Abstract
Orofacial pain is among the most common chronic pain conditions and can result from temporomandibular disorders (TMDs) of the temporomandibular joint (TMJ). Matrix metalloproteinases (MMPs) drive degeneration of TMJ tissues and likely mediate pain in TMJ disorders given their role in nociception. However, few studies have assessed MMPs in the TMJ innervated tissues nor in the context of pain. This study defined the extent of MMP-1, MMP-9, and MMP-2 in TMJ tissues from patients undergoing total joint replacement (TJR) or arthroplasty discectomy for painful TMJ disorders. Protein expression was probed by Western blot in TMJ disc and capsular ligaments taken during TJR (n=6) or discectomy (n=3) for osteoarthritis or internal derangement in an IRB-approved study. Pro- and active MMP-1, active MMP-9, and pro- and active MMP-2 are detectable. MMP-1 and MMP-9 correlate positively to each other (Kendall’s τ=0.63; p=0.01), strengthening the hypothesis that they are mechanistically related in regulatory cascades. Active MMP-1 and active MMP-9 correlate positively with self-reported pain scores (τ≥0.51; p≤0.04) suggesting their involvement in peripheral nociception. Overall, neither MMPs nor pain correlate with functional vertical opening of the jaw. MMP-1 varies with the observed stage of degeneration during surgery (p=0.04). Neither overall MMPs nor pain correlate with the overall MRI scores, corroborating the longstanding, but confounding, clinical observation that pain and radiological evidence of joint damage are not always related. Clinical significance: These findings suggest that MMPs mediate pain in innervated soft tissues and may be targets for diagnosing disease stage and treatments in painful TMJ disorders.
Keywords: ligament, temporomandibular joint, matrix metalloproteinase, degeneration, osteoarthritis
Introduction
Chronic pain is a substantial public health challenge and is commonly associated with diseases of synovial joints.1 Temporomandibular disorders (TMDs) are a complex set of diseases related to alterations in the structure, function, and/or physiology of the masticatory system and are associated with a diffuse set of symptoms including pain.2 A subset of TMDs include disorders which arise from pathologies of the temporomandibular joint (TMJ).2 TMJ disorders are the second leading musculoskeletal condition resulting in pain and occur with degenerative diseases like internal derangement (ID) and osteoarthritis (OA).2–4 The TMJ forms the articulation between the mandibular glenoid fossa and the condyle and is separated into two synovial cavities by a fibrocartilaginous disc and encapsulated by a ligamentous capsule (Fig 1A). The mandibular branch of the trigeminal nerve supplies the sensory innervation of the TMJ, with nociceptive fibers innervating the capsular ligament, the peripheral articular disc, the synovial membrane, and the periosteum.5,6 Nociceptor innervation of the capsular ligament and disc varies regionally, with the anterior portion of the joint capsule being most densely innervated, followed by the posterior, lateral, and medial portions5. The disc has more nerves in the peripheral portion and no fibers in the central disc band5. Pathologic repeated loading that occurs in ID and OA activates the nociceptive fibers that innervate the TMJ capsular ligament and disc;5,7 as such, innervated tissues act as pain sensors in TMJ disorders.
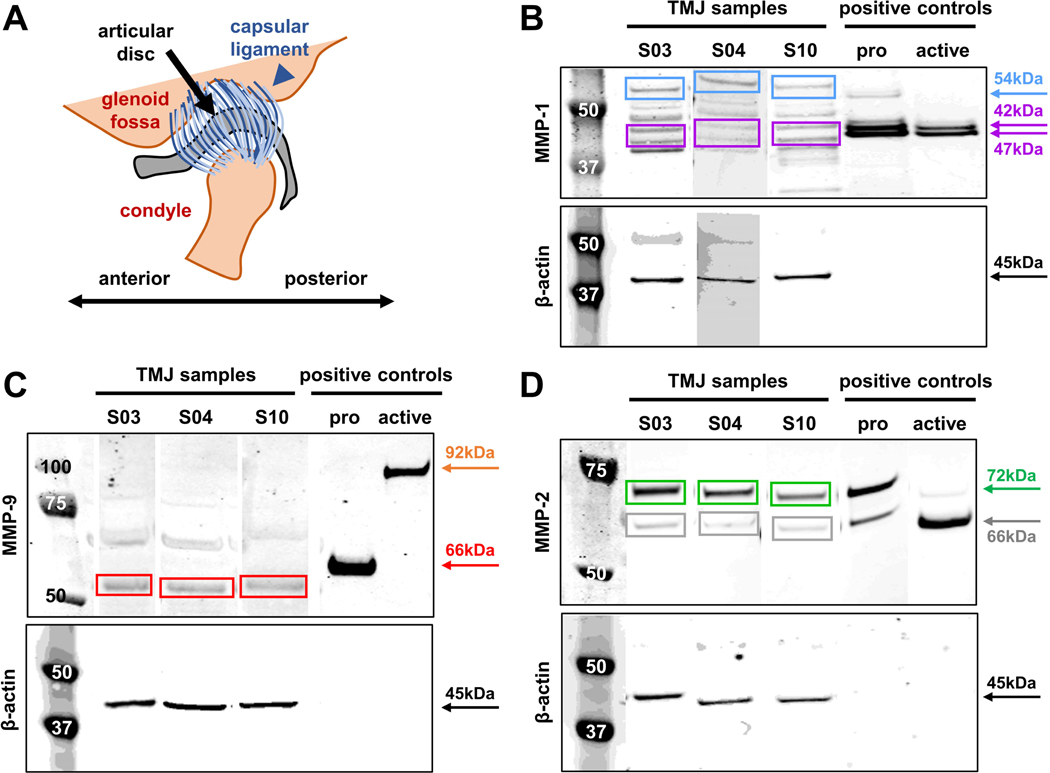
Figure 1.
(A) Schematic of the TMJ anatomy showing the capsular ligament that surrounds the joint space and the articular disc. Harvested ligament and disc samples were finely dissected and processed separately. Representative Western Blots are shown and labeled for (B) MMP-1, (C) MMP-9, or (D) MMP-2. For each blot (B-C), the left-most lane shows the molecular weight standards (in kDa) and the next three lanes show exemplary protein bands, grouped together for visualization and taken from different gels, from a TMJ disc sample (S03) and ligament sample (S10) taken from TJR surgeries and a ligament sample taken from an arthroplasty discectomy (S04). All brightness adjustments applied for visualization were applied equally for each gel image. Labeling for β-actin (45kDa) is shown under each MMP label and was used to normalize MMP labeling intensity for each sample. Also shown are the pro- and APMA-activated recombinant proteins that serve as positive controls. Sample band intensity is quantified using the positive controls of (B) pro-MMP-1 (54kDa), active MMP-1 (double bands at 42kDa & 47kDa; purple), (C) pro-MMP-9 (92kDa; orange), active MMP-9 (66kDa), (D) pro-MMP-2 (72kDa), and active MMP-2 (66kDa). (B) The intensity of both bands of active MMP-1 (42kDa & 47kDa) were summed. (C) Active MMP-9 was quantified at 60kDa based on differences in intracellular post-processing between the human and recombinant protein11, as confirmed by the manufacturer. All pro- and active MMPs were detectable except for pro-MMP-9 at 92kDa.
The resulting persistent pain and severe jaw dysfunction that occur with TMJ degeneration and end-stage disease can be treated surgically with open joint arthroplasty, discectomy, and/or total joint replacement (TJR).2 Although patients experience positive outcomes and long-term stability after surgical intervention,8,9 invasive surgery is only used after other treatments fail and can only correct a subset of TMJ abnormalities.2 Furthermore, pharmacologic treatments often fall short since they are adapted from other musculoskeletal and/or pain disorders rather than derived from evidence-based studies of individuals with painful TMJ disorders.2 Defining those molecular mechanisms related to severe TMJ degeneration can identify better therapeutic targets for earlier intervention and disease prediction.10
Matrix metalloproteinases (MMPs) have many roles in physiological and pathological processes including degenerative pathogenesis with painful TMJ disorders.11–13 MMPs are broadly categorized into subgroups based on their substrate specificity and domain structure;11 the collagenases (MMP-1, MMP-8, MMP-13) and gelatinases (MMP-2, MMP-9) increase in TMJs with ID and OA.14–22 Although there is overlap in the extracellular matrix (ECM) substrates across MMPs, collagenases and gelatinases preferentially degrade fibrillar (triple-helical) and denatured collagens, respectively.11 MMPs are classically described for their catabolism of ECM constituents and, thus, in association with tissue destruction in TMJ disorders. Yet, MMPs are also implicated in diseases of the nervous system,23 pain transmission,24 and in receptor-mediated and intercellular signaling pathways that regulate nociception.25,26 Accordingly, although MMPs may drive TMJ disorders pain and/or joint tissue degeneration, their role is unknown.
MMP-1 is a likely mediator of TMJ pain given its role in nociception and its elevated levels in TMJ disorders.11,12,14,15,25,26 MMP-1 is secreted as a catabolically inactive zymogen, pro-MMP-1, that is extracellularly activated through disruption of its cysteine-zinc interaction by proteases like plasmin or other MMPs.11,12 Since MMPs can only cleave ECM substrates after they are activated, they cannot participate in matrix remodeling in their pro-forms. However, in both active and pro- forms, many MMPs have non-ECM regulatory roles. This is true for MMP-1, which binds to receptors implicated in nociception, including β1-integrin and proteinase activated receptor-125,26 and non-ECM substrates like pro-inflammatory cytokines and neuropeptides involved in pain signaling.11,12 Moreover, MMP-1 activates gelatinases (pro-MMP-9, pro-MMP-2) that are heavily implicated in transmitting pain.11,23,24 MMP-1, MMP-9, and MMP-2 are detected together in TMJ synovial fluid (SF) with ID14 and OA15, supporting increases in gelatinases’ possible mechanistic involvement due to upstream MMP-1. Yet if, and how, MMP-1 relates to pain in TMJ disorders, via MMP-9, MMP-2, or other pathways, is unknown.
Although MMPs and their relationship to joint damage have been probed,14,16–20 results are mixed and depend on the damage metric used. In most cases, patients are compared by disease severity using Wilkes staging, which combines clinical, radiologic, and surgical findings on a gross anatomical level.27 Overall, those studies show that MMP-9 and MMP-2 generally increase in joint tissues with the presence of pathology compared to asymptomatic states,19,20 but that MMP levels do not always increase with disease severity. For instance, MMP-9 and MMP-2 are reported to be higher in the synovial fluid from TMJs with mild ID than with severe ID;14 yet, other studies report the opposite result, with increased gelatinase levels in more severe disease states.16,17 Unlike tissue damage metrics, very few studies have assessed MMPs in the context of pain scores despite patient-reported pain being the primary reason for seeking treatment. There is some evidence of elevated MMP-3 levels in TMJ SF with greater pain;18 but, if and how MMPs relate to pain and clinical degenerative signs in TMJ disorders is unknown.
This study defined the extent of MMP-1, MMP-9, and MMP-2 in the innervated capsular ligament and disc of TMJs from patients undergoing TJR or arthroplasty discectomy surgery for painful TMJ disorder. Despite those tissues being innervated and acting as pain sensors,5,6 most studies characterizing MMPs in TMJ disorders evaluate SF levels.14–18,20 Given the different tissue compositions and innervation patterns,5,28,29 and factors used in surgical decision making about recommending TJR or discectomy,2,7 MMPs were evaluated by tissue and surgery type. Severity of disease progression using Wilkes staging27 and dysfunction by quantification of maximal incisal opening (MIO)7 were also included in analyses. The pro- and active forms were probed since both forms are involved in joint destruction and/or nociception.11,23–26 Since active MMP-1 converts pro-MMP-9 and pro-MMP-2 to active protease states,11,12 we hypothesized that active MMP-1 correlates with active MMP-9 and MMP-2. We also hypothesized that MMPs are involved in ECM catabolism and pain transmission in painful TMJ disorders; so, we tested relationships separately between each MMP and each of clinical pain score, Wilkes stage,27 and MRI damage scores.30 Since there is still debate about whether structural degeneration and pain are related,31,32 we tested that relationship using MRI damage and pain scores for these subjects.
Methods
Patient population
This study was designed as a case-control study of evidence level 3. All procedures were performed with approval from the Institutional Review Board (protocol #828997) of the University of Pennsylvania. Written informed consent was granted from all subjects undergoing either TJR (n=6) or arthroplasty surgery for discectomy (ART) (n=3) (Table 1). Inclusion criteria were age above 18 years, surgery for OA and/or ID, and failing prior medical management of at least 6 weeks of physical therapy, medication, and use of an occlusal appliance.7 Individuals were excluded from the study if they underwent TMJ surgery for conditions other than ID or OA and/or had surgical site infection, history of facial congenital abnormalities, or prior acute facial fractures. Patient-reported pain scores were collected before surgery and quantified by a Likert scale from 0 (no pain) to 10 (most severe pain).33 Maximal incisal opening (MIO) was used to quantify functional range of motion and measured the vertical distance between the edges of the central incisors on the maxillary and mandibular surfaces.7 The TheraBite Measuring Scale (Atos Medical Company; New Berlin, WI), a range of motion device, was used to measure MIO by placing the scale’s notch on the central mandibular incisor and measuring the extent of maximum unassisted mouth opening by the patient in millimeters.8,34,35 A single surgeon (EJG co-author) performed all surgeries for the patient cohort and assigned a Wilkes stage with scores from 1 (least severe) to 5 (most severe).27 Wilkes stage classifications were assigned based on observations of the TMJ gross pathology made in the operating room and findings on imaging available for each patient; all Wilkes stage assessment was performed independent from and before any blinded tissue assays were performed. TMJ tissue samples were extracted at the time of surgery; capsular ligament tissue was harvested from all anatomical regions that were available, and the entirety of the disc was extracted (Fig. 1). Ligament and/or disc tissue was immediately finely dissected separately based on tissue type and stored at −80ºC until protein extraction.
Table 1:
Summary of subject clinical & imaging data
| Subject ID | Age (yrs) | Sex | Surgery type | Tissue type | Wilkes stage | Pain score | MIO (mm) | MRI score total | MRI score osseous |
|---|---|---|---|---|---|---|---|---|---|
| S01 | 53 | M | TJR | disc | 5 | 10 | 20 | 2** | 2 |
|
| |||||||||
| S02 | 52 | F | ART | ligament | 4 | 9 | 48 | ||
|
| |||||||||
| S03 | 61 | F | TJR | disc | 5 | 6 | 40 | 4** | 2 |
|
| |||||||||
| S04 | 39 | F | ART | disc ligament | 5 | 8 | 26 | ||
|
| |||||||||
| S06 | 33 | F | TJR | ligament | 5 | 10 | 28 | 3 | 3 |
|
| |||||||||
| S09 | 29 | F | ART | ligament disc | 3 | 6 | 28 | 0^^ | 0 |
|
| |||||||||
| S10 | 66 | F | TJR | ligament | 5 | 6 | 35 | ||
|
| |||||||||
| S12 | 44 | F | TJR | disc | 5 | 8 | 33 | ||
|
| |||||||||
| S13 | 29 | F | TJR | ligament disc | 5 | 9 | 44 | 3^^ | 3 |
M, male; F, female; TJR, total joint replacement; ART, arthroplasty; MIO, maximal incisal opening.
Shading indicates no MRI report available.
All MRI reports contain evaluation of bone;
indicates MRI reports with comments on inflammation.
MR score for these subjects are correlated to MMP levels for ligament & disc samples, separately
Tissue processing, MMP Western Blot & analyses
Tissue (n=12 from 9 subjects; Table 1) was finely chopped, homogenized in T-PER protein extraction buffer with 1X Halt protease inhibitor cocktail (Thermo Fisher; Waltham, MA), and centrifuged to isolate supernatant for 5min at 10,000g. A BCA kit (Thermo Fisher) quantified protein. Human recombinant forms of MMP-1 (Anaspec; Fremont, CA), MMP-9, and MMP-2 (both R&D Systems; Minneapolis, MN) were fully activated by incubation with 1mM 4-Aminophenylmercuric acetate (APMA) at 37ºC for 3hr, 5hr, and 2hr, respectively. Pro-MMP-1 (30ng/μL), APMA-activated MMP-1 (30ng/μL), pro-MMP-9 (50ng/μL), APMA-activated MMP-9 (400ng/μL), pro-MMP-2 (20ng/μL), and APMA-activated MMP-2 (50ng/μl) were loaded as positive controls (15mg/mL) and underwent SDS gel electrophoresis at 150V for 80mins. Protein was transferred to a PVDF membrane using the iBlot2 system (Thermo Fisher), blocked for 1hr in Intercept blocking buffer (Li-Cor; Lincoln, NE), and triple-washed in TBS-T for 5min each. Separate membranes were incubated in either a primary antibody for MMP-1 (4μg/mL), MMP-9 (4μg/mL), or MMP-2 (1μg/mL) (all from R&D Systems) overnight at 4ᵒC. The next day membranes underwent several TSB-T washes and were incubated in secondary antibody (1:10,000; Li-Cor) for 2hr at room temperature. After additional TSB-T washes, membranes were imaged using an Odyssey imaging system (Li-Cor). To account for any variation in sample loading, membranes were stripped in stripping buffer (Li-Cor), labeled with β-actin (1:1,000; Cell Signaling; Danvers, MT) using the same protocol, and imaged again. Protein expression was quantified using the pixel intensity of bands matching the positive controls using Image Studio Lite (VR5.2; Li-Cor) and normalized to β-actin intensity for each sample (Fig. 1).
Scoring of MRI reports
MRI reports were available for some patients (n=5 subjects) and rated using the EuroTMjoint research network progressive score30 (Table 1). That scale separately assesses inflammation and osseous deformity, with each domain evaluated from 0 (normal) to 4 (severe pathology).30 Although bone abnormalities were evaluated in all reports, only two contained comments on inflammation (Table 1); the absence of comments about inflammation was taken as normal (grade 0). For each subject with an MRI report, scores were tallied for each domain separately, as well as summed for a total MRI score (scale of 0–8).
Statistical analyses
All statistical analyses were performed with α=0.05 using JMP-Prov14 (SAS Institute Inc.; Cary, NC). Since a Shapiro-Wilk test revealed non-normal distribution for MMP protein data, they were treated as non-normal continuous numeric variables and compared using non-parametric statistics. Both pain and MRI scores were treated as ordinal numeric variables. MIO was treated as a continuous numeric variable. A Kendall’s τ correlation tested significance of relationships between active MMP-1 and each of active MMP-9 and active MMP-2, between each MMP and pain score, and between each MMP and MIO. Correlations were performed for tissue samples from the entire patient population (Table 1). A separate set of correlations were also performed using only those tissue samples that were obtained from TJR surgeries (Table 1) in order to test the relationships between MMPs, and between MMPs and each of pain score and MIO, in a homogenous sample set coming from a single surgery type (TJR) and Wilkes stage classification (Wilkes stage of 5) (Table 1). A non-parametric Wilcoxon signed rank test tested differences in MMPs between disc and ligament tissues; the same analysis compared surgery type (TJR, ART). A Wilcoxon signed rank test assessed MMP differences between joints with most severe Wilkes rating (stage 5) and all other scores. Separate Kendall’s correlations tested relationships between each MMP and MRI scores, between pain and MIO, and between pain and MRI scores.
Results
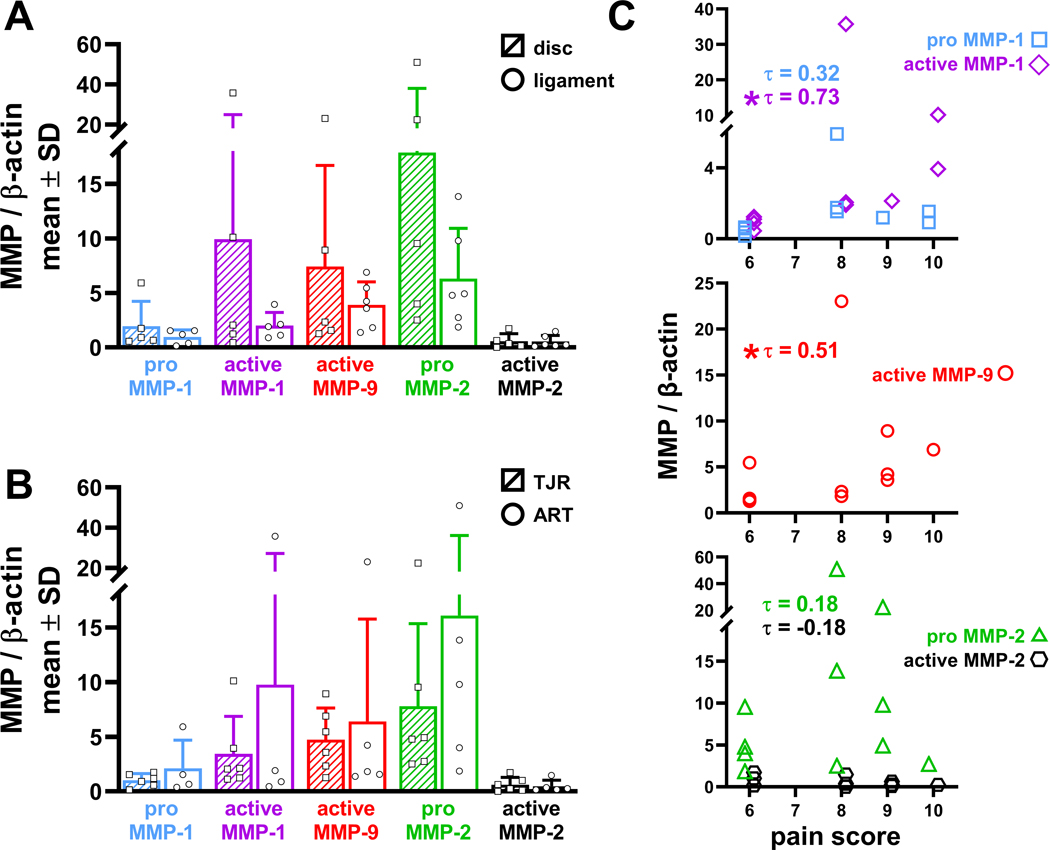
Nearly all joints (from subjects age 45.1±13.7years) have the most severe Wilkes stage (5), with only 2 subjects that underwent arthroplasty discectomy surgeries exhibiting lower stages (Table 1). All patients report pain scores in the top half of the Likert scale and have a MIO of 33.55±9.11mm before surgery (Table 1). MMPs are detected in nearly all samples, with MMP-1 detected in 10 samples and MMP-9 and MMP-2 in 11 samples. Although both pro- and active forms of MMP-1 and MMP-2 are detected across samples, only active MMP-9 is evident (Fig. 1). Expression is greatest for active MMP-1 (5.96±10.84), active MMP-9 (5.49±6.32), and pro-MMP-2 (11.57±14.43), although each exhibit substantial variation (Fig. 2). In contrast, pro-MMP-1 (1.46±1.65) and active MMP-2 (0.55±0.58) are more tightly clustered across samples (Fig. 2). Consistently, the outlier sample with the highest expression levels of each of pro- and active MMP-1, active MMP-9, and pro-MMP-2 is from the same patient sample (S04; Table 1) (Fig. 2).
Figure 2.
Relative MMP expression for each tissue sample. The pro- and active forms of MMP-1 and MMP-2, and the active form of MMP-9, are expressed in most tissue samples, with single data points shown for each sample along with the mean and standard deviation (SD). Active MMP-1 and active MMP-9 are significantly correlated (*p=0.01; τ=0.63; squares); but, there is no correlation between active MMP-1 and active MMP-2 (p=1.00; τ=0.00; circles). The τ correlation coefficient, ranging from −1 indicating a negative association to +1 indicating a positive association, between active MMP-1 and MMP-9 is 0.63 and indicates an increase in MMP-9 with greater expression of MMP-1. The τ correlation coefficient between active MMP-1 and MMP-2 is 0.00 and indicates no relationship between the two variables. The inset shows a close-up of the data points closest to the origin.
For the entire population of tissue samples, active MMP-1 and active MMP-9 are significantly positively correlated (p=0.01, τ=0.63) to each other (Fig. 2). Yet, active MMP-1 and active MMP-2 are not correlated (p=1.00, τ=0.00) (Fig. 2). In the TJR samples, active MMP-1 is not correlated with active MMP-9 (p=0.25, τ=0.41), nor with active MMP-2 (p=0.44, τ=−0.27). Although MMP levels appear higher in disc than ligament, with increases from 1.06–4.97 fold (Fig. 3A), those increases are not significant. Similarly, expression levels of each MMP are not different whether extracted during arthroplasty discectomy or a TJR procedure (Fig. 3B).
Figure 3.
MMP levels for data clustered by (A) tissue type (disc (hashed & squares); ligament (unfilled & circles) and by (B) surgery type (TJR (hashed & squares); ART (unfilled & circles) show no differences for any MMPs (Wilcoxon rank sum test). Bar plots depict mean±standard deviation (SD) of data with single data points for each sample superimposed. (C) Both active MMP-1 (*p<0.01; τ=0.73) and active MMP-9 (*p=0.04; τ=0.51) correlate with patient-reported pain. The plots for MMP-1 and MMP-2 against pain score show data points staggered on either side of the ordinal pain scores to enable visualizing each of the data points for the pro- and active forms.
Although MMP levels do not differ by tissue or surgery type, some MMPs are related to clinical data (Figs. 3 & 4). For the entire population of tissue samples, active MMP-1 is significantly correlated (p<0.01, τ=0.73) with pain score; the same relationship is also detected between pain and active MMP-9 (p=0.04, τ=0.51) (Fig. 3C). In the TJR samples, a significant correlation with pain exists only for active MMP-1 (p<0.01, τ=0.95). Despite positive correlations with pain (Fig. 3C), no relationships are detected between any of the MMPs with MIO (Fig. 4A), using all tissue samples (p≥0.43) or just the TJR samples (p≥0.05). Further, MIO and pain do not correlate (p=0.58, τ=−0.15).
Figure 4.
(A) Scatter plot of MMP level versus the maximal incisal opening. No correlations are detected between any of the MMPs with MIO (p≥0.43). (B) MMP levels for samples from joints with a most severely degenerated Wilkes stage of 5 (hashed bars; squares) and those with a Wilkes stage less than 5 (unfilled bars; circles). Tissues from joints with a Wilkes stage 5 score have greater expression of active MMP-1 than tissues from less degenerated joints (*p=0.04; Wilcoxon rank sum test). Bar plots depict mean±standard deviation (SD) of data with single data points shown for each sample. (C) Exemplary T2-weighted MR images (S06) in the closed position of the healthy, unoperated right TMJ and degenerated left TMJ prior a TJR. Substantial condylar degeneration and flattening is evident on the degenerated TMJ which was assigned a Wilkes stage of 5, an osseous MRI score of 3 (out of 4), and a total MRI score of 3 (out of 4); no inflammatory changes were noted. Scatter plots of MMP level versus the EuroTMjoint total score from subjects (n=5) with MR imaging. MMP levels for each sample with an accompanying MR report were included separately; so, MMP levels of both the ligament and disc samples for subjects S09 and S13 were included as unique data points in correlation analyses with MR scores. Data points are staggered around discrete MRI scores for data visualization. There are no correlations detected with total MRI score and any of the MMPs probed (p≥0.1502; τ≤0.54). (D) There is no relationship between total (circles) or osseous (squares) MRI score with pain.
MMP levels in samples with the most severe stage of Wilkes pathology (5) are higher than those from joints with less severe pathology (Fig. 4B). While this trend exists for all MMPs, it is only significant for active MMP-1 (p=0.04). Despite substantial degeneration evident on MR images and quantified by the EuroTMJoint scoring (Table 1; Fig. 4C), total MRI score is not correlated with any of the MMPs (Fig. 4C). MRI scores do not correlate with pain, regardless of whether inflammation is used in the scoring (Fig. 4D).
Discussion
MMP-1, MMP-9, and MMP-2 are detected in both the TMJ disc and capsular ligament, with MMP-1 and MMP-2 detected in both pro- and active forms (Figs. 1&2). Although immunolabeling has shown MMP-1 in the ligament and MMP-9 and MMP-2 in the disc of patients with TMJ disorders,19,21,22 those studies did not distinguish between the pro- and active forms, limiting their ability to determine the degree to which MMP levels are catabolically active. In fact, detecting more active forms of MMP-1 and MMP-9 than their catabolically inactive pro-forms (Fig. 2) suggests that a majority of those MMPs may be available to cleave triple helical collagen and gelatin, respectively.11 Since triple helical Type I collagen is the primary ECM component, with negligible gelatin, of both the disc and ligament,28,29 both tissues are susceptible to degradation by active MMP-1 (Fig. 3A). MMP-1 degradation of Type I collagen may affect the tissue’s macro- and microenvironment; tissue degradation can lead to whole joint destabilization and subsequent degeneration.36,37 Joint destabilization can further impair joint function and decrease range of motion in some patients (Table 1), although active MMP-1 does not appear to relate to MIO (Fig. 4A).36,37 Since tissue-level degradation by MMP-1 can produce joint instability, elevated active MMP-1 may cause the more severe degeneration evident in the Wilkes stage 5 joints (Fig. 4B). On a microscale, MMP-1-cleavage of Type I collagen could disrupt the matrix surrounding innervating afferents embedded in TMJ tissues,5 breaking afferent-collagen binding sites like integrin adhesions. Since axonal nociceptive signaling depends on β1-integrin binding sites,38 collagen degradation by MMP-1 could initiate and/or mediate pain signaling, and may explain the correlation between active MMP-1 and pain (Fig. 3C).
Although all MMPs are detected, MMP-9’s pro-form is not detected (Figs. 1&2) and only the active forms of MMP-1 and MMP-9 relate to pain (Fig. 3C). The positive correlation between active MMP-1 and MMP-9 further suggests their mechanistic relationship to one another in painful TMJ disorders broadly (Fig. 2). Yet, the fact that active MMP-1 and MMP-9 do not correlate in the tissue samples from TJR patients suggests that the two enzymes may not be mechanistically related in that subset of patients, and possibly with others with highly severe degenerative disease. Nonetheless, since active MMP-1 catalyzes pro-MMP-9 by cleaving its bait region,11 upstream regulation of MMP-9 by MMP-1 may be responsible for their correlation (Fig. 2). It is also possible that MMP-1 activates any of the pro-MMP-9 that is present in soft tissues to an extent that makes only the active form detectable and explaining why pro-MMP-9 is not detected (Fig. 1C). The lack of pro-MMP-9 could also be an artefact of this population’s late stage degeneration (Table 1), since pro-MMP-9 in the SF is higher in mildly degenerated TMJs than those with severe degeneration.14
The ECM-independent and nociceptive-related mechanisms by which active MMP-1 and MMP-9 directly bind to neuronal receptors may explain their relationships to pain (Fig. 3C).11,12,24–26,39 For example, MMP-1 stimulates intracellular calcium-dependent signaling via Gi protein-coupled receptors on neurons,26 which can stimulate neurotransmitter release,39 propagating neuronal excitability and sensitivity4 (Table 1). Co-localizing MMPs to neuronal receptors in TMJ disc and ligament would help determine regulatory pathways in these soft tissues. Furthermore, MMPs are quantified in whole tissue homogenates here. Since the sensory innervation of the capsular ligament and disc both have regional variability,5 findings using the whole tissue homogenates do not capture that variability since they contain all anatomical tissue regions regardless of their degree of innervation. As such, any variance in the extent of MMP expression with respect to the degree of tissue innervation is not possible. Localization by anatomical tissue region would provide insight into whether MMPs aggregate in nerve-rich regions.
The association between MMP-9, but not MMP-2, and pain (Fig. 3C) may reveal that inflammatory stimuli to afferents drives TMJ pain. This notion is supported by MMP-9 being rapidly upregulated in the dorsal root ganglion after nerve injury24 and trigeminal ganglion after an injection of an inflammatory agent in the TMJ.40 Moreover, the fact that the correlation between MMP-9 and pain is not maintained when using only tissues from TJR patients may implicate inflammatory mechanisms more strongly in those patients undergoing arthroplasty discectomy and implicate neuropathic mechanisms more strongly in syndromes requiring TJR. This notion is supported by evidence of a “bi-phasic” physiological response in animal models of joint degeneration whereby inflammatory cascades are necessary for early-onset behavioral sensitivity and neuropathic mechanisms dominate late-onset behavioral sensitivity those patients.41–43 No association between MMP-2 and pain score (Fig. 3C) contradicts our original hypothesis since MMP-2 maintains chronic pain24 and every subject has chronic pain based on their high pain scores (Table 1) and the inclusion criteria of our study. Although active MMP-2 levels are low (Fig. 2), a relationship between pro-MMP-2 and pain could exist with more samples (Fig. 3C). Indeed, a power analysis using sample size charts for Kendall’s τ correlations indicates that approximately 35 samples (which is 3 times the sample size used here; Table 1) are required to disprove the null hypothesis 44. It is also likely that MMP-9 and MMP-2 vary with disease progression since expression levels and functional roles of MMPs vary according to degenerative stage.14,16,17 Finally, MMP-2 has been shown to be undetectable in the TMJ disc from healthy patients without any evidence of TMJ disorders.22 So, our detection of MMP-2 in both its pro- and active forms (Figs. 1 & 2) suggests its elevation in TMJ disorders may have a role in driving pathology despite its lack of correlation to pain (Fig. 3C).
The increased active MMP-1 in joints at the most severe stages of degenerative disease progression (Fig. 4B) indicates the occurrence of pathological remodeling in joint tissues, since MMP-1 is undetectable during healthy homeostasis.12 Yet, the lack of correlation between active MMP-1 with either MIO or MRI damage score (Fig. 4) suggests that MMP-1 role in matrix remodeling may not contribute to TMJ functional deficits or to tissue-level evidence of tissue damage on MRI. The lack of association between damage scores and pain (Fig. 4D) is consistent with the discordance between imaging evidence of joint pathology and pain clinically.31,32 Yet, no association between MIO and pain is surprising given that greater pain scores are often associated with a decrease in functional outcomes.45 Nonetheless, six of the nine patients exhibit a functional deficiency in range of motion defined by having an MIO ≤40mm (Table 1).46 Together, these results (Figs. 3C&4) suggest that MMP-1’s role in pain may be independent of its mediation of the structure-function relationship of the TMJ.
Overall, none of the MMPs relate to total MRI scores (Fig. 4C), which may be due to our techniques and/or the small number of MR reports available for our patient population (Table 1). The correlations investigated here relate MMP levels in specific tissues to whole joint imaging metrics (Fig. 4C).30 However, since MMP-3 levels in SF and arthroscopically-detected joint synovitis are related,18 it is possible that the imaging features and tissue assays used here are too coarse to detect relationships. Analyses of these MMPs in the context of higher specificity imaging features would be helpful. Although the EuroTMjoint scale was originally developed for juvenile idiopathic arthritis, it was used here for its inclusion of inflammatory features (e.g. edema, effusion).30 Yet, the MRI reports are themselves limited by the documented practitioner impressions, particularly with regards to remarks about evidence of inflammation. Yet, this does not preclude the possibility that synovitis and/or effusion may contribute to the pain reported by patients. Additional studies analyzing more specific features of the MR images by multiple blinded scorers would more fully define relationships between imaging evidence of inflammation and/or degenerative changes with the magnitude of MMP protein in joint tissues.
Although this dataset allows for the analysis of MMP levels across multiple clinical metrics (Figs. 3 & 4), this study lacks a control group of tissue from healthy and non-symptomatic patients and/or from patients prior to disease progression. This limitation is due, in part, to the fact that capsular ligament and disc tissue can only be acquired during late-stage disease surgery for TMJ disorders, and not during less invasive and earlier interventions in disease progression. However, the findings that active MMP-1 and pro-MMP-1 are approximately 6- and 1.5-fold over β-actin (Fig. 2) align with the reported MMP-1 changes in innervated capsular ligament from degenerated spinal facet joints.47 That study also found that the increase in MMP-1 was significantly greater in capsular ligaments from degenerated facet joints than from non-degenerated facet joints,47 suggesting that MMP-1 levels in TMJ tissues may also be elevated over tissues from healthy TMJs if a control group was also probed. Quantifying MMPs in soft tissue taken during mandibular surgeries in the absence of painful TMDs (such as for fracture or tumor removal) or from post-mortem for controls would help confirm such speculation.19,22 Furthermore, MMPs were assayed in tissues from patients experiencing unmanageable pain for at least six weeks, biasing the findings toward higher pain scores (Table 1). The addition of a control group of individuals who report no, or only low levels, of pain from the masticatory region would determine whether the positive correlations between each of MMP-1 and MMP-9 and pain (Fig. 3C) exist for pain scores lower than 6.
The diagnosis of a TMJ disorder in this patient population was based on intra-articular lidocaine injections confirming the TMJ as the etiology for pain in patients that underwent TJR. For all patients, regardless of surgery, diagnoses of OA and/or ID were made using bimanual muscle and joint palpation in conjunction with MRI. Although those tools confirm the TMJ as the source for pain, the dual-axis Diagnostic Criteria for Temporomandibular Disorders (DC/TMD)2,46 was not used to categorize the type of TMD in this patient population. Given the retrospective nature of this study, the DC/TMD was not formally used. In addition, validity of the DC/TMD has been shown to be inconsistent for certain TMJ disorders, including degenerative joint diseases such as osteoarthritis,2,46,48 and concerns remain about the utility of the DC/TMD to differentiate between myofascial and arthritic pain.48,49 However, TMD categorization with the DC/TMD would better define certain aspects of the TMJ disorders diagnosed in this patient population, particularly components defined in Axis II of the criteria which assess psychosocial status and pain-related disability.2,46 Investigating whether Axis II components relate to MMP levels would also provide insight into whether MMP levels track with broader pain responses, psychological status, and/or other co-morbidities. For example, the very high MMP expression levels for the outlier sample (Fig. 2; S04) may be due, in part, to other medical conditions, including depression, anemia, and/or substance abuse12. Incorporating the DC/TMD would also allow for study outcomes to be better placed in the broader context of the complex set of TMDs.
Medications taken at the time, or in advance, of surgery could alter MMPs and may confound these findings, especially if they localize to the disc and/or ligament at the time of sample harvest. For example, all but one of the subjects in this study reported using NSAIDs, which can alter MMP levels.11 Moreover, S04, the sample in which MMP expression levels are the highest (Fig. 2), listed current medications including an opioid, an anti-convulsant, and an analgesic. Since all of those medications mediate nociceptive pathways and MMP expression and signaling50, it is possible that they confound the MMP responses here. Collectively, these results suggest that MMPs in innervated soft tissues may have a role in TMJ pain in addition to mediating ECM destruction. Specifically, MMP-1 and MMP-9 may be useful indicators of painful disease and may be helpful as diagnostic predictors of pain.2 Since probing MMPs in soft tissues requires invasive surgery, evaluating more accessible physiological samples (SF, serum, saliva) could not only help elucidate mechanistic pathways, but also aid in prognosis and treatment. Regardless, findings implicate MMPs in pain mediation and highlight them as useful clinical tools and/or targets for painful TMJ disorders.
Acknowledgements
This project was performed using funding from the NCCIH (AT010326–07) and the Catherine Sharpe Foundation. The study sponsors had no role in study design, collection, analysis, and interpretation of data, manuscript drafting, nor in the decision to submit this article for publications.
Footnotes
Conflict of Interest Disclosure
Dr. Granquist is a consultant for Zimmer Biomet but has no conflict. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this manuscript.
References
- 1.Institute of Medicine. 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC. [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine. 2020. Temporomandibular Disorders: Priorities for Research and Care. Washington, DC. [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A, et al. 2019. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160(1):19–27. [DOI] [PubMed] [Google Scholar]
- 4.Slade GD, Ohrbach R, Greenspan JD, et al. 2016. Clinical Review Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res 95(10):1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kido MA, Kiyoshima T, Kondo T, et al. 1993. Distribution of substance P and calcitonin gene-related peptide-like immunoreactive nerve fibers in the rat temporomandibular joint. J. Dent. Res 72(3):592–598. [DOI] [PubMed] [Google Scholar]
- 6.Sessle BJ. 2011. Peripheral and central mechanisms of orofacial inflammatory pain. In: International Review of Neurobiology. Academic Press Inc. p 179–206. [DOI] [PubMed] [Google Scholar]
- 7.Scrivani SJ, Keith DA, Kaban LB. 2008. Temporomandibular disorders. N. Engl. J. Med 359(25):2693–2705. [DOI] [PubMed] [Google Scholar]
- 8.Wolford LM, Mercuri LG, Schneiderman ED, et al. 2015. Twenty-Year Follow-up Study on a Patient-Fitted Temporomandibular Joint Prosthesis: The Techmedica/TMJ Concepts Device. J. Oral Maxillofac. Surg 73(5):952–960. [DOI] [PubMed] [Google Scholar]
- 9.Abramowicz S, Dolwick MF. 2010. 20-Year Follow-Up Study of Disc Repositioning Surgery for Temporomandibular Joint Internal Derangement. J. Oral Maxillofac. Surg 68(2):239–242. [DOI] [PubMed] [Google Scholar]
- 10.Kusiak JW, Veasley C, Maixner W, et al. 2018. The TMJ Patient-Led Round Table: A History and Summary of Work. 1–37. Available from: http://mdepinet.org/wp-content/uploads/TMJ-Patient-RoundTable-Briefing-Report_9_25_18.pdf.
- 11.Visse R, Nagase H. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res 92(8):827–839. [DOI] [PubMed] [Google Scholar]
- 12.Sbardella D, Fasciglione GF, Gioia M, et al. 2012. Human matrix metalloproteinases: An ubiquitarian class of enzymes involved in several pathological processes. Mol. Aspects Med 33:119–208. [DOI] [PubMed] [Google Scholar]
- 13.Kartha S, Zhou T, Granquist EJ, Winkelstein BA. 2016. Development of a Rat Model of Mechanically Induced Tunable Pain and Associated Temporomandibular Joint Responses. J. Oral Maxillofac. Surg 74(1):54.e1–e10. [DOI] [PubMed] [Google Scholar]
- 14.Srinivas R, Sorsa T, Tjäderhane L, et al. 2001. Matrix metalloproteinases in mild and severe temporomandibular joint internal derangement synovial fluid. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 91(5):517–525. [DOI] [PubMed] [Google Scholar]
- 15.Kanyama M, Kuboki T, Kojima S, et al. 2000. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J. Orofac. Pain 14(1):20–30. [PubMed] [Google Scholar]
- 16.Mizui T, Ishimaru JI, Miyamoto K, Kurita K. 2001. Matrix metalloproteinase-2 in synovial lavage fluid of patients with disorders of the temporomandibular joint. Br. J. Oral Maxillofac. Surg 39(4):310–314. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka A, Kumagai S, Kawashiri S, et al. 2001. Expression of matrix metalloproteinase-2 and −9 in synovial fluid of the temporomandibular joint accompanied by anterior disc displacement. J. Oral Pathol. Med 30(1):59–64. [DOI] [PubMed] [Google Scholar]
- 18.Fujita H, Morisugi T, Tanaka Y, et al. 2009. MMP-3 activation is a hallmark indicating an early change in TMJ disorders, and is related to nitration. Int. J. Oral Maxillofac. Surg 38(1):70–78. [DOI] [PubMed] [Google Scholar]
- 19.Almeida LE, Caporal K, Ambros V, et al. 2015. Immunohistochemical expression of matrix metalloprotease-2 and matrix metalloprotease-9 in the disks of patients with temporomandibular joint dysfunction. J. Oral Pathol. Med 44(1):75–79. [DOI] [PubMed] [Google Scholar]
- 20.Kubota T, Kubota E, Matsumoto A, et al. 1998. Identification of matrix metalloproteinases (MMPs) in synovial fluid from patients with temporomandibular disorder. Eur. J. Oral Sci 106(6):992–998. [DOI] [PubMed] [Google Scholar]
- 21.Gho WG, Choi Y, Park KH, Huh JK. 2018. Expression of collagenases (Matrix metalloproteinase-1, 8, 13) and tissue inhibitor of metalloproteinase-1 of retrodiscal tissue in temporomandibular joint disorder patients. J. Korean Assoc. Oral Maxillofac. Surg 44(3):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti C, Cornaglia I, Casasco A, et al. 1999. Immunolocalization of gelatinase-A (matrix metalloproteinase-2) in damaged human temporomandibular joint discs. Arch. Oral Biol 44:297–304. [DOI] [PubMed] [Google Scholar]
- 23.Yong VW, Power C, Forsyth P, Edwards DR. 2001. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci 2(7):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawasaki Y, Xu Z-Z, Wang X, et al. 2008. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med 14(3):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumin JA, Dickeson SK, Stricker TP, et al. 2001. Pro-collagenase-1 (Matrix Metalloproteinase-1) Binds the α2β1 Integrin upon Release from Keratinocytes Migrating on Type I Collagen. J. Biol. Chem 276(31):29368–29374. [DOI] [PubMed] [Google Scholar]
- 26.Conant K, Haughey N, Nath A, et al. 2002. Matrix metalloproteinase-1 activates a pertussis toxin-sensitive signaling pathway that stimulates the release of matrix metalloproteinase-9. J. Neurochem 82(4):885–893. [DOI] [PubMed] [Google Scholar]
- 27.Wilkes CH. 1989. Internal Derangements of the Temporomandibular Joint. Arch Otolaryngol Head Neck Surg 115(2):469–477. [DOI] [PubMed] [Google Scholar]
- 28.Milam SB, Klebe RJ, Triplett RG, Herbert D. 1991. Characterization of the extracellular matrix of the primate temporomandibular joint. J. Oral Maxillofac. Surg 49(4):381–391. [DOI] [PubMed] [Google Scholar]
- 29.Burgeson RE, Nimni ME. 1992. Collagen types. Molecular structure and tissue distribution. Clin. Orthop. Relat. Res (282):250–272. [PubMed] [Google Scholar]
- 30.Kellenberger CJ, Junhasavasdikul T, Tolend M, Doria AS. 2018. Temporomandibular joint atlas for detection and grading of juvenile idiopathic arthritis involvement by magnetic resonance imaging. Pediatr. Radiol 48(3):411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emshoff R, Brandlmaier I, Gerhard S, et al. 2003. Magnetic resonance imaging predictors of temporomandibular joint pain. J. Am. Dent. Assoc 134(6):705–714. [DOI] [PubMed] [Google Scholar]
- 32.Koh K-J, List T, Petersson A, Rohlin M. 2009. Relationship Between Clinical and Magnetic Resonance Imaging Diagnoses and Findings in Degenerative and Inflammatory Temporomandibular Joint Diseases: A Systematic Literature Review. J. Orofac. Pain 23(2):123–139. [PubMed] [Google Scholar]
- 33.Leonardi R, Perrotta RE, Almeida L-E, et al. 2016. Lubricin in synovial fluid of mild and severe temporomandibular joint internal derangements- bricin in synovial fluid of mild and severe temporomandibular joint internal derangements. Med Oral Patol Oral Cir Bucal 21(6):e793–e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumari S, Reddy D, Paul S. 2019. The normal range of maximal incisal opening in pediatric population and its association with physical variables. Ann. Afr. Med 18(3):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercuri LG, Edibam NR, Giobbie-Hurder A. 2007. Fourteen-Year Follow-Up of a Patient-Fitted Total Temporomandibular Joint Reconstruction System. J. Oral Maxillofac. Surg 65(6):1140–1148. [DOI] [PubMed] [Google Scholar]
- 36.Varady NH, Grodzinsky AJ. 2016. Osteoarthritis year in review 2015: mechanics. Osteoarthr. Cartil 24:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otterness IG, Bliven ML, Eskra JD, et al. 2000. Cartilage damage after intraarticular exposure to collagenase 3. Osteoarthr. Cartil 8(5):366–373. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Zhao E, Winkelstein BA. 2017. A Nociceptive Role for Integrin Signaling in Pain After Mechanical Injury to the Spinal Facet Capsular Ligament. Ann. Biomed. Eng 45(12):2813–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basbaum AI, Bautista DM, Scherrer G, Julius D. 2009. Cellular and Molecular Mechanisms of Pain. Cell 139(2):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nascimento GC, Rizzi E, Gerlach RF, Leite-Panissi CRA. 2013. Expression of MMP-2 and MMP-9 in the rat trigeminal ganglion during the development of temporomandibular joint inflammation. Brazilian J. Med. Biol. Res 46(11):956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong J-I, Park IY, Kim HA. 2020. Understanding the Molecular Mechanisms Underlying the Pathogenesis of Arthritis Pain Using Animal Models. Int. J. Mol. Sci 21(2):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J-S, Kroin JS, Buvanendran A, et al. 2011. Characterization of a new animal model for evaluation and treatment of back pain due to lumbar facet joint osteoarthritis. Arthritis Rheum. 63(10):2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller RE, Tran PB, Ishihara S, et al. 2020. Microarray analyses of the dorsal root ganglia support a role for innate neuro-immune pathways in persistent pain in experimental osteoarthritis. Osteoarthr. Cartil 28(5):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.May JO, Looney SW. 2020. Biometrics & Biostatistics Sample Size Charts for Spearman and Kendall Coefficients. J. Biom. Biostat 11(2). [Google Scholar]
- 45.Armijo-Olivo S, Pitance L, Singh V, et al. 2016. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther 96(1):9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffman E, Ohrbach R, Truelove E, et al. 2014. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group †. J Oral Facial Pain Headache 28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J-S, Ali MH, Wydra F, et al. 2015. Characterization of degenerative human facet joints and facet joint capsular tissues. Osteoarthr. Cartil 23(12):2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steenks M, Türp J, de Wijer A. 2018. Reliability and Validity of the Diagnostic Criteria for Temporomandibular Disorders Axis I in Clinical and Research Settings: A Critical Appraisal. J. Oral Facial Pain Headache 32(1):7–18. [DOI] [PubMed] [Google Scholar]
- 49.Sperry MM, Kartha S, Winkelstein BA, Granquist EJ. 2019. Experimental Methods to Inform Diagnostic Approaches for Painful TMJ Osteoarthritis. J. Dent. Res 98(4):388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji RR, Xu ZZ, Wang X, Lo EH. 2009. MMP regulation of neuropathic pain. Trends Pharmacol. Sci 30(7):336. [DOI] [PMC free article] [PubMed] [Google Scholar]