitral regurgitation, and it is not limited to patients with MVP. This study was to evaluate the association of LGE of the LV papillary muscles or ventricular wall on cardiac magnetic resonance imaging (CMR) and ventricular arrhythmia in patients with mitral regurgitation.
Materials and Methods
This study included 88 patients (mean age ± standard deviation, 58.3 ± 12.0 years; male, 42%) with mitral regurgitation who underwent CMR. They were allocated to the MVP (n = 43) and non-MVP (n = 45) groups, and their LGE images on CMR, clinical characteristics, echocardiographic findings, and presence of arrhythmia were compared.
Results
LV myocardial wall enhancement was more frequent in the MVP group than in the non-MVP group (28% vs. 11%, p = 0.046). Papillary muscle enhancement was observed in 7 (7.9%) patients. Of the 43 patients with MVP, 15 (34.8%) showed LGE in the papillary muscles or LV myocardium, including 12 (27.9%) with LV myocardial wall enhancement and 4 (9.3%) with papillary muscle enhancement. One patient with bilateral diffuse papillary muscle enhancement experienced sudden cardiac arrest due to ventricular fibrillation. Univariable logistic regression analysis showed that high systolic blood pressure (BP; odds ratio [OR], 1.05; 95% confidence interval [CI], 1.01–1.09; p = 0.027) and ventricular arrhythmia (OR, 6.84; 95% CI, 1.29–36.19; p = 0.024) were significantly associated with LGE of the papillary muscles.
Conclusion
LGE of the papillary muscles was present not only in patients with MVP, but also in patients with other etiologies of mitral regurgitation, and it was associated with high systolic BP and ventricular arrhythmia. Papillary muscle enhancement on CMR should not be overlooked.
Keywords: Mitral regurgitation, Mitral valve prolapse, Late gadolinium enhancement, Arrhythmia
INTRODUCTION
The function of the mitral valve relies on the coordination of several components, including the mitral annulus, mitral valve leaflets, chordae tendineae, papillary muscles, left ventricle (LV), and left atrium [1]. Discoordination or defects of any component can cause mitral regurgitation. Among the conditions associated with mitral regurgitation, mitral valve prolapse (MVP) is associated with sudden cardiac death and ventricular arrhythmias [2,3,4,5]. In addition, late gadolinium enhancement (LGE) of the papillary muscles on cardiac magnetic resonance imaging (CMR) was found to be associated with complex ventricular arrhythmia in patients with MVP [6]. Arrhythmogenic MVP is characterized by fibrosis of the papillary muscles and the inferobasal LV wall [7]. Moreover, LGE of the papillary muscles was correlated with the origin of electrophysiologically determined ventricular arrhythmia [8].
However, the mechanism underlying the association between papillary muscle enhancement and ventricular arrhythmia in patients with MVP remains unclear despite several hypotheses suggesting that arrhythmogenic MVP may be caused by mechanical stretching of the papillary muscles and LV wall, improper autonomic tone, or endocardial friction lesions [9,10,11,12]. Moreover, it is not fully known if patients with mitral regurgitation other than MVP may present with LGE of the LV wall or papillary muscles, which could be associated with arrhythmia. The present study investigated the CMR findings of patients with mitral regurgitation, which is not limited to MVP, and evaluated the clinical factors, including arrhythmia. The goal of this study was to determine whether the LGE of the papillary muscle or LV wall in patients with mitral regurgitation is associated with ventricular arrhythmia.
MATERIALS AND METHODS
Patient Selection
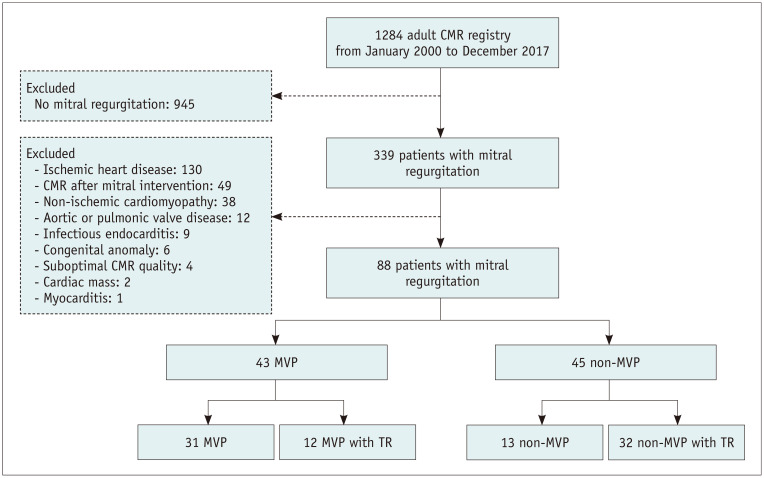
This retrospective observational study was approved by the Institutional Review Board of our hospital (approval number: 2018-0058), which waived the requirement for informed consent. Between January 2000 and December 2017, 1284 adult patients underwent CMR at our tertiary medical center. The decision to perform CMR was based on the clinician's opinion. Of these 1284 patients, 339 were diagnosed with mitral regurgitation on echocardiography. Patients with ischemic heart disease (n = 130) and non-ischemic cardiomyopathy (n = 38), such as sarcoidosis, amyloidosis, hypertrophic cardiomyopathy, concomitant aortic or pulmonic valve disease (n = 12), infectious endocarditis (n = 9), congenital anomalies such as mitral cleft (n = 6), cardiac mass (n = 2), and myocarditis (n = 1), as well as those with suboptimal CMR image quality (n = 4), were excluded because these conditions are thought to directly affect the evaluation of the mitral apparatus or LV myocardium (Fig. 1). Patients with moderate (50–69%) and severe (≥ 70%) coronary artery stenosis diagnosed by invasive coronary angiography or coronary artery CT angiography, defined as ischemic heart disease, were excluded (n = 130). Patients who underwent CMR after cardiac interventions, such as mitral valve surgery or cardiac ablation, were also excluded (n = 49). Finally, 88 patients with mitral regurgitation were included in the study. Clinical findings, including the presence of arrhythmia, use of antiarrhythmic drugs, echocardiography results, presence of comorbidities, and follow-up periods, were thoroughly reviewed. Follow-up data, including mortality, were obtained from patients' electronic medical records and by reviewing the nationwide data on deaths provided by the National Statistics Office.
Fig. 1. Study flow chart.
CMR = cardiac magnetic resonance imaging, MVP = mitral valve prolapse, TR = tricuspid regurgitation
Echocardiography
All patients underwent transthoracic echocardiography using commercially available ultrasound machines with 3–5 MHz real-time transducers (iE33, EPIC, Philips Medical Systems; Vivid 7, E9, General Electric Healthcare). Comprehensive two-dimensional and Doppler images were obtained by expert cardiologists according to the recommendations of the European Association of Cardiovascular Imaging [13] and stored. End-systolic volume, end-diastolic volume, and LV ejection fraction were obtained using the biplane Simpson method. LV mass, LV mass indexed to body surface area, LV cavity dimension, and LV wall thickness were calculated at end-diastole. Functional mitral regurgitation was defined as a disorder of LV remodeling or annular dilatation in which anatomically normal leaflets did not show adequate coaptation on echocardiography [14].
Electrocardiography
Electrocardiography (ECG) was performed using a 12-lead event ECG or a 24 hours Holter monitoring system on admission or during follow-up. The ECG data were obtained before or after CMR, but during the same episode of care. Complex ventricular arrhythmias, including multiform ventricular premature beat (VPB), repetitive VPB, ventricular tachycardia, and ventricular fibrillation, were categorized as grade III or higher [6,15]. The use of an implantable cardioverter-defibrillator (ICD) for arrhythmia after the date for CMR was also recorded.
CMR Protocol
All patients underwent CMR using a 1.5T scanner (Magnetom Avanto, Siemens) with a standard protocol. Cine images, including the vertical long axis, short axis, four-chamber, and LV outflow tract views, were acquired using ECG-gated steady-state free precession imaging. The CMR parameters included repetition time/echo time of 37.1/1.9 ms, flip angle of 68°, and a matrix of 256 × 256. A single bolus dose of 0.1 mmoL/kg gadobutrol (Gadovist, Bayer Shering Pharma) was injected intravenously at a rate of 2 mL/s using an automatic injector, followed by a saline flush of 20–30 mL. LGE images were acquired 15–20 minutes after the injection of contrast media using ECG-gated magnitude-only inversion recovery and phase-sensitive inversion recovery sequences. Complete two-dimensional short-axis images of the heart, from the heart apex to above the valve plane, were obtained at a thickness of 8 mm without an intersection gap. Two-chamber, four-chamber, and LV outflow tract views were also obtained.
CMR Image Analysis
The visual quality of the CMR images was assessed [16]: images without significant artifacts were classified as grade 0; images for which CMR interpretation was not affected by artifacts were classified as grade 1; images for which reliable CMR interpretation was possible despite artifacts were classified as grade 2. No CMR images could not be evaluated due to severe artifacts. Examples of each are shown in Supplementary Figure 1.
We compared the numbers of the cases with LGE of the papillary muscles reported during daily practice and the detected cases in our study after recognizing the importance of papillary muscle evaluation using a McNemar test. We also determined the difference in the number of patients with LGE of the LV wall detected through quantitative evaluation to demonstrate the limitations of visual analysis. LGE on CMR was independently analyzed by two radiologists, and interobserver agreement was evaluated. The final decision was reached by consensus. Parts of the myocardium showing definite higher signal intensity than the adjacent myocardium presumed to be normal on the LGE images were considered enhanced on visual analysis. After that, LGE of the LV myocardium, except papillary muscle, was quantified independently by two radiologists (with 4 years of experience in diagnostic radiology; with 4 years of experience in diagnostic radiology and 5 years of experience in CMR analysis) using a signal intensity threshold of 5 standard deviations (SDs), above that of the reference myocardium. The LGE images were analyzed slice by slice, and the volume of the fibrotic tissue showing LGE as a percentage of the total LV volume was calculated. LGE of the LV myocardium was quantitatively analyzed using commercially available software (CMR42, version 5.6.4; Circle Cardiovascular Imaging Inc.), and the final analysis outcomes were verified by consensus of the two radiologists.
Statistical Analysis
The continuous variables are presented as mean ± SD or median and interquartile range, and they were analyzed using Student's t test. The categorical variables were analyzed using chi-squared tests. Echocardiographic results, including mitral regurgitation grade and CMR findings, were presented descriptively. Interobserver reliability for LGE on CMR was determined using kappa statistics. The differences between the two readers in the detection of LGE on CMR were also tested using McNemar's test. The number of patients with LGE of the papillary muscles in daily practice and the study reports after recognizing the importance of papillary muscle evaluation were also compared using McNemar's test. The intra-class correlation coefficient (ICC) of the percent LV myocardial fibrosis in 17 patients, reported as a continuous variable, was determined using a two-way random and consistency method. The clinical parameters associated with myocardial or papillary LGE on CMR were assessed using univariable logistic regression analysis. Factors that were statistically significant during univariable analysis (p < 0.05) were included in the multivariable logistic regression analysis with the forward conditional method. Additional univariable and multivariable logistic regression analyses were performed to identify the factors associated with ventricular arrhythmia as a dependent variable. All statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM Corp.), and a p value of < 0.05 was considered statistically significant.
RESULTS
Study Population
Among 88 patients with mitral regurgitation (mean age ± SD, 58.3 ± 12.0 years; 42% males), 43 (49%) had MVP, and 45 (51%) presented with other causes of mitral regurgitation. Regarding the comorbidities, 31 patients with hypertension, 13 patients with diabetes mellitus, and 1 patient with chronic kidney disease underwent normal saline infusion and hemodialysis within 4 hours of contrast-enhanced CMR examination. Overall, 55 (63%) patients had taken antiarrhythmic drugs, including beta-blockers, digoxin, and non-dihydropyridine calcium channel blockers, for atrial fibrillation (n = 54) or paroxysmal supraventricular tachycardia (n = 1). Four patients with atrial fibrillation were not taking any antiarrhythmic drugs. The mean followup period ± SD from the date of acquisition of CMR imaging data to the last follow-up date was 48.7 ± 26.2 months.
In our study, LGE of the papillary muscles was detected in seven patients. We found that we had significantly underestimated LGE in our routine clinical practice, including the detection of papillary muscle enhancement (p = 0.031) and LV wall enhancement (p = 0.006) (Supplementary Table 1). The number of patients with LGE of the LV myocardium detected by quantitative measurement was 17, which was higher than the seven previously detected by only visual analysis.
LGE of the LV myocardium was more common in the MVP group than in the non-MVP group (28% vs. 11%, p = 0.046) (Table 1). The causes of non-MVP mitral regurgitation are listed in Table 2. MVP was the most common cause of mitral regurgitation, followed by rheumatic mitral disease. Among the 43 MVP patients, 12 had concurrent tricuspid regurgitation (TR), whereas among the 45 non-MVP patients, 32 had TR. The clinical characteristics of these four subgroups, consisting of patients with MVP alone, MVP with TR, non-MVP, and non-MVP with TR, are shown in Supplementary Table 2.
Table 1. Demographic and Clinical Characteristics of All Patients and of Patients with and without MVP.
| Variables | All Patients (n = 88) | MVP (n = 43) | Non-MVP (n = 45) | P | |
|---|---|---|---|---|---|
| Age | 58.3 ± 12.0 | 56.6 ± 12.4 | 59.9 ± 11.5 | 0.190 | |
| Male sex, % | 37 (42) | 28 (65) | 9 (20) | < 0.001 | |
| BSA, m2 | 1.63 ± 0.20 | 1.68 ± 0.20 | 1.58 ± 0.20 | 0.013 | |
| Heart failure, % | 20 (23) | 12 (28) | 8 (18) | 0.257 | |
| Diabetes, % | 13 (15) | 3 (7) | 10 (22) | 0.044 | |
| Dyslipidemia, % | 1 (1) | 1 (2) | 0 (0) | 0.304 | |
| Hypertension, % | 28 (32) | 14 (33) | 14 (31) | 0.884 | |
| Systolic BP, mm Hg | 117.6 ± 18.0 | 121.3 ± 18.9 | 114.0 ± 16.6 | 0.058 | |
| Diastolic BP, mm Hg | 75.8 ± 12.3 | 78.1 ± 12.9 | 72.2 ± 11.2 | 0.027 | |
| Heart rhythm, %* | |||||
| Sinus rhythm | 23 (26) | 17 (40) | 6 (13) | 0.005 | |
| PAC or SVT | 5 (6) | 4 (9) | 1 (2) | 0.152 | |
| Afib | 56 (64) | 19 (44) | 37 (82) | < 0.001 | |
| AV block | 5 (6) | 4 (9) | 1 (2) | 0.152 | |
| PVC or VT | 10 (11) | 6 (14) | 4 (9) | 0.454 | |
| Vfib | 1 (1) | 1 (2) | 0 (0) | 0.304 | |
| Echocardiography | |||||
| LVEF, % | 61.0 (54.3–66.0) | 63.0 (58.0–69.0) | 58.0 (49.5–64.0) | 0.034 | |
| LVEDVI, mL/m2 | 85.1 (65.5–113.7) | 99.3 (77.2–116.4) | 70.8 (52.5–96.3) | 0.018 | |
| LVESVI, mL/m2 | 35.7 (24.6–45.2) | 37.4 (30.4–47.6) | 32.1 (21.2–40.7) | 0.252 | |
| LVMI, g/m2 | 129.8 (106.7–160.7) | 142.1 (111.6–166.4) | 122.0 (103.7–157.7) | 0.346 | |
| MVP site, % (n = 38) | |||||
| Anterior leaflet | 10 (11) | 10 (23) | 0 (0) | < 0.001 | |
| Posterior leaflet | 19 (22) | 19 (44) | 0 (0) | < 0.001 | |
| Bi-leaflet | 9 (10) | 9 (21) | 0 (0) | 0.001 | |
| LGE location on CMR, % | |||||
| PM | 7 (8) | 4 (9) | 3 (7) | 0.648 | |
| LV myocardium | 17 (19) | 12 (28) | 5 (11) | 0.046 | |
| PM or LV myocardium | 22 (25) | 15 (35) | 7 (16) | 0.036 | |
Data are mean ± standard deviation, number of patients (%), or median (interquartile range). *Eight patients in the MVP group and four in the non-MVP group had two different types of heart rhythm. Afib = atrial fibrillation, AV block = atrioventricular block, BP = blood pressure, BSA = body surface area, CMR = cardiac magnetic resonance imaging, LGE = late gadolinium enhancement, LV = left ventricle, LVEDVI = left ventricular end-diastolic volume index, LVEF = left ventricular ejection fraction, LVESVI = left ventricular end-systolic volume index, LVMI = left ventricular mass index, MVP = mitral valve prolapse, PM = papillary muscles, PAC = premature atrial complex, PVC = premature ventricular complex, SVT = supraventricular tachycardia, TR = tricuspid regurgitation, Vfib = ventricular fibrillation, VT = ventricular tachycardia
Table 2. Patient Classification and Echocardiography Results.
| No. of Patients | LVEF (%) | LVEDVI (mL/m2) | LVESVI (mL/m2) | LVMI (g/m2) | MR Grade | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| MVP group | ||||||||||
| MVP alone | 31 | 64.0 (60.0–69.0) | 93.8 (77.2–114.5) | 36.3 (29.6–45.9) | 133.2 (111.6–159.0) | 0 | 0 | 0 | 31 | |
| MVP with TR | 12 | 58.0 (49.0–67.5) | 113.1(74.8–126.9) | 42.6 (31.8–53.3) | 158.8 (110.5–182.8) | 0 | 0 | 0 | 12 | |
| Non-MVP group | ||||||||||
| Rheumatic MR/MSR | 13 | 54.0 (47.5–64.0) | 102.6 (67.4–138.0) | 41.3 (23.8–65.5) | 131.0 (96.9–190.5) | 0 | 2 | 1 | 10 | |
| Rheumatic MR/MSR with TR | 16 | 59.5 (55.5–64.5) | 69.7 (59.6–85.9) | 30.7 (22.1–37.0) | 111.1 (101.0–124.1) | 3 | 1 | 2 | 10 | |
| Functional MR with TR | 16 | 56.5 (47.3–64.0) | 61.5 (44.6–86.0) | 25.6 (17.8–35.4) | 132.2 (104.1–156.9) | 3 | 2 | 3 | 8 | |
Data are median (interquartile range), mean ± standard deviation, or number of patients (%). LVEDVI = left ventricular end-diastolic volume index, LVEF = left ventricular ejection fraction, LVESVI = left ventricular end-systolic volume index, LVMI = left ventricular mass index, MR = mitral regurgitation, MSR = mitral stenosis with regurgitation, MVP = mitral valve prolapse, TR = tricuspid regurgitation
All MVP patients and 28 of 45 (62%) non-MVP patients had grade 4 mitral regurgitation (Table 2). Atrial fibrillation was significantly less common in the MVP group (44% vs. 82%, p < 0.001). Ventricular arrhythmias, including premature ventricular complex (PVC) (n = 9), ventricular tachycardia (n = 1), and ventricular fibrillation (n = 1), were observed in 11 (12.5%) patients.
CMR Findings
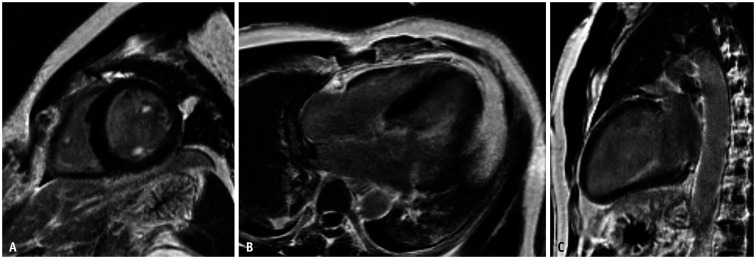
Visual assessment of the quality of the CMR images showed that all the images were interpretable. Of the 88 patients, 31 had grade 0, 41 had grade 1, and 16 had grade 2 images. The interobserver reliabilities for LGE of the LV myocardium (k = 0.82, p < 0.001) and papillary muscle (k = 0.73, p < 0.001) were satisfactory. There was no significant difference between the findings of the two radiologists on LGE on CMR, including papillary muscle enhancement (p = 0.625) and LV wall enhancement (p = 1.000). The final decision was based on the consensus of the two radiologists. The interobserver agreement for the extent of LGE of the LV myocardium was high (ICC = 0.98, 95% confidence interval [CI], 0.94–0.99). The presence and absence of LGE and ventricular arrhythmia in all patients are shown in Table 3. Of all patients, seven (8%) had LV papillary muscle enhancement, and 17 (19%) had LV wall enhancement. Of the 31 patients with MVP alone, one (3%) had papillary muscle enhancement and eight (26%) had LV wall enhancement (Supplementary Table 2). The patient with MVP alone, who presented with complex ventricular arrhythmia, showed diffuse LGE on both papillary muscles but not on LV myocardium (Fig. 2). This patient had been hospitalized for sudden cardiac arrest due to ventricular fibrillation and had undergone ICD insertion after defibrillation and cardiopulmonary resuscitation.
Table 3. Presence of LGE on Cardiac Magnetic Resonance Imaging according to the Valvular Disease Classification.
| No. of Patients | PM Enhancement (%) | LV Enhancement (%) | Ventricular Arrhythmia (%) | LGE at PM or LV Myocardium and Combined Ventricular Arrhythmia (%) | ||
|---|---|---|---|---|---|---|
| MVP group | ||||||
| MVP alone | 31 | 1 (3) | 8 (26) | 3 (10) | 2 (6) | |
| MVP with TR | 12 | 3 (25) | 4 (33) | 4 (33) | 2 (17) | |
| Non-MVP group | ||||||
| Rheumatic MR/MSR | 13 | 1 (8) | 3 (23) | 1 (8) | 0 (0) | |
| Rheumatic MR/MSR with TR | 16 | 1 (8) | 0 (0) | 2 (13) | 1 (6) | |
| Functional MR with TR | 16 | 1 (6) | 2 (13) | 1 (6) | 0 (0) | |
LGE = late gadolinium enhancement, LV = left ventricle, MR = mitral regurgitation, MSR = mitral stenosis with regurgitation, MVP = mitral valve prolapse, PM = papillary muscle, TR = tricuspid regurgitation
Fig. 2. CMR images of a 48-year-old male with no previous medical history, except for dyslipidemia, who presented with convulsion and loss of consciousness.
A-C. Event electrocardiography monitor in the ambulance showed ventricular fibrillation, and the patient was resuscitated after defibrillation. Echocardiography showed severe mitral regurgitation due to diffuse prolapse of the anterior mitral leaflet. Short-axis view (A), four-chamber view (B), and two-chamber view (C) of CMR images showing intense enhancement of both papillary muscles. The patient was treated with implantable cardioverter-defibrillator insertion and mitral valve repair. CMR = cardiac magnetic resonance imaging
Factors associated with LGE
Systolic BP was significantly higher in the group with LGE of the papillary muscles than in the group with negative LGE (133 vs. 116 mm Hg, p = 0.017) (Supplementary Table 3). The LV mass index was greater in the group with positive LGE of the papillary muscles; however, the result was not statistically significant. Univariable logistic regression analysis showed that high systolic blood pressure (BP) (odds ratio [OR], 1.04; 95% CI, 1.01–1.07; p = 0.005), high diastolic BP (OR, 1.05; 95% CI, 1.01–1.09; p = 0.021), and the presence of MVP (OR, 2.91; 95% CI, 1.05–8.08; p = 0.041) were significantly associated with LV myocardial or papillary muscle LGE on CMR (Table 4). Systolic BP was included, instead of diastolic BP, to prevent multicollinearity during multivariable logistic regression analysis. Multivariable logistic regression analysis showed that systolic BP (OR, 1.04; 95% CI, 1.01–1.07; p = 0.013) was significantly associated with LGE on CMR.
Table 4. Univariable and Multivariable Logistic Regression Analyses of Factors Significantly associated with the Presence of LGE at Any Location and Specifically in the PM.
| Presence of LGE in PM or LV Myocardium | Presence of LGE in PM | ||||||
|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | |||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Age | 0.99 (0.95–1.03) | 0.535 | 1.02 (0.95–1.09) | 0.645 | |||
| Sex | 0.51 (0.19–1.35) | 0.174 | 0.52 (0.11–2.46) | 0.406 | |||
| BSA, m2 | 2.70 (0.25–29.49) | 0.415 | 2.28 (0.05–98.48) | 0.668 | |||
| Heart failure | 1.13 (0.46–4.22) | 0.558 | 1.40 (0.25–7.83) | 0.702 | |||
| Diabetes | 0.50 (0.10–2.46) | 0.385 | 0.96 (0.11–8.68) | 0.999 | |||
| Hyperlipidemia | 0.00 (0.00–0.00) | 1.000 | 0.00 (0.00–0.00) | 1.000 | |||
| Hypertension | 2.22 (0.82–6.03) | 0.115 | 3.17 (0.66–15.24) | 0.145 | |||
| Systolic BP, mm Hg | 1.04 (1.01–1.07) | 0.005 | 1.04 (1.01–1.07) | 0.013 | 1.05 (1.01–1.09) | 0.027 | |
| Diastolic BP, mm Hg | 1.05 (1.01–1.09) | 0.021 | 1.05 (0.99–1.11) | 0.111 | |||
| Atrial fibrillation | 0.47 (0.18–1.25) | 0.129 | 1.47 (0.27–8.06) | 0.657 | |||
| Ventricular arrhythmia* | 2.94 (0.80–10.83) | 0.105 | 6.84 (1.29–36.19) | 0.024 | |||
| Echocardiography | |||||||
| LVEF, % | 1.00 (0.97–1.04) | 0.826 | 1.02 (0.98–1.07) | 0.359 | |||
| LVEDVI, mL/m2 | 1.00 (0.99–1.02) | 0.602 | 1.01 (0.98–1.03) | 0.664 | |||
| LVESVI, mL/m2 | 1.02 (1.00–1.04) | 0.163 | 1.03 (1.00–1.06) | 0.070 | |||
| LVMI, g/m2 | 1.01 (1.00–1.02) | 0.466 | 1.01 (0.99–1.03) | 0.404 | |||
| Presence of MVP | 2.91 (1.05–8.08) | 0.041 | 2.38 (0.82–6.91) | 0.112 | 1.44 (0.30–6.83) | 0.649 | |
| Anterior leaflet | 3.59 (0.93–13.86) | 0.064 | 3.65 (0.61–21.97) | 0.157 | |||
| Posterior leaflet | 1.53 (0.50–4.67) | 0.456 | 0.00 (0.00–0.00) | 0.998 | |||
| Bi-leaflet | 1.19 (0.23–6.17) | 0.839 | 1.52 (0.16–14.28) | 0.714 | |||
*Ventricular arrhythmia includes premature ventricular complex, ventricular tachycardia, and ventricular fibrillation. BP = blood pressure, BSA = body surface area, CI = confidence interval, LVEDVI = left ventricular end-diastolic volume index, LVEF = left ventricular ejection fraction, LVESVI = left ventricular end-systolic volume index, LVMI = left ventricular mass index, MVP = mitral valve prolapse, OR = odds ratio, PM = papillary muscle
Univariable logistic regression analysis showed that systolic BP (OR, 1.05; 95% CI, 1.01–1.09; p = 0.027) and ventricular arrhythmia (OR, 6.84; 95% CI, 1.29–36.19; p = 0.024) were significantly associated with papillary muscle LGE. However, multivariable regression analysis of the factors associated with LGE of the papillary muscles was not performed because of the inevitable limitation caused by the small sample of patients with LGE of the papillary muscles (n = 7). Univariable logistic regression analysis, with ventricular arrhythmia as a dependent variable, showed that heart failure (OR, 8.62; 95% CI, 2.20–33.75; p = 0.002) and LGE of the papillary muscles (OR 6.84; 95% CI, 1.29–36.19; p = 0.024) were associated factors with ventricular arrhythmia (Supplementary Table 4). Multivariable analysis was also performed to test the possible association of ventricular arrhythmia with LGE in papillary muscles (Supplementary Table 4), which has already been considered a relevant factor in previous studies [6,7,8]. However, the results of the multivariable analysis may have been overestimated because of there were few patients with ventricular arrhythmia.
DISCUSSION
Of the 88 patients with mitral regurgitation included in this study, 22 (25%) showed LGE of the papillary muscles or LV walls, including seven (8%) with LGE of the LV papillary muscles. LGE of the LV myocardium was more frequent in patients with MVP than in those without MVP (28% vs. 11%, p = 0.046). Multivariable logistic regression analysis showed that high systolic BP was associated with LGE of the LV wall or papillary muscles. Ventricular arrhythmia was associated with LGE of the papillary muscles in patients with mitral regurgitation.
The percentage of MVP patients with papillary muscle enhancement in our study was lower than that in previous reports. For example, of 16 MVP patients, ten (63%) and eight (50%) showed LGE of the papillary muscles on three-dimensional (3D) CMR and 2D CMR, respectively [6]. Another study reported LGE of the papillary muscles on 3D CMR in six (46%) of 13 patients with MVP [17]. Of the 30 patients with MVP, 25 (83%) showed LGE of the papillary muscles [7]. Of nine MVP patients with PVC of papillary muscle origin, four (44%) showed papillary muscle enhancement on CMR. Nine (36%) of 25 patients had PVC-related cardiomyopathy that resolved after ablation [18]. These discrepancies may be due to the differences in the inclusion criteria. Previous studies included patients who presented with arrhythmia or cardiac events. For example, one earlier study evaluated patients who experienced sudden cardiac death using a cardiac pathology registry [7], whereas our study used a CMR database for a general patient population. Although our study did not include 3D LGE images, the proportion of patients with papillary muscle enhancement was much lower than that in previous studies. In addition, complex ventricular arrhythmia was detected in only one (3%) of our patients, which was much lower than the percentages previously reported [6]. Although each of the earlier studies included a few patients, suggesting a possible selection bias, no study to date, including ours, has determined the true incidence of LGE of the papillary muscles or LV myocardium. However, our study, using a general CMR database, found that LGE of the papillary muscles was associated with ventricular arrhythmia, even in patients with mitral regurgitation other than MVP. This suggests that these CMR abnormalities should not be overlooked.
The discrepancies among studies on the prevalence of papillary muscle enhancement may also be due to difficulties with distinguishing the tip of the papillary muscles from the chordae tendineae, as well as true lesions from artifacts. Three confidence levels of papillary muscle enhancement have been reported: no fibrosis, possible fibrosis, and definite fibrosis, with possible fibrosis defined as enhancement of the point of choral insertion into the papillary muscles [17]. This reflects the difficulty in determining papillary muscle enhancement in these patients. A prospective study using CMR reported the prevalence and location of LV fibrosis in patients with chronic mitral regurgitation; however, they excluded papillary muscles from their analysis, considering that the detection of papillary muscle LGE is limited [19]. In this study, the LV basal or mid inferolateral wall or basal inferior wall, with segments adjacent to the posteromedial papillary muscle, showed significant LGE in the patients with MVP than in those without MVP (32.8% vs. 1.1%; p < 0.001) [19]. We are aware that the evaluation of papillary muscles on LGE images is difficult because of the high signal intensity of the LV cavity. Therefore, we thoroughly reviewed the LGE images and compared them with the cine images and carefully detected papillary muscle LGE in our study after recognizing the importance of papillary muscle evaluation.
Our study did not exclude patients with concomitant TR. Several patients had both mitral regurgitation and TR, perhaps because increased left atrial pressure can cause pulmonary hypertension and TR [20]. Mitral regurgitation can induce left atrial enlargement and atrial fibrillation, resulting in TR, suggesting that concurrent TR represents disease progression in patients with mitral regurgitation. The inclusion of these patients showed that LGE of the papillary muscles was present not only in patients with MVP, but also in patients with functional mitral regurgitation, rheumatic mitral regurgitation, and mitral regurgitation with TR. Because LGE of the papillary muscle may be associated with ventricular arrhythmia, further studies of disease entities other than MVP should be performed.
We also found that high BP was associated with LGE of the LV and papillary muscles. LGE is frequently detected in patients with arterial hypertension and has been reported in up to 50% of patients with LV hypertrophy [21]. In our study, hypertension, which was present in 32% of the patients, was not associated with LGE of the LV or papillary muscles. However, BP was significantly associated with LGE as a continuous parameter. Pressure overload resulting from arterial hypertension is thought to induce LV hypertrophy and may result in the development of fibrosis. The relationship between LGE of the papillary muscles and hypertension may be similar, but it has not been addressed previously. CMR may help assess the risk of ventricular arrhythmia in patients with papillary enhancement or arterial hypertension and guide patient management.
The present study had several limitations. This study was retrospective, and it used a CMR database at a single tertiary center, suggesting a possible selection bias. Because not all patients with ventricular arrhythmia or sudden cardiac death underwent CMR, the proportion of patients with LGE may have been underestimated. Moreover, because most patients had severe mitral regurgitation, patients with mild mitral regurgitation who had not undergone CMR were not included. We also included patients with concomitant TR, as TR was regarded as a consequence of mitral regurgitation. Thus, LGE at the right ventricular insertion points in two patients, possibly due to pulmonary hypertension, may have been misinterpreted as being directly associated with mitral regurgitation. However, regardless of the concurrent TR, CMR for the detection of papillary muscle enhancement may predict ventricular arrhythmia, considering that LGE of the papillary muscles, but not the LV myocardium, could be a source of ventricular arrhythmia in patients with mitral regurgitation. Lastly, although the association between ventricular arrhythmia and papillary muscle enhancement was statistically significant, the confidence interval was wide, indicating the limitation of the small sample. Further studies are necessary to evaluate the clinical impact of papillary muscle enhancement on CMR.
In conclusion, LGE of the LV papillary muscles or LV myocardial walls is not uncommon in patients with mitral regurgitation. LGE of the papillary muscles was present not only in patients with MVP, but also in patients with other etiologies of mitral regurgitation. Ventricular arrhythmia is associated with LGE of the LV papillary muscles.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Su Jin Lim, Hyun Jung Koo, Dong Hyun Yang.
- Data curation: all authors.
- Formal analysis: Su Jin Lim, Hyun Jung Koo, Dong Hyun Yang.
- Investigation: all authors.
- Methodology: Su Jin Lim, Hyun Jung Koo, Dong Hyun Yang.
- Project administration: Hyun Jung Koo, Dong Hyun Yang.
- Resources: all authors.
- Software: Su Jin Lim, Hyun Jung Koo, Joon-Won Kang, Dong Hyun Yang.
- Supervision: Hyun Jung Koo, Dong Hyun Yang.
- Validation: Hyun Jung Koo.
- Visualization: Su Jin Lim, Hyun Jung Koo.
- Writing—original draft: Su Jin Lim, Hyun Jung Koo.
- Writing—review & editing: all authors.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2020.1485.
Comparison of the Numbers of Patients with Late Gadolinium Enhancement of the LV Wall or PMs between the CMR Report Acquired during Daily Practice and Re-Evaluation Data in the Study Cohort
Demographic and Clinical Characteristics of Patient Subgroups with and without MVP, and with and without TR
Comparison of Echocardiographic LV Mass and Systolic Blood Pressure between Patients with and without LGE of the Papillary Muscles
Univariable and Multivariable Logistic Regression Analyses of Factors associated with Ventricular Arrythmia
Assessment of the visual quality of cardiac magnetic resonance images in representative patients.
References
- 1.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Vecchia L, Ometto R, Centofante P, Varotto L, Bonanno C, Bozzola L, et al. Arrhythmic profile, ventricular function, and histomorphometric findings in patients with idiopathic ventricular tachycardia and mitral valve prolapse: clinical and prognostic evaluation. Clin Cardiol. 1998;21:731–735. doi: 10.1002/clc.4960211007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HY. Relationship of heart rate turbulence, heart rate variability and the number of ventricular premature beats in patients with mitral valve prolapse and non-significant regurgitation. Int J Cardiol. 2009;135:269–271. doi: 10.1016/j.ijcard.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 4.Turker Y, Ozaydin M, Acar G, Ozgul M, Hoscan Y, Varol E, et al. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging. 2010;26:139–145. doi: 10.1007/s10554-009-9514-6. [DOI] [PubMed] [Google Scholar]
- 5.Narayanan K, Uy-Evanado A, Teodorescu C, Reinier K, Nichols GA, Gunson K, et al. Mitral valve prolapse and sudden cardiac arrest in the community. Heart Rhythm. 2016;13:498–503. doi: 10.1016/j.hrthm.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 8.Fulton BL, Liang JJ, Enriquez A, Garcia FC, Supple GE, Riley MP, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 2018;29:146–153. doi: 10.1111/jce.13374. [DOI] [PubMed] [Google Scholar]
- 9.Chesler E, King RA, Edwards JE. The myxomatous mitral valve and sudden death. Circulation. 1983;67:632–639. doi: 10.1161/01.cir.67.3.632. [DOI] [PubMed] [Google Scholar]
- 10.Farb A, Tang AL, Atkinson JB, McCarthy WF, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am J Cardiol. 1992;70:234–239. doi: 10.1016/0002-9149(92)91281-8. [DOI] [PubMed] [Google Scholar]
- 11.Sniezek-Maciejewska M, Dubiel JP, Piwowarska W, Mroczek-Czernecka D, Mazurek S, Jas´kiewicz J, et al. Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin Cardiol. 1992;15:720–724. doi: 10.1002/clc.4960151029. [DOI] [PubMed] [Google Scholar]
- 12.Wilde AA, Düren DR, Hauer RN, deBakker JM, Bakker PF, Becker AE, et al. Mitral valve prolapse and ventricular arrhythmias: observations in a patient with a 20-year history. J Cardiovasc Electrophysiol. 1997;8:307–316. doi: 10.1111/j.1540-8167.1997.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 14.Gillam LD. Is it time to update the definition of functional mitral regurgitation?: structural changes in the mitral leaflets with left ventricular dysfunction. Circulation. 2008;118:797–799. doi: 10.1161/CIRCULATIONAHA.108.795781. [DOI] [PubMed] [Google Scholar]
- 15.Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44:130–142. doi: 10.1161/01.cir.44.1.130. [DOI] [PubMed] [Google Scholar]
- 16.Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505–514. doi: 10.1081/jcmr-120022267. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Peters DC, Kissinger KV, Goddu B, Yeon SB, Manning WJ, et al. Evaluation of papillary muscle function using cardiovascular magnetic resonance imaging in mitral valve prolapse. Am J Cardiol. 2010;106:243–248. doi: 10.1016/j.amjcard.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enriquez A, Shirai Y, Huang J, Liang J, Briceño D, Hayashi T, et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: electrophysiologic substrate and catheter ablation outcomes. J Cardiovasc Electrophysiol. 2019;30:827–835. doi: 10.1111/jce.13900. [DOI] [PubMed] [Google Scholar]
- 19.Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72:823–834. doi: 10.1016/j.jacc.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009;53:401–408. doi: 10.1016/j.jacc.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the Numbers of Patients with Late Gadolinium Enhancement of the LV Wall or PMs between the CMR Report Acquired during Daily Practice and Re-Evaluation Data in the Study Cohort
Demographic and Clinical Characteristics of Patient Subgroups with and without MVP, and with and without TR
Comparison of Echocardiographic LV Mass and Systolic Blood Pressure between Patients with and without LGE of the Papillary Muscles
Univariable and Multivariable Logistic Regression Analyses of Factors associated with Ventricular Arrythmia
Assessment of the visual quality of cardiac magnetic resonance images in representative patients.