Abstract
Humoral allogeneic immunity driven by anti-HLA donor-specific antibodies (DSAs) and antibody-mediated mediated rejection (ABMR) significantly impede prolonged survival of organ allografts after transplantation. Although the importance of T follicular helper (TFH) cells in controlling antibody responses has been long established, their role in directing DSA generation leading to ABMR was only recently appreciated in the clinical setting of organ transplantation. In this review, we provide a comprehensive summary of the current knowledge on the biology of human TFH cells as well as their circulating (cTFH) counterparts and describe their pivotal role in driving humoral alloimmunity. In addition, we discuss the intrinsic effects of current induction therapies and maintenance immunosuppressive drugs as well as of biotherapies on TFH cells; and provide future directions and novel opportunities of biotherapeutic targeting of TFH cells that have the potential of bringing the prophylactic and curative treatments of ABMR towards personalized and precision medicine.
Introduction
The immune conflict triggered by the development of anti-HLA donor-specific antibodies (DSAs) in genetically incompatible organ recipients with their donors has been recognized as a major limitation to prolonged survival of allografts, across all solid organ transplants including kidney, heart, lung and liver transplants.1,2 Antibody-mediated rejection (ABMR) is the clinical manifestation of DSA pathogenicity and results from binding of DSAs to the allogeneic endothelium, which triggers microvascular inflammation and intimal arteritis through antibody-dependent cell-mediated cytotoxicity mediated by effector NK cells and monocytes, as well as complement activation through complement-dependent cytotoxicity.3,4 ABMR represents a main immunological cause of allograft failure. Yet, adequate predictive biomarkers of humoral alloimmunity are still lacking, mostly because of the poor understanding of the complex cellular and molecular mechanisms underlying DSA and ABMR development. As a consequence, patients developing ABMR are still in need of adequate treatments.
Given that DSAs are isotype switched IgG antibodies directed against protein antigens, it is postulated that they are generated through T cell-dependent B cell responses. Although it could have been largely anticipated that T follicular helper (TFH) cells, the CD4 T cell subset specialized in the control of B cell responses,5 would play a major role in the induction of DSA generation and their pathogenicity leading to ABMR, it was only in the past few years that their role was elucidated. A growing number of studies have demonstrated a pivotal role for TFH cells in transplantation in animal models but also, more recently, in humans.6,7
This review aims to summarize the most recent and relevant findings regarding the contribution of human TFH cells to health and disease, and to humoral alloimmunity against organ transplants; and how these cells represent amenable therapeutic targets for treatment of ABMR.
Human TFH cells in health and disease
a). Germinal center TFH cells
Germinal center (GC) TFH cells are pivotal in the optimal development of humoral immunity by controlling cognate antigen-specific B cell responses and the formation of GCs. While B cells may undergo Ig class-switch recombination prior to their differentiation into GC B cells, they proliferate, undergo somatic hypermutations and generate memory B cells as well as high-affinity plasma cells in GCs with the crucial help of TFH cells.8 GC TFH cells exhibit salient cellular, molecular and tissue-dynamic features that are essential to their development and function. These unique features markedly demarcate GC TFH cells from the classical paradigm of effector CD4 T cell activation differentiation.
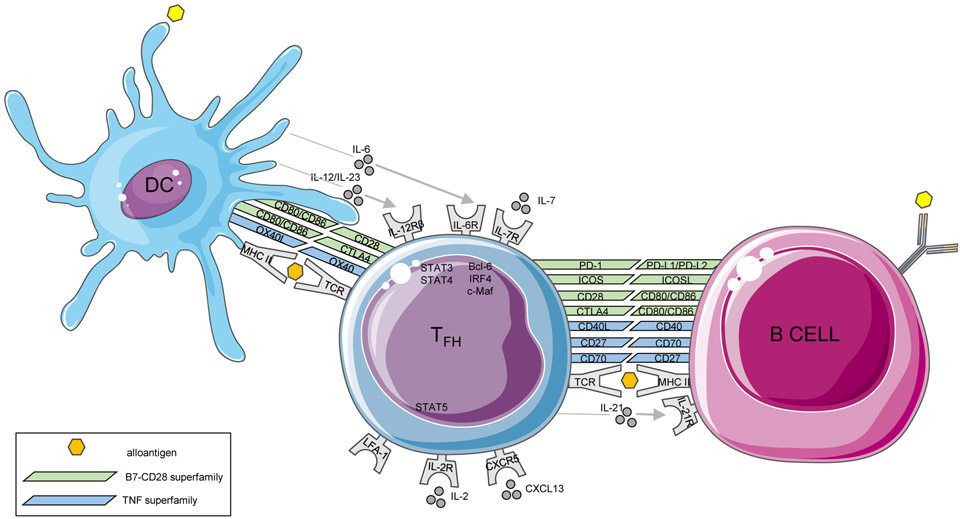
Differentiation of TFH cells is a complex and highly controlled process that involves a succession of coordinated spatio-temporal interactions with different cell populations of antigen presenting cells (APCs), and unlike other populations of CD4 T cell effectors, no single event allows their complete differentiation.9,10 Full differentiation of TFH cells requires a multi-step process, with an initial activation mediated by dendritic cells (DCs) in the T cell zones of secondary lymphoid organs (SLO), a subsequent B cell interaction at the T-B border and then within GCs.11 In the T cell zone of SLO, the indirect presentation of antigen by DCs to naive CD4 T cells, concomitant with the engagement of the costimulatory molecule ICOS, induces the expression of the transcription factor Bcl-6 and consecutively that of CXCR5.12 A complex combination of cytokines including IL-6, IL-12, IL-21, IL-23 and TGF-β upstream STAT3/STAT4 pathways promotes human TFH cell fate and counteracts the non-TFH ones.9,13 In contrast, IL-2 is a potent inhibitor of commitment to TFH cell fate and the IL-2/STAT5 axis cooperates with the transcription factor Blimp-1 (a Bcl-6 antagonist) to inhibit TFH cell differentiation.14 The initial dose of antigen and potent TCR signals in conjunction with the finely tuned costimulatory and cytokine signals provided by DCs are also strong determinants of TFH cell generation and polarization.15,16 In addition, sustained antigenic stimulation by B cells is also necessary for TFH cell differentiation17 and once committed, TFH cells continue to require continuous cognate GC B cell exposure for their maintenance (Figure 1).
Figure 1.
TFH cell crosstalk with dendritic cell and B cell.
The phenotype and transcriptional profiles of TFH cells are now well established. In addition to Bcl-6, several other transcription factors contribute to TFH cell differentiation and maintenance including c-Maf, IRF4, Baft, LEF1 and TCF1.10,18 STAT3 and STAT4 are of particular importance as they promote the expression and production of IL-21, the hallmark cytokine of TFH cells.19 In addition, epigenetic changes, including histone modification and DNA methylation that control the expression of the abovementioned transcriptional programs, are increasingly recognized.20 Regarding surface molecules, TFH cells express a range of costimulatory receptors, that reinforce their cognate interaction with B cells and promote B cell activation (e.g. CD28, CD40L, ICOS, PD-1, OX40), and multiples cytokine receptors (IL-6R, IL-7Rα, IL-12Rβ, IL-21R) allowing self-response to cytokines, cell renewal, as well as activation and differentiation into memory TFH cells (Figure 1).
The human deficiency of TFH cells in numbers or function have highlighted their crucial role in defense against infection and cancer, as individuals displaying genetic deficiency for hallmark molecules lack TFH cells and GCs, and exhibit increased susceptibility to infections and cancers. Conversely, increased TFH cells in numbers or function due to genetic mutations or polymorphism of IL-21 can lead to increase susceptibility to autoimmunity.21,22 Also, exponential number of studies have documented that the increase frequencies/numbers and function of TFH cells with activation phenotypes (expressing increased ICOS, PD-1 or IL-21) promote protective antibody response against pathogens after vaccination or infection and can also promote deleterious autoantibody generation and their pathogenicity leading to autoimmunity and tissue damage.
b). Circulating TFH cells
Because of difficulties in accessing lymphoid tissues and GCs in humans, the analysis of circulating TFH (cTFH) cells has proved valuable in understanding alterations of TFH cell response that contribute to human diseases. cTFH cells are blood memory CD4 T cells that express CXCR5 that are in a resting state in the absence of antigenic stimulation. Although the developmental origins of cTFH cells remain debated, the phenotypic, transcriptional and epigenetic relationships between cTFH and GC TFH cells have been outlined by studies performed in genetically mutant mice and specimens from patients with primary immunodeficiency.23,24 These cTFH memory cells are mainly generated from cells either committed to the TFH lineage, or from recirculated TFH of the GC. Some of these cTFH cells may develop before GC formation and therefore represent precursors of GC TFH,25 while others may emerge from memory GC TFH cells at the resolution of GC responses.26-28 However, more definitive arguments for a pre- or post-GC emergence and differentiation of cTFH cells are still needed. Unlike GC TFH, cTFH cells display markedly distinct features: (i) absence of Bcl-6 expression; (ii) lower expression of CXCR5 and of (iii) CD40L, ICOS and PD-1 at resting state.29,30 However, cTFH similar to GC TFH cells display common functional attributes such as the capacity to help B cells and promote their activation and differentiation into plasma cells.31 This helper capacity appears to be more evident for PD-1+ compared to PD-1− cTFH subsets and to other CD4+CXCR5− T cell subsets. There is also substantial overlap in the transcriptional programs of cTFH and GC TFH cells, including the common expression of c-MAF, IRF4 and POU2AF.25,29-33
Activated (PD-1+ICOS+) cTFH cells with effector (CD45RO+CD62L−) memory phenotype are robust surrogate biomarkers of ongoing GC and antibody responses in multiple clinical settings including vaccination, acute and chronic infections, allergies and cancers. They emerge before antibodies in blood, as do plasmablasts, and therefore predict the magnitude and the pathogenicity of the antibodies generated. cTFH cells have been shown to correlate with systemic CXCL13 levels, antibody titers and disease manifestations.34 cTFH cells are composed of heterogeneous subsets, and depending on the antigenic trigger, co-stimulation and inflammatory environment, can be composed of predominant cTFH-Th1 (CXCR3+CCR6−), cTFH-Th2 (CXCR3−CCR6−), cTFH-Th1/17 (CXCR3+CCR6+) or cTFH-Th17 (CXCR3−CCR6+) subsets. In addition to producing IL-21, these subsets have distinct capacities to secrete IFNg, IL-4 or IL-17 helper cytokines as well as to instruct naive or memory B cells to differentiate into plasma cells and produce Ig of different subclasses.35
c). T peripheral helper (TPH) cells.
More recently the spectrum of TFH-like cells with capacity to provide B cell help has substantially expanded. Very recent studies have identified a novel subset of PD-1hiCXCR5− CD4+ T cells with B helper capacities, localized in inflamed join tissues of patients, and were therefore named T peripheral helper (TPH) cells.36 As for TFH cells, TPH cells were described as expressing CXCL13, CD40L, ICOS and the transcription factor c-Maf, but not Bcl-6,37,38 and exerting B helper activities in a manner largely dependent on IL-21 and SLAMF5.36 Because of their hallmark capacity to produce CXCL13 and their frequent co-localization with B cells, it is likely that TPH cells play a pivotal role in the formation of tertiary lymphoid structures in target tissues. As TFH cells, TPH cells can recirculate and can be detected as circulating TPH (cTPH) cells in peripheral blood of healthy individuals and are found expanded in patients with lupus, Sjogren’s syndrome, IgG4-related disease, systemic sclerosis, IgA nephropathy and type I diabetes.39,40 Intriguingly, unlike cTFH cells that provide strong B cell help to naive B cells to produce IgG, IgE and IgA, cTPH cells can only induce naive B cells to produce IgM.37 cTPH cells appear to strongly correlate with the accumulation of blood extrafollicular/atypical CD11c+ CD21− memory B cells that also lack the expression of CXCR5.41,42 Both CD11c+ CD21− CXCR5− B cells and TPH cells are found in inflamed tissues such as rheumatoid arthritis synovial or lupus nephritis tissues.36,43 As CD11c+ CD21− CXCR5− B cells, cTPH cells display marked Th1-biased features such as the expression of T-bet and the chemokine receptors CCR2, CCR5 and CX3CR1, and thus likely respond to chemokines secreted by targets tissues, that attract both cTPH cells and atypical B cells away from secondary lymphoid organs.36,42 Interestingly, cTPH cells may have cell cytotoxicity potential, as indicated by the expression of GZMA, GZMB and PRF1 in specific disease settings.42,44
However, it still remains to understand: (i) the molecular control of TPH versus TFH cell differentiation, (ii) the transcriptional differences between TPH and TFH cells, (iii) whether there can be plasticity between TPH and TFH cells, and (iv) whether TPH cells are only poised to help atypical memory B cells. Also, data regarding the existence of TPH and cTPH cells in patients mounting DSAs and developing ABMR are missing and warranted.
d). T follicular regulatory (TFR) cells
Another TFH-like cell subset with capacity to suppress GC and antibody responses is represented by T follicular regulatory (TFR) cells. TFR cells share both TFH cell features (Bcl-6, CXCR5 and PD-1) and T regulatory (TREG) cell markers (CD25, CTLA4 and FoxP3) and function (IL-10 and TGFβ).45,46 Pre-TFR cells are derived from thymic TREGs and are CXCR5+ FoxP3+ cells localized at the T-B border.47 While the initial stage of TFR cell differentiation appears B cell independent, B cells are necessary for stabilization of the TFR cell program, including the expression of TCF1 and Bcl-6.48 CD25 signaling helps to inhibit TFH cell program and favors a TFR cell fate by promoting Blimp-1 and STAT5.49 However, a distinct subset of CD25− TFR cells can be found in GCs and differs from the CD25+ TFR cells by the downregulation of IL-2-dependent canonical TREG features, but nonetheless retains suppressive capacities.50,51
Circulating TFR (cTFR) cells, similar to cTFH cells, appear to be less activated than those found in the SLOs, generally lacking the expression of PD-1, ICOS and Bcl-6 but retaining CXCR5, and expressing CD45RA and CCR7.52 Due to their relative resting state compared to the activated TFR cells found in SLOs, cTFR cells have weaker suppressive activity unless they are pre-activated.31,52 cTFR cells are shown to be generated at an early stage of the GC response prior to the involvement of B cells, as they are still generated in individuals genetically lacking B cells.52 cTFR cells were found to be expanded 7 days after flu vaccination and to correlate with auto-antibody responses during Sjogren’s syndrome, suggesting that their expansion is indeed indicative of ongoing GC responses.52
Some mechanisms of the suppression of humoral immunity by TFR and cTFR cells are similar to those used by TREGS and are dependent on: (i) CTLA4, by regulation of CD28 signaling to TFH cells via direct control of CD80 and CD86 expression on B cells,53,54 (ii) upregulation of CD25 and FoxP3.52
However, other mechanisms of suppression such as granzyme B-mediated cytotoxicity55 and through the action of the ectoenzymes CD39 and CD7356 were shown for TREGS but not for TFR cells. In addition, recent studies have demonstrated the potent TFR cell capacity to regulate the early, but not late, GC responses and to control Ig responses to vaccines, allergens and autoantigens.57,58
Role of TFH cells in transplantation
The pivotal role of TFH cells during humoral alloimmunity against organ transplants has been increasingly documented during the past 5 years. The first studies were performed using mouse59,60 and nonhuman primate61,62 models of allosentization and ABMR. These studies have demonstrated that ABMR can be used as an experimental model to study TFH cell response to alloantigen and have allowed to identify hypertrophic GCs and increase in TFH cell numbers and activation during ABMR. Also, these models are of particular interest to test whether existing or novel therapeutic agents have intrinsic effects on GC and TFH cell responses. In transplant recipients, using blood cTFH cells as a proxy for estimating TFH cell functions in GCs proved promising for diagnostic and immune monitoring purposes. However, this approach has limitations and warrant further clinical investigations to measure to what extent these cells reflect what is occurring within GCs and their utility to monitor the intrinsic effects of therapies and immune response to treatments in ABMR.
a). Role of cTFH cells in DSA generation
More recently, increasing number of studies have exemplified the implication of TFH cells during humoral alloimmunity in humans. De Graav et al were the first to document the functional capacity of cTFH cells from kidney recipients to promote B cell activation and Ig production, and the increased number of cTFH cells post transplant in patients with preformed DSA6 It was next shown by other groups that cTFH cells with activation features (PD-1+ICOS+ or PD-1hiCCR7lo) correlated with DSA generation and their MFI levels.63 Of note, frequencies of PD-1+ICOS+ cTFH in the first year was predictive of de novo DSA development after 1 year post transplant.64 These findings nicely corroborate those in preclinical kidney transplantation models showing that TFH cells are required for de novo DSA responses, as well as augmentation of secondary (memory) DSA responses following presensitization,65 and that PD-1+ICOS+ cTFH cells are biomarkers of DSA formation.66 In contrast, PD-1+ICOS+ cTFH cells were substantially decreased in frequency and functionally impaired in patients displaying allograft operational tolerance, who very rarely mount DSAs post transplant.67
Like in animal models, human cTFH cells display potent capacity to respond to donor antigen by producing IL-21. Increased IL-21+ cTFH cells can be readily identified pretransplant in patients who further develop DSAs, unlike in those who do not develop DSAs posttransplant,68,69 indicating that preformed T cell memory to donor is significantly enriched within cTFH cell compartment.
b). Role of cTFH cells in ABMR
Although the association between cTFH cell activation, their reactivity to donor and DSA generation was established, their relationship to DSA pathogenicity and ABMR was unclear. Our group has recently shown that there was an expansion of overall cTFH cell compartment during ABMR but also an enrichment for activated/proliferating Ki67+ICOS+ and surprisingly of early memory precursors CD45RO+CCR7+CD127+ cells that may replenish and sustain effector TFH response during chronic alloantigen trigger, which was not previously documented.70 Frequencies of Ki67+ICOS+ cTFH cells coincided with concomitant expansion of blood Ki67+ activated (CD20+CD38lo) B cells and antibody-secreting (CD20−CD38hi) cells, indicative of coordinated cTFH-B cell responses. Ki67+ICOS+ cTFH cells also correlated with plasma CXCL13 and DSA MFI levels, suggesting a robust GC reaction that contribute to pathogenic DSA generation during ABMR. This finding is consistent with recent data in preclinical models showing the crucial role of TFH cells in promoting DSA responses and ABMR, as deletion of TFH cells at the time of transplant resulted in significantly less severe allograft ABMR in mice.65
Our transcriptional analyses of cTFH cells further supported an enrichment for both TFH precursors (LEF1 and TCF1) and effector TFH (CD28, CD40L, IL-21R) gene signatures, that were selective to patients with DSA+ABMR+, unlike those who did not develop DSA (DSA-) or developed DSA but did not progress to ABMR (DSA+ABMR-) patients.70 This distinct transcriptional programing of cTFH cells in ABMR resulted, not surprisingly, in their increased functional capacity to provide help to B cells, that differentiated into plasma cells in response to this vigorous cTFH cell help and generated DSAs enriched in IgG3 isotype. Although previous studies have found that both CXCR3+ (Th1) and CXCR3− (Th2 and Th17) cTFH subsets were found increased in patients mounting DSAs,64,68 it was unclear how these distinctly polarized cTFH cells correlated with differential pathogenicity of DSAs and what polarized subsets were observed during ABMR. Our recent study showed that while heterogeneous expansion of Th1, Th2 and Th17 cTFH subsets were present during ABMR, only Th2 and Th17 subsets were predominantly increased within Ki67+ICOS+ cTFH, and these were associated with increased IgG3 DSA generation. This is consistent with the known roles of IL-17 and IL-21 in isotype switching towards IgG3.71,72 While previous studies have shown an association between increased frequencies of activated cTFH cells and allograft rejection,6,63 it was unclear whether this increase in cTFH cells was associated with specific patterns of allograft injury and with clinical outcome. Analyzing frequencies of cTFH cells with histological and clinical profiles, we found that expansion of Ki67+ICOS+ as well as of CD45RO+CCR7+CD127+ cTFH were associated with a more severe form of ABMR with poorer kidney allograft survival. These severe ABMR were characterized by increased arteritis and by more interstitial inflammation and tubulitis, that are features of T-cell mediated rejection (TCMR).70 Thus, cTFH cell phenotypic and functional profiles represent valuable surrogate biomarkers to predict and distinguish less robust phenotype (DSA+ABMR−) from pathogenic (DSA+ABMR+) humoral responses after transplantation.
c). Role of TFH cells within allograft tissues
It is tempting to speculate that inflammatory cellular infiltrates found in allografts undergoing ABMR could be composed of increased cTFH cells that would have migrated to target tissues. Indeed, it was previously found that TFH cells could be detected within allografts from patients with acute TCMR6 and in those with mixed ABMR with TCMR lesions.73,74 These TFH cells can also be part of ectopic/tertiary lymphoid structures formed by dense cell clusters of activated B cells.75 Our group also found that TFH cells can be detected within allografts with chronic ABMR (Figure 2). Interestingly, a substantial number of CD4 T cells that were CXCR5− could be also observed, suggesting a TPH or CD4+ T conventional (non-TFH) phenotype of these cells, concomitant with the presence of CXCR5+ (TFH) cells during chronic ABMR (Figure 2C). As TFH cells can be observed in cases showing TCMR, it remains to be investigated whether cTFH cell transcriptional profile and function in patients with TCMR would significantly differ from those with ABMR.
Figure 2. TFH cells within kidney allograft showing chronic ABMR.
Representative hematoxylin and eosin, and multiplex immunofluorescence staining performed on a kidney allograft biopsy (transplant explant biopsy) from a patient with incurable chronic ABMR, refractory to immunosuppressive treatments. Scale bars indicate 50μm.
d). Role of cTFR cells in transplantation
In transplantation, cTFR cells are increasingly described76,77 and their frequency were found to be diminished in the context of acute and chronic rejection.78 Moreover, using inducible TFR cell deletion strategies, a recent study revealed that TFR cells significantly inhibited de novo DSA formation, but had a minor role in controlling kidney allograft ABMR in mice.65 However, in the context of DSA and ABMR responses in humans, their frequency, function and whether these cells can migrate to allograft tissues remains to be resolved. Thus, further attention on TFR cells is needed in future studies to understand how to promote their development and suppressive function, as they could be instrumental to optimally control TFH cell-dependent humoral responses.
Effects of current therapies on TFH cells
Although the current induction therapies and maintenance immunosuppressive (IS) drugs used nowadays in the clinic were primarily designed to target and alter T cell responses, their intrinsic effects on TFH cell differentiation and functions were not initially known but are now increasingly documented (Table 1). The effects of such therapies were measured by assessing cTFH cells in patients but these effects on TFH cells in GCs are also supported by multiple experimental models.
Table 1.
Effects of current therapies on human TFH cells
| Type of drug | Drug | Intrinsic effects on TFH cells | References |
|---|---|---|---|
| Induction therapy | Thymoglobulin | reduces cTFH numbers | 63 |
| reduces cTFH numbers, induces proliferation of cTFH and promotes an effector memory (CD62L−) phenotype and their overexpression of CXCR3 and PD-1 | 68 | ||
| reduces cTFH numbers and their activation-induced upregulation of CD40L and ICOS | 79 | ||
| reduces cTFH numbers and induces increased frequencies of PD-1+, ICOS+PD-1+ and PD1+CXCR3− cTFH at 12-months post transplant | 64 | ||
| Basiliximab | no effect on cTFH numbers | 63 | |
| no effect on cTFH numbers and induces a central memory (CD62L+) cTFH phenotype | 68 | ||
| no effect on cTFH numbers | 79 | ||
| Maintenance immunosuppression | Tacrolimus | reduces cTFH, GC-TFH precursor and TFH memory numbers. No effects on GC-TFH numbers. Inhibits PD-1 expression on cTFH and plasma cell formation in cTFH-B cell co-cultures | 86 |
| inhibits naive CD4 T cell differentiation into TFH, reduces TFH expression of ICOS and PD-1, and their production of IL-21. Inhibits TFH-dependent proliferation and differentiation of B cells into plasma cells in TFH-B cell co-cultures | 90 | ||
| Mycophenolate mofetil | reduces T cell expression of CD40L. inhibits T cell-dependent proliferation and differentiation of B cells into plasma cells in T-B cell co-cultures and in IL-21 stimulated B cells | 96 | |
| reduces T cell expression of CD40L and ICOS. inhibits T cell-dependent B cell production of IgG in T-B cell co-cultures | 97 | ||
| Sirolimus | inhibits naive CD4 T cell differentiation into TFH. reduces TFH expression of ICOS and PD-1, and their production of IL-21. Inhibits TFH-dependent proliferation and differentiation of B cells into plasma cells in TFH-B cell co-cultures | 90 | |
| reduces total cTFH, PD-1+ cTFH and STAT3+ cTFH numbers | 178 | ||
| Corticosteroids | reduces cTFH and Th1, Th2 and Th17-cTFH subset numbers | 102 |
a). Effects of induction therapies on TFH cells
Thymoglobulin, basiliximab and alemtuzumab, as compared to absence of induction therapy, have drastically reduced the incidence of acute rejection during the first year post transplant.
Thymoglobulin is a mixture of polyclonal IgGs recovered from the sera of rabbits or horses immunized with human thymocytes and therefore mostly aims to deplete the T cell compartment. Its intrinsic effects on cTFH cells include a prolonged depletion in cell numbers, but also a shift from central (CD62L+) to effector (CD62L−) memory phenotype as well as the overexpression of PD-1 and CXCR3 consistent with pre-activated and Th1 phenotypes.63,64,68 These effects are maintained throughout the first year post transplant. Conversely, the lymphopenia-induced proliferation of cTFH cells was transient and maximum at 1-month post transplant and was observed selectively in patients further developing DSA in the first year post transplant, indicating that in these patients, thymoglobulin may promote a pre-activation state that may favor DSA development.68 However, regardless of DSA development, thymoglobulin was able to diminish activation-induced upregulation of CD40L and ICOS on cTFH cells, indicative of a partial control of cTFH cell helper function.79
Basiliximab is a monoclonal chimeric mouse-human antibody and nondepleting agent targeting the α-chain (CD25) of IL-2R. It was designed to reduce IL-2 binding to its receptor and therefore reduce IL-2 induced capacity for T cells to proliferate following activation. Unlike thymoglobulin, basiliximab does not induce cTFH cell depletion nor proliferation, and maintain cTFH cells in a central memory differentiation.68,79 Given that IL-2 prevent TFH cell differentiation and function, it could have been anticipated that basiliximab would promote increase cTFH cell numbers and activation state via blockade of IL-2R, however this was not observed in the recent studies. IL-2/IL-2R is crucial for TREG and TFR optimal cell development and functions.80 Is it therefore likely that basiliximab would also block the functions of TREG and TFR cells, thus preventing the suppression of TFH cell response by these cells.
Alemtuzumab is a humanized monoclonal antibody directed against CD52, an immune receptor widely present on the surface of T and B cells. Alemtuzumab is known to induce rapid and profound T and B cell depletion,81 and to be associated with increased incidence of ABMR in patients, when compared to thymoglobulin.82,83 This mechanism includes increased GC TFH differentiation and promotion of IL-21 production by GC TFH.84
b). Effects of maintenance IS drugs on TFH cells
In addition to induction therapy, the IS drug combination including calcineurin inhibitor (or mTOR inhibitor) with purine synthesis inhibitors (mycophenolate mofetil or azathioprine) and with corticosteroids, is currently considered as the standard-of-care maintenance IS regimen for organ transplant recipients. This IS combination regimen has dramatically reduced the incidence of acute rejection in the first year post transplant and transformed the clinical picture and spectrum of allograft rejection with increased incidence of subclinical and chronically evolving forms of ABMR. Although the T cell response is the central target of maintenance IS drugs, their effects on TFH cell differentiation and functions were only recently described.
Calcineurin inhibitors (tacrolimus and cyclosporine) were designed to suppress calcineurin activity and activation of NFAT in T cells, which are induced after the engagement of TCR. Compared to cyclosporine and mTOR inhibitors, tacrolimus is more effective at preventing de novo DSA development in transplant patients.85 This may be explained by the reduced numbers of CXCR5+PD-1+ cTFH cells and CXCR5+PD-1+ GC TFH precursors in patients treated with tacrolimus, while no effects were observed on B cells, TFR and TREGS. In vitro, tacrolimus is associated with significant reduction of PD-1 expression on cTFH and of plasma cell formation in cTFH-B cell co-cultures.86 Tacrolimus also significantly reduces TFH cell function and expression of Bcl-6 and of BATF/JUN/IRF4 complex, and reduces IL-6 and IL-21 production.87
mTOR inhibitors (rapamycin and everolimus) engage FK binding proteins, forming a complex that inhibits mTOR in T cells. This inhibition prevents translation of mRNA-encoding proteins needed to enter the cell cycle and therefore impairing T cell proliferation and cytokine production.88 In addition to this nonspecific mechanism, rapamycin is able to inhibit naive CD4 T cell differentiation into TFH cells,89,90 to reduce TFH cell expression of ICOS and PD-1, and their production of IL-21.90 Rapamycin also inhibits TFH cell-dependent proliferation and diminished differentiation of B cells into plasma cells and antibody production in TFH-B cell co-cultures to a degree similar to that achieved with TFR cells.90,91 This effect was also observed experimentally in vivo by increased heart allograft survival and reduced TFH and B cell numbers post transplant in mice recipients treated with rapamycin compared to vehicle.92 Moreover, genetic studies have shown that while mTOR kinase complexes 1 and 2 may act distinctly, they are both essential for TFH cell differentiation and GC responses under steady state and after antigen immunization.93,94 However, in heart-transplanted mice treated with alemtuzumab, rapamycin promoted increased in PD-1+ICOS+ TFH cells in lymph nodes, suggesting paradoxical effects on TFH cells in post depletion settings.95
Mycophenolate mofetil blocks cell division by inhibiting DNA synthesis and acts as a selective inhibitor of inosine-59-monophosphate dehydrogenase, a key enzyme in de novo synthesis of purine. While mycophenolate mofetil is known to be a significant inhibitor of B cell activation and proliferation, no studies have specifically investigated the effects of mycophenolate mofetil on TFH cells, some have reported its effect on CD4 T-cell help to B cells. Two studies have shown that mycophenolate mofetil significantly reduced CD40L and ICOS expression on polyclonally activated T cells, and inhibited T cell-dependent proliferation and differentiation of B cells into plasma cells in T-B cell co-cultures and in IL-21 stimulated B cells.96,97 This is consistent with clinical studies suggesting that mycophenolate mofetil may be efficient in preventing de novo DSA formation, when compared to azathioprine, another inhibitor of DNA synthesis.98,99
Corticosteroids are among the oldest and most commonly used immune-modulatory drugs. Their mechanisms of action on T cells are numerous and include interference with cell receptors and their downstream signaling pathways and transcription factors, including TCR and its downstream kinases.100,101 Specifically, corticosteroids were shown to markedly reduce cTFH, Th1-, Th2- and Th17-cTFH subset numbers in individuals with IgG4-related disease.102 Investigating the effects of corticosteroids on cTFH cells in transplant patients are warranted, as a recent study showed an association between lack of corticosteroids and increased risk of subclinical ABMR.103
Biotherapeutic targeting of TFH cells
The success of biotherapy in organ transplantation has been recently illustrated by the use of belatacept, the first targeted biotherapy that received FDA approval for prophylaxis of organ rejection in kidney transplantation, which significantly diminish the incidence of de novo DSA and improve long-term allograft survival, when compared to cyclosporine.104 It was then demonstrated that this decreased risk of de novo DSA development was explained by a direct effect of belatacept on TFH cells and their crosstalk with cognate B cells.105 In addition, the IL-6R antagonist tocilizumab, a biotherapy developed and FDA approved for treatment of rheumatoid arthritis, was also used in recent clinical trials in ABMR.106 Its administration in patients with chronic ABMR resulted in stabilization of allograft function and DSA reduction. While biotherapeutic targeting of TFH cells may result in complex immune modulation beyond TFH cell responses, these successes further encourage and open the way for understanding the intrinsic effects of biotherapies on TFH cells, and for developing novel biotherapies targeting these cells in organ transplantation (Table 2).
Table 2.
Biotherapeutic targeting of human TFH cells
| Type of drug | Drug | INN | Clinical setting | Trial registration |
References |
|---|---|---|---|---|---|
| Costimulatory molecules agonists and antagonists | CTLA4-Ig | belatacept | transplantation | NCT00256750 | 104 |
| Anti-CD28 | lulizumab | transplantation, Sjogren’s syndrome, lupus | NCT04066114, NCT02265744, NCT02843659 | – | |
| Anti-CD40 | bleselumab | transplantation | NCT01780844, NCT01279538 | 121, 122 | |
| Anti-CD40L | dapirolizumab | lupus | NCT01764594 | 179 | |
| Anti-ICOS | MEDI-570 | lupus, lymphoma | NCT01127321, NCT02520791 | – | |
| Anti-OX40 | GBR 830 | atopic dermatitis | NCT02683928 | 141 | |
| Anti-CD70 | cusatuzumab | acute myeloid leukemia | NCT03030612 | 147 | |
| Cytokines and cytokine receptors antagonist | Anti-IL-6 | clazakizumab | transplantation, rheumatoid arthritis, COVID-19 | NCT03444103, NCT01373151, NCT04363502 | – |
| Anti-IL-6R | tocilizumab | transplantation, rheumatoid arthritis, giant-cell arteritis, COVID-19 | NCT01594424, NCT01119859, NCT01791153, NCT04356937 | 180, 181, 182, 183 | |
| Anti-IL-21 | BOS161721 | lupus | NCT03371251 | – | |
| Anti-IL-21 | NNC114–0005 | healthy subjects, rheumatoid arthritis | NCT01208506 | 163 | |
| Anti-IL-21R | ATR-107 | healthy subjects | NCT01162889 | 164 | |
| Anti-IL-7Rα | OSE-127 | healthy subjects | NCT03980080 | – | |
| Anti-IL-7Rα | RN168 | type 1 diabetes | NCT02038764 | 168 | |
| Anti-IL-12/IL-23 | ustekinumab | psoriasis, Crohn's disease, ulcerative colitis | NCT00454584, NCT00771667, NCT02407236 | 170, 171, 172 |
INN, international nonproprietary name
a). Costimulatory molecules agonists and antagonists
a1. CD28/CTLA4/B7 axis.
Therapeutic targeting of CD28/CTLA4/B7 has always been a primary goal in transplantation.107 The use of a recombinant CD28-Ig that binds CD80/CD86 and blocks CD28 signaling was ineffective due to insufficient affinity for its B7 ligands, leading to the development of another recombinant protein: the CTLA4-Ig (belatacept).108 Belatacept is a human fusion protein combining the extracellular portion of CTLA4, which has been mutated to confer greater binding avidity to CD80 (B7-1) and CD86 (B7-2), and the constant region fragment of human IgG1. CTLA4 binds CD80 and CD86 on APCs, preventing their interaction with CD28 and therefore inhibiting T cell activation. Although belatacept has been primarily developed to target DC-mediated priming of T cells, preclinical and recently human models have shown that belatacept could also unexpectedly inhibited antibody responses.62 Nonhuman primates treated with belatacept showed decreased in follicle size, GC formation and IL-21 production, indicating a role for belatacept in disruption of TFH-B cell interaction.62 In mice models, delayed belatacept could inhibit ongoing antibody response, even though priming of allogenic T cells had already occurred. The administration of belatacept 14 days after sensitization successfully inhibited DSA production and collapsed GC responses.109,110 These results suggested that CTLA4-Ig action on TFH-B cell responses were prominent and independent of initial T cell priming by DCs. Leibler at al. confirmed that this mechanism also existed in humans.105,111 Belatacept inhibited TFH-B cell crosstalk in vitro by decreasing TFH cell activation and decreasing B cell differentiation into plasma cells. Interestingly, belatacept-treated kidney recipients had lower frequencies of PD-1+ICOS+ cTFH and effectors B cells than recipients on calcineurin inhibitors, indicating that the inhibition mechanism proposed also existed in vivo.105 Consistently, interventional trials further confirmed that belatacept was associated with decreased incidence of de novo DSA, decreased DSA levels and also prevented the occurrence of ABMR.112,113
More recently, agents more specifically inhibiting CD28 were developed, while leaving CTLA4 intact, to better control alloreactive T cell responses and prevent the undesirable T-cell mediated rejections observed in patients treated with belatacept. The use of such selective CD28 blockers in murine and primate models of transplantation has indeed revealed superior allograft survival results as compared to belatacept, an effect potentially dependent on the preservation of negative signaling through CTLA4.107 Indeed, preservation of CTLA4-mediated signals with anti-CD28 is associated with enhanced TREG functionality114 and better control of the proliferative response of preexisting TFH cells than belatacept.115 In addition, anti-CD28 significantly reduces cTFH, ICOS+PD-1+ cTFH and GC TFH cell numbers and inhibits GC TFH cell proliferation, and GC TFH cell-dependent B cell differentiation into plasma cells in GC TFH-B cell co-cultures.66,116 However, as loss of CD28 signals would also lead to alteration of TREG and TFR cell functions, the effects of anti-CD28 on these cells should be considered. The efficacy of the anti-CD28 lulizumab in combination with other adjunct therapies is currently tested in clinical trials in living donor kidney transplant recipients, or in patients with lupus or Sjogren’s syndrome (Table 2).
a2. CD40/CD40L axis.
CD40/CD40L molecules remain promising therapeutic targets in transplantation as blockade of this pathway has demonstrated strong effects on humoral responses. In a myriad of experimental models, inhibition of the CD40/CD40L axis resulted in markedly increased allograft survival and represents a potential way of inducing a tolerogenic environment.117 In mice, anti-CD40L therapy drastically reduces DSA production via terminating ongoing GC reactions.109 In humans, interventional trials evaluating the safety and efficiency of anti-CD40L reagents were prematurely halted because of severe thromboembolic complications (related to CD40L expression on human platelets).118 Anti-human CD40, are now developed as safer and potential alternatives. Importantly, anti-CD40 also effectively disrupts GCs by reducing GC TFH cell numbers and their IL-21 production, and attenuated DSA production in kidney transplanted monkeys.62,119 Moreover, bleselumab (ASKP1240) abolished DSAs production in a monkey model of pancreatic islet transplantation.120 Several clinical trials using bleselumab in kidney transplantation and lupus are ongoing (Table 2).121,122
a3. LFA-1/ICAM axis.
LFA-1 (CD11a) is an integrin critical for trafficking of TFH cells to GCs but also for delivering potent T cell costimulatory signals that augment Bcl-6 and their effector function.123 In animals, anti-LFA-1 inhibits priming of naive alloreactive T cell responses, prolongs allogeneic cardiac and islet allograft survival124,125 and is effective in mitigating donor-specific memory T cells that are independent of CD28 and CD40L.126,127 Moreover, anti-LFA-1 reduces GC TFH cell formation, serum IL-21 and DSA generation in a cardiac model of chronic ABMR following alemtuzumab induction.84 In 2 clinical trials in allogeneic islet transplantation and 1 in kidney transplantation, patients were treated with the anti-LFA-1 efalizumab.128-130 However, further trials using efalizumab were halted because of excessive incidence of progressive multifocal leukoencephalopathy and posttransplant lymphoproliferative disease.131
a4. ICOS/ICOSL axis.
ICOS/ICOSL axis can be blocked using recombinant ICOS-Ig or anti-ICOS antibody. So far, preclinical studies in nonhuman primates evaluating the efficacy of ICOS-Ig have shown unsuccessful results in preventing allograft rejection.132,133 Anti-ICOS, however, appears to more effectively inhibit humoral responses; its administration resulted in decreased DSA generation in islet xenografts recipients,134 and its combination with anti-CD40, also led to decrease in DSAs and improved histology as compared to anti-CD40 alone, in a cardiac model of chronic ABMR.135 Anti-ICOS treatment appears promising, particularly when combined with other costimulatory blockade therapies, as compensatory pathways in B cells may overrule its requirement.136 Several clinical trials in lupus and lymphoma using anti-ICOS are ongoing (Table 2).
a5. OX40/OX40L axis.
OX40 is a member of TNF superfamily of high interest in transplantation, particularly because of its prominent role in regulating alloreactive memory T cell and TFH cell responses.137 Although specific effects on TFH cells were not evaluated, promising results showed that anti-OX40 significantly reduces alloreactive CD4 T cell survival and prevented skin allograft rejection in mice.138,139 Moreover, alloreactive memory CD4 T cells that were relatively insensitive to either CD28 or CD40L blockade could be attenuated via anti-OX40L after skin allograft transplantation.140 While the anti-OX40 GBR 830 is currently evaluated in atopic dermatitis, its role in clinical transplantation requires further investigation.141
a6. CD70/CD27 axis.
CD70/CD27 are other members of the TNF superfamily and mutually expressed on TFH and B cells. CD70 activates PI3K/AKT pathway and stimulates cytokine production in TFH cells,142 while CD70 synergize with the CD40 pathway143 and play a role in B cell activation and antibody production in B cells.144 CD27, the ligand of CD70, is expressed on activated/memory TFH and B cells as well as plasma cells. In rats, anti-CD70 administration diminished memory T cells in recipient lymph nodes and prolonged corneal allograft survival.145 Similar findings were observed in heart-transplanted mice treated with anti-CD70.146 While there are ongoing clinical trials with the anti-CD70 cusatuzumab in acute myeloid leukemia, studies in transplant recipients are lacking.147
a7. PD-1/PD-L1 axis.
PD-1 is the ligand for PD-L1, and is known to dampen TCR and CD28 signaling, to diminish the accumulation of TFH cells and to control TFH cell localization in GC.148,149 In contrast to CTLA4-Ig that blocks TFH cells from receiving costimulatory signals, or to anti-PD1 antagonists used in cancer immunotherapy, PD-L1-Ig induces an inhibitory signal through PD-1. PD-L1-Ig with or without anti-CD40L, was shown to prevent allograft rejection and prolong allograft survival in 2 experimental models; islet150 and cardiac transplantation.151 However, as loss of PD-1 signals is also expected to enhance TREG and TFR cell functions,152 the effects of PD-L1-Ig on these cells should be considered. Clinical trials using PD-L1-Ig are lacking and warranted to validate these findings in humans.
b). Cytokines and cytokine receptor antagonists
b1. IL-6/IL-6R axis.
IL-6 plays a major role in TFH cell differentiation and function153 and in B cells, it promotes antibody production of all IgG subclasses154 and plasmablast/plasma cell survival.155 Plasmablasts produce large amounts of IL-6, which in turn, stimulate TFH cells via IL-6R.156 Anti–IL-6R (tocilizumab) administration resulted in reduction in TFH and Th17 cells, and augmentation in TREGS157 in experimental models. In patients with chronic ABMR, tocilizumab resulted in stabilization of kidney allograft function and significant decreased in DSA MFI levels.106 Anti-IL-6 (clazakizumab) recently showed promising results in late ABMR patients, including reduction of DSA levels and modulation of ABMR gene and histological activity (Table 2).
b2. IL-21/IL-21R axis.
While IL-21 is a pivotal cytokine for optimal TFH-B cell crosstalk,43 it may have double-edge sword effects in clinical settings.158 IL-21R is highly expressed on TFH cells but also on naive and activated memory B cells.159 Donor-reactive cTFH cells were shown to produce IL-2168 and IL-21 is detected at high levels in serum in experimental models of ABMR.84 In mice, anti-IL-21R administration could significantly delay skin allograft rejection160 and anti-IL-21 significantly prolonged islet graft survival.161 In humans, anti-IL-21R led to reduction in STAT3 phosphorylation in cTFH cells, increased plasma cells differentiation and IgG production in a coculture model of cTFH-B cells stimulated with donor antigen.162 Safety and efficacy data are accumulating in clinical trials in lupus and rheumatoid arthritis for anti-IL-21,163 and in healthy subjects for anti-IL-21R (Table 2).164 Thus, these 2 blocking agents are not yet ready for prime time in transplantation.
b3. IL-7/IL-7R axis.
IL-7 is crucial for memory CD4 T cell development, homeostatic proliferation and long-live maintenance. It also plays a role in TFH cell generation in vivo, and anti-IL-7 antibody markedly impairs the development of IgG responses.165 Importantly, IL-7Rα (CD127) was shown to be significantly upregulated on cTFH cell subset with a memory precursor phenotype during ABMR.70 In mice, anti-IL7Rα has been shown to induce long-term skin allograft survival or islet allograft tolerance when combined with prior T cell depletion, by delaying T cell reconstitution, decreasing allospecific memory T cells and decreasing DSAs.166 Conversely, in baboons, anti-IL-7Rα combined with thymoglobulin and tacrolimus did not promote increased allograft survival nor delayed T cell reconstitution.167 Currently, clinical trials using anti-IL-7Rα are ongoing in healthy subjects and in type 1 diabetes.168
b4. IL-12/IL-23/IL-12Rβ axis.
Unlike in mice, IL-12/IL-23 play an important role in the differentiation and polarization of human TFH cells, therefore these cytokines represent potential targets to control humoral responses.169 IL-12/IL-23 can be simultaneously targeted using anti-IL-12/IL-23 (ustekinumab), which has shown clinical efficacy in psoriasis, Crohn’s disease and ulcerative colitis.170-172 Ustekinumab could be repurposed in transplantation, given that TFH cell differentiation and IL-21 production strongly rely on both IL-12 and IL-23 signals.
c). Combination of targeted biotherapies
Targeting multiple cellular components and stages of the humoral alloresponse appears crucial for optimal control of DSA generation and ABMR. Of note, proteasome inhibitor (bortezomib), along with plasma cell depletion, paradoxically triggers homeostatic proliferation of GC TFH and B cells, indicative of humoral compensation, a plausible explanation for the failure of the treatment to sustainably decrease DSA levels and reverse ABMR.173 Therefore, most recent studies have investigated the combined effects of proteasome inhibitor (carfilzomib) combined with anti-CD28 (lulizumab) and showed reduction in lymph node TFH cells, DSA levels and improved kidney allograft survival in allosensitized nonhuman primates.174 Similar beneficial effects on TFH cells and DSAs were observed using carfilzomib combined with belatacept175 and using bortezomib combined with belatacept and anti-CD40176 in the same primate transplant model. In humans, bortezomib combined with belatacept also resulted in decreased PD-1+ICOS+CD38+ cTFH cells and DSAs in highly sensitized heart transplant candidates.177 Thus, mitigating both upstream TFH cell responses and terminal effector plasma cell responses appears promising for increase efficacy in depletion of DSAs and for prevention of rebound effects.
In conclusion, the recent recognition of the pivotal role of TFH cells in directing DSA generation leading to ABMR has significantly improved our understanding of the cellular and molecular cues underlying the pathophysiology of ABMR. Although current maintenance IS drugs and induction therapies appear to have beneficial effects in tempering TFH cell responses, further efforts are needed to develop efficient combinatorial biotherapies targeting TFH cells, as well as other cellular participants to the humoral immune response for the prophylaxis and curative treatment of ABMR. This will represent an important step towards personalized and precision medicine in the field of organ transplantation.
Acknowledgments
Funding
This work was supported by the following grants: R21-AI116746 (DM), R01-AI130010 (DM), 5T32AI074490-12 (KL) and the Human Immunology Program at the Starzl Transplantation Institute.
Abbreviations
- ABMR
antibody-mediated rejection
- Bcl-6
B cell lymphoma-6
- Blimp-1
B lymphocyte-induced maturation protein-1
- cTFH
circulating T follicular helper cell
- cTFR
circulating T follicular regulatory cell
- CTLA4
cytotoxic T-lymphocyte-associated antigen 4
- DC
dendritic cell
- DSA
donor-specific antibody
- GC
germinal center
- HLA
human leucocyte antigen
- ICOS
inducible T cell co-stimulator
- IFN-g
interferon-gamma
- Ig
immunoglobulin
- IL
interleukin
- IRF4
interferon regulatory factor 4
- LFA-1
lymphocyte function-associated antigen 1
- mTOR
mammalian target of rapamycin
- PD-1
programmed cell death-1
- STAT
signal transducer and activator of transcription
- TFH
T follicular helper cell
- TFR
T follicular regulatory cell
- TPH
T peripheral helper cell
- TREG
T regulatory cell
- TNF
tumor necrosis factor
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Loupy A, Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379(120):1150–1160. [DOI] [PubMed] [Google Scholar]
- 2.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665–673. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies. J Clin Invest. 2017;127(7):2492–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis K, Hertig A, Taupin J-L, et al. Markers of graft microvascular endothelial injury may identify harmful donor-specific anti-HLA antibodies and predict kidney allograft loss. Am J Transplant. 2019;19(9):2434–2445. [DOI] [PubMed] [Google Scholar]
- 5.Crotty S T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Graav GN, Dieterich M, Hesselink DA, et al. Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol. 2015;180(2):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Besouw NM, Mendoza Rojas A, Baan CC. The role of follicular T helper cells in the humoral alloimmune response after clinical organ transplantation. HLA. 2019;94(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roco JA, Mesin L, Binder SC, et al. Class-switch recombination occurs infrequently in Germinal centers. Immunity. 2019;51(2):337–350.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinuesa CG, Linterman MA, Yu D, et al. Follicular helper T cells. Annu Rev Immunol. 2016;34(4):335–368. [DOI] [PubMed] [Google Scholar]
- 11.Deenick EK, Ma CS, Brink R, et al. Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr Opin Immunol. 2011;23(1):111–118. [DOI] [PubMed] [Google Scholar]
- 12.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt N, Liu Y, Bentebibel S-E, et al. Molecular mechanisms regulating T helper 1 versus t follicular helper cell differentiation in humans. Cell Rep. 2016;16(4):1082–1095. [DOI] [PubMed] [Google Scholar]
- 14.Ballesteros-Tato A, León B, Graf BA, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazilleau N, McHeyzer-Williams LJ, Rosen H, et al. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10(4):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tubo NJ, Pagán AJ, Taylor JJ, et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153(4):785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumjohann D, Preite S, Reboldi A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38(3):596–605. [DOI] [PubMed] [Google Scholar]
- 18.Choi YS, Gullicksrud JA, Xing S, et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. 2015;16(9):980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt N, Liu Y, Bentebibel S-E, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15(9):856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu KT, Kanno Y, Cannons JL, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35(4):622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma CS, Uzel G, Tangye SG. Human T follicular helper cells in primary immunodeficiencies. Curr Opin Pediatr. 2014;26(6):720–726. [DOI] [PubMed] [Google Scholar]
- 22.Sawalha AH, Kaufman KM, Kelly JA, et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67(4):458–461. [DOI] [PubMed] [Google Scholar]
- 23.Tangye SG, Deenick EK, Palendira U, et al. T cell-B cell interactions in primary immunodeficiencies. Ann N Y Acad Sci. 2012;1250:1–13. [DOI] [PubMed] [Google Scholar]
- 24.Vella LA, Buggert M, Manne S, et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest. 2019;129(8):3185–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39(4):770–781. [DOI] [PubMed] [Google Scholar]
- 26.Choi YS, Yang JA, Yusuf I, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190(8):4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asrir A, Aloulou M, Gador M, et al. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat Commun. 2017;8:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suan D, Nguyen A, Moran I, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. 2015;42(4):704–718. [DOI] [PubMed] [Google Scholar]
- 29.Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hale JS, Youngblood B, Latner DR, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sage PT, Alvarez D, Godec J, et al. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124(12):5191–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasheed A-U, Rahn H-P, Sallusto F, et al. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36(7):1892–1903. [DOI] [PubMed] [Google Scholar]
- 33.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–244. [DOI] [PubMed] [Google Scholar]
- 34.Havenar-Daughton C, Lindqvist M, Heit A, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A. 2016;113(10):2702–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morita R, Schmitt N, Bentebibel S-E, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortea-Gordo P, Nuño L, Villalba A, et al. Two populations of circulating PD-1hiCD4 T cells with distinct B cell helping capacity are elevated in early rheumatoid arthritis. Rheumatology. 2019;58(9):1662–1673. [DOI] [PubMed] [Google Scholar]
- 38.Ekman I, Ihantola E-L, Viisanen T, et al. Circulating CXCR5-PD-1hi peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia. 2019;62(9):1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christophersen A, Lund EG, Snir O, et al. Distinct phenotype of CD4+ T cells driving celiac disease identified in multiple autoimmune conditions. Nat Med. 2019;25(5):734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshitomi H, Ueno H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol Immunol. 2020;18:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenks SA, Cashman KS, Zumaquero E, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49(4):725–739.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bocharnikov AV, Keegan J, Wacleche VS, et al. PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight. 2019;4(20):e130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ettinger R, Sims GP, Fairhurst A-M, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175(12):7867–7879. [DOI] [PubMed] [Google Scholar]
- 44.Yabe H, Kamekura R, Yamamoto M, et al. Cytotoxic Tph-like cells are involved in persistent tissue damage in IgG4-related disease. Mod Rheumatol. 2021;31(1):249–260. [DOI] [PubMed] [Google Scholar]
- 45.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing JB, Lim EL, Sakaguchi S. Control of foreign Ag-specific Ab responses by Treg and Tfr. Immunol Rev. 2020;296(1):104–119. [DOI] [PubMed] [Google Scholar]
- 47.Sayin I, Radtke AJ, Vella LA, et al. Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J Exp Med. 2018;215(6):1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang B-H, Wang K, Wan S, et al. TCF1 and LEF1 control Treg competitive survival and Tfr development to prevent autoimmune diseases. Cell Rep. 2019;27(12):3629–3645.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen E, Rabe H, Luo L, et al. Control of germinal center localization and lineage stability of follicular regulatory T Cells by the Blimp1 transcription factor. Cell Rep. 2019;29(7):1848–1861.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wing JB, Kitagawa Y, Locci M, et al. A distinct subpopulation of CD25- T-follicular regulatory cells localizes in the germinal centers. Proc Natl Acad Sci U S A. 2017;114(31):E6400–E6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Botta D, Fuller MJ, Marquez-Lago TT, et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol. 2017;18(11):1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fonseca VR, Agua-Doce A, Maceiras AR, et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol. 2017;2(14):eaan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wing JB, Ise W, Kurosaki T, et al. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–1025. [DOI] [PubMed] [Google Scholar]
- 54.Sage PT, Paterson AM, Lovitch SB, et al. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao D-M, Thornton AM, DiPaolo RJ, et al. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107(10):3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clement RL, Daccache J, Mohammed MT, et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol. 2019;20(10):1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu W, Liu X, Lin X, et al. Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med. 2018;215(3):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alsughayyir J, Chhabra M, Qureshi MS, et al. Relative frequencies of alloantigen-specific helper CD4 T cells and B cells determine mode of antibody-mediated allograft rejection. Front Immunol. 2018;9:3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badell IR, La Muraglia GM, Liu D, et al. Selective CD28 blockade results in superior inhibition of donor-specific T follicular helper cell and antibody responses relative to CTLA-4-Ig. Am J Transplant. 2018;18(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burghuber CK, Kwun J, Page EJ, et al. Antibody-mediated rejection in sensitized nonhuman primates: modeling human biology. Am J Transplant. 2016;16(6):1726–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim EJ, Kwun J, Gibby AC, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. 2014;14(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cano-Romero FL, Laguna Goya R, Utrero-Rico A, et al. Longitudinal profile of circulating T follicular helper lymphocytes parallels anti-HLA sensitization in renal transplant recipients. Am J Transplant. 2019;19(1):89–97. [DOI] [PubMed] [Google Scholar]
- 64.Danger R, Chesneau M, Delbos F, et al. CXCR5+PD1+ICOS+ circulating T follicular helpers are associated with de novo donor-specific antibodies after renal transplantation. Front Immunol. 2019;10:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammed MT, Cai S, Hanson BL, et al. Follicular T cells mediate donor-specific antibody and rejection after solid organ transplantation [published online ahead of print January 9, 2021]. Am J Transplant. doi: 10.1111/ajt.16484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.La Muraglia GM, Wagener ME, Ford ML, et al. Circulating T follicular helper cells are a biomarker of humoral alloreactivity and predict donor-specific antibody formation after transplantation. Am J Transplant. 2020;20(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chenouard A, Chesneau M, Bui Nguyen L, et al. Renal operational tolerance is associated with a defect of blood Tfh cells that exhibit impaired B cell help. Am J Transplant. 2017;17(6):1490–1501. [DOI] [PubMed] [Google Scholar]
- 68.Macedo C, Hadi K, Walters J, et al. Impact of induction therapy on circulating t follicular helper cells and subsequent donor-specific antibody formation after kidney transplant. Kidney Int Rep. 2019;4(3):455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Besouw NM, Yan L, de Kuiper R, et al. The number of donor-specific IL-21 producing cells before and after transplantation predicts kidney graft rejection. Front Immunol. 2019;10:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Louis K, Macedo C, Bailly E, et al. Coordinated circulating T follicular helper and activated B cell responses underlie the onset of antibody-mediated rejection in kidney transplantation. J Am Soc Nephrol. 2020;31:2457–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitsdoerffer M, Lee Y, Jäger A, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107(32):14292–14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pène J, Gauchat J-F, Lécart S, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172(9):5154–5157. [DOI] [PubMed] [Google Scholar]
- 73.Thaunat O, Patey N, Caligiuri G, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185(1):717–728. [DOI] [PubMed] [Google Scholar]
- 74.Liarski VM, Kaverina N, Chang A, et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med. 2014;6(230):230ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Leur K, Clahsen-van Groningen MC, van den Bosch TPP, et al. Characterization of ectopic lymphoid structures in different types of acute renal allograft rejection. Clin Exp Immunol. 2018;192(2):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niu Q, Mendoza Rojas A, Dieterich M, et al. Immunosuppression has long-lasting effects on circulating follicular regulatory T cells in kidney transplant recipients. Front Immunol. 2020;11:1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wallin EF. T follicular regulatory cells and antibody responses in transplantation. Transplantation. 2018;102(10):1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W, Bai J, Huang H, et al. Low proportion of follicular regulatory T cell in renal transplant patients with chronic antibody-mediated rejection. Sci Rep. 2017;7(1):1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen C-C, Koenig A, Saison C, et al. CD4+ T cell help is mandatory for naive and memory donor-specific antibody responses: impact of therapeutic immunosuppression. Front Immunol. 2018;9:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wing JB, Lim EL, Sakaguchi S. Control of foreign Ag-specific Ab responses by Treg and Tfr. Immunol Rev. 2020;296(1):104–119. [DOI] [PubMed] [Google Scholar]
- 81.Lee F, Luevano M, Veys P, et al. The effects of CAMPATH-1H on cell viability do not correlate to the CD52 density on the cell surface. PloS One. 2014;9(7):e103254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todeschini M, Cortinovis M, Perico N, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol. 2013;191(5):2818–2828. [DOI] [PubMed] [Google Scholar]
- 83.Noureldeen T, Albekioni Z, Machado L, et al. Alemtuzumab induction and antibody-mediated rejection in kidney transplantation. Transplant Proc. 2014;46(10):3405–3407. [DOI] [PubMed] [Google Scholar]
- 84.Kwun J, Park J, Yi JS, et al. IL-21 Biased Alemtuzumab Induced Chronic Antibody-Mediated Rejection Is Reversed by LFA-1 Costimulation Blockade. Front Immunol. 2018;9:2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wiebe C, Rush DN, Nevins TE, et al. Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol. 2017;28(11):3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallin EF, Hill DL, Linterman MA, et al. The calcineurin inhibitor tacrolimus specifically suppresses human T follicular helper cells. Front Immunol. 2018;9:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang T, Xu T, Liu X, et al. Roles of BATF/JUN/IRF4 complex in tacrolimus mediated immunosuppression on Tfh cells in acute rejection after liver transplantation. J Cell Physiol. 2021;236(3):1776–1786. [DOI] [PubMed] [Google Scholar]
- 88.Kahan BD, Chang JY, Sehgal SN. Preclinical evaluation of a new potent immunosuppressive agent, rapamycin. Transplantation. 1991;52(2):185–191. [DOI] [PubMed] [Google Scholar]
- 89.Ye L, Lee J, Xu L, et al. mTOR promotes antiviral humoral immunity by differentially regulating CD4 helper T cell and b cell responses. J Virol. 2017;91(4):e01653–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kraaijeveld R, Li Y, Yan L, et al. Inhibition of T helper cell differentiation by tacrolimus or sirolimus results in reduced B-Cell activation: effects on T follicular helper cells. Transplant Proc. 2019;51(10):3463–3473. [DOI] [PubMed] [Google Scholar]
- 91.Sage PT, Ron-Harel N, Juneja VR, et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17(12):1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fantus D, Dai H, Ono Y, et al. Influence of the novel ATP-competitive dual mTORC1/2 Inhibitor AZD2014 on immune cell populations and heart allograft rejection. Transplantation. 2017;101(12):2830–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeng H, Cohen S, Guy C, et al. mTORC1 and mtorc2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 2016;45(3):540c554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J, Lin X, Pan Y, et al. Critical roles of mTOR Complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. eLife. 2016;5:e17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oh B, Yoon J, Farris A, et al. Rapamycin interferes with postdepletion regulatory T cell homeostasis and enhances DSA formation corrected by CTLA4-Ig. Am J Transplant. 2016;16(9):2612–2623. [DOI] [PubMed] [Google Scholar]
- 96.Karnell JL, Karnell FG, Stephens GL, et al. Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J Immunol. 2011;187(7):3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heidt S, Roelen DL, Eijsink C, et al. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol. 2010;159(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4(3):438–443. [DOI] [PubMed] [Google Scholar]
- 99.Hourmant M, Cesbron-Gautier A, Terasaki PI, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16(9):2804–2812. [DOI] [PubMed] [Google Scholar]
- 100.Van Laethem F, Baus E, Smyth LA, et al. Glucocorticoids attenuate T cell receptor signaling. J Exp Med. 2001;193(7):803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Löwenberg M, Tuynman J, Bilderbeek J, et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood. 2005;106(5):1703–1710. [DOI] [PubMed] [Google Scholar]
- 102.Akiyama M, Yasuoka H, Yamaoka K, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. 2016;18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bertrand D, Gatault P, Jauréguy M, et al. Protocol biopsies in patients with subclinical de novo donor-specific antibodies after kidney transplantation: a multicentric study. Transplantation. 2020;104(8):1726–1737. [DOI] [PubMed] [Google Scholar]
- 104.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770–781. [DOI] [PubMed] [Google Scholar]
- 105.Leibler C, Thiolat A, Hénique C, et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B Cells and impaired T follicular helper-B cell crosstalk. J Am Soc Nephrol. 2018;29(3):1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17(9):2381–2389. [DOI] [PubMed] [Google Scholar]
- 107.Ford ML. T cell cosignaling molecules in transplantation. Immunity. 2016;44(5):1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esensten JH, Helou YA, Chopra G, et al. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13(9):2280–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young JS, Chen J, Miller ML, et al. Delayed cytotoxic t lymphocyte-associated protein 4-immunoglobulin treatment reverses ongoing alloantibody responses and rescues allografts from acute rejection. Am J Transplant. 2016;16(8):2312–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leibler C, Thiolat A, Elsner RA, et al. Costimulatory blockade molecules and B-cell-mediated immune response: current knowledge and perspectives. Kidney Int. 2019;95(4):774–786. [DOI] [PubMed] [Google Scholar]
- 112.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333–343. [DOI] [PubMed] [Google Scholar]
- 113.Leibler C, Matignon M, Moktefi A, et al. Belatacept in renal transplant recipient with mild immunologic risk factor: a pilot prospective study (BELACOR). Am J Transplant. 2019;19(3):894–906. [DOI] [PubMed] [Google Scholar]
- 114.Poirier N, Azimzadeh AM, Zhang T, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med. 2010;2(17):17ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ville S, Poirier N, Branchereau J, et al. Anti-CD28 antibody and belatacept exert differential effects on mechanisms of renal allograft rejection. J Am Soc Nephrol. 2016;27(12):3577–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.La Muraglia GM, Zeng S, Crichton ES, et al. Superior inhibition of alloantibody responses with selective CD28 blockade is CTLA-4 dependent and T follicular helper cell specific. Am J Transplant. 2021;21(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pinelli DF, Ford ML. Novel insights into anti-CD40/CD154 immunotherapy in transplant tolerance. Immunotherapy. 2015;7(4):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawai T, Andrews D, Colvin RB, et al. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. [DOI] [PubMed] [Google Scholar]
- 119.Okimura K, Maeta K, Kobayashi N, et al. Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects. Am J Transplant. 2014;14(6):1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Watanabe M, Yamashita K, Suzuki T, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13(8):1976–1988. [DOI] [PubMed] [Google Scholar]
- 121.Harland RC, Klintmalm G, Jensik S, et al. Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am J Transplant 2020;20(1):159–171. [DOI] [PubMed] [Google Scholar]
- 122.Vincenti F, Klintmalm G, Yang H, et al. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation. Am J Transplant. 2020;20(1):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meli AP, Fontés G, Avery DT, et al. The integrin LFA-1 controls T follicular helper cell generation and maintenance. Immunity. 2016;45(4):831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nicolls MR, Gill RG. LFA-1 (CD11a) as a therapeutic target. Am J Transplant. 2006;6(1):27–36. [DOI] [PubMed] [Google Scholar]
- 125.Setoguchi K, Schenk AD, Ishii D, et al. LFA-1 antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am J Transplant. 2011;11(5):923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Badell IR, Russell MC, Thompson PW, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120(12):4520–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poston RS, Robbins RC, Chan B, et al. Effects of humanized monoclonal antibody to rhesus CD11a in rhesus monkey cardiac allograft recipients. Transplantation. 2000;69(10):2005–2013. [DOI] [PubMed] [Google Scholar]
- 128.Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant. 2010;10(8):1870–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Turgeon NA, Avila JG, Cano JA, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010;10(9):2082–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vincenti F, Mendez R, Pescovitz M, et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007;7(7):1770–1777. [DOI] [PubMed] [Google Scholar]
- 131.Oberholzer J, Kinzer K, Fiorina P. The anti-LFA-1 trial in islet transplantation. Am J Transplant. 2010;10(8):1725–1726. [DOI] [PubMed] [Google Scholar]
- 132.Lo DJ, Anderson DJ, Song M, et al. A pilot trial targeting the ICOS-ICOS-L pathway in nonhuman primate kidney transplantation. Am J Transplant. 2015;15(4):984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Neill NA, Zhang T, Braileanu G, et al. Pilot study of delayed ICOS/ICOS-L blockade with αCD40 to modulate pathogenic alloimmunity in a primate cardiac allograft model. Transplant Direct. 2018;4(2):e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nabeyama K, Yasunami Y, Toyofuku A, et al. Beneficial effects of costimulatory blockade with anti-inducible costimulator antibody in conjunction with CTLA4Ig on prevention of islet xenograft rejection from rat to mouse. Transplantation. 2004;78(11):1590–1596. [DOI] [PubMed] [Google Scholar]
- 135.Guillonneau C, Aubry V, Renaudin K, et al. Inhibition of chronic rejection and development of tolerogenic T cells after ICOS-ICOSL and CD40-CD40L co-stimulation blockade. Transplantation. 2005;80(2):255–263. [PubMed] [Google Scholar]
- 136.Weinstein JS, Bertino SA, Hernandez SG, et al. B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen. J Immunol. 2014;192(7):3166–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ward-Kavanagh LK, Lin WW, Šedý JR, et al. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44(5):1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kinnear G, Wood KJ, Marshall D, et al. Anti-OX40 prevents effector T-cell accumulation and CD8+ T-cell mediated skin allograft rejection. Transplantation. 2010;90(12):1265–1271. [DOI] [PubMed] [Google Scholar]
- 139.Kinnear G, Wood KJ, Fallah-Arani F, et al. A diametric role for OX40 in the response of effector/memory CD4+ T cells and regulatory T cells to alloantigen. J Immunol. 2013;191(3):1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vu MD, Clarkson MR, Yagita H, et al. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176(3):1394–1401. [DOI] [PubMed] [Google Scholar]
- 141.Guttman-Yassky E, Pavel AB, Zhou L, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(2):482–493.e7. [DOI] [PubMed] [Google Scholar]
- 142.García P, De Heredia AB, Bellón T, et al. Signalling via CD70, a member of the TNF family, regulates T cell functions. J Leukoc Biol. 2004;76(1):263–270. [DOI] [PubMed] [Google Scholar]
- 143.Jacquot S, Kobata T, Iwata S, et al. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159(6):2652–2657. [PubMed] [Google Scholar]
- 144.Arens R, Nolte MA, Tesselaar K, et al. Signaling through CD70 regulates B cell activation and IgG production. J Immunol. 2004;173(6):3901–3908. [DOI] [PubMed] [Google Scholar]
- 145.Narimatsu A, Hattori T, Usui Y, et al. Blockade of costimulatory CD27/CD70 pathway promotes corneal allograft survival. Exp Eye Res. 2020;199(21):108190. [DOI] [PubMed] [Google Scholar]
- 146.Dai H, Chen J, Shao W, et al. Blockade of CD27/CD70 pathway to reduce the generation of memory T cells and markedly prolong the survival of heart allografts in presensitized mice. Transpl Immunol. 2011;24(4):195–202. [DOI] [PubMed] [Google Scholar]
- 147.Riether C, Pabst T, Höpner S, et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med. 2020;26:1459–1467. [DOI] [PubMed] [Google Scholar]
- 148.Good-Jacobson KL, Szumilas CG, Chen L, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shi J, Hou S, Fang Q, et al. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity. 2018;49(2):264–274.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao W, Demirci G, Strom TB, et al. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76(6):994–999. [DOI] [PubMed] [Google Scholar]
- 151.Dudler J, Li J, Pagnotta M, et al. Gene transfer of programmed death ligand-1.Ig prolongs cardiac allograft survival. Transplantation. 2006;82(12):1733–1737. [DOI] [PubMed] [Google Scholar]
- 152.Sage PT, Francisco LM, Carman CV, et al. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14(2):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma CS, Suryani S, Avery DT, et al. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87(8):590–600. [DOI] [PubMed] [Google Scholar]
- 154.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994;153(11):4948–4958. [PubMed] [Google Scholar]
- 155.Nutt SL, Hodgkin PD, Tarlinton DM, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–171. [DOI] [PubMed] [Google Scholar]
- 156.Chavele K-M, Merry E, Ehrenstein MR. Cutting edge: circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. J Immunol. 2015;194(6):2482–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim I, Wu G, Chai N, et al. Anti-interleukin 6 receptor antibodies attenuate antibody recall responses in a mouse model of allosensitization. Transplantation. 2014;98(12):1262–1270. [DOI] [PubMed] [Google Scholar]
- 158.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13(5):379–395. [DOI] [PubMed] [Google Scholar]
- 159.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177(8):5236–5247. [DOI] [PubMed] [Google Scholar]
- 160.de Leur K, Luk F, van den Bosch TPP, et al. The effects of an IL-21 receptor antagonist on the alloimmune response in a humanized mouse skin transplant model. Transplantation. 2019;103(10):2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petrelli A, Carvello M, Vergani A, et al. IL-21 is an antitolerogenic cytokine of the late-phase alloimmune response. Diabetes. 2011;60(12):3223–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Leur K, Dor FJMF, Dieterich M, et al. IL-21 receptor antagonist inhibits differentiation of B cells toward plasmablasts upon alloantigen stimulation. Front Immunol. 2017;8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ignatenko S, Skrumsager BK, Mouritzen U. Safety, PK, and PD of recombinant anti-interleukin-21 monoclonal antibody in a first-in-human trial. Int J Clin Pharmacol Ther. 2016;54(4):243–252. [DOI] [PubMed] [Google Scholar]
- 164.Hua F, Comer GM, Stockert L, et al. Anti-IL21 receptor monoclonal antibody (ATR-107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol. 2014;54(1):14–22. [DOI] [PubMed] [Google Scholar]
- 165.Seo YB, Im SJ, Namkoong H, et al. Crucial roles of interleukin-7 in the development of T follicular helper cells and in the induction of humoral immunity. J Virol. 2014;88(16):8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mai H-L, Boeffard F, Longis J, et al. IL-7 receptor blockade following T cell depletion promotes long-term allograft survival. J Clin Invest. 2014;124(4):1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mai HL, Nguyen TVH, Branchereau J, et al. Interleukin-7 receptor blockade by an anti-CD127 monoclonal antibody in nonhuman primate kidney transplantation. Am J Transplant. 2020;20(1):101–111. [DOI] [PubMed] [Google Scholar]
- 168.Herold KC, Bucktrout SL, Wang X, et al. Immunomodulatory activity of humanized anti-IL-7R monoclonal antibody RN168 in subjects with type 1 diabetes. JCI Insight. 2019;4(24):e126054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol. 2015;34:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. [DOI] [PubMed] [Google Scholar]
- 171.Sandborn WJ, Gasink C, Gao L-L, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16):1519–1528. [DOI] [PubMed] [Google Scholar]
- 172.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;382(1):1201–1214. [DOI] [PubMed] [Google Scholar]
- 173.Kwun J, Burghuber C, Manook M, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017;28(7):1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schroder PM, Schmitz R, Fitch ZW, et al. Preoperative Carfilzomib and Lulizumab based desensitization prolongs graft survival in a sensitized non-human primate model. Kidney Int. 2021;99(1):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ezekian B, Schroder PM, Mulvihill MS, et al. Pretransplant desensitization with costimulation blockade and proteasome inhibitor reduces DSA and delays antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients. J Am Soc Nephrol. 2019;30(12):2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burghuber CK, Manook M, Ezekian B, et al. Dual targeting: combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant. 2019;19(3):724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alishetti S, Farr M, Jennings D, et al. Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition. Am J Transplant. 2020;20(12):3620–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li YM, Li Y, Shi YY, et al. Impact of immunosuppressive drugs on circulating Tfh cells in kidney transplant recipients: A pilot study. Transpl Immunol. 2018;46:1–7. [DOI] [PubMed] [Google Scholar]
- 179.Chamberlain C, Colman PJ, Ranger AM, et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis. 2017;76(11):1837–1844. [DOI] [PubMed] [Google Scholar]
- 180.Vo AA, Choi J, Kim I, et al. A phase I/II trial of the interleukin-6 receptor-specific humanized monoclonal (tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation. 2015;99(11):2356–2363. [DOI] [PubMed] [Google Scholar]
- 181.Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–1550. [DOI] [PubMed] [Google Scholar]
- 182.Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–328. [DOI] [PubMed] [Google Scholar]
- 183.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]