Abstract
The global incidence of malignant melanoma, the leading cause of skin cancer death, has steadily increased in recent years. Surgical excision is the treatment of choice for early-stage melanoma. However, 40–60% of patients with high-risk melanoma or with nodal involvement eventually experience loco-regional relapse or tumor progression. Adjuvant therapy aims to reduce the rate of recurrence in radically operated high-risk patients with melanoma and thus improves survival. Interferon-α has long been the only approved drug for adjuvant melanoma therapy, despite an unclear survival benefit. The landmark success of immune-checkpoint inhibitors and BRAF/MEK-directed targeted therapies in the treatment of patients with stage IV melanoma led to the initiation of clinical trials in the adjuvant setting. These trials demonstrated the efficacy of immune-checkpoint inhibitors and targeted therapies for the adjuvant treatment of high-risk patients with melanoma, as shown both by an increase in recurrence-free survival and the emergence of long-term survivors, finally resulting in the approval of the cytotoxic T-lymphocyte antigen 4 inhibitor ipilimumab, PD1 inhibitors (nivolumab, pembrolizumab), and BRAF/MEK inhibitors for adjuvant melanoma therapy. This review aims to delineate the advances in adjuvant melanoma therapy, issuing particularly recent results from clinical trials. Moreover, we also discuss pending issues and future challenges, which comprise the adequate selection of adjuvant regimens for patient subgroups and the identification of markers likely to predict the individual response to adjuvant treatments. Last, we outline the role of emerging neoadjuvant approaches, which may complement adjuvant strategies and are currently investigated in clinical trials.
Key Points
| Adjuvant therapy for high-risk patients with stage IIIA–IIID melanoma shows high efficacy and improves relapse-free survival. |
| Adjuvant therapy with nivolumab or pembrolizumab demonstrated a significant prolongation of relapse-free survival while providing a good safety profile for patients with high-risk melanoma. |
| Adjuvant therapy with BRAF/MEK inhibitors should be preferred for patients with BRAF-mutant non-ulcerated stage III melanoma. |
Introduction
The incidence of malignant melanoma has increased dramatically in the past decades, and melanoma still has one of the fastest growing incidence rates among all malignant tumors in the Western world. At the same time, melanoma is responsible for over 90% of skin cancer deaths owing to its tendency to metastasize early [1]. In clinical practice, melanomas usually present in early clinical stages (stages I and II), which are defined by the absence of lymph node (LN) metastasis in the American Joint Committee on Cancer classification [2, 3]. Surgical resection with an appropriate safety margin and subsequent sentinel LN biopsy provide a good prognosis particularly for stage I (melanoma-specific 5-year survival rate: 97–99%). Notably, however, patients with early-stage II melanoma may eventually experience a tumor relapse in the course of longer follow-up periods and show a melanoma-specific 5-year survival of 87–94% [3]. Hence, in order to allow for an objective evaluation of the risk for tumor relapse after initial surgical excision, longer follow-up periods may be required for these patients. Meanwhile, patients with thick ulcerated primary melanomas (IIC) and those patients showing regional LN metastases are classified as high-risk melanomas (stage III) and are associated with higher mortality [4]. Radical LN dissection and subsequent radiotherapy (RT) have long been considered the common scheme in the case of existing LN metastases [5, 6]. However, the concept of RT in the treatment of completely resected nodal or primary melanoma has been challenged by randomized controlled trials and little evidence supports the risk/benefit ratio for adjuvant RT in these patients today [7, 8]. Hence, prior to the introduction of adjuvant systemic therapy, radical LN dissection remained the treatment of choice for patients with regional LN metastasis, while RT was considered a valuable treatment option only for patients showing unresectable melanoma or when surgery was not radical [9].
Following LN metastases, there is a dramatic increase in the risk for the occurrence of organ metastases, regardless of whether surgical resection of the primary tumor or the affected LN has taken place previously [10-12]. In this regard, it has been shown that patients with stage IIID have a melanoma-specific 5-year survival of approximately 32% and a 1-year rate of recurrence-free survival (RFS) of 42.1% [13] [3, 10, 14]. Because of the increasing risk of tumor progression in the case of LN metastases, the adjuvant application of systemic therapies, which aim to minimize the risk of tumor progression [15] is an attractive concept. These considerations similarly apply to patients with stage IIC melanoma, who are at a higher risk of disease progression compared with patients at stage IIIA (5-year survival 82% vs 93%) [16, 17].
Adjuvant melanoma therapy was initially investigated using dacarbazine and bacillus Calmette-Guerin. This treatment, however, did not significantly improve overall (OS) or RFS of patients with melanoma [18]. Hence, the systemic application of interferons (IFN) represented the only effective therapeutic option used in the dermato-oncological routine until the advent of immune-checkpoint inhibitors (ICI) and BRAF/MEK-directed targeted therapy (TT) [19]. Interferons exert diverse effects both on immune cells, including myeloid and lymphoid cells, as well as on tumor cells, endothelial cells, and others. Depending on the particular cell type, IFN-α was found to induce distinct gene expression profiles, resulting in innate immune activation [20], dendritic cell stimulation, and subsequent induction of Th1-type T-helper cells [21], decreased secretion of vascular endothelial growth factor [22], and overexpression of major histocompatibility complex I [23]. First trials investigating adjuvant therapy with IFN-α 2b, 2a, or pegylated IFN showed a significant sustained effect on RFS, but only marginal improvements in OS, while revealing a marked concomitant toxicity [24]. In particular, the EORTC 18991 phase III trial demonstrated a significant improvement in RFS (hazard ratio [HR]: 0.82, p = 0.01) for patients with stage III melanoma receiving adjuvant pegylated IFN-α 2b as compared with placebo, finally leading to US Food and Drug Administration approval [24]. In further trials, the best response to adjuvant IFN treatment was being observed in patients with ulcerated melanoma, suggesting that ulceration of the primary tumor may serve as an overriding predictive factor for IFN sensitivity [25, 26]. Moreover, long-term follow-up results from the EORTC 18991 trial revealed a diminished impact of adjuvant pegylated IFN-α 2b as compared with the benefit seen in the initial trial results and showed only a marginally significant impact on RFS, whereas no increase in OS or distant metastasis-free survival has been observed [27]. Therefore, the use of IFN-α has significantly declined in recent years and is currently only available in the pegylated form for off-label use [28, 29]. In the following, we provide a comprehensive review on the advances in adjuvant melanoma therapy that have been achieved since the advent of ICI and TT (Fig. 1; Table 1).
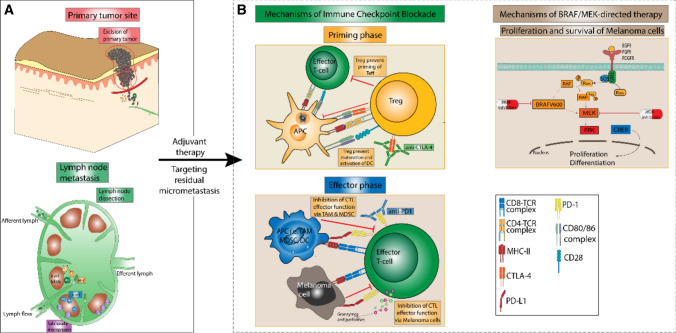
Fig. 1.
Schematic overview of the concept of adjuvant melanoma therapy and its underlying mechanisms of action. Following the initial excision of the primary tumor and if necessary existing lymph node metastasis (A), the adjuvant application of either immune-checkpoint inhibitors or BRAF/MEK-directed targeted therapy can be considered for patients with stage IIC–IIID melanoma (B). Immune-checkpoint inhibitors reinforce the anti-tumor immune response to melanoma cells both in peripheral lymph nodes and the tumor microenvironment (left panel, B). Here, the anti- cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab mainly affects the priming and activation of T cells in lymph nodes (top center). By contrast, anti- programmed cell death protein 1 (PD-1) antibodies mainly serve to restore effector cell function of T cells within the tumor microenvironment via blockade of the T-cell bound checkpoint molecule PD-1 on effector T cells (bottom center). Targeted therapy confers anti-tumor activity via the blockade of the RAF-MEK-ERK-signaling cascade, thus disrupting melanoma cell proliferation and differentiation (right panel, B). Notably, both targeted therapy and immune-checkpoint inhibitor therapy target residual melanoma cells and micrometastases, which have not been cleared by initial excision, thus reducing the risk of melanoma progression in adjuvant therapy. APC antigen-presenting cells, CTL , DC dendritic cell, MDSC , MHC II major histocompatibility complex II, PD-L1 programmed death-ligand 1, TAM , Treg regulatory T cell
Table 1.
Summary of the results from the randomized clinical trials reported for adjuvant melanoma therapy
| Authors | Trial | Regimen | Patients (N) | AJCC 7th Edition stages | HR RFS |
HR OS | 18-months RFS | 3-year RFS |
|---|---|---|---|---|---|---|---|---|
| Immunomodulatory therapy | ||||||||
| Eggermont et al. [46] | EORTC 18071 | Ipilimumab 10 mg/kg vs placebo | 951 | III (> 1 mm) | 0.75 | 0.72 | – | 46.5% vs 34.8% |
| Weber et al. [48] | CheckMate 238 | Nivolumab 3 mg/kg vs ipilimumab 10 mg/kg | 906 | IIIB–IIIC/IV | 0.65 | NA | 66.4% vs 52.7% | – |
| Eggermont et al. [73] | KeyNote 054 | Pembrolizumab 200 mg vs placebo | 1019 | III (> 1 mm) | 0.57 | NA | 71.4% vs 53.2% | – |
| Tarhini et al. [47] | ECOG 1609 | Ipilimumab 10 mg/kg vs Ipilimumab 3 mg/kg vs High-dose IFN-α 2b | 1670 | IIIB, IIIC, IV, M1a-b |
1.0 0.85 (0.66–1.09) |
– 0.78 |
– 59% 52% |
– 53% 46% |
| Targeted therapy | 2-year RFS | |||||||
| Long et al. [76] | COMBI-AD | Dabrafenib/trametinib vs placebo | 870 | III (LN metastasis > 1 mm) | 0.47 | 0.57 | 67% vs 44% | 58% vs 39% |
| Maio et al. [77] | BRIM8 | Vemurafenib vs placebo | 498 |
IIC, IIIA, IIIB IIIC |
0.54 | NA | 72.3% vs 56.5% | - |
AJCC American Joint Committee on Cancer, HR hazard ratio, IFN interferon, LN lymph node, NA not available, OS overall survival, RFS recurrence-free survival
Adjuvant Immunomodulatory Therapy
The advent of ICI-based immunotherapy has significantly improved the treatment of metastatic malignancies [10]. Checkpoint molecules are fundamental components for maintaining immunologic self-tolerance [30], controlling the duration and magnitude of immune responses and thus preventing tissue damage from an excessive immune response [31]. Notably, malignant tumors may mimic this immune regulatory mechanism by expressing checkpoint molecules themselves and via the accumulation of immunoregulatory cells, which show a strong expression of checkpoint molecules. Checkpoint molecules may be discriminated by their ability to affect [1] the priming and activation of T cells in the LN or [2] whether they specifically inhibit an effective anti-tumor immune response in the tumor microenvironment [32-35].
The checkpoint molecule cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a member of the B7/CD28 family that controls T-cell functions. It is constitutively expressed by regulatory T cells but can also be upregulated by CD4+ T cells upon activation. Cytotoxic T-lymphocyte antigen 4 competes with the co-stimulatory receptor CD28 for the ligation to CD80/86, which is expressed on antigen-presenting cells [36]. Because of the higher affinity of CTLA-4 to CD80/86, it serves as a critical regulator limiting CD28-mediated signaling during antigen presentation, which results in an increased activation threshold for T cells and thus balances T-cell-mediated immune responses to antigens, such as tumor cell antigens [37-39]. The pivotal role of CTLA-4 as a molecule of immunologic self-tolerance [39] was impressively demonstrated in mice with CTLA-4 knockouts, which have shown severely exaggerated autoimmune responses resulting in lethal outcomes [40, 41]. Consistent with this finding, inhibition of CTLA-4 restored effective anti-tumor activity in both murine tumor models and humans [42-44].
Ipilimumab
In the first clinical trials investigating the effects of the CTLA-4 antibody ipilimumab (IPI), a substantial prolongation of OS has been observed in more than 20% in patients with metastatic melanoma with some patients even showing durable tumor responses [45]. In this regard, the EORTC study 18071 confirmed the efficacy of IPI in the adjuvant setting for patients with stage III melanoma. In particular, the initial application of IPI in four cycles of 10 mg/kg followed by 3 years of maintenance therapy with 10 mg/kg resulted in a significantly prolonged RFS and OS compared with placebo control (5-year RFS 40.8% vs 30.3%; HR: 0.76 and 5-year OS 65.4 vs 54.4%; HR: 0.58). However, a high incidence of severe treatment-related adverse events (CTCAE > grade 3/4; treatment-associated adverse events [trAE]) and a treatment-related mortality of 1% were observed [46]. Regarding the occurrence of trAE, the subsequent E1609 study demonstrated that IPI at a lower dose of 3 mg/kg was less toxic and resulted in a reduction of trAE (IPI 10 mg/kg 58% vs IPI 3 mg/kg 36.4%). Moreover, this trial demonstrated a significant improvement in OS and a non-significant trend towards a better RFS of IPI 3 mg/kg as compared with high-dose IFN treatment as an active control regimen. Notably, adjuvant treatment with IPI 10 mg/kg was more toxic and was not found superior in efficacy as compared to adjuvant IFN [14, 47]. Based on the efficacy data of IPI, the US Food and Drug Administration approved IPI 10 mg/kg as an adjuvant therapy for patients with stage III melanoma by 2015. Because of its correlation with an increased incidence of trAE and the emergence of programmed cell death protein 1 (PD-1) antibodies as a more effective adjuvant therapy of patients with stage III melanoma, IPI 10 mg/kg has, however, not been granted approval in an adjuvant therapy setting in Europe [48, 49].
Nivolumab
Next to the blockade of CTLA-4 via IPI, anti-PD-1 antibodies pembrolizumab (Pembro) and nivolumab (Nivo) were found to significantly improve RFS and OS in the treatment of metastatic melanoma. In comparison to IPI, both PD-1 antibodies are characterized by higher response rates >40% and a better side-effect profile. Notably, some patients even show durable tumor responses after cessation of anti-PD1 therapy, suggesting the ongoing restoration of an effective anti-tumor immune response upon application of PD-1 inhibitors [29, 50].
The checkpoint molecule PD-1 is mainly expressed by T cells in the course of their activation and subsequently regulates their activity state [51–54]. Ligation of PD-1 to programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), which are expressed by antigen-presenting cells, such as monocytes, macrophages, and dendritic cells, but also melanoma cells [55–58] results in the inhibition of T-cell effector function [36]. This occurs via intracellular activation of immunoreceptor tyrosine-based inhibitory motifs [59], decreased secretion of interleukin-2, IFN-γ, and tumor necrosis factor-α [60, 61], decelerated cell-cycle progression [62–64], and a reduced expression of survival-promoting proteins. In the course of chronic inflammation (i.e., chronic infections, tumor formation and progression), an upregulation of PD-1 on T cells, B cells, and myeloid cells is frequently being observed, resulting in a profound regulation of the adaptive immune response [65, 66].
The efficacy of the anti-PD-1 antibody Nivo for adjuvant melanoma therapy was investigated in the CheckMate 238 study. This randomized double-blind trial demonstrated the superiority of adjuvant therapy with Nivo compared with IPI in stage IIIB/C and IV. A total of 906 patients were enrolled in the study and were treated either with Nivo 3 mg/kg or IPI 10 mg/kg for 12 months. Discontinuation of therapy occurred in the event of tumor progression or severe side effects. The primary endpoint of the CheckMate 238 study was RFS, secondary endpoints included OS and the incidence of trAE. Adjuvant therapy with Nivo resulted in a significantly improved RFS compared with IPI (RFS at 12 months: 70% vs 60%; 24 months: 63% vs 50%; ), a better tolerability with fewer severe side effects (14.4% vs 45.9%), and less treatment discontinuations because of therapy-related side effects (9.72% vs 42.6%). Particularly referring to the enrollment of a less favorable population of patients in terms of clinicopathological factors (stage IIIB, C, and IV), the results of the Checkmate238 trial may be considered even more remarkable [48, 67-69]. The recently published 4-year follow-up of the Checkmate 238 trial demonstrated sustained RFS with fewer irAE in patients with stage III/IV melanoma, who received adjuvant therapy with Nivo vs IPI (RFS 51.7% vs 41.2%; HR: 0.71 [95% confidence interval (CI) 0.60–0.86]; p = 0.0003; OS 77.9% [95% CI 73.7–81.5] vs 76.6% [72.2–80.3]; HR: 0.87 [95% CI 0.66–1.14]; p = 0.31) [69]. However, no significant differences in OS have been observed between IPI and Nivo, which might be attributed to the variable impact of subsequent follow-up therapies upon tumor progression. Because the RFS currently serves as the primary endpoint for evaluating the efficacy of adjuvant therapies, the adjuvant application of Nivo can be considered more effective and compatible compared with IPI [13, 70].
Pembrolizumab
The efficacy of adjuvant Pembro therapy was evaluated in patients with clinical stage IIIA–IIIC melanoma and sentinel LN metastases > 1 mm (KEYNOTE-054 study). After randomization, 1019 patients received either Pembro 200 mg or placebo every 3 weeks for 12 months or 18 cycles. Discontinuation criteria included tumor progression or the occurrence of non-tolerable side effects. Adjuvant therapy with Pembro resulted in a significant prolongation of RFS compared with placebo. The recent published 3.5-year follow-up of the KEYNOTE-054 clinical trial demonstrated a significantly improved RFS and distant-metastasis free survival (DMFS) in patients treated with Pembro in comparison with placebo (RFS 59.8% [95% CI 55.3–64.1] vs 41.4% [37.0–45.8]; DMFS, 65.3% [95% CI 60.9–69.5] vs 49.4% [44.8–53.8]) [71]. Interestingly, the analysis of prespecified subgroups related to age (> 65 years), tumor stage (IIIA–C), PD-L1 expression (PD-L1 positive 65.3% vs PD-L1 negative 56.9%), or BRAF mutation status (BRAF-V600E/K positive 62.0% vs BRAF wild-type 61.8%) did not show significant differences in terms of RFS [72]. Therapy-related adverse events, such as fatigue (37.1%), skin reactions (28.3%), diarrhea (19.1%), hypothyroidism (14.3%), arthralgia (12.0%), nausea (11.4%), or hyperthyroidism (10.2%), and treatment discontinuations were more frequently found in the Pembro arm (31.6% vs 18.5% and 13.0% vs 2.2% for trAE) [73].
Overall, clinical trials with adjuvant Pembro and Nivo demonstrated a significant prolongation of RFS for high-risk patients with melanoma. Therefore, the current guidelines of adjuvant melanoma therapy recommend the application of either a PD-1 inhibitor for a limited period of 12 months or until the occurrence of intolerable side effects or tumor progression [74]. Notably, both PD-1 inhibitors can be administered using higher dosages and with longer time intervals between each cycle without increasing the risk of side effects or loss of efficacy [75]. However, it should be noted that further prospective randomized controlled trials, comparing the high-dose regimen vs the currently established low-dose regimen, will be needed in order to establish the adjuvant safety and efficacy of the proposed treatment regimen.
Combined Checkpoint-Inhibitor Therapy
Considering the results of adjuvant ICI monotherapy in patients with resected stage III melanoma and the well-known clinical response rates of a combined checkpoint inhibitor therapy (cICI) with IPI and Nivo in non-resectable stage III or IV melanoma, the indication for adjuvant cICI is currently being intensely discussed. A recently published phase II study demonstrated the superiority of cICI with Nivo 1 mg/kg plus IPI 3 mg/kg in stage IV patients compared with monotherapy with Nivo after complete resection of melanoma and without evidence of currently existing metastasis (no evidence of disease). Here, a total of 167 patients were randomized into three treatment arms: (1) IPI 3 mg/kg plus Nivo 1 mg/kg, (2) Nivo 3 mg/kg, and (3) placebo. After a median follow-up of 28.4 months, the median RFS was not reached in the IPI plus Nivo group, whereas median RFS was 12.4 months (95% CI 5.3–33.3) in the Nivo group and 6.4 months (95% CI 3.3–9.6) in the placebo group. In the same vein, the HR for recurrence in the IPI plus Nivo group vs placebo was 0.23 (97.5% CI 0.12–0.45; p < 0.0001), and for the Nivo group vs placebo was 0.56 (0.33–0.94; p = 0.011). However, significantly more patients with cICI therapy experienced grade 3/4 trAE (70.9% vs 26.8% for monotherapy and 5.9% in the placebo group). Discontinuation of therapy because of intolerable side effects occurred in 62% of patients receiving cICI therapy and in 13% of patients receiving Nivo monotherapy [83]. However, these positive results favoring IPI plus Nivo vs Nivo monotherapy in an adjuvant therapy setting in patients with stage III or IV melanoma could not be reproduced in the Checkmate 915 trial (NCT03068455), a larger scale, commercially sponsored, international phase III trial that compared the adjuvant therapy of IPI plus Nivo with Nivo monotherapy. In total, 1844 patients with completely resected stage IIIB–IV melanoma were randomized in a 1:1 ratio into a cohort receiving IPI 1 mg/kg every 6 weeks plus Nivo 240 mg every 2 weeks or a cohort receiving Nivo monotherapy 480 mg every 4 weeks. First results published in 2020 showed that the dual primary endpoints of this clinical trial were not met. While the RFS rate at the 3-year follow-up for cohort A was 70% (95% CI 45–85) vs 75% (95% CI 50–89) for cohort B. The median RFS was not reached in either group and did not reveal a significant difference between the two cohorts (HR: 0.92, 95% CI 0.77–1.09, p = 0.269). Moreover, the current data showed similar outcomes after breaking down the patients into the melanoma stages III and IV. The RFS in patients with stage III melanoma after the 2-year follow-up was 64.7% in the cohort treated with IPI plus Nivo and 63.6% in the cohort treated with Nivo monotherapy. In the subgroup with stage IV melanoma, the 3-year and 2-year RFS rates were 74% (95% CI 48–88) and 80% (95% CI 55–92), respectively. Additionally, the treatment duration between the treatment cohorts was notably different, as patients who received the combination regimen had a median duration of therapy of 7.6 months vs 11.1 months in the Nivo-alone arm. Overall, these preliminary results show that IPI plus Nivo did not significantly improve the RFS or DMFS in patients with stage IIIB–IV melanoma and currently reaffirm Nivo monotherapy as the treatment regimen in an adjuvant setting [83].
Adjuvant BRAF/MEK-Directed Targeted Therapy
Next to ICI therapy, the clinical application of combined BRAF/MEK inhibitors has led to a widespread improvement in the therapeutic landscape for patients with metastatic melanoma in recent years. Of all patients with melanoma, 40–60% harbor a BRAF mutation, most commonly affecting codon 600 (BRAF V600E) [84]. This mutation results in the constitutive activation of the RAF-MEK-ERK signal transduction pathway, which is critical for melanoma development and progression [85].
Following the approval of BRAF/MEK inhibitor treatment with dabrafenib (Dab) and trametinib (Tram) for metastatic melanoma, their application has also been extended to the adjuvant setting for patients with melanoma stage III by 2018. The approval is based on a double-blind, placebo-controlled, phase III study that enrolled 870 patients with completely resected, high-risk stage III melanoma and BRAF V600E or V600K mutations. Adjuvant therapy was given for 12 months with Dab (300mg) plus Tram [2 mg] or placebo. Recurrence-free survival was defined as the primary endpoint, whereas OS, DMFS, RFS, and safety were defined as secondary endpoints. Combined BRAF/MEK inhibitor therapy showed a significantly improved RFS and DMFS as compared with the placebo group after a minimal follow-up of 59 months (58% vs 39%; HR: 0.47). However, the safety profile remained acceptable, largely showing similar results to those previously reported for patients with metastatic melanoma treated with Dab plus Tram [76]. The 5-year follow-up confirmed the longer survival without tumor reoccurrence or distant metastasis in patients treated with Dab plus Tram than placebo-treated patients (RFS 52% vs 36% [HR for relapse or death: 0.51; 95% CI 0.42–0.61]; DMFS 65% vs 54% [HR for distant metastasis or death: 0.55; 95% CI 0.44–0.70]). Notably, no apparent long-term irAE have been observed [86]. A second study tested the efficacy and tolerability of vemurafenib 960 mg in the adjuvant setting for patients with stage IIC and III BRAFV600-positive melanoma [77]. Here, 498 patients were enclosed and randomized into two cohorts. The primary endpoint was defined as RFS, and secondary endpoints included OS, DMFS, tolerability, and quality of life. Although both cohorts showed advantages for vemurafenib (median RFS 23 months vs 15 months), these results were not considered significant because the pre-specified endpoint was not met in one cohort.
Outlook and Future Concepts
Approximately 90% of all melanomas are diagnosed without evidence of existing distant metastasis. Following the occurrence of LN metastasis, melanoma-specific 10-year survival decreases from nearly 88% (IIIA) to 24% (IIID) [87]. Unfavorable prognostic factors for metastasis are tumor thickness and ulceration; accordingly, the recurrence rate of resected primary melanomas increases from approximately 5% for non-ulcerated small tumors (< 1 mm) to over 40% for melanomas with tumor thickness > 4 mm and existing ulceration. In this regard, it has been shown that the melanoma-specific 10-year survival and the risk for tumor recurrence is worse for patients with stage IIC (without LN metastasis) compared with stage IIIA (evidence of LN metastases) [88, 89].
Adjuvant Therapy for Patients with Stage II Melanoma
The beneficial results from adjuvant melanoma therapy in stage III patients provide hope that stage IIC patients may also benefit from adjuvant therapy [90, 91]. Hence, several phase III trials are currently investigating whether adjuvant ICI therapy using PD-1 antibodies improves RFS in stage II patients: these include the KEYNOTE-716 study (NCT03553836), which investigates the efficacy of a 12-month adjuvant therapy with Pembro 200 mg for stage IIB/C patients. Here, RFS is defined as a primary endpoint, and secondary endpoints include OS and distant metastasis-free survival [84].
The efficacy of Nivo in an adjuvant setting for melanoma stage IIB/C is currently being analyzed in the CheckMate 76k phase III trial (NCT04099251). Similar to the KEYNOTE-716 trial, 12 months of therapy with Nivo 480mg or placebo will follow a 2:1 randomization. In the case of progression for patients included in the placebo arm, a cross-over to Nivo therapy is possible.
The NivoMela study (NCT04309409) additionally allows for the enrollment of stage IIA patients alongside stage IIB–C patients. Using biomarker-based risk stratification, patients with high scores and thus an increased risk of recurrence receive Nivo or placebo. A low stratification leads to inclusion in the observation arm. Randomization is based on tumor stage (IIA vs IIB vs IIC), sex (female vs male), and location of the primary tumor (extremities vs trunk vs head and neck).
Concepts of Neoadjuvant Melanoma Therapy
Next to the promising results from adjuvant melanoma trials, the application of ICI in the neoadjuvant setting is currently under investigation, which might pose a new therapeutic alternative for patients with advanced melanoma (see Table 2). The efficacy of neoadjuvant therapy has already been demonstrated for various tumor entities [92–95]. The benefit of neoadjuvant treatment includes the reduction in tumor burden, thus resulting in an improved operability and control of locoregional metastasis. Additionally, it has been suggested that a neoadjuvant approach might enable the assessment of the individual responsiveness towards ICI therapy, thus gaining further information on potential mechanisms of tumor resistance [96, 97].
Table 2.
Summary of preliminary results and ongoing clinical trials on the safety and efficacy of (neo)adjuvant melanoma therapy
| Authors | Regimen | Patients (N) | AJCC 7th Edition stages | ORR | RFS | Adverse events (> grade 3 CTCAE) | Other findings |
|---|---|---|---|---|---|---|---|
| Huang et al. [78] | Neoadjuvant single-dose Pembro, surgery after 3 weeks, adjuvant Pembro for 12 months | 27 | Resectable stage III/IV | pCR 19%; near pCR 11% | Nearly 100% in patients with pCR or near pCR | No irAE > grade 3 reported | Mechanisms of resistance (e.g., immune suppression) |
|
Amaria et al. [79] |
Cohort A: neoadjuvant Nivo 3 mg/kg for 4 doses, surgery, adjuvant Nivo 3 mg/kg for 24 weeks (q2w) Cohort B: IPI 3 mg/kg with Nivo 1 mg/kg for up to 3 doses, surgery adjuvant Nivo 3 mg/kg for 24 weeks (q2w) |
23 | Recectable stage III/IV |
Cohort A: pCR 25% Cohort B: pCR 45% |
Cohort A 56% Cohort B 81% [20 months median follow-up] |
Cohort A 8% grade 3 trAE Cohort B 73% grade 3 trAE |
Response to ICB was associated with an increased infiltration of TIL and clonal diversity |
|
Blank et al. [80] |
Cohort A: surgery, adjuvant IPI 3 mg/kg with Nivo 1 mg/kg for up to 4 doses Cohort B neoadjuvant IPI 3 mg/kg + Nivo 1 mg/kg for up to 2 doses, surgery, adjuvant IPI 3 mg/kg + Nivo 1 mg/kg for up to 2 doses |
20 | Clinical stage III | Cohort B: 78% (pCR 33%, near pCR 33%) | No relapse in patients with pCR/near pCR at 25.6 months follow-up | Nearly 90% grade 3 trAE | Neoadjuvant IPI + Nivo increased infiltration of T-cell clones in TME |
|
Rozeman et al. [81] |
Cohort A: neoadjuvant IPI 3 mg/kg + Nivo 1 mg/kg for up to 2 doses (q3w), surgery Cohort B: neoadjuvant IPI 1 mg/kg + Nivo 3 mg/kg for up to 2 doses (q3w), surgery Cohort C: vs IPI 3 mg/kg for up to 2 doses, followed by Nivo 1 mg/kg for up to 2 doses, surgery |
86 | Clinical stage III |
Cohort A: 80% Cohort B: 77% Cohort C: 65% |
100% of pCR and near pCR patients on all arms at 8.3 months median follow-up |
Cohort A: 40% Cohort B: 20% Cohort C: 50% |
High TMB and IFN-γ signature were associated with pathological response and RFS |
|
Rozeman et al. [81] Experimental: PRADO extension cohort |
Marker placement in ILN, neoadjuvant IPI 1 mg/kg with Nivo 3 mg/kg for up to 2 doses (q3w), ILN resection after 6 weeks Surgery and adjuvant therapy: •pCR/near pCR no CLND or adjuvant treatment, only follow-up (e.g., CT) •pPR CLND no adjuvant treatment, only follow-up •pNR will undergo CLND and start at week 12 with adjuvant treatment for 52 weeks + follow-up |
144 | III (LN metastasis >1 mm) | 71% pRR the IL, 61% MPR, TLND was omitted in 58 (97%) of the MPR patients | - | 24% | Neoadjuvant treatment induced a high pRR with tolerable toxicity |
|
Long et al. [82] |
Neoadjuvant Dab 300 mg with Tram 2 mg daily for 12 weeks, surgery, adjuvant Dab 300 mg with Tram 2 mg daily for 40 weeks | 35 | Resectable IIIB–C | 86% at resection, 46% CR | Median 23 months | 29% | Neoadjuvant TT led to a high proportion of patients with pCR, no PD during neoadjuvant therapy |
CLND complete lymph node dissection, CR complete response, CT computed tomography, Dab dabrafenib, ICB , IFN interferon, ILN index lymph node, IPI ipilimumab, irAE immune-related adverse events, LN lymph node, Nivo nivolumab, MPR , ORR overall response rate, pCR pathological complete response, PD progressive disease, pNR no response, pRR pathological response rate, q2w every 2 weeks, q3w every 3 weeks, RFS recurrence-free survival, TLND , TMB tumor mutational burden, TME, trAE treatment-related adverse events, Tram trametinib, TT targeted therapy
A first trial investigating the efficacy of neoadjuvant treatment reported a good anti-tumor immune response with a complete or partial response after a single dose of Pembro 200 mg in a total of 8 of 27 patients with resectable stage IIIB/C or IV melanoma (no residual tumor, n = 5; <10% viable tumor cells, n = 3). The treatment regimen involved a single dose of Pembro 3 weeks prior to curative surgery, followed by 12 months of adjuvant Pembro therapy. After a median follow-up of 25 months, all eight patients remained recurrence free. Recurrence-free survival and OS for the entire study cohort were estimated at 63% and 93%, respectively [78].
Consistent with these results, another trial investigating the efficacy of cICI therapy in the neoadjuvant setting demonstrated strong overall response rates (ORR 73%, pathological complete response [pCR] 45%) as compared with Nivo monotherapy (ORR 25%, pCR 25%). Here, patients received either four doses of Nivo or three doses of combined ICI prior to surgical resection of the metastases. Surgery was followed by 6 months of adjuvant Nivo therapy (3 mg/kg every 2 weeks) in both groups. Despite the better ORR, grade 3 trAE have been more common for patients receiving cICI therapy as compared with monotherapy (73% vs 38%) [79].
More recently, the OpACIN trial (NCT02437279) evaluated the response to cICI therapy (Nivo 1 mg/kg plus IPI 3 mg/kg) in the neoadjuvant setting (two doses prior to surgery and two doses thereafter) or the adjuvant setting (four doses) for patients with palpable LN metastases and clinical stage III. Neoadjuvant therapy with cICI resulted in good response rates in the affected LN metastases (ORR 80%, pCR 60% < 10% viable tumor cells) and an estimated 3-year RFS of 80% for the neoadjuvant group and 60% for the adjuvant group. However, the application of cICI therapy has been accompanied by increased toxicity (trAE grade 3/4 90%), leading to discontinuation of therapy after the second or third cycle. Taking into consideration the high-grade toxicity found in the OpACIN trial, the OpACIN-neo trial (NCT02977052) provided neoadjuvant therapy with reduced doses of IPI and Nivo in patients with stage III melanoma with palpable LN metastases. In this trial, patients were randomized into three neoadjuvant study arms (Arm A: two doses of Nivo 1 mg/kg plus IPI 3 mg/kg; Arm B: two doses of Nivo 3 mg/kg plus IPI 1 mg/kg; Arm C: two doses of IPI 3 mg/kg followed by Nivo 3 mg/kg), which was followed by radical LN dissection at 6 weeks. Primary endpoints were toxicity, pathological response, and clinical response. Within the first 12 weeks, the highest rate of trAE was found in patients receiving sequential therapy (Arm C: 50% vs Arm B: 20% vs Arm A: 40%). Hence, this study arm was closed early. The strongest response was reported for conventional cICI therapy (Arm A: 63% showing a clinical response and 80% pathological response vs Arm B: 57% and 77% vs Arm C: 35% and 65%). Although the median RFS of the OpACINneo trial has not been reached yet, 18-month RFS of all patients was estimated with 85% (Arm A: 90%, Arm B: 82%, Arm C: 83%). Underscoring the general efficacy of neoadjuvant melanoma treatment using ICI is the observation that tumor progression was only found in 1.4% of patients showing an initial pathological response after a follow-up of 36 months in the OpACIN or OpACINneo trial.
Overall, the OpACIN trial demonstrated for the first time the potential benefit of neoadjuvant vs adjuvant ICI, while the OpACINneo trial demonstrated the good response of cICI. In particular, using lower doses of IPI (1 mg/kg) plus Nivo (3 mg/kg), a significantly better tolerability with 20% of patients showing grade 3/4 trAE has been observed, while response rates (pathological response rate 77%) remained similar compared to conventional cICI with Nivo 1 mg/kg plus IPI 3 mg/kg [81, 98, 99].
Next to the efficacy of a neoadjuvant therapy within the conventional therapeutic concept, as analyzed by the OpACIN trial, it remains to be determined whether patients with an initial response to neoadjuvant cICI therapy (Nivo 3 mg/kg plus IPI 1 mg/kg) benefit from subsequent LN dissection. This issue will be addressed by the PRADO (Personalized Response-driven Adjuvant Combination of IPI and Nivo in Stage IIIIB/C melanoma) [NCT02977052] trial. The protocol included two cycles of neoadjuvant cICI with Nivo 3 mg/kg plus IPI 1 mg/kg, which is followed by LN dissection after 6 weeks. In the case of no pathological response to neoadjuvant treatment (> 50% tumor cells), patients received LN dissection and 12 months of adjuvant therapy, which comprised either ICI or BRAF/MEK inhibitor therapy. By contrast, patients with pCR or almost complete response (detection of < 10% tumor cells) did not undergo LN dissection. Overall, pathological response was seen in 71%, and pCR (< 10% tumor cells in the index LN) was observed in 60% [100, 101]. Further data regarding the efficacy of either treatment are currently pending.
Last, the NeoCombi trial (NCT01972347) investigated the efficacy of neoadjuvant BRAF/MEK inhibitor therapy for stage IIIB/C patients. To this end, patients received 12 weeks of neoadjuvant therapy with Dab (300 mg) plus Tram (2 mg) followed by radical LN dissection and 40 weeks of adjuvant therapy. Preliminary data revealed that 86% of patients showed a pathological response (pCR: 43%, pPR: 40%) at the time of LN dissection. These data suggest, that neoadjuvant BRAF/MEK inhibitor therapy might also be considered a feasible therapeutic option; however, therapeutic outcome of adjuvant TT was not as favorable as after neoadjuvant immunotherapy, as after a median follow-up of 12.1 months, tumor progression was being observed in 36% of patients [82]. Consistent with these data, interim data from the Combi-Neo (NCT02231775) trial revealed a significantly longer RFS (median RFS 19.7 months vs 2.9 months) for neoadjuvant Dab (300 mg) plus Tram (2 mg) treatment as compared with initial LN dissection followed by adjuvant therapy. Here, it has been investigated whether 8 weeks of neoadjuvant Dab/Tram therapy prior to LN dissection plus adjuvant BRAF/MEK inhibitor therapy might be beneficial as compared to LN dissection and subsequent adjuvant therapy only. After a median follow-up of 18.6 months, 10/14 patients receiving neoadjuvant BRAF/MEK inhibitor therapy showed an ongoing tumor response as compared with 0/7 patients for conventional treatment [102].
Conclusions and Implications for Clinical Practice
Recently, the advent of ICI therapy and TT has radically changed the concept of adjuvant melanoma therapy [14]: adjuvant therapy protocols involving ICI and BRAF/MEK inhibitors have proven to result in a significant reduction in the risk for tumor relapse in patients with stage III melanoma. Accordingly, adjuvant ICI or BRAF/MEK inhibitor therapy is an evidence-based therapeutic alternative and must be discussed with patients. Because a direct comparison of the two adjuvant regimens in patients with the BRAF mutation has not been performed to date, the treatment decision involves considerations on the various side-effect profiles, the preferred mode of application, and the individual efficacy in a physician-patient discussion [91]. Because of the different criteria for patient enrollment in trials involving ICI or BRAF/MEK inhibitor therapy, cross-over comparisons of primary endpoints should be avoided [48, 73, 76]. Hence, pre-existing conditions and treatment feasibility are the most common criteria included in the decision whether to choose adjuvant ICI or targeted therapy for patients with BRAF mutation [70]. For BRAF wild-type patients, by contrast, ICI remains the most promising adjuvant therapeutic option. Treatment with either Nivo or Pembro did not show significant differences in terms of RFS or ORR [72].
Although there is culminating evidence on factors that may predict a response to BRAF/MEK inhibitor therapy for patients with stage IV melanoma, such as normal levels of lactate dehydrogenase, low tumor burden, or lack of cerebral metastasis [103], there are currently no predictive markers that might serve as guideposts for adjuvant therapy in stage III patients. Further complicating the decision is the observation inferred from longer follow-up trials, which suggest that adjuvant TT might be inferior to ICI therapy in terms of long-term outcomes [91]. This finding does however somewhat contrast with recent reports showing a statistically significant prolongation of OS after adjuvant BRAF/MEK inhibitors but not after anti-PD-1, at least when compared to IPI. Therefore, existing comorbidities and tolerability play a significant role in decision making. While pre-existing cardiac conditions are of primary concern in BRAF/MEK inhibitor therapy [104, 105], the presence of autoimmune diseases should be clarified before initiating ICI therapy [106, 107]. The high incidence of mild therapy-associated side effects during targeted therapy, some of which may persist throughout the duration of therapy [76], should be considered before initiating adjuvant BRAF/MEK inhibitor treatment. By contrast, side effects during ICI therapy are less frequent, but more often result in treatment cessation and can sometimes cause serious complications, including death [67]. Thus, discontinuation of therapy because of trAE is more common with ICI therapy [73].
As 40–60% of patients with melanoma harbor a BRAF mutation, therapeutic decisions may additionally take into account the need for therapeutic sequencing [108]. Sequencing of BRAF/MEK inhibitor and ICI therapy might be particularly relevant regarding potential immunomodulatory effects found in patients with the BRAF mutation and conferred by BRAF/MEK inhibitors. In this context, it has been observed that patients with the BRAF mutation frequently show an immunosuppressive TME, which is characterized by a decreased activity of antigen-presenting cells and an overexpression of inhibitory cytokines such as interleukin-6 and interleukin-10 [109–111]. BRAF/MEK inhibitor therapy, by contrast, may promote a shift toward an immunogenic TME (Fig. 2) by favoring the infiltration of CD8+ T cells, the expression of tumor antigens, or PD-L1 expression on melanoma cells, while reducing the infiltration by regulatory T cells and MDSC [111–113]. Accordingly, it is debated whether sequential BRAF/MEK therapy followed by ICI therapy may result in a better response to systemic treatment [114, 115].
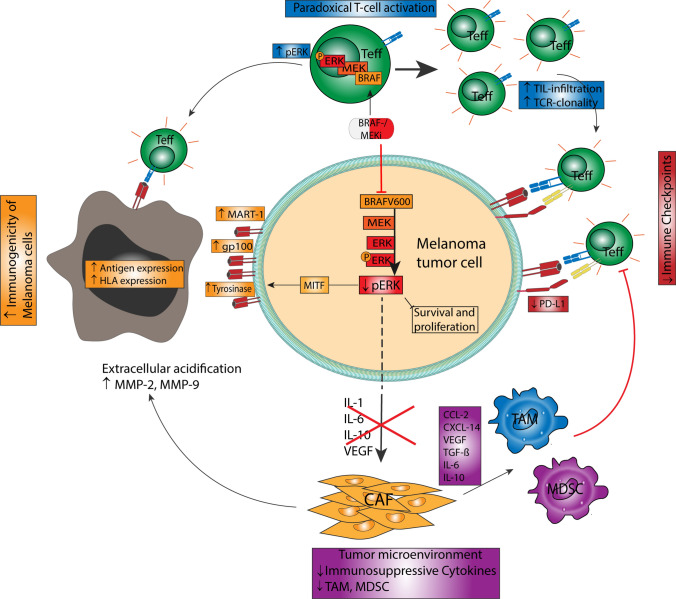
Fig. 2.
Immunomodulatory effects of BRAF/MEK inhibitors in the context of melanoma therapy. Next to the direct antiproliferative effects conferred by targeted therapy, it has been found that these might further exert immunomodulatory properties within the tumor microenvironment. In particular, BRAF inhibition resulted in a stronger expression of tumor antigens, thus favoring the immunological recognition of melanoma cells. Furthermore, BRAF inhibitors were initially shown to reduce programmed death-ligand 1 (PD-L1) expression on melanoma cells. More importantly, BRAF inhibition may result in a paradoxical activation of effector T cells thus enhancing anti-melanoma immune response. Last, BRAF-MEK inhibitors were found to reduce the secretion of cytokines such as interleukin (IL)-1, IL-6, or IL-10, which impairs the infiltration of immunosuppressive tumor-associated macrophages and myeloid derived suppressor cells. Overall, BRAF/MEK inhibitors may therefore tip the scale towards an inflamed tumor microenvironment favoring an anti-melanoma immune response. CAF, HLA human leukocyte antigen, MDSC, MMP, TAM, TCR, Teff, TIL, TGF-β transforming growth factor-β, VEGF vascular endothelial growth factor
However, the concept of neoadjuvant melanoma therapy represents another promising option for patients with advanced melanoma, as it has been found to efficiently reduce the tumor burden and thus improve operability. In this regard, preliminary clinical data revealed that patients with a pCR after neoadjuvant therapy were very likely to show persistent tumor responses [116]. Furthermore, neoadjuvant therapy allows for the early identification of mechanisms of tumor resistance, thus improving the subsequent therapeutic strategy [117]. However, it remains to be determined whether the initial data of the OpACIN study will be confirmed by subsequent trials and whether neoadjuvant treatment may additionally improve RFS as compared to adjuvant therapy. Another issue concerns the duration of neoadjuvant ICI therapy, which may be addressed in future trials [118]. Despite these yet unresolved issues, the introduction of ICI and BRAF/MEK inhibitor therapy has opened new possibilities for both adjuvant and neoadjuvant melanoma therapy. Further studies now need to clarify which patients might particularly benefit from neoadjuvant or adjuvant therapy and which treatment protocols might show the best efficacy for individual patient subgroups.
Declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. S.G. is funded by the German Research Council (SFB 1066, B4, B5) and by the German Research Council (SFB TR156, B11). H.S. and M.H. are supported by an intramural research funding of the UMC Mainz. M.H. is supported by the Clinician Scientist Fellowship “TransMed Jumpstart Program: 2019_A72” supported by the Else Kröner Fresenius Foundation.
Conflict of interest
H.S., M.H., U.N., M.S., S.P., J.H., C.L., and S.G. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Writing and editing by HS, MH, UN, MS, SP, JH, CL, and SG; figures designed by MH and HS; supervision by SG and CL. All authors have read and agreed to the version of the manuscript.
Footnotes
Henner Stege, Maximilian Haist, Carmen Loquai and Stephan Grabbe contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126(6):1166–1174. doi: 10.1002/cncr.32585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 5.Karakousis CP, Balch CM, Urist MM, Ross MM, Smith TJ, Bartolucci AA. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol. 1996;3(5):446–452. doi: 10.1007/BF02305762. [DOI] [PubMed] [Google Scholar]
- 6.Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson MA, Burmeister BH, Ainslie J, Fisher R, Di Iulio J, Smithers BM, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–1060. doi: 10.1016/S1470-2045(15)00187-4. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–597. doi: 10.1016/S1470-2045(12)70138-9. [DOI] [PubMed] [Google Scholar]
- 9.Strojan P. Role of radiotherapy in melanoma management. Radiol Oncol. 2010;44(1):1–12. doi: 10.2478/v10019-010-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi CR, Mozzillo N, Maurichi A, Pasquali S, Quaglino P, Borgognoni L, et al. The number of excised lymph nodes is associated with survival of melanoma patients with lymph node metastasis. Ann Oncol. 2014;25(1):240–246. doi: 10.1093/annonc/mdt510. [DOI] [PubMed] [Google Scholar]
- 11.Joo JH, Kim YS, Nam JH. Prognostic significance of lymph node ratio in node-positive cervical cancer patients. Medicine. 2018;97(30):11711. doi: 10.1097/MD.0000000000011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandro P, Andrea M, Nicola M, Simone M, Giuseppe M, Lorenzo B, et al. Lymph-node ratio in patients with cutaneous melanoma: a multi-institution prognostic study. Ann Surg Oncol. 2015;22(7):2127–2134. doi: 10.1245/s10434-014-4132-5. [DOI] [PubMed] [Google Scholar]
- 13.Ascierto PA, Borgognoni L, Botti G, Guida M, Marchetti P, Mocellin S, et al. New paradigm for stage III melanoma: from surgery to adjuvant treatment. J Transl Med. 2019;17(1):266. doi: 10.1186/s12967-019-2012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testori AAE, Chiellino S, van Akkooi ACJ. Adjuvant therapy for nelanoma: past, current, and future developments. Cancers. 2020;12(7):1994. doi: 10.3390/cancers12071994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggermont AMM, Dummer R. The 2017 complete overhaul of adjuvant therapies for high-risk melanoma and its consequences for staging and management of melanoma patients. Eur J Cancer. 2017;86:101–105. doi: 10.1016/j.ejca.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20(1):1–17. doi: 10.1016/j.soc.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yushak M, Mehnert J, Luke J, Poklepovic A. Approaches to high-risk resected stage II and III melanoma. Am Soc Clin Oncol Educ Book. 2019;39:e207–e211. doi: 10.1200/EDBK_239283. [DOI] [PubMed] [Google Scholar]
- 18.Agarwala SS, Neuberg D, Park Y, Kirkwood JM. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer. 2004;100(8):1692–1698. doi: 10.1002/cncr.20166. [DOI] [PubMed] [Google Scholar]
- 19.Kim KB, Legha SS, Gonzalez R, Anderson CM, Johnson MM, Liu P, et al. A randomized phase III trial of biochemotherapy versus interferon-alpha-2b for adjuvant therapy in patients at high risk for melanoma recurrence. Melanoma Res. 2009;19(1):42–49. doi: 10.1097/CMR.0b013e328314b84a. [DOI] [PubMed] [Google Scholar]
- 20.Flood BA, Higgs EF, Li S, Luke JJ, Gajewski TF. STING pathway agonism as a cancer therapeutic. Immunol Rev. 2019;290(1):24–38. doi: 10.1111/imr.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaupp L, Muth S, Rogell L, Kofoed-Branzk M, Melchior F, Lienenklaus S, et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell. 2020;181(5):1080–9619. doi: 10.1016/j.cell.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Raig ET, Jones NB, Varker KA, Benniger K, Go MR, Biber JL, et al. VEGF secretion is inhibited by interferon-alpha in several melanoma cell lines. J Interferon Cytokine Res. 2008;28(9):553–561. doi: 10.1089/jir.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28(3–4):239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 24.Eggermont AMM, Suciu S, Santinami M, Testori A, Kruit WHJ, Marsden J, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372(9633):117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 25.Daud A, Soon C, Dummer R, Eggermont AM, Hwu WJ, Grob JJ, et al. Management of pegylated interferon alpha toxicity in adjuvant therapy of melanoma. Expert Opin Biol Ther. 2012;12(8):1087–1099. doi: 10.1517/14712598.2012.694421. [DOI] [PubMed] [Google Scholar]
- 26.Eggermont AMM, Suciu S, Rutkowski P, Kruit WH, Punt CJ, Dummer R, et al. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB–III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer. 2016;55:111–121. doi: 10.1016/j.ejca.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Eggermont AMM, Suciu S, Testori A, Santinami M, Kruit WHJ, Marsden J, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon Alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30(31):3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 28.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 29.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol. 2020;1248:201–226. doi: 10.1007/978-981-15-3266-5_9. [DOI] [PubMed] [Google Scholar]
- 31.Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47(5):765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 32.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ai M, Curran MA. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother. 2015;64(7):885–892. doi: 10.1007/s00262-014-1650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrov DA, Shi W, Schwartz JC, Almo SC, Nathenson SG. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290(5492):816–819. doi: 10.1126/science.290.5492.816. [DOI] [PubMed] [Google Scholar]
- 38.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily: CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 39.Walunas TL, Bluestone JA. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998;160(8):3855–3860. [PubMed] [Google Scholar]
- 40.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–5791. [PubMed] [Google Scholar]
- 41.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 42.Christodoulou MI, Zaravinos A. New clinical approaches and emerging evidence on immune-checkpoint inhibitors as anti-cancer therapeutics: CTLA-4 and PD-1 pathways and beyond. Crit Rev Immunol. 2019;39(5):379–408. doi: 10.1615/CritRevImmunol.2020033340. [DOI] [PubMed] [Google Scholar]
- 43.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 44.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 47.Tarhini AA, Lee SJ, Hodi FS, Rao UNM, Cohen GI, Hamid O, et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J Clin Oncol. 2020;38(6):567–575. doi: 10.1200/JCO.19.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 49.Khunger A, Buchwald ZS, Lowe M, Khan MK, Delman KA, Tarhini AA. Neoadjuvant therapy of locally/regionally advanced melanoma. Ther Adv Med Oncol. 2019;11:1758835919866959. doi: 10.1177/1758835919866959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao C, Tian J, Liu H, Li F, Niu H, Zhu B. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2017;96(26):7325. doi: 10.1097/MD.0000000000007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaFleur MW, Muroyama Y, Drake CG, Sharpe AH. Inhibitors of the PD-1 pathway in tumor therapy. J Immunol. 2018;200(2):375–383. doi: 10.4049/jimmunol.1701044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 57.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33(10):2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 59.Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 2014;20(4):265–271. doi: 10.1097/PPO.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirsch I, Janovec V, Stranska R, Bendriss-Vermare N. Cross talk between inhibitory immunoreceptor tyrosine-based activation motif-signaling and Toll-like receptor pathways in macrophages and dendritic cells. Front Immunol. 2017;8(394):394. doi: 10.3389/fimmu.2017.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell KS, Dessing M, Lopez-Botet M, Cella M, Colonna M. Tyrosine phosphorylation of a human killer inhibitory receptor recruits protein tyrosine phosphatase 1C. J Exp Med. 1996;184(1):93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boonen GJ, van Dijk AM, Verdonck LF, van Lier RA, Rijksen G, Medema RH. CD28 induces cell cycle progression by IL-2-independent down-regulation of p27kip1 expression in human peripheral T lymphocytes. Eur J Immunol. 1999;29(3):789–798. doi: 10.1002/(SICI)1521-4141(199903)29:03<789::AID-IMMU789>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 63.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18(22):2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 64.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9(3):338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67(10):1481–1489. doi: 10.1007/s00262-018-2226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 67.Weber JS, Del Vecchio M, Mandala M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab (NIVO) versus ipilimumab (IPI) in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase III CheckMate 238 trial. Ann Oncol. 2019;30(Suppl_5):533. [Google Scholar]
- 68.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ascierto PA, Del Vecchio M, Mandala M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 70.Cohen JV, Buchbinder EI. The evolution of adjuvant therapy for melanoma. Curr Oncol Rep. 2019;21(12):106. doi: 10.1007/s11912-019-0858-3. [DOI] [PubMed] [Google Scholar]
- 71.Eggermont AMM, Blank CU, Mandalà M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(5):643–654. doi: 10.1016/S1470-2045(21)00065-6. [DOI] [PubMed] [Google Scholar]
- 72.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 Trial. J Clin Oncol. 2020;38(33):3925–3936. doi: 10.1200/JCO.20.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 74.Tarhini AA. The current state of adjuvant therapy of melanoma. Lancet Oncol. 2020;21(11):1394–1395. doi: 10.1016/S1470-2045(20)30544-1. [DOI] [PubMed] [Google Scholar]
- 75.Long GV, Tykodi SS, Schneider JG, Garbe C, Gravis G, Rashford M, et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29(11):2208–2213. doi: 10.1093/annonc/mdy408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long GV, Hauschild A, Santinami M, Atkinson V, Mandala M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 77.Maio M, Lewis K, Demidov L, Mandala M, Bondarenko I, Ascierto PA, et al. Adjuvant vemurafenib in resected, BRAF(V600) mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018;19(4):510–520. doi: 10.1016/S1470-2045(18)30106-2. [DOI] [PubMed] [Google Scholar]
- 78.Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 81.Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960. doi: 10.1016/S1470-2045(19)30151-2. [DOI] [PubMed] [Google Scholar]
- 82.Long GV, Saw RPM, Lo S, Nieweg OE, Shannon KF, Gonzalez M, et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF(V600) mutation-positive melanoma (NeoCombi): a single-arm, open-label, single-centre, phase 2 trial. Lancet Oncol. 2019;20(7):961–971. doi: 10.1016/S1470-2045(19)30331-6. [DOI] [PubMed] [Google Scholar]
- 83.Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395(10236):1558–1568. doi: 10.1016/S0140-6736(20)30417-7. [DOI] [PubMed] [Google Scholar]
- 84.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 85.Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A, et al. Melanoma. Nat Rev Dis Primers. 2015;1:15003. doi: 10.1038/nrdp.2015.3. [DOI] [PubMed] [Google Scholar]
- 86.Dummer R, Hauschild A, Santinami M, Atkinson V, Mandalà M, Kirkwood JM, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2020;383(12):1139–1148. doi: 10.1056/NEJMoa2005493. [DOI] [PubMed] [Google Scholar]
- 87.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 88.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28(18):3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Madu MF, Franke V, Van de Wiel BA, Klop WMC, Jozwiak K, van Houdt WJ, et al. External validation of the American Joint Committee on Cancer 8th edition melanoma staging system: who needs adjuvant treatment? Melanoma Res. 2020;30(2):185–192. doi: 10.1097/CMR.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 90.Kanaki T, Stang A, Gutzmer R, Zimmer L, Chorti E, Sucker A, et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur J Cancer. 2019;119:18–29. doi: 10.1016/j.ejca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 91.Kwak M, Farrow NE, Salama AKS, Mosca PJ, Hanks BA, Slingluff CL, et al. Updates in adjuvant systemic therapy for melanoma. J Surg Oncol. 2019;119(2):222–231. doi: 10.1002/jso.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Estevez LG, Gradishar WJ. Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res. 2004;10(10):3249–3261. doi: 10.1158/1078-0432.CCR-03-0133. [DOI] [PubMed] [Google Scholar]
- 93.Wang M, Hou L, Chen M, Zhou Y, Liang Y, Wang S, et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Sci Rep. 2017;7:44673. doi: 10.1038/srep44673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huber K. Cancer of the esophagus and gastroesophageal junction: improved survival with neoadjuvant radiochemotherapy. Strahlenther Onkol. 2013;189(2):161–162. doi: 10.1007/s00066-012-0249-2. [DOI] [PubMed] [Google Scholar]
- 95.Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 96.Dede K, Lang I, Porneczi B, Mester G, Fekete A, Koszegi G, et al. Preoperative chemotherapy in the surgical treatment of colorectal liver metastases. Magy Seb. 2013;66(6):325–330. doi: 10.1556/MaSeb.66.2013.6.4. [DOI] [PubMed] [Google Scholar]
- 97.Amaria RN, Menzies AM, Burton EM, Scolyer RA, Tetzlaff MT, Antdbacka R, et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019;20(7):e378–e389. doi: 10.1016/S1470-2045(19)30332-8. [DOI] [PubMed] [Google Scholar]
- 98.Carlino MS, Long GV, Schadendorf D, Robert C, Ribas A, Richtig E, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: a randomised clinical trial. Eur J Cancer. 2018;101:236–243. doi: 10.1016/j.ejca.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 99.Blank CU, Versluis JM, Rozeman EA, Menzies AM, Reijers IL, Krijgsman O, et al. Abstract 3412: 36-months and 18-months relapse-free survival after (neo)adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma patients: update of the OpACIN and OpACIN-neo trials. Cancer Res. 2020;80(Suppl_16):3412. [Google Scholar]
- 100.Tetzlaff MT, Messina JL, Stein JE, Xu X, Amaria RN, Blank CU, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861–1868. doi: 10.1093/annonc/mdy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blank CU, Reijers ILM, Pennington T, Versluis JM, Saw RPM, Rozeman EA, et al. First safety and efficacy results of PRADO: a phase II study of personalized response-driven surgery and adjuvant therapy after neoadjuvant ipilimumab (IPI) and nivolumab (NIVO) in resectable stage III melanoma. J Clin Oncol. 2020;38(15):10002. [Google Scholar]
- 102.Amaria RN, Prieto PA, Tetzlaff MT, Reuben A, Andrews MC, Ross MI, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181–193. doi: 10.1016/S1470-2045(18)30015-9. [DOI] [PubMed] [Google Scholar]
- 103.Long GV, Eroglu Z, Infante J, Patel S, Daud A, Johnson DB, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2018;36(7):667–673. doi: 10.1200/JCO.2017.74.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meirson T, Asher N, Bomze D, Markel G. Safety of BRAF+MEK inhibitor combinations: severe adverse event evaluation. Cancers (Basel). 2020;12(6):1650. doi: 10.3390/cancers12061650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bronte E, Bronte G, Novo G, Rinaldi G, Bronte F, Passiglia F, et al. Cardiotoxicity mechanisms of the combination of BRAF-inhibitors and MEK-inhibitors. Pharmacol Ther. 2018;192:65–73. doi: 10.1016/j.pharmthera.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 106.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123(11):1904–1911. doi: 10.1002/cncr.30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Granier C, Karaki S, Roussel H, Badoual C, Tran T, Anson M, et al. Cancer immunotherapy: rational and recent breakthroughs. Rev Med Interne. 2016;37(10):694–700. doi: 10.1016/j.revmed.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 108.Testori AAE, Chiellino S, van Akkooi ACJ. Adjuvant therapy for melanoma: past, current, and future developments. Cancers (Basel) 2020;12(7):1994. doi: 10.3390/cancers12071994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ho PC, Meeth KM, Tsui YC, Srivastava B, Bosenberg MW, Kaech SM. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNgamma. Cancer Res. 2014;74(12):3205–3217. doi: 10.1158/0008-5472.CAN-13-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ott PA, Henry T, Baranda SJ, Frleta D, Manches O, Bogunovic D, et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother. 2013;62(4):811–822. doi: 10.1007/s00262-012-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7(279):279ra41. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson DB, Pectasides E, Feld E, Ye F, Zhao S, Johnpulle R, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-PD-1 before and after BRAF inhibition. J Immunother. 2017;40(1):31–35. doi: 10.1097/CJI.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pavlick AC, Fecher L, Ascierto PA, Sullivan RJ. Frontline therapy for BRAF-mutated metastatic melanoma: how do you choose, and is there one correct answer? Am Soc Clin Oncol Educ Book. 2019;39:564–571. doi: 10.1200/EDBK_243071. [DOI] [PubMed] [Google Scholar]
- 116.Menzies AM, Rozeman EA, Amaria RN, Huang ACC, Scolyer RA, Tetzlaff MT, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC) J Clin Oncol. 2019;37(Suppl15):9503. doi: 10.1038/s41591-020-01188-3. [DOI] [PubMed] [Google Scholar]
- 117.Garutti M, Buriolla S, Bertoli E, Vitale MG, Rossi E, Schinzari G, et al. “To anticipate”: neoadjuvant therapy in melanoma with a focus on predictive biomarkers. Cancers (Basel) 2020;12(7):1941. doi: 10.3390/cancers12071941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keung EZ, Amaria RN, Sondak VK, Ross MI, Kirkwood JM, Wargo JA, et al. Neoadjuvant systemic therapy for high-risk melanoma patients. In: Balch CM, Atkins MB, Garbe C, Gershenwald JE, Halpern AC, Kirkwood JM, et al., editors. Cutaneous melanoma. Cham: Springer International Publishing; 2020. pp. 767–793. [Google Scholar]