Abstract
With the recent developments in the field of nanotechnology, the biosynthesis of nanoparticles has increased tremendously. Silver nanoparticles (SNPs) are among the most synthesized nanoparticles and this extensive synthesis can elevate the amounts of SNPs in the environment, which, consequently, pose a serious threat to the ecosystem and can bring unwanted environmental effects. As plants are an important part of ecosystem, investigation of toxic effects of SNPs on plants is particularly interesting. This study evaluates the potential risk of SNPs interaction with plants. For this, seeds of Vigna radiata L. were screened in presence of SNPs (20 mgL−1) using the germination, growth, and biochemical parameters as a phototoxicity criterion. The 19.57 nm average-sized SNPs were synthesized via the biosynthesis method. These biosynthesized SNPs were then applied on two varieties of V. radiata (Azri and High cross 404) and found to have variety dependent toxic effects on seed germination, growth, and biochemical parameters. Seed germination, root length, shoot length, fresh weight, chlorophyll, carotenoid, sugar content, and total proteins were reduced by 20, 46, 50, 18, 55, 62, 82, and 67%, respectively, in High cross 404, when compared with control (distilled water). The variety Azri was less sensitive than the variety High cross 404. In conclusion, the results demonstrated that SNPs affect seed germination and seedling growth when internalized and accumulated in plants, revealing that SNPs were responsible for the side effects. More in-depth research is required, in the form of different concentrations of SNPs or different plant species, to draw a logical conclusion and develop legislation about the safe use of biosynthesized SNPs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01073-4.
Keywords: SNPs, Seed germination, Growth, Crop
Introduction
Nanoparticles include a heterogeneous variety of materials (Santos et al. 2015), but only a few are significantly used. Environment is at a threat by widely used nanoparticles. Silver nanoparticles (SNPs) are one of the most traded and used nanoparticles in the world because of their unique physical and chemical properties (Ma et al. 2011; Wang et al. 2012). Some examples of the products that contain nanosilver are food supplements, food packaging, cosmetics, textiles, household appliances, electronic compounds, and water disinfectants. The extensive and widespread use of nanosilver-containing products are shifting worldwide attention to nanotoxicity research (Panyala et al. 2008; Brar et al. 2010; Hamed-Chaman et al. 2012; Zahir et al. 2012; Yang et al. 2013; Ribeiro et al. 2014; Colman et al. 2014; Hedberg et al. 2014; Nam et al. 2014; Tripathi et al. 2015, 2016, 2017a). Therefore, a serious concern is the discharge of metal nanoparticles into the atmosphere, as such discharge may harm the environment, and consequently also on the health of humans (Cvjetko et al. 2018). Currently, it has been observed that metal nanoparticles can enter into the plants and accumulate in the food chains, and thus, in turn, possibly reach the consumer (Pittol et al. 2017). Numerous studies have shown many lethal effects of metal nanoparticles on growth, flowering, fruiting, and other physiological processes that can be hazardous for the production of sustainable agriculture worldwide (Tripathi et al. 2017b).
In this situation, the interpretation of the interactions between the plants and nanoparticles is vital because plants are an essential part of the ecosystem and function in an ecosystem as primary producers. However, the impact of nanoparticles on plants depends on the type of plant species as well as the morphological and physicochemical properties of nanoparticles. Nanoparticles can enter into the living tissues of plants and thus migrate to different parts of the plants. They may also be absorbed by the roots and through vascular bundles transported to the aerial part (Feichtmeier et al. 2015). It has been shown in different studies that nanoparticles can inhibit germination and growth of plants (Pradhan et al. 2015; Singh and Kumar 2015). During the literature survey, it has been observed that SNPs affect the plant physiological, chemical, and metabolic processes by altering growth, water absorption, nutrient uptake, transpiration, photosynthesis, and respiration, and by producing reactive oxygen species (ROS) and generating antioxidant responses (Qian et al. 2013; Nair and Chung 2014; Hossain et al. 2015; Tripathi et al. 2017b). Both positive and negative responses are reported upon exposure to SNPs (Qian et al. 2013; Syu et al. 2014). Mehmood and Murtaza reported enhanced yields in pea plants when exposed to 60 ppm SNPs (Mehmood and Murtaza 2016). An induced growth rate was reported in soybean when 15 nm SNPs were applied (Mustafa et al. 2015). Similarly, when cucumbers were exposed to 500 ppm SNPs, an increased fruit yield and weight was reported (Shams et al. 2013). Sadak (2019) stated increased growth of fenugreek plant using 20, 40, and 60 mg/L SNPs. On the other hand, several reports exhibit the negative effects of SNPs on plants. For instance, a 2–7 times lower growth rate was observed in tomatoes when exposed to SNPs (Noori et al. 2020) or a reduced photosynthetic activity was recorded in wheat upon exposure to SNPs (Rastogi et al. 2019). An inhibited growth and cytotoxicity were found in Allium cepa under SNPs (Kumari et al. 2009; Scherer et al. 2019). Different size SNPs (20–80 nm) caused toxic effects on Arabidopsis thaliana seedlings (Ma et al. 2010). Inhibition in root growth and damaged cell wall or cell morphology was reported in A. thaliana and Asian rice, respectively, exposed to SNPs (Geisler-Lee et al. 2014; Mirzajani et al. 2014). From literature survey, we observed that lower concentrations of SNPs induce positive effect, while high concentrations cause phytotoxicity. For example, the growth, biomass, and chlorophyll content of Brassica rapa were increased at 1 mg/L SNPs and decreased at 5 and 10 mg/L SNPs (Thiruvengadam et al. 2015). Parveen and Rao (2014) studied the effect of 20 and 50 mg/L SNPs on Pennisetum glaucum and found that higher concentration of SNPs decreased the root, shoot, and seedling length. Kim et al. (2018) also reported toxic effect of higher concentrations of SNPs as compared to lower concentrations on Triticum aestivum and Phaseolus mungo. From all these studies, it appears that the effects of SNPs on plants vary based on morphology and concentration of SNPs, as well as on the type of plant, growth conditions, and availability of SNPs in the soil (Holden et al. 2016; Verma et al. 2018). Based on these facts that higher concentrations of SNPs produce toxic effect, we assessed the phytotoxic effect of biosynthesized SNPs (20 mg/L) from Medicago polymorpha in two varieties of important crop plant Vigna radiata. Our focus was to check the effect of biosynthesized SNPs, because the effect of chemically engineered SNPs of 20 mg/L has already been reported on V. radiata (Nair and Chung 2015). It is very crucial to examine whether biosynthesized SNPs harm seed germination, seedling growth, and biochemical contents of V. radiata because a major part of phytotoxicity studies reported so far has used chemically engineered SNPs. Only few studies are available that reported the phytotoxicity of biosynthesized SNPs in plants such as in Lupinus termis (Al-Hugail et al. 2018), Linum flavum, and Lepidium sativum (Dobrucka et al. 2019), and Phaseolus vulgaris (Verma et al. 2020), but in comparison to them our additional aim was to check the role of plant extract in phytotoxicity effect. This study provides novel information on the intra-varietal effect of SNPs using two varieties of V. radiata, which can additionally be proven more helpful in understanding the species-dependent effects of SNPs. Furthermore, the results of this study can play an important role in the identification of various environmental risks associated with biosynthesized SNPs.
Material and methods
Biosynthesis of SNPs
The SNPs were synthesized by using the aqueous extract of Medicago polymorpha that was collected from Rawalakot, Azad Kashmir, Pakistan. To prepare the aqueous extract, 10 g powder of M. polymorpha was soaked in 250 mL distilled water with continuous shaking for 24 h at room temperature (25 ± 2 °C). After filtration, the filtrate was immediately used for the synthesis of SNPs. To synthesize SNPs, the already described synthesis procedure was used, a 20 mL of plant filtrate was mixed with 80 mL of 1 mM AgNO3 solution and kept at room temperature (25 ± 2 °C) in dark for 24 h (Yousaf et al. 2020).
Characterization of SNPs
For the UV absorption spectrum, the reaction solution was scanned in the range of 300–800 nm using Perkin-Elmer lambda 750 spectrophotometers. For scanning electron microscopy (SEM), x-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR), the reaction solution was centrifuged at 13,000 rpm for 10 min and powder form of SNPs was studied using MIRA 3 XM Field Emission Scanning Electron Microscopes, PANalytical X’pert PRO X-Ray Diffractometer, and Perkin Elmer Spectrum 100 FT-IR spectrometer respectively.
Phytotoxicity assay
The phytotoxic effect of biosynthesized SNPs was checked in two varieties of V. radiata (Azri and High Cross 404). The seeds of each variety were obtained from the Agriculture Department, Government of Azad Jammu and Kashmir. All the seeds were surface sterilized in 5% sodium hypochlorite for 10 min before application. Then the seeds were washed with distilled water. For seed treatment, seeds were soaked in 20 mg/L SNPs for 24 h in a beaker and kept at room temperature in dark (Kushwah et al. 2018). For comparison, some seeds were also soaked in control (distilled water), plant filtrate, and reaction solution (plant filtrate plus AgNO3) and placed under similar conditions as used for SNPs treated seeds. Afterward, the seeds were washed with distilled water. The seeds of each treatment were then transferred to Petri plates containing a wet blotting paper with 1 mL treatment daily supply and allowed to germinate in natural conditions (temperature 25 ± 2 °C and photoperiod 12 h light). There were 10 seeds of every treatment in each Petri plate with three replicates. The seed germination parameters were measured after 5 days, and growth and biochemical attributes were measured after 10 days. Seed germination percentage (GP) was calculated as
where GN represents the total number of seeds germinated and SN is the total seeds tested in each treatment.
Another parameter calculated was relative germination percentage (RGP) and calculated by the formula.
where GP treatment is the germination percentage of seeds in each treatment and GP control is the germination percentage of seeds in control (distilled water).
In addition to this, another important parameter related to seed germination calculated was vigor index (VI), represented as.
VI = germination% × seedling length (root + shoot).
In the case of growth parameters, the root and shoot length, and fresh weight were measured for 10 days old seedlings. The root and shoot length of 5 plants from each treatment was measured in centimeters (cm) using a school ruler and the average was calculated. The fresh weight of 5 plants from each treatment was measured in grams (g) using digital balance and the average was calculated.
For biochemical parameters, 10 days old seedlings were subjected to analysis of total chlorophyll, carotenoid, phenol, soluble sugar, and protein contents. For the estimation of total chlorophyll and carotenoid, 10 days old of fresh seedlings of each treatment were collected and washed with distilled water. A 0.5 g finely cut pieces of fresh seedlings were homogenized in 10 mL of 80% acetone with the help of mortar and pestle. The homogenate was then centrifuged with a speed of 5000 rpm for 25 min at 4 °C. 0.5 mL of the supernatant was diluted with 4.5 mL of 80% acetone and subjected to spectrophotometric analysis at 663.2, 664.8, and 470 nm using Perkin-Elmer lambda 750 spectrophotometers. The total chlorophyll and carotenoid were calculated through the equation given below (Lichtenthaler 1987).
Chlorophyll a (mg/g) = [(12.25 × A663.2) – (2.79 × A646.8)] × mL acetone/mg leaf tissue.
Chlorophyll b (mg/g) = [(21.50 × A646.8) – (5.10 × A663.2)] × mL acetone/mg leaf tissue.
Total Chlorophyll = Chlorophyll a + Chlorophyll b.
Carotenoids (mg/g) = (1000 A470 – 1.8Chlorophyll a – 85. 02 Chlorophyll b)/198.
The total phenolic content (TFC) in the plant extract was measured using Folin–Ciocalteu’s phenol reagent (Pourmorad et al. 2006). In brief, 0.2 mL of plant extract was added with 5 mL of Folin–Ciocalteu’s phenol reagent (10 times diluted) in a test tube and mixed properly. The mixture was allowed to stand for 4 min and then it was added with 4 mL sodium carbonate (7.5 percent w/v) followed by dilution up to the volume of 25 mL with deionized distilled water. All the contents were mixed thoroughly and kept at room temperature (25 ± 2 °C) for 90 min. The test tubes were allowed to stand for 90 min at room temperature and the absorbance of each sample was recorded at 760 nm using Shimadzu UV-1601 spectrophotometer. The total phenols were assessed through gallic acid (GA) calibration curve and presented as the equivalent of mg GA/g fresh weight (FW).
Total soluble sugar was determined by using anthrone reagent (DuBois et al. 1956). In short, 25 mg fresh seedlings were crushed in 10 mL of ethanol (80%). The homogenate was centrifuged at 15,000 rpm for 10 min and in 0.1 mL supernatant, 4 mL anthrone reagent was added followed by a 10 min heating in a water bath. After that, the absorbance of each sample was measured at 625 nm using a Shimadzu UV-1601 spectrophotometer. The total soluble sugar was assessed through the glucose (Glu) calibration curve and presented as the equivalent of µg Glu/g FW.
The total protein content in the fresh seedlings was estimated through the Bradford reagent method (Bradford 1976). For protein extraction, 0.5 g fresh seedlings were homogenized in 100 mM Tris-HCl buffer (pH 8.0) containing 150 mM NaCl and 1 ml-1 a-mercapto-ethanol and kept in dark for 15 min followed by centrifugation at 15,000 rpm for 20 min at 4C. 0.5 mL supernatant was taken in test tubes, added with 1.5 mL Bradford reagent, and mixed well. After keeping at room temperature for 5 min and then absorbance was measured at 595 nm using Shimadzu UV-1601 spectrophotometer. the total protein content was assessed through bovine serum albumin (BSA) calibration curve and presented as the equivalent of mg BSA/g FW.
Statistical analysis
The experiment was carried out in a completely randomized design with three replicates. Data were analyzed using ANOVA followed by least significance difference tests. The p-value < 0.05 was considered as significant difference. The results presented are the means of three replicates ± standard deviation (SD). Statistix software version 8.0 was used for statistical analysis.
Results and discussion
Preparation and characterization of SNPs
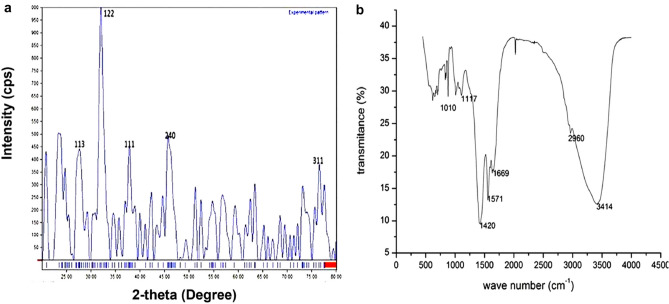
The biosynthesized SNPs were used in this study, as the biosynthesis method of SNPs is one of the leading and most frequently used methods nowadays and it is expected that SNPs are accumulating in the ecosystem. Hence it is very important to check the phytotoxicity of biosynthesized SNPs in crops. In this study, SNPs were synthesized from the aqueous extract of M. polymorpha by reacting with AgNO3 solution. The formation of SNPs was monitored from the color change and UV–visible spectroscopy of the reaction solution. The initial color of the reaction solution was yellowish which later on developed into brown as the reaction is continued to 24 h (Fig. 1). The UV–visible spectrum of the reaction solution (after 24 h) was recorded in the range of 200–800 nm wavelength and a well-developed peak was observed at 426 nm (Fig. 2). This change in color from yellowish to brown and the absorption peak at 426 nm is caused by stimulation of surface plasmon resonance vibrations in the SNPs (Zilberberg et al. 2015; Shankar et al. 2004) and can be interpreted as a signal of reduction of silver ions to SNPs. There are numerous findings that support the claim that SNPs exhibit brown color with absorption peaks below 700 nm (Kumar et al. 2014; Zou et al. 2007; Kathiravan et al. 2014). The morphology, distribution, and size of SNPs were determined through SEM and TEM analysis as illustrated in Supplementary Fig. 1. The SEM and TEM micrographs reveal the presence of spherical and irregular shaped SNPs, as shown by Jemal et al. (2017) and Vishwakarma et al. (2017). The size distribution histogram shows that most of the particles are 5–10 nm in size with an average size of 19.57 nm. The agglomeration of SNPs arise may be due to interaction with biochemical substances (Cedervall et al. 2007), attached to SNPs as capping agents. In support of our results, Zhang et al. (2012) also suggested that SNPs can have a spherical or ovoid shape. The characteristics of SNPs such as size and shape directly affect NPs toxicity (Nguyen et al. 2017). The XRD pattern confirms the crystalline nature of SNPs with 5 diffraction peaks observed at 2θ values of 27.81°, 32.16°, 38.12°, 46.21° and 76.45° that could correspond to (113), (122), (111), (240) and (311) planes of the face-centered cubic structure of SNPs (Fig. 3a). It is assumed as described above that reduction of silver ion to SNPs or the agglomeration of SNPs was due to biomolecules present in the plant extract and attached to SNPs. It is here confirmed by FTIR analysis of SNPs that shows transmission peaks at 1010-1117, 1420, 1571, 1669, 2960 and 3414 cm−1 representing O–H group of alcohols, –C=C– group of the aromatic ring, –C=O group of Nitro compounds, –C–H vibrations and –O–H stretch vibration respectively (Fig. 3b). These functional groups suggest that alcohol, carboxyl, or aldehyde groups were mainly involved in the capping and stabilizing of SNPs. These types of functional groups are already being reported in different studies (Bagherzade et al. 2017; Ahmed et al. 2016).
Fig. 1.

Change in color after the addition of plant extract in AgNO3 solution; a plant extract; b AgNO3 solution; c plant extract plus AgNO3 solution at zero-time; d and plant extract plus AgNO3 sol after 24 h. A 20 mL of plant extract was added in 80 mL of 1 mM AgNO3 solution and kept at room temperature
Fig. 2.
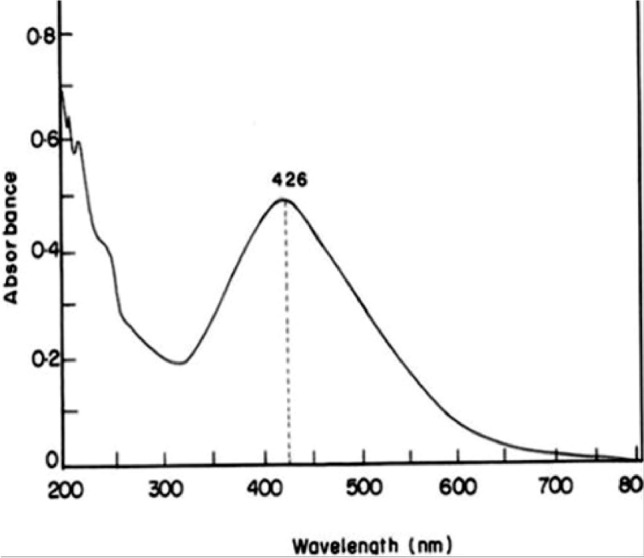
UV/Vis absorption spectrum of colloidal suspension of SNPs after 24 h presenting the characteristic plasmonic band at 426 nm
Fig. 3.
Characterization of SNPs; a X-ray diffraction (XRD) of SNPs presenting crystalline nature of SNPs; b Fourier transform infra-red spectrometry of SNPs presenting the functional groups of biomolecules capped the SNPs
Phytotoxicity of SNPs
Plants are producers and serve as constituents of the basic organization of any ecosystem. They can uptake, transfer, and collect SNPs from their adjacent growing medium (Monica and Cremonini 2009). When SNPs are discharged into the atmosphere, they bargain their move into the plants via the food chain and cause phytotoxicity. To verify whether biosynthesized SNPs had any phytotoxic effect, 20 mg/L SNPs were tested on V. radiata along with control, plant extract, and plant extract plus AgNO3 (reaction solution). The effect was measured on seed germination, seedling growth, and seedling biochemical contents of V. radiata. More or less, the pattern of effect of different treatments was similar i.e., negative in SNPs and plant extract + AgNO3 in all the parameters studied except phenolic content that increased under these treatments. There was no statistical difference between the control and plant extract, indicating that plant extract did not affect germination and growth of V. radiata. The plant extract + AgNO3 nevertheless showed a toxic effect but less than SNPs on seed germination and growth of V. radiata. This effect of plant extract + AgNO3 may be due to the formation of SNPs in the reaction solution as the SNPs alone were found to have a more toxic effect. Figure 4 depicts the impact of several treatments on seed germination in V. radiata varieties (control, SNPs, plant extract, and plant extract + AgNO3). Because of the ease, sensitivity, cost-effectiveness, and appropriateness of toxic compounds, seed germination measures are often employed in phytotoxicity tests. Furthermore, the germination of seed has a direct relationship with the density of plants (Baalbaki et al. 1990). Seed GP, RGP, and VI were all considerably lower in seeds treated with SNPs and plant extract + AgNO3 in our research. With SNPs, seed GP was reduced by 78% in Azri and 76% in High cross 404, whereas plant extract + AgNO3 reduced GP by 86% in Azri and 82% in High cross 404 when both were compared to a control group, where Azri had a GP of 98% and High Cross 404 had a GP of 94%. RGP was likewise considerably reduced in SNP-treated cultivars (86 percent in Azri and 83% in High cross 404). Plant extract with AgNO3 resulted in an RGP of 90% in Azri and 88% in High cross 404. Plant extract with AgNO3 resulted in an RGP of 90% in Azri and 88% in High cross 404. In a controlled setting, the RGP in Azri and High cross 404 was 96 and 98%, respectively. When compared to a control with VI of 466.4 and 496.4, the VI was lowered to 350.6 and 390.6 in Azri and 275.2 and 378 in High cross 404 under the treatments of SNPs and plant extract + AgNO3, respectively. In every case, the plant extract was determined to be insignificant. Our findings support earlier study that found that, depending on the plant species and SNP concentration, SNPs might have undesirable consequences such as a decrease in seed germination and plant growth (Dimkpa et al. 2013; Yin et al. 2012; Nair and Chung 2014; Scherer et al. 2019). Because NPs enter through the seed coat, alter cellular structures including the membrane, and inhibit germination, it has been observed in previous studies that the effect of SNPs varies in the germination of different plant seeds, depending on the selective permeability of the seed coat (Wierzbicka and Obidzinska 1998; Blaser et al. 2008). Earlier studies (Abdel-Azeem and Elsayed 2013; Giordani et al. 2012; Castiglione and Cremonine 2009; Lin and Xing 2007; Yin et al. 2012) have shown no toxic effects on seed germination when exposed to Ag, Zn, and Al nanoparticles. On the other hand, including our study, SNPs have shown a toxic effect on seed germination. For example, 100 ppm SNPs significantly inhibited seed germination in Lactuca sativa (Pinheiro et al. 2020). Verma et al (2020) applied 0, 15, 30, 60, 120, 240, and 480 mg/L biosynthesized SNPs on Phaseolus vulgaris seeds and found that seed GP was reduced at high concentration than in low concentration. The seed GP was 100% in 0–60 mg/L SNPs and then started to decrease in 120–480 mg/L SNPs. A particle size-dependent study (Scherer et al. 2019), showed that small-sized SNPs were more toxic as they used 10, 20, 51, and 73 nm SNPs on Allium cepa and found reduced germination index only with 10 nm SNPs. A declined germination was also found in Vicia faba (Abd-Alla et al. 2016) and Oryza sativa (Thuesombat et al. 2014) under the application of SNPs.
Fig. 4.

Effect of SNPs on seed germination of V. radiata varieties; a seed germination percentage (GP); b relative germination percentage (RGP); c vigor index (VI). Data represent the mean ± standard deviation of means. Means having different letters are significantly different from each other. Data were analyzed using ANOVA followed by least significance difference tests, p < 0.05, n = 3)
The seedling growth phase such as RL, SL, and FW was also selected to check the effect of SNPs because it is easy to perceive SNPs toxicity at early seedling growth phases (Yin et al. 2012). Figure 5 represents the effect of SNPs, plant extract, and plant extract plus AgNO3 on RL, SL, and FW of 10 days old seedlings of V. radiata varieties. The RL was reduced by 21 and 46% in Azri and High cross 404 with SNPs and 5 and 36% with plant extract + AgNO3, respectively, as compared to control. A significant reduction was observed in SL with SNPs, which was reduced by 34 and 50% in Azri and High cross 404. Whereas, with plant extract + AgNO3, SL was reduced by 22 and 32% in Azri and High cross 404, respectively, which is significantly lower than SNPs. Similar to root and shoot length, a significant reduction in FW was observed with SNPs. FW was reduced by 22 and 18% in Azri and High cross 404 compared to the plant extract + AgNO3 where it was reduced only by 12.5 and 2.5%. Similar to our results, A significant reduction was observed in root and shoot elongation of wheat seedling when treated with 5 mM SNPs (Rastogi et al. 2019). In addition to this, several studies demonstrate the toxic effect of SNPs on the growth of plants. It was also observed that these effects were dose dependent. An inhibited seed germination and reduced biomass were observed in Cucrubita pepo at more than 100 mg/L SNPs (Stampoulis et al. 2009). A dose-dependent reduction in root and shoot length of wheat was recorded (Dimkpa et al. 2013). Similar to this, a significantly reduced root elongation and fresh weight were found in rice (Nair and Chung 2014). In Arabidopsis, a reduction in biomass was observed when the concentration of SNPs was increased from 5 to 20 mg/L (Kaveh et al. 2013). The root and shoot length in Phaseolus vulgaris decreased at higher concentration of biosynthesized SNPs (120–480 mg/L) than in a lower concentration (0–60 mg/L) (Verma et al. 2020). In another study, a high concentration of SNPs (300–500 ppm) decreased shoot and root elongation while a low concentration (100 ppm) stimulated the growth (Al-Hugail et al. 2018). In addition to the size and concentration of SNPs, the type of plant is also a major factor in demonstrating the effect of SNPs. In our study, a significant reduction occurred in FW at 20 mg/L SNPs, and in contrast to this, at 30 mg/L SNPs, there was no reduction was found in FW of Arabidopsis thaliana (Zhang et al. 2019). The toxic effects of SNPs are due to their high uptake by plants (Harris and Bali 2008) that lead to their accumulation in high amounts and cause a reduction in root length and biomass (Yin et al. 2011). According to Singh et al. (2014), SNPs enter through seeds and modify the cell division and cell elongation process. In sunflower, it was studied that SNPs deformed the epidermis and anatomical properties, resulted in decreased FW (Krizkova et al. 2008).
Fig. 5.

Effect of SNPs on growth parameters of V. radiata varieties; a root length (cm); b shoot length (cm); c fresh weight (g). Data represent the mean ± standard deviation of means. Means having different letters are significantly different from each other. Data were analyzed using ANOVA followed by least significance difference tests, p < 0.05, n = 3)
Previously, it has been reported that SNPs negatively influence the photosynthetic system by reducing chlorophyll contents (Tripathi et al. 2017b). In this regard, we have checked the effect of SNPs on chlorophyll and carotenoid contents in V. radiata. Exposure to SNPs and plant extract plus AgNO3 induced a significant difference in total chlorophyll and carotenoid contents in seedlings of V. radiata varieties in comparison to control plants (Fig. 6). A significant decrease in chlorophyll contents by 51% in Azri and 55% in High cross 404 was observed with SNPs. When treated with plant extract + AgNO3, the chlorophyll contents were decreased by 45% Azri and 35% in High cross 404, which was lower than SNPs. The negative effect of SNPs was also observed on carotenoid content, which significantly decreased by 62 and 63% in Azri and High cross 404 respectively. In plant extract + AgNO3 treatment, the carotenoid content was reduced by 36 and 31% in Azri and High cross 404, respectively. The reduction in photosynthetic pigment under SNPs has already been recorded in different plants. A decrease in chlorophyll and carotenoid contents were observed in O. stiva under SNPs exposure (Nair and Chung 2014). Qian et al. (2013) reported that SNPs accumulated in Arabidopsis leaves where they damaged the thylakoid membrane, and reduced chlorophyll content, resulting in plant growth retardation. Jiang et al. (2012) also found that SNP treatment resulted in a substantial reduction in chlorophyll levels. It has already been proposed that nanoparticles attach to the cell walls of plant cells, where they provide a shading effect and limit the light to reach the photosystem, resulting in poor photosynthetic pigment and activity (Wei et al. 2010).
Fig. 6.

Effect of SNPs on photosynthetic pigments of V. radiata varieties; a chlorophyll content (mg/g); b carotenoid content (mg/g). Data represent the mean ± standard deviation of means. Means having different letters are significantly different from each other. Data were analyzed using ANOVA followed by least significance difference tests, p < 0.05, n = 3)
The impact of SNPs, plant extract, and plant extract + AgNO3 on the phenolic, sugar, and protein content of V. radiata varieties is shown in Fig. 7. In contrast to all other parameters investigated, SNPs and plant extract with AgNO3 resulted in a modest increase in phenolic content. Plant responses to oxidative stress caused by SNPs may be linked to an increase in phenolic content. It is a well-known fact that SNPs induce oxidative stress in plants because Ag ions produce free radicals, which cause oxidative stress in plant cells (Nair et al. 2010). In Azri and High cross 404, total soluble sugar contents were decreased by 60 and 82% when SNPs were used, however, soluble sugars were decreased by 9 and 20% when treated with plant extract + AgNO3, indicating that SNPs had the most severe effect on sugar content. In Azri and High cross 404, the SNPs and plant extract + AgNO3 likewise decreased protein content by 66 and 67% and 39 and 41%, respectively. The harmful effect of SNPs on biochemical characteristics of V. radiata seedlings is revealed by the well-observed reduction in soluble sugars and protein content. SNPs have previously been discovered to alter the expression of several proteins involved in the cell's primary metabolism and defence system (Ma et al. 2010). SNPs cause biological changes after entering cells and cellular organelles, and essential macrobiotic components such as protein are altered (Pham et al. 2012; Griffitt et al. 2009). Biosynthesized SNPs were shown to be more hazardous than chemically produced SNPs in our investigation, specifically on shoot growth and chlorophyll content of V. radiata. Previously, 20 mg/L chemically produced SNPs had no influence on shoot length, fresh weight, or chlorophyll content of V. radiata (Nair and Chung 2015), however 20 mg/L biosynthesized SNPs dramatically decreased shoot growth and chlorophyll levels in our study. This shows that biosynthesized SNPs may have different effect as compared to engineered SNPs. In general, some reported suggestions can help in understating the toxicity of SNPs in plants. For example, SNPs increase the production of hydrogen peroxide in the cells that destroy the plant cells and ultimately result in reduced growth and development of plants (Tripathi et al. 2017b; Monica and Cremonini 2009). SNPs also decrease the membrane potential of mitochondria in plants (Hsin et al. 2008). SNPs could cause modifications in proteins related to redox regulation, sulfur metabolism, endoplasmic reticulum, and vacuole (Vannini et al. 2013). One of the leading reasons for SNPs phytotoxicity is the generation of oxidative stress. It is a well-known fact that metals enhance the production of reactive oxygen species (ROS) that cause oxidative stress in plant cells (Tkalec et al. 2014; Balen et al. 2011). Several researchers have already mentioned that SNPs induce oxidative stress in plant cells (Nair and Chung 2014; Barbasz et al. 2016; Jiang et al. 2014). In oxidative stress, the extensive production of ROS causes lethal effects in the form of cell destruction, chromosomal abnormalities, micronucleus induction, and demolishing gene expression and DNA (Pokhrel and Dubey 2013). The destruction of DNA by ROS includes the formation of chromatin bridges, adhesiveness, disordered metaphase, and various chromosomal breaks (Anjum et al. 2013; Patlolla et al. 2012; Panda et al. 2011). Overproduction of ROS generated by SNPs may also damage chloroplasts, constrain plant growth, and decrease plant cellular viability (Karami et al. 2015; Oukarroun et al. 2013; Sosan et al. 2016). Beyond all these reasons, different studies show the different responses of plants towards SNPs. Some of them have shown retarded growth under SNPs application and in some plants, a positive effect of SNPs was observed. These different types of effects may be linked to the cellular penetrability of the involved plant and also to the size, shape, and concentration of SNPs (Tripathi et al. 2017b) because different researchers used different types of plants as well as different sized SNPs.
Fig. 7.
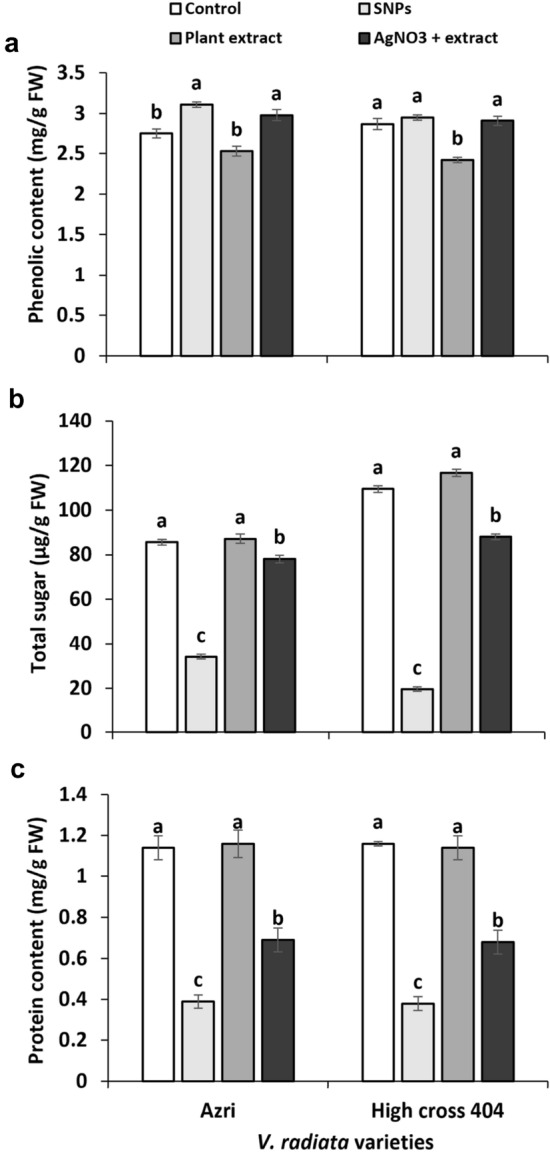
Effect of SNPs on biochemical contents of V. radiata varieties; a phenolic content (mg/g FW); b soluble sugar (µg/g FW); c total protein (mg/g FW). Data represent the mean ± standard deviation of means. Means having different letters are significantly different from each other. Data were analyzed using ANOVA followed by least significance difference tests, p < 0.05, n = 3)
Conclusion
This study demonstrated that biosynthesized SNPs induced toxic effects on seed germination, seedling growth, and biochemical attributes of V. radiata varieties. The effects were variety-dependent because the variety of High Cross 404 was found to be more sensitive than the variety Azri. The results revealed that SNPs caused a decrease in seed germination percentage, vigor index, root and shoot length, fresh weight, chlorophyll, carotenoid, sugar, and protein, possibly due to the overproduction of ROS triggered by SNPs. In conclusion, the results demonstrated that SNPs might affect the seed germination and seedling growth when internalized and accumulated in the plants, revealing that SNPs were responsible for the harmful effects. Thus, stimulation of the tolerance mechanism is very vital so that plant cells should be protected from stress conditions. Moreover, these biosynthesized Ag nanoparticles can be used in herbicide materials due to their phytotoxic properties.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We honestly acknowledge the support and assistance extended by the Institute of Space Technology (IST) and High-tech Laboratory of the University of Azad Jammu and Kashmir for FESEM, XRD, FTIR, and UV-vis spectroscopy analysis.
Authors' contribution
NA: Investigation, Data curation, Writing—original draft. AM: Methodology, Conceptualization, Writing—review & editing, Supervision. KSA: Writing—review & editing. KH: Writing—review & editing.
Funding
The authors did not receive support from any organization for the submitted work.
Availability of data and material
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have approved the final version for submission.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Alla MH, Nafady NA, Khalaf DM. Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: implications for induction of autophagy process in root nodule. Agric Ecosyst Environ. 2016;218:163–177. doi: 10.1016/j.agee.2015.11.022. [DOI] [Google Scholar]
- Abdel-Azeem EA, Elsayed BA. Phytotoxicity of silver nanoparticles on Vicia faba seedlings. NY Sci J. 2013;6:148–156. [Google Scholar]
- Ahmed S, Ahmad SM, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9:1–7. doi: 10.1016/j.jrras.2015.06.006. [DOI] [Google Scholar]
- Al-Huqail AA, Hatata MM, Al-Huqail AA, Ibrahim MM. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J Biol Sci. 2018;25(2):313–319. doi: 10.1016/j.sjbs.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum NA, Gill SS, Duarte AC, Pereira E, Ahmad I. Silver nanoparticles in soil–plant systems. J Nano Res. 2013;15:1–26. [Google Scholar]
- Baalbaki RZ, Zurayk RA, Bleik SN, Talhuk A. Germination and seedling development of drought susceptible wheat under moisture stress. Seed Sci Technol. 1990;17:291–302. [Google Scholar]
- Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop Biomed. 2017;7(3):227. doi: 10.1016/j.apjtb.2016.12.014. [DOI] [Google Scholar]
- Balen B, Tkalec M, Sikic S, Tolic S, Cvjetko P, Pavlica M, Vidakovic-Cifrek Z. Biochemical responses of Lemna minor experimentally exposed to cadmium and zinc. Ecotoxicology. 2011;20:815–826. doi: 10.1007/s10646-011-0633-1. [DOI] [PubMed] [Google Scholar]
- Barbasz A, Kreczmer B, Oćwieja M. Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3) Acta Physiol Plant. 2016;38:76. doi: 10.1007/s11738-016-2092-z. [DOI] [Google Scholar]
- Blaser SA, Scheringer M, Macleod M, Hungerbühler K. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ. 2008;390:396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brar SK, Verma M, Tyagi R, Surampalli R. Engineered nanoparticles in wastewater and wastewater sludge-evidence and impacts. Waste Manag. 2010;30(3):504e520. doi: 10.1016/j.wasman.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Castiglione MR, Cremonine R. Nanoparticles and higher plants. Caryologia. 2009;62:161–165. doi: 10.1080/00087114.2004.10589681. [DOI] [Google Scholar]
- Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman BP, Espinasse B, Richardson CJ, Matson CW, Lowry GV, Hunt DE, Wiesner MR, Bernhardt ES. Emerging contaminant or an old toxin in disguise? Silver nanoparticle impacts on ecosystems. Environ Sci Technol. 2014;48(9):5229e5236. doi: 10.1021/es405454v. [DOI] [PubMed] [Google Scholar]
- Cvjetko P, Zovko M, Peharec-Stefanic P, Biba R, Tkalec M, Domijan AM, Vinkovic Vrcek I, Letofsky-Papst I, Sikic S, Balen B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ Sci Pollut Res. 2018;25:5590–5602. doi: 10.1007/s11356-017-0928-8. [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ. Silver nanoparticles disrupt Wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol. 2013;47:1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- Dobrucka R, Szymanski M, Przekop R. The study of toxicity effects of biosynthesized silver nanoparticles using Veronica officinalis extract. Int J Environ Sci Technol. 2019;16:8517–8526. doi: 10.1007/s13762-019-02441-0. [DOI] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Feichtmeier NS, Walther P, Leopold K. Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles. Environ Sci Pollut Res Int. 2015;22(11):8549–8558. doi: 10.1007/s11356-014-4015-0. [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, Brooks M, Gerfen J, Wang Q, Fotis C, Sparer A, Ma X, Berg R, Geisler M. Reproductive toxicity and life history study of silver nanoparticle effect, uptake and transport in Arabidopsis thaliana. Nanomaterials. 2014;4(2):301–318. doi: 10.3390/nano4020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani T, Fabrizi A, Guidi L, Natali L, Giunti G, Ravasi F, Cavallini A, Pardossi A. Response of tomato plants exposed to treatment with nanoparticles. EQA-Environ Qual. 2012;8:27–38. [Google Scholar]
- Griffitt RJ, Hyndman K, Denslow ND, Barber DS. Comparison of molecular and histological changes in zebra fish gills exposed to metallic nanoparticles. Toxicol Sci. 2009;107:404–415. doi: 10.1093/toxsci/kfn256. [DOI] [PubMed] [Google Scholar]
- Hamed-Chaman S, Arab M, Roozban M, Ahmadi N (2012) Postharvest longevity and quality of cut carnations ‘pax’ and ‘tabor’, as affected by silver nanoparticles. In: VII International Postharvest Symposium 1012:527e532.
- Harris AT, Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res. 2008;10:691–695. doi: 10.1007/s11051-007-9288-5. [DOI] [Google Scholar]
- Hedberg J, Skoglund S, Karlsson ME, Wold S, OdnevallWallinder I, Hedberg Y. Sequential studies of silver released from silver nanoparticles in aqueous media simulating sweat, laundry detergent solutions and surface water. Environ Sci Technol. 2014;48(13):7314e7322. doi: 10.1021/es500234y. [DOI] [PubMed] [Google Scholar]
- Holden PA, Gardea-Torresdey JL, Klaessig F, Turco RF, Mortimer M, Hund-Rinke K, Hubal E, Avery D, Barcelo D, Behra R. Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ Sci Technol. 2016;50(12):6124–6145. doi: 10.1021/acs.est.6b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain Z, Mustafa G, Komatsu S. Plant responses to nanoparticle stress. Int J Mol Sci. 2015;16:26644–26653. doi: 10.3390/ijms161125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Jemal K, Sandeep BV, Pola S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J Nanomater. 2017;2017:4213275. doi: 10.1155/2017/4213275. [DOI] [Google Scholar]
- Jiang HS, Li M, Chang FY, Li W, Yin LY. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem. 2012;31:1880–1886. doi: 10.1002/etc.1899. [DOI] [PubMed] [Google Scholar]
- Jiang HS, Qiu XN, Li GB, Li W, Yin LY. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem. 2014;33:1398–1405. doi: 10.1002/etc.2577. [DOI] [PubMed] [Google Scholar]
- Karami S, Reza M, Fatemeh H. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J Plant Physiol. 2015;20:257–263. doi: 10.1007/s40502-015-0171-6. [DOI] [Google Scholar]
- Kathiravan V, Ravi S, Ashokkumar S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their invitro anticancer activity. Spectroch Acta Part A: Mol Biomol Spectro. 2014;130:116–121. doi: 10.1016/j.saa.2014.03.107. [DOI] [PubMed] [Google Scholar]
- Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol. 2013;47:10637–10644. doi: 10.1021/es402209w. [DOI] [PubMed] [Google Scholar]
- Kim D, Saratale RG, Shinde S, Syed A, Ameen F, Ghodake G. Green synthesis of silver nanoparticles using Laminaria japonica extract: characterization and seedling growth assessment. J Clean Prod. 2018;172:2910–2918. doi: 10.1016/j.jclepro.2017.11.123. [DOI] [Google Scholar]
- Krizkova S, Ryant P, Krystofova O, Adam V, Galiova M, Beklova M, Babula P, Kaiser J, Novotny K, Novotny J, Liska M, Malina R, Zehnalek J, Hubalek J, Havel L, Kizek R. Multi-instrumental analysis of tissues of sunflower plants treated with silver (i) ions – plants as bioindicators of environmental pollution. Sensors. 2008;8:445–463. doi: 10.3390/s8010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Smita K, Cumbal L, Debut A, Pathak RN. Sonochemical synthesis of silver nanoparticles using starch: a comparison. Bioinorg Chem Appl. 2014;8:784268. doi: 10.1155/2014/784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Mukherjee A, Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 2009;407:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Kushwah KS, Verma RC, Patel S, Jain NK. Colchicine induced polyploidy in Chrysanthemum carinatum L. J Phylogenet Evol Biol. 2018;6(193):2. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of Photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Lin D, Xing B. Pytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408:3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Ma J, Lu X, Huang Y. Genomic analysis of cytotoxicity response to nanosilver in human dermal fibroblasts. J Biomed Nanotechnol. 2011;7:263–275. doi: 10.1166/jbn.2011.1286. [DOI] [PubMed] [Google Scholar]
- Mehmood A, Murtaza G. Application of SNPs to improve yield of Pisum sativum L. (pea) IET Nanobiotechnol. 2016;11(4):390–394. doi: 10.1049/iet-nbt.2016.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzajani F, Askari H, Hamzelou S, Schober Y, Reompp A, Ghassempour A, Spengler B. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol Environ Saf. 2014;108:335–339. doi: 10.1016/j.ecoenv.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Monica RC, Cremonini R. Nanoparticles and higher plants. Caryologia. 2009;62:161–165. doi: 10.1080/00087114.2004.10589681. [DOI] [Google Scholar]
- Mustafa G, Sakata K, Hossain Z, Komatsu S. Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J Proteomics. 2015;122:100–118. doi: 10.1016/j.jprot.2015.03.030. [DOI] [PubMed] [Google Scholar]
- Nair PMG, Chung IM. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere. 2014;112:105–113. doi: 10.1016/j.chemosphere.2014.03.056. [DOI] [PubMed] [Google Scholar]
- Nair PMG, Chung IM. Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.) Acta Physiol Plant. 2015;37:1719. doi: 10.1007/s11738-014-1719-1. [DOI] [Google Scholar]
- Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nano particulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- Nam DH, Lee BC, Eom IC, Kim P, Yeo MK. Uptake and bioaccumulation of titanium-and silver nanoparticles in aquatic ecosystems. Mol Cell Toxicol. 2014;10(1):9e17. doi: 10.1007/s13273-014-0002-2. [DOI] [Google Scholar]
- Nguyen THD, Vardhanabhuti B, Lin M, Mustapha A. Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control. 2017;77:17–24. doi: 10.1016/j.foodcont.2017.01.018. [DOI] [Google Scholar]
- Noori A, Donnelly T, Colbert J, Cai W, Newman LA, White JC. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: physiological and molecular response. Int J Phytoremed. 2020;22(1):40–51. doi: 10.1080/15226514.2019.1634000. [DOI] [PubMed] [Google Scholar]
- Oukarroum A, Barhoumi L, Pirastru L, Dewez D. Silver nanoparticle toxicity effect on growth and cellular Viability of the aquatic plant Lemna gibba. Environ Toxicol Chem. 2013;32:902–907. doi: 10.1002/etc.2131. [DOI] [PubMed] [Google Scholar]
- Panda KK, Achary VMM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Pandaa BB. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol Invitro. 2011;25:1097–1105. doi: 10.1016/j.tiv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Panyala NR, Pena-Mendez EM, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed. 2008;6(3):117e129. doi: 10.32725/jab.2008.015. [DOI] [Google Scholar]
- Parveen A, Rao S. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J Clust Sci. 2014;26(3):693–701. doi: 10.1007/s10876-014-0728-y. [DOI] [Google Scholar]
- Patlolla AK, Berry A, May L, Tchounwou PB. Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. Int J Environ Res Public Health. 2012;9:1649–1662. doi: 10.3390/ijerph9051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CH, Yi J, Gu MB. Biomarker gene response in male Medaka (Oryzias latipes) chronically exposed to silver nanoparticle. Ecotoxicol Environ Saf. 2012;78:239–245. doi: 10.1016/j.ecoenv.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Pinheiro SKP, Chaves MM, Miguel TBAR, Barros FC, Farias CP, Ferreira OP, Miguel EC. Toxic effects of silver nanoparticles on the germination and root development of lettuce (Lactuca sativa) Aust J Bot. 2020;68:127–136. doi: 10.1071/BT19170. [DOI] [Google Scholar]
- Pittol M, Tomacheski D, Simoes DN, Ribeiro VF, Santana RMC. Macroscopic effects of silver nanoparticles and titanium dioxide on edible plant growth. Environ Nanotechnol Monit Manage. 2017;8:127–133. doi: 10.1016/j.enmm.2017.07.003. [DOI] [Google Scholar]
- Pokhrel LR, Dubey B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ. 2013;452–453:321–332. doi: 10.1016/j.scitotenv.2013.02.059. [DOI] [PubMed] [Google Scholar]
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotech. 2006;5:1142–1145. [Google Scholar]
- Pradhan S, Patra P, Mitra S, Dey KK, Basu S, Chandra S, Palit P, Goswami A. Copper nanoparticle (CuNP) nanochain arrays with a reduced toxicity response: a biophysical and biochemical outlook on Vigna radiata. J Agric Food Chem. 2015;63:2606–2617. doi: 10.1021/jf504614w. [DOI] [PubMed] [Google Scholar]
- Qian H, Peng X, Han X, Ren J, Sun L, Fu Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci. 2013;25:1947–1956. doi: 10.1016/S1001-0742(12)60301-5. [DOI] [PubMed] [Google Scholar]
- Rastogi A, Zivcak M, Tripathi DK, Yadav S, Kalaji HM, Brestic M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica. 2019;57:209–216. doi: 10.32615/ps.2019.019. [DOI] [Google Scholar]
- Ribeiro F, Gallego-Urrea JA, Jurkschat K, Crossley A, Hassellov M, Taylor C, Soares A, Loureiro MVMS. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ. 2014;466:232e241. doi: 10.1016/j.scitotenv.2013.06.101. [DOI] [PubMed] [Google Scholar]
- Sadak MS. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum) Bull Natl Res Cent. 2019;43:38. doi: 10.1186/s42269-019-0077-y. [DOI] [Google Scholar]
- Santos CSC, Gabriel B, Blanchy M, Menes O, Garcia D, Blanco M, Arconada N, Neto V. Industrial applications of nanoparticles – a prospective overview. Mater Today-Proc. 2015;2:456–465. doi: 10.1016/j.matpr.2015.04.056. [DOI] [Google Scholar]
- Scherer MD, Sposito JCV, Falco WF, Grisolia AB, Andrade LHC, Lima SM, Machado G, Nascimento VA, Gonçalves DA, Wender H, Oliveira SL, Caires ARL. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci Total Environ. 2019;660:459–467. doi: 10.1016/j.scitotenv.2018.12.444. [DOI] [PubMed] [Google Scholar]
- Shams G, Ranjbar M, Amiri A. Effect of silver nanoparticles on concentration of silver heavy element and growth indexes in cucumber (Cucumis sativus L. negeen) J Nanopart Res. 2013;15:1630. doi: 10.1007/s11051-013-1630-5. [DOI] [Google Scholar]
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au. Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Singh D, Kumar A. Effects of nano silver oxide and silver ions on growth of Vigna radiata. Bull Environ Contam Toxicol. 2015;95:379–384. doi: 10.1007/s00128-015-1595-4. [DOI] [PubMed] [Google Scholar]
- Singh VP, Kumar J, Singh S, Prasad SM. Dimethoate modifies enhanced UV-B effects on growth, photosynthesis and oxidative stress in mung bean (Vigna radiata L.) seedlings: implication of salicylic acid. Pestic Biochem Phys. 2014;116:13–23. doi: 10.1016/j.pestbp.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Sosan A, Svistunenko D, Straltsova D, Tsiurkina K, Smolich I, Lawson T, Subramaniam S, Golovko V, Anderson D, Sokolik A, Colbeck I, Demidchik V. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J. 2016;85(2):245–257. doi: 10.1111/tpj.13105. [DOI] [PubMed] [Google Scholar]
- Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;43:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- Syu Y, Hung JH, Chen JC, Chuang H. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem. 2014;83:57–64. doi: 10.1016/j.plaphy.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Thiruvengadam M, Gurunathan S, Chung IM. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.) Protoplasma. 2015;252:1031–1046. doi: 10.1007/s00709-014-0738-5. [DOI] [PubMed] [Google Scholar]
- Thuesombat P, Hannongbua S, Akasit S, Chadchawan S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf. 2014;104:302–309. doi: 10.1016/j.ecoenv.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Tkalec M, Peharec SP, Cvjetko P, Sikic S, Pavlica M, Balen B. The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLoS ONE. 2014;9:e87582. doi: 10.1371/journal.pone.0087582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem. 2015;96:189e198. doi: 10.1016/j.plaphy.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK, Dubey NK. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultivar and hybrid differing in arsenate tolerance. Front Environ Sci. 2016;4:46. doi: 10.3389/fenvs.2016.00046. [DOI] [Google Scholar]
- Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP, Singh S, Prasad SM, Singh PK, Dubey NK, Pandey AC, Chauhan DK. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem. 2017;110:167–177. doi: 10.1016/j.plaphy.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Tripathi DK, Tripathi A, Guar S, Singh S, Singh Y, Vishwakarma K, Yadav G, Sharma S, Singh VK, Mishra RK, Upadhyay RG, Dubey NK, Lee Y, Chauhan DK. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: a concentric review. Front Microbiol. 2017;8:7. doi: 10.3389/fmicb.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini C, Domingo G, Onelli E, Prinsi B, Marsoni M, Espen L, Bracale M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE. 2013;8:68752. doi: 10.1371/journal.pone.0068752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Das AK, Patel MK, Shah A, Kumar V, Gantait S. Engineered nanomaterials for plant growth and development: a perspective analysis. Sci Total Environ. 2018;630:1413–1435. doi: 10.1016/j.scitotenv.2018.02.313. [DOI] [PubMed] [Google Scholar]
- Verma DK, Patel S, Kushwah KS. Green biosynthesis of silver nanoparticles and impact on growth, chlorophyll, yield and phytotoxicity of Phaseolus vulgaris L. Vegetos. 2020 doi: 10.1007/s42535-020-00150-5. [DOI] [Google Scholar]
- Vishwakarma K, Shweta UN, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci. 2017;8:1501. doi: 10.3389/fpls.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Westerhoff P, Hristovski KD. Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J Hazard Mater. 2012;201:16e22. doi: 10.1016/j.jhazmat.2011.10.086. [DOI] [PubMed] [Google Scholar]
- Wei C, Zhang Y, Guo J, Han B, Yang X, Yuan J. Effects of Silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus. J Environ Sci (china) 2010;22:155–160. doi: 10.1016/S1001-0742(09)60087-5. [DOI] [PubMed] [Google Scholar]
- Wierzbicka M, Obidzinska J. The effect of lead on seed inhibition and germination in different plant species. Plant Sci. 1998;137:155–171. doi: 10.1016/S0168-9452(98)00138-1. [DOI] [Google Scholar]
- Yang Y, Zhang C, Hu Z. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ Sci Process Impacts. 2013;15(1):39e48. doi: 10.1039/C2EM30655G. [DOI] [PubMed] [Google Scholar]
- Yin L, Cheng Y, Espinasse B, Colman PB, Auffan M, Wiesner M, Rose Liu J, Bernhardt ES. More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol. 2011;45:2360–2367. doi: 10.1021/es103995x. [DOI] [PubMed] [Google Scholar]
- Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE. 2012 doi: 10.1371/journal.pone.0047674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf H, Mehmood A, Ahmad KS, Raffi M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater Sci Eng C. 2020;112:110901. doi: 10.1016/j.msec.2020.110901. [DOI] [PubMed] [Google Scholar]
- Zahir AA, Bagavan A, Kamaraj C, Elango G, Rahuman AA. Efficacy of plant-mediated synthesized silver nanoparticles against Sitophilus oryzae. J Biopest. 2012;5(Suppl):95e102. [Google Scholar]
- Zhang Z, Kong F, Vardhanabhuti B, Mustapha A, Lin M. Detection of engineered silver nanoparticle contamination in pears. J Agri Food Chem. 2012;60:10762–10767. doi: 10.1021/jf303423q. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Jiang HS, Gu SP, Zhou XH, Lu ZW, Kang XH, Yin L, Huang J. Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity. Environ Pollut. 2019;252:1539e1549. doi: 10.1016/j.envpol.2019.06.032. [DOI] [PubMed] [Google Scholar]
- Zilberberg L, Mitlin S, Shankar H, Asscher M. Buffer layer assisted growth of Ag nanoparticles in titania thin films. J Phys Chem C. 2015;119:28979–28991. doi: 10.1021/acs.jpcc.5b09621. [DOI] [Google Scholar]
- Zou J, Xu T, Hou B, Wu D, Sun Y. Controlled growth of silver nanoparticles in a hydrothermal process. China Particuol. 2007;5:206–212. doi: 10.1016/j.cpart.2007.03.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Not applicable.