Abstract
Plant growth-promoting rhizobacteria (PGPR) represent a set of microorganisms that play significant role in improving plant growth and controlling the phytopathogens. Unpredictable performance after the application of PGPR has been observed when these were shifted from in-vitro to in-vivo conditions due to the prevalence of various abiotic stress conditions. During growing period, the potato crop is subjected to a combination of biotic and abiotic stresses. Rhizoctonia solani, a soil-borne plant pathogen, causes reduced vigor and yield of potato crop worldwide. In the current study, multi-stress-tolerant rhizobacterial strain, Bacillus subtilis PM32, was isolated from field-grown potato with various plant growth promoting (PGP) traits including zinc and potassium solubilization, biological nitrogen fixation, ammonia and siderophore, as well as extracellular enzyme productions (cellulase, catalase, amylase, protease, pectinase, and chitinase). The strain PM32 exhibited a distinct potential to support plant growth by demonstrating production of indole-3-acetic acid (102.6 μM/mL), ACC-deaminase activity (1.63 μM of α-ketobutyrate/h/mg protein), and exopolysaccharides (2.27 mg/mL). By retarding mycelial growth of R. solani the strain PM32 drastically reduced pathogenicity of R. solani. The strain PM32 also suppressed the pathogenic activity significantly by impeding mycelial expansion of R. solani with inhibition co-efficient of 49.87. The B. subtilis PM32 also depicted significant tolerance towards salt, heavy metal (Pb), heat and drought stress. PCR based amplification of ituC and acds genes coding for iturin and ACC-deaminase activity respectively indicated potential of strain PM32 for lipopeptides production and ACC deaminase enzyme activity. Results of both in-vitro and pot experiments under greenhouse conditions depicted the efficiency of B. subtilis PM32 as a promising bio-control agent for R. solani infection together with enhanced growth of potato plants as deciphered from biomass accumulation, chlorophyll a, b, and carotenoid contents. Therefore, it was envisioned that application of indigenous multi-stress tolerant PGPR may serve to induce biotic and abiotic stress tolerance in crops/plants for pathogen control and sustainable global food supply.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01067-2.
Keywords: Food security, Biocontrol, Bacillus subtilis, Abiotic stress, Iturin
Introduction
Soil-borne plant pathogens are serious threat to agriculture sector worldwide. Soil-borne diseases pose a great challenge for the plant protection department. Despite the massive application of fungicides, fungal pathogens are responsible for 20% yield loss across the world every year (Rahman et al. 2018; Lucas 2020). R. solani is a soil-borne plant pathogen, a prominent threat to agriculture, and can infect a broad range of crops and trees (Chávez et al. 2020). It causes black scurf and stem cankers in potato crop while affecting yield in several plants like potato (20%), sugar beet (50%), and lettuce by 70% (Bokhari et al. 2015; Esfahani 2020; Hussain and Khan 2020). Furthermore, R. solani reduces the yield and deteriorates the quality of export-oriented potato which leads to massive economic losses (Daami et al. 2008a, b). This pathogen is difficult to control because there are no commercially available resistant cultivars of potato against pathogen. In addition, R. solani has extensive host range and higher persistence of its sclerotia even under unfavorable environmental conditions (Kang et al. 1998; Chávez et al. 2020; Hussain and Khan 2020). Therefore, the exploration of innovative control measures is crucial to minimize the spread of R. solani.
The commonly practiced method to manage R. solani is the use of synthetic fungicides, but owing to their acute toxicity, long presence in soil, accumulation in the food chain, toxicity to other organisms, and the demand for chemical free food, it has become undesirable (Gupta 2018; Lamichhan et al. 2020). Due to all these undesirable attributes, most of the fungicides have been banned for use in agriculture sector (Lamichhan et al. 2020). Moreover, resistance in R. solani has been reported against many synthetic fungicides used for potato crop in various regions under field conditions (Tarhouni 2007). Therefore, prime importance should be given to find a biological control of R. solani which might be an effective, environment-friendly, lucrative, and feasible control option (Rahman et al. 2018).
Controlling fungal diseases through the utilization of PGPR has been considered an efficient approach for sustainable crop production (Bonanomi et al. 2018; Ali et al. 2020; Dong et al. 2021). Bacterial inoculum and its formulations have dual effects, i.e., increasing plant development and control of the fungal infection, which can ultimately reduce reliance on synthetic fungicides (Compant et al. 2013; Arora et al. 2020). Bacterial inoculations makeup almost two-thirds of the agrobiological industry (Lugtenberg et al. 2002; Singh et al. 2020a,b). The biocontrol agents like bacteria can efficiently minimize fungal disease attacks (Amna et al. 2020). In an investigation, two Bacillus sp. showed significant control of apple tree rot disease (Wang et al. 2014). In another study Bacillus subtilis had a promising impact on control of tomato wilt (Chen et al. 2013). In recent times, inoculation of Bacillus species to control soil-borne diseases has gained attention (Xiao et al. 2021). The strains of the Bacillus genus are distributed extensively in soil and plants. In rhizosphere, these bacteria exist and can enhance plant growth by direct and indirect mechanisms (Santoyo et al. 2016; Thakur et al. 2020; Glick 2020). The former involves the acquisition of several nutrients for the plants and production of phyto-hormones (Tahir et al. 2020), while the later involves the safety of plants from fungal pathogens and abiotic stress (Santoyo et al. 2016; Afridi et al. 2019; Tahir et al. 2019a, b; Moncada et al. 2020). The bio-control mechanisms involve the synthesis of various antagonistic compounds including antibiotics, HCN, siderophore, and hydrolytic enzymes (Costa et al. 2014; Keswani et al. 2020; Tilocca et al. 2020). So far, various genera of bacteria have been studied widely to control various pathogens and to enhance nutrients availability (Suárez et al. 2019; Amna et al. 2020; Xiao et al. 2021). To the best of our knowledge, it was noted that there is insufficient data relevant to native microflora to suppress potato stem canker disease. In this context, the less-explored and less-applied B. subtilis PM32 accentuates its importance as a feasible and sustainable alternative for the development of need-based sustainable agriculture, and therefore, can be deemed as novel owing to its trend setting of being explored and being applied since the recent past. Besides, data regarding use of multi-stress tolerant phyto-beneficial rhizobacteria inhabiting ituC and acds genes responsible for iturin and 1-aminocyclopropane-1-carboxylate deaminase synthesis was limited.
Moreover, the key concern in cropping system is the prevalence of multiple abiotic stresses including salt, heat, drought, and heavy metal, where resilience of biocontrol becomes a profound concern. Biocontrol agents need to be adapted to abiotic stresses including salt, heat, droughts, heavy metals, and oxidative stress. As a result, choosing a multi-stress-tolerant PGPR is critical to get promising results under in-vivo conditions. Hence, the aims of the present research were (1) to isolate the PGPR strain for dual-use as plant growth promoter and bio-control mediator (2) to analyze the antagonism of PGPR strain PM32 against R. solani in-vitro and extracellular enzyme production and assessment of abiotic stress endurance of strain PM32 under in-vivo conditions. The ultimate objective was to optimize a blend of multiple stress tolerant PGPR strains for enhanced yield of potato and other crops, and protection from soil-borne pathogens.
Materials and methods
Soil sample collection and isolation of bacterial strains from the rhizosphere of potato plant
Rhizospheric soil samples were collected in September 2018 randomly from a potato (Solanum tuberosum L.) field located in Chitral, Pakistan (35.427583 ° N, 71.773732 ° E) where potato is grown on a large scale. Isolation of bacteria was carried out by using serial dilution technique (Ali et al. 2020). One gram of soil, firmly adhered to potato roots, was suspended into 10 mL of sterile deionized water in a conical flask (50 mL) and kept on shaking at 120 rpm for 1 h. The bacterial suspension was spread equally on LB agar medium, Petri dishes were incubated at 32 ± 2 °C for 1 day. Bacterial grown colonies were isolated and transferred to new Petri plates for purification. Pure bacterial cultures were preserved in 18% glycerol at − 20 °C until the execution of further analysis. The R. solani was isolated from infected potato tubers collected from Chitral, Pakistan (35.427583 ° N, 71.773732 ° E). Pathogenicity of R. solani was also checked through pathogenicity test (Mejdoub et al. 2012).
Plant growth-promoting attributes of bacterial strain PM32
The strains were grown in nutrient medium for 24 h to examine plant growth-promoting activities, and 109 CFU/mL were maintained during the current research work. Luria–Bertani -broth medium (LB; g L−1) consisted of tryptone, 10 g L−1; NaCl, 5 g L−1; yeast extract, 5 g L−1; tryptophan, 1 g L−1 for indole acetic acid (IAA) assay. Inoculation was carried out in the test tubes and kept in shaker incubator (120 rpm) for 3–4 d at 30 °C. After centrifugation, 1 mL supernatant was mixed with 2 mL of Salkowski reagent and incubated for 30 min in the dark at room temperature. The change in color and the optical density were recorded at 530 nm by using a spectrophotometer. A standard curve was built with known concentrations of commercial IAA by using Salkowski reagent. IAA concentration was assessed by using the standard curve (Rfaki et al. 2020). The respective zinc medium (NH4)2SO4, 1.0 g L−1; glucose, 10 g L−1; KCl, 0.2 g L−1; MgSO4, 0.2 g L−1; K2HPO4, 0.1 g L−1 and agar 15 g L−1 at pH 7.0, supplemented with insoluble zinc oxide 1) was utilized to assess zinc solubilization. The strain was spot inoculated on agar plate and incubation was carried out for 7 d at 32 °C. The sign of Zn solubilization was the presence of the halo zone around the colony (Gontia et al. 2017). Alexandrov agar medium (glucose, 5 g L−1; MgSO4.7H2O, 0.5 g L−1; CaCO3, 0.1 g L−1; FeCl3, 0.006 g L−1; Ca3PO4, 2 g L−1; Feldspar, 3 g L−1; agar 15 g L−1 at pH 7.5) was utilized to assess the solubilization of insoluble potassium by bacterial strains. The strain PM32 was spot inoculated at central position of plate, and incubation was carried out at 32 °C for 7–10 d. Potassium solubilization was indicated by the development of a halo zone around the colony (Parmar and Sindhu 2013). The nitrogen-fixing ability of B. subtilis PM32 was checked by using nitrogen-free medium (Dahala et al. 2017).
Assay for siderophore production by bacterial strain was done by spot inoculation of strain on blue agar plates (Chrome azurol S agar medium). For blue agar medium, Chrome azurol S (60.5 mg) was dissolved in distilled water (50 mL) and added to 10 mL iron (III) solution (1 mM FeCl3. 6H2O, and 10 mM HCl). That prepared solution was thoroughly mixed in 40 mL of distilled water with a second solution consisting of hexadecyl trimethyl ammonium. The spot inoculated blue agar plates were incubated for 2 to 3 d at 32 °C. The presence of an orange to yellow colored district zone around the colony indicated siderophore synthesis (Amna et al. 2019). To assess ammonia production, bacterial strains were inoculated in test tubes containing the peptone water medium and incubated at 30 °C for 24 to 48 h in a shaker incubator at 120 rpm. The change in media color from yellow to brown after adding 0.5 mL Nessler's reagent was an indicator of ammonia production (Amna et al. 2019). The ATCC medium No. 14 was used to evaluate the exopolysaccharides (EPS) production by the bacterial strain (Amna et al. 2019). Bacterial streaked plates were incubated for 3 d at 32 °C. The development of slimy layers surrounding the colony verified the production of EPS after three days of incubation. Quantification of EPS was done by following Ali et al. (2014).
Production of extracellular enzymes
The bacterial strains were assessed to produce extracellular enzymes including protease, amylase, pectinase, and catalase (Amna et al. 2019). Chitinase and cellulase production assay by bacterial strains were evaluated by following Amna et al. (2020) and Sethi et al. (2013) respectively. Production of ACC-deaminase enzyme by the bacterial strains was detected by supplying ACC as the only nitrogen supply in the medium and quantification of ACC deaminase was also executed (Ali et al. 2014).
Molecular profiling (ituC, acds and 16S rRNA gene)
The DNA of strain PM32 was extracted following the methodology of Ahmed et al. (2014). To amplify iturin gene (ituC) in strain PM32, PCR cycling conditions maintained were 95 °C for 4 min subsequently 40 cycles of 94 °C for 1 min, 58 °C for 1 min and 70 °C for 1 min and last step (extension) for 5 min at 70 °C. The primer pair used for ituC included ITUC-F1 (CCCCCTCGG TCAAGTGAATA) and ITUC-R1 (TTGGTTAAGCCCTGATGCTC) (Amna et al. 2020). To amplify acds gene universal primers were utilized (5′-GGC AAGGTCGACATCTATGC-3′ and 5′-GGCTTGCCATTCAGCTATG-3). The PCR conditions for acds gene included: initial temperature at 94 °C for 3 min subsequently 30 cycles of denaturation (1 min); annealing at 58 °C (1 min); extension at 72 °C (3 min) with further final extension at same temperature for 5 min (Mehmood et al. 2021). 16S rRNA gene was amplified by busing universal primer [27F (5-AGAGTTTGATCACTGGCTCAG-3) and 1492R (5-CGGCTTACCTTGTTACGACTT-3)] (Tehmeena et al. 2020). The PCR products were sent to Macrogen, Korea for sequencing. BLAST analysis on the NCBI database was used to determine the sequence homology.
Biocontrol of R. solani in dual culture, and measurement of inhibition coefficient of strain PM32
Antagonistic activity of strain PM32 was checked through dual culture technique (Amna et al. 2020). In the center of the plate containing PDA and LB medium(1:1) a 5 mm freshly grown fungal disc was placed. At an equal distance from the fungal plug, the strain PM32 was spot inoculated. Incubation of cultured plates was done at 28 ± 2 °C. Control was considered with only fungal inoculation. After incubation of 7 d, the mycelial growth inhibition of fungi was assessed by a scoring system. The percentage inhibition of fungus was evaluated with equation given below
The “C” denotes the fungal growth in control while “T” represents growth of fungus alongside the strain PM32.
After calculating percentage inhibition, the inhibition co-efficient of strain PM32 against R. solani was determined (Cray et al. 2015). By estimation of growth of fungus and estimating radial growth measurements alongside the antagonistic biocontrol agent, the inhibition coefficient was calculated over time and the highest growth rate was determined (A). It indicates percentage of the rate of radial increase of fungus in control (B). Outward extension of the fungus alongside the antagonistic strain PM32 was also used to estimate the expansion of plant-pathogen in the form of percentage of the distance among the positions of inoculation of biocontrol agent and fungus (i.e., value C). Furthermore, the radial measurements were also taken when fungus came across the biocontrol agent. These calculations were additionally utilized to find fungal growth rates in mixed culture zone (i.e., value D). In the same way this value was specified as a percentage of the rate of radial expansion of fungus in the control (i.e., value E). The inhibition coefficient was then calculated by following formula;
Inhibition coefficient: [(100—B) × 0.4] + [(100 − C) × 0.4] + [(100 − E) × 0.2].
The [(100 − B) × 0.4] denotes a potential 40% role of distal inhibition (before coming in interaction) of the growth rate of the fungus; [(100 − C) × 0.4] denotes potential 40% role of inhibition of fungal colony alongside the biocontrol agent; and [(100 − E) × 0.2] denotes a potential 20% role of the capability to constrain fungal growth rate when in a zone of mixed culture.
Determination of abiotic stress tolerance of bacterial strain PM32
For salinity stress tolerance assay, the B. subtilis PM32 was grown in LB broth medium with various salt stress levels (1 and 2 Molar NaCl) to determine the salt stress potential of strain PM32. Bacterial growth in broth culture was observed by taking optical density (O.D.) at 600 nm daily for seven days. At a particular stress level, the OD 0.1 of bacterial broth was regarded as tolerant of salt stress (Amna et al. 2019). The nutrient agar medium was supplemented with varying degrees of heavy metal for the heavy metal stress tolerance assay (Pb, 50 ppm to 1000 ppm). Then strain PM32 was also assessed in nutrient broth containing 100 mg/L to 300 mg/L level of Pb. For high temperature tolerance assay the Bacillus subtilis PM32 was cultured in nutrient broth medium and incubated at several temperatures ranges i.e., 30 °C–50 °C for 48 h. Optical density more than 0.2 was found to be thermo-tolerant at 600 nm wavelength at 45 ° C (Ali et al. 2009). The strain PM32 was assessed to grow in controlled water supply through Trypticase Soy Broth supplemented with various level of PEG-8000 (T1 = − 0.05 Mpa, T2 = − 0.15 Mpa, T3 = − 0.30 Mpa, T4 = − 0.49 Mpa, T5 = − 0.73 Mpa, T6 = − 0.97 Mpa) (Marulanda et al. 2007). The experiment was arranged in triplicate in shaking incubator (120 rpm) at 32 °C for 2 d and optical density was noted by using a spectrophotometer (Agilent 8453 UV–visible Spectroscopy System) at wave length of 600 nm.
Experimental setup: Biocontrol of stem canker
A pot experiment was designed in a greenhouse to assess the bio-control efficiency of bacterial strain PM32 against R. solani responsible for stem canker of potato. Semi-controlled conditions were maintained for pot experiment including temperature 24–28 °C, photoperiod of 10 h, and light intensity 80 μmol m−2 s−1. The variety of potato used in experiment was Desiree. The soil used for experimentation was taken from the Land Resource Research Institute, Islamabad (LRRI, 33.676343°N, 73.125549°E), Pakistan. The analysis of soil was carried out for various characteristics and was observed to have an electrical conductivity of 0.38 dS m−1, organic matter of 0.79%, total nitrogen, 72.25 ppm, available phosphorus, 1.18 ppm, 103 ppm available potassium, and pH of 7.4. Whole experiment was performed in triplicate with 4 treatments (1) Control without any inoculation of bacteria or fungus (2) T1; the only inoculation of B. subtilis PM32 (3) T2; dual application of B. subtilis PM32 and R. solani (4) T3; plants infected with R. solani (5) T4; plants inoculated with R. solani and fungicide (KOCIDE 3000, FMC). Harvesting of potato plants was carried out after 65 d of germination and kept in freezer at − 1 °C for further study.
Estimation of PGP traits, relative water contents, electrolyte leakage (EL), chlorophyll contents, disease severity and antioxidant enzyme activity
The shoot and root length, number of stems, and fresh weights were recorded just after plant harvesting. Relative water contents and EL were assessed (Ahmad et al., 2018).
Leaf material (0.05 g) was crushed and homogenized in 10 mL of 80% acetone with mortar and pestle and incubated in the dark for 60 min to extract the pigments including Chlorophyll a, Chlorophyll b and carotenoids). The supernatant was tested for photosynthetic pigments with a spectrophotometer after 10 min, centrifugation at 6000. For chlorophyll a and b, absorbance was recorded at 650 nm and 665 nm, respectively. To estimate carotenoids contents, the extract was amended with 5 mL of NaOH (1 M) and 15 mL dimethyl ether and absorbance was recorded at 450 nm (Muneer et al. 2020a, b). Antioxidative enzymes activities, including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were also determined (Afridi et al. 2019).
During the course of the experiment, potato plants were regularly monitored for appearance of symptoms of soil-borne diseases like Rhizoctonia stem canker (Stem discoloration) (Schoonhoven 1987). After harvesting, each plant containing multiple stems was observed for stem discoloration symptoms. Severity of symptoms was ranked by using 0–5 evaluation scale where 0 was considered with zero symptoms; 1 represent minor discoloration with minor lesion; 2 significant lesions with necrosis on < 50% of stem or stolon area; 3 represent lesion on > 50% stem or stolon diameter; 4 with large lesion area on stem (80%) and 5 stem fully covered with lesion or plant completely dead.
Statistical analysis
The experimental data were analyzed statistically in triplicates. One-way analysis of variance (ANOVA) was used to analyze the data in statistical package Statistix 8.1. Further, the least significant difference (LSD) test was used for the detection of differences among the treatment means p ≤ 0.05). Whole experimental data was subjected to Shapiro–Wilk test (Table S1) on SPSS software (IBM SPSS Statistics 21) to check normal distribution (p > 0.05) for all the studied parameters (Wang and Riffel 2011). R software was used to perform principal component analysis (PCA) and Pearson correlation analysis.
Results
Isolation, screening, characterization, and biochemical identification of bacterial strains
A total of 15 antagonistic bacterial strains were isolated from the rhizospheric soil samples of potato plants. All isolated strains were screened for a variety of biochemical activities, including IAA production, zinc, and potassium solubilization, nitrogen fixation, siderophore, ammonia and EPS production, cellulase, catalase, amylase, protease, pectinase, chitinase, and ACC deaminase enzyme activities (Table S2). Ultimately, only one strain PM32 was selected for comprehensive studies based on qualitative plant growth promoting activities and antifungal activities (Table 1, Table S2, Table S3). Based on various PGP traits and extracellular enzyme assays, the strain PM32 was categorized as a PGPR (Table 2, Table S2, Table S4). As regarding 16S rRNA sequencing and homology search, the strain was confirmed as Bacillus subtilis and was submitted to NCBI with the accession number of MW316740. PCR was used to amplify genes (ituC and acds) possibly involved in the biosynthesis of lipopeptides and ACC deaminase enzyme which could be produced by the strain PM32. The primer pairs intended to detect genes involved in the expression of iturin and the ACC deaminase enzyme yielded amplicons with the expected sizes. (Figure S1).
Table 1.
Inhibition coefficient of R. solani by strain PM32
| Bacterial strain | Bacillus subtilis PM32 |
|---|---|
| Expansion of fungal strain (mm day−1); [A]c | 0.29 ± 0.16 |
| Growth rate A as a percentage of control; [B] | 54.29 ± 2.88 |
| Expansion of fungal strain as a percentage of the gap in the middle points of inoculation; [C]c | 63.50 ± 0.00 |
| Duration prior to exposure of fungus and bacterial strain (days) | 3.00 ± 0.47 |
| Expansion of fungus in zone of mixed culture (mm day_1); [D]d | 0 |
| Growth-rate D as a percentage of control; [E] | 0 |
| Inhibition coefficient e | 49.87 ± 1.29 |
Maximum growth rate is represented by A. Value B denotes growth rate of fungus in dual culture as a % of fungal growth in control. Value c denotes distance covered by the R. solani as a percentage of the gap between the positions of inoculation of fungus and strain PM32. Value D denotes the growth rate of R. solani in the zone of mixed culture and value E denotes percentage radial growth rate of R. solani
Table 2.
Morphological and biochemical features of bacterial strain PM32
| Bacterial traits | Results |
|---|---|
| Morphological and colony feature | Large size, irregular wrinkled growth, cream-coloured colonies on LB agar at 32 °C 24 h of incubation |
| Cell traits | Gram-negative, under microscope exhibited Cells of rod shape with scattered display |
Quantitative determination of IAA, ACC-deaminase, exopolysaccharides, production
Upon qualitative confirmation of IAA, ACC-deaminase, exopolysaccharides, synthesized by strain PM32 the quantitative analysis for these parameters was also carried out. The bacterial strain PM32 exhibited IAA production by 102.6 µM/mL. ACC-deaminase and exopolysaccharide production by PM32 was found to be 1.63 µM/mg protein/h and 2.27 mg/mL, respectively (Table S4).
Antifungal activity, inhibition percentage, and measurement of inhibition co-efficient
Mycelial expansion of R. solani was minimized substantially (p ≤ 0.05) with inoculation of strain PM32 in dual culture assay as compared to control plants. Inhibition of R. solani was observed 52% as compared to control. Furthermore, inhibition co-efficient for PM32 against R. solani was calculated as 49.8 after 15 d of incubation period (Table 1, Figure S2).
Abiotic stress tolerance assay for strain PM32
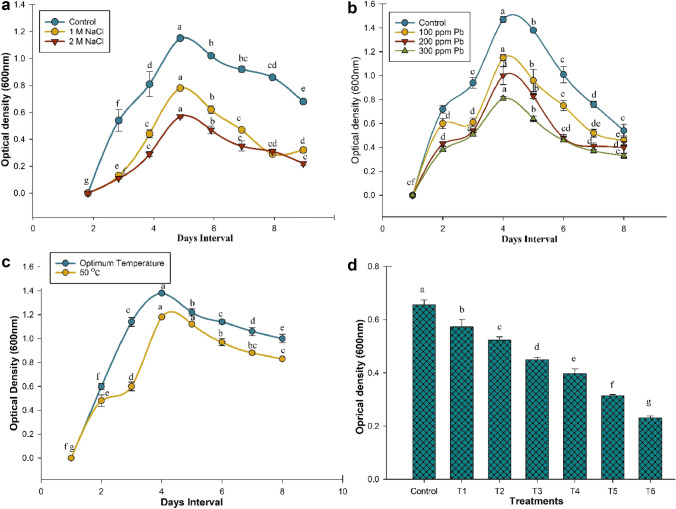
The studied bacterial strain PM32 showed resistance to salinity stress level 2 (2 M NaCl) (Fig. 1a). Besides, the strain PM32 also uncovered a significant (p ≤ 0.05) growth and tolerance at 300 mg/L level of Pb (Fig. 1b). Upon high temperature assay highest OD (600 nm) of bacterial cells was 1.38 observed at ideal temperature and at 50 °C observed OD (600 nm) was 1.17 (Fig. 1c). The drought tolerance assay for B. subtilis PM32 revealed a plentiful bacterial growth at all levels of PEG-8000 stress (Fig. 1d), demonstrating the osmo-adaptive capability of strain. But bacterial growth was dramatically decreased as the stress increased.
Fig. 1.
Seven-days growth of strain PM32 at two levels of salinity control; T1 = 1molar NaCl, T2 = 2molar NaCl (a) Bacterial growth against various levels of heavy Pb stress (b) Growth of strain PM32 at elevated temperature (c). At several drought stress levels induced through PEG-8000 (T1 = − 0.05 Mpa, T2 = − 0.15 Mpa, T3 = − 0.30 Mpa, T4 = − 0.49 Mpa, T5 = − 0.73 Mpa, T6 = − 0.97 Mpa) (d)
Effects of strain PM32 on potato growth, chlorophyll contents and electrolyte leakage
In the pot experiment, differences in biomass, relative water, and chlorophyll contents were observed under normal and biotic stress of R. solani (Table 3). All these growth traits were enhanced significantly (p ≤ 0.05) with the application of strain PM32 under both conditions, normal and under stress of R. solani. Inoculation of strain PM32 significantly (p ≤ 0.05) boosted shoot length by 20%, the root length by 49%, relative water contents by 20%, fresh weight by 25%, number of stems by 39%, under treatment conditions as compared to control plants. When R. solani was applied, a significant reduction in these parameters was observed. Inoculation with strain PM32 significantly (p ≤ 0.05) improved shoot length by 24%, root length by 60%, relative water contents by 21%, fresh weight by 32% and number of stems by 54% relative to the R. solani infected plants. After inoculation with strain PM32, electrolyte leakage was dramatically (p ≤ 0.05) reduced under both normal and fungal stress conditions (Table 3).
Table 3.
Effect of B. subtilis PM32 on length of shoot and root, number of stems, fresh weight, relative water contents, electrolyte leakage chlorophyll a, b, and carotenoids content of potato plants under green house
| Treatments | Shoot length (cm) | Root length (cm) | No of stems | Fresh weight (gram) | Relative water content (%) | Electrolyte leakage (%) | Chlorophyll content | ||
|---|---|---|---|---|---|---|---|---|---|
| Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Carotenoids (mg/g) | |||||||
| Control | 61.01 ± 0.81b | 3.77 ± 0.52b | 2.66 ± 2.39b | 56.60 ± 0.47c | 51.36 ± 1.06c | 41.40 ± 2.79b | 0.43 ± 0.01c | 0.65 ± 0.02b | 3.93 ± 0.12c |
| T1 | 76.66 ± 0.47a | 7.53 ± 0.41a | 4.33 ± 1.81a | 76.13 ± 0.47a | 62.23 ± 2.00a | 35.20 ± 0.91c | 0.72 ± 0.03a | 0.84 ± 0.02a | 4.66 ± 0.20a |
| T2 | 69.01 ± 1.63ab | 6.66 ± 0.47a | 3.66 ± 2.09a | 56.96 ± 0.47c | 59.4 ± 1.47ab | 33.80 ± 1.07c | 0.55 ± 0.03b | 0.67 ± 0.02b | 4.26 ± 0.08b |
| T3 | 51.80 ± 1.88c | 2.66 ± 0.30c | 1.66 ± 0.94c | 38.66 ± 0.47d | 46.83 ± 1.16d | 57.66 ± 3.29a | 0.33 ± 0.02d | 0.33 ± 0.03c | 2.83 ± 0.12d |
| T4 | 70.84 ± 5.51a | 6.99 ± 0.24a | 4.01 ± 1.94a | 65.99 ± 0.00b | 58.63 ± 1.38b | 33.40 ± 1.44c | 0.60 ± 0.01b | 0.83 ± 0.04a | 4.77 ± 0.08a |
Effects of different treatments on growth of potato plants in green house. Control= uninoculated; T1 = B. subtilis PM32 inoculation; T2 = B. subtilis PM32 and R. solani inoculation; T3 = R. solani inoculation; T4 = R. solani + Fungicide (KOCIDE 3000). The treatments exhibit dissimilar letters within rows represent significance at P ≤ 0.05 level
Estimation of disease severity and antioxidants (SOD, POD, and catalase)
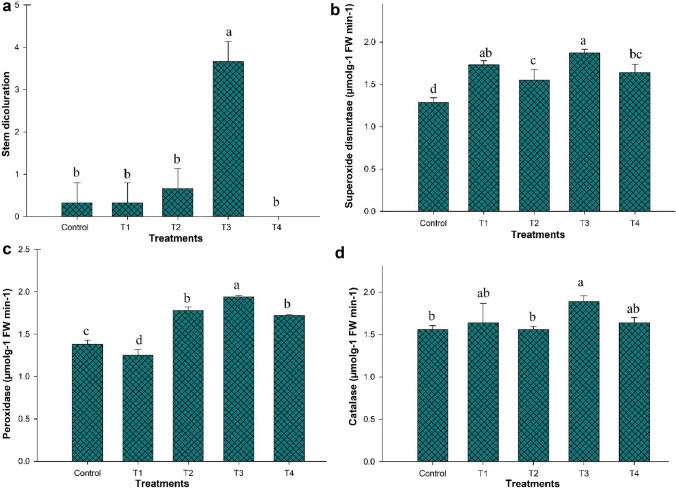
In control and bacterial treated plants, antioxidant enzymes (SOD, POD, and CAT) were evaluated under disease stress and normal conditions. With R. solani infestation, all antioxidant enzyme attributes, comprising of SOD, POD, and CAT, were improved in contrast to control plants, but strain PM32 inoculation reduced the activities of the antioxidative enzymes. Disease incidence symptoms were significantly (p ≤ 0.05) declined with application of strain PM32 as compared to fungal infected plants. Stem discoloration of potato plant was minimized significantly (p ≤ 0.05) by 81% relatives to fungal infected plants after inoculation of strain PM32 (Fig. 2).
Fig. 2.
Effect of B. subtilis PM32 on stem discoloration, SOD, POD and CAT of potato plants under biotic stress R. solani. Control (un-inoculated plants), T1 (B. subtilis PM32 inoculation), T2 (B. subtilis PM32 and R. solani infection), T3 (R. solani infection), T4 (Fungicide (KOCIDE 3000) and R. solani infection). The means with same letter(s) are not significantly different at p ≤ 0.05. The bars show standard error of mean values (n = 3)
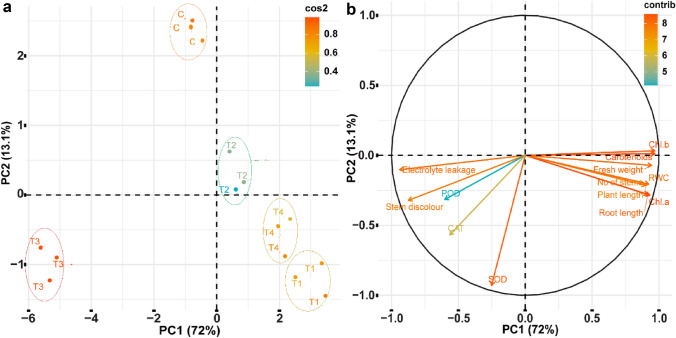
Principal component analysis
The principal component analysis clusters the input and response variables into distinct groups based on analogy/variance and correlations. In the present study, PCA was performed to compare pot experiment treatments for their accumulative effect on plant response traits. (Fig. 3a). The PCA divided all the five treatments into distinct divisions, indicating dissimilar effects of these treatments from each other for various plant response traits. The PCA divided response variables of potato plant into different groups under bacterial strain PM32 application (Fig. 3b). The chlorophyll pigments and growth parameters of potato plant were clustered together, implying the same trend of increasing response under strain PM32 inoculation and R. solani stress. While the antioxidant enzymes were categorized with stem discoloration and EL showing increasing response under pathogenic fungal stress. Further, various growth, physiological, membrane leakage, and antioxidant activities of potato plant were positively or negatively correlated as shown in Fig. 4.
Fig. 3.
The PCA biplots showing correlation among various treatments (a) and among potato variables of pot experiment
Fig. 4.
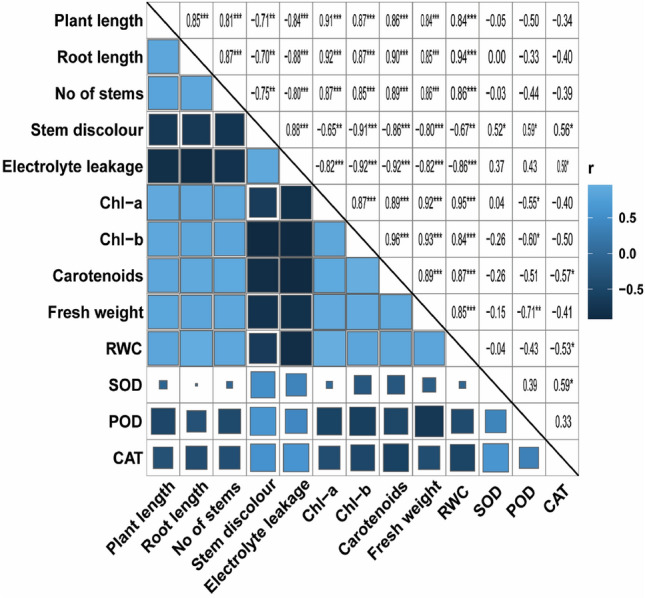
Pearson correlation analysis of applied treatments with morpho-physio-biochemical and antioxidative attributes of potato plant
Discussion
The use of multi-functional PGPR in agricultural practices is a viable solution to problems such as biotic and abiotic restrictions, climate variation, low crop yield, and increasing food demand (Ahmad et al. 2018; Amna et al. 2019; Mehmood et al. 2021). These environment-friendly strategies can minimize the noxious impacts originating from synthetic agrochemicals residues in food chain (Huang et al. 2020; Amna et al. 2021; Bhatt et al. 2021). The current study was an attempt to explore multi-stress-tolerant PGPR B. subtilis as bio-control agent against R. solani accompanied by plant growth-promoting traits. Outcomes of current research revealed that strain PM32 harboring several PGP attributes and antagonistic activity have the potential to improve plant growth in numerous ways. The important PGP traits including IAA, zinc, and potassium solubilization, siderophore, and ammonia production displayed by strain PM32 can help in promoting plant growth under stressed and non-stressed conditions (Khan et al. 2020; Arora et al. 2020). The strain PM32 showed high IAA (102.6 μM/mL), EPS (2.27 mg/mL), and ACC-deaminase (1.63 μM/mg protein/h) production which depicts its potential to promote plant growth. In an investigation by Khan et al. (2016) the IAA production by B. subtilis LK14 was observed as 8.7 μM/mL. The strain PM32 produced IAA, 11 folds as compared to Bacillus subtilis LK14, and that shows the potential of strain PM32 to promote plant growth. Production of phytohormone IAA, an important trait being involved in improving root and shoot cell division and elongation which ultimately leads to increased root surface area and length that provides the plant easier access to soil nutrients (Ghosh et al. 2011). Production of EPS by bacteria improves water retention and uptake in the rhizospheric region through biofilm formation and is very helpful to stabilize soil aggregates as well as to regulate sources of nutrients and organic carbon. Exopolysaccharides produced by Bacillus subtilis MKU SERB2 were recorded as 0.617 mg/mL, but the strain PM32 produced 2.27 mg/mL exopolysaccharides that is four folds more than strain MKU SERB2 Fig. 5.
Fig. 5.
Effect of strain PM32 on growth of potato plants under biotic stress of R. solani. Control (un-inoculated plants), T1 (B. subtilis PM32 inoculation), T2 (B. subtilis PM32 and R. solani infection), T3 (R. solani infection), T4 (Fungicide (KOCIDE) and R. solani). Arrows indicate the disease symptoms of potato including stem discoloration and wilting due to R. solani
Moreover, the siderophore production trait displayed by strain PM32 is a vital feature that can perform a significant role in improving plant growth under both biotic and abiotic stress conditions by forming bonds with iron in the rhizospheric region which becomes unavailable to the phytopathogens (Sasirekha and Srividya 2016). This process is efficient since PGPR-generated siderophores have more attraction for iron than fungus. Iron is thus limited for the use of pathogens and hence they are unable to proliferate in the rhizosphere (Glick 2012). Siderophore production is, therefore, an essential factor for the antagonism against phytopathogens and plant development.
Biocontrol of soil-borne plant pathogens is linked to the synthesis of extracellular enzymes including amylase, protease, catalase, cellulase, pectinase, and chitinase by bacteria. These enzymes have capacity to lyse the fungal cell wall (Olanrewaju et al. 2019). Extracellular enzymes including ACC-deaminase support plant growth under both normal and stress conditions (Ali et al. 2021). The ACC-deaminase synthesized by bacterial strains minimizes ethylene level through cleavage of ACC to α-ketobutyrate and ammonia under biotic and abiotic stress (Amna et al. 2020; Ali et al. 2021). The strain B. subtilis PM32 has ability to produce significant ACC-deaminase (1.63 μM/mg protein/h) that shows its potential to adapt to stress situations. Production of ACC-deaminase enzymes by strain PM32 is also shown in earlier investigations (Zainab et al. 2020; Amna et al. 2020; Afridi et al. 2021; Maqbool et al. 2021). Inhibition of fungal mycelia could be owing to a variety of antimicrobial diffusible and volatile metabolites that act against phytopathogenic fungi by a variety of mechanisms, including retarded germination, inhibition, and lysis of fungal mycelium. On the other hand, inhibition of fungal mycelia might be due to various antimicrobial diffusible and volatile metabolites which may act against phytopathogenic fungi through various channels which include reduced germination, inhibition as well as the lysis of fungal mycelium (Cray et al. 2015; Haidar et al. 2016; Bibi et al. 2020). Furthermore, amplification of ituC in the bacterial strain PM32 responsible for iturin an antimicrobial lipopeptide compounds is well known with potential uses in biocontrol (Cao et al. 2012; Amna et al. 2020). In another study, lipopeptides production by bacterial strain portrayed a significant role in controlling Fusarium wilt of cucumber (Cao et al. 2012).
The strain PM32 had a strong antifungal activity with inhibition percentage of 52% and inhibition coefficient of 49.9 for R. solani. Some previous investigations revealed that mere percentage inhibition of fungus cannot solely determine biocontrol potential of bacterium (Gontia et al. 2017). Here, the inhibition coefficient was evaluated that quantified the effectiveness of antagonistic bacteria alongside fungal pathogens and is based on the gap between the biocontrol agent and fungal pathogen, the size of biocontrol agent colony and the radial extension of the fungal pathogen. On the other hand predominance of abiotic stressors is one of the key restrictions in agriculture cropping systems, where the resilience of applied biocontrol agents has become a major concern (Singh and Jha 2017; Mahmoud et al. 2020). The strain PM32 also showed abiotic stress tolerance to salinity (2 M), heavy metal (Pb 300 ppm), heat (50 °C), and drought (30%). Multi-stress tolerant PGPR would be an essential tool to improve the farming system and to quell the issues of low yearly crop yield and global climate change (Ferreira et al. 2019; Din et al. 2020). Sugar cane growth was dramatically boosted when multi-stress resistant (salinity, heavy metal, heat, and drought) bacteria were inoculated under biotic stress of red rot disease (Amna et al. 2020). Under this scenario, inoculation of abiotic stress resistant bacterial strains for dual purpose usage (fungal and abiotic stress including salinity, heavy metal, high temperature, and drought tolerance) technique can make a major contribution to sustainable food production (Amna et al. 2019).
The efficacy of B. subtilis PM32 as antagonistic agent against R. solani and growth promoter was evaluated by using potato as a test plant (Table 3). We observed increase in shoot and root length of potato plants with inoculation of strain PM32 under infestation of R. solani might be associated with the PGP traits exhibited by the strain as well as production of ACC deaminase is also associated with biotic and abiotic stress tolerance in plants (Chen et al. 2017). The underlying mechanisms presented by PGPR possibly include the reduction of endogenous ethylene, therefore assisting improved uptake of nutrients by improving root and shoot development (Shahzad et al. 2013). Significant improvement in potato growth with regards to biomass accumulation after inoculation of strain PM32 either under infected or non-infected conditions probably results from stimulation of photosynthetic pigments resulting from improved nutrient acquisitions.
Disease symptoms including stem discoloration were significantly declined with inoculation of strain PM32 as compared to infected fungal plants. The biocontrol of fungal disease is attained by synthesis of lytic enzymes which lyse the fungal cell wall, competition for nutrients in rhizospheric region, synthesis of antifungal compounds and initiation of systemic resistance to counter pathogenic infection. The strain PM32 has strong inhibition co-efficient and capacity to produce various hydrolytic enzymes that might degrade fungal cell wall as reported previously (Nalini and Parthasarathi 2018; Amna et al. 2020). The generation of reactive oxygen species (ROS) is normally the instant response of plant after a pathogenic fungal attack. The production of ROS under stressed conditions causes less uptake of water and nutrients by roots and enhanced the leakage of membrane electrolytes. Under such stress conditions, the metabolic system of the host plant is shifted towards disease stress. In the same way, less chlorophyll pigments were recorded in R. solani infected potato plants and might be owing to increased buildup of ROS. Deactivation of enzymes responsible for chlorophyll biosynthesis might be another reason for less chlorophyll contents under fungal stress (Kalaji et al., 2016). Increased levels of SOD, POD and CAT activity might be attributed to initiation of plant protection mechanisms and confers resistance against pathogenic attack to plant (Warnakumari et al. 2011; Afridi et al. 2019).
Combined PCA analysis of all the data of plant parameters was carried out that showed that the general effect of strain PM32 and R. solani on plant responses differed from each other and control treatments. Grouping of antioxidants with EL and stem discoloration depicted an increasing response under pathogenic fungal stress. Increase in antioxidant enzymes with EL and stem discoloration might be due to protective response of plant in the form of reactive oxygen species (ROS) under fungal stress (Warnakumari et al. 2011; Batool et al. 2019). The use of multivariate analysis to discover possible trends and correlations among data sets has gained substantial importance (Natasha et al. 2018; Shahid et al. 2020). It was also stated that in some cases, ordinary statistical analysis may not be sufficient to identify significance among various treatments and factors, however multivariate analysis, such as PC analysis, splits data sets based on their overall effect on various response variables.
Conclusion
It is concluded that B. subtilis PM32 is a potential biocontrol agent against R. solani with its various PGP traits including IAA, phosphate, zinc, and potassium solubilization, nitrogen fixation, siderophore, extracellular enzymes production. Moreover, strain PM32 also had tolerance towards NaCl (2 M), Pb (300 ppm), heat (50 °C), and drought stress. Overall results of the study revealed that multi-stress tolerant bacterial strain PM32 has excellent biocontrol potential for the management of R. solani responsible for stem canker in potato. Additional investigations should be performed under in-vivo conditions for multi-stress-tolerant strain PM32 in potato and other arable crops to support commercial formulation development.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful towards National Agricultural Research Center (NARC), Islamabad, Pakistan to provide space for cultivation of potato plants to carry out the experimental work. We are grateful to Quaid-i-Azam University Islamabad for providing funds (URF-2019).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afridi MS, Mahmood T, Salam A, Mukhtar T, Mehmood S, Ali J, Khatoon Z, Bibi M, Javed MT, Sultan T, Chooudhary HJ. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol Biochem. 2019;139:569–577. doi: 10.1016/j.plaphy.2019.03.041. [DOI] [PubMed] [Google Scholar]
- Afridi MS, Van Hamme JD, Bundschuh J, Khan MN, Salam A, Waqar M, Chaudhary HJ. Biotechnological approaches in agriculture and environmental management-bacterium Kocuria rhizophila 14ASP as heavy metal and salt-tolerant plant growth-promoting strain. Biologia. 2021;76:3091–3105. doi: 10.1007/s11756-021-00826-6. [DOI] [Google Scholar]
- Ahmad I, Akhtar MJ, Mehmood S, Akhter K, Tahir M, Saeed MF, Hussain S. Combined application of compost and Bacillus sp. CIK-512 ameliorated the lead toxicity in radish by regulating the homeostasis of antioxidants and lead. Ecotoxicol Environ Saf. 2018;148:805–812. doi: 10.1016/j.ecoenv.2017.11.054. [DOI] [PubMed] [Google Scholar]
- Ahmed OB, Asghar AH, Elhassan MM. Comparison of three DNA extraction methods for polymerase chain reaction (PCR) analysis of bacterial genomic DNA. Afr J Microbiol Res. 2014;8(6):598–602. doi: 10.5897/AJMR2013.6459. [DOI] [Google Scholar]
- Ali SZ, Sandhya V, Grover M, Kishore N, Rao LV, Venkateswarlu B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol Fert Soil. 2009;46:45–55. doi: 10.1007/s00374-009-0404-9. [DOI] [Google Scholar]
- Ali SZ, Sandhya V, Rao LV. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann Microbiol. 2014;64(2):493–502. doi: 10.1007/s13213-013-0680-3. [DOI] [Google Scholar]
- Ali S, Hameed S, Shahid M, Iqbal M, Lazarovits G, Imran A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol Res. 2020;232:126389. doi: 10.1016/j.micres.2019.126389. [DOI] [PubMed] [Google Scholar]
- Ali J, Ali F, Ahmad I, Rafique M, Munis MFH, Hassan SW, Chaudhary HJ. Mechanistic elucidation of germination potential and growth of Sesbania sesban seedlings with Bacillus anthracis PM21 under heavy metals stress: An in vitro study. Ecotoxicol Environ Saf. 2021;208:111769. doi: 10.1016/j.ecoenv.2020.111769. [DOI] [PubMed] [Google Scholar]
- Amna XY, Farooq MA, Javed MT, Kamran MA, Mukhtar T, Ali J, Tabassumf T, Rehmang S, Munis MFH, Sultan T, Chaudhary HJ. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol Biochem. 2020;151:640–649. doi: 10.1016/j.plaphy.2020.04.016. [DOI] [PubMed] [Google Scholar]
- Amna, Din BU, Sarfraz S, Xia Y, Kamran MA, Javed MT, Sultan T (2019) Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol Environ Saf, 183:109466 [DOI] [PubMed]
- Amna, Mahmood T, Khan UN, Amin B, Javed MT, Mehmood S, Farooq MA, Chaudhary HJ (2021) Characterization of bio‐fabricated silver nanoparticles for distinct anti‐fungal activity against sugarcane phytopathogens. Microsc Res Tech, 10.1002/jemt.23708 [DOI] [PubMed]
- Arora NK, Fatima T, Mishra J, Mishra I, Verma S, Verma R. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J Adv Res. 2020;26:69–82. doi: 10.1016/j.jare.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batool R, Rehman SU, Rafique M, Amna Ali J, Mukhtar T, Mahmood S, Sultan T, Munis FH, Chaudhary HJ. Biocontrol potential of Bacillus gibsonii and Brevibacterium frigoritolerans in suppression of Fusarium stalk rot of maize: a sustainable approach. Asian J Agric Biol. 2019;7:320–333. [Google Scholar]
- Bhatt P, Sharma A, Rene ER, Kumar AJ, Zhang W, Chen S. Bioremediation of fipronil using Bacillus sp. FA3: Mechanism, kinetics and resource recovery potential from contaminated environments. J Water Process Eng. 2021;39:101712. doi: 10.1016/j.jwpe.2020.101712. [DOI] [Google Scholar]
- Bibi F, Yasir M, Al-Sofyani A, Naseer MI, Azhar EI. Antimicrobial activity of bacteria from marine sponge Suberea mollis and bioactive metabolites of Vibrio sp. EA348. Saudi J Biol Sci. 2020;4:1139–1147. doi: 10.1016/j.sjbs.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari N, Siddiqui I, Perveen K, Siddique I, Soliman D. Mycocidal ability of Toona ciliata against Rhizoctonia salani. Janimal Plant Sci. 2015;25:1477–1481. [Google Scholar]
- Bonanomi G, Lorito M, Vinale F, Woo SL. Organic amendments, beneficial microbes, and soil microbiota: toward a unified framework for disease suppression. Ann Rev Phytopathol. 2018;56:1–20. doi: 10.1146/annurev-phyto-080615-100046. [DOI] [PubMed] [Google Scholar]
- Cao Y, Xu Z, Ling N, Yuan Y, Yang X, Chen L, Shen B, Shen Q. Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Sci Hortic. 2012;135:32–39. doi: 10.1016/j.scienta.2011.12.002. [DOI] [Google Scholar]
- Chávez-Ramírez B, Kerber-Díaz JC, Acoltzi-Conde MC, Ibarra JA, Vásquez-Murrieta MS, Estrada-de Los Santos P. Inhibition of Rhizoctonia solani RhCh-14 and Pythium ultimum PyFr-14 by Paenibacillus polymyxa NMA1017 and Burkholderia cenocepacia CACua-24: a proposal for biocontrol of phytopathogenic fungi. Microbiol Re. 2020;230:126347. doi: 10.1016/j.micres.2019.126347. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH. Biocontrol of tomato wilt disease by B acillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol. 2013;15(3):848–864. doi: 10.1111/j.1462-2920.2012.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xin K, Liu H, Cheng J, Shen X, Wang Y, Zhang L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci Rep. 2017;7:41564. doi: 10.1038/srep41564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Brade G, Muzammil S, Sessitsch A, Mathieu LA, F, Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. Biocontrol. 2013;58:435–455. doi: 10.1007/s10526-012-9479-6. [DOI] [Google Scholar]
- Costa PBD, Granada CE, Ambrosini A, Moreira F, de Souza R, dos Passos JFM, Arruda L, Passaglia LMP. A model to explain plant growth promotion traits: a multivariate analysis of 2211 bacterial isolates. PLoS ONE. 2014;9:116020. doi: 10.1371/journal.pone.0116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray JA, Houghton JD, Cooke LR, Hallsworth JE. A simple inhibition coefficient for quantifying potency of biocontrol agents against plant-pathogenic fungi. Biocontrol. 2015;81:93–100. [Google Scholar]
- Daami-Remadi M, Zammouri S, El-Mahjoub M. Effect of the level of seed tuber infection by Rhizoctonia solaniat planting on potato growth and disease severity. Afr J Plant Sci Biotechnol. 2008;2:34–38. [Google Scholar]
- Daami-Remadi M, Zammouri S, El-Mahjoub M. Relative susceptibility of nine potato (Solanum tuberosum L.) cultivars to artificial and natural infection by Rhizoctonia solanias measured by stem canker severity, black scurf and plant growth. Afr J Plant Sci Biotechnol. 2008;2:57–66. [Google Scholar]
- Dahala B, NandaKaflea G, Perkins L, Brözel VS. Diversity of free-Living 519 nitrogen fixing Streptomyces in soils of the bad lands of South Dakota. Microbiol Res. 2017;195:31–39. doi: 10.1016/j.micres.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Din BU, Rafique M, Javed MT, Kamran MA, Mehmood S, Khan M, Chaudhary HJ. Assisted phytoremediation of chromium spiked soils by Sesbania Sesban in association with Bacillus xiamenensis PM14: a biochemical analysis. Plant Physiol Biochem. 2020;146:249–258. doi: 10.1016/j.plaphy.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Dong X, Fang L, Ye Z, Zhu G, Lai Q, Liu S. Screening of biocontrol bacteria against soft rot disease of Colocasia esculenta (L.) schott and its field application. PLoS ONE. 2021;16(7):e0254070. doi: 10.1371/journal.pone.0254070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani MN. Genetic variability and virulence of some Iranian Rhizoctonia solani isolates associated with stem canker and black scurf of potato (Solanum tuberosum L.) J Plant Prot Res. 2020;60:21–30. [Google Scholar]
- Ferreira CM, Soares HM, Soares EV. Promising bacterial genera for agricultural practices: an insight on plant growth-promoting properties and microbial safety aspects. Sci Total Environ. 2019;682:779–799. doi: 10.1016/j.scitotenv.2019.04.225. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ghosh P, Maiti TK. Production and metabolism of indole acetic acid (IAA) by root nodule bacteria (Rhizobium): a review. J Pure Appl Microbiol. 2011;5:523–540. [Google Scholar]
- Glick BR. Introduction to plant growth-promoting bacteria. In Beneficial plant-bacterial interactions. Cham: Springer; 2020. [Google Scholar]
- Gontia-Mishra I, Sapre S, Tiwari S. Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere. 2017;3:185–190. doi: 10.1016/j.rhisph.2017.04.013. [DOI] [Google Scholar]
- Gupta PK. Toxicity of fungicides. In Veterinary toxicology. Cambridge: Academic Press; 2018. [Google Scholar]
- Haidar R, Fermaud M, Calvo-Garrido C, Roudet J, Deschamps A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol Mediterr. 2016;55:301–322. [Google Scholar]
- Huang Y, Zhang W, Pang S, Chen J, Bhatt P, Mishra S, Chen S. Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ Res. 2020;194:110660. doi: 10.1016/j.envres.2020.110660. [DOI] [PubMed] [Google Scholar]
- Hussain T, Khan AA. Bacillus subtilis HussainT-AMU and its Antifungal activity against Potato Black scurf caused by Rhizoctonia solani on seed tubers. Biocatal Agric Biotechnol. 2020;23:101443. doi: 10.1016/j.bcab.2019.101443. [DOI] [Google Scholar]
- Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- Kang Y, Carlson R, Tharpe W, Schell MA. Characterization of genes involved in biosynthesis of a novel antibiotic from Burkholderia cepacia BC11 and their role in biological control of Rhizoctonia solani. Appl Environ Microbiol. 1998;64:3939–3947. doi: 10.1128/AEM.64.10.3939-3947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani C, Singh HB, García-Estrada C, Caradus J, He YW, Mezaache-Aichour S. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl Microbiol Biotechnol. 2020;104:1013–1034. doi: 10.1007/s00253-019-10300-8. [DOI] [PubMed] [Google Scholar]
- Khan AL, Halo BA, Elyassi A, Ali S, Al-Hosni K, Hussain J, Lee IJ. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Elec J Biotechnol. 2016;21:58–64. doi: 10.1016/j.ejbt.2016.02.001. [DOI] [Google Scholar]
- Khan N, Bano A, Ali S, Babar MA. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth and Reg. 2020;90:189–203. doi: 10.1007/s10725-020-00571-x. [DOI] [Google Scholar]
- Lamichhane JR, You MP, Laudinot V, Barbetti MJ, Aubertot JN. Revisiting Sustainability of Fungicide Seed Treatments for Field Crops. Plant Disease. 2020;104(3):610–623. doi: 10.1094/PDIS-06-19-1157-FE. [DOI] [PubMed] [Google Scholar]
- Lucas JA. Plant pathology and plant pathogens. USA: Wiley; 2020. [Google Scholar]
- Lugtenberg BJ, Chin-A-Woeng TF, Bloemberg GV. Microbe–plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek. 2002;81:373–383. doi: 10.1023/A:1020596903142. [DOI] [PubMed] [Google Scholar]
- Mahmoud OMB, Hidri R, Talbi-Zribi O, Taamalli W, Abdelly C, Djébali N. Auxin and proline producing rhizobacteria mitigate salt-induced growth inhibition of barley plants by enhancing water and nutrient status. S Afr J Bot. 2020;128:209–217. doi: 10.1016/j.sajb.2019.10.023. [DOI] [Google Scholar]
- Maqbool S, Amna A, Mehmood S, Suhaib M, Sultan T, Munis MFH. Interaction of Acc deaminase and antioxidant enzymes to induce drought tolerance in enterobacter Cloacae 2wc2 inoculated maize genotypes. Pak J Bot. 2021;53:3. doi: 10.30848/PJB2021-3(28). [DOI] [Google Scholar]
- Marulanda A, Porcel R, Barea JM, Azcón R. Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb Ecol. 2007;54(3):543. doi: 10.1007/s00248-007-9237-y. [DOI] [PubMed] [Google Scholar]
- Mehmood S, Khan AA, Shi F, Tahir M, Sultan T, Munis MFH, Chaudhary HJ. Alleviation of salt stress in wheat seedlings via Multifunctional Bacillus aryabhattai PM34: an In-Vitro Study. Sustainability. 2021;13(14):8030. doi: 10.3390/su13148030. [DOI] [Google Scholar]
- Mejdoub-Trabelsi B, Jabnoun-Khiareddine H, Daami-Remadi M. Effect of Fusarium species and temperature of storage on the susceptibility ranking of potato cultivars to tuber dry rot. Pest Technol. 2012;6:41–46. [Google Scholar]
- Moncada A, Miceli A, Vetrano F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci Hortic. 2020;275:109733. doi: 10.1016/j.scienta.2020.109733. [DOI] [Google Scholar]
- Muneer MA, Wang P, Zhang J, Li Y, Munir MZ, Ji B. Formation of common mycorrhizal networks significantly affect plant biomass and soil properties of the neighboring plants under various nitrogen levels. Microorganisms. 2020;8:230. doi: 10.3390/microorganisms8020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer MA, Wang P, Lin C, Ji B. Potential role of common mycorrhizal networks in improving plant growth and soil physicochemical properties under varying nitrogen levels in a grassland ecosystem. Glob Ecol Conserv. 2020;24:e01352. doi: 10.1016/j.gecco.2020.e01352. [DOI] [Google Scholar]
- Nalini S, Parthasarathi R. Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Ann Agric Sci. 2018;16:108–115. [Google Scholar]
- Natasha SM, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI. A criticalreview of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollu. 2018;234:915–934. doi: 10.1016/j.envpol.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO. Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol Biotechnol. 2019;103:1155–1166. doi: 10.1007/s00253-018-9556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar P, Sindhu SS. Potassium solubilization by rhizosphere bacteria: influence of nutritional and environmental conditions. J Microbiol Res. 2013;3:25–31. [Google Scholar]
- Rahman SFS, Singh E, Pieterse CM, Schenk PM. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–111. doi: 10.1016/j.plantsci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Rfaki A, Zennouhi O, Aliyat FZ, Nassiri L, Ibijbijen J. Isolation, selection and characterization of root-associated rock phosphate solubilizing bacteria in moroccan wheat (Triticum aestivum L.) Geomicrobiol J. 2020;37(3):230–241. doi: 10.1080/01490451.2019.1694106. [DOI] [Google Scholar]
- Santoyo GG, Moreno-Hagelsieb MC, Orozco-Mosqueda GBR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Sasirekha B, Srividya S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric Nat Resour. 2016;50:250–256. [Google Scholar]
- Van Schoonhoven A (1987) Standard system for the evaluation of bean germplasm. CIAT
- Sethi S, Datta A, Gupta BL, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. International Scholarly Research Notices
- Shahid M, Farooq ABU, Rabbani F, Khalid S, Dumat C. Risk assessment and biophysiochemical responses of spinach to foliar application of lead oxide nanoparticles: a multivariate analysis. Chemosphere. 2020;245:125605. doi: 10.1016/j.chemosphere.2019.125605. [DOI] [PubMed] [Google Scholar]
- Shahzad SM, Arif MS, Riaz M, Iqbal Z, Ashraf M. PGPR with varied ACCdeaminase activity induced different growth and yield response in maize (Zea mays L.) under fertilized conditions. Eur J Soil Biol. 2013;57:27–34. doi: 10.1016/j.ejsobi.2013.04.002. [DOI] [Google Scholar]
- Singh RP, Jha PN. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front Microbiol. 2017;8:1945. doi: 10.3389/fmicb.2017.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Singh P, Li H, Song QQ, Guo DJ, Solanki MK. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: a comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020;20:1–21. doi: 10.1186/s12870-020-02400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kumari R, Yadav AN, Mishra S, Sachan A, Sachan SG (2020a) Tiny microbes, big yields: Microorganisms for enhancing food crop production for sustainable development. In New and Future Developments in Microbial Biotechnology and Bioengineering (pp. 1–15). Elsevier, USA
- Suárez-Moreno ZR, Vinchira-Villarraga DM, Vergara-Morales DI, Castellanos L, Ramos FA, Guarnaccia C. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front Microbiol. 2019;10:290. doi: 10.3389/fmicb.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir M, Ahmad I, Shahid M, Shah GM, Farooq ABU, Akram M, Zakir A. Regulation of antioxidant production, ion uptake and productivity in potato (Solanum tuberosum L.) plant inoculated with growth promoting salt tolerant Bacillus strains. Ecotoxicol Environ Saf. 2019;178:33–42. doi: 10.1016/j.ecoenv.2019.04.027. [DOI] [PubMed] [Google Scholar]
- Tahir M, Khalid U, Khan MB, Shahid M, Ahmad I, Akram M, Ahmad N. Auxin and 1-Aminocyclopropane-1-carboxylate deaminase activity exhibiting rhizobacteria improved maize quality and productivity under drought conditions. Int J Agric Biol. 2019;21:943–954. [Google Scholar]
- Tahir M, Naeem MA, Shahid M, Khalid U, Farooq ABU, Ahmad N, Waqar A. Inoculation of pqqE gene inhabiting Pantoea and Pseudomonas strains improves the growth and grain yield of wheat with a reduced amount of chemical fertilizer. J Appl Microbiol. 2020;129(3):575–589. doi: 10.1111/jam.14630. [DOI] [PubMed] [Google Scholar]
- Tarhouni B (2007) Chemical control of Rhizoctonia salani. Annual Report of the Technical Center of Potato, Essaida, Tunisia, pp. 81e86 (in Arabic)
- Thakur N, Kaur S, Tomar P, Thakur S, Yadav AN (2020) Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering 243–282.
- Tilocca B, Cao MQ. Scent of a Killer: microbial volatile and its role in the biological control of plant pathogens. Front Microbiol. 2020;11:41. doi: 10.3389/fmicb.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Riffel M. Making the right conclusions based on wrong results and small sample sizes: interpretation of statistical tests in ecotoxicology. Ecotoxicol Environm Saf. 2011;74:684–692. doi: 10.1016/j.ecoenv.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu B, Xue Y, Chen Z, Liang X. Identification and antifungal activity of the antagonistic bacteria of Cytospora spp. Zhongguo Shengtai Nongye Xuebao/chinese J Eco-Agriculture. 2014;22(10):1214–1221. [Google Scholar]
- Warnakumari NS, Ramanan D, Kanniappan M. Host – pathogen interaction between the soil borne fungi, Fusarium moniliforme and maize plants (Zea mays L.) J Ecobiotechnol. 2011;3:01–05. [Google Scholar]
- Xiao J, Guo X, Qiao X, Zhang X, Chen X, Zhang D. Activity of fengycin and iturin A isolated from Bacillus subtilis Z-14 on Gaeumannomyces graminis var tritici and soil microbial diversity. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.682437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainab N, Din BU, Javed MT, Afridi MS, Mukhtar T, Kamran MA, Chaudhary HJ. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol Biochem. 2020;152:90–99. doi: 10.1016/j.plaphy.2020.04.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.