INTRODUCTION
A time-to-event analysis is an analysis of any dichotomous outcome (i.e., events vs. no events) occurring over time. Survival analysis is used practically as a synonym for time-to-event analysis although time-to-event analysis is not restricted to death (as the event) and survival. Survival analysis has been increasingly used in imaging research studies. Examples include studies evaluating the association between imaging findings/biomarkers and patient survival and image-based modelling studies to predict survival [1,2,3,4]. We have noticed numerous such studies reporting the methods and results incompletely or unclearly, either among manuscripts submitted to the Korean Journal of Radiology or those published in other journals and other fields of medical research [5,6,7,8,9]. The purpose of this review is to list the relatively frequent mistakes in reporting survival analysis observed in research studies in the field of imaging research. This article focuses on the adequacy of description and clarity in reporting survival analysis. It does not intend to discuss more fundamental issues regarding the methodological appropriateness of survival analysis, such as non-informative censoring, proportional hazards assumption, time-dependent covariates and coefficients, immortal time bias, and competing risks [10,11,12,13]. Researchers should confirm in the first place whether their analyses considered the fundamental methodological issues well. This article also does not cover reporting of the studies to evaluate the performance of survival prediction models, for which the methodologic guide can be found elsewhere [14].
The Basics
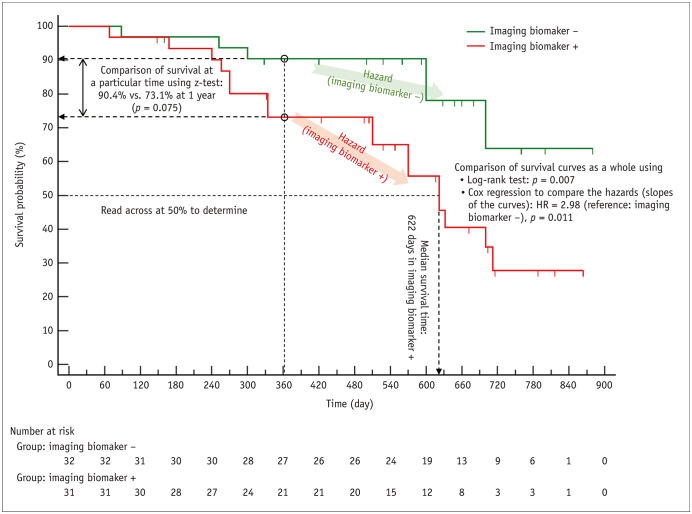
Survival analysis observes the development of events of interest (such as death) as follow-up time elapses, and the survival curve is a plot of the probability (%) of staying free of events until a certain follow-up time, referred to as survival probability or cumulative survival, on the y-axis against the follow-up time on the x-axis (Fig. 1). An alternative plot of ‘100% – survival probability’ referred to as cumulative incidence of events or incidence proportion [15], against the follow-up time can also be drawn. Patients may drop out of study observation before developing events, and they are referred to as censored patients. Although we do not know what happened to the censored patients after the censored time, we know that they were free of events until the time of censoring. Therefore, they still contribute useful information that should be included when analyzing survival. The Kaplan-Meier method is a popular method to create a survival curve considering the censored patients (Fig. 1). More explanations about how to construct a Kaplan-Meier survival curve can be found elsewhere [14]. The related statistical parameters and analytic methods commonly used for survival analysis are summarized in Figure 1.
Fig. 1. Example Kaplan-Meier survival curves and a graphic summary of the related statistical parameters and statistical methods commonly used for survival analysis.
Two Kaplan-Meier survival curves, one each for patients with (red) and without (green) the imaging biomarker, are shown. The Kaplan-Meier method recalculates the survival probability every time a new patient develops an event, which decreases as shown by a downward step in the curve. Downward blips represent the censored patients. When a patient is censored, the survival curve does not dip down. The mean survival time in the sense of the mean length of time a subject can be expected to survive cannot be calculated until the last patient has developed an event. The median survival time can be obtained if the survival probability has dropped to 50%. Therefore, the median survival can be obtained for patients who are the imaging biomarker + (red); however, not for patients who are the imaging biomarker − (green). To determine the median survival time, draw a horizontal line at 50% survival, see where it crosses the curve, and look down at the x-axis to read off the time. The median survival time is 622 days for the group with the imaging biomarker (red). The survival of the two groups can be compared in several different ways. The log-rank test and the Cox proportional hazards regression are commonly used to compare the survival curves as a whole across the entire follow-up time. Patients without the imaging biomarker (green) shows significantly better survival according to both methods (p = 0.007 and p = 0.011, respectively). The Cox proportional hazards regression calculates HR. Hazard has the meaning of the slope of a survival curve, which is the rate of developing events in a time period, and the HR (i.e., the ratio of hazards of two survival curves) estimated by the Cox regression is essentially a relative risk. The HR of 2.98 indicates that the risk of death is 2.98 times greater in the patients with the imaging biomarker (red) compared to those without the imaging biomarker (green, the reference category). If one wants to compare the survival probability at a specific follow-up time, for example, at 1 year (90.4% vs. 73.1%), the z-test is commonly used (p = 0.075). HR = hazard ratio
Common Mistakes
Mistake 1: Unclear Definition of Events
A sound survival analysis starts with a clear definition of events. The definitions are well-known for some circumstances, such as the analysis of overall survival in cancer patients, for which the events are death from any cause [16]. However, the definitions may often vary according to research questions, clinical settings, or cancer types [17,18,19,20,21,22]. For example, even if disease-free survival in oncologic survival analysis considers disease recurrence or death from any cause as events, the exact definition of disease recurrence may vary across studies. Therefore, providing a clear description of the definition of events accompanied by references when available is helpful [23,24,25,26,27,28,29,30,31,32]. Some examples are shown below.
• “the earliest signs of HCC progression (LTP, intrahepatic distant recurrence, gross vascular invasion, or extrahepatic distant metastasis) as determined by CT or MR imaging using the modified RECIST criteria, or death from any cause (22, 23)”[23]
• “major adverse cardiovascular event (MACE) defined as cardiac death, acute myocardial infarction (AMI), CAD requiring coronary revascularization, or stroke/transient ischemic attack (TIA)” [25]
Mistake 2. Reporting a Comparison between Patients with and without Events to Explore Factors associated with Survival
This approach [29,33,34,35,36,37,38,39,40,41,42] may sound reasonable at a glance but is generally invalid. This analysis is logical only when the follow-up time is fixed and specified for the grouping of patients (e.g., patients developing events by 6 months after treatment vs. patients free of events until 6 months) and all patients have completely been followed until the specified time point (e.g., all have been followed until 6 months without dropouts unless they had events) [36,37,38,43]. Complete follow-up is difficult to achieve in clinical research, especially in retrospective studies or studies that involve long periods of follow-up. Patients who dropped out before the specified time cannot be categorized into either events or no events as there is no way to know if they would have developed events if they had been followed further. Some investigators then exclude dropouts for the analysis [39]; however, such exclusion is inappropriate and may cause selection biases. Even if the two conditions are met, other issues remain, such as 1) whether events do not occur after the specified follow-up time, 2) if events can still occur after the specified time, is it okay to ignore them by categorizing them into no events group, and 3) why does the specific time matter instead of other follow-up time points. Unless there are explicit sound explanations for these questions, the analysis will be unsatisfactory.
Accordingly, this analytic approach is more reasonable if a study is looking for events that occur within relatively short periods. For example, one study [36] divided patients with glioblastoma multiforme into those who had early progression after treatment (i.e., progression before 6 months) and those who did not because the study had a specific purpose of finding factors associated with the early progression after the treatment. All study patients could be followed completely without dropouts as the follow-up period was relatively short.
For the same reason, case-control design, i.e., separate collection of patients who had events and those who did not, is generally inappropriate for survival analysis. As an exception, case-control design combined with some other specialized methodological features may be used for a huge epidemiological study to determine factors associated with the occurrence of rare events [44,45,46].
Mistake 3. Inappropriately Reporting the Mean Survival Time
The mean survival time, reported in some studies [39,47], can be misleading. The mean survival time in the sense of the mean length of time a subject can be expected to survive cannot be calculated until the survival time for every patient is known as every patient has died. We simply do not know the survival time of a patient who is not dead yet. Clinical studies where all study patients had events are rare and, therefore, the mean survival time is generally not obtainable. The “mean” survival times reported in clinical research studies are typically the area under the survival curve between time zero to the finish of study observation that statistical software programs calculate [48] or, maybe, merely the mean of the follow-up times, both of which should not be mistaken for the mean length of time a subject can be expected to survive. The authors should first consider if such “mean” value is truly informative in the study or redundant only creating confusion. Generally, it is more appropriate to present the median survival time as a statistic that represents the survival lengths of the study patients. The median survival time is the length of time that half of the patients have developed events (Fig. 1). If fewer than half the subjects have developed events by the end of the study, the median survival cannot be determined, either.
Mistake 4. Not Clarifying the Unit Amount When Reporting Hazard Ratio for a Continuous Variable
With a continuous variable, the hazard ratio (HR) indicates the change in the risk of events if the parameter rises by one-unit amount. Therefore, it is important to state in the report what was considered one-unit amount. For example, one study reported an HR of 1.34 for systolic right ventricular mass index measured on cardiac MRI for the development of major adverse cardiac and cerebrovascular events [33]. The systolic right ventricular mass index was a continuous variable measured in g/m2. The study specifically describes that the HR of 1.34 is per increase of 5 g/m2. Without the xplanation, one might inadvertently misinterpret it as an HR of 1.34 for a 1 g/m2 increase in the index value, which would erroneously make the HR for a 5 g/m2 increase 4.32 (= 1.345).
Mistake 5. Making Imprecise Reference to the p Values from the Log-Rank Test and the Cox Regression
Some studies cite p values from the log-rank test or the Cox proportional hazards regression alongside when contrasting survival probabilities at a particular follow-up time or the median survival times between groups [23,47,49,50,51,52,53]. Some examples are shown below:
• “The 5-year OS rate was 100% (no event) for mrTRG 1, 92.7% for mrTRG 2, 89.6% for mrTRG 3, 80.1% for mrTRG 4, and 40.0% for mrTRG 5 (p = 0.024 by Cox proportional hazards regression)” [47]
• “The 2-year LTP-free survival rates of patients in the DSM-RFA and SSM-RFA groups were 90.0% and 94.4%, respectively (p = 0.331 by log-rank test), and the 2-year recurrence-free survival rates were 54.9% and 75.7%, respectively (p = 0.265 by log-rank test)” [49]
• “The median overall survival time in the validation set were 137.5 months, 76.1 months, and 44.0 months for low-, intermediate-, and high-risk groups, respectively (p < 0.001 by log-rank test)” [23]
Caution is needed in the reporting to prevent it from being interpreted as if the statistical analyses specifically refer to the comparison of survival probabilities at a particular time or the comparison of the median survival times because the log-rank test and the Cox proportional hazards regression compare the survival curves as a whole for the entire follow-up time (Fig. 1).
If the investigators want to specifically compare the survival probability at a specific time, the z-test is commonly used (Fig. 1) [54]. Methods to specifically compare median survival times have also been proposed [55], if the comparison is particularly needed for reasons such as crossing survival curves. However, such statistical testing is rarely used in clinical research studies. Instead, presenting the median survival with its 95% confidence interval would be clear enough as shown below.
• “The multiple Cox's proportional hazard analysis showed that the location of distal end of biliary stent was the only independent predictor of biliary stent patency (hazard ratio, 3.771; 95% CI, 1.157–12.283). The median biliary stent patency rate was significantly longer in patients in whom the distal end of biliary stent was beyond the distal end of the duodenal stent (median, 327 days; 95% CI, 249–405 days), compared with cases in which the distal end of the biliary stent was within the duodenal stent (median, 170 days; 95% CI, 115–225 days)” [56]
Mistake 6: Multivariable Cox Regression Followed by Univariable Log-Rank Test
The Cox proportional hazards regression applies regression methodology to the analysis of survival data. It has an advantage over the log-rank test, which is a univariable analysis, that it can compare the survival between groups after adjusting for other variables, i.e., multivariable analysis. The multivariable Cox regression analysis is typically used to further interrogate the variables that are identified as significant at univariable analyses which can be the univariable Cox regression or the log-rank test [57]. Therefore, the results from the multivariable Cox regression are considered more conclusive than the results from the univariable analysis. The HR from multivariable Cox regression is referred to as adjusted HR to distinguish it from unadjusted (or crude) HR from the univariable analysis. Some investigators perform a multivariable Cox regression to identify a factor associated with survival. They, then report crude Kaplan-Meier survival curves segregated by the factor identified and additionally compare them using the log-rank test [29,35,58]. This reporting may deliver an incorrect message as if the crude Kaplan-Meier curves and the log-rank test provide more ultimate results. If one wants to show the Kaplan-Meier curves regarding a risk factor identified by multivariable Cox regression, adjusted Kaplan-Meier curves can be presented accompanied by adjusted HR [59,60,61].
CONCLUSION
Paying attention to avoid the mistakes listed above would help make the research report more accurate and transparent. Referring to published papers that report survival analysis relatively adequately [26,56,62,63,64,65,66,67] would also be helpful.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Writing—original draft: Seong Ho Park.
- Writing—review & editing: Kyunghwa Han, Seo Young Park.
References
- 1.Lee G, Park H, Bak SH, Lee HY. Radiomics in lung cancer from basic to advanced: current status and future directions. Korean J Radiol. 2020;21:159–171. doi: 10.3348/kjr.2019.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH, Park H, Ko ES. Radiomics in breast imaging from techniques to clinical applications: a review. Korean J Radiol. 2020;21:779–792. doi: 10.3348/kjr.2019.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park HJ, Park B, Lee SS. Radiomics and deep learning: hepatic applications. Korean J Radiol. 2020;21:387–401. doi: 10.3348/kjr.2019.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JE, Kickingereder P, Kim HS. Radiomics and deep learning from research to clinical workflow: neuro-oncologic imaging. Korean J Radiol. 2020;21:1126–1137. doi: 10.3348/kjr.2019.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Zhou X, Zhang Y, Sun X, Liu H, Zhang Y. Reporting and methodological quality of survival analysis in articles published in Chinese oncology journals. Medicine (Baltimore) 2017;96:e9204. doi: 10.1097/MD.0000000000009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai-Adisaksopha C, Iorio A, Hillis C, Lim W, Crowther M. A systematic review of using and reporting survival analyses in acute lymphoblastic leukemia literature. BMC Hematol. 2016;16:17. doi: 10.1186/s12878-016-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batson S, Greenall G, Hudson P. Review of the reporting of survival analyses within randomised controlled trials and the implications for meta-analysis. PLoS One. 2016;11:e0154870. doi: 10.1371/journal.pone.0154870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraira V, Muriel A, Emparanza JI, Pijoan JI, Royuela A, Plana MN, et al. Reporting quality of survival analyses in medical journals still needs improvement. A minimal requirements proposal. J Clin Epidemiol. 2013;66:1340–1346.e5. doi: 10.1016/j.jclinepi.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. Br J Cancer. 1995;72:511–518. doi: 10.1038/bjc.1995.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey T, Mukherjee A, Chakraborty S. A practical overview and reporting strategies for statistical analysis of survival studies. Chest. 2020;158:S39–S48. doi: 10.1016/j.chest.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Tolles J, Lewis RJ. Time-to-event analysis. JAMA. 2016;315:1046–1047. doi: 10.1001/jama.2016.1825. [DOI] [PubMed] [Google Scholar]
- 12.Therneau T, Crowson C, Atkinson E. Using time dependent covariates and time dependent coefficients in the cox model. [Published April 25, 2021]. [Accessed July 15, 2021]. Cran.r-project.org Web site. https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf.
- 13.Lee H, Nunan D. Immortal time bias. [Accessed July 15, 2021]. Catalogofbias.org Web site. https://catalogofbias.org/biases/immortal-time-bias/
- 14.Park SY, Park JE, Kim H, Park SH. Review of statistical methods for evaluating the performance of survival or other time-to-event prediction models (from conventional to deep learning approaches) Korean J Radiol. 2021;22:1697–1707. doi: 10.3348/kjr.2021.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Lesson 3, Section 2: morbidity frequency measures. [Accessed July 15, 2021]. CDC. gov Web site. https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section2.html.
- 16.FDA. Clinical trial endpoints for the approval of cancer drugs and biologics: guidance for industry. [Published December 2018]. [Accessed July 15, 2021]. FDA.gov Web site. https://www.fda.gov/media/71195/download.
- 17.Cohen R, Vernerey D, Bellera C, Meurisse A, Henriques J, Paoletti X, et al. Guidelines for time-to-event end-point definitions in adjuvant randomised trials for patients with localised colon cancer: results of the DATECAN initiative. Eur J Cancer. 2020;130:63–71. doi: 10.1016/j.ejca.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellera CA, Penel N, Ouali M, Bonvalot S, Casali PG, Nielsen OS, et al. Guidelines for time-to-event end point definitions in sarcomas and gastrointestinal stromal tumors (GIST) trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann Oncol. 2015;26:865–872. doi: 10.1093/annonc/mdu360. [DOI] [PubMed] [Google Scholar]
- 19.Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann Oncol. 2015;26:2505–2506. doi: 10.1093/annonc/mdv478. [DOI] [PubMed] [Google Scholar]
- 20.Kramar A, Negrier S, Sylvester R, Joniau S, Mulders P, Powles T, et al. Guidelines for the definition of time-to-event end points in renal cell cancer clinical trials: results of the DATECAN project†. Ann Oncol. 2015;26:2392–2398. doi: 10.1093/annonc/mdv380. [DOI] [PubMed] [Google Scholar]
- 21.Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)†. Ann Oncol. 2015;26:873–879. doi: 10.1093/annonc/mdv478. [DOI] [PubMed] [Google Scholar]
- 22.Bonnetain F, Bonsing B, Conroy T, Dousseau A, Glimelius B, Haustermans K, et al. Guidelines for time-to-event end-point definitions in trials for pancreatic cancer. Results of the DATECAN initiative (Definition for the Assessment of Time-to-event End-points in CANcer trials) Eur J Cancer. 2014;50:2983–2993. doi: 10.1016/j.ejca.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Park C, Kim JH, Kim PH, Kim SY, Gwon DI, Chu HH, et al. Imaging predictors of survival in patients with single small hepatocellular carcinoma treated with Transarterial chemoembolization. Korean J Radiol. 2021;22:213–224. doi: 10.3348/kjr.2020.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong EK, Choi SH, Shin DJ, Jo SW, Yoo RE, Kang KM, et al. Comparison of genetic profiles and prognosis of high-grade gliomas using quantitative and qualitative MRI features: a focus on G3 gliomas. Korean J Radiol. 2021;22:233–242. doi: 10.3348/kjr.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung H, Kim BY, Kim HS, Kim HO, Lee JM, Woo JS, et al. Long-term clinical effects of carotid intraplaque neovascularization in patients with coronary artery disease. Korean J Radiol. 2020;21:900–907. doi: 10.3348/kjr.2019.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park B, Park J, Lim JK, Shin KM, Lee J, Seo H, et al. Prognostic implication of volumetric quantitative ct analysis in patients with COVID-19: a multicenter study in Daegu, Korea. Korean J Radiol. 2020;21:1256–1264. doi: 10.3348/kjr.2020.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JW, Kim JH, Byun SS, Kang JM, Shin JH. Paclitaxel-coated balloon versus plain balloon angioplasty for dysfunctional autogenous radiocephalic arteriovenous fistulas: a prospective randomized controlled trial. Korean J Radiol. 2020;21:1239–1247. doi: 10.3348/kjr.2020.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo J, Choi SI, Kim YK. Subclinical coronary atherosclerosis: implication of coronary computed tomography angiography findings among statin candidates according to the 2013 ACC/AHA Cholesterol Management Guidelines. Korean J Radiol. 2019;20:1156–1166. doi: 10.3348/kjr.2018.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon SH, Kim E, Jeon Y, Yi SY, Bae HJ, Jang IK, et al. Prognostic value of coronary CT angiography for predicting poor cardiac outcome in stroke patients without known cardiac disease or chest pain: the assessment of coronary artery disease in stroke patients study. Korean J Radiol. 2020;21:1055–1064. doi: 10.3348/kjr.2020.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim JH, Kim SY, Won HJ, Shin YM, Kim PN. Percutaneous radiofrequency ablation for metachronous hepatic metastases after curative resection of pancreatic adenocarcinoma. Korean J Radiol. 2020;21:316–324. doi: 10.3348/kjr.2019.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Kim DY, An C, Han K, Won JY, Kim GM, et al. Evaluation of early response to treatment of hepatocellular carcinoma with yttrium-90 radioembolization using quantitative computed tomography analysis. Korean J Radiol. 2019;20:449–458. doi: 10.3348/kjr.2018.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang Y, Hong EK, Rhim JH, Yoo RE, Kang KM, Yun TJ, et al. Prognostic value of dynamic contrast-enhanced MRI-derived pharmacokinetic variables in glioblastoma patients: analysis of contrast-enhancing lesions and non-enhancing T2 high-signal intensity lesions. Korean J Radiol. 2020;21:707–716. doi: 10.3348/kjr.2019.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn Y, Koo HJ, Kang JW, Choi WJ, Kim DH, Song JM, et al. Prognostic implication of right ventricle parameters measured on preoperative cardiac MRI in patients with functional tricuspid regurgitation. Korean J Radiol. 2021;22:1253–1265. doi: 10.3348/kjr.2020.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MY, Park EA, Lee W, Lee SP. Cardiac magnetic resonance feature tracking in aortic stenosis: exploration of strain parameters and prognostic value in asymptomatic patients with preserved ejection fraction. Korean J Radiol. 2020;21:268–279. doi: 10.3348/kjr.2019.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn Y, Koo HJ, Lee S, Kim DH, Song JM, Kang DH, et al. Preoperative cardiac computed tomography characteristics associated with recurrent aortic regurgitation after aortic valve re-implantation. Korean J Radiol. 2020;21:181–191. doi: 10.3348/kjr.2019.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jo SW, Choi SH, Lee EJ, Yoo RE, Kang KM, Yun TJ, et al. Prognostic prediction based on dynamic contrast-enhanced MRI and dynamic susceptibility contrast-enhanced MRI parameters from non-enhancing, T2-high-signal-intensity lesions in patients with glioblastoma. Korean J Radiol. 2021;22:1369–1378. doi: 10.3348/kjr.2020.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SH, Song BI, Kim HW, Won KS, Son YG, Ryu SW. Prognostic value of restaging F-18 fluorodeoxyglucose positron emission tomography/computed tomography to predict 3-year post-recurrence survival in patients with recurrent gastric cancer after curative resection. Korean J Radiol. 2020;21:829–837. doi: 10.3348/kjr.2019.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim EK, Lee GY, Jang SY, Chang SA, Kim SM, Park SJ, et al. The extent of late gadolinium enhancement can predict adverse cardiac outcomes in patients with non-ischemic cardiomyopathy with reduced left ventricular ejection fraction: a prospective observational study. Korean J Radiol. 2021;22:324–333. doi: 10.3348/kjr.2020.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, Jeong WK, Kim JH, Kim JM, Kim TY, Choi GS, et al. Serial observations of muscle and fat mass as prognostic factors for deceased donor liver transplantation. Korean J Radiol. 2021;22:189–197. doi: 10.3348/kjr.2019.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao D, Gao Q, Cheng Y, Du DY, Wang SY, Wang SX. The prognostic value of 18F-fluorodeoxyglucose PET/CT in the initial assessment of primary tracheal malignant tumor: a retrospective study. Korean J Radiol. 2021;22:425–434. doi: 10.3348/kjr.2020.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho YH, Do KH, Chae EJ, Choi SH, Jo KW, Lee SO, et al. Association of chest CT-based quantitative measures of muscle and fat with post-lung transplant survival and morbidity: a single institutional retrospective cohort study in Korean population. Korean J Radiol. 2019;20:522–530. doi: 10.3348/kjr.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeon EK, Cho YD, Yoo DH, Lee SH, Kang HS, Cho WS, et al. De novo intracranial aneurysms detected on imaging follow-up of coiled aneurysms in a Korean population. Korean J Radiol. 2019;20:1390–1398. doi: 10.3348/kjr.2018.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.BMJ. 12. Survival analysis. [Accessed July 15, 2021]. bmj.com Web site. https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/12-survival-analysis.
- 44.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 45.Ganna A, Reilly M, de Faire U, Pedersen N, Magnusson P, Ingelsson E. Risk prediction measures for case-cohort and nested case-control designs: an application to cardiovascular disease. Am J Epidemiol. 2012;175:715–724. doi: 10.1093/aje/kwr374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaubel DE, Zhang H, Kalbfleisch JD, Shu X. Semiparametric methods for survival analysis of case-control data subject to dependent censoring. Can J Stat. 2014;42:365–383. [Google Scholar]
- 47.Yoen H, Park HE, Kim SH, Yoon JH, Hur BY, Bae JS, et al. Prognostic value of tumor regression grade on MR in rectal cancer: a large-scale, single-center experience. Korean J Radiol. 2020;21:1065–1076. doi: 10.3348/kjr.2019.0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. 2nd ed. New York: Springer; 2003. [Google Scholar]
- 49.Choi JW, Lee JM, Lee DH, Yoon JH, Kim YJ, Lee JH, et al. Radiofrequency ablation using a separable clustered electrode for the treatment of hepatocellular carcinomas: a randomized controlled trial of a dual-switching monopolar mode versus a single-switching monopolar mode. Korean J Radiol. 2021;22:179–188. doi: 10.3348/kjr.2020.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im DJ, Hur J, Han K, Suh YJ, Hong YJ, Lee HJ, et al. Prognostic value of dual-energy CT-based iodine quantification versus conventional CT in acute pulmonary embolism: a propensity-match analysis. Korean J Radiol. 2020;21:1095–1103. doi: 10.3348/kjr.2019.0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong DY, Kang TW, Min JH, Song KD, Lee MW, Rhim H, et al. Effect of perfluorobutane microbubbles on radiofrequency ablation for hepatocellular carcinoma: suppression of steam popping and its clinical implication. Korean J Radiol. 2020;21:1077–1086. doi: 10.3348/kjr.2019.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee B, Choi YJ, Kim SO, Lee YS, Hong JY, Baek JH, et al. Prognostic value of radiologic extranodal extension in human papillomavirus-related oropharyngeal squamous cell carcinoma. Korean J Radiol. 2019;20:1266–1274. doi: 10.3348/kjr.2018.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee DH, Kim SH, Lee SM, Han JK. Prediction of treatment outcome of chemotherapy using perfusion computed tomography in patients with unresectable advanced gastric cancer. Korean J Radiol. 2019;20:589–598. doi: 10.3348/kjr.2018.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Zhang G. Comparing survival curves based on medians. BMC Med Res Methodol. 2016;16:33. doi: 10.1186/s12874-016-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon JH, Gwon DI, Kim JW, Chu HH, Kim JH, Ko GY, et al. Percutaneous biliary metallic stent insertion in patients with malignant duodenobiliary obstruction: outcomes and factors influencing biliary stent patency. Korean J Radiol. 2020;21:695–706. doi: 10.3348/kjr.2019.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim R, Kim CK, Park JJ, Kim JH, Seo SI, Jeon SS, et al. Prognostic significance for long-term outcomes following radical prostatectomy in men with prostate cancer: evaluation with prostate imaging reporting and data system version 2. Korean J Radiol. 2019;20:256–264. doi: 10.3348/kjr.2018.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang HJ, Lee SM, Seo JB, Kim JE, Choi HY, Kim N, et al. Quantitative vertebral bone density seen on chest CT in chronic obstructive pulmonary disease patients: association with mortality in the Korean obstructive lung disease cohort. Korean J Radiol. 2020;21:880–890. doi: 10.3348/kjr.2019.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 60.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z, Jin C, Wu CC, Liang T, Zhao H, Wang Y, et al. Association between initial chest CT or clinical features and clinical course in patients with coronavirus disease 2019 pneumonia. Korean J Radiol. 2020;21:736–745. doi: 10.3348/kjr.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Won SY, Park HS, Kim EK, Kim SI, Moon HJ, Yoon JH, et al. Survival rates of breast cancer patients aged 40 to 49 years according to detection modality in Korea: screening ultrasound versus mammography. Korean J Radiol. 2021;22:159–167. doi: 10.3348/kjr.2019.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang T, Yang Y, Liu X, Deng J, Wu J, Hou L, et al. Primary invasive mucinous adenocarcinoma of the lung: prognostic value of CT imaging features combined with clinical factors. Korean J Radiol. 2021;22:652–662. doi: 10.3348/kjr.2020.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi WS, Yoon CJ, Lee JH. Percutaneous enteral stent placement using a transhepatic access for palliation of malignant bowel obstruction after surgery. Korean J Radiol. 2021;22:742–750. doi: 10.3348/kjr.2020.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng Y, Xiao A, Yu X, Zhao Y, Lu Y, Li X, et al. Development and validation of a prognostic nomogram based on clinical and CT features for adverse outcome prediction in patients with COVID-19. Korean J Radiol. 2020;21:1007–1017. doi: 10.3348/kjr.2020.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim NH, Lee SR, Kim YH, Kim HJ. Diagnostic performance and prognostic relevance of FDG positron emission tomography/computed tomography for patients with extrahepatic cholangiocarcinoma. Korean J Radiol. 2020;21:1355–1366. doi: 10.3348/kjr.2019.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh J, Lee JM, Park J, Joo I, Yoon JH, Lee DH, et al. Hepatocellular carcinoma: texture analysis of preoperative computed tomography images can provide markers of tumor grade and disease-free survival. Korean J Radiol. 2019;20:569–579. doi: 10.3348/kjr.2018.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]