Abstract
Increasing temperature poses a serious threat to rice productivity. This study investigated the impact of various biochar treatments and phosphorous (P) fertilization on osmolyte accumulation, ROS development, and antioxidant activity in two rice cultivars (IR-64 and Huanghuazhan) under high-temperature stress. All plants of both cultivars were grown in a controlled environment under ambient temperatures (AT), high day temperatures (HDT) or high night temperatures (HNT). The different fertilization treatments were biochar alone, P alone and biochar + P with control. In the leaves and xylem sap of both rice cultivars, particularly in the susceptible cv. IR-64, high-temperature stress increased the production of MDA and H2O2. HDT and HNT decreased total soluble sugars, protein, and proline levels in both rice cultivars. HNT was observed as more harmful compared to HDT during most of the studied characteristics. The response of antioxidant enzyme activities, viz, SOD, POD, CAT, APX, ASC, GSH, GR, and GSSC activities, to the temperature treatments varied between the two cultivars. Antioxidant activities decreased in the leaves and xylem sap of IR-64 but increased in those of Huanghuazhan upon exposure to high-temperature stress. Huanghuazhan exhibited better heat tolerance compared to IR-64, which was linked to its increased antioxidant enzyme activation and metabolite synthesis. As compared to the control, all soil fertilization treatments considerably reduced the adverse impacts of high temperature on the rice cultivars. The combination of biochar and P resulted in better performance compared to the other treatments in terms of all studied attributes.
Keywords: Antioxidants, Biochar, high-temperature stress, Phosphorus fertilization, Rice cultivar, Reactive oxygen species
Introduction
The heat stress due to increasing temperature is the central dilemma in agriculture across the globe these days. Plants experience several morpho-anatomical, biochemical and physiological changes resulting from transiently or chronically elevated temperatures that affect plant growth and development which can lead to decrease in e economic yield considerably. The Earth's atmosphere is projected to warm by 2–4 °C by the end of the twenty-first century due to anthropogenic and natural factors (IPCC 2007; Eitzinger et al. 2010). Greenhouse gases (GHGs), such as nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4), released by agricultural processes are reportedly some of the significant contributors to global warming.
Increasing temperature can lead to changes in agricultural crops' geographical distribution and growing seasons, allowing earlier seasonal threshold temperatures and crop maturity (Porter 2005). Schöffl et al. (1999) reported that catastrophic cell breakdown, extreme cell injury, and even death can occur in minutes at very high temperatures. Injury or death occurs only after prolonged exposure to relatively high temperatures. Protein denaturation and increased membrane lipid fluidity and aggregation are direct injuries that can be caused by high temperatures. Mitochondrial and chloroplast enzyme inactivation, membrane integrity loss, protein synthesis inhibition, and degradation inhibition are examples of indirect or slower-acting heat injuries (Howarth, 2005). The microtubule organization in plants is also affected by heat stress, which causes them to break and elongate, phragmoplast microtubule elongation and microtubule aster development in mitotic cells (Smertenko et al. 1997). Finally, such damage leads to growth inhibition, malnutrition, ion flux reduction, and reactive oxygen species (ROS) and toxic compound generation (Schöffl et al. 1999; Howarth 2005).
The accretion of certain organic compounds with low molecular mass, known as compatible osmolytes, is a critical adaptive mechanism for abiotic stresses such as extreme temperature, water deficiency, and salinity. Quaternary ammonium compounds, osmolytes such as alcohols (polyols), tertiary sulfonium compounds, tertiary compounds, and proline accumulate under heat. Such solutes will increase plants' stress tolerance by accumulating these solutes (Hare et al. 1998; Sakamoto and Murata 2002; Sairam and Tyagi 2004). Tolerant plants have evolved various enzyme and non-enzyme ROS scavenging and detoxification processes to protect themselves from ROS's harmful effects (Apel and Hirt 2004). The activities of different antioxidant enzymes are highly susceptible to temperature, and their activation occurs at various temperatures. Plants are also protected from oxidative stress by antioxidant metabolites such as ASA, GSH, tocopherol, and carotene. As a result of increased ASA and GSH synthesis, heat-acclimated turf grass was shown to develop fewer ROS (Sairam et al. 2000; Xu et al. 2006).
The majority of the world's crops have been subjected to heat stress at different stages of their life cycle due to rising temperatures. Rice production has also been under pressure as a result of rising temperatures. Rice (Oryza sativa) is a major cereal crop that is grown throughout the world. Both wet and dry land cultivation (upland) systems are very susceptible to drought and other unpredictable weather conditions. Rice production has already been increased to satisfy the human populations' growing demands. The response of rice growth to high temperatures are still poorly understood, despite rice being used as a model plant for many years (Cassman and Wood 2005; Nagai and Makino 2009).
Rice production can be adversely affected by variations in temperature during the day. Daytime temperatures above the critical level can have a negative impact on photosynthesis by disrupting photosynthetic system II and altering the thylakoid structural organization. (Zhang et al. 2005). This increases the production of reactive oxygen species, resulting in the loss of cell material leakage, cell membrane integrity, and, finally, cell death (Howarth, 2005).
Global interest in agriculture's role in mitigating climate change by sequestering carbon in stable soil organic matter forms is increasing (Stavi and Lal, 2013). The development and application of biochar in agriculture will help mitigate climate change while also improving the quality and management of agricultural and forestry waste materials. Biochar is a carbonaceous material created from the thermal decomposition of residual biomass under low temperatures and low oxygen levels (Lehmann et al. 2006). Certain biochar properties are responsible for its high cation exchange capacity, high porosity, and high water retention ability; these properties prevent nutrient loss and favour nutrient retention, provide a direct nutrient supply depending on the biochar form, and give biochar the ability to serve as a habitat for beneficial microorganisms which help plants absorb and release nutrients (Atkinson et al. 2010).
Plant tolerance to various biotic stresses is also improved by using biochar. Since biochar increases the osmotic values of leaves, it improves plant's water status, resulting in greater resistance to future water stress conditions (Barker et al. 1993; Gonzalez et al. 2009; Kammann et al. 2011). Because of decreased transpiration, with the application of BC, higher plant growth was observed. This, combined with increased osmolality, could improve plant drought tolerance (Kammann et al. 2011). Kammann et al. (2011) also reported that biochar-treated plants used slightly less water despite their larger leaf areas. Graber et al. (2010) hypothesized that plants such as Arabidopsis thaliana in soil modified with biochar could respond with a resistance mechanism to the presence of low ( ±)-catechin levels causing stress due to the lower levels of phytotoxic compounds within the root zone (Prithiviraj et al. 2007). A. thaliana growth exhibited an inverted U-shaped reaction to inundation with ( ±)-catechins, which are phytotoxic at high concentrations. At low ( ±)-catechin concentrations, which promote growth, the leaves of plants inoculated with Pseudomonas syringae pv. tomato, a pathogen of A. thaliana, developed limited lesions only at inoculation sites. The control plants exhibited widespread infection, indicating systemically induced resistance to phytotoxic compound development (Prithiviraj et al. 2007).
To maintain the plant structural integrity and vital physiological processes, adequate nutrition is needed. P is an essential plant macronutrient that accounts for approximately 0.2% of a plant's dry weight. It is present in nucleic acids, phospholipids, phosphoproteins, dinucleotides, and adenosine triphosphate, among other substances. Phosphorus is also necessary for energy production and storage (Waraich et al. 2012; Hasanuzzaman et al. 2013). The application of exogenous protectants (potassium, K, calcium, Ca, nitrogen, N; phosphorus, P;, etc.) has been found in recent years to effectively reduce damage caused to plants by HT stress (Waraich et al. 2011).
Few studies have examined the effect of phosphorus and biochar on plants under different stress conditions. Moreover, no data are available regarding stress from high day and night temperatures, which creates a significant gap in the literature on this crucial subject. This study examined how the antioxidant protection system and osmolyte and ROS accumulation in the plant leaves and xylem sap of two rice cultivars under high day and night temperatures were affected by biochar and P fertilization.
Materials and methods
Crop husbandry
Pot experiments were conducted using two cultivars of rice (Oryza sativa L.), an indica type, viz, Huanghuazhan (HHZ), and IR-64. Both cultivars exhibit different response to temperature stress but have similar plant architecture (medium stature). IR-64 is highly temperature sensitive, while Huanghuazhan is tolerant of high-temperature stress. The rice plants were grown under natural conditions. Both cultivars' seeds were retained for two days in a wet towel to accelerate germination. After germination, the seeds were planted in seedling growing trays (1 seed per cell). The seedling plants were transferred into plastic pots three weeks after sowing. The plants were grown in the plastic pots (upper interior diameter, 27.2 cm; height, 27.2 cm; and thickness, 0.15 cm) with air-dried soil and sand mixed at a ratio of 2:1 (21.6 cm) after transplantation. A compound (NPK) fertilizer was applied at a rate of 10 g per pot. The trial was conducted using standard pot test procedures, and no disease or pest problems were found.
Greenhouse conditions
Three indoor temperature-controlled growth chambers were established, i.e., HDT (high day temperature, 35 ± 2 °C), HNT (high night temperature, 32 ± 2 °C), and AT (high ambient temperature, 28 ± 2 °C daily), for the three temperature treatments (Table 1). The day temperatures were applied from 7 am to 7 pm, and the night temperatures were applied from 7 pm to 7 am (12 h duration). The control group plants were cultivated at 29 °C (12-h-day/12-h-night cycles). The heat treatment was applied before the booting phase since most rice damage caused by high temperatures occurs during that period. During the experiment, the humidity was 75%. In the growth chamber, the photosynthetic flow density was maintained at 1000 μmol mm−2 s–1. The CO2 concentration was not measured inside the chamber.
Table 1.
Summary of analyses of variance (ANOVA) for the influence of high temperature, soil fertilization treatments, and their interactions on metabolites, antioxidants, and ROS production in leaves of two rice cultivars
| Source of Variation | Sugar | Protein | Proline | SOD | POD | CAT | APX | GR | GSH | GSSG | ASC | MDA | H2O2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR-64 | |||||||||||||
| Temperature treatments | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Soil fertilization treatments | ** | ** | * | ** | Ns | ns | ns | ** | ** | ** | ** | ** | ** |
| Temperature × SFT | ns | ns | ns | ns | Ns | ns | ns | Ns | ns | ns | ** | ns | ns |
| CV | 12.70 | 12.81 | 7.07 | 5.16 | 7.24 | 10.84 | 6.98 | 11.06 | 5.37 | 9.11 | 7.92 | 6.79 | 6.06 |
| HHZ | |||||||||||||
| Temperature treatments | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| Soil fertilization treatments | ** | ** | ** | * | ns | ns | ns | ** | * | ** | ** | ** | ** |
| Temperature × SFT | ns | ns | ns | ns | ns | ns | ns | Ns | ns | ns | ns | ns | ns |
| CV | 7.81 | 11.33 | 5.98 | 5.22 | 6.56 | 7.91 | 5.37 | 6.43 | 6.o8 | 7.66 | 6.95 | 5.99 | 4.62 |
** and * denote significance at the 0.01 and 0.05 probability level, respectively
ns non-significant, CV Coefficient of variation, PGR Plant growth regulators, SOD superoxide dismutase, POD peroxidase, CAT catalase, APX ascorbate peroxidase, GR glutathione reductase, GSH glutathione, GSSG glutathione disulfide, ASC ascorbate, HHZ Huanghuazhan (heat tolerant), IR-64 (heat susceptible)
Biochar and phosphorous fertilizer treatments
Rice husks were chosen as the biomass waste material for the biochar samples because they are commonly found in majority of areas of the World. Sigma-Aldrich in Shanghai, China, supplied the phosphate fertilizer in the form of TSP (triple superphosphate [Ca(H2PO4)2.2H2O]). Before being used in the plant growth experiment, the biochar samples were ground to a thickness of 2 mm in a stainless steel mill. All of the biochar (300 g) and phosphorous fertilizer (at a rate of 2 g) combinations were applied to each pot prior to transplantation. Biochar and phosphorous were not applied to the control pots.
Observations
Collection of xylem sap
The xylem sap was collected as described in Rahayu et al. (2005) and Dodd et al. (2004), with slight modifications. The root-shoot interface beneath the cotyledons was cut to 2 cm above the root-shoot interface for xylem sap collection. To prevent contamination of the wounded cells and phloem sap, each cut stem was washed with distilled water and then blotted with filter paper after 3 min.
In accordance with Rahayu et al. (2005), a tube of silicon was then placed over the stem. The xylem sap was driven into the tube using a xylem syringe, removed, and stored on ice for a short period immediately to avoid tube overflow. The xylem sap collection effort lasted for 3 h, and the sap samples were then frozen at − 20 °C before analysis. The sap was then analysed.
Biochemical analyses of rice leaves and xylem sap
The total soluble sugars were calculated with the ethanol extract from the plant leaves and xylem sap using the phenol–sulfuric acid method (Dubois et al. 1956). Two grams of freshly ground sample was precisely weighed out, and 5 ml of xylem sap solution was measured and boiled in 80% neutral aquatic ethanol for six hours.
Whatman No. 1 filter paper was used to filter the extract. The clear solution was filtered and then diluted with ethanol to a known amount. An aliquot of ethanol extract (10 ml) was transferred to a clean, dry beaker in a water bath and heated to dryness. The residues were then dissolved in water and transported quantitatively to the desired level in a volumetric flask of 25 ml. The mixture consisted of 1 mL of water extract, 1 mL of phenol solution, and 5 mL of sulfuric acid (96%). Measurements taken with a Nano-quant Infinite M 200 spectrophotometer were compared against measurements of a blank prepared with water instead of the sample and used to determine the O.D. at 490 nm. A plot of the O.D. values for different standard glucose solutions was used as a standard curve.
Bradford's (1976) method was used to determine the protein content of the leaves and xylem sap, while the Gilmour et al. (2000) method was used to assess the proline content. The plant leaves and panicles of every species were homogenized in 1 mL of 3% (w/v) room temperature sulfosalicylic acid and then stored at 4 °C overnight. In addition, acid ninhydrin and glacial acetic acid were mixed into the supernatant. The mixture was heated to 100 °C in a water bath for 45 min. An ice bath was then used to halt the reaction. Toluene was used to extract the mixture, and the absorbance was measured at 519 nm wavelength with a Nano-quant Infinite M 200 spectrophotometer. A calibration curve was used to measure the sample's proline concentration, which was expressed as mg proline g−1 FW.
Lipid peroxidation
The complete thiobarbituric acid-reactive substances (TBARS) content was used to estimate the oxidative damage to membrane lipids, which was expressed as MDA equivalents. The level of MDA was calculated according to Hendry et al. (1993).
Hydrogen peroxide content
The process described in Velikova et al. (2000) with modifications was used to measure the hydrogen peroxide content. Rice leaf extract (0.25 g) and a 5 ml xylem sap solution were mixed at 0 °C with 3 ml trichloroacetic acid and 0.1 g activated charcoal. A supernatant aliquot of 0.5 mL (pH 7.1) was applied to 10 mM potassium phosphate buffer (PP 7.0) with 1 M KI at 0.5 mL. The content of H2O2 was expressed in Nmol−1 FW, and the absorbance was measured at 390 nm.
Antioxidants
Leaves (0.5 g) and 5 ml of xylem sap were homogenized in a mortar and pestle for enzyme extraction in 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA, 3 mM 2-mercaptoethanol, and 2% (w/v) polyvinyl-polypyrrolidone. After centrifugation at 16,000 g, the enzyme activity in the supernatant was measured at 4 °C for 30 min.
Peroxidase activity (POD) and catalase activity (CAT) were also evaluated using the procedures of Bai et al. (2009). The solution for the POD test contained 50 mM (pH 7.8) phosphate buffer, 25 mM guaiacol, 20 mM (H2O2) and 0,50 ml of a 3 ml enzyme extract reaction solution. One unit of POD activity was expressed as a 0.01-unit/min shift in absorbance. The CAT reaction solution contained 50 mM (pH 7.0) phosphate buffer, 200 mM H2O2, and 50 L enzyme extract (3 ml). The enzyme extract was used to start the reaction. To measure the reaction solution uptake at 240 nm every 30 s, a Nano-quant (Infinite M 200 spectrophotometer was used. As for POD, absorbance shifts of 0.01 units min-1 in the CAT reaction solution were used to express CAT activity. Ascorbate peroxidase activity was determined as described in Nakano and Asada (1981). To assess ascorbate peroxidase activity, the decrease in ascorbate absorption at 290 nm was detected. The enzyme extract, 50 mM (cool, pH 7), 0.5 mM ascorbate, 0.5 mM H2O2, and 0.1 mM EDTA were included in the reaction mixture, which had a 0.3 ml final volume. The reaction started after the hydrogen peroxide was added. A molar extinction coefficient of 2.8 mM−1 cm−1 was used to calculate the ascorbate peroxidase activity. The enzyme activity was measured in units of mg−1 protein. One enzyme unit at 25 °C was required to break down 1 mol of the substrate per minute.
Glutathione reductase (GR) activity was assessed using the Foster and Hess (1980) system. The amounts of enzyme required to degrade 1 mol H2O2 per minute, resulting in the formation of 1 mol tetraguaiacol (50% controlled), and to degrade 1 mol ASA per minute as well as the reduction in A340 per minute were measured to describe the GR activity. A 3% sulfosalicylic acid extract was used to assess the contents of GSH and GSSG, as described in Smith's method (1985). The contents of GSH and GSSG were spectrophotometrically calculated with an enzyme recycling test at 412 nm. 5-dithiobis-2-nitrobenzoic acid and NADPH reduction in known amounts of GR involves the sequential oxidation of GSH. The GSSG content in the extracts was measured using 2-vinyl pyridine.
Monodehydroascorbate reductase (MDHAR) activity was calculated by monitoring NADH oxidation at 340 nm (Hossain et al. 1984). The assay used involved 50 mM K2HPO4 (pH 7.7), 150 µM NADH, 500 µM ASC, 0.4 U ascorbate, and 50 µl enzyme extract. The difference in the oxidation rate in the absence of ascorbate oxidase was determined. At 340 nm, the MDHAR activity was measured with a 6.22 mM−1 cm−1 coefficient for NADH.
Monodehydroascorbate reductase (DHAR) activity was calculated as defined by Hossain and Asada (1984), with slight modifications. The sample was then centrifuged for 20 min at 18,000 g at a temperature of 4 °C in an extraction buffer (50 mM Tris HCl, pH 7.4, 100 mM NaCl, 2 mM EDTA, and 1 mM MgCl2). The test result was calculated as the ASC output by measuring the increase in absorption at 265 nm of 50 mM K2HPO4/KH2PO4, pH 6.5, 0.5 mM DHA and 1 mM GSH. An extinguishing coefficient of 14 mM-1 cm-1 was used to calculate the DHAR activity for ASC at 265 nm.
Ascorbate (ASC) and Dehydroascorbate (DHA) enzyme assays for the rice leaves and xylem sap
The ascorbate content was determined following the Foyer et al. (1983) method, with some modifications. Ground samples were added to liquid nitrogen with 0.25 g of perchloric acid, and 1 ml 0.25 M perchloric acid was added to 5 ml of sap. The crude extract was centrifuged at 4 °C at 10.000 g for ten minutes, and an aliquot of the supernatant (0.5 ml) and 0.1 ml 0.12 M NaH2PO4 were decanted into a different test tube (pH 5.6). Afterward, K2CO3 was added drop wise to raise the pH to 5–6. In this case, centrifugation was performed, and the supernatant was removed to test for ASC, DHA and insoluble KClO4. The ASC content was calculated spectrophotometrically at 265 nm in 1 M NaH2PO4 buffer, pH 5.6, with 1 U ascorbate oxidase. After incubating with 50 mM dithiothreitol, the overall ascorbate content was measured (DTT). The difference in ascorbate content from that in the reaction with DHA was taken as the total ascorbate contents.
Statistical analysis
All treatments were replicated four times. The data collected were analysed using Statistics 9.0 software (Analytical Software, Tallahassee, FL, USA) in a completely randomized three-factor design with the least significant difference (LSD) test. The links between various attributes were calculated using a SIGMA plot with polynomial regression (Systat Software Inc, San Jose, CA, USA).
Results
Production of Osmolytes in leaves and xylem sap
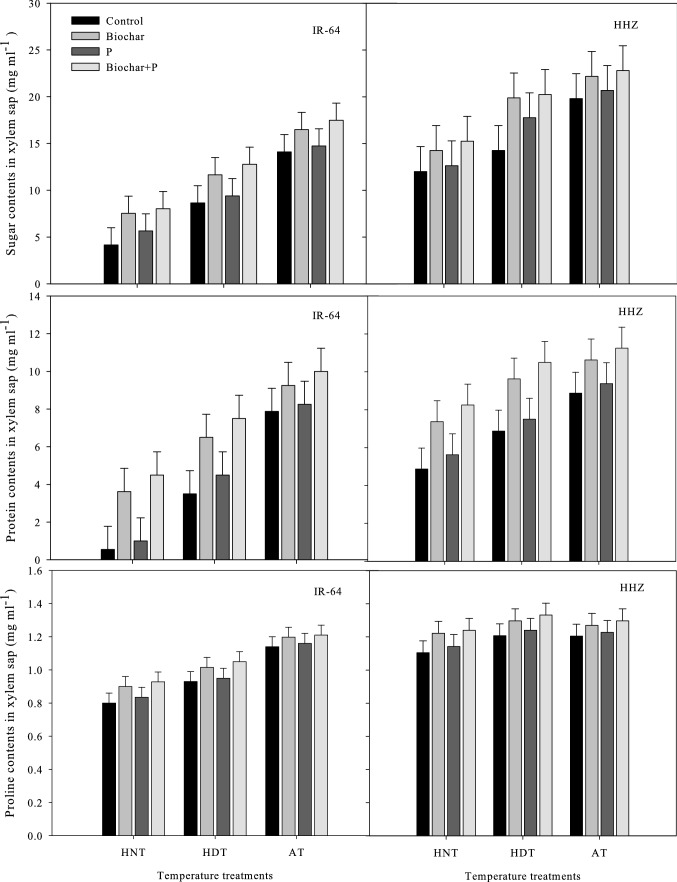
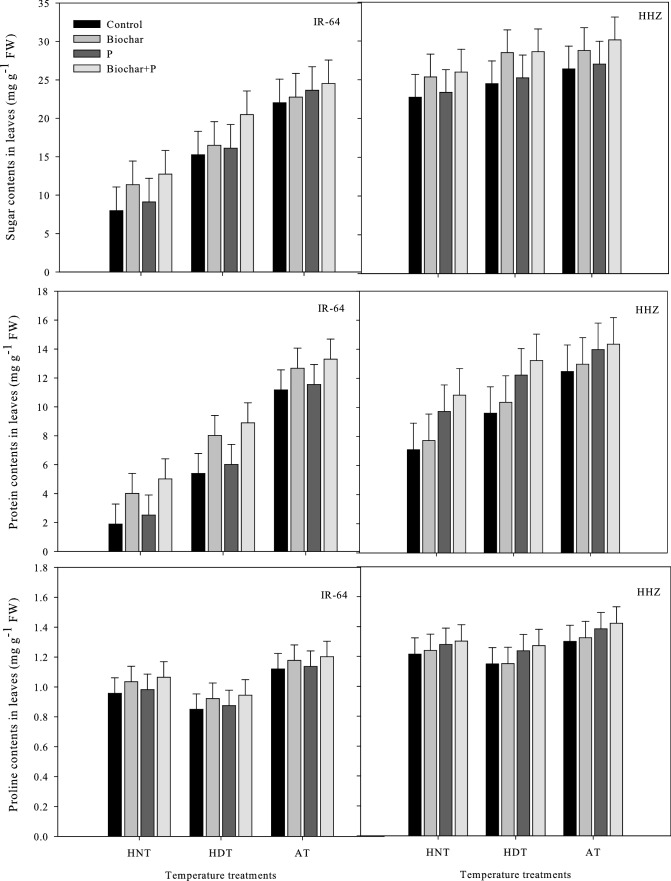
In the xylem sap (Fig. 1) and leaves (Fig. 2) of both rice cultivars, significant variations in the total soluble sugars, soluble protein, and soluble proline contents were observed under high-temperature stress and various soil fertilization treatments. However, the interactive effects of high-temperature stress and soil modifications were not significant (Tables 1 and 2).
Fig. 1.
Influence of high temperature stress and soil fertilization treatments on the osmolyte production in xylem sap of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
Fig. 2.
Influence of high temperature stress and soil fertilization treatments on the osmolyte production in leaves of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
Table 2.
Summary of analysis of variance (ANOVA) for the influence of high temperature, soil fertilization treatments, and their interactions on metabolites, antioxidants, and ROS production in xylem sap of two rice cultivars
| Source of Variation | Sugar | Protein | Proline | SOD | POD | CAT | As | GR | GSH | GSSG | ASC | MDA | H2O2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR-64 | |||||||||||||
| Temperature treatments | ** | ** | ** | ** | ** | * | ** | ** | ** | ** | ** | ** | ** |
| Soil fertilization treatments | ** | ** | ** | ** | * | ** | ** | ** | ** | ** | ** | ** | ** |
| Temperature × SFT | ns | ns | ns | * | ns | ns | ns | ** | ns | ns | ** | Ns | ns |
| CV | 11.79 | 11.38 | 4.18 | 5.67 | 7.24 | 5.84 | 7.78 | 12.53 | 5.37 | 9.11 | 7.92 | 6.79 | 6.06 |
| HHZ | |||||||||||||
| Temperature treatments | ** | ** | ** | ** | * | ** | ** | ** | ** | ** | ** | ** | ** |
| Soil fertilization treatments | ** | ** | ** | * | * | ** | ** | ** | * | ** | ** | ** | ** |
| Temperature × SFT | ns | ns | ns | ns | * | ns | ns | ns | ns | ns | ns | ns | Ns |
| CV | 10.50 | 6.88 | 4.04 | 4.78 | 6.56 | 7.91 | 6.97 | 7.38 | 6.08 | 7.66 | 6.95 | 5.99 | 4.62 |
** and * denote significance at the 0.01 and 0.05 probability level, respectively
ns non-significant, CV Coefficient of variation, PGR Plant growth regulators, SOD superoxide dismutase, POD peroxidase, CAT catalase, APX ascorbate peroxidase, GR glutathione reductase, GSH glutathione, GSSG glutathione disulfide, ASC ascorbate, HHZ Huanghuazhan (heat tolerant), IR-64 (heat susceptible)
Compared to AT, the high-temperature treatments significantly decreased the soluble proline content, soluble sugar content, and soluble protein content in leaves and xylem sap (p ≤ 0.01). However, high nocturnal temperatures decreased the osmolyte contents of both cultivars more compared to HDT, though the xylem sap of both cultivars was shown to be lower in osmolytes under HDT than under AT and HNT (Fig. 2). The combined use of biochar and P was the best treatment for osmolyte production of all the other soil modification treatments. Biochar alone was the second best treatment for the leaves and xylem sap of both cultivars, except with regard to the proline content of young leaves. P resulted in higher proline contents than biochar alone under HNT, followed by the combined use of biochar + P.
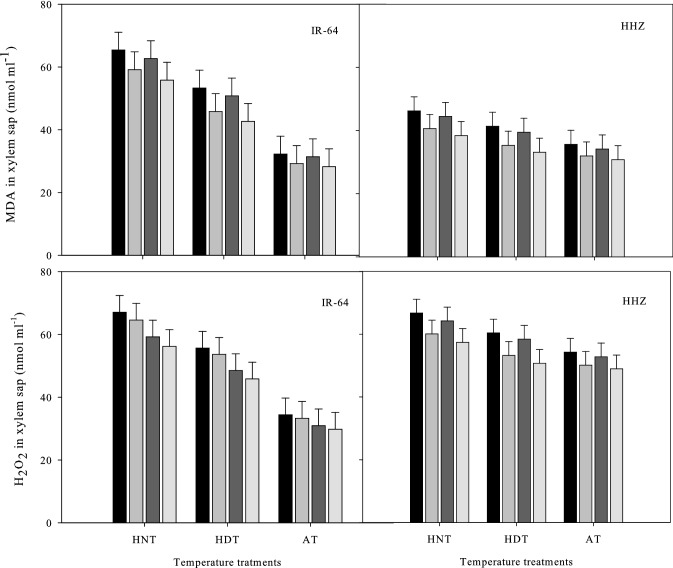
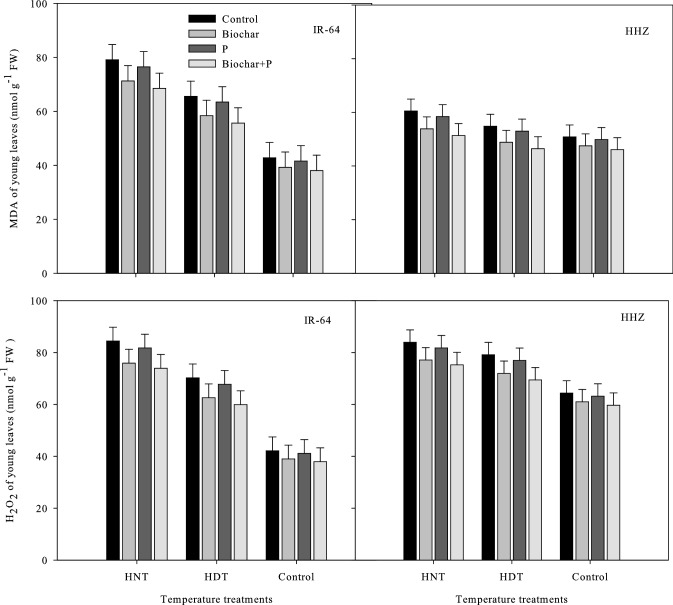
Lipid peroxidation and Hydrogen peroxide production in leaves and xylem sap
The contents of H2O2 and MDA in the xylem sap (Fig. 3) and leaves (Fig. 4) of the two rice cultivars were significantly influenced by the high-temperature stress and soil treatments, as shown in Tables 1 and 2, respectively. In the leaves and xylem sap of both cultivars, higher MDA and H2O2 levels were observed under HNT compared to under HDT and AT (Figs. 3 and 4). Additionally, the H2O2 content in IR-64 seedlings under the high-temperature treatments ranged between 84–47 nmol/g FW and 37–42 nmol/g FW, whereas that in sap ranged from 29–12 nmol/g FW to 67–43 nmol/g FW. H2O2 levels were between 59.66 and 83.94 nmol/g FW in leaves and between 48.95 and 66.57 nmol/g FW in xylem sap. The combination of biochar and P was shown to be far more effective at reducing the adverse impacts of high temperatures compared to the other soil modification treatments. Of all treatments, this treatment resulted in the lowest MDA and H2O2 levels. Biochar alone was the second best treatment with regard to these characteristics (Figs. 3 and 4).
Fig. 3.
Influence of high temperature stress and soil fertilization treatments on MDA and H2O2 contents in xylem sap of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
Fig. 4.
Influence of high temperature stress and soil fertilization treatments on MDA and H2O2 contents in leaves of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
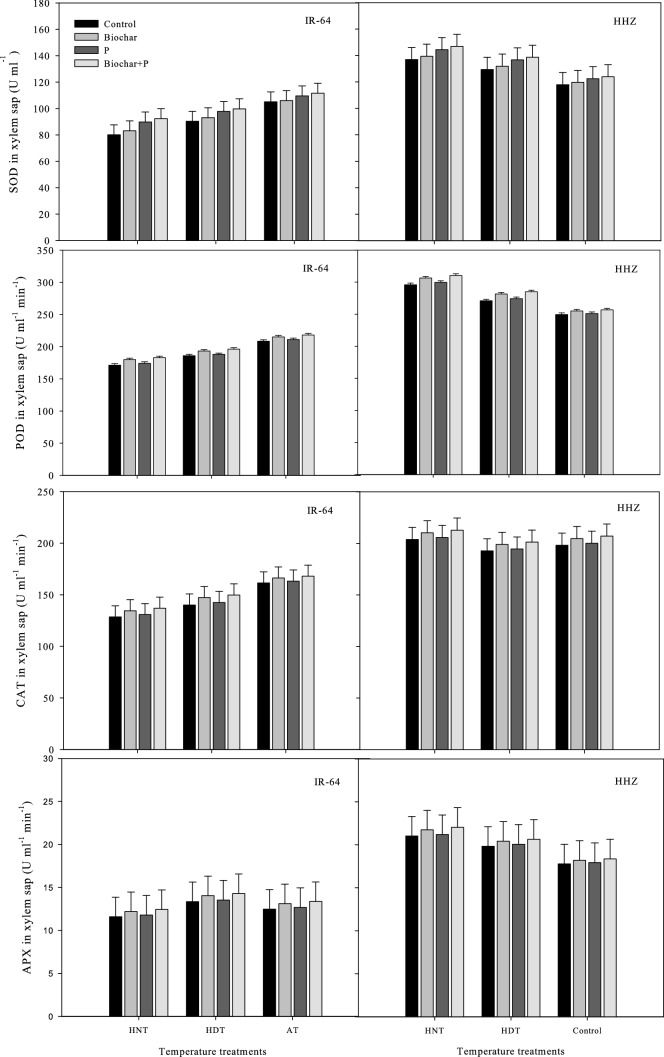
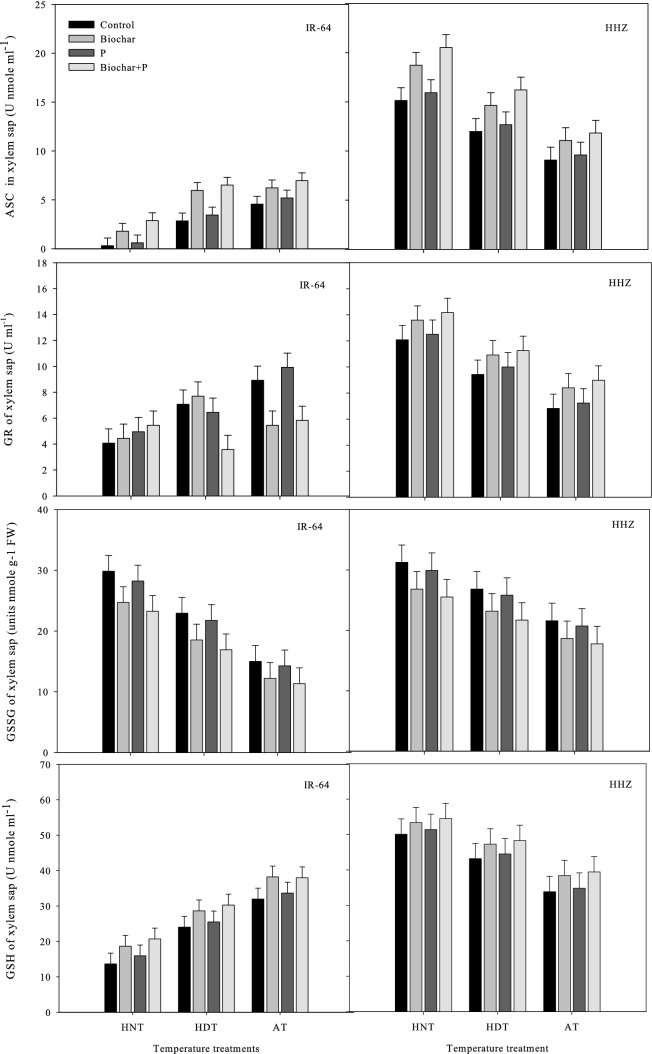
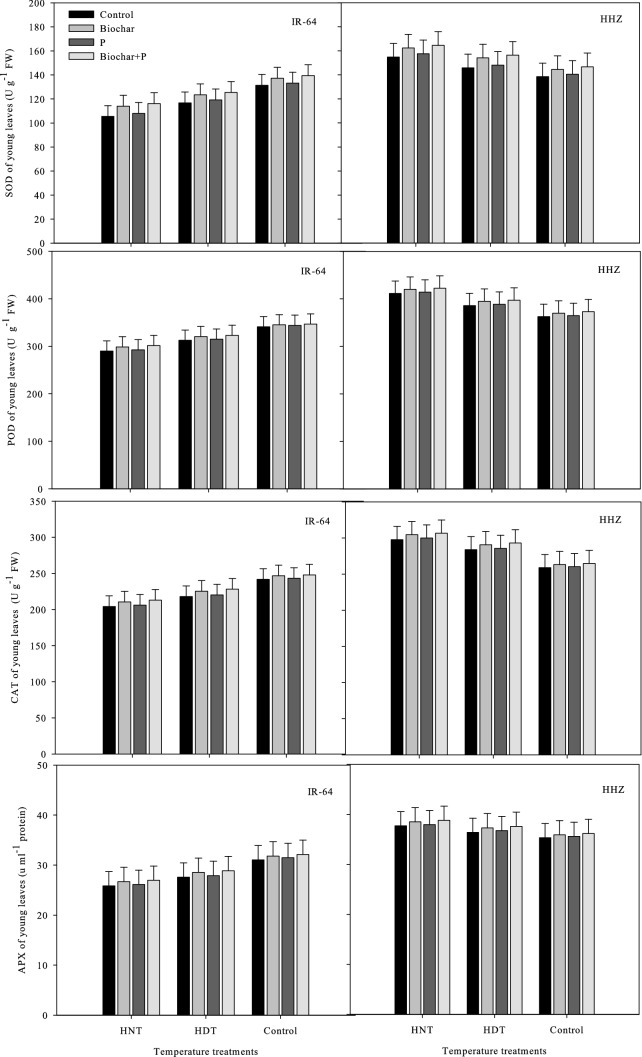
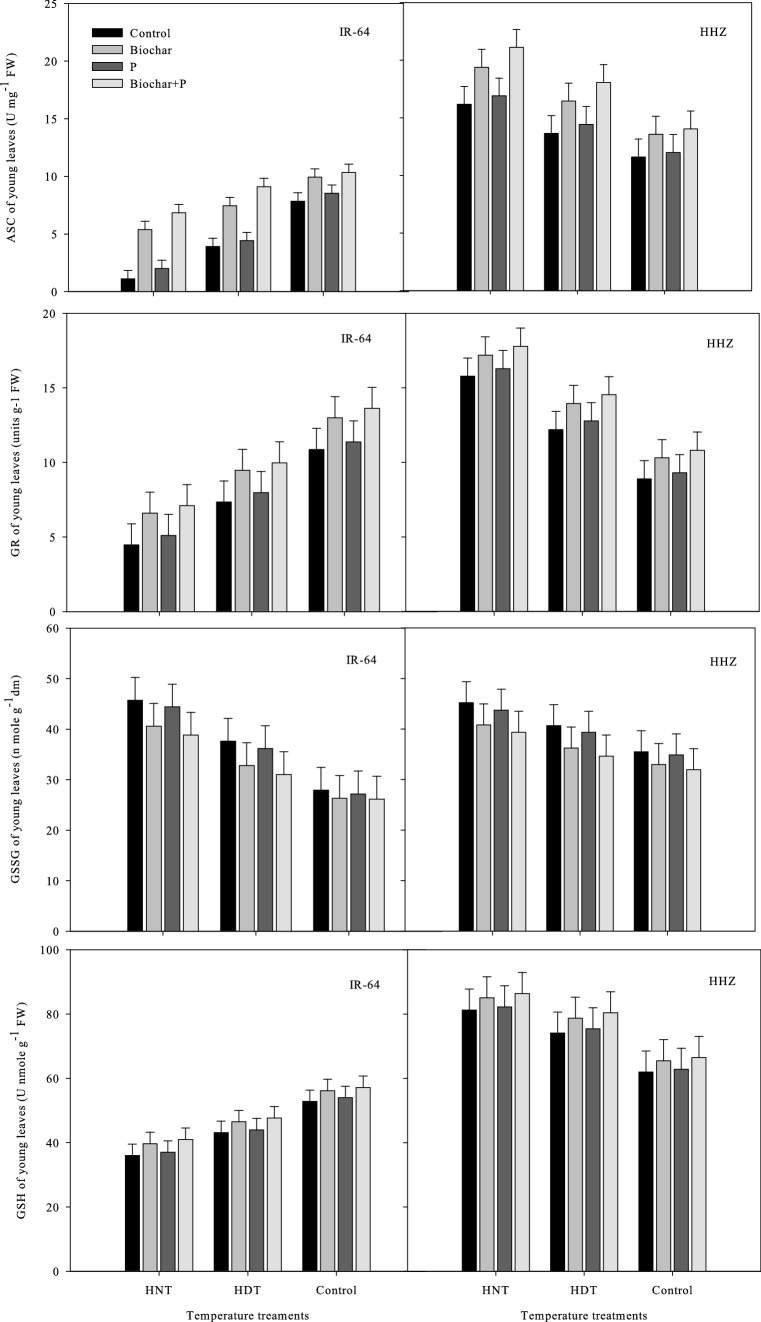
Antioxidant production in leaves and xylem sap
In the leaves and xylem sap of the rice cultivars, the concentrations of antioxidant enzymes such as ASC, GR, GSSG, SOD, APX, POD, CAT, and GSH were significantly different under high-temperature stress and the soil treatments (Figs. Figs. 5,6,7and8). Nonetheless, there was an insignificant interactive effect of both factors on the rice cultivar IR-64, on the SOD and GR concentrations in sap in HHZ, and in the POD activity in xylem sap (p ≤ 0.05) (Tables 1 and 2).
Fig. 5.
Influence of high temperature stress and soil fertilization treatments on SOD, POD, CAT and APX activities in xylem sap of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
Fig. 6.
Influence of high temperature stress and soil fertilization treatments on ASC, GSH, GR and GSSC activities in xylem sap of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
Fig. 7.
Influence of high temperature stress and soil fertilization treatments on SOD, POD, CAT and APX activities in leaves of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
Fig. 8.
Influence of high temperature stress and soil fertilization treatments on ASC, GSH, GR and GSSC activities in leaves of two rice cultivars. Error bars above means denote LSD of interaction at the 0.05 probability level. HHZ: Huanghuazhan (heat tolerant), IR-64 (heat susceptible). HDT: high day temperature, HNT: high night temperature, AT: ambient temperature (control)
The antioxidant activity was higher in young leaves, but not in xylem sap, under HNT in both IR-64 and HHZ. Additionally, cv. IR-64 produced fewer antioxidants under HNT compared to under AT and produced the least under HDT. HHZ showed the opposite results, exhibiting higher antioxidant activity under HNT. These results suggest that HHZ has a more efficient ROS scavenging mechanism than IR-64. The most effective soil amendment treatment for minimizing the adverse effects of high-temperature stress and activating antioxidants in the leaves and xylem sap of both cultivars was the combination of biochar and P (Figs. 5,6,7and8).
Under biochar + P application, all antioxidants were highly regulated in the leaves and xylem sap of both cultivars, and this treatment resulted in higher antioxidant activity in both cultivars compared to other treatments. In both cultivars, applying biochar alone was observed as the second best treatment in terms of antioxidant activity. GSSG was the only antioxidant that exhibited contrasting activity patterns; it showed higher activity under the control treatment and P alone. This indicates that the use of biochar can decrease the activity of certain antioxidants while increasing the activity of other antioxidants.
Discussion
In this study, the impact of biochar use on the accumulation of osmolytes, the production of ROS, and antioxidation in leaves and xylem sap in two rice cultivars were examined with and without P under high-temperature stress. In both rice cultivars, the accumulation of various metabolites, such as protein, free proline, and total soluble sugar, was significantly reduced by high-temperature stress. Jain et al. (2007) have also reported similar results. By reducing carbohydrate metabolism in some genes, high-temperature regimens can alter carbon metabolising enzymes, sucrose synthesis, and starch accumulation. Furthermore, most upstream molecular malfunctions that result in altered carbohydrate metabolism and deficiencies under high-temperature conditions will disrupt invertase-mediated saccharose hydrolysis in the cell wall, resulting in saccharose biosynthesis failure. Previous studies have shown that the leading substances for regulating osmotic damage and protecting the cell membrane structure under different stress conditions are the soluble sugars and protein that are excreted by plants (Jain et al. 2007; Zhang et al. 2007; Ruan et al. 2010).
We have observed significant variation in antioxidant activity in the young leaves and xylem sap of both cultivars when they were exposed to various high-temperature treatments. Of the two cultivars, the antioxidant activity of HHZ, i.e., the GSH, GR, ASC, APX, CAT, POD, and SOD activities, were higher in the leaves and xylem sap at high temperatures. In contrast, the antioxidant activity of IR-64 at high temperatures was lower compared to that at AT. HHZ was more resistant to high temperatures, which may be attributable to its ability to maintain higher antioxidant activity even at high temperatures. The high sensitivity of IR-64 to high-temperature stress could be due to the low antioxidant activity in its young leaves and xylem sap. SOD is a metalloenzyme involved in the catabolism of O2- to O2 and H2O2. It is formed in several cell compartments. Changes in SOD activity and the upregulation of this enzyme are used as indicators of changes in O2• output (Alscher et al. 2002; Faize et al. 2011). The higher SOD activity in HHZ leaves and xylem sap could indicate that the O2- output of Huanghuazhan was lower compared to that of IR-64 or that HHZ plants were better able to sustain SOD detoxification activity under HNT and HDT stress. POD, CAT and H2O2 are also essential antioxidants which directly remove O2. The leaves and xylem of the heat-stressed HHZ plants in our sample showed higher POD, CAT, APX, ASC, GR, GSSG, and GSH activities compared to those of IR-64. Additionally, in our experiments, the activities of GSH, GSSG, CAT, ASC, APX, GR, and POD were higher in the leaves and xylem sap of the heat-stressed HHZ plants compared to IR-64. ROS development was accelerated in some plants in this study, as indicated by GR, ASC, POD, GSSG, APX, GSH, and CAT behaviour. In several studies, CAT and POD levels in various crops have been observed as higher under high-temperature stress (Liu and Huang, 2000; Sairam et al. 2000; Almeselmani et al. 2006; França et al. 2007; Faize et al. 2011). Increased POD and CAT activities can detoxify H2O2 due to stress and in respond to the increased accumulation of H2O2 during stress. However, in the case of wheat, increased POD activity did not prevent excessive ROS production; a decrease in POD activity was reported under high-temperature stress (Almeselmani et al. 2006; Kumar et al. 2008).
Similarly, an increase in CAT activity under heat stress was reported by Demiral and Turkan (2004), while CAT activity was reduced in rice roots under cadmium stress, as observed by Hu et al. (2009). The role of APX in H2O2 scavenging in chloroplasts without CAT is well known (Asada and Takahashi, 1987). Several studies of annual crops have shown that APX activities have increased due to heat stress and that heat-tolerant cultivars have increased in popularity more rapidly compared to heat-sensitive cultivars (Dash and Mohanty, 2002; Sairam et al. 2000; Dash and Mohanty 2002; Almeselmani et al. 2006).
Our results showed that APX activity in rice leaves and xylem increased under HDT and HNT stress, possibly because chloroplasts increased H2O2 scavenging mechanisms and prevented the accumulation of H2O2, which led to less heat damage. Another important antioxidant, GSSG, is highly regulated when plants are exposed to stress. However, in our study, this antioxidant was generated by IR-64 more under HNT compared to under AT and was more abundant in HHZ under HNT. This may have occurred because the ratio of ROS production to antioxidant activation was very high ROS production resulted in excessive ROS accumulation. HHZ showed HNT tolerance, on the other hand, which may be due to the higher GSSG output in its new leaves and xylem sap. GSH is also a vital sulfur source and an important antioxidant. It maintains a cellular redox balance and thereby scavenges ROS (such as H2O2). GSH was shown to reduce ROS and regenerate an additional antioxidant, ASC, during the ascorbate glutathione cycle. Through the ascorbate peroxidase reaction, the GSH reduces ROS and converts H2O2 to H2O with ASC. Our work suggests that HHZ counteracts oxidation caused by high-temperature stress by increasing the output of these antioxidants (Shi et al. 2001). Similar findings have also been reported by Jha et al. (2014) which suggests that crop plant tolerance to high-temperature stress is linked to increased antioxidant enzyme activity.
The treatments with various soil amendments, such as biochar + P application, biochar application alone, P application alone were used to reduce the adverse effects of high-temperature stress. The biochar + P treatment was the most effective soil amendment treatment for preventing oxidation in rice plants by modulating antioxidant activity and osmolyte accumulation (Glaser et al. 2002; Beesley et al. 2010). Previous research has shown that biochar has a positive impact on plant growth and regulation under drought and salt stress and in polluted soil, but this study aimed to determine how biochar affects rice plants under high-temperature stress (Parvage et al. 2013). Our results revealed that the accumulation of proline, soluble protein, and soluble sugars in the leaves and xylem sap of both rice cultivars increased, but IR-64 plants exhibited lower accumulation than HHZ plants. This may have been due to differences in the cultivars' genetic capacity to withstand high temperatures, as well as differences in osmolyte accumulation in the rice plant leaves and xylem sap. Under water-stressed conditions, biochar may be used as an ameliorating agent to improve tomato crop productivity (Akhtar et al. 2014). According to recent studies, the application of biochar may be a viable tactic for increasing the productivity of crops (Major et al. 2010; Ventura et al. 2013; Basso et al. 2013). Some studies have shown that high temperatures minimize enzyme activity and result in metabolome reconfiguration (i.e., alterations in signalling molecules, hormones, and other metabolic intermediates) (Kaplan et al. 2004; Cook et al. 2004). Our observations suggests the efficiency of biochar and resulted in improved thermal stability and high-temperature production in rice.
Increased levels of antioxidants can help protect enzymes and cell membrane integrity from damage caused by heat stress-induced ROS. We observed that MDA and H2O2 production in the xylem sap and leaves of both cultivars was lower in the control treatment compared to when biochar was applied alone. Compared to the control, P application alone resulted in a significant reduction in peroxidation in the young leaves and xylem sap. This may be associated with the increased production and up regulation of many ROS antioxidants. Rice plants respond to increased oxidation in leaves and xylem sap by accumulating osmolytes for osmotic regulation and producing more antioxidants to scavenge ROS. However, compared to a control to which no soil amendments were applied for amelioration, our soil amendment treatments accelerated the process of osmolyte accumulation. By increasing osmolyte accumulation and antioxidant activity, biochar + P was observed as the most suitable combination for preventing the oxidation of various biological membranes and organelles. This is supported by our results showing that after biochar + P, biochar alone was the second most effective treatment, followed by P alone. This indicates that biochar may have a synergistic effect on P consumption, which would increase ATP production and provide the plants with energy to resist high-temperature stress (Cui et al. 2011; Parvage et al. 2013). Similar results have been published in other studies on the application of biochar to soils, indicating that biochar can increase or decrease P uptake (Rondon et al. 2007).
Furthermore, the use of biochar can increase the activity of certain enzymes as well as mineral absorption. Our observations are consistent with those of Wang et al. (2014), who suggested that use of biochar increased GPX, CAT, POX, and SOD activity. They also observed that biochar improved plant antioxidant capacity and mitigated oxidative stress damage by lowering phenolic acid concentrations.
Conclusion
High-temperature stress reduced osmolyte synthesis, increased ROS production, and altered the antioxidative defence systems of both rice cultivars, particularly at night. Compared to the control, the biochar and phosphorus (P) fertilization treatments significantly reduced the harmful effects of high-temperature stress. For the majority of the tested characteristics, biochar + P application outperformed the other treatments. Compared to IR-64, Huanghuazhan performed better and accumulated more osmolytes and fewer ROS under high-temperature stress. The better tolerance of Huanghuazhan was correlated with increased antioxidant activities under HDT and HNT stress.
Acknowledgements
This project was funded by the deanship of scientific research (DSR) at King Abdulaziz University, Jeddah, under Grant No. G: 463-130-1440. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Author’s contributions
Hesham Alharby;: Conceptualization, Data curation, Visualization. Atif Bamagoos: Supervision, Project administration, Funding acquisition Investigation, Methodology, Resources. Atif Bamagoos: Investigation, Software, Validation. Shah Fahad: Methodology, Validation, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the deanship of scientific research (DSR) at King Abdulaziz University, Jeddah, under Grant No. G: 463-130-1440. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Consent to publish
Our manuscript does not contain data from any individual person, so it is “Not applicable.”
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Atif Bamagoos and Shah Fahad are the co-first author names and contributed equally to this work.
Contributor Information
Atif Bamagoos, Email: abamagoos@kau.edu.sa.
Hesham Alharby, Email: halharby@kau.edu.sa.
Shah Fahad, Email: shah_fahad80@yahoo.com.
References
- Akhtar SS, Li G, Andersen MN, Liu F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric Water Manag. 2014;138:37–44. doi: 10.1016/j.agwat.2014.02.016. [DOI] [Google Scholar]
- Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006;171:382–388. doi: 10.1016/j.plantsci.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331e1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Ann Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam, The Netherlands: Elsevier; 1987. pp. 227–287. [Google Scholar]
- Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits frombiochar application to temperate soils: a review. Plant Soil. 2010;33:1–18. doi: 10.1007/s11104-010-0464-5. [DOI] [Google Scholar]
- Bai TH, Li CY, Ma FW, Shu HR, Han MY. Exogenous salicylic acid alleviates growth inhibition and oxidative stress induced by hypoxia stress in Malus robusta Rehd. J Plant Growth Regul. 2009;28:358–366. doi: 10.1007/s00344-009-9104-9. [DOI] [Google Scholar]
- Barker DJ, Sullivan CY, Moser LE. Water deficit effects on osmotic potential, cell wall elasticity, and proline in five forage grasses. Agron J. 1993;85:270–275. doi: 10.2134/agronj1993.00021962008500020020x. [DOI] [Google Scholar]
- Basso AS, Miguez FE, David AL, Robert H, Westgate M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy. 2013;5(2):132–143. doi: 10.1111/gcbb.12026. [DOI] [Google Scholar]
- Beesley L, Moreno-Jimenez E, Gomez-Eyles JL. Effects of biochar and green waste compost amendments on mobility, bioavailability, and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut. 2010;158:2282e2287. doi: 10.1016/j.envpol.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassman KG, Wood S (2005) Cultivated Systems. In: Ecosystem and human well-being: Current state and trends: findings of the Conditions and Trends Working Group/ edited by Rashid Hassan, Robert Scholes, Neville Ash. pp 747–787.
- Cook D, Fowler S, Fiehn O, Thomashow MF. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci U S A. 2004;101:15243–15248. doi: 10.1073/pnas.0406069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui HJ, Wang MK, Fu ML, Ci E. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J Soils Sediments. 2011;11(7):1135–1141. doi: 10.1007/s11368-011-0405-9. [DOI] [Google Scholar]
- Dash S, Mohanty N. Response of seedlings to heat-stress in cultivars of wheat: Growth temperature-dependent differential modulation of photosystem 1 and 2 activity, and foliar antioxidant defense capacity. Plant Physiol. 2002;159:49–59. doi: 10.1078/0176-1617-00594. [DOI] [Google Scholar]
- Demiral T, Turkan I. Does exogenous glycine betaine affect antioxidative system of rice seedlings under NaCl treatment? J Plant Physiol. 2004;161:1089–1100. doi: 10.1016/j.jplph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Ngo C, Turnbull CGN, Beveridge CA. Effects of nitrogen supply on xylem cytokinin delivery, transpiration and leaf expansion of pea genotypes differing in xylem cytokinin concentration. Funct Plant Biol. 2004;31(9):903–911. doi: 10.1071/FP04044. [DOI] [PubMed] [Google Scholar]
- Dubois M, Smith F, Gilles KA, Hamilton JK, Rebers PA. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Eitzinger J, Orlandini S, Stefanski R, Naylor REL. Climate change and agriculture: introductory editorial. J Agric Sci Camb. 2010;148:499–500. doi: 10.1017/S0021859610000481. [DOI] [Google Scholar]
- Faize ML, Burgos L, Faize A, Piqueras E, Nicolas G, Barba-Espin MJ, Alcobendas C-M, Artlip T, Hernandez JA. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599e2613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- Foster JG, Hess JL. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980;66:482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Rowell J, Walker D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- França MB, Panek AD, Eleutherio ECA. Oxidative stress and its effects during dehydration. Comp Biochem Physiol Part A. 2007;146:621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. Over expression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000;124:1854–1865. doi: 10.1104/pp.124.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B, Lehmann J, Zech W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils. 2002;35:219–230. doi: 10.1007/s00374-002-0466-4. [DOI] [Google Scholar]
- Gonzalez JA, Gallardo M, Hila LM, Rosa M, Prado FE. Physiological responses of quinoa (Chenopodium quinoa Willd.) to drought and waterlogging stresses: dry matter partitioning. Bot Stud. 2009;50:35–42. [Google Scholar]
- Graber ER, Meller-Harel Y, Kolton M, Cytryn E, Silber A, Rav David D, Tsechansky L, Borenshtein M, Elad Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil. 2010;337:481–496. doi: 10.1007/s11104-010-0544-6. [DOI] [Google Scholar]
- Hare PD, Cress WA, Staden JV. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998;21:535–553. doi: 10.1046/j.1365-3040.1998.00309.x. [DOI] [Google Scholar]
- Hasanuzzaman M, Nahar K, Fujita M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz MM, Prasad MNV, editors. Ecophysiology and responses of plants under salt stress. New York, NY, USA: Springer; 2013. pp. 25–87. [Google Scholar]
- Hendry GAF, Thorpe PC, Merzlyak MN. Stress Indicators: Lipid Peroxidation. In: Hendry GAF, Grime JP, editors. Methods in comarative plant ecology. London: Chapman and Hall; 1993. pp. 85–92. [Google Scholar]
- Hossain MA, Asada K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol. 1984;25:85–92. [Google Scholar]
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. [Google Scholar]
- Howarth CJ. Genetic improvements of tolerance to high temperature. In: Ashraf M, Harris PJC, editors. Abiotic stresses: plant resistance through breeding and molecular approaches. New York: Howarth Press Inc; 2005. [Google Scholar]
- Hu YL, Ge Y, Zhang CH, Ju T, Cheng W. Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen per oxide pretreatment. Plant Growth Regul. 2009;59:51–61. doi: 10.1007/s10725-009-9387-7. [DOI] [Google Scholar]
- Jain M, Prasad PVV, Boote KJ, Allen LH, Jr, Chourey PS. Effects of season long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench) Planta. 2007;227:67–79. doi: 10.1007/s00425-007-0595-y. [DOI] [PubMed] [Google Scholar]
- Jha UC, Bohra A, Singh NP. Heat stress in crop plants: its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breeding. 2014;133(6):679–701. doi: 10.1111/pbr.12217. [DOI] [Google Scholar]
- Kammann CI, Linsel S, Gößling JW, Koyro HW. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil. 2011;345:195–210. doi: 10.1007/s11104-011-0771-5. [DOI] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. Exploring the temperature stress metabolome of Arabidopsis. Plant Physiol. 2004;136:4159–4168. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Tewari RK, Sharma PN. Cadmium enhances generation of hydrogen peroxide and amplifies activities of catalase, peroxidases and superoxide dismutase in maize. J Agron Crop Sci. 2008;194:72–80. doi: 10.1111/j.1439-037X.2007.00285.x. [DOI] [Google Scholar]
- Lehmann J, Gaunt J, Rondon M. Biochar sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Chang. 2006;11:395–419. doi: 10.1007/s11027-005-9006-5. [DOI] [Google Scholar]
- Liu X, Huang B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000;40:503–510. doi: 10.2135/cropsci2000.402503x. [DOI] [Google Scholar]
- Major J, Rondon M, Molina D, Riha SJ, Lehmann J. Maize yield and nutrition during 4 years after biochar application to a Colombian savannah oxisol. Plant Soil. 2010;333:117e128. doi: 10.1007/s11104-010-0327-0. [DOI] [Google Scholar]
- Nagai T, Makino A. Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant Cell Physiol. 2009;50:744–755. doi: 10.1093/pcp/pcp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Parvage MM, Ulen B, Eriksson J, Strock J, Kirchmann H. Phosphorus availability in soils amended with wheat residue char. Biol Fertil Soils. 2013;49:245–250. doi: 10.1007/s00374-012-0746-6. [DOI] [Google Scholar]
- Porter JR. Rising temperatures are likely to reduce crop yields. Nat. 2005;436:174. doi: 10.1038/436174b. [DOI] [PubMed] [Google Scholar]
- Prithiviraj B, Perry LG, Badri DV, Vivanco JM. Chemical facilitation and induced pathogen resistance mediated by a root-secreted phytotoxin. New Phytol. 2007;173:852–860. doi: 10.1111/j.1469-8137.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) (2007). Climate change and its impacts in the near and long term under different scenarios. In: The Core Writing Team, RK Pachauri & A Reisinger (Eds) Climate Change 2007: Synthesis Report, pp 43–54. Geneva, Switzerland: IPCC.
- Rahayu YS, Walch-Liu P, Neumann G, Römheld V, Wirén NV, Bangerth F. Root-derived cytokinins as longdistance signals for NO3 −induced stimulation of leaf growth. J Exp Bot. 2005;56(414):1143–1152. doi: 10.1093/jxb/eri107. [DOI] [PubMed] [Google Scholar]
- Rondon MA, Lehmann J, Ramírez J, Hurtado M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils. 2007;43:699–708. doi: 10.1007/s00374-006-0152-z. [DOI] [Google Scholar]
- Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS. Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant. 2010;3:942–955. doi: 10.1093/mp/ssq044. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–421. [Google Scholar]
- Sairam RK, Srivastava GC, Saxena DC. Increased antioxidant activity under elevated temperatures, a mechanism of heat stress tolerance in wheat genotypes. Biol Plant. 2000;43:245–251. doi: 10.1023/A:1002756311146. [DOI] [Google Scholar]
- Sakamoto A, Murata N. The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 2002;25:163–171. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- Schöffl F, Prandl R, Reindl A. Molecular responses to heat stress. In: Shinozaki K, Yamaguchi-Shinozaki K, editors. Molecular responses to cold, drought, heat and salt stress in higher plants. Austin, Texas: R. G. Landes Co; 1999. pp. 81–98. [Google Scholar]
- Shi WM, Muramoto Y, Ueda A, Takabe T. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene. 2001;273(1):23–27. doi: 10.1016/S0378-1119(01)00566-2. [DOI] [PubMed] [Google Scholar]
- Smertenko A, Draber P, Viklicky V, Opatrny Z. Heat stress affects the organization of microtubules and cell division in Nicotiana tabacum cells. Plant Cell Environ. 1997;20:1534–1542. doi: 10.1046/j.1365-3040.1997.d01-44.x. [DOI] [Google Scholar]
- Smith IK. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 1985;79:1044–1047. doi: 10.1104/pp.79.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavi I, Lal R. Agroforestry and biochar to offset climate change: a review. Agron Sustain Dev. 2013;33:81–96. doi: 10.1007/s13593-012-0081-1. [DOI] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- Ventura M, Sorrenti G, Panzacchi P, George E, Tonon G. Biochar Reduces short-term nitrate leaching from a horizon in an apple orchard. J Environ Qual. 2013;42:76–82. doi: 10.2134/jeq2012.0250. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin R, Liu R. Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J Anal Appl Pyrol. 2014;110:375–381. doi: 10.1016/j.jaap.2014.10.006. [DOI] [Google Scholar]
- Waraich EA, Ahmad R, Ashraf MY, Saifullah AM. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric Scand Sect B Plant Soil Sci. 2011;61(4):291–304. [Google Scholar]
- Waraich EA, Ahmad R, Halim A, Aziz T. Alleviation of temperature stress by nutrient management in crop plants: a review. J Soil Sci Plant Nutr. 2012;12:221–244. doi: 10.4067/S0718-95162012000200003. [DOI] [Google Scholar]
- Xu S, Li J, Zhang X, Wei H, Cui L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot. 2006;56:274–285. doi: 10.1016/j.envexpbot.2005.03.002. [DOI] [Google Scholar]
- Zhang JH, Huang WD, Liu YP, Pan QH. Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under crosstemperature stresses. J Integr Plant Biol. 2005;47:959–970. doi: 10.1111/j.1744-7909.2005.00109.x. [DOI] [Google Scholar]
- Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aus J Agric Res. 2007;58:811–815. doi: 10.1071/AR06253. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.