Abstract
Introduction
In the USA, psoriasis affects approximately 3% of the population and costs more than $110 billion annually. The development of targeted biologics has revolutionized psoriasis management, but at an increasing cost. According to Joint AAD/NPF guidelines, an important need exists to identify biomarkers that can predict the appropriate biologic agent for patients.
Methods
A survey of community dermatologists was developed to address (1) significant factors influencing biologic therapy utilization in psoriasis; (2) the clinical utility of a test stratifying biologic response.
Results
Respondents confirmed that trial and error leads to frequent biologic switching. The survey indicated that 82% of dermatologists switch 10–30% of their patients in the first year and 98% switch intra-class for at least 50% of non-responding patients. The trial and error is due, in part, to formularies influencing the physician 77% of the time, with only 14% reporting that their first choice and the formulary alignment is greater than 75%. Compounding trial and error, 93% of the physicians report that they wait at least 12 weeks before determining non-response, in alignment with AAD/NPF guidelines. The lack of precision medicine and this trial-and-error approach result in unnecessary wasted spending and suboptimal patient outcomes. After being given an overview of Mind.Px, a dermal biomarker patch used to predict therapeutic response to a biologic class, survey participants expressed that:
93% would utilize Mind.Px results to determine first-line therapy even if this differed from initial clinical choice
100% would utilize Mind.Px if part of the prior authorization process
98% say Mind.Px would improve patient outcomes
81% reported Mind.Px would help with prior authorization process
Conclusions
Surveyed dermatologists believe a test that predicts psoriasis treatment response to a class of biologic drugs would lessen trial and error, provide a tool for physicians to make more informed decisions about drug selection, improve patient outcomes, and significantly reduce wasted spending.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00573-1.
Keywords: Biologic therapy, Cost-effectiveness, Physician survey, Psoriasis, Use patterns
Key Summary Points
| This study represents a survey of 43 community dermatologists |
| 77% considered insurance formulary a moderate to major influence on first-line utilization of biologic drugs for treating psoriasis and the formulary was compatible with their first-line choice at least 75% of the time for only 14% of respondents |
| Non-response at 12–16 weeks was used to determine switching therapy, in alignment with AAD/NPF published guidelines |
| Mind.Px is a dermal biomarker patch used to predict therapeutic response to biologic class |
| If the Mind.Px diagnostic test was available and could accurately predict biologic response, 98% of responders would use it, and if Mind.Px was incorporated into the prior authorization process, 100% of responders would use it |
| 93% of responders would utilize Mind.Px prediction results to determine first-line therapy even if this differed from their initial clinical choice |
| 98% of physicians agreed that Mind.Px would improve patient outcomes |
Introduction
In the USA, psoriasis affects approximately 3% of the population and the costs of managing the disease totaled $112 billion in 2013. Approximately half of these costs were direct costs (e.g., medication, phototherapy, and provider visits) [1] with direct psoriasis costs ranging from $51.7 billion to $63.2 billion, indirect costs ranging from $23.9 billion to $35.4 billion, and medical comorbidities estimated to contribute $36.4 billion annually in 2013 US dollars [2]. New entrants to the market, including the popular anti-interleukin-23 (IL-23) biologics, have higher costs and likely have increased the total economic burden of disease.
The development of targeted biologics has significantly changed the management of moderate-to-severe psoriasis. Patient quality of life has been dramatically improved by the high efficacy and relatively low toxicity of biologics [3]. However, this improvement has resulted in a dramatic increase in spending on higher-priced targeted biologic drugs. The ability of biologics to clear, or almost clear, the disease has changed the outcomes and expectations of many patients with psoriasis. Moderate‐to‐severe psoriasis, a chronic, lifelong condition, has a negative impact on quality of life and therefore it is important to select safe and effective treatments early during the treatment course [4].
From its initial approval by the US Food and Drug Administration (FDA) in December of 2002, adalimumab has become the top-selling drug in the world [5]. In 2020, 99% of commercial patients in the USA had access to adalimumab as a preferred, first-line, targeted immunomodulator on payer formularies [6]. The commercial success of adalimumab has paved the way for other biologic agents to successfully enter the market, including the interleukin (IL) inhibitors that target IL-17, IL-23, and IL-12/23. Capitalizing on the higher published efficacy rates, the manufacturers of IL-17 and IL-23 inhibitors price these drugs significantly higher than the tumor necrosis factor-alpha (TNFα) inhibitors. As a result, most payers have retained TNFα inhibitors as their first-line biologic agents. Although most payers include one IL-17 or IL-23 inhibitor as first-line therapy, many relegate these more expensive drugs to second- or even third-line therapy depending on rebates provided by the manufacturers [7, 8].
Complex formulary decisions and rebates are based on multiple factors, particularly with drugs approved for a wide variety of indications. However, a formulary limited by decisions regarding the best treatment for inflammatory disorders such as rheumatoid arthritis and Crohn’s disease may not adequately address the needs of patients with psoriasis [1]. Moreover, many comorbidities must be considered when selecting the best agent for any given patient [9, 10]. This strategy may not align with the appropriate first-line targeted biologic for patients with psoriasis based on clinical factors, leading to poor clinical response and the need to switch drugs following the initial phase of treatment.
The early phase of treatment with a biologic is often lengthy. As the Joint American Academy of Dermatology–National Psoriasis Foundation (AAD-NPF) guidelines of care for the management and treatment of psoriasis with biologics have stated, definitive response (positive or negative) to treatment with almost all of the biologics used in the treatment of psoriasis is best ascertained after 12–16 weeks of continuous therapy [11]. Thus, in this paradigm, patients who fail first-line TNFα inhibitor therapy move on to second-line IL-17 and IL-23 inhibitors, creating a trial-and-error approach to biologic therapy that is extremely costly to payers as well as frustrating and burdensome to patients and their physicians. In addition, given the expanding number of drugs across several biologic classes in the treatment of psoriasis, the cost effectiveness of the newer agents has not been fully characterized [12].
Predictive biomarkers can be used in clinical practice in identifying risk for and diagnosing a disease, stratifying patients, assessing disease severity or progression, predicting prognosis, or guiding appropriate treatment. Indeed, biomarkers have played a very important role in understanding the pathogenesis of psoriasis and have facilitated the development of biological therapies [13]. As stated in the Joint NPF/AAD guidelines [11], an important need exists to identify biomarkers that can potentially predict the appropriate biologic agent for individual patients with psoriasis prior to initiating treatment.
Mindera Corporation (San Diego, CA) has developed a platform that utilizes a simple, minimally invasive, painless patch for the collection of a biomarker sample in a matter of minutes. Furthermore, the ability to collect patient data at scale, combined with high-precision molecular testing, results in a powerful platform where machine learning tools can be brought to bear, resulting in clinically actionable algorithms that possess excellent sensitivity and specificity. Use of this platform can potentially translate into substantial cost savings for healthcare systems, particularly when applied to the prediction of response to expensive treatments, effectively eliminating the current trial-and-error approach to treatment.
Using the Mindera platform, a predictive test has been developed (Mind.Px) that uses collected mRNA biomarkers to predict patient response to biologic prior to initial dosing. The role of mRNA in chronic skin diseases is well characterized and has been exploited by the test to provide the missing predictive link between a patient’s genetic markers and responsiveness to different drug classes. After capture of mRNA from a patient’s psoriatic lesion, next-generation sequencing is used to evaluate more than 7000 biomarkers per test sample. The results of this biomarker analysis can be used by healthcare providers and payers to predict an individual patient’s response to a mechanism of action class of biologic drugs to optimize treatment selection. The use of Mind.Px could result in better outcomes and significantly reduced costs to the healthcare system.
To further characterize the utilization of biologic therapy in the real-world management of psoriasis, a survey was conducted to evaluate prescribing behavior of physicians as well as their response to the concept of Mind.Px. The survey addressed two key questions:
What are the significant factors influencing the utilization of biologic therapy?
What is the perceived clinical utility of a test stratifying response to biologic therapy?
Methods
This study was determined to be exempt from IRB oversight as per 45 CFR 46.104(d)(2) by the Institutional Review Board (Advarra). All of the participating dermatologists consented to participate in the study electronically. The survey respondents gave consent to participate and were aware that the results of the study would be used for potential publication. Each participant was pre-screened for eligibility and their responses verified. The group was given a unique login and completed the survey electronically. The survey was conducted under a secure link provided by our vendor that was password protected. The study was performed in accordance with the declaration of Helsinki 1964 and its later amendments.
A list of physicians was generated from a database that included biologic, systemic, and topical use by tiers. From this database the top tier 200 biologic users were selected to conduct the pre-screening phase of the survey. Dermatologists were asked to provide details about their practice, their psoriasis patient population, including the number of patients with moderate/severe psoriasis being treated, the biologic agents used to treat patient with moderate/severe psoriasis, and the geographic location of their practice. The physicians were not compensated for this screening.
On the basis of these findings, a cohort of 45 dermatologists was selected to respond to a survey consisting of 40 questions; 43 of the 45 dermatologists completed the survey. The physicians who completed the remaining surveys were compensated for their participation. A majority (84%) of respondents were from private practice and 16% from academia. The respondents were well distributed across the USA, with 11 from the Midwest, 8 from the Northeast, 14 for the Southeast, 9 from the Southwest, and 1 from the Northwest. This cohort of dermatologists treated 10–50 patients with psoriasis per month, of whom 25% were being treated for mild psoriasis, 43% for moderate psoriasis, and 29% for severe psoriasis. More than 75% of patients were being treated with biologics. The respondent dermatologists reported using multiple biologics with their patient base. Twenty six percent (26%) used more than 10 different biologics, 51% used 7–9 different biologics, and 22% reported using fewer than 6 different biologics.
The 43 dermatologists who completed the survey then participated in a webinar describing response rates for biologics, loss of response over time, and the estimated costs to society relating to lack of/loss of therapeutic response. Following the webinar, the participants were asked to complete a post-survey questionnaire comprising eight questions; 43 of the 43 participants completed both the pre- and post-survey questionnaires.
Results
Selection of First-Line Therapy
The selection of an agent for first-line therapy can be driven by multiple factors. In this survey, 88% of the physicians indicated that perceived response rates were a moderate to most important factor influencing their choice of biologic for a patient (Table 1). Alternatively, adverse events (81%) and comorbidities (82%) also were reported as moderate to most important factors in the decision-making process for first-line biologic. Other areas of influence including patient preference (44%), dermatologist familiarity (61%), ease of use (67%), and cost (63%) were also reported as moderate to most important. The factors driving clinical decisions regarding selection of first-line therapy, as determined through the physician survey, are shown in Table 1. Additionally, 54% of respondents stated greater than 50% of the time that formulary determined first-line choice of biologics. Of physicians surveyed, for first-line use, 2% would preferentially choose a TNFα inhibitor, 75% IL-23 inhibitors, 21% IL-17 inhibitors, and 2% IL-12/23 inhibitors. However, 77% of the physicians surveyed indicated that they would make their first-line selection based on the patient’s insurance formulary regardless of their personal preference for that patient based on the various clinical factors they considered important.
Table 1.
Dominant factors driving clinical decisions regarding selection of first-line therapy. In all cases, respondents listed the factor as either “moderately” or “mostly” influencing their decision
| Factor | % of respondents |
|---|---|
| Response rates | 88 |
| Patient diagnosed with psoriatic arthritis | 86 |
| Presence of comorbidities | 82 |
| Side effects/adverse events | 81 |
| Formulary of the patient’s insurance company | 77 |
| Ease of use | 67 |
| Cost | 63 |
| Physician familiarity | 61 |
| Patient preference | 44 |
Treatment Failure and Switching
The 43 respondents were asked a series of questions regarding treatment duration and timing of treatment decisions. The first patient follow-up visit is scheduled at 4 weeks by 37% of the respondents, between 6 and 8 weeks for 28% of respondents, and at 12 weeks by 30% of the respondents; the remaining 5% stated that the first follow-up is scheduled for 3 weeks. Of the respondents, 51% saw patients for follow-up at 12 weeks and 49% determined non-response at 12 weeks while 30% determined non-response at 16 weeks and 14% waited 6 months. This was independent of the chosen biologic agent, and regardless of the published rates of onset and degree of response.
The respondents indicated that there is a high rate of failure of the first-line biologic therapy and the need for switching to a second-line agent is frequent. Seventy-four percent (74%) of the respondents reported that 10–75% of patients required switching in the first year of treatment as a result of either primary or secondary failure. Most switching occurs within the first 2 years of treatment, with respondents reporting that for those patients that require switching, 47% occurs in the first year and 40% in the second year. Seventy-nine percent (79%) of the physicians switch treatments at 12 or 16 weeks, which coincides with the timing of follow-up visits to assess response to first-line therapy. When switching does occur, most physicians switch to a different class of biologic (82% use the same class less than 25% of the time). Notably, very few physicians discontinue use of biologic therapy altogether. These results confirm that trial-and-error treatment paradigms dominate prescribing behaviors.
Formulary Complexity and Misalignment with Physician Preference
Physicians in the survey reported that payer formulary status had moderate to most influence in their treatment decision 77% of the time, and 54% indicated that formulary status determined their first-line choice more than 50% of the time. As an example, in a world with no formulary restrictions 2% of respondents chose TNFα inhibitors in the first line, yet TNFα inhibitors are, in fact, the first-line formulary choice for these physicians 44% of the time. If there were no formulary restrictions, 75% of the respondents chose an anti-IL-23 as their first-line agent, and with formulary restrictions this number reduced to 45% of the time (Fig. 1a). Interestingly, in selecting second-line biologics, there was somewhat greater agreement between physician preference and formulary-driven selection. This finding may be indicative of the lack of response seen with first-line formulary-driven selections (Fig. 1b).
Fig. 1.
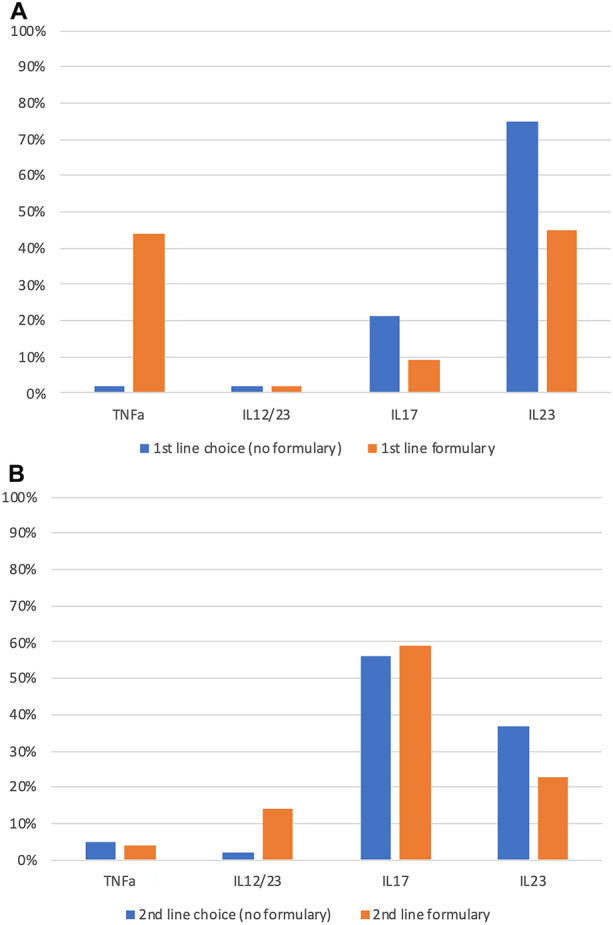
Misalignment of a first-line and b second-line selection by class based on formulary. IL interleukin, TNF tumor necrosis factor
Impact of Mind.Px Correctly Predicting Patient Biologic Response
The post-survey webinar described Mind.Px as a tool that can be used to prospectively predict response to biologics in patients with psoriasis, resulting in improved patient outcomes. Use of Mind.Px also has the potential to significantly reduce the waste of billions of annual healthcare dollars because of patient non-response to initial treatment.
Following the webinar, participants were asked to respond to questions relating to the Mind.Px platform. Respondents indicated that the most important problem that a predictive diagnostic test such as Mind.Px addresses is determining the class of drug to which an individual patient will respond. Of respondents, 79% said that a test that stratifies patients for response solves the important problem of matching individual patient treatment response with specific biologic class (Table 2). The majority of respondents (81%) also believed that a test such as Mind.Px would help with the prior authorization process, with 14% indicating this was the most important benefit to them and their patients.
Table 2.
Results following the post-survey webinar to the question “How would Mind.Px improve clinical practice?”
| How would Mind.Px improve clinical practice? | % of respondents |
|---|---|
| My patients would see me as more informed and helpful | 79 |
| Mind.Px would help patients determine the right therapy despite biologic manufacturer influence and advertising | 88 |
| Mind.Px would reduce office visits by frustrated patients | 67 |
Table 3 presents the results following the post-survey webinar. Importantly, 98% of respondents indicated that they would use Mind.Px, and 93% of respondents stated that they would use it to determine their first-line therapy even if it differed from their initial choice. In addition, 98% indicated that Mind.Px would improve patient outcomes by helping physicians determine the correct therapy, which in turn would make their patients see them as more informed and helpful.
Table 3.
Results following the post-survey webinar
| Question | % responding “yes” |
|---|---|
| If the Mind.Px diagnostic test was available, would you use it? | 98 |
| If Mind.Px was incorporated into the prior authorization process, would you use it? | 100 |
| Would you utilize Mind.Px prediction results to determine first-line therapy if this differed from your initial clinical choice? | 93 |
Positive Impact of Mind.Px on Physician Prior Authorization Process and Office Workflow
The prior authorization process for biologic approval has become a required, burdensome, and frequently costly piece of the psoriasis treatment paradigm. Of respondents, 82% indicated that prior authorization was required in more than 50% of treatment decisions. Prior authorization requires considerable additional resources to manage, with 35% of the respondents indicating they appealed the payer decision 50% or more of the time and 49% indicating they appealed the decision 25–50% of the time. One hundred percent (100%) of respondents would use Mind.Px if it were incorporated into payer prior authorization protocols and 81% stated Mind.Px would help with the prior authorization process along with 67% responding that Mind.Px would reduce office visits by frustrated patients and 79% of physicians indicated that they would be perceived by their patients to be more informed and helpful (Tables 2 and 3).
Discussion
Surveys from the National Psoriasis Foundation collected from 2003 to 2011 found that 23.6–35.5% of patients with moderate psoriasis, and 9.4–29.7% of patients with severe psoriasis, were considered undertreated and only 47.7% of patients were satisfied with their treatment [14]. With a wider variety of biologic therapies now available, physicians now can significantly and safely impact the lives of patients with psoriasis. However, access to the optimal therapy may be challenging because of trial-and-error prescribing behavior occurring owing to the lack of a predictive tool to align patients with the appropriate biologic class. This creates treatment failures and leads to massive wasted healthcare spending as well as poorer patient outcomes.
This survey confirmed that trial-and-error prescribing behavior dominates biologic usage by dermatologists who often treat psoriasis. These results also showed that response rates, comorbidities, and the risk of adverse events do play a role in clinician preference. However, it was interesting to note that formulary considerations were nearly as important as comorbidities and potential adverse events in determining selection of therapy. Furthermore, 84% of physicians reported that they appeal prior authorization greater than 25% of the time, with 35% stating that they appeal greater than 50% of the time and that 82% reported more than 50% of the time a start or switch of biologic requires a prior authorization.
As indicated in this survey, physician preferences and formulary considerations may not align, especially with initial therapy selections. In addition to clinical factors, various issues may be considered when selecting appropriate therapy: changing insurance policies, patient age, Medicare eligibility, stability of response to current therapy, or patient preference. These factors are frequently beyond the scope of a payer decision, resulting in perpetuation of the currently misaligned treatment paradigm. Ultimately, all of these factors lead to significant failure rates, switching of therapies initiated by both patients and physicians, delayed efficacy, and considerably higher costs. A simple test that would predict response to treatment and optimize outcomes would reduce treatment failures and decrease the costs incurred while waiting to determine if the therapy selected in a trial-and-error manner is going to be effective.
The time to assessing response to therapy has been widely accepted as 12–16 weeks, as established by the AAD/NPF guidelines, and was validated in the majority of survey respondents. Remarkably, 14% of respondents waited until 6 months after initiating treatment before assessing response to biologic therapy. However, because the rate of failure can be significant, especially with non-biologic-naïve patients, the time to assessing response is costly in terms of healthcare dollars, as well as physician and patient frustration. Indeed, an extended induction phase greater than that recommended by the AAD/NPF guidelines magnifies the per patient wasted spend.
Conclusions
Via this survey it has been confirmed that there are multiple factors that influence the selection of biologic for patients with psoriasis, including formulary status, response rates, comorbidities, potential adverse events, cost, familiarity and ease of use, and patient preference. Overwhelmingly, survey respondents perceived the potential use of the Mind.Px test as potentially having a positive impact on their clinical practice, including improved patient outcomes, reduced patient frustration, and increased efficiencies in the prior authorization process. These physicians also appreciated that the availability of such a test might give their patients more confidence in the drug they are receiving. Indeed, 93% of responding physicians affirmed that they would follow the recommendation of Mind.Px, even if this differed from their initial biologic choice.
Mind.Px could eliminate the trial-and-error process and introduce a new treatment paradigm where patients are matched to the most appropriate biologic at the onset of treatment. By removing unnecessary induction periods from the patient treatment journey, wasted spend by the system is dramatically reduced. Mind.Px potentially brings precision medicine to psoriasis, aligning the goals and necessities of patients, physicians, and payers.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully thank the physicians who participated in this survey.
Funding
Support for this survey and the journal’s Rapid Service fee was provided by Mindera Corporation.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Evelyn Albu of e4 Health Group. Support for this assistance was funded by Mindera Corporation.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Bruce Strober is a consultant (honoraria) for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Immunic Therapeutics, Bristol Myers Squibb, Connect Biopharma, Dermavant, Equillium, Janssen, Leo, Eli Lilly, Maruho, Meiji Seika Pharma, Mindera, Novartis, Pfizer, GlaxoSmithKline, UCB Pharma, Sun Pharma, Ortho Dermatologics, Regeneron, Sanofi-Genzyme, Ventyxbio, and vTv Therapeutics. He has received speaker honoraria from AbbVie, Eli Lilly, Janssen, and Sanofi-Genzyme. Dr. Strober is the co-Scientific Director of the Cor-Evitas (Corrona) Psoraisis Registry, the Editor-in-Chief (honoraria) of the Journal of Psoriasis and Psoriatic Arthritis, and has received research support from Dermavant, AbbVie, Corrona Psoriasis Registry, Dermira, Cara, and Novartis. David Pariser is a consultant (honoraria) for Atacama Therapeutics, Bickel Biotechnology, Biofrontera AG, Bristol Myers Squibb, Celgene, Dermira, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Sanofi, TheraVida, and Valeant. Dr. Pariser has also received research funding from Almirall, Amgen, AOBiome, Asana Biosciences, Bickel Biotechnology, Celgene, Dermavant Sciences, Dermira, Eli Lilly, LEO Pharma, Menlo Therapeutics, Merck, Novartis, Novo Nordisk, Ortho Dermatologics, Pfizer, and Regeneron. Ann Deren-Lewis is an employee of Mindera Corporation. Ms. Deren-Lewis is a Board member of Afecta Pharmaceuticals. She is a consultant (honoraria) for Park Perfection and Ellis Day Skin Science. Tobin Dickerson is an employee and shareholder of Mindera Corporation. Mark Lebwohl is an employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB, Inc. Dr. Lebwohl is also a consultant for Aditum Bio, Almirall, AltruBio Inc., AnaptysBio, Arcutis, Inc., Aristea Therapeutics, Arrive Technologies, Avotres Therapeutics, BiomX, Boehringer-Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Dr. Reddy’s Laboratories, Evelo Biosciences, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Helsinn Therapeutics, Hexima Ltd., LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, and Verrica. Alan Menter is a consultant (honoraria) for Abbott Labs, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen Biotech, LEO Pharma, Mindera, Novartis, SunPharma, and UCB, Inc. Dr. Menter has also received grant funding from Abbott Labs, Amgen, Boehringer Ingelheim, Celgene, Janssen Biotech, LEO Pharma, Merck, and SunPharma.
Compliance with Ethics Guidelines
This study was determined to be exempt from IRB oversight as per 45 CFR 46.104(d)(2) by the Institutional Review Board (Advarra). All of the participating dermatologists consented to participate in the study electronically. The survey respondents gave consent to participate and were aware that the results of the study would be used for potential publication. Each participant was pre-screened for eligibility and their responses verified. The group was given a unique login and completed the survey electronically. The survey was conducted under a secure link provided by our vendor that was password protected. The study was performed in accordance with the declaration of Helsinki 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available as the data are proprietary to the sponsor of the study.
References
- 1.Malatestinic W, Amato D, Feldman SR. Formulary decisions and the evolution of psoriasis treatment. J Clin Pathways. 2015;1:43–47. [Google Scholar]
- 2.Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151(6):651–658. doi: 10.1001/jamadermatol.2014.3593. [DOI] [PubMed] [Google Scholar]
- 3.Trettin B, Feldman SR, Andersen F, Danbjørg DB, Agerskov H. A changed life: the life experiences of patients with psoriasis receiving biological treatment. Br J Dermatol. 2020;183(3):516–523. doi: 10.1111/bjd.18876. [DOI] [PubMed] [Google Scholar]
- 4.Strohal R, Prinz JC, Girolomoni G, Nast A. A patient-centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol. 2015;29(12):2390–2398. doi: 10.1111/jdv.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasure S. BioSpace. BioSpace feature: a look at miracle drug Humira’s journey to proven efficacy. 2018. https://www.biospace.com/article/biospace-feature-a-look-at-miracle-drug-humira-s-journey-to-proven-efficacy-/. Accessed Mar 12, 2021.
- 6.AbbVie. Humira. Access for your patients. https://www.humiradermpro.com/humira-formulary-coverage. Accessed Mar 12, 2021.
- 7.Wu JJ, Feldman SR, Rastogi S, Menges B, Linghohr-Smith M, Lin J. Comparison of the cost-effectiveness of biologic drugs used for moderate-to-severe psoriasis treatment in the United States. J Dermatolog Treat. 2018;29(8):769–774. doi: 10.1080/09546634.2018.1466022. [DOI] [PubMed] [Google Scholar]
- 8.Stoll D, Gutschmidt D, Dulitz S. New paths in predictive payer coverage. PolicyReporter™. http://policyreporter.com. Accessed Mar 12, 2021.
- 9.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient. Psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40. doi: 10.1016/j.jaad.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 10.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient. Focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43–53. doi: 10.1016/j.jaad.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 11.Menter A, Strober B, Kaplan D, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho AVE, Duquia RP, Horta BL, Bonamigo RR. Efficacy of immunobiologic and small molecule inhibitor drugs for psoriasis: a systematic review and meta-analysis of randomized clinical trials. Drugs R D. 2017;17(1):29–51. doi: 10.1007/s40268-016-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 2016;6:7–32. doi: 10.2147/PTT.S64950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl M. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation Surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as the data are proprietary to the sponsor of the study.


