Abstract
Uterine endometrioid adenocarcinomas (UEC) are known for their morphologic plasticity. In addition to a multiplicity of metaplasias, UECs may also undergo high-grade divergent differentiation in the form of high-grade neuroendocrine carcinoma, neuroectodermal differentiation or carcinosarcoma; others may dedifferentiate completely. Here we describe 5 cases of UECs with high-grade divergent differentiation showing a striking morphologic and immunophenotypic resemblance to cutaneous pilomatrix carcinoma. Specifically, the high-grade component in all cases exhibited solid, basaloid morphology with conspicuous tumor cell necrosis and the presence of shadow cells, accompanied by diffusely aberrant (nuclear and cytoplasmic) ß-catenin expression as well as variably diffuse CDX2 expression. Additionally, the high-grade component in all cases showed loss of ER and PAX8 expression, retained MMR expression, wild-type p53 expression, patchy p16 expression and diffusely positive cytokeratin expression (AE1/AE3 and CK7); at least focal neuroendocrine marker expression was present in all cases. CK20 was negative in all cases, with the exception of very focal staining in a single case (2% of tumor cells). All 5 of our tumors had at least a focal conventional FIGO grade 1 component. In all 4 cases tested, the low-grade component retained both PAX8 and ER expression and had, at best, focally aberrant ß-catenin expression. Two of our cases had molecular analysis performed and both harbored mutations in exon 3 of CTNNB1 as expected; molecular analysis also revealed that both cases lacked POLE or TP53 mutations and showed no microsatellite instability. The tumors in this series were uniformly aggressive. Four of the 5 patients in our cohort had available follow up information; of these, 3/4 died of their disease within 14 months of diagnosis and the fourth patient had distant metastatic disease at presentation and is alive with disease 1 month following diagnosis. The one patient without follow up information also had distant metastatic disease at presentation and was lost to follow up 17 months later.
The cases described in this series 1) represent a highly aggressive CTNNB1-mutated subset of the “no specific molecular profile” category of endometrioid adenocarcinomas; 2) illustrate a form of high-grade divergent differentiation resembling cutaneous pilomatrix carcinoma already described in carcinomas at other anatomic sites; and 3) underscore the difficulty in recognizing this phenotype at distant metastatic sites, which are frequent even at the time of presentation, given the consistent loss of ER and PAX8 expression and concurrent CDX2 expression.
Keywords: ß-catenin, CTNNB1 mutation, FIGO grade 3, Endometrioid adenocarcinoma, TCGA, Pilomatrix carcinoma, No specific molecular profile
Introduction
There is an increasing interest in the molecular classification of uterine endometrial carcinomas, especially uterine endometrioid adenocarcinomas (UEAs), based on The Cancer Genome Atlas (TCGA) largely due to the divergent clinical outcomes associated with each of the different categories proposed in this system1. In this context, mutations in the gene encoding ß-catenin, CTNNB1, may constitute an independent risk factor in UEAs as they have been associated with a poor prognosis, even in low-grade (FIGO grade 1-2), low-stage (uterus-confined) tumors, and even within the otherwise low-risk TCGA categories such as the copy number low (CN-L)/no specific molecular profile (NSMP) group2. In these tumors, aberrant nuclear expression of ß-catenin by immunohistochemistry has been shown to be a robust surrogate marker of CTNNB1 mutation2,3.
Previous work on CTNNB1-mutated UEAs has, for good reason, focused on the prognostic importance of this molecular marker in lower-grade tumors, while high-grade tumors have been relatively underrepresented2,4,5. Here we describe 5 additional cases of grade 3 UEAs all of which showed strong, diffusely aberrant ß-catenin expression. All 5 tumors also showed a consistent clinicopathologic profile in that they had uniformly aggressive clinical behavior as well as histological and immunophenotypic features resembling those of cutaneous pilomatrix carcinoma, another tumor highly associated with diffusely aberrant ß-catenin expression6,7 and aggressive clinical behavior8,9. In this report, we discuss the pathogenetic and diagnostic features of these tumors.
Materials and Methods
Case Selection
Following an index case (case 3), we screened cases from a cohort of FIGO grade 3 uterine endometrioid adenocarcinomas (UEAs) diagnosed at our institution between 2003 and 2015. Specifically, we screened for UEAs showing features similar to those of our index case (i.e. features resembling cutaneous pilomatrix carcinoma, including solid growth of basaloid tumor cells with conspicuous tumor cell necrosis and shadow cell formation). Tumors with these features were selected for further immunohistochemical workup. An additional case (case 5) was recently prospectively identified during routine clinical practice.
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue sectioned at 5 μm increments was stained with hematoxylin and eosin (H&E) and immunohistochemical stains. Immunohistochemistry was performed on a Benchmark Ultra autostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA), using commercially available antibodies (see Table 1).
Table 1.
Antibodies used in this study
| Antibody | Clone | Dilution | Source |
|---|---|---|---|
| AE1/AE3 | AE1/AE3 | 1:100 | Dako (Denmark) |
| ß-catenin | 14 | Predilute | Cell Marque (CA, USA) |
| CDX2 | EPR2764Y | Predilute | Cell Marque (CA, USA) |
| Chromogranin | LKZH10 | Predilute | Ventana (AZ, USA) |
| CK7 | SP52 | Predilute | Ventana (AZ, USA) |
| CK20 | SP33 | Predilute | Ventana (AZ, USA) |
| ER | SP1 | Predilute | Ventana (AZ, USA) |
| INSM1 | A-8 | 1:200 | Invitrogen (CA, USA) |
| MSH6 | 44 | Predilute | Cell Marque (CA, USA) |
| PAX8 | MRQ-50 | Predilute | Cell Marque (CA, USA) |
| PMS2 | EPR3947 | Predilute | Cell Marque (CA, USA) |
| P16 | E6H4 | Predilute | Ventana (AZ, USA) |
| p53 | DO-7 | Predilute | Ventana (AZ, USA) |
| P63 | BC4A4 | Predilute | Biocare (CA, USA) |
| Synaptophysin | SP11 | Predilute | Ventana (AZ, USA) |
ß-catenin was considered aberrant if nuclear and cytoplasmic staining were present; membranous expression was considered normal (i.e. not aberrant). The extent of aberrant ß-catenin expression was qualitatively assessed as being either diffusely present (>95% of cells) or only focally present (any amount of aberrant staining less than diffuse); of note, staining patterns close to, but falling short of the definition for diffuse staining were not seen. Any amount of nuclear staining for CDX2, p63, or INSM1 or cytoplasmic staining for synaptophysin, chromogranin, cytokeratin AE1/AE3, cytokeratin 7 or cytokeratin 20 was considered positive and the percentage of positive cells was recorded; however, diffusely (>90%) positive cases were simply scored as “positive”. Any amount of nuclear staining for ER, PAX8, PMS2, or MSH6 was considered positive (“retained” in the case of PMS2 and MSH6). P53 was considered aberrant if there was either diffuse strong nuclear staining (>70%), a complete lack of staining in the presence of a working internal positive control cell population (i.e. null pattern staining), or diffuse cytoplasmic staining with or without nuclear positivity; wild-type staining was defined as scattered weak to moderate nuclear staining in less than 70% of tumor cells. P16 was considered as positive if diffuse, strong nuclear and cytoplasmic staining were present; staining patterns falling short of this were recorded as “patchy”.
Molecular Testing
DNA profiling was performed on a clinical basis using StrataNGS, which uses multiplex polymerase chain reaction (PCR) to evaluate mutations and copy number alterations in cancer genes, as previously described10.
Results
Case Selection
5 patients with pilomatrix carcinoma-like features (see methods section for details) were identified from our cohort.
Clinical Features
Patients ranged in age from 38-73 years old (mean: 58 years old). Clinically, all 5 cases showed very aggressive behavior. Three cases had distant metastatic disease at the time of presentation. Distant metastatic sites in these patients included the lungs (in 2 cases), mediastinal lymph nodes (in 1 case) liver (in 1 case) and soft tissue (in 1 case). An additional case was stage T3 at presentation with regional lymph node metastases, including the para-aortic lymph nodes. Only 1 patient had uterus-confined disease at presentation; however, even this patient recurred shortly afterwards with distant metastases (to the lung and liver), resulting in her death 12 months following diagnosis. Four of our 5 patients had follow up information available. Three of these 4 patients died of their disease in less than a year-and-a-half following their diagnosis (range 10 – 14 months; mean: 12 months); the fourth patient is a recently-diagnosed patient that had distant metastatic disease at presentation and is alive with disease, at 1 month following diagnosis. The one patient without available follow up information also presented with distant metastatic disease and was lost to follow up 17 months later. All patients received chemotherapy. The patients’ clinical features are summarized in Table 2
Table 2.
Clinical and pathological characteristics
| Age (Years) |
Size in uterus (cm) |
LVI | CTNNB1 mutation |
Stage at Presentation |
Treatment | Site(s) of Distant Metastatic Disease |
Length of Follow up (months) |
Survival | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 60 | 1.2 | N | ND | T1bN0M0 | S, C, R | Liver, chest wall, brain | 12 | DOD |
| Case 2 | 65 | 1.7 | Y | CTNNB1 (D32Y) | T1bN0M1 | S, C, R | Bone, lung, soft tissue | 17 | LFU |
| Case 3 | 56 | U | U | ND | M1 | S, C | Liver | 10 | DOD |
| Case 4 | 73 | 3.5 | Y | CTNNB1 (S37F) | T3aN2aM0 | S, C, R | Lung, liver | 14 | DOD |
| Case 5 | 38 | U | U | ND | M1 | NAC | Lung, mediastinal lymph nodes | 1 | AWD |
Abbreviations: AWD: alive with disease; C: chemotherapy; CA: carcinoma; DOD: died of disease; LFU: lost to follow-up; N: no; NAC: neoadjuvant chemotherapy; ND: not done; S: surgery; R: radiation therapy; U: unknown; Y: yes.
Pathological Features
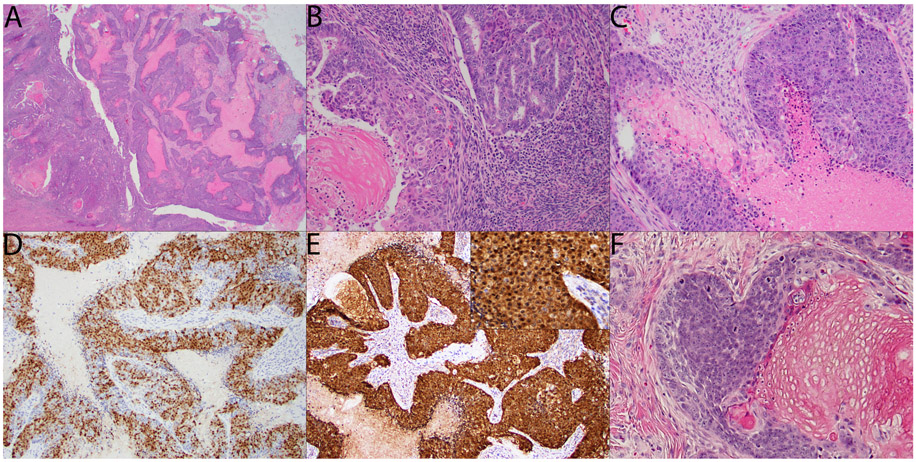
Morphologically, the tumors all showed very similar features. All tumors included at least a focal component of FIGO grade 1 endometrioid adenocarcinoma (Figures 1A (left side) and 1B). In one case (case 1), this component showed both focal shadow cell formation as well as focal morular and squamous metaplasia (shadow cell formation shown in Figure 1B). All cases also had a dominant FIGO grade 3 component composed of high-grade, basaloid tumor cells with a nested and/or solid growth pattern and conspicuous tumor cell necrosis (Figures 1A (right side) and 1C). Additionally, shadow cells were present in this high-grade component in all cases (Figure 1C). Trichohyaline granules were also present in a subset of cases. By immunohistochemistry (IHC), the high-grade component in all 5 cases showed diffusely aberrant (i.e. nuclear and cytoplasmic) staining for ß-catenin (Figure 1E and inset). In addition, all 5 cases also showed at least focal staining for CDX2 with 3/5 cases showing staining in >50% of the tumor cells (Figure 1D). ER and PAX8 were completely negative in all 5 cases. Cytokeratins AE1/AE3 and CK7 were diffusely positive in all 5 cases, while CK20 was negative in all cases, with the exception of focal staining (2% of cells) in a single case. Mismatch repair proteins (PMS2 and MSH6) both showed retained nuclear expression in all 5 cases, p53 showed a wild-type staining pattern in all 5 cases and p16 showed only patchy staining in all 5 cases. Synaptophysin was variably expressed in all 5 cases, with the percent of positive tumor cells ranging from 20-60%; chromogranin was also positive in all cases, but only focally so (positive staining ranged from 2-10% of tumor cells), while INSM1 was entirely negative, with the exception of one case with 2% of tumor cells showing positive staining. P63 was negative in 3/5 cases and focally positive in the other two. The immunophenotype for the high-grade component of these tumors is summarized in Table 3.
Figure 1.
A: Tumor at scanning magnification showing the transition between the FIGO grade 1 component on the left and the FIGO grade 3 component on the right; conspicuous tumor cell necrosis is already visible in the FIGO grade 3 component at this power (H&E, 20x). B: FIGO grade 1 component showing focal shadow cell formation (H&E, 200x). C: FIGO grade 3 component showing nests of basaloid tumor cells with tumor cell necrosis on the lower right and shadow cell formation on the upper left (H&E, 200x). D: CDX2 in the FIGO grade 3 component showing nuclear staining in >50% of the tumor cells (DAB, 100x). E: ß-catenin in the FIGO grade 3 component showing diffusely aberrant nuclear and cytoplasmic staining; inset at higher magnification (DAB, 100x; inset 200x). F: Example of cutaneous pilomatrix carcinoma for comparison, courtesy of Dr. Klaus Busam (H&E, 300x).
Table 3.
Immunohistochemical staining results for the FIGO grade 3 component with diffusely aberrant ß-catenin expression
| Case | CDX2 | PAX8 | ER | AE1/AE3 | CK7 | CK20 | Syn | Chr | INSM1 | p63 | p53 | p16 | PMS2 | MSH6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | >50% | N | N | P | P | N | 30% | 5% | N | N | WT | patchy | R | R |
| 2 | >50% | N | N | P | P | 2% | 40% | 10% | N | 15% | WT | patchy | R | R |
| 3 | >50% | N | N | P | P | N | 20% | 2% | 2% | N | WT | patchy | R | R |
| 4 | 40% | N | N | P | P | N | 40% | 5% | N | N | WT | patchy | R | R |
| 5 | 40% | N | N | P | P | N | 60% | 10% | N | 5% | WT | patchy | R | R |
Abbreviations: Chr: chromogranin; N: negative; P: positive (diffuse); R: retained; Syn: synaptophysin; WT: wild-type staining pattern.
The low-grade component was tested in 4/5 tumors; in all cases, ER and PAX8 were both retained. Additionally, while one case (case 1) showed focally aberrant expression of ß-catenin in a background of predominantly normal (membranous) staining, the remaining cases showed only membranous staining in their low-grade components. CDX2 mirrored the ß-catenin expression pattern, showing at best focal expression in the low-grade component.
Molecular testing
Molecular testing was performed in 2/5 cases and revealed an exon 3 mutation in CTNNB1 in both cases: In case 2 p.(D32Y) and in case 4 p.(S37F). Additionally, in case 4, two mutations in PTEN [p.(T319fs) and p.T319fs] and two mutations in PIK3CA [p.(E453K) and p.(R38H)] were also seen. Additionally, both tumors had no mutations in either POLE or TP53 and both tumors showed a microsatellite stable profile.
Discussion
Here we describe a subset of FIGO grade 3 uterine endometrioid adenocarcinomas (UEAs) with diffusely aberrant ß-catenin expression. All 5 tumors showed remarkably consistent morphologic and immunophenotypic features. Morphologically, they all resembled cutaneous pilomatrix carcinomas. For comparison, a case of cutaneous pilomatrix carcinoma is shown in Figure 1F. Much like pilomatrix carcinoma, all our cases showed a solid growth pattern with high-grade basaloid tumor cells with conspicuous tumor cell necrosis and shadow cell formation9 (Figures 1A (right side) and 1C). In addition to diffusely aberrant ß-catenin expression (Figure 1E and inset) a consistent immunophenotypic feature of cutaneous pilomatrix carcinomas6, all the tumors also showed at least focal CDX2 expression (Figure 1D), another marker associated with pilomatrix carcinomas7. Concurrent with this high-grade divergent differentiation, all 5 tumors also showed complete loss of ER and PAX8 expression, both of which showed retained expression in the low-grade components of these tumors. In two of the five cases, next-generation sequencing was performed and revealed the expected exon 3 CTNNB1 mutation in both cases.
All 5 cases showed very aggressive clinical features. Most significantly, three of the 5 patients died of their distant metastatic disease in less than 1.5 years from diagnosis and the remaining 2 patients already had distant metastatic disease at presentation – one of these 2 patients was lost to follow up and the other was diagnosed very recently (see Table 2 for further details).
The cases in this series illustrate three main points. First, as predicted by others2,11, it is likely that these high-grade CTNNB1-mutated tumors are at least partially responsible for the high-risk subset of the “no-specific molecular profile” (NSMP) category of tumors, whose distant metastasis and 5-year recurrence-free survival rates are on par with that of the TP53-mutant group2,11. We realize that we are taking some liberty applying the TCGA molecular classification here, since only two of our five cases were evaluated for POLE mutations (both lacked POLE mutations); that being said, these tumors were uniform in all other respects, including their retained MMR expression and wild-type pattern p53 staining, making the NSMP group the most likely designation.
Second, these cases illustrate a genotype-phenotype correlation similar to that reported in tumors from other anatomic sites. In all of these examples, the respective tumors have shown morphologic and immunophenotypic features identical to those seen in our cases, including overtly high-grade carcinomas with basaloid morphology and conspicuous tumor cell necrosis, shadow cell formation and (in the more recent reports) diffusely aberrant ß-catenin expression. Well-documented carcinomas showing these features include urothelial12, gastric13, pulmonary14 and ovarian carcinomas15-17, among others. This suggests that a divergent pilomatrix carcinoma-like phenotype is somewhat agnostic of the anatomic site at which a carcinoma has arisen and is more a consequence of the underlying molecular genetic signature involving, at least in part, exon 3 mutations in CTNNB1. Interestingly, however, in the endometrium, while numerous cases of endometrioid adenocarcinoma “with shadow cell differentiation” or with “pilomatrixoma-like” features have been described in the literature, many of these were low-grade tumors showing features similar to the low-grade component of case 1 (Figure 1B). Conversely, a frankly high-grade tumor phenotype similar to the cases described in this series was either not well documented or not emphasized in these reports18-22.
Lastly, the ER-negative/PAX8-negative/CDX2-positive immunoprofile of these tumors, together with their poorly-differentiated morphology, could lead to diagnostic confusion at metastatic sites. This is especially pertinent since the rate of early distant metastasis, even at the time of presentation, is so high in these tumors, at least in this small series. Metastasis to the dermis or superficial soft tissue could perfectly mimic cutaneous pilomatrix carcinoma. In such cases, perhaps the only means to resolve this differential diagnosis would be to suggest that an endometrial biopsy be obtained in order to demonstrate the presence of a concurrent endometrioid adenocarcinoma. In this scenario, even an exclusively low-grade endometrioid adenocarcinoma in the endometrial biopsy is supportive. Alternatively, the CDX2 expression in these tumors could prompt a search for a poorly-differentiated primary gastrointestinal adenocarcinoma. As our cases were uniformly positive for CK7 and nearly negative for CK20 (one case showed focal staining in 2% of the tumor cells), the CK7/CK20 profile of these tumors may useful in excluding a gastrointestinal source, at least from the lower gastrointestinal tract. The variable synaptophysin expression seen in our cases could also suggest a large cell neuroendocrine carcinoma, a diagnosis made even more compelling by the presence of basaloid tumor cells with abundant necrosis. Application of additional neuroendocrine markers, such as chromogranin and INSM1 may be of assistance in this scenario: in our cases, chromogranin staining was focal at best, being present in 2-10% of tumor cells, while INSM1 was entirely negative in all cases, with the exception of very focal staining (2% of tumor cells) in a single case. Finally, the poorly-differentiated morphology of these cases, together with their loss of ER and PAX8 expression, is reminiscent of dedifferentiated endometrial carcinoma; however, the diffuse expression of AE1/AE3 and CK7 and retained MMR protein expression militate against dedifferentiated endometrial carcinoma, which tends to show loss or diminution of epithelial marker expression as well as a high rate of MMR protein deficiency. In any of these scenarios, knowledge of this particular morphology and immunoprofile can help to suggest a gynecologic workup, including an endometrial biopsy and imaging studies in order to ensure that patients with these tumors get the appropriate subspecialty referral for prompt treatment of their aggressive disease.
In summary, we describe a series of uterine endometrioid adenocarcinomas with pilomatrix carcinoma-like high-grade divergent differentiation accompanied by diffusely aberrant ß-catenin expression, at least focal CDX2 expression and loss of ER and PAX8 expression. This form of divergent differentiation likely accounts for a subset of the high-risk tumors in the “no specific molecular profile tumors” TCGA category with highly aggressive clinical behavior. We underscore both the genotype-phenotype correlation exemplified by these tumors as well as their potential for misclassification at distant metastatic sites, which may already be present at presentation.
Acknowledgements
The authors would like to thank Dr. Klaus Busam for contributing the image of cutaneous pilomatrix carcinoma used in figure 1. This study was funded by the University of Wisconsin School of Medicine and Public Health Department of Pathology and Laboratory Medicine. This study was also funded in part by the NIH/NCI Support Grant P30 CA008748 for Memorial Sloan Kettering Cancer Center.
Footnotes
Sources of Support: Department of Pathology and Laboratory Medicine, University of Wisconsin Hospital and Clinics, Madison, WI
Financial disclosure: All authors declare no conflict of interest
References
- 1.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costigan DC, Dong F, Nucci MR, Howitt BE. Clinicopathologic and Immunohistochemical Correlates of CTNNB1 Mutated Endometrial Endometrioid Carcinoma. Int J Gynecol Pathol. 2020;39(2):119–127. [DOI] [PubMed] [Google Scholar]
- 3.Kim G, Kurnit KC, Djordjevic B, et al. Nuclear beta-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Mod Pathol. 2018;31(10):1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurnit KC, Kim GN, Fellman BM, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. 2017;30(7):1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers A, Barry WT, Hirsch MS, Matulonis U, Lee L. beta-Catenin mutations in recurrent FIGO IA grade I endometrioid endometrial cancers. Gynecol Oncol. 2014;134(2):426–427. [DOI] [PubMed] [Google Scholar]
- 6.Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32(2):148–157. [DOI] [PubMed] [Google Scholar]
- 7.Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45(5):318–324. [DOI] [PubMed] [Google Scholar]
- 8.Jones C, Twoon M, Ho W, Portelli M, Robertson BF, Anderson W. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45(1):33–38. [DOI] [PubMed] [Google Scholar]
- 9.Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71(8):2491–2498. [DOI] [PubMed] [Google Scholar]
- 10.Miller TI, Zoumberos NA, Johnson B, et al. A genomic survey of sarcomas on sun-exposed skin reveals distinctive candidate drivers and potentially targetable mutations. Hum Pathol. 2020;102:60–69. [DOI] [PubMed] [Google Scholar]
- 11.Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28(6):836–844. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T Bladder carcinoma with shadow cell differentiation: a case report with immunohistochemical analyses. Int J Clin Exp Pathol. 2012;5(8):840–844. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T Gastric carcinoma with shadow cell differentiation in metastatic lymph nodes. Human Pathology: Case Reports. 2017;7:19–22. [Google Scholar]
- 14.Garcia-Escudero A, Navarro-Bustos G, Jurado-Escamez P, Rios-Martin J, Gonzalez-Campora R. Primary squamous cell carcinoma of the lung with pilomatricoma-like features. Histopathology. 2002;40(2):201–202. [DOI] [PubMed] [Google Scholar]
- 15.Lalich D, Tawfik O, Chapman J, Fraga G. Cutaneous metastasis of ovarian carcinoma with shadow cells mimicking a primary pilomatrical neoplasm. Am J Dermatopathol. 2010;32(5):500–504. [DOI] [PubMed] [Google Scholar]
- 16.Zamecnik M, Jando D, Kascak P. Ovarian basaloid carcinoma with shadow cell differentiation. Case Rep Pathol. 2014;2014:391947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J, Keh P, Katz L, Rao MS. Pilomatricoma-like endometrioid adenosquamous carcinoma of the ovary with neuroendocrine differentiation. Gynecol Oncol. 1996;61(2):291–293. [DOI] [PubMed] [Google Scholar]
- 18.Zamecnik M, Bartos P, Kascak P. Shadow cell differentiation in endometrioid carcinomas of the uterus. Its frequent occurrence and beta-catenin expression. Cesk Patol. 2015;51(3):123–126. [PubMed] [Google Scholar]
- 19.Nakamura T Shadow cell differentiation from squamoid morule in endometrial adenoacanthoma. Int J Clin Exp Pathol. 2015;8(10):13120–13124. [PMC free article] [PubMed] [Google Scholar]
- 20.Squillaci S, Marchione R, Piccolomini M, Chiudinelli M, Fiumano E, Ungari M. Uterine endometrioid adenocarcinoma with extensive pilomatrixoma-like areas. A case report. Pathologica. 2013;105(1):8–10. [PubMed] [Google Scholar]
- 21.Scheck SM, Bethwaite P, Johnson C, Mogensen O. Metastatic endometrial endometrioid carcinoma mimicking pilomatrixoma of the distal vagina. BMJ Case Rep. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamecnik M, Michal M. Shadow cell differentiation in tumours of the colon and uterus. Zentralbl Pathol. 1995;140(6):421–426. [PubMed] [Google Scholar]