Abstract
Production of Physalis peruviana L. has gained prominence in Northeastern Brazil. However, salinity limits the crop development in the Brazilian semiarid. Thus, this research aimed to evaluate the application of Acadian® biostimulant as mitigant of the deleterious effects of salinity on growth and gas exchange of P. peruviana plants. The experiment was combining different electrical conductivity of irrigation water (0.50, 1.23, 3.00, 4.44, and 5.50 dS m−1) and biostimulant doses (0.00, 1.45, 5.00, 8.55, and 10.00 mL L−1). The main variables evaluated were plant height, stem diameter, number of leaves, root length, leaf area, specific leaf area, leaf area ratio, absolute and relative growth rate for plant height, and gas exchange. Experimental results showed that an increase in electrical conductivity of irrigation water had negatively affected the growth components and gas exchange in P. peruviana. Also, the application of seaweed-based biostimulant improves the photosynthetic capacity (43.3%), reduces transpiration rate (26.5%) and water loss by this process, further it attenuated the deleterious effects of salinity on specific leaf area, leaf area ratio, and stomatal conductance. To further elucidate the effectiveness of biostimulant application as a mitigant of salt stress, research aimed at the biochemical and enzyme activities of the plant's antioxidant system should be conducted to better understand this process.
Keywords: Acadian®, Horticulture, Photosynthesis, Plant physiology, Salt stress
Introduction
Cape gooseberry (Physalis peruviana L.) is an herbaceous plant from the Solanaceae family, native to the South American Andes regions, occurring in Brazil, Chile, Colombia, Ecuador, and Peru (Fischer and Melgarejo 2020). It is considered a high potential species for national horticulture, due to its nutritional value and economic viability of production (Rodrigues et al. 2021). It is largely consumed as fresh fruits, which are rich in bioactive compounds beneficial to human health, such as vitamin C, minerals, phenolic compounds, chlorophylls, and carotenoids (Mezzalira et al. 2017; Puente et al. 2021; Rodrigues et al. 2021).
However, in northeastern Brazil, mainly in the semiarid region, abiotic factors limit P. peruviana cultivation, which has a limit salinity of 3.0 dS m−1. Despite the species may be moderately tolerant to salinity, quality of irrigation water is essential for plants to express their productive potential, since high salt content in water becomes a limiting factor for plant growth and productivity (Monroy-Velandia and Coy-Barrera 2021).
Also, inadequate irrigation management increases the salt content in the soil and consequently in the different organs of the plant, which damages several metabolic and physiological processes (Lofti et al. 2018). Thus, search for chemical products capable of minimizing the adverse effects of salinity on plants is of prime importance. In this sense, application of biostimulants have been used in this regard (Elansary et al. 2016). The application of biostimulants promotes root growth and fast and uniform establishment of seedlings (Souza et al. 2020), which benefit plants under stress conditions. The beneficial effect of biostimulants has already been observed by some researchers, such as Popescu (2020) in the growth of cucumber (Cucumis sativus L.) and tomato (Solanum lycopersicum L.) plants under saline water, Souza et al. (2020) in the treatment of zucchini (Cucurbita pepo L.) seeds, and Souza Neta et al. (2018) in gherkin (Cucumis anguria L.) plants under salt stress.
In previous studies, Acadian® is an Ascophyllum nodosum seaweed-based biostimulant rich in carbohydrates and macro and micronutrients that induces the synthesis of phytohormones (auxins, gibberellins, cytokines, and abscisic acid) on plants (Silva et al. 2016). These growth regulators in the biostimulant induces defense gene signaling and hormonal homeostasis, thereby increasing the plant tolerance to salinity (Hadia et al. 2020). Thus, this study aimed to evaluate the the application of Acadian® biostimulant as a mitigant of the deleterious effects of salinity on growth and gas exchange of P. peruviana plants.
Material and methods
Site description and experimental design
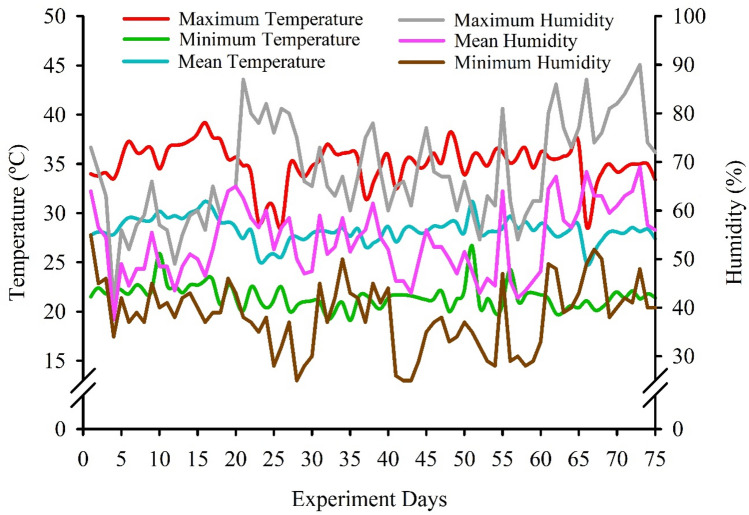
The experiment was carried out from January to April 2019 under greenhouse conditions (Fig. 1) at the Department of Crop and Environmental Sciences, Center of Agrarian Sciences, Federal University of Paraíba (UFPB), Areia city, Paraíba State, Brazil. The experimental site is located at the geographical coordinates 6°58′1.45'' S, 35°42′48.90'' W, and 575 m above the sea level.
Fig. 1.
Temperature and humidity in the greenhouse during the experiment
Treatments were arranged in a randomized block design at the incomplete factorial scheme, combining different electrical conductivity of irrigation water (ECw) and seaweed-based biostimulant doses (BD). The minimum (−α) and maximum (α) values ranged respectively from 0.5 to 5.5 dS m−1 and 0.0 to 1.0%, totaling nine treatments generated through the center composite design (Mateus 2001), with four replicates and two plants per plot (Table 1).
Table 1.
Treatments generated through the center composite design (CCD) matrix
| Treatments | ECw (dS m−1) | BD (mL L−1) |
|---|---|---|
| T1 | 1.23 | 1.45 |
| T2 | 1.23 | 8.55 |
| T3 | 4.77 | 1.45 |
| T4 | 4.77 | 8.55 |
| T5 | 0.50 | 5.00 |
| T6 | 5.50 | 5.00 |
| T7 | 3.00 | 10.00 |
| T8 | 3.00 | 0.00 |
| T9 | 3.00 | 5.00 |
Conducting the experiment
Cape gooseberry seeds were obtained from mother plants produced under greenhouse conditions at Federal University of Campina Grande, Pombal city, Paraíba State, Brazil. Four seeds collected from the same plant were sown per polyethylene pot. Then, a thinning was performed leaving one seedling, the most vigorous one. Pots of 1.2 dm−3 capacity were filled with substrate formulated with Dystrophic Regolithic Neosol (Embrapa 2018), cattle manure, and washed sand at the 3:1:1 ratio. The substrate was evaluated for physicochemical attributes (fertility and salinity of saturated extract shown in Table 2) following the methodologies of Embrapa (2017) and Richards (1954).
Table 2.
Physicochemical characterization of the substrates used in the experiment
| Physical | Value | Fertility | Value | Salinity | Value |
|---|---|---|---|---|---|
| Sand (g kg−1) | 874 | pH in water (1: 2.5) | 8.1 | pH | 7.40 |
| Silt (g kg−1) | 91 | P (mg dm−3) | 65.16 | CEe (dS m−1) | 2.00 |
| Clay (g kg−1) | 35 | K+ (mg dm−3) | 423.97 | SO4−2 (mmolc L−1) | 2.17 |
| Texture | Sandy | Na+ (cmolc dm−3) | 0.24 | Ca+2 (mmolc L−1) | 6.50 |
| Al+3 (cmolc dm−3) | 0.00 | Mg+2 (mmolc L−1) | 17.50 | ||
| H+ + Al+3 (cmolc dm−3) | 0.99 | Na+ (mmolc L−1) | 3.67 | ||
| Ca+2 (cmolc dm−3) | 2.88 | K+ (mmolc L−1) | 7.67 | ||
| Mg+2 (cmolc dm−3) | 0.96 | CO3−2 (mmolc L−1) | 0.00 | ||
| SB (cmolc dm−3) | 5.17 | HCO3−2 (mmolc L−1) | 17.50 | ||
| CEC (cmolc dm−3) | 6.16 | Cl− (mmolc L−1) | 10.00 | ||
| SOM (cmolc dm−3) | 15.00 | SAR (mmolc L−1) | 1.06 | ||
| ESP (%) | 0.30 | ||||
| Classification | Normal |
P, K, Na: extracted by Mehlich 1; SB: Sum of exchangeable bases; H + Al: extracted by calcium acetate extractor 0.5 M pH 7.0; CEC: Cation exchange capacity; Al, Ca, Mg: extracted by KCl 1 M; SOM: Soil organic matter content by the Walkley–Black method; C.E.: Electrical conductivity at 25ºC; SAR: Sodium adsorption ratio; ESP: Exchangeable sodium percentage
The irrigation waters with different electrical conductivities were prepared by adding sodium chloride (NaCl) at the required proportions to the potable water from the UFPB supply system, which had 0.5 dS m−1 ECw. EC was measured by using a portable conductivity meter (CD-860 model, Instrutherm®). Irrigation was performed daily, and saline water was applied 15 days after sowing (DAS), maintaining the soil at field capacity (FC) to ensure seed emergence and plant development. The water blade applied was determined by the drainage lysimetric method, as proposed by Bernardo et al. (2006), using three separate replicates per treatment.
The biostimulant, an A. nodosum seaweed-based extract (Acadian®, Agritech, Canada), was applied 20 DAS. The extract had the following characteristics: 8.12, 6.82, 12.00, 1.60, 2.03, and 8.16 g kg−1 N, P, K, Ca, Mg, and S, respectively; 5.74, 13.60, 11.5, 0.04, 24.40, and 20,000 mg kg−1 B, Cu, Fe, Mn, Zn, and Na respectively; potassium hydroxide, with 61.48 g L−1 water-soluble K2O; 69.60 g L−1 total organic carbon; and 1.16 g dm−3 density (Arrais et al., 2016). The seaweed extract was diluted with water to concentrations of 1.45, 5.00, 8.55, and 10.00 mL L−1, then 100 mL per plant of each solution was sprayed in leaves in the late afternoon. Six applications were performed weekly.
Growth and gas exchange analyses
Also, at 75 DAS the effect of treatments on plant growth was evaluated. Plant height (PH) and root length (RL) were measured by using a millimetric ruler; stem diameter (SD) by using a digital caliper; and the number of leaves (NL) by counting the fully expanded leaves. Moreover, leaf area (LA), specific leaf area (SLA), and leaf area ratio (LAR) were calculated according to Benincasa (2003) by the following equations, respectively: LA = (L × W) × f, where L is the length of each leaf, W is the width of each leaf, and f is the correction factor (0.66) according to Piesanti et al. (2018); SLA = LA/LDW, where LDW is the leaf dry weight; and LAR = LA/SDW, where SDW is the shoot dry weight.
From 15 to 75 DAS, the absolute and relative growth rates for plant height (AGRPH and RGRPH, respectively) were determined adapting the methodology proposed by Benincasa (2003), as described in the following equations: AGRPH = (PH2−PH1)/(t2−t1), where PH1 is the plant height at 15 DAS, PH2 is the plant height at 75 DAS, t1 is the number of days at the first evaluation (15 DAS), and t2 is the number of days at the second evaluation (75 DAS); and RGRPH = (InPH2−InPH1)/(t2−t1), where lnPH1 is the natural logarithm of plant height at 15 DAS, and lnPH2 is the natural logarithm of plant height at 75 DAS.
At 75 DAS, plant gas exchange was measured using a portable infrared gas analyzer—IRGA (LI-6400XT model, LI-COR®, Nebraska, USA) with 300 mL min−1 airflow and coupled-light source of 1200 µmol m−2 s−1. The following variables were measured: stomatal conductance (gs; mol H2O m−2 s−1), net assimilation rate of CO2 (A; μmol of CO2 m−2 s−1), transpiration rate (E; mmol H2O m−2 s−1), internal concentration of CO2 (Ci; μmol CO2 mol−1), water use efficiency (WUE and iWUE = A/E), and instantaneous carboxylation efficiency (Ecar = A/Ci). Analyses were performed at 9–10 a.m. in fully expanded leaves.
Statistical analyses
Data were submitted to analysis of variance by the F test and, when significant, regression analysis was performed. Statistical analysis was performed in R software version 2.13.1. (R Core Team 2016).
Results
According to the analysis of variance, analyzing the isolated factors, biostimulant doses (BD) significantly affected all the analyzed variables, except by stem diameter (SD). In turn, the electrical conductivity of irrigation water (ECw) significantly affected plant height (PH), leaf area (LA), number of leaves (NL), absolute growth rate for plant height (AGRPH), and root length (RL). However, a significant interaction between factors was observed only for specific leaf area (SLA) and leaf area ratio (LAR) (Table 3).
Table 3.
Mean square of the analysis of variance for growth components of Physalis peruviana cultivated under irrigation water with different electrical conductivity (ECw) and seaweed-based biostimulant doses (BD)
| Source of variation | DF | Mean squares | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PH | SD | LA | NL | SLA | LAR | AGRPH | RGRPH | RL | ||
| Block | 3 | 10.23ns | 0.06ns | 1236.6ns | 2.74ns | 261ns | 80.0ns | 0.005ns | 0.00001ns | 4.06ns |
| Treatments | 8 | 39.74** | 0.11ns | 2713.5** | 9.97** | 4401** | 878.3** | 0.027** | 0.00008** | 20.44** |
| BD (L) | 1 | 8.26** | 0.22ns | 50.49** | 3.38** | 25.36* | 18.59* | 0.214** | 0.01234** | 1.12ns |
| BD (Q) | 1 | 0.98ns | 0.28ns | 29.29* | 0.25ns | 23.35ns | 16.22* | 0.031ns | 0.00120ns | 2.22** |
| ECw (L) | 1 | 2.32* | 0.02ns | 37.54** | 1.88* | 11.82ns | 6.25ns | 0.072** | 0.00119ns | 0.28ns |
| ECw (Q) | 1 | 0.08ns | 0.95ns | 27.06** | 0.87ns | 15.77ns | 13.71ns | 0.005ns | 0.00156ns | 4.83** |
| BD (L) x ECw (L) | 1 | 0.19ns | 0.10ns | 3.05ns | 0.06ns | 9.72** | 4.24** | 0.003ns | 0.00011ns | 0.37ns |
| CV | 8.90 | 5.70 | 10.40 | 11.00 | 9.30 | 14.20 | 10.10 | 8.50 | 5.80 | |
PH: plant height; SD: stem diameter; LA: leaf area; NL: number of leaves; SLA: specific leaf area; LAR: leaf area ratio; AGRPH: absolute growth rate for plant height; RGRPH: relative growth rate for plant height; RL: root length; L: linear model; Q: quadratic model; DF: degrees of freedom; CV: coefficient of variation. *, **: significant at p < 0.05 and p < 0.01, respectively, by the F test; ns: non-significant
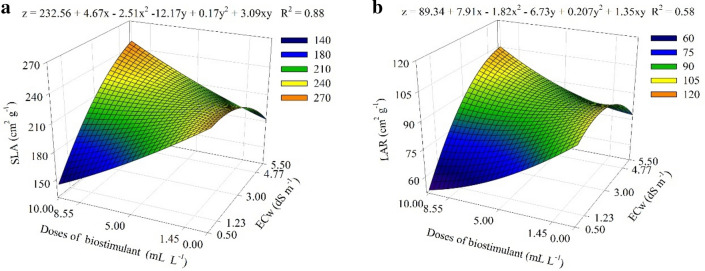
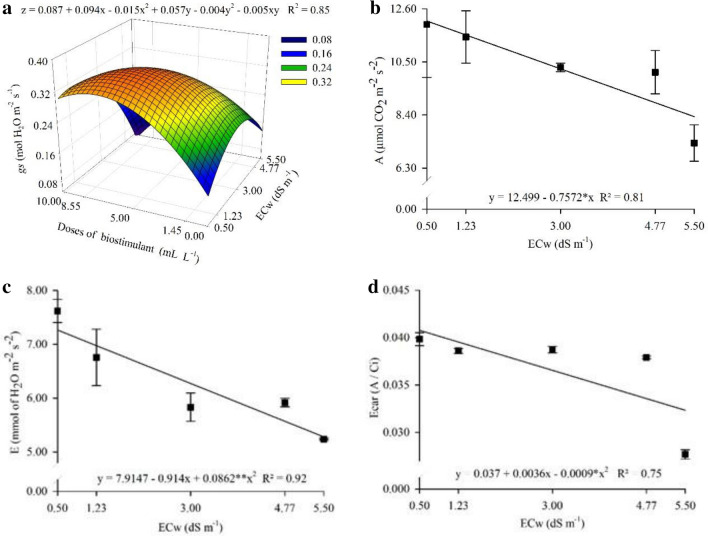
It was observed that the biostimulant applied up to 9.95 mL L−1 dose attenuated the deleterious effect of salinity by up to 5.48 dS m−1, in which a maximum specific leaf area of 247.3 cm2 g−1 was obtained (Fig. 2a). Likewise, a maximum leaf area ratio of 104.8 cm2 g−1 was obtained with the application of 9.93 mL L−1 biostimulant under 5.43 dS m−1 (Fig. 2b).
Fig. 2.
Specific leaf area a and leaf area ratio b of Physalis peruviana L. plants submitted to saline stress and seaweed-based biostimulant doses
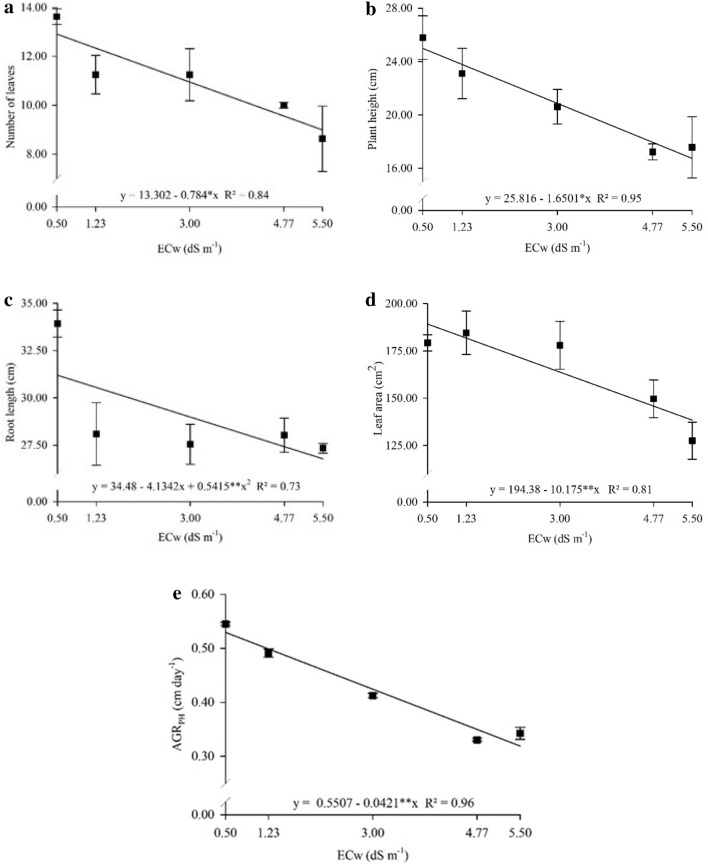
Salinity of irrigation water negatively influenced the NL and PH of P. peruviana plants (Fig. 2). The NL (Fig. 3a) and PH (Fig. 3b) decreased respectively by 30.7% and 32.9% in plants under 5.5 dS m−1 as compared to control (0.5 dS m−1). Root length decreased with increasing salinity up to 3.82 dS m−1, in which roots measured 26.6 cm, 18.2% smaller as compared to control (0.5 dS m−1) (Fig. 3c). Moreover, saline water irrigation at 5.5 dS m−1 reduced LA by 26.8% (Fig. 3d) and AGRPH by 39.6% (Fig. 3e) relative to control (Table 4).
Fig. 3.
Number of leaves a, plant height b, root length c, leaf area d, and absolute growth rate for plant height e of Physalis peruviana L. plants cultivated under irrigation water with different electrical conductivities
Table 4.
Mean square of the analysis of variance for gas exchange of Physalis peruviana L. cultivated under irrigation water with different electrical conductivity (ECw) and seaweed-based biostimulant doses (BD)
| Source of variation | DF | Mean squares | ||||||
|---|---|---|---|---|---|---|---|---|
| gs | Ci | E | A | WUE | iWUE | Ecar | ||
| Block | 3 | 0.016** | 3009.2** | 2.09* | 1.84ns | 0.31ns | 408.3ns | 0.00006ns |
| Treatments | 8 | 0.018** | 987.1ns | 4.10** | 18.96** | 0.20ns | 212.7ns | 0.00026** |
| BD (L) | 1 | 0.130** | 37.47** | 1.78** | 2.91** | 0.05ns | 8.02ns | 0.00182ns |
| BD (Q) | 1 | 0.042ns | 1.31ns | 0.70ns | 0.48ns | 0.22ns | 5.53ns | 0.00299ns |
| ECw (L) | 1 | 0.039ns | 15.80ns | 0.09ns | 0.51ns | 0.02ns | 5.63ns | 0.00153ns |
| ECw (Q) | 1 | 0.034ns | 11.95ns | 1.56** | 2.29* | 0.01ns | 1.82ns | 0.00644* |
| BD (L) x ECw (L) | 1 | 0.017* | 0.64ns | 0.19ns | 0.23ns | 0.08ns | 2.96ns | 0.00114ns |
| CV | 15.8 | 5.70 | 10.8 | 13 | 15.8 | 20.8 | 11.1 | |
gs: stomatal conductance (mol m−2 s−1); A: net CO2 assimilation rate (μmol CO2 m−2 s−1); E: transpiration (mmol H2O m−2 s−1); Ci: internal concentration of CO2 (μmol CO2 m−2 s−1); WUE: water use efficiency (A/E); Ecar: instantaneous carboxylation efficiency (A/Ci); L: linear model; Q: quadratic model; DF: degrees of freedom; CV: coefficient of variation. *, **: significant at p < 0.05 and p < 0.01, respectively, by the F test; ns: non-significant
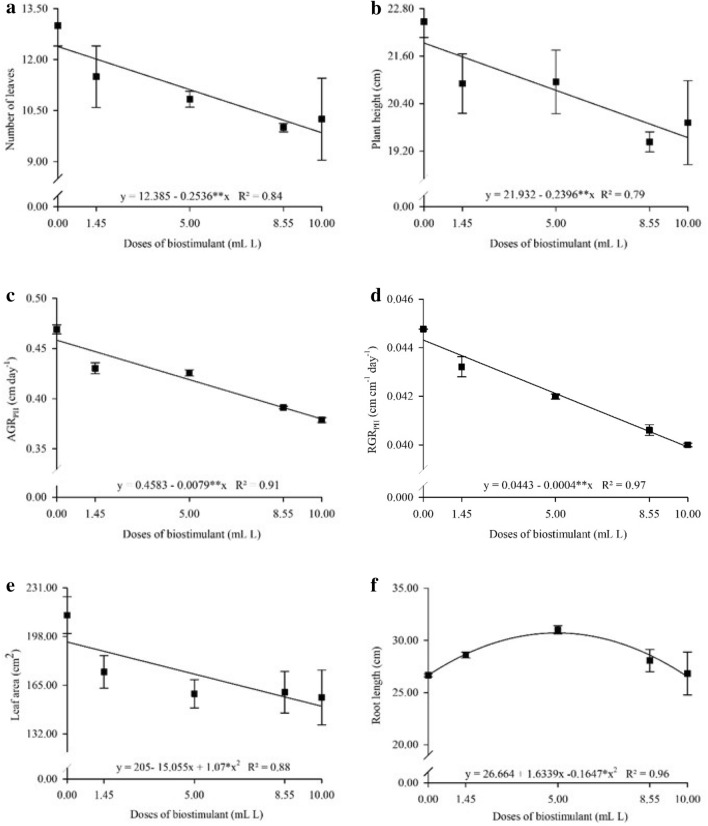
The seaweed-based biostimulant negatively influenced the NL, PH, AGRPH, and RGRPH of P. peruviana, with reductions of 20.9, 10.9, 17.3, and 9%, respectively (Fig. 4a–d). LA reduced by 26.5% as the biostimulant dose increased up to 7.04 mL L−1 (Fig. 4e). In contrast, RL increased by 15.4% with increasing biostimulant dose up to 4.96 mL L−1, in which roots measured 30.72 cm in length (Fig. 4f).
Fig. 4.
Number of leaves a, plant height b, root length c, leaf area d and absolute e and relative f growth rate for plant height in Physalis peruviana L. plants cultivated under seaweed-based biostimulant doses
Based on the analysis of variance for gas exchanges, saline water irrigation significantly affected transpiration rate (E), net assimilation rate of CO2 (A), and instantaneous carboxylation efficiency (ICE), while biostimulant doses affected E and A. Also, a significant interaction between the factors was observed for stomatal conductance (gs) (Table 3).
Acadian® biostimulant applied at 6.30 mL L−1 dose provided positive effects on gs in plants cultivated under up to 1.93 dS m−1, in which a maximum gs of 0.359 mol de H2O m−2 s−1 was observed (Fig. 5a). In turn, A decreased by 31.3% per unitary increase in ECw, with values of 12.12 and 8.33 µmol de CO2 m−2 s−2 (Fig. 5b). Also, E and ICE decreased by 26.5% and 25%, respectively, per unitary increase in ECw (Figs. 5c–d).
Fig. 5.
Stomatal conductance a, net CO2 assimilation rate b, transpiration c, and instantaneous carboxylation efficiency d in Physalis peruviana L. plants cultivated under seaweed-based biostimulant doses
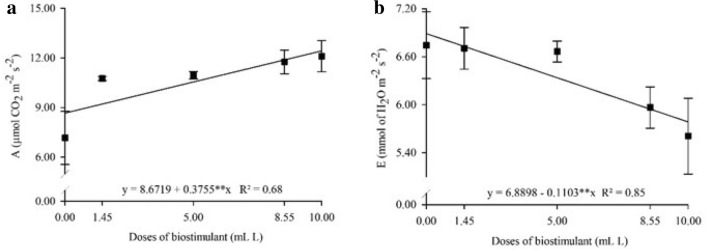
Biostimulant doses only affected A and E (Fig. 6). The application of 10.00 mL L−1 biostimulant increased A by 43.4% as compared to control (0.00 mL L−1), in which plants increased from 8.67 to 12.43 µmol CO2 m−2 s−2 the assimilation rate (Fig. 6a). In contrast, E decreased with increasing biostimulant dose. Plants transpired up to 16% less relative to control plants, with values of 6.88 and 5.78 mmol of H2O m−2 s−2 (Fig. 6b).
Fig. 6.
Net CO2 assimilation rate a and transpiration b in Physalis peruviana L. plants cultivated under seaweed-based biostimulant doses
Discussion
The beneficial effect of biostimulant for SLA and LAR may be due to these seaweed extracts, from natural or synthetic origin, induce plants to express their genetic potential under abiotic stress conditions, by promoting an effective hormonal and nutritional balance (Oliveira et al. 2016). Such results showed that bioactive compounds present in seaweed extracts were effective in improving plant performance under saline stress conditions (Battacharyya et al. 2015).
Such a decrease in NL may act as a plant adaptation mechanism for salt tolerance to minimize water loss by transpiration. Reduced LA is associated with reduced NL, which evidence the sensitivity of leaves to salinity. Also, reduced plant growth in height may be due to excessive uptake of toxic ions as well as the low capacity of the plant to perform osmotic adjustment (Nobre et al. 2013). Excessive salt concentration in the soil solution alters cellular metabolic activities, restricting cell wall elasticity and thus limiting cell elongation and, therefore, the vegetative growth of the plant (Taiz et al. 2017). Similar results were found in P. peruviana (Miranda et al. 2014) and watermelon (Citrullus lanatus (Thunb.)) plants (Silva Junior et al. 2020) under salt stress conditions.
Salt accumulation in the soil due to saline water irrigation results in increased sodium and chlorine uptake by plants. Accumulation of these ions inside the cell reduces root growth, for instance, due to inhibition of cotyledon reserve depletion (Marques et al. 2011), which explains the reduction in root length. Likewise, Oliveira et al. (2015) observed that roots of Jatropha plants grew 28% less under 5.0 dS m−1 ECw. However, the negative effect of biostimulant on plant growth can be attributed to excessive accumulation of toxic ions, such as Na+ since seaweed extracts contain 3 to 5% of this ion in composition (Garcia et al. 2014). The previous study also reported similar results in cashew (Anacardium occidentale L.) seedlings (Garcia et al. 2014). On the other hand, Oliveira et al. (2017) found that the application of biostimulants can improve root development by 56.5%, using a concentration of 10 mL in gherkin (Cucumis anguria L.) seedlings.
The positive effect of the biostimulant on stomatal conductance of saline-irrigated plants occurs because the biostimulant improves leaf water relations, thus maintaining cell turgidity and reducing stomatal closure, as reported by Xu and Leskovar (2015) in spinach (Spinacia oleracea L.). Kałużewicz et al. (2017) observed the same behavior in broccoli (Brassica oleracea var. Italica) under water stress. However, salinity reduced the net assimilation rate of CO2 due to excessive accumulation of salts, which damage the photosynthetic apparatus and thus change CO2 assimilation (Rouphael et al. 2012). In addition, a decrease in transpiration rate acts as a stress acclimatization mechanism in plants, because low transpiration results in low water loss.
In turn, a decrease in instantaneous carboxylation efficiency may be related to reduced net photosynthesis, indicating a low carbon fixation rate by Rubisco. As reported in the present study, the negative effects of saline water on plant gas exchange have been also reported by many authors, such as Azizian and Sepaskhah (2014) in corn (Zea mays L.), Yarami and Sepaskhah (2015) in saffron (Crocus sativus L.), and Huang et al. (2015) in ramie (Boehmeria nivea L.). However, Abideen et al. (2014) stated that moderate salinity stimulates growth and photosynthesis in Phragmites karka L.
The main effect of biostimulant application showed that plants increased photosynthetic activity. It was due to the content of active compounds in seaweed extracts, like cytokines or other similar (Battacharyya et al. 2015). Reduction in E, on the other hand, can be attributed to improved leaf water status induced by the biostimulant (Xu and Leskovar 2015). Similar results were found by Xu and Leskovar (2015) in spinach (Spinacia oleracea L.) plants.
Conclusions
An increase in electrical conductivity of irrigation water negatively affects the growth components and gas exchange of P. peruviana. Application of seaweed-based biostimulant improves the photosynthetic capacity, reduces transpiration, and attenuates the deleterious effects of salinity in specific leaf area, leaf area ratio and stomatal conductance in P. peruviana plants. Therefore, results obtained here allow developing future research under field conditions, which can directly benefit the local farmers.
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) from Brazil for their financial assistance in conducting the experiment.
Declarations
Conflict of interest
The authors state that there is no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francisco Romário Andrade Figueiredo, Email: romarioagroecologia@yahoo.com.br.
Jackson Silva Nóbrega, Email: jacksonnobrega@hotmail.com.
Reynaldo Teodoro de Fátima, Email: reynaldo.t16@gmail.com.
Jean Télvio Andrade Ferreira, Email: jeantelvioagronomo@gmail.com.
Márcia Paloma da Silva Leal, Email: palomalealagro@gmail.com.
Marlenildo Ferreira Melo, Email: marlenildo-melo@hotmail.com.
Thiago Jardelino Dias, Email: thiagojardelinodias@gmail.com.
Manoel Bandeira de Albuquerque, Email: bandeira1977@gmail.com.
References
- Abideen Z, Koyro HW, Huchzermeyer B, Ahmed MZ, Gul B, Khan MA. Moderate salinity stimulates growth and photosynthesis of Phragmites karka by water relations and tissue specific ion regulation. Environ Exp Bot. 2014;105:70–76. doi: 10.1016/j.envexpbot.2014.04.009. [DOI] [Google Scholar]
- Arrais IG, Almeida JPN, Dantas LLGR, Silva FSO, Silva CC, Mendonça V. Extrato da alga Ascophyllum nodosum (L.) Le Jolis na produção de porta-enxertos de Anonna glabra L. Revista de Ciências Agrárias. 2016;39:234–241. doi: 10.19084/RCA15057. [DOI] [Google Scholar]
- Azizian A, Sepaskhah AR. Maize response to water, salinity and nitrogen levels: physiological growth parameters and gas exchange. Int J Plant Prod. 2014;8:131–162. doi: 10.22069/IJPP.2014.1376. [DOI] [Google Scholar]
- Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants in horticulture. Sci Hortic. 2015;196:39–48. doi: 10.1016/j.scienta.2015.09.012. [DOI] [Google Scholar]
- Benincasa MMP. Análise de crescimento de plantas, noções básicas. 2. Jaboticabal: FUNEP; 2003. [Google Scholar]
- Bernardo S, Soares AA, Mantovani EC. Manual de irrigação. 8. Viçosa: UFV; 2006. [Google Scholar]
- Elansary HO, Skalicka-Woźniakc K, King IW. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol Biochem. 2016;105:310–320. doi: 10.1016/j.plaphy.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Embrapa (2018). Sistema brasileiro de classificação de solos. 5 ed., Brasília, DF: Embrapa
- Embrapa (2017). Manual de métodos de análise de solo. Manual de análises químicas de solos, plantas e fertilizantes. 3 ed. Brasília, DF: Embrapa
- Fischer G, Melgarejo LM. The ecophysiology of cape gooseberry (Physalis peruviana L.)—An Andean fruit crop. A review. Revista Colombiana de Ciencias Hortícolas. 2020;14:76–89. doi: 10.17584/rcch.2020v14i1.10893. [DOI] [Google Scholar]
- Garcia KGV, Silva CP, Cunha MCS, Nascimento CDV, Tosta MS. Extrato da alga Ascophyllum nodosum (L.) no desenvolvimento de porta enxertos de cajueiro. Enciclopédia Biosfera. 2014;10:1706–1715. [Google Scholar]
- Hadia EH, Slama A, Romdhame L, M’hamed HC, Abodoma AH, Fahej MAS, Radhoune L. Morpho-physiological and molecular responses of two Libyan bread wheat cultivars to plant growth regulators under salt stress. Italian J Agron. 2020;15:246–253. doi: 10.4081/ija.2020.1633. [DOI] [Google Scholar]
- Huang CJ, Wei G, Jie YC, Xu JJ, Zhao SY, Wang LC, Anjum SA. Responses of gas exchange, chlorophyll synthesis and ROS-scavenging systems to salinity stress in two ramie (Boehmeria nivea L.) cultivars. Photosynthetica. 2015;53:455–463. doi: 10.1007/s11099-015-0127-0. [DOI] [Google Scholar]
- Kaluzewicz A, Krzesinski W, Spizewki T, Zaworka A. Effect of bioestimulants on several physiological characteristics and chlorophyll contente in broccoli under drought stress and Re-watering. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2017;45:197–202. doi: 10.15835/nbha45110529. [DOI] [Google Scholar]
- Lofti R, Ghassemi-Golezani K, Najafi N. Grain filling and yield of mung bean affected by salicylic acid and silicon under salt stress. J Plant Nutr. 2018;41:1778–1785. doi: 10.1080/01904167.2018.1457686. [DOI] [Google Scholar]
- Mateus NB, Barbin D, Conagin A. Viabilidade de uso do delineamento composto central. Acta Sci Agron. 2001;23:1537–1546. doi: 10.4025/actascitechnol.v23i0.2795. [DOI] [Google Scholar]
- Monroy-Velandia D, Coy-Barrera E. Effect of salt stress on growth and metabolite profiles of cape gooseberry (Physalis peruviana L.) along three growth stages. Mollecules. 2021;26:2756. doi: 10.3390/molecules26092756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques EC, Freitas VS, Bezerra MA, Prisco JT, Gomes Filho E. Efeitos do estresse salino na germinação, emergência e estabelecimento da plântula de cajueiro anão precoce. Revista Ciência Agronômica. 2011;42:993–999. doi: 10.1590/S1806-66902011000400023. [DOI] [Google Scholar]
- Mezzalira EJ, Villa F, Piva AL, Santin A, Melgarejo MA. Initial development of physalis species under growing environments. Revista de Ciências Agroveterinárias. 2017;16:293–301. doi: 10.5965/223811711632017293. [DOI] [Google Scholar]
- Miranda D, Fischer G, Mewis I, Rohn S, Ulrichs C. Salinity effects on proline accumulation and total antioxidant activity in leaves of the cape gooseberry (Physalis peruviana L.) J Appl Botany Food Qual. 2014;87:67–73. doi: 10.5073/JABFQ.2014.087.010. [DOI] [Google Scholar]
- Nobre RG, Lima GS, Gheyi HR, Lourenço GS, Soares LAA. Emergência, crescimento e produção da mamoneira sob estresse salino e adubação nitrogenada. Revista Ciência Agronômica. 2013;44:76–85. doi: 10.1590/S1806-66902013000100010. [DOI] [Google Scholar]
- Oliveira FA, Guedes RAA, Gomes LP, Bezerra FMS, Lima LA, Oliveira MKT. Interação entre a salinidade e bioestimulante no crescimento inicial de pinhão-manso. Revista Brasileira De Engenharia Agrícola e Ambiental. 2015;19:204–210. doi: 10.1590/1807-1929/agriambi.v19n3p204-210. [DOI] [Google Scholar]
- Oliveira FA, Medeiros JF, Cunha RC, Souza MWL, Lima LA. Uso de bioestimulante como agente amenizador do estresse salino na cultura do milho pipoca. Revista Ciência Agronômica. 2016;47:307–315. doi: 10.5935/1806-6690.20160036. [DOI] [Google Scholar]
- Oliveira FA, Oliveira JM, Souza Neta ML, Oliveira MKT, Alves RC. Substrato e bioestimulante na produção de mudas de maxixeiro. Horticultura Besileira. 2017;35:141–146. doi: 10.1590/S0102-053620170122. [DOI] [Google Scholar]
- Piesanti S, Lima AP, Tonietto S, Souza R, Schubert R, Morsseli T. Crescimento inicial e qualidade de frutos de plantas de Physalis, adubadas com diferentes doses de vermicomposto ovino. Cadernos De Agroecologia. 2018;13:1–8. [Google Scholar]
- Popescu M. The role of seaweeds extracts as alleviators of salt stress in cucumber and tomato plants. Curr Trends Nat Sci. 2020;9:201–204. doi: 10.47068/ctns.2020.v9i17.024. [DOI] [Google Scholar]
- Puente L, Vega-Gálvez A, Fuentes I, Stucken K, Rodríguez A, Pastén A. Effects of drying methods on the characterization of fatty acids, bioactive compounds and antioxidant capacity in a thin layer of physalis (Physalis peruviana L.) pulp. J Food Sci Technol. 2021;58:1470–1479. doi: 10.1007/s13197-020-04659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R-Core-Team . R: a language and environment for statistical computing. Viena: R. Foundation for Satatical Computing; 2016. [Google Scholar]
- Richards LA. Diagnosis and improvement of saline and alkaline soils. Washington: United States Salinity Laboratory Staff; 1954. [Google Scholar]
- Rodrigues MHBS, Lopes KP, Bomfim MP, Pereira NAE, Silva JG, Paiva FJP, Santos AS. Characterization of physiological maturity of Physalis peruviana L. fruits. Semina Ciências Agrárias. 2021;42:929–948. doi: 10.5433/1679-0359.2021v42n3p929. [DOI] [Google Scholar]
- Rouphael Y, Cardarelli M, Bassal A, Leonardi C, Giuffrida F, Colla G. Vegetable quality as affected by genetic, agronomic and environmental factors. J Food Agric Environ. 2012;10:680–688. doi: 10.1234/4.2012.3485. [DOI] [Google Scholar]
- Silva CC, Arrais IG, Almeida JPN, Dantas LLGR, Silva FSO, Mendonça V. Extrato da alga Ascophyllum nodosum (L. Le Jolis na produção de porta-enxertos de Anonna glabra L. Rev Ciênc Agrá. 2016;39:234–241. doi: 10.19084/RCA15057. [DOI] [Google Scholar]
- Silva Junior FB, Sousa GG, Sousa JTM, Lessa CIN, Silva FDB. Salt stress and ambience on the production of watermelon seedlings. Revista Caatinga. 2020;33:518–528. doi: 10.1590/1983-21252020v33n224rc. [DOI] [Google Scholar]
- Souza MWL, Oliveira FA, Torres SB, Souza Neta ML, Sá FVS, Leal CCP. Exogenous application of biostimulant in zucchi (Cucurbina pepo L.) subjected to salt stress. Revista Ciência Agronômica. 2020;51:e20207116. doi: 10.5935/1806-6690.20200055. [DOI] [Google Scholar]
- Souza Neta ML, Oliveira FA, Torres SB, Souza AAT, Silva DDA, Santos ST. Gherkin cultivation in saline medium using seeds treated with a biostimulant. Acta Scientiarum Agronomy. 2018;40:e35216. doi: 10.4025/actasciagron.v40i1.35216. [DOI] [Google Scholar]
- Taiz L, Zeiger E, Moller IM, Murphy A. Fisiologia e Desenvolvimento Vegetal. 6. Porto Alegre: Artmed; 2017. [Google Scholar]
- Yarami N, Sepaskhah AR. Physiological growth and gas exchange response of saffron (Crocus sativus L.) to irrigation water salinity, manure application and planting method. Agric Water Manag. 2015;154:43–51. doi: 10.1016/j.agwat.2015.03.003. [DOI] [Google Scholar]
- Xu C, Leskovar DI. Effect of A. nodosum extracts on spinach growth, physiology and nutrition value under drought stress. Sci Hortic. 2015;183:39–47. doi: 10.1016/j.scienta.2014.12.004. [DOI] [Google Scholar]