Abstract
Localized provoked vulvodynia (LPV) is the most common cause of chronic dyspareunia in premenopausal women, characterized by pain with light touch to the vulvar vestibule surrounding the vaginal opening. The devastating impact of LPV includes sexual dysfunction, infertility, depression, and even suicide. Yet, its etiology is unclear. No effective medical therapy exists; surgical removal of the painful vestibule is the last resort. In LPV, the vestibule expresses a unique inflammatory profile with elevated levels of pro-nociceptive proinflammatory mediators prostaglandin E2 (PGE2) and interleukin-6 (IL-6), which are linked to lower mechanical sensitivity thresholds. Specialized pro-resolving mediators (SPMs), lipids produced endogenously within the body, hold promise as an LPV treatment by resolving inflammation without impairing host defense. Ten of 13 commercially available SPMs reduced IL-6 and PGE2 production by vulvar fibroblasts, administered either before or after inflammatory stimulation. Using a murine vulvar pain model, coupling proinflammatory mediator quantification with mechanical sensitivity threshold determination, topical treatment with the SPM, maresin 1, decreased sensitivity and suppressed PGE2 levels. Docosahexaenoic acid (DHA), a precursor of maresin 1, was also effective in reducing PGE2 in vulvar fibroblasts and rapidly restored mouse sensitivity thresholds. Overall, SPMs and their precursors may be a safe and efficacious for LPV.
Keywords: SPMs, vulvodynia, pain, inflammation, polyunsaturated fatty acids
Introduction
Vulvodynia afflicts 9-18% of women during their lifetime18, 32–34, 58, 67. Most patients suffer from localized provoked vulvodynia (LPV), which causes severe and lasting pain with touch to the vestibule, the vulvar tissue immediately surrounding the vaginal opening32, 67. Outer dermal portions of the vulva (external vulva), although only a few centimeters away, are relatively pain-free, while light touch to the mucocutaneous vestibule can result in lasting pain (allodynia)18, 26–28. This impairs a woman’s ability to engage in sexual intercourse, use a tampon, or even walk18, 36, 51, 58, 64, resulting in a substantial decline in quality of life7, 8.
There are no prescribed medical therapies for vulvodynia that outperform placebo18. The only reliable therapy to eliminate pain is surgical removal of the vestibule following unsuccessful trials of less invasive therapies18, 32, 58. A clinical trial has demonstrated that this perineoplasty procedure is more effective than behavioral therapy6. However, it is considered by many to be disfiguring and comes with significant risks71.
A key reason current vulvodynia therapies fall short is that the origins of the disease are poorly understood; vulvodynia is defined as unexplained vulvar pain lasting for ≥3 months18, 32, 67. To address this unmet need, our research team has embarked upon mechanism-based drug development for LPV. We have implemented two models to study the underlying mechanisms of LPV: 1) an in vitro vulvodynia model utilizing fibroblasts isolated from the anatomic site of LPV pain and 2) a new in vivo mouse vulvodynia model described herein. Compelling evidence, including our work and others, establishes a link between inflammation and vulvodynia1, 3, 4, 18–21, 26, 28, 41, 42, 55, 70, 73, 79. Fibroblasts from painful areas are inherently sensitive to inflammatory stimuli and will respond to stimuli that normally do not elicit a response, such as the resident microbial flora18–21, 26. In LPV patients, vestibular fibroblasts produce more pro-nociceptive interleukin-6 (IL-6) and prostaglandin E2 (PGE2) than fibroblasts from non-painful vulvar or regionally sampled fibroblasts from women without disease, which is linked to lower pain thresholds18–21, 26. Fibroblasts play an important role in the immune response, maintain their phenotypes in culture, and produce proinflammatory mediators, which makes them a useful model for investigating what is unique about the vestibule (site of pain) in LPV patients18–21, 26–28, 57.
A mechanistic approach to LPV therapeutic development might therefore target inflammation18, 26, 52 and could be extended to other painful chronic diseases, including fibromyalgia74. In vestibular fibroblasts, inhibiting nuclear kappa factor B (NFκB), a master regulator of inflammation, ablates proinflammatory signaling18–21. However, even local inhibition of NFκB risks impairing inflammatory defenses at a site that must respond to infectious challenge35, 40, 44. Topical steroids have been used to treat other vulvar conditions, such as lichen sclerosus69, but they pose the same risks as NFκB inhibitors and have not shown efficacy for vulvodynia31, 58, 75.
Inflammation is a dynamic process modulated by naturally derived products, referred to as specialized pro-resolving mediators (SPMs)9, 12, 13. Dietary omega fatty acids are converted into SPMs that help resolve inflammation and have anti-nociceptive effects for other chronic pain conditions, including migraine headaches and fibromyalgia13, 37, 66. All SPM classes (lipoxins, resolvins, protectins, and maresins) have been shown to impart analgesic effects in rodent pain models, including models of acute and chronic inflammatory, neuropathic, post-operative, and cancer pain68. Furthermore, SPMs have virtually no toxicity, and several are in clinical trials for other indications11, 14, 29, 30, 38, 54, 63. SPMs are not traditional anti-inflammatory agents and are not immunosuppressive; they are a critical component of the resolution machinery, deficits of which are associated with chronic pain2, 9, 10, 59–61, 68.
Therefore, we investigated whether SPMs have efficacy for vulvodynia treatment. In addition to testing SPMs in our human fibroblast in vitro model20, 26, 28, we optimized a mouse model that mimics key features of vulvodynia. We found that SPMs, and even the polyunsaturated fatty acids from which they are derived, are effective in reducing pro-nociceptive IL-6 and PGE2 levels and restoring pain thresholds to baseline levels in mice.
Materials and Methods
Patient/Sample Selection.
LPV-afflicted cases (fullfilling Friedrich’s Criteria5) and age/race-matched pain-free controls were recruited from the Division of General Obstetrics and Gynecology clinical practice at the University of Rochester between December 2012 and November 2019. All subjects provided informed consent, and the research was approved by the University of Rochester Institutional Review Board (RSRB #42136). Expanded details on our selection criteria and sampling procedures have been previously published26, 28. In brief, all subjects denied the use of corticosteroids and non-steroidal anti-inflammatory medications and had no chronic inflammatory illnesses other than LPV. Prior to biopsy of the vestibular and external vulvar sites, sampling sites underwent Wagner™ mechanical algometry. We used a Method of Limits technique for vulvodynia mechanical pain threshold initially described by Zolnoun et al.81 and replicated in our earlier publication26. Using the Wagner™ algometer, an increasing 0.5 N per second force (range 0 to 5 N) was applied perpendicular to the mucocutaneous surface by a moistened dacron tipped swab affixed to the Wagner™ algometer. Force was terminated at the point of pain development (subject signaled by hand-held clicker) or when the mucocutaneous force reached 5 N. Algometer-site tissue was sampled and used to create fibroblast strains as previously described26, 28. A total of 2 existing paired (vulvar vestibule and external vulva) case and 2 paired control fibroblast strains (8 total) were used for this study. Key patient characterists are listed in Table 1. These strains are representative examples of a much larger panel of cases and controls; they respond consistently and predictably from experiment to experiment19–21. Fibroblasts are a useful model for vulvodynia, because they 1) participate in the immune response and secrete proinflammatory mediators into the media that can be readily quantified, 2) maintain their phenotype in culture, and 3) are viable over a number of passages without immortalization18–21, 26–28, 57.
Table 1.
Patient Characteristics for Fibroblast Strains
| Strain | Age | Race | Vestibule Threshold | Vulva Threshold |
|---|---|---|---|---|
| Case A | 20 | Caucasian | 0.53 | 1.13 |
| Case B | 61 | Caucasian | 0.63 | 1.15 |
| Control A | 41 | Caucasian | 2.64 | 3.50 |
| Control B | 36 | Caucasian | 1.80 | 4.00 |
Fibroblast culture.
Previously established primary fibroblast strains (each obtained from a different patient or healthy control) were cultured in Minimum Essential Medium (MEM) supplemented with 10% FBS, GlutaMAX, gentamicin, and antibiotic/antimycotic solution (Gibco/Invitrogen/Thermo Fisher Scientific, Grand Island, NY). Early passage (4-10) external vulvar and vestibular fibroblast strains were seeded at 2.5 X 104 cells/cm2. After cultures reached full confluence, fibroblasts were typically serum-starved for 48 h in MEM lacking FBS prior to stimulation with inflammatory agonists (e.g. IL-1β). Fibroblast cellular identity was previously confirmed by microscopic inspection and with fibroblast-specific markers (e.g. vimentin, collagen). At the same time, the cells were confirmed to be negative for epithelial cell markers (e.g. cytokeratin), smooth muscle and myofibroblast markers (e.g. α-smooth muscle actin), endothelial cell markers (e.g. CD34), and bone marrow derived cell markers (e.g. CD45)39. These markers remain constant across passages, and the cells consistently respond to stimuli throughout culture.
Impact of SPM pre- and post-treatment on proinflammatory mediator production in vulvar fibroblasts.
Cells were grown to confluency in 24 well plates then serum started for 48 h prior to any subsequent treatments. Two representative paired case strains were used. All treatments were administered to quadruplicate wells. For SPM pre-treatment (prior to the initiation of proinflammatory signaling), cells were treated with 5 nM RvD1, AT-RvD1, 17S-HDHA, RvD2, RvD3, RvD4, RvD5, RvE1, LXA4, LXB4, maresin 1,7(S) epi-Maresin 1, protectin D1, or protectin DX for 18 h (Cayman Chemical Company, Ann Arbor, MI, catalogue numbers 10012554, 13060,10007280, 10007279, 13834, 13835, 10007280, 10007848, 90410, 90420, 10878, 13161, 10010390, and 10008128, respectively). After 18 h, cells were treated a second time with SPMs, 30 minutes prior to stimulation with 5 pg/ml IL-1β (R&D Systems, Minneapolis, MA). Cells received a final SPM dose 18 h later and supernatants were collected 48 h after IL-1β stimulation. For post-treatment (after the initiation of proinflammatory signaling) cells were first treated with 5 pg/ml IL-1β for 30 minutes prior to treatment with 5 nM SPMs. After 24 h, samples received a booster dose of SPMs, and supernatants were collected 48 h after IL-1β stimulation. Previous dose ranging experiments were conducted to determine 5 nM was the lowest effective dose of SPMs that significantly reduced IL-6 and PGE2 production. For the few SPMs that did not reduce IL-6 and PGE2 levels, 10 or even 100-fold higher concentrations were ineffective. Standard sandwich ELISAs were performed to measure production of IL-6 (BD Biosciences, Franklin Lakes, NJ) and competitive EIA assays were performed to measure PGE2 production (Cayman Chemical Company, Ann Arbor, MI).
3D tissue culture of mouse vulvar biopsies.
Six mm punch biopsies were collected, encompassing the entire vulvar area immediately posterior to the vaginal opening, and each biopsy tissue was oriented to permit non-tangential cross-sectioning and was equally bisected to create two samples. Each biopsy half was washed several times in sterile saline prior to being placed in a 24 well plate. Twelve mice donated a total of 24 biopsies pieces, which were divided among treatments; 3 pieces from different individuals (n=3) were used for each treatment. Each well, containing a single biopsy piece, was flooded with 0.5 ml serum-free MEM, supplemented with GlutaMAX, gentamicin, and antibiotic/antimycotic (Gibco). 3D cultures were pre-treated with lipoxin A4, resolvin D2, or maresin 1 at a concentration of 1, 10, or 100 nM for 24 h. Each treatment was applied to three pieces of biopsy tissue. After 24 h, cells were treated a second time with lipoxin A4, resolvin D2, or maresin 1 30 minutes prior to treatment with 10 pg/ml murine IL-1β (R&D Systems) for another 24 h before harvesting supernatants and assaying for PGE2, as described earlier.
Effect of docosahexaenoic acid (DHA) treatment on vulvar fibroblasts.
To study the proinflammatory signal modulating effects of DHA, cells were grown to confluency, then treated with 200 nM or 1 μM DHA (Cayman Chemical, Ann Arbor, MI) for 72 h prior to stimulation with 10 pg/ml IL-1β and a second dose of DHA for another 72 h. Two paired case and control strains were used. Supernatants were then assayed for PGE2.
Maresin and DHA Testing in Mice.
All procedures involving mice were approved by the University of Rochester Committee on Animal Resources (UCAR protocol 2016-006). Zymosan, a proinflammatory yeast cell wall preparation (MilliporeSigma, St. Louis, MO) was used to induce sustained vulvar allodynia, measured by pain threshold testing as described by Farmer et al.22. The Farmer model used live yeast infection or zymosan as the provoking stimuli to elicit vulvar allodynia; we elected to use zymosan because it is more highly reproducible and reduces the technical complexity of the model versus infection with a live pathogen. We initially used a manual von Frey that employs a series of “hairs” of different thicknesses/rigidity that exert differing forces when applied to the injection site, located at the midline posterior vulva (between the vaginal opening and anus) and then transitioned to an electronic system. Details on model development can be found in the Supplement (Figs S1–4).
During the Phase 1 of the experiment, 12 8-week-old female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were acquired and trained to facilitate electronic von Frey determination of vulvar pain thresholds. During training, the mice were given access to a preferred drinking solution containing 3% dextrose and 0.125% saccharin76 (in tap water), which was filter sterilized before administration. The mice were given the solution in a standard water bottle (replacing their normal water supply) daily for ~6 h/day in their home cage for ~1 week until they began to steadily drink the solution, at which point they were transferred to the testing apparatus. Once transferred to the testing apparatus (Fig 1), they were given a steady drip (50 μl droplet/s) of solution for ~2 h/day, 2-3 days/week, for a period of ~2 weeks. Once the mice were able to find the solution in the test cage, the flow was tapered to one 50 μl droplet/30 s interval, and the mice were again exposed for ~2 h/day, 2-3 days/week, for a period of ~2 weeks to this non-contingent fixed time schedule of delivery. Once the mice held relatively still in the testing apparatus upon introduction to the cage, they began baseline mechanical sensitivity threshold testing. The mice were shaved and tails were inked weekly. Four colors were used to distinguish each mouse in a cage; the same 4 colors were used for each cage, so mice from different cages could not be distinguished. A series of three individual threshold tests were conducted to determine the baseline threshold before zymosan injections commenced.
Figure 1: Treatment scheme and testing environment.
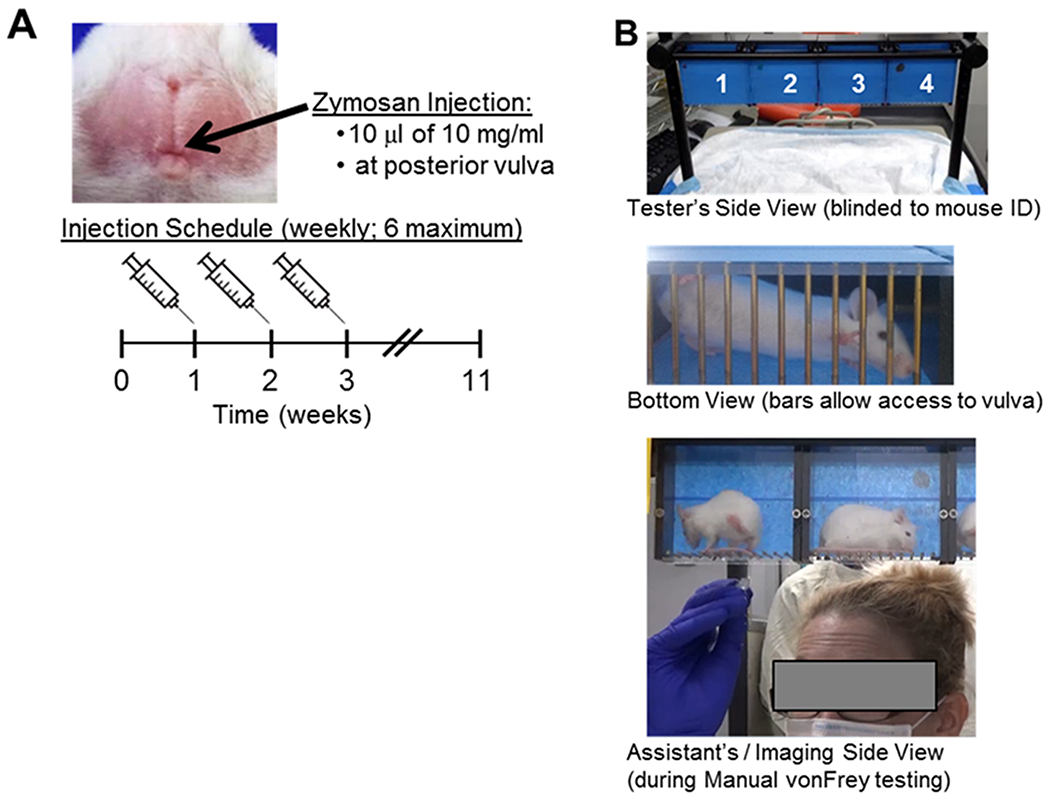
Panel A: Image of mouse vulva. Arrow indicates injection site. Below image, there is a schematic of in vivo mouse model to establish vulvar allodynia. Panel B: Images of allodynia testing environment. Four mice are placed in separate compartments that permit access to the vulva from below. The front side is masked to reduce visual cues during testing, while permitting imaging from the rear.
For testing using the electronic von Frey, a cage of 4 mice was tested at once, alternating the mice stimulated, working from left to right and repeating the series until 5 values were collected for each mouse. The electronic von Frey device was gently rotated upwards at approximately 1 g/s, increasing the pressure, until the mouse stepped or jumped off the hair, at which point the peak force was automatically recorded (mechanical sensitivity threshold). The investigator was blinded to cage and mouse identity. Blinding was maintained by reading cage barcodes into custom LabVIEW software for use with the Mousemet electronic von Frey device (TopCat Metrology, Ltd., Cambridge, UK). The software reads the barcode and keeps it blinded from the investigator during testing, after which the data, retaining mouse identities, is exported to an Excel file.
The mice were permitted access to the preferring drinking solution throughout testing at a rate of one 50 μl droplet/30 s interval. The inter-trial interval is at least 30 s to prevent acute desensitization from repetitive stimulation. The tester assesses the validity of each test and records test values to each mouse based on test cage position using the button on the electronic von Frey device or the computer keyboard. To be a valid test, all the following must be true: 1) the force increased ~1 g/s across within an acceptable range of 0.5-4 g/s, 2) the hair contacted the injection site, 3) the hair did not leave the injection site prior to mouse response, and 4) the hair and device arm were not depressed by anything other than contact with the mouse. With our custom software, the device automatically records the peak value when pressure on the hair is relieved (e.g. the mouse steps off the hair), eliminating the need for the tester to make a determination of if/when the mouse reacts to the stimulus.
During the induction phase, the mice received up to 6 weekly injections to the midline posterior vulvar under isoflurane anesthesia and underwent weekly threshold testing. The number of injections administered was based on threshold; injections ceased after a ≥33% drop in threshold (vs. baseline threshold) for any 2 weeks. Four mice received saline and 8 mice received zymosan injection. The person conducting the pain threshold testing was blinded to treatment assignment. Mechanical sensitivity threshold testing and injections occurred at the same day and time each week; thresholds were determined the day before injection each week. Mice were shaved weekly the day prior to testing. A final threshold was determined on the seventh week, following the final injection on week 6. At the end of the seventh week, mice that had at least two consecutive pain tests (any two weeks) with a greater than 33% reduction in threshold were eligible for drug testing. Any mice not developing allodynia, as defined by these criteria, remained in the analysis to serve as sentinels for any ill effects of treatment and to reduce testing bias. Mice were monitored for several weeks after induction to determine if their allodynia was sustained prior to testing the effects of maresin 1 treatment.
Mice with persistent allodynia were block randomized to topical maresin 1 or vehicle treatment. Due to the risk of cross-contamination with topical treatment, only one treatment type was administered per cage (block randomization). Mice received once daily application of the treatment seven days a week for 4 weeks. Treatments were prepared fresh daily with sterile ingredients. Mice received 1 μg of maresin 1 each day, suspended in ethanol and diluted in phosphate buffered saline (PBS) with 5% DMSO to a final volume of 30 μl. The vehicle treatment contained the same amount of ethanol, PBS, and DMSO. Mice were held by the tail, and the liquid was slowly and gently applied to the entire shaved area using a pipettor. Mice were held for a total of two minutes prior to release into the home cage to allow the liquid to dry prior to release to avoid having the treatment rub off on the bedding. Threshold testing was performed blinded each week as described. Mice also underwent vulvovaginal lavage each week (while under anesthesia for injection) to determine vulvovaginal concentrations of PGE2 for each mouse.
To assess the possible influence of conditioned behavior, mice were randomly selected to receive either topical lidocaine/prilocaine or saline. A second individual not performing threshold testing selected which mice would receive either solution and applied the solutions to the mice immediately prior to threshold testing. A pea-sized amount of lidocaine/prilocaine cream was applied to a sterile swab, which was rolled over the entire vulvar surface. For mice receiving saline, the swab was submerged in saline, and then the moistened swab was rolled across the entire vulvar surface. The mice were released into their home cage immediately after application. The person performing the threshold testing was blinded to cage and mouse identity. Electronic von Frey threshold testing was performed as previously described. Testing for all mice was completed in less than 1 h from the time of lidocaine/prilocaine application, ensuring all mice were still adequately numbed by the solution during threshold testing.
For DHA testing, 36 8-week-old female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were divided into three treatment groups. A topical formulation (cream) of DHA was prepared containing the following: 3% glyceryl monostearate, 3% cetyl alcochol, 2.5% polyoxyl-40, 3.5% isopropyl myristate, 4% white petrolatum, 1% benzyl alcohol, 0.5% vitamin E acetate, 15% neosorb 70/20 B, 65.5% purified water, and 2% highly purified fish oil enriched for DHA (Omegatex®, Solutex GC, SL, Madrid, Spain; ~70% DHA by volume). To prepare the cream, the oil and water phases were heated to 70°C and combined by high speed homogenization for 5 min, and then mixed while cooling. Active phase ingredients were pre-mixed with antioxidant vitamin E and preservative benzyl alcohol and added to the emulsion at 45°C. A vehicle cream containing all the elements, save DHA, was also prepared. Creams were prepared in a small batch under non-GMP conditions by the Ferndale Pharma Group (Detroit, MI). Mice were randomized into three groups: vehicle, DHA, and mock. Mice were held by the tail, and a pea-sized amount of cream was applied to the entire shaved area by rolling a sterile cotton tipped swab over the area. For mock treatment, a saline-moistened swab was used. Threshold testing was performed blinded each week as described.
Statistical Methods.
The following statistical programs were used: SAS 9.4 (SAS, Cary, NC), STATA (Stata Crop, LLC, College Station, TX), and Graph Pad Prism 7 (Graph Pad Software, San Diego, CA). Differences in IL-6 and PGE2 production in human vulvar fibroblasts in response to treatment with SPMs was found to be non-normally distributed by Shapiro-Wilk testing. We therefore utilized the non-parametric Wilcoxon Rank sum test. Based upon the testing of 10 SPM candidates, significance was Bonferroni corrected to a P < 0.006 of a two-tailed distribution. In cultured mouse tissue biopsies, differences in PGE2 were determined via one-way ANOVA. In live mice, differences in pain thresholds were analyzed with one-way ANOVA, while differences in PGE2 levels were analyzed via paired t-test. Spearman Rank correlation was performed to examine the relationship between PGE2 levels and threshold in mice. According to the Shapiro-Wilk testing, pain threshold values were found normally distributed but PGE2 values significantly differed from normality and therefore Spearman Rank was selected. Significance was defined at P ≤ 0.05 of a two-sided distribution.
Results
SPM treatment reduces human vulvar fibroblast proinflammatory signaling.
To assess whether SPMs reduce proinflammatory signaling linked to pain in vulvodynia26, we evaluated the effects of all commercially available SPMs on IL-6 and PGE2 production in vulvar fibroblasts from two representative cases used in previous studies19–21, 26. We employed two treatment strategies: 1) a pre-treatment strategy where cells were treated with SPMs prior to exposure to the inflammatory activator interleukin-1 beta (IL-1 β) and 2) a post-treatment strategy where inflammatory signaling was initiated with IL-1 β for 30 minutes prior to SPM treatment. We tested lipoxin A4, lipoxin B4, resolvin D1, aspirin-triggered resolvin D1, resolvin D2, resolvin D3, resolvin D4, resolvin D5, resolvin E1, maresin 1, 7S-epi-maresin 1, protectin D1, and protectin Dx. Ten of the 13 SPMs reduced IL-6 and/or PGE2, as a pre-treatment and/ora post-treatment (Fig 2). Resolvin D1, aspirin-triggered D1, and lipoxin B4 were the only SPMs that were ineffective in reducing IL-6 or PGE2 levels. The magnitude of reduction by pre-treatment was generally greater than post-treatment, and more SPMs were able to significantly reduce IL-6 versus PGE2(Fig 2). Nonetheless, most SPMs tested were capable of reducing proinflammatory mediator levels associated with vulvodynia pain.
Figure 2. Modulation of proinflammatory mediator production by SPMs.
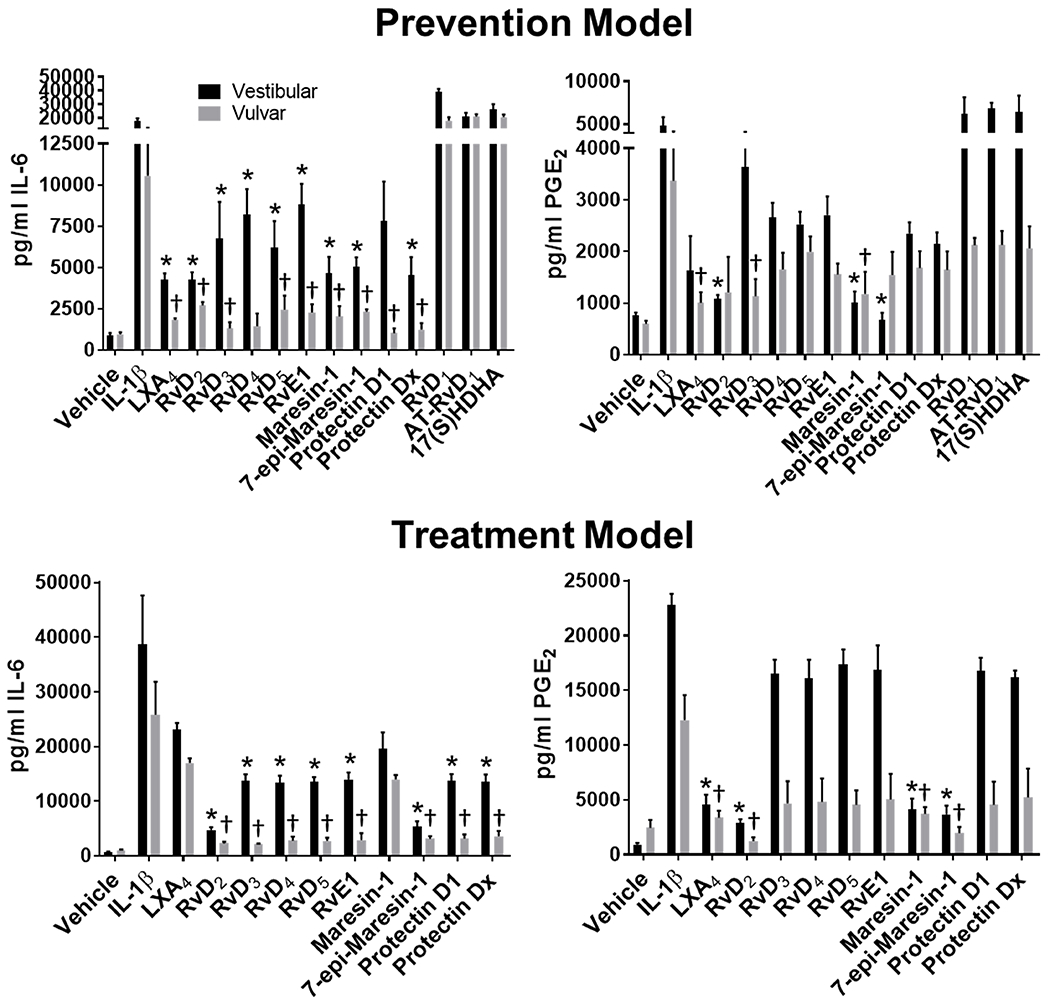
Patient vestibular and external vulvar fibroblasts underwent pre-treatment for 10 h with the indicated SPMs at a 5 nM concentration, then activation with IL-1β (10 pg/ml) for 48 h (prevention model) or underwent activation first with IL-1β for 30 min, then treatment with 5 nM SPMs for 18 h, followed by a booster dose for 6 h (treatment model). Culture media were collected and analyzed for IL-6 or PGE2 content. Levels in SPM + IL-1β treated samples were compared to IL-1β only treated levels via Wilcoxon rank sum with Bonferroni correction (P<0.006 *vestibular cells, †fvulvar cells). Mean values +/− SEM are plotted, n=4, Vest = vulvar vestibule, Vulv = external vulva.
To parse out the most significant differences, we plotted a concordance bubble chart (Fig 3) depicting the concordance of the degree of reduction in both IL-6 and PGE2 levels across all vulvar samples, including external vulvar and vestibular fibroblasts, both with pre- and post-treatment dosing. This strategy enabled the use of high stringency statistical methods to better rank the ability of each SPM to reduce proinflammatory mediator production to identify the most promising hits for further testing. The highest concordance was evident for lipoxin A4, 7S-epi-maresin 1, and resolvin D2, indicating they have the strongest suppressive effects for both IL-6 and PGE2. The relative bubble size reflects specificity of the suppressive response for the vulvar vestibule (painful area), confirming resolvin D2 and 7S-epi-maresin 1 are highly effective in reducing proinflammatory mediator release. Overall, exogenous SPM treatment has the capacity to reduce proinflammatory signaling associated with vulvar pain, while the maresins, lipoxin A4, and resolvin D2 were especially effective in reducing IL-6 and PGE2 levels in human vulvar fibroblasts.
Figure 3. PGE2 and IL 6 production is reduced by SPM treatment.
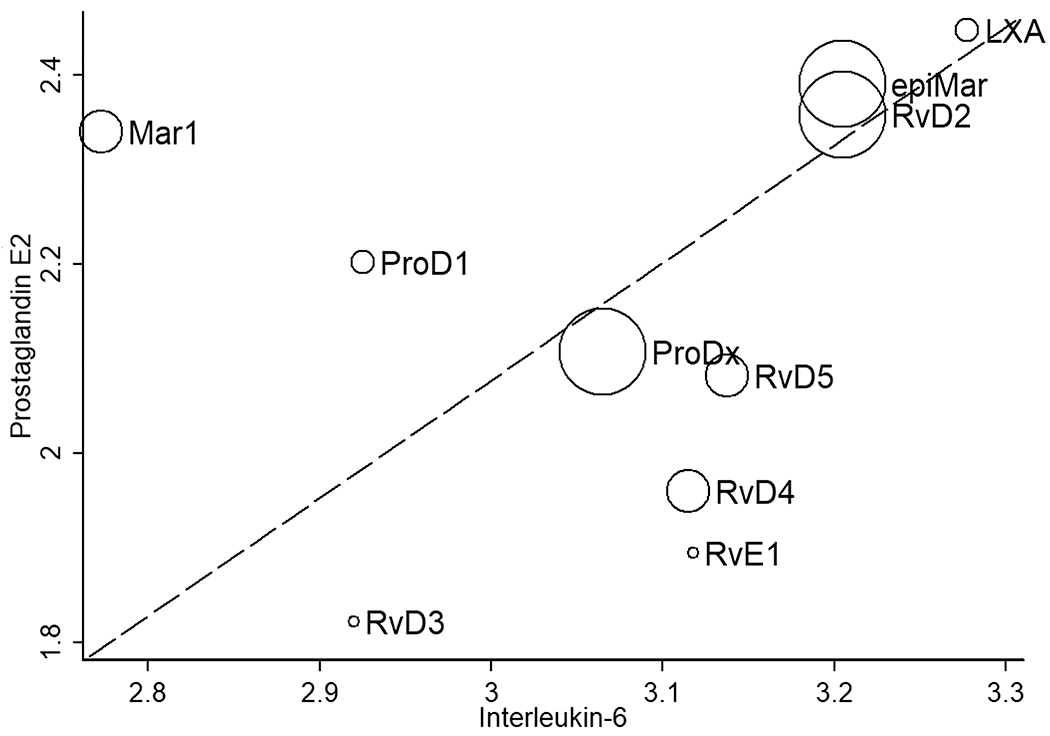
This graph shows the concordance of the degree of reduction in the two proinflammatory biomarkers, IL-6 and PGE2 following SPM inflammatory suppression in human vulvar fibroblasts. The scatterplot displays IL-6 and PGE2 associated Z scores (Wilcoxon rank sum) for suppressive effects, analyzing all data points, including pre- and post-treatment. The bubble size qualitatively reflects statistically significant suppressive effects. LXA = lipoxin A4, RvD = resolvin D-series, RvE = resolvin E-series. Three additional SPMs (resolvin D1, aspirin-triggered resolvin D1, and lipoxin B4) did not significantly reduce IL-6 or PGE2 levels (not shown), while ten depicted in this figure had a significant effect.
SPM treatment reduces proinflammatory mediator levels in cultured mouse biopsy tissues.
Prior to testing a response to SPM treatment in mice, we tested the effects of SPM treatment on PGE2 production in a three-dimensional tissue culture model using mouse vulvar biopsies. Pre-treatment with either maresin 1, lipoxin A4, or resolvin D2, SPMs that profoundly reduced both IL-6 and PGE2 production in human vulvar fibroblasts (Figs 2&3), also reduced PGE2 release from mouse vulvar tissue (Fig 4). All three SPMs produced dose-related effects over a 1-100 μM concentration. Maresin 1 was then selected for further testing in mice, because it was highly effective in reducing IL-6 and PGE2 levels in both models, especially in the 3D mouse culture model. In addition, exogenous maresin 1 has been shown to reduce inflammatory signals, including IL-6 and alleviate neuropathic pain and allodynia in other mouse models2, 23, 62. Reduced levels of endogenous maresin 1 may be even be linked to the development of chronic pain2. These observations, coupled with our in vitro data, led us to further investigate the analgesic properties of maresin 1 in a mouse model of vulvar allodynia.
Figure 4. SPM treatment reduces PGE2 levels in cultured CD-1 mouse vulvar biopsies.
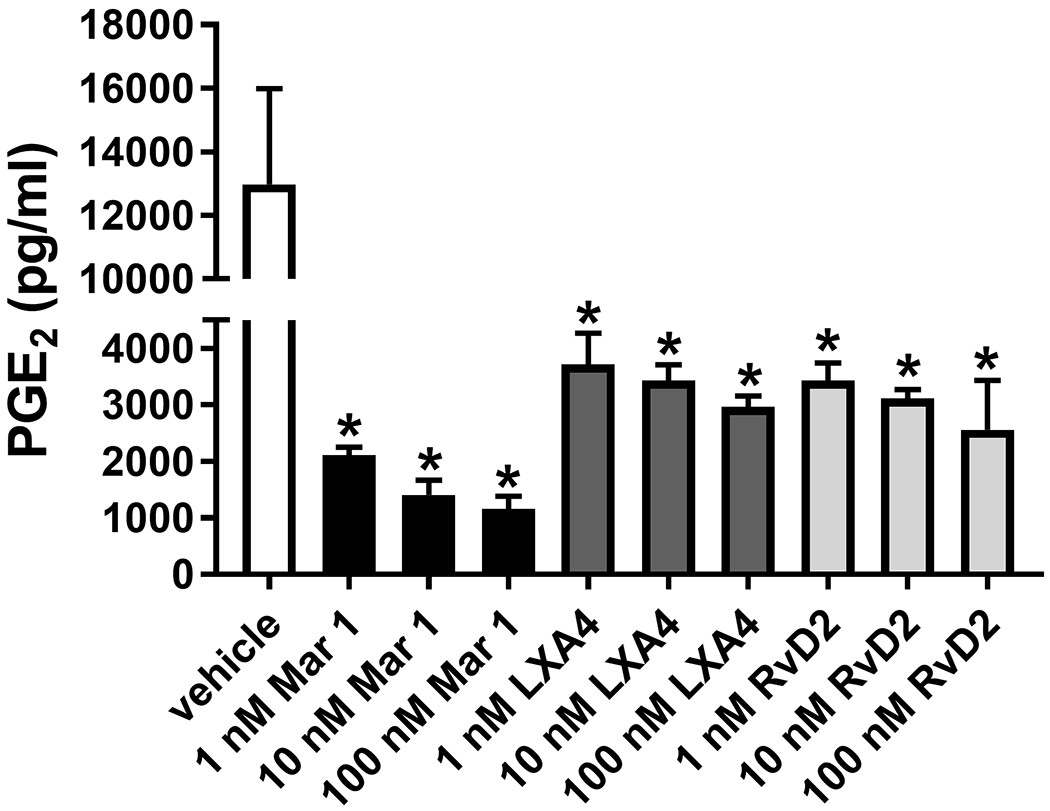
Mouse vulvar tissues (4 mm punch biopsies) from 12 CD-1 mice were collected, bisected, divided into treatment groups with 3 samples per group (n=3), and then pre-treated in culture medium with maresin 1 (Mar 1), lipoxin A4 (LXA4), or resolvin D2 (RvD2) for 18 h, followed by an additional 18 h stimulation with IL-1β (10 pg/ml). Culture medium was collected and analyzed for PGE2 content. Mean +/− SEM, n=3 replicate cultures, one-way ANOVA, P<0.05 vs. vehicle for all SPM treatments. Maresin 1 reduced PGE2 levels to the strongest degree in a dose dependent manner.
Maresin 1 reduces increases pain thresholds in mice.
Using the model of vulvar allodynia described by Farmer et al.22 as a starting point (Fig 1, S1), we developed a robust and reproducible model for testing the efficacy of potential topical SPM-based therapies for vulvodynia. Feasibility trials in mice demonstrated once daily treatment with maresin 1 can raise mechanical sensitivity thresholds and reduce PGE2 levels (Fig S2). Enhanced model development (Fig S3) produced reproducible and consistent threshold measurements (Fig S4).
Following the model development stage, we tested the efficacy of maresin 1 in increasing mechanical sensitivity thresholds. A reliable progressive reduction in mechanical sensitivity threshold values was produced by repeated zymosan injections (Fig 5). Furthermore, reduced thresholds persisted through and beyond the induction period. After four injections, most mice developed allodynia that was sustained for several weeks, creating a window to evaluate analgesic effects. After four weeks of maresin 1 treatment, there was an increase in threshold, near or above baseline. Thresholds were increased above the baseline; threshold values increased over time for both maresin 1 and vehicle treated mice but threshold values were higher for maresin 1 treated mice during treatment (P<0.05) (Fig 5). However, a vehicle effect was evident; DMSO has analgesic properties that may contribute to the observed increase in thresholds49. This increase is nonetheless enhanced by maresin 1, demonstrating maresin 1 has analgesic properties beyond the effects of DMSO, contributing to a faster and fuller recovery.
Figure 5. Increased PGE2 levels are accompanied by a lower mechanical sensitivity threshold; maresin 1 increases mechanical sensitivity thresholds in C57BL/6 mice.
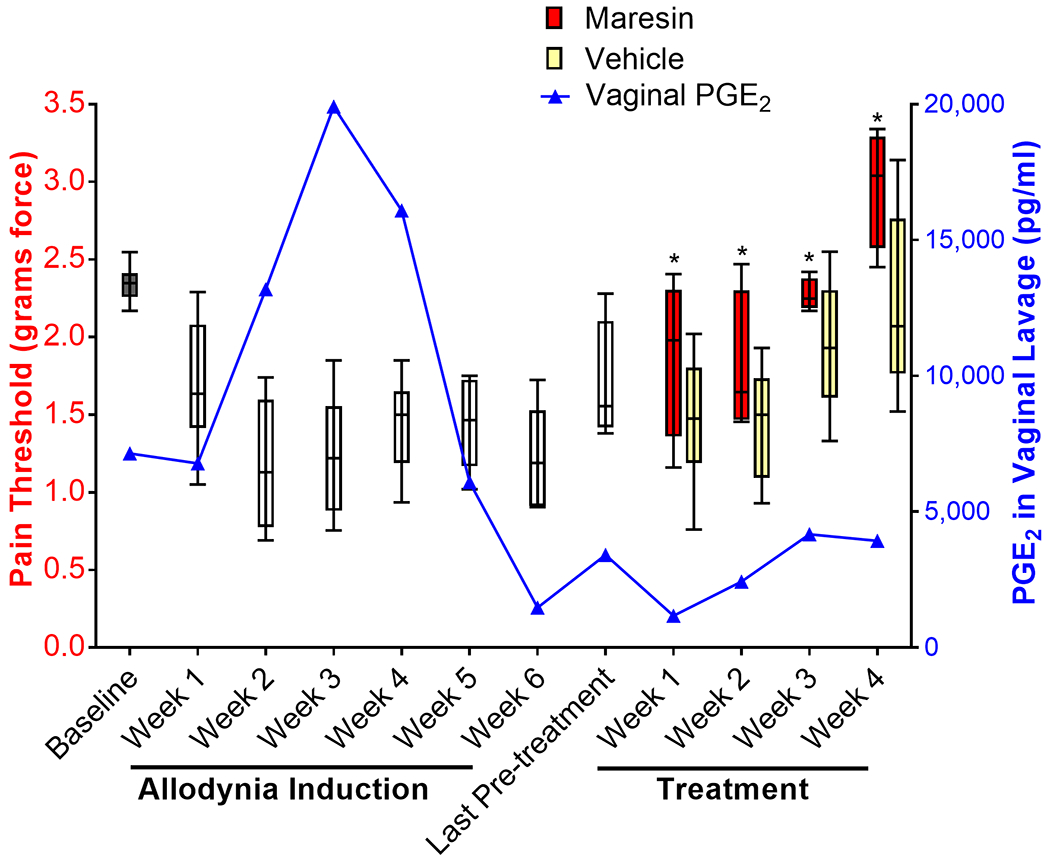
This graph depicts the average mechanical sensitivity thresholds for all C57BL/6 mice that developed allodynia (box and whisker plots, showing maximum, minimum, and median values). Baseline thresholds were established prior to allodynia induction by averaging three separate testing sessions during which an average of 5 threshold measures were taken per session (gray bar). Allodynia was then induced through a series of up to 6 weekly zymosan injections (white bars). A final assessment was taken on week 7 (last white bar) before mice were treated with maresin 1 (red bars) or vehicle (yellow bars) for 4 consecutive weeks. The threshold values are plotted as the average of 5 threshold tests for each mouse, with the exception of the baseline (average of 3 test or 15 values), while the PGE2 levels are the average of the entire group with one weekly sampling per mouse (n=7 mice). Over the induction period, pain thresholds declined and remained low, while PGE2 levels increased and eventually tapered off as acute inflammation was resolved, consistent with visible inflammation during the induction period (erythema, edema), which resolved prior to initiating treatment. There was an inverse relationship between thresholds and PGE2 levels during the induction phase (rho=0.56; P<0.002), similar to that seen in vulvodynia. During the treatment phase, PGE2 levels remained low and thresholds increased. Thresholds were higher in maresin 1 treated mice (*P<0.05 maresin vs. vehicle), but thresholds also increased with vehicle. Overall, maresin 1 exhibited effects beyond the vehicle and enhanced recovery in mice.
PGE2 is highly conserved between mice and humans24 and is a surrogate marker for pain in women with LPV; high levels of PGE2 in vitro predict reduced pain thresholds26. Therefore, we also assayed vulvovaginal levels of PGE2 in mice weekly. The pattern in PGE2 levels was consistent with an acute inflammatory response preceding sustained allodynia. PGE2 levels peaked after the fourth injection and decreased as acute inflammation resolved, at which point maresin 1 treatment did not further reduce these levels (Fig 5). Mice receiving zymosan had signs of erythema and edema during the induction phase, which resolved after the cessation of the injection series. Plotting average PGE2 levels against average thresholds showed a concomitant decrease in threshold value. Spearman Rank correlation demonstrated an inverse relationship between threshold values and PGE2 levels (rho=0.56; P<0.002), recapitulating what occurs in women with vulvodynia where high PGE2 levels (produced by fibroblasts in vitro) are associated with low threshold values26. In this aspect, the mouse model recapitulates the human condition; repeated vulvar insults (e.g. chronic yeast infection) produce acute inflammation that resolves over time but leaves a lasting hypersensitivity to normally non-painful stimuli. However, vulvodynia PGE2 levels were not reliable in distinguishing treatment effects.
Mice do not exhibit conditioned behavior in response to von Frey testing.
To validate the specificity of threshold testing in C57BL/6 mice, we devised a method to evaluate behavioral conditioning to mechanical sensitivity threshold assessment. Because frequent touching of the vulva with a probe might result in avoidance behavior, a subset of mice received topical lidocaine/prilocaine prior to threshold testing. Without evidence of a conditioned response to the von Frey filament, lidocaine/prilocaine should minimize pain responsive behaviors.
C57BL/6 mice showed a significant increase in threshold values following application of the lidocaine/prilocaine solution (Fig 6). Baseline thresholds were ~3 g force and were reduced to < 1 g after six weeks of zymosan injection, but restored to ~3 g with lidocaine/prilocaine application. Baseline thresholds were indistinguishable from post-lidocaine thresholds. Therefore, avoidance conditioning did not occur or interfere with mechanical sensitivity threshold assessment.
Figure 6. Reduced mechanical sensitivity threshold and lidocaine reversal are not avoidance responses in C57BL/6 mice.
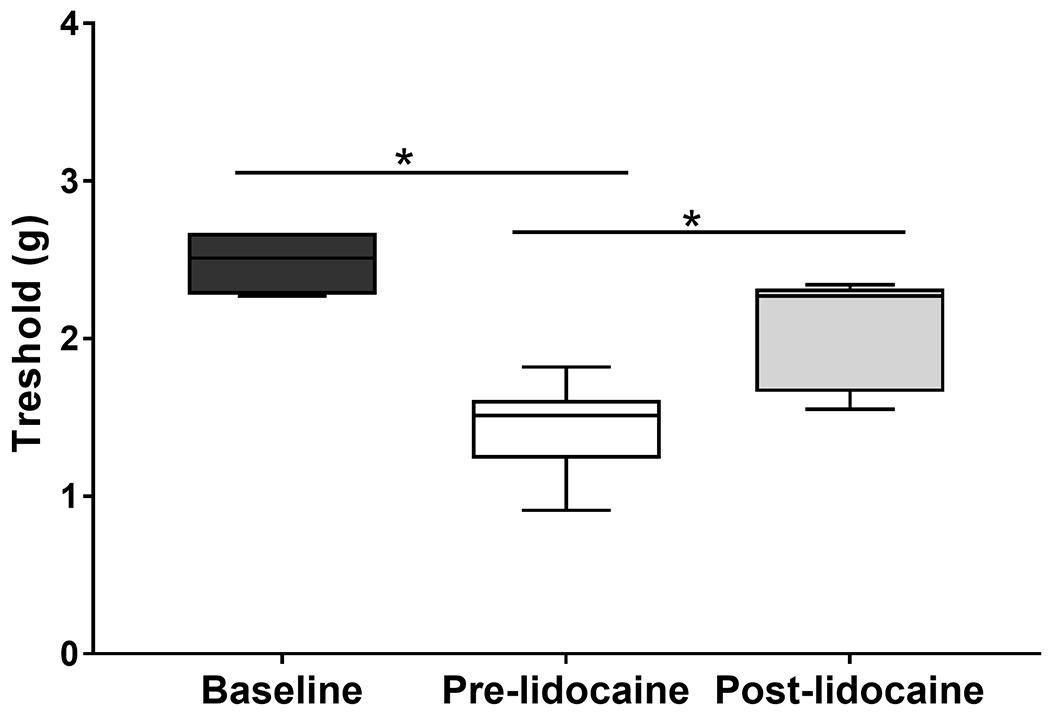
Box and whisker plots with maximum, minimum, and median values show baseline touch sensitivity and sensitivity after zymosan treatment before and after lidocaine treatment for C57BL/6 mice. Data are based on 5 determinations in each of 6 mice (one-way ANOVA, *P<0.05). Thresholds were reduced with zymosan and increased or were restored to baseline levels with lidocaine. Baseline thresholds were indistinguishable from post-lidocaine thresholds. Therefore, altered mechanical sensitivity threshold was not conditioned avoidance of contact.
Vulvar fibroblasts can use docosahexaenoic acid (DHA) as a substrate for SPM production, which reduces PGE2 levels in vitro.
Natural products rich in omega-3 fatty acids (e.g. fish oil) could represent a more efficient option for therapeutic development. Therefore, we sought to determine if human vulvar fibroblasts could utilize polyunsaturated fatty acid (PUFA) precursors to produce SPMs and if PUFA supplementation would be sufficient to reduce proinflammatory mediator levels. Preliminary analysis showed DHA, arachidonic acid (AA), and eicosapentaenoic acid (EPA) are substrates for the production of SPMs while the greatest number of and most abundant SPMs were DHA derived (Figure S5). Therefore, we also examined the effects of DHA on proinflammatory mediator production. DHA treatment significantly reduced PGE2 levels dose responsively and was effective even at very low nanomolar concentrations (Fig 7). Taken together, these observations suggest DHA is effective in reducing proinflammatory signaling in vulvar fibroblasts through the production of SPMs.
Figure 7. DHA reduces proinflammatory mediator levels in human vulvar fibroblasts.
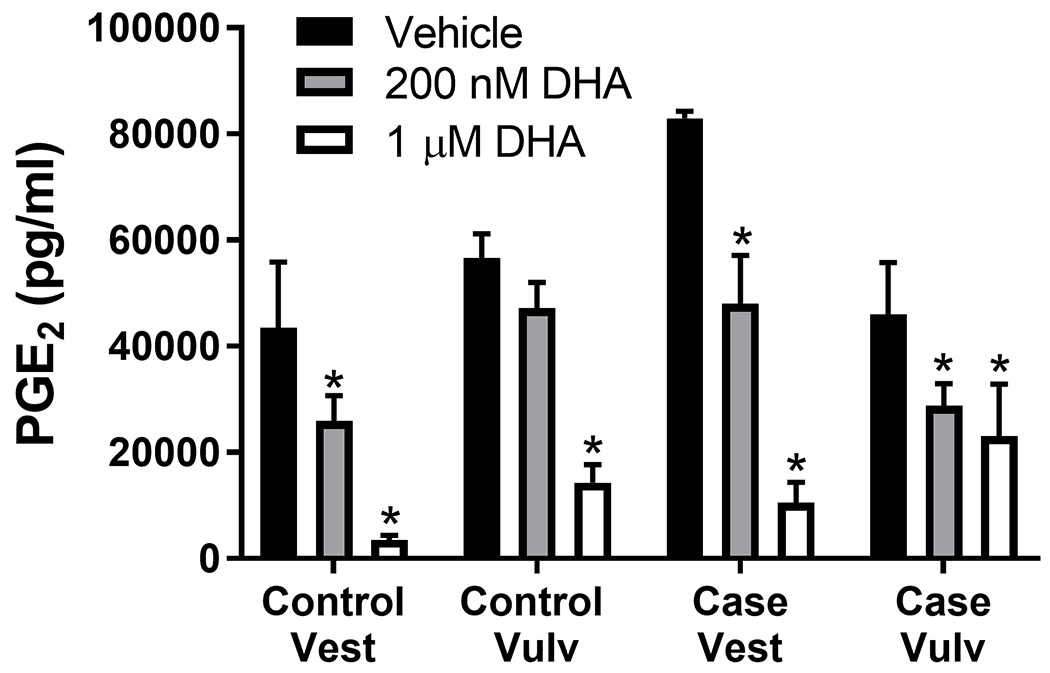
Human vestibular and external vulvar fibroblasts from 2 case and 2 control strains were pre-treated with DHA for 72 h, then activated with IL-1β (10 pg/ml) for another 72 h. Culture media were collected and analyzed for PGE2. DHA reduced PGE2 levels in the presence of IL-1β, a strong inflammatory stimulus. Mean +/− SEM of n=4. ANOVA, *P<0.05 vs. corresponding vehicle treatment.
DHA increases sensitivity thresholds in mice.
We went on to test a topical formulation of DHA in mice using formulations comparable to other topical applications currently approved for human use. Mice were treated twice daily for a total of 7 weeks, at which point the majority of the mice in the vehicle and DHA treated groups had recovered (75%), while only half the mice recovered in the mock treated group (Fig 8AB). Mice in the DHA group recovered fastest; 50% recovered after 2 weeks of treatment, while it took 4 weeks of vehicle treatment and 6 weeks of mock treatment for 50% to recover. With no recovery in the mock treatment group in the first 4 weeks, these early affects can be attributed to DHA and to some extent the vehicle; a vehicle effect was apparent after 3 weeks. We used the threshold data to calculate the percent improvement score, which represents the percent increase in threshold over the last threshold prior to initiating treatment (Fig 8C). The average percent improvement in mechanical sensitivity threshold was significantly lower in the mock and vehicle treated groups versus the DHA treated group over the first four weeks of treatment, again demonstrating that mice treated with DHA recovered more quickly than vehicle or mock treated mice.
Figure 8. Topical DHA treatment increases pain threshold and reduces time to recovery in C57BL/6 mice.
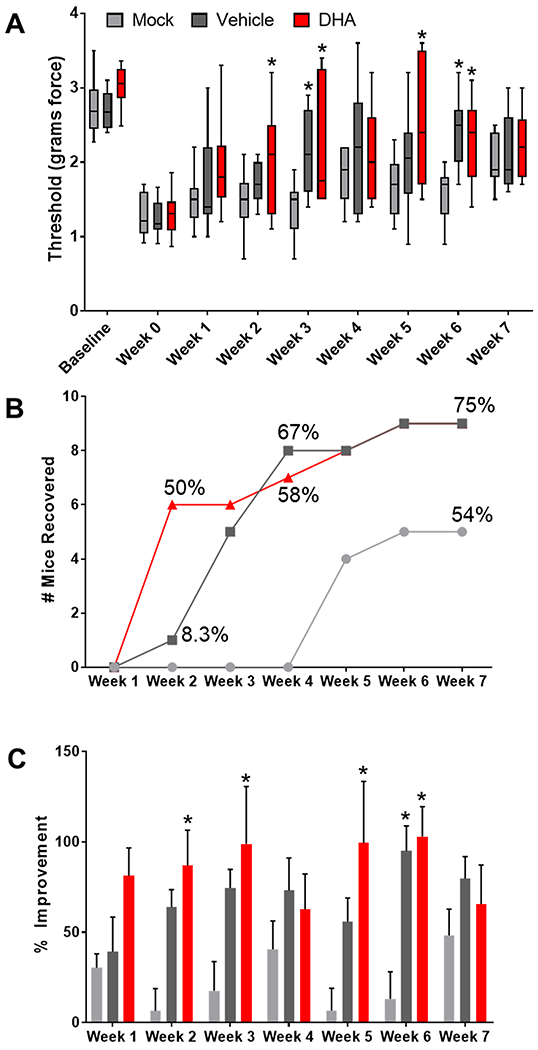
Panel A depicts the average mechanical sensitivity thresholds for all C57BL/6 mice that developed allodynia (box and whisker plots, showing maximum, minimum, and median values). Mice were treated with DHA, vehicle, or mock treatment for 7 weeks after establishing allodynia (n=11-12 mice/group). The starting baseline prior to allodynia induction and the last threshold prior to initiating treatment (week 0) are also plotted. At week 0, thresholds were significantly lower than at baseline, but increased over the course of treatment, approaching levels similar to baseline. ANOVA, *P<0.05 vs. mock treatment. Panel B shows mouse recovery overtime with treatment, which takes into account individual improvements for each mouse. Mice were considered to have recovered if they showed at least a 70% improvement in their pain threshold for two consecutive weeks. Mice began to recover as early as week 2 in the DHA group. By week 4, >50% of the mice had recovered in the DHA and vehicle groups, while no mice had recovered in the mock treated group. Panel C shows the mean percent improvement (+/− SEM) over the last pre-treatment threshold throughout seven weeks of treatment, which compares each mouse’s threshold to their pre-treatment threshold. The percent improvement in pain threshold was significantly higher in the DHA versus mock treated group at weeks 2, 3, 5, and 6, while vehicle was only significantly higher than mock after 6 weeks of treatment, Two-way ANOVA, *P<0.05, n=12 for vehicle and DHA, n=11 for mock.
Discussion
Developing new therapies for pain is particularly difficult, because there is a paucity of mechanistic pain research in this area, and although there are quantifiable endpoints, the experience can vary from person to person and animal models present complex challenges16, 47, 48, 77, 78. New therapies for chronic vulvar pain are direly needed; vulvodynia patients may undergo treatment for years before symptoms resolve18, 58. Often, this resolution is achieved by surgical removal of the vestibule, encompassing a substantial portion of the vulvar tissue6, 18, 58, 72.
The etiology of vulvodynia is poorly understood, and as a result the current therapies are not mechanism-based18. Therefore, our work has focused on identifying mechanisms of disease to achieve targeted efficacious therapies. Our findings to date18–21, 26, 28, along with the findings of other groups41, 42, 70, 73, implicate aberrant proinflammatory signaling in this mechanism. Here, we tested the plausibility of using SPMs as a therapy for vulvodynia; their roles in the resolution of inflammation suggest they might be ideal therapeutics for LPV. In addition, recent publications indicate SPMs may alleviate pain in other inflammatory conditions2, 25, 43, 62, 68, 80.
Consistent with our hypothesis, we found that most commercially available SPMs can reduce the levels of one or both proinflammatory mediators previously associated with vulvodynia pain26, while a few SPMs have particularly profound effects. We came to focus on the maresins, because both 7S-epi-maresin and maresin 1 were among the SPMs that had striking effects in reducing IL-6 and PGE2 in fibroblasts, as well as PGE2 levels in mouse vulvar tissue. In addition, we found that vulvar fibroblasts are capable of making SPMs, most of which are DHA-derived, among them maresin 1. DHA was also effective in reducing PGE2 levels in fibroblasts, which could be explained by an indirect effect involving the production of SPMs, namely maresins or D-series resolvins. The D-series resolvins detected via lipidomic analysis have no or weaker effects on PGE2, suggesting maresin 1 may be responsible for reductions in PGE2. Our in vitro fibroblast model represents an efficient way to prescreen potential therapeutics for vulvodynia, even beyond SPMs. Here, we identified 10 SPMs and one PUFA (DHA) that were highly effective in suppressing pro-nociceptive proinflammatory signaling, which could impart analgesic effects.
Proinflammatory cytokines have long been implicated in the elicitation of pain in numerous conditions65. As denoted, IL-6 and PGE2 are surrogate measures of LPV pain, as measured in our in vitro fibroblast model26. However, this model does not allow evaluation of the potential analgesic effects of SPMs. Studying putative therapeutics in in vitro and in vivo testing systems enhances the likelihood of success in subsequent clinical trials. To this end, we optimized a validated murine model to test new therapeutic agents for vulvodynia22. This model recapitulates important clinical aspects of human vulvodynia, eliminates subjective evaluations of pain, permits randomization and blinding, and assesses mechanical sensitivity in mice as is done in human settings18, 26. A lone investigator can conduct blinded testing through the use of appropriate software that exploits barcode scanning for blinding. The software and systems developed here can be readily incorporated in hind paw models commonly used to study pain15, 47, 48, 56, 82. We incorporated both measures of sensitivity and the ability to track inflammatory end points. Although lavage fluid may not completely reflect the profile in the tissue, using vulvovaginal lavage fluid is noninvasive and can be repeated throughout the course of the experiment.
We cannot be certain that repeated bouts of inflammation, such as weekly zymosan injection in the mouse model, are clinically linked to vulvodynia. However, several pieces of evidence suggest that inflammatory insults may elicit or at minimum contribute to vulvar allodynia. Greater than 70% of women with vulvodynia report a previous history of chronic or recurrent yeast infection17, and at least some women with vulvodynia exhibit cutaneous hypersensitivity to yeast53. The Farmer model established that repeated injection of zymosan or infection with live Candida albicans resulted in vulvar allodynia that remained after infection and inflammation were resolved, very similar to what is observed in women with LPV disease22. In addition, fibroblasts from the vulvar vestibule are exquisitely sensitive to proinflammatory stimuli18–21, 26, 28, there is an increased abundance of and altered organization of inflammatory cells in the painful vestibule of LPV patients41, 42, 70, 73, and there is a correlation between mechanical thresholds in patients and the production of proinflammatory mediators by fibroblasts cultured from these painful areas26. Here, we demonstrate that agents that help to resolve inflammation also impart analgesic effects in mice with vulvar allodynia, suggesting inflammation is involved in the vulvar pain mechanism and is a suitable target for analgesic therapy.
Using this mouse model, we tested first the effects of maresin 1 and then DHA on vulvar sensitivity and found that both were effective in raising sensitivity thresholds reflective of increased tolerance of force and presumably reduced sensitivity. For maresin 1, we also assayed PGE2 levels in vulvovaginal lavage fluid, but did not find a significant treatment effect, although we did find a correlation between threshold and PGE2 levels, similar to what is observed in women with LPV. The absence of a treatment effect on PGE2 levels could reflect the differences in anatomical sampling, or the levels detected during the treatment phase may already be too low to further suppress. The primary outcome measure, change in mechanical sensitivity threshold, denotes significant drug effects. For both maresin 1 and DHA treatment, there was a vehicle effect, although mice receiving active treatment recovered more quickly and fully than mice receiving vehicle. The DMSO in the maresin 1 vehicle and the long chain alcohols in the DHA vehicle have known analgesic effects, which would account for their vehicle effects46, 49. This vehicle effect could also explain failures to detect differences in vulvovaginal PGE2 levels. Nonetheless, the effects of maresin 1 and DHA clearly surpassed their respective vehicles, confirming their potential efficacy for the treatment of vulvodynia. These observations are consistent with at least one other study reporting analgesic effects for maresin 123.
In the context of translating these findings to patient applications, such vehicle effects could further enhance the therapeutic response in women. However, careful design of the treatment vehicle in future mouse studies would be helpful in eliminating background effects to better evaluate the effects of SPM or PUFA treatment alone. As far as deciding which approach is best, a chemically synthesized SPM or highly purified PUFA-enriched oil, both offer advantages and disadvantages. A pure SPM is more likely to have direct and potent effects, but would require lengthy drug development steps, while fish oil, which is already consumed in diet, could lead to a considerably faster translation. Determining optimal strategies for SPM-based drug development would be a next logical step and could easily be evaluated in our mouse model.
In summary, combined in vitro fibroblast and in vivo mouse modeling represents an effective strategy for testing novel mechanism-based therapies for vulvodynia and possibly other pain conditions. Using this approach, we have identified a class of molecules, the SPMs, that may be highly effective in reducing vulvar pain in the clinic. The pathogenesis of vulvodynia involves a hypersensitivity to inflammatory stimuli, which may be overcome by exogenous SPM treatment, which helps to resolve inflammation without inhibiting this vital process. Many of the SPMs tested reduced both IL-6 and PGE2 levels in vulvar fibroblasts. Topical application of maresin 1 or the PUFA from which is it derived, DHA, are highly effective in reducing measures of pain and inflammation in a mouse model of vulvodynia. Overall, SPMs and their precursors are potentially potent and safe treatments for vulvodynia that address at least part of the disease mechanism.
Supplementary Material
Highlights.
Vulvodynia reduces a woman’s quality of life, and new therapies are direly needed.
Inflammation likely plays a role in vulvodynia and represents a therapeutic target.
Agents that resolve inflammation reduce pro-nociceptive signals in human vulvar fibroblasts.
Pro-resolving agents have analgesic effects in a mouse model of vulvodynia.
Pro-resolving agents are a potentially noninvasive and safe therapy for vulvodynia.
Perspective.
Vulvodynia, like many pain conditions, is difficult to treat because disease origins are incompletely understood. Here, we applied our knowledge of more recently discovered vulvodynia disease mechanisms to screen novel therapeutics. We identified several specialized pro-resolving mediators as likely potent and safe for treating LPV with potential for broader application.
Acknowledgements
This work was funded by NIH-NICHD R01 HD092334. The Wayne State Lipidomics Core is supported in part by the National Center for Research Resources, National Institutes of Health Grant S10RR027926se mechanism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflicts of interest.
References
- 1.Akopians AL, Rapkin AJ. Vulvodynia: The Role of Inflammation in the Etiology of Localized Provoked Pain of the Vulvar Vestibule (Vestibulodynia). Semin Reprod Med. 33:239–245, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Allen BL, Montague-Cardoso K, Simeoli R, Colas RA, Oggero S, Vilar B, McNaughton PA, Dalli J, Perretti M, Sher E, Malcangio M. Imbalance of proresolving lipid mediators in persistent allodynia dissociated from signs of clinical arthritis. Pain. 161:2155–2166, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker D, Peresleni T, Kocis C. Inflammatory Markers in Vestibulodynia. Obstetrics and gynecology. 127:1S–2S, 2016 [Google Scholar]
- 4.Barry CM, Matusica D, Haberberger RV. Emerging Evidence of Macrophage Contribution to Hyperinnervation and Nociceptor Sensitization in Vulvodynia. Front Mol Neurosci. 12:186, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI. Vulvar vestibulitis syndrome: reliability of diagnosis and evaluation of current diagnostic criteria. Obstetrics and gynecology. 98:45–51,2001 [DOI] [PubMed] [Google Scholar]
- 6.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI, Meana M, Amsel R. A randomized comparison of group cognitive--behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 91:297–306, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bergeron S, Likes WM, Steben M. Psychosexual aspects of vulvovaginal pain. Best practice & research. Clinical obstetrics & gynaecology. 28:991–999, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Bond KS, Weerakoon P, Shuttleworth R. A literature review on vulvodynia and distress. Sex Relatsh Ther. 27:46–62, 2012 [Google Scholar]
- 9.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 40:315–327, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nature reviews. Immunology. 13:59–66, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Cherpokova D, Jouvene CC, Libreros S, DeRoo EP, Chu L, de la Rosa X, Norris PC, Wagner DD, Serhan CN. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 134:1458–1468, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 58:114–129, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 64:443–462, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 8:353ra111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chundi V, Challa SR, Garikapati DR, Juvva G, Jampani A, Pinnamaneni SH, Venigalla S. Biochanin-A attenuates neuropathic pain in diabetic rats. J Ayurveda Integr Med. 7:231–237, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doleys DM. Philosophical Issues and Psychological Variables that Influence the Determination of Opioid Effectiveness: A Narrative Review. Pain Physician. 20:E1091–E1105, 2017 [PubMed] [Google Scholar]
- 17.Donders G, Bellen G. Characteristics of the pain observed in the focal vulvodynia syndrome (VVS). Med Hypotheses. 78:11–14, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Falsetta ML, Foster DC, Bonham AD, Phipps RP. A review of the available clinical therapies for vulvodynia management and new data implicating proinflammatory mediators in pain elicitation. BJOG : an international journal of obstetrics and gynaecology. 124:210–218, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Haidaris CG, Phipps RP. A role for bradykinin signaling in chronic vulvar pain. The journal of pain : official journal of the American Pain Society. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Haidaris CG, Stodgell CJ, Phipps RP. Identification of novel mechanisms involved in generating localized vulvodynia pain. American journal of obstetrics and gynecology. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Piekna-Przybylska D, Maggirwar SB, Haidaris CG, Phipps RP. Toll-Like Receptor Signaling Contributes to Proinflammatory Mediator Production in Localized Provoked Vulvodynia. Journal of lower genital tract disease. 22:52–57, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM, Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med. 3:101ra191,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, Verri WA Jr. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. British journal of pharmacology. 176:1728–1744, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher BS, Kujubu DA, Perrin DM, Herschman HR. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. The Journal of biological chemistry. 267:4338–4344, 1992 [PubMed] [Google Scholar]
- 25.Fonseca FC, Orlando RM, Turchetti-Maia RM, de Francischi JN. Comparative effects of the omega3 polyunsaturated fatty acid derivatives resolvins E1 and D1 and protectin DX in models of inflammation and pain. J Inflamm Res. 10:119–133, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster D, Falsetta M, Woeller C, Pollock S, Song K, Bonham A, Haidaris C, Stogell C, Messing S, Iadarola M, Phipps R. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia afflicted and pain-free women. Pain. 156 :386–396, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster DC, Hasday JD. Elevated tissue levels of interleukin-1 beta and tumor necrosis factor-alpha in vulvar vestibulitis. Obstetrics and gynecology. 89:291–296, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. American journal of obstetrics and gynecology. 196:346 e341–348, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 437:185–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilligan MM, Gartung A, Sulciner ML, Norris PC, Sukhatme VP, Bielenberg DR, Huang S, Kieran MW, Serhan CN, Panigrahy D. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proceedings of the National Academy of Sciences of the United States of America. 116:6292–6297, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groysman V Vulvodynia: new concepts and review of the literature. Dermatol Clin. 28:681–696, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann ED, Kaufman RH, Lynch PJ, Margesson LJ, Moyal-Barracco M, Piper CK, Reed BD, Stewart EG, Wilkinson EJ. The vulvodynia guideline. Journal of lower genital tract disease. 9:40–51, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. American journal of obstetrics and gynecology. 210:40 e41–48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? Journal of the American Medical Women’s Association. 58:82–88, 2003 [PubMed] [Google Scholar]
- 35.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes & development. 18:2195–2224, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kingdon J Vulvodynia: a comprehensive review. Nurs Womens Health. 13:48–57; quiz 58, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Klein CP, Sperotto ND, Maciel IS, Leite CE, Souza AH, Campos MM. Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology. 86:57–66, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, Tamvakopoulos C, Sideras P, Serhan CN, Andreakos E. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 5:762–775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol. 159:925–935, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence T The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor perspectives in biology. 1:a001651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstetrics and gynecology. 117:1307–1313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leclair CM, Leeborg NJ, Jacobson-Dunlop E, Goetsch MF, Morgan TK. CD4-positive T-cell recruitment in primary-provoked localized vulvodynia: potential insights into disease triggers. Journal of lower genital tract disease. 18:195–201,2014 [DOI] [PubMed] [Google Scholar]
- 43.Luo X, Gu Y, Tao X, Serhan CN, Ji RR. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Frontiers in pharmacology. 10:745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytotherapy research : PTR. 24:949–963, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, Kusanovic JP, Munoz H, Honn KV. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 28:4835–4846, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magrioti V, Hadjipavlou-Litina D, Constantinou-Kokotou V. Synthesis and In vivo anti-inflammatory activity of long-chain 2-amino-alcohols. Bioorg Med Chem Lett. 13:375–377, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 10:283–294, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 151:12–17, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Morris RW. Analgesic and local anesthetic activity of dimethyl sulfoxide. J Pharm Sci. 55:438–440, 1966 [DOI] [PubMed] [Google Scholar]
- 50.Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, Serhan CN, Maddipati KR. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci Rep. 8:18050, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nunns D, Mandal D, Byrne M, McLelland J, Rani R, Cullimore J, Bansal D, Brackenbury F, Kirtschig G, Wier M, British Society for the Study of Vulval Disease Guideline G. Guidelines for the management of vulvodynia. The British journal of dermatology. 162:1180–1185, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Okajima K, Harada N. Regulation of inflammatory responses by sensory neurons: molecular mechanism(s) and possible therapeutic applications. Curr Med Chem. 13:2241–2251,2006 [DOI] [PubMed] [Google Scholar]
- 53.Ramirez De Knott HM, McCormick TS, Do SO, Goodman W, Ghannoum MA, Cooper KD, Nedorost ST. Cutaneous hypersensitivity to Candida albicans in idiopathic vulvodynia. Contact Dermatitis. 53:214–218, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martinez-Sobrido L, Topham DJ, Phipps RP. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? Journal of immunology. 193:6031–6040, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reed BD, Plegue MA, Sen A, Haefner HK, Siddiqui J, Remick DG. Nerve Growth Factor and Selected Cytokines in Women With and Without Vulvodynia. Journal of lower genital tract disease. 22:139–146, 2018 [DOI] [PubMed] [Google Scholar]
- 56.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Preclinical Pain C, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 139:243–247, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2:e119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadownik LA. Etiology, diagnosis, and clinical management of vulvodynia. Int J Womens Health. 6:437–449, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510:92–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in immunology. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor perspectives in biology. 7:a016311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26:1755–1765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sham HP, Walker KH, Abdulnour RE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito-Figueroa S, Colby JK, Serhan CN, Levy BD. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. Journal of immunology. 200:2757–2766, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simonelli C, Eleuteri S, Petruccelli F, Rossi R. Female sexual pain disorders: dyspareunia and vaginismus. Current opinion in psychiatry. 27:406–412, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience letters. 361:184–187, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell metabolism. 19:21–36, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stockdale CK, Lawson HW. 2013 Vulvodynia Guideline update. Journal of lower genital tract disease. 18:93–100, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Tao X, Lee MS, Donnelly CR, Ji RR. Neuromodulation, Specialized Proresolving Mediators, and Resolution of Pain. Neurotherapeutics. 17:886–899, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thorstensen KA, Birenbaum DL. Recognition and management of vulvar dermatologic conditions: lichen sclerosus, lichen planus, and lichen simplex chronicus. J Midwifery Womens Health. 57:260–275, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Tommola P, Butzow R, Unkila-Kallio L, Paavonen J, Meri S. Activation of vestibule-associated lymphoid tissue in localized provoked vulvodynia. American journal of obstetrics and gynecology. 212:476 e471–478, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Tommola P, Unkila-Kallio L, Paavonen J. Surgical treatment of vulvar vestibulitis: a review. Acta obstetricia et gynecologica Scandinavica. 89:1385–1395, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Tommola P, Unkila-Kallio L, Paavonen J. Long-term follow up of posterior vestibulectomy for treating vulvar vestibulitis. Acta obstetricia et gynecologica Scandinavica. 90:1225–1231,2011 [DOI] [PubMed] [Google Scholar]
- 73.Tommola P, Unkila-Kallio L, Paetau A, Meri S, Kalso E, Paavonen J. Immune activation enhances epithelial nerve growth in provoked vestibulodynia. American journal of obstetrics and gynecology. 215:768 e761–768 e768, 2016 [DOI] [PubMed] [Google Scholar]
- 74.Trouvin AP, Perrot S. New concepts of pain. Best Pract Res Clin Rheumatol. 33:101415, 2019 [DOI] [PubMed] [Google Scholar]
- 75.Updike GM, Wiesenfeld HC. Insight into the treatment of vulvar pain: a survey of clinicians. American journal of obstetrics and gynecology. 193:1404–1409, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 157:552–554, 1967 [DOI] [PubMed] [Google Scholar]
- 77.van Amerongen G, de Boer MW, Groeneveld GJ, Hay JL. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br J Clin Pharmacol. 82:903–922, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 140:441–451,2004 [DOI] [PubMed] [Google Scholar]
- 79.Zanotta N, Campisciano G, Scrimin F, Ura B, Marcuzzi A, Vincenti E, Crovella S, Comar M. Cytokine profiles of women with vulvodynia: Identification of a panel of proinflammatory molecular targets. European journal of obstetrics, gynecology, and reproductive biology. 226:66–70, 2018 [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Terrando N, Xu ZZ, Bang S, Jordt SE, Maixner W, Serhan CN, Ji RR. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain After Bone Fracture in Mice. Frontiers in pharmacology. 9:412, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. The journal of pain : official journal of the American Pain Society. 13:910–920, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zulazmi NA, Gopalsamy B, Farouk AA, Sulaiman MR, Bharatham BH, Perimal EK. Antiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic pain. Fitoterapia. 105:215–221, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


