Abstract
Plant annexins are a kind of conserved Ca2+-dependent phospholipid-binding proteins which are involved in plant growth, development and stress tolerance. Radish is an economically important annual or biennial root vegetable crop worldwide. However, the genome-wide characterization of annexin (RsANN) gene family remain largely unexplored in radish. In this study, a comprehensive identification of annexin gene family was performed at the whole genome level in radish. In total, ten RsANN genes were identified, and these putative RsANN proteins shared typical characteristics of the annexin family proteins. Phylogenetic analysis showed that the RsANNs together with annexin from Arabidopsis and rice were clustered into five groups with shared similar motif patterns. Chromosomal localization showed that these ten RsANN genes were distributed on six chromosomes (R3-R8) of radish. Several cis-elements involved in abiotic stress response were identified in the promoter regions of RsANN genes. Expression profile analysis indicated that the RsANN genes exhibited tissue-specific patterns at different growth stages and tissues. The Real-time quantitative PCR (RT-qPCR) revealed that the expression of most RsANN genes was induced under various abiotic stresses including heat, drought, salinity, oxidization and ABA stress. In addition, stress assays showed that overexpression of RsANN1a improved plant’s growth and heat tolerance, while artificial microRNAs (amiRNA)-mediated knockdown of RsANN1a caused dramatically decreased survival ratio of Arabidopsis plants. These findings not only demonstrate that RsANN1a might play a critical role in the heat stress response of radish, but also facilitate clarifying the molecular mechanism of RsANN genes in regulating the biological process governing plant growth and development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01056-5.
Keywords: Radish, annexin gene family, RsANN1a, amiRNA, Gene function validation
Introduction
Annexins are evolutionarily conserved and Ca2+-dependent phospholipid-binding proteins, which are defined as a kind of multigene family and exist in all eukaryotes and some prokaryotes (Laohavisit and Davies, 2011). The plant annexin gene was first identified in tomato (Boustead et al. 1989). So far, only a few annexin gene families have been identified in plants including Arabidopsis (Clark et al. 2001), mustard (Jami et al. 2009), rice (Jami et al. 2012b), tomato (Lu et al. 2012), maize (Zhang et al. 2015), peanuts (He et al. 2015) and wheat (Xu et al. 2016).
Compared with the animal annexin gene family, the plant annexin gene family is relatively small and the structure is simple (Delmer and Potikhha, 1997), as well as the molecular weight of plant annexins range from 32 to 42 kD (Talukdar et al. 2009). Similar to animal annexins, plant annexins also have a variable N-terminus and a conserved C-terminus. The typical C-terminal structure usually consists of four annexin repeats (annexin Repeat I-IV) of approximately 70 amino acids (aa). It was found that at least three or four annexin repeats are highly conserved in animals, while only one or two repeats are conserved in plants (Gerke et al. 2005). Sequence analysis revealed that there are many conserved motifs in the plant annexins. For example, the conserved motif of GxGT-(38 residues)-D/E for calcium binding only exists in the Repeat I and Repeat IV (Mortimer et al. 2008). Plant annexins also possess an actin binding site in Repeat III and a GTP binding site (DXXG) in Repeat IV. In addition, there are also some post-translational modification sites in plant annexins, which might act as regulators for Ca2+-dependent signaling (Konopka-Postupolska et al. 2011; Laohavisit and Davies, 2011; Mortimer et al. 2008).
Increasing evidences indicated that plant annexin genes show differential expression profiles in many tissues at different development stages. For example, in Arabidopsis, AtANN1 was highly expressed in the stem, but AtANN2, AtANN3 and AtANN4 were hardly expressed in the stem while their expression level was higher in the root, AtANN5, AtANN6 and AtANN7 were abundantly expressed in the flowers (Clark et al. 2001). AtANN5 was hardly expressed in vegetative organs such as roots and rosette leaves at the vegetative growth stage, after transforming to the reproductive period, the expression level of AtANN5 showed a slight increase in the developing stem, and high expression level was observed in young developmental siliques. As compared with pistils, it showed the highest AtANN5 transcript abundance occurring's in mature pollen of stamens (Lichocka et al. 2018).
Several annexin genes were found to play a critical role in plant response to a variety of abiotic stresses. The AnnMs2 gene was first identified to be dramatically up-regulated under osmotic stress, ABA and drought stress in alfalfa (Kovacs et al. 1998). Subsequently, in many species including Arabidopsis (Cantero et al. 2006; Konopka-Postupolska et al. 2009), mimosa (Hoshino et al. 2004), Brassica (Jami et al. 2008; 2009; Ahmed et al. 2018), and rice (Shen et al. 2017), plant annexin genes were found to be involved in response to various abiotic stresses, and the expression level was regulated by many stress conditions including heat, cold, drought, salinity, and oxidative stresses as well as phytohormones. Overexpression of OsANN1 showed improved growth and enhanced heat stress tolerance (Qiao et al. 2015), while CRISPR/Cas9-mediated knock-out of the OsANN3 resulted in dramatically decreased survival ratio in loss-of-function mutants for OsAnn3 in rice under cold stress, indicating that OsANN3 could play a critical role in response to cold tolerance of rice (Shen et al. 2017). Furthermore, heterologous expression of BjANN2 could enhance salt tolerance and confer ABA insensitivity in transgenic tobacco seedlings (Ahmed et al. 2018). In maize, ZmANN33 and ZmANN35 were also proved to play a positive role during the plantʼs recovery from chilling injury (He et al. 2019).
Radish (Raphanus sativus L.), a member of Brassicaceae family, is an important root vegetable crop cultivated worldwide (Xu et al. 2013). The yield and quality of radish taproot are seriously affected by various stresses including heat, drought and salinity. It was reported that plant annexins play important roles in response to abiotic stresses (Konopka-Postupolska et al. 2009; Szalonek et al. 2015; Ijaz et al. 2017). However, the information of the genome-wide characterization of annexin gene family in radish is still unavailable. In this study, ten RsANN genes were first identified from radish genome and the phylogenetic analysis of annexin proteins was performed. Besides, chromosomal localization, putative cis-elements, gene structures and conserved motifs of these RsANN genes were systematically analyzed, and the expression profiles of RsANN genes in different developmental stages and tissues were obtained according to the RNA-seq data. In addition, RT-qPCR was conducted to analyze the expression patterns of RsANN genes in response to abiotic stresses including heat, salinity, drought, ABA and hydrogen peroxide. Furthermore, the biological function of RsANN1a gene was explored by overexpression and suppression in Arabidopsis. These results could contribute to gain insight into function of annexin family genes in radish.
Materials and methods
Plant material and stress treatments
The seeds of radish advanced inbred line ‘NAU-XBC’ were surface-sterilized with 1% (v/v) sodium hypochlorite, and incubated in dark for two days. Germinated seeds were sown in plastic pots and cultivated in the greenhouse with day/night temperature of 25/16°C (14/10 h). For salt and drought treatments, 3-week-old radish seedlings were placed in a Hoagland nutrient solution containing 250 mM NaCl (Wang et al. 2020b) and 15% PEG6000 (w/v) (Mahesh et al. 2013), respectively. For oxidative and ABA treatments, 10 mM H2O2 (Richards et al. 2014) and 100 µM ABA (Xu et al. 2016; Lu et al. 2012) were sprayed directly onto radish seedlings and control seedlings were treated with water. For heat treatment, the seedlings were grown at 40°C (Wang et al. 2018b) and the control ones were grown at 25°C. After 24 h of each treatment, the leaves were collected from the seedlings for RT-qPCR analysis.
Wild-type (WT) Arabidopsis (Col-0) was employed for gene biological functional analysis in this study. Seeds were surface-sterilized and then kept in darkness at 4°C for 48 h. Subsequently, the seeds were sown on 1/2 MS medium. The seedlings were growing in a growth chamber prior to treatment with day/night temperature of 22/18°C (16/8 h).
Identification of annexin genes in radish
The Genome Database of radish (http://radish-genome.org/) (Jeong et al. 2016) was employed to identify the radish annexin genes. Firstly, the members of annexin gene family were identified according to annexin domain (PF00191) using HMMER software version 3.0 search tool (Finn et al. 2011). Subsequently, each of the putative sequences was verified to confirm its reliability via the public databases including Pfam (http://pfam.xfam.org/), SMART (http://smart.embl.de/) (Letunic et al. 2012) and NCBI (https://www.ncbi.nlm.nih.gov/).
Sequence alignment and phylogeny analysis
Clustal W (https://pir.georgetown.edu/pirwww/search/multialn.shtml) was used to generate the multiple sequence alignments of RsANN protein sequences with gap opening penalty of 10.00, gap extension penalty of 0.05 and delay divergent sequences of 40% (Aiyar et al. 2000). Furthermore, the evolutionary relationship of annexin among radish, Arabidopsis and rice was investigated. The annexin protein sequences of Arabidopsis and rice were downloaded from TAIR database (http://www.arabidopsis.org/index.jsp) and Rice Genome Annotation Project (RAP) database (http://rice.plantbiology.msu.edu/index.shtml), respectively. An un-rooted phylogenetic tree was generated with the neighbor-joining (NJ) method using MEGA X software with: p-distance (Model/Method), pairwise deletion (Gaps/Missing Data treatment) and 1000 bootstrap replicates (Test of phylogeny) (Kumar et al. 2018; Fan et al. 2019). The phylogenetic tree was visualized using Evolview (http://www.evolgenius.info/evolview/) (He et al. 2016).
Chromosomal localization and cis-element analysis of RsANN genes
In order to reveal the distribution of RsANN genes, genome localization details for the putative RsANNs were collected from the annotation information, and a map with the distribution of RsANNs was generated with TBtools software (Chen et al. 2020). Totally 2000 bp of genomic DNA sequences upstream of translation start site (TSS) of RsANNs was obtained and the cis-elements in promoter region of RsANNs were investigated using the PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Rombauts et al. 1999).
Analysis of protein properties, conserved motifs and gene structure
PROSITE database (http://www.expasy.org/ScanProsite) was employed to analyze the sites of the post-translational modifications of the deduced amino acid sequences. The conserved annexin protein motifs among radish, Arabidopsis and rice were identified using the MEME 5.0.2 (http://meme.sdsc.edu/meme/cgi-bin/meme.cgi) with the parameters settings as follows: the number of different motifs as 10, minimum motif width as 6 and a maximum motif width as 50. Subsequently, the functional annotation of these putative conserved motifs was identified by SMART databases (http://smart.embl.de/) and InterProScan (http://www.ebi.ac.uk/interpro/scan.html). Furthermore, the intron/exon structures of RsANN genes were investigated using the online software GSDS (http://gsds.cbi.pku.edu.cn/) by comparing the full length cDNA sequences with the genomic sequences (Guo et al. 2007).
Expression analysis of RsANN genes
The expression behaviors of RsANN genes were investigated in the different developmental stages and tissues according to Illumina RNA sequencing data which were downloaded from NODAI radish genome database (Mitsui et al. 2015). In this study, we analyzed the transcriptional profiles of RsANN genes in five tissues (cortical, cambium, xylem, root tip and leaf) and six different developmental stages of leaf and root [7, 14, 20, 40, 60 and 90 days after sowing (DAS)] based on the RNA-Seq data with three replicates. Furthermore, the Reads Per Kb per Million reads (RPKM) method was adopted to present the expression level of each RsANN gene (Mitsui et al. 2015). Finally, heat map was generated with TBtools software according to the manufacturer’s instructions.
Total RNA extraction and RT-qPCR analysis
Total RNA was extracted from different samples using Trizol reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Then, the Superscript III First-Strand Synthesis System (Invitrogen) was used to synthesize the first-strand cDNA, and the specific primers (Table S1) of RsANN genes for RT-qPCR were designed by Beacon Designer 7.7 (Premier Biosoft International, Palo Alto, CA, USA). RT-qPCR was carried out on a Roche LightCycler 480 (LC480) Real-Time PCR System. RsActin gene was chosen as the reference gene (Xu et al. 2013). Three biological and technical replicates were conducted, and the relative quantification of each sample was analyzed with the 2−∆∆CT method (Livak and Schmittgen. 2001).
Cloning and vector construction and plant transformation
RsANN1a was isolated from the cDNA of ‘NAU-XBC’ using the primers RsANN1a-F/R (Table S1). The amplified PCR fragment was cloned into pMD18-T vector and transformed into DH5α E. coli cells, and the positive clone was selected for sequencing. The Xba I and Sma I restriction sites were selected to construct the vector of overexpression-RsANN1a. To select artificial microRNA (amiR) sequences, the conserved sequences of RsANN1a and AtANN1 gene were used to design the amiR-RsANN1a primers by the WMD3 tool (http://wmd3.weigelworld.org/). Subsequently, the precursor of miR319a was used as the backbone of amiRNA expression, which was further exchanged with the amiR-RsANN1a by the overlapping PCR method (Schwab et al. 2006). The specific sequence was inserted with the Xba I and Sac I digestion sites and then transferred into the pCAMBIA2301 vector (Xie et al. 2021). To overexpress RsANN1a and express amiR-RsANN1a in WT Arabidopsis, the pCAMBIA2301 vector containing the target gene was introduced into the A.tumefaciens strain GV3101 and then transformed into the plants using floral dipping method.
Heat stress tolerance assays
To investigate the biological function of RsANN1a during heat stress tolerance, stress assays were conducted at the seedling stages on transgenic and WT lines. All Arabidopsis seeds from WT, OE and amiR lines were sown in 1/2 MS solid medium and grew at 25°C/18°C and 16 h light/8 h dark cycle for 15 days. Subsequently, the seedlings were incubated in a growth chamber at 37°C for 8 h in the dark. After 5 days recovery under normal conditions, the plants were photographed and survival ratio was measured.
Results
Identification of annexin proteins in radish
To define the candidate annexin proteins in radish, the annexin domain (PF00191) was used to search against protein sequences of the radish genome. In total, ten genes were defined as members of annexin gene family, which were named as RsANN1a, RsANN1b, RsANN2a, RsANN2b, RsANN3a, RsANN3b, RsANN4a, RsANN4b, RsANN7 and RsANN8 according to the homology to Arabidopsis. Being similar to other plant annexins, these RsANN proteins contained several conserved motifs (Fig. 1). For example, eight deduced RsANN proteins contained two type II Ca2+-binding sites (G-X-G-T-(38)-D/E) in the first and fourth repeat (Fig. 1). Except for Ca2+-binding site, there were five RsANN proteins containing conserved His residue, and eight contained the predicted IRI motif. Moreover, except for RsANN4a, RsANN4b and RsANN8, the remaining seven RsANN proteins also contained GTP-binding regions (DXXG) in the fourth repeat. In addition, the RsANN proteins contained some conserved residues that were involved in the formation of S3 cluster.
Fig. 1.
Multiple sequence alignment of deduced protein sequences of 10 RsANN proteins. The yellow and pink indicates type II Ca2+-binding sites and putative S3 clusters, respectively. The red, blue and green indicates the conserved His residue, the IRI motif, and the putative GTP binding motif, respectively
Phylogeny and structure of RsANN genes
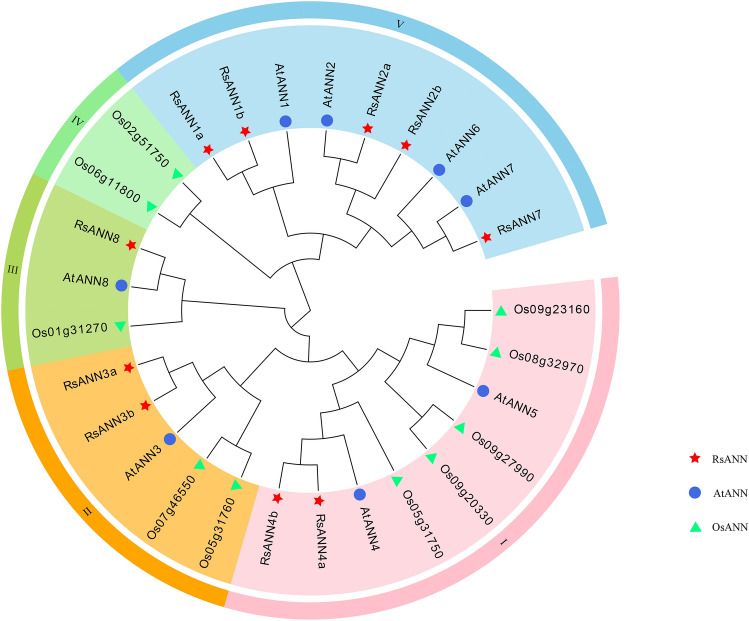
In order to better understand the phylogenetic relationships of the annexins among radish, Arabidopsis and rice with 10, 8 and 10 members, respectively, the deduced protein sequences of 28 annexin members were aligned, and an un-rooted phylogenetic tree was constructed subsequently with the neighbor-joining (NJ) method (Fig. 2). It was obvious that these annexin proteins could be clustered into five different groups, the group I contained 9 members (with 2, 2, and 5 members of radish, Arabidopsis and rice, respectively), the group II had 5 members and the group III had 3 members. Only 2 rice annexins were included in group IV, and the group V contained 9 members including 5 radish and 4 Arabidopsis annexins, indicating annexins in radish had higher homology with that in Arabidopsis.
Fig. 2.
The un-rooted phylogenetic tree of radish, Arabidopsis and rice annexins. This tree is generated by MEGA X with 1000 bootstrap replicates
To analyze the features of RsANN protein sequences, the 10 conserved motifs identified from these 28 annexins were used for phylogenetic study using the MEME motif search tool (Bailey and Elkan 1994; Bailey and Gribskov 1998). Each of these ten conserved motifs containing 6-50 aa was submitted to the SMART and InterProScan for further identification. Totally five motifs including motif 1, 2, 3, 5 and 6 were found to be annexin domains and associated with calcium ion binding/calcium-dependent phospholipid binding. Motif analysis showed that the majority of RsANN proteins in the same group shared similar motif distribution based on the phylogenetic analysis (Fig. 3), indicating that the annexin proteins from the same group probably had similar functions. However, the different motif patterns between the groups demonstrated the functional divergence across groups.
Fig. 3.
Phylogenetic relationship and motif distribution of annexin proteins in radish, Arabidopsis and rice
Additionally, in order to investigate the structural diversity of RsANN genes, the exon–intron patterns were analyzed. The number of exons of the RsANN genes ranged from four to six, and the number of introns of the RsANN genes varied from three to five (Fig. 4). Moreover, the members of the same evolutionary branch of the RsANN gene family had roughly similar exon–intron structures, and the number of exons and introns was basically similar, however, the lengths of exons and introns were quite diverse.
Fig. 4.
Phylogenetic relationship and gene structure of RsANN genes in radish
Chromosomal localization and cis-element analysis of RsANN genes
All these ten RsANN genes were distributed on six radish chromosomes (R3-R8) with no one localized on R1, R2 and R9 (Fig. 5). Among the six radish chromosomes, four chromosomes (R3, R4, R5 and R7) contained two RsANN genes, while the R6 and R8 only contained one RsANN gene.
Fig. 5.
Chromosomal localization of RsANN genes
The cis-elements could play a critical role in the transcriptional regulation of genes. The 5’-upstream putative promoter regions (2 kb upstream of translation start site) of RsANN genes were isolated for searching the potential cis-elements using the PlantCARE. The results showed that at least 20 putative cis-elements like CAAT-box and TATA-box and a series of light responsive cis-elements like ACE, Box I and GA-motif could be found. Furthermore, the potential stress-responsive cis-elements such as ABA responsiveness element (ABRE), MYB binding site (MBS), heat shock element (HSE), low temperature responsiveness element (LTR) and TC-rich repeats could also be identified, and all of these ten RsANN genes contained at least two of these five putative cis-elements (Table S2), while seven RsANN genes contained ABRE within their promoter sequences. Except for RsANN4b and RsANN8, the remaining ones contained the MBS for drought response. In addition, five RsANN genes contained HSE, LTR and TC-rich repeats.
Characterization of deduced RsANN proteins
Some post-translational modification sites were detected in the deduced RsANN protein sequences using ScanProsite (http://ca.expasy.org/tools/scanprosite/). For instance, there were putative phosphorylation sites in all the deduced RsANN protein sequences in the form of protein kinase C (PKC), casein kinase II (CK II), cAMP-cGMP dependent protein kinase (cAMP-cGMP) and tyrosine kinase (Tyr). Additionally, N-Myristoylation (N-Myr) and N-Glycosylation (N-Glyc) were also found in certain deduced RsANN protein sequences. Interestingly, some RsANN protein sequences like RsANN1a/b and RsANN2a/b had the similar post-translational modification sites, respectively (Table S3), suggesting that these post-translational modifications might regulate the activity of RsANN proteins.
Expression profiling of RsANN genes in radish
To estimate the expression profiles of RsANN genes, reads per kilobase per million reads (RPKM) of ten RsANN genes in leaves and roots from six different development stages was obtained from the published database (Fig. 6). There were three RsANN genes, RsANN1a, RsANN1b and RsANN2a, maintaining a relatively high transcriptional level, while the remaining ones either maintained a relatively low level or had no expression in root and leaf tissue. In addition, the expression profiles of some RsANN genes varied with the growth of radish. For example, the transcript level of RsANN3a and RsANN4a showed a substantial decrease as the root grew from 7 to 14-day-old stage (Fig. 6A). Similarly, significantly higher expression level of RsANN4a was observed in 14-day-old leaf tissue, compared with that in other development stages (Fig. 6B). The differences in the expression profiles of RsANN genes suggested that the RsANNs may play an important role in the root and leaf development.
Fig. 6.
Expression profiles of RsANN genes in six stages of root and leaf. A and B represents the expression profile of RsANN gene at six stages (7d, 14d, 20d, 40d, 60d and 90d) of root and leaf, respectively. The expression values were calculated by RPKM. The scale represents relative expression value
The expression levels of ten RsANN genes in five tissues (cortical, cambium, xylem, root tip and leaf) from three stages (40d, 60d and 90d) were also explored (Fig. 7). It was shown that the expression profiles of RsANN genes were generally similar across three different stages. For instance, the expression level of RsANN1a, RsANN1b and RsANN2a was generally higher than that of other RsANN genes in five tissues across three different stages. RsANN7 was specifically expressed in cortical, whereas RsANN2b was specifically expressed in root tip. Moreover, with the growth of radish from 40 to 60-day-old stage, the transcript abundance of RsANN4a decreased in cortical, cambium, root tip and leaf, while the expression level of RsANN4a showed a slight increase as the xylem grew from 60 to 90-day-old stage. In summary, these results indicated that the expression of RsANN genes was spatial-and temporal-specific, and they could play a vital role in the growth and development process.
Fig. 7.
Expression profiles of RsANN genes in different tissues. A, B and C represents the expression profile of RsANN genes in five tissues (cortical, cambium, xylem, root tip and leaf) from three stages (40d, 60d and 90d), respectively. The expression values were calculated by RPKM. The scale represents relative expression value
Expression profile of RsANN genes under abiotic stresses
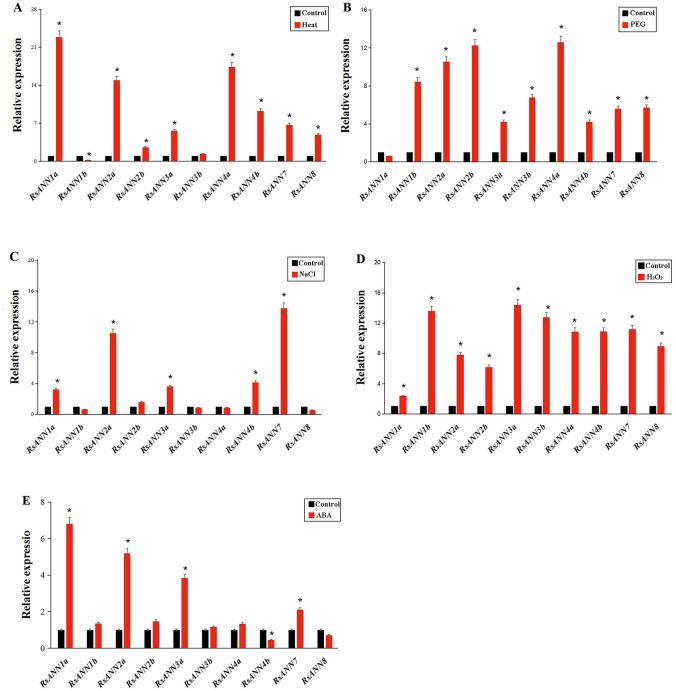
To investigate whether RsANN genes are responsive to abiotic stresses, RT-qPCR was conducted to analyze the expression level of RsANN genes after treatment with five abiotic stresses including high temperature, drought, salinity, oxidization and ABA stress. For heat stress, RsANN1b showed only a significant down-regulation compared with the untreated control. In addition to the slight up-regulation of RsANN3b, the other eight RsANN genes showed significant up-regulation, particularly in RsANN1a, RsANN2a and RsANN4a (22.9-, 14.9- and 17.4-fold, respectively) (Fig. 8A). The expression of RsANN genes showed a significant up-regulation except for RsANN1a under drought stress (Fig. 8B). Exposure to NaCl induced a significant increase in the expression level in five RsANN genes (Fig. 8C). Moreover, all the ten RsANN genes showed a significant up-regulation after treated by 100 mM H2O2 for 24 h (Fig. 8D). In addition, a significant decrease in the transcript abundance of RsANN4b and RsANN8 was observed under ABA treatment, while the expression level of the others showed a significant increase (Fig. 8E). Especially, the expression level of RsANN1a increased to 22.9-fold under the heat stress. The transcript levels of RsANN genes were differentially regulated by various stresses, indicating that the RsANN genes might play an important role in response to abiotic stresses in radish.
Fig. 8.
The expression of RsANN genes after 24 h treatment in response to abiotic stresses. A Heat treatment (40°C); B PEG6000 (15% w/v); C NaCl (250 mM); D H2O2 (10 mM); E ABA (100 µM). Bar represents mean ± SD, P < 0.05
RsANN1a plays a positive role in heat tolerance
To gain further insight into the function of RsANN1a in the heat stress responses of radish, a total of seven OE and five amiR transgenic T3 lines were obtained, respectively. The transgenic T1 lines were identified by PCR detection (Fig. S2). Moreover, no segregation of T3 seeds were observed using 100 mg·L−1 kanamycin (Fig. S3), confirming that the positive transgenic lines were homozygous. For stress assays at the seedling stages, the phenotypes among two transgenic lines and WT were compared under heat stress condition, and a significant difference was detected in terms of survival ratio (Fig. 9C). The survival ratio of overexpression-RsANN1a transgenic plants was significantly higher compared to that of WT with survival ratio of 66.7% and 30.7% in overexpressed RsANN1a and WT line, respectively (Fig. 9A), while lower survival ratio was detected in amiR-RsANN1a transgenic plants with survival ratio of 20.8% (Fig. 9B). Taken together, these results demonstrated that RsANN1a might play a positive role in heat tolerance in radish.
Fig. 9.
Plants of WT and two transgenic lines after heat stress. A Stress assay between WT and overexpressed RsANN1a transgenic (OE) plants. B Stress assay between WT and amiR-RsANN1a transgenic (amiR) plants. C Statistical analysis of survival ratio after heat stress. Each bar shows the mean ± SD of the triplicate assay. The value with different letters indicates significant difference at P < 0.05 according to Duncan’s multiple range test
Discussion
With the rapid development of the sequencing technology, more and more plant genomes were sequenced. Annexin families have been identified from several plant species. It may be noted that that two versions of radish genome database were used in this study. The chromosome-level genome released by Jeong et al. (2016) was used for the identification and localization of RsANN genes. However, this genome version lacks the temporal and spatial gene expression profile in radish. Interestingly, the global gene expression profile was reported in the NODAI Radish Genome Database (Mitsui et al. 2015), which was used to investigate the comprehensive expression profile of RsANN genes in different tissues and developmental stages.
The annexin gene family and their structures in radish
The annexins was one of the evolutionarily conserved, Ca2+-dependent phospholipid-binding proteins. In plants, the C-terminal region of annexin proteins consists of four internal repeats, and each repeat is approximately 70 amino acids in length as well as the conserved motif of (G-X-G-T-(38)-D/E) only present in the first and forth repeats (Mortimer et al. 2008). In the present study, the sequence analysis of the RsANN proteins showed that eight RsANN proteins contained two type II Ca2+-binding sites (G-X-G-T-(38)-D/E) in the first and fourth repeats. Functional prediction showed that these Ca2+-binding sites implicated in phospholipid binding. Previous evidences suggested that the conserved H residue was related to the peroxidase activity just like the heme-binding domain of horseradish peroxidase and played an important role in maintaining the secondary structure (Konopka-Postupolska et al. 2009; Mortimer et al. 2008). The IRI motif was involved in F-actin binding and the GTP-binding region (DXXG) and was related to the phosphodiesterase activity (Jami et al. 2012b). Some conserved residues involved in the formation of S3 clusters were also found in the RsANN proteins, which have been proven to play an important role in redox reactions (Hofmann et al. 2003; Konopka-Postupolska et al. 2009). In addition, post-translational modification was essential for stress tolerance in Arabidopsis (Mizoi et al. 2019; Wang et al. 2020a), and many post-translational modification sites were detected in the deduced RsANN protein sequences, suggesting that the RsANN gene family might be involved in some different biological processes.
Based on the genomic locations and amino acid sequence similarities, it could be speculated that all annexins may have a common ancestor, and the increased gene number may be due to the gene duplication events. Moreover, genome analysis revealed that gene duplication in the ancestral grass occurred approximately 70 million years ago (Paterson et al. 2004). It’s worthy to note that an unrooted phylogenetic tree could describe the relatedness of a group of organisms, and it was extensively used in the phylogenetic analysis, particularly when studying the phylogenetic relationship between different species (Jami et al. 2012a; He et al. 2020). Therefore, an un-rooted phylogenetic tree was constructed for better understanding of the phylogenetic relationship of annexin genes among radish, Arabidopsis and rice. As shown in Fig. 2, group I, II and III contained annexin proteins from both the monocot and the dicots plants, suggesting that annexins from the monocots and eudicots showed conservation before the monocot-dicot split (Jami et al. 2012b). The structural and phylogenetic analysis indicated that annexins within a group might have similar function.
The roles of RsANN genes in response to abiotic stresses
Accumulating evidences from various species including Arabidopsis, tomato and rice has demonstrated that the up-regulation of annexin genes might play a vital role in response to abiotic stress conditions (Clark et al. 2001; Lu et al. 2012; Jami et al. 2012b). However, there was no such available information about RsANN genes. In this study, most of RsANN genes showed the induced expression pattern in response to abiotic stress treatments including heat (40°C), drought (PEG6000), salinity (NaCl), ABA and oxidative stress (H2O2). Specially, almost all RsANN genes showed a significant up-regulation level under PEG6000 and H2O2 treatment (Fig. 8). Overexpression of AtANN1 could improve the growth greatly and enhance drought tolerance of Arabidopsis, while the AtANN1 knockout mutant showed more sensitivity to drought stress. Moreover, the H2O2 content showed a significant increase in OE-AtANN1 line compared to that in WT, suggesting a feedback mechanism between AtANN1 and H2O2 content may exist in Arabidopsis under drought stress (Konopka-Postupolska et al. 2009), which was similar to rice in response to heat stress (Qiao et al. 2015). Therefore, it could be speculated that ANN1 might play a key role in response to drought stresses in plant.
Different plants may differ in the tolerance to high temperature and different pathways were involved in heat stress response within plant species (Lohani et al. 2020). In this study, two different temperatures were chosen for heat stress in radish and Arabidopsis, which was similar to several previous studies (Zang et al. 2017; Geng et al. 2018). For heat stress treatment of radish, the temperature was set to 40°C according to previous studies (Yang et al. 2019; Wang et al. 2018a), while 37°C was set for thermotolerance assays in Arabidopsis referring to several studies (Ambastha et al. 2020, Friedrich et al. 2021). Over the past few years, several miRNAs have been proven to be involved in heat stress response in some plant species (Sharma et al. 2021). Recently, 1802 heat-responsive miRNAs were identified (Yang et al. 2019), and 26 known and 19 novel miRNAs were identified as differentially expressed under heat stress in radish (Wang et al. 2015). Importantly, miR854 was reported to target annexin 1 under heat stress (Wang et al. 2018a). These findings provided useful data for further investigating the molecular mechanism underlying heat stress response in radish. The majority members of annexin gene family showed heat-induced expression in Arabidopsis (Cantero et al. 2006). Overexpression of OsANN1 showed improved growth and it interacted with OsCDPK24 to enhance heat stress tolerance (Qiao et al. 2015). Surprisingly, the CDS and peptide sequences of RsANN1a were similar with that of OsANN1 gene (Fig. S1, Table S4), indicating that the RsANN1a and OsANN1 gene might play conserved functions in enhancing heat stress tolerance in plants. Based on the association analysis of heat stress-responsive differentially expressed genes (DEGs) and differential abundance protein species (DAPS) in radish taproot, the expression of RsANN1 was significantly up-regulated in response to heat stress at transcriptomic and proteomic levels (Wang et al. 2018a, b). Moreover, previous proteomic work reported that an annexin-like protein was accumulated in response to heat stress (Zhang et al. 2013). In this study, heat treatment could also result in differential expression of RsANN genes. Notably, the expression of RsANN1a was strongly induced by heat stress compared to the control (22.9-fold) (Fig. 8A) and the survival ratio of amiR-RsANN1a plants has been observed as significantly lower compared with that in OE-RsANN1a plants and WT (Fig. 9). Interestingly, stress-responsive cis-elements analysis of the promoter region showed that RsANN1a contained four HSE cis-elements (Table S2). Therefore, it could be speculated that when plants are exposed to high temperature, RsANN1a might sense stress factors through Ca2+ signal transduction and regulate the peroxide content in plants because of its structural features. Moreover, RsANN1a contained four HSE cis-elements, which could interact with other transcription factors such as CDPK to respond heat stress. Taken together, our results indicated that RsANN1a might play an important role in radish thermotolerance and lay the foundation for further investigation on the mechanism underlying heat tolerance process in radish.
Conclusion
In summary, a total of ten RsANN genes were first identified from the radish genome, which were localized on six radish chromosomes. A comparative phylogenetic analysis of annexin among Arabidopsis, radish and rice showed that 28 annexin proteins were clustered into five different groups and each group shared similar motif patterns. Moreover, a number of cis-elements involved in abiotic stress response were detected in the promoter regions and post-translational modification sites were found in the RsANN proteins. Furthermore, these RsANN genes exhibited tissue-specific expression patterns, and they were differentially regulated by various stresses. Under high temperature treatment, overexpression and amiRNA-mediated knockdown of RsANN1a gene in Arabidopsis indicated that it might play a key role in the heat stress response in radish.
Supplementary Information
Below is the link to the electronic supplementary material.
Author’s contributions
SF, XL and LL conceived and designed the experiments. SF, YJ and SX performed the experiments. SF and YJ wrote the manuscript. WJ, WY, MY and ZY reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This current work was funded by grants from the Jiangsu Agricultural Science and Technology Innovation Fund (JASTIF, CX(19)3032), National Natural Science Foundation of China (NSFC 32102399) and the project funded by the priority academic program development of Jiangsu Higher Education Institutions (PAPD).
Declarations
Conflict of interest
All authors declared that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feng Shen, Jiali Ying and Liang Xu these authors have contributed equally to this work.
References
- Ahmed I, Yadav D, Shukla P, Kirti PB. Heterologous expression of Brassica juncea annexin, AnnBj2 confers salt tolerance and ABA insensitivity in transgenic tobacco seedlings. Funct Integr Genomics. 2018;18:569–579. doi: 10.1007/s10142-018-0614-z. [DOI] [PubMed] [Google Scholar]
- Aiyar A. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. Methods Mol Biol. 2000;132:221–241. doi: 10.1385/1-59259-192-2:221. [DOI] [PubMed] [Google Scholar]
- Ambastha V, Leshem Y. Differential cell persistence is observed in the Arabidopsis female gametophyte during heat stress. Plant Reprod. 2020;33(2):111–116. doi: 10.1007/s00497-020-00390-0. [DOI] [PubMed] [Google Scholar]
- Bailey TL, Gribskov M. Combining evidence using p-values: application to sequence homology searches. Bioinformatics. 1998;14(1):48–54. doi: 10.1093/bioinformatics/14.1.48. [DOI] [PubMed] [Google Scholar]
- Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- Boustead CM, Smallwood M, Small H, Bowles DJ, Walker JH. Identification of calcium-dependent phospholipid-binding proteins in higher plant cells. FEBS Lett. 1989;244(2):456–460. doi: 10.1016/0014-5793(89)80582-4. [DOI] [Google Scholar]
- Cantero A, Barthakur S, Bushart TJ, Chou S, Morgan RO, Fernandez MP. Expression profiling of the Arabidopsis Annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol Biochem. 2006;44(1):13–24. doi: 10.1016/j.plaphy.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas H, Frank M, He Y, Xia R. TBtools - an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;S1674–2052(20):30187–30188. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Clark GB, Sessions A, Eastburn DJ, Roux SJ. Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol. 2001;126(3):1072–1084. doi: 10.1104/pp.126.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Potikha TS. Structures and functions of annexins in plants. Cellular and Molecular Life Sciences (CMLS) 1997;53(6):546–553. doi: 10.1007/s000180050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Xu L, Wang Y, Tang M, Liu L. Genome- and transcriptome-wide characterization of bZIP gene family identifies potential members involved in abiotic stress response and anthocyanin biosynthesis in radish (Raphanus sativus L.) Int J Mol Sci. 2019;20(24):6334. doi: 10.3390/ijms20246334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T, Oberkofler V, Trindade I, Altmann S, Brzezinka K, Lämke J, Gorka M, Kappel C, Sokolowska E, Skirycz A, Graf A, Bäurle I. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat Commun. 2021;12(1):3426. doi: 10.1038/s41467-021-23786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Zang X, Li H, Liu Z, Zhao A, Liu J, Peng H, Yao Y, Hu Z, Ni Z, Sun Q, Xin M. Unconventional splicing of wheat TabZIP60 confers heat tolerance in transgenic Arabidopsis. Plant Sci. 2018;274:252–260. doi: 10.1016/j.plantsci.2018.05.029. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6(6):449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Guo AY, Zhu QH, Chen X, Luo JC. GSDS: A gene structure display serve. Hereditas. 2007;29(8):1023–1026. doi: 10.1360/yc-007-1023. [DOI] [PubMed] [Google Scholar]
- He M, Yang X, Cui S, Mu GJ, Hou MY, Chen HY, Liu LF. Molecular cloning and characterization of annexin genes in peanut (Arachis hypogaea L.) Gene. 2015;568(1):40–49. doi: 10.1016/j.gene.2015.05.004. [DOI] [PubMed] [Google Scholar]
- He F, Gao C, Guo G, Liu J, Gao Y, Pan R, Guan Y, Hu J. Maize annexin genes ZmANN33 and ZmANN35 encode proteins that function in cell membrane recovery during seed germination. J Exp Bot. 2019;70(4):1183–1195. doi: 10.1093/jxb/ery452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Liao L, Xie S, Yao M, Xie P, Liu W, Kang Y, Huang L, Wang M, Qian L, Liu Z, Guan C, Guan M, Hua W. Comprehensive analyses of the annexin (ANN) gene family in Brassica rapa, Brassica oleracea and Brassica napus reveals their roles in stress response. Sci Rep. 2020;10(1):4295. doi: 10.1038/s41598-020-59953-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhang H, Gao S, Lercher MJ, Chen WH, Hu S. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016;44:W236–W241. doi: 10.1093/nar/gkw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A, Delmer DP, Wlodawer A. The crystal structure of annexin Gh1 from Gossypium hirsutum reveals an unusual S3 cluster. Eur J Biochem. 2003;270(12):2557–2564. doi: 10.1046/j.1432-1033.2003.03612.x. [DOI] [PubMed] [Google Scholar]
- Hoshino D, Hayashi A, Temmei Y, Tsuchiya KT. Biochemical and immunohistochemical characterization of Mimosa annexin. Planta. 2004;219(5):867–875. doi: 10.2307/23388601. [DOI] [PubMed] [Google Scholar]
- Ijaz R, Ejaz J, Gao S, Liu T, Imtiaz M, Ye Z, Wang T. Overexpression of annexin gene AnnSp2, enhances drought and salt tolerance through modulation of aba synthesis and scavenging ROS in tomato. Sci Rep. 2017;7(1):12087. doi: 10.1038/s41598-017-11168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami SK, Dalal A, Divya K, Kirti PB. Molecular cloning and characterization of five annexin genes from Indian mustard (Brassica juncea L. Czern and Coss) Plant Physiol Biochem. 2009;47(11–12):977–990. doi: 10.1016/j.plaphy.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Jami SK, Clark GB, Ayele BT, Ashe P, Kirti PB. Genome-wide comparative analysis of annexin superfamily in plants. PLoS one. 2012;7(11):e47801. doi: 10.1371/journal.pone.0047801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami SK, Clark GB, Ayele BT, Roux SJ, Kirti PB. Identification and characterization of annexin gene family in rice. Plant Cell Rep. 2012;31(5):813–825. doi: 10.1007/s00299-011-1201-0. [DOI] [PubMed] [Google Scholar]
- Jami SK, Clark GB, Turlapati SA, Handley C, Roux SJ, Kirti PB. Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol Biochem. 2008;46(12):1019–1030. doi: 10.1016/j.plaphy.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Jeong YM, Kim N, Ahn BO, Oh M, Chung WH, Chung H, Jeong S, Lim KB, Hwang YJ, Kim GB, Baek S, Choi SB, Hyung DJ, Lee SW, Sohn SH, Kwon SJ, Jin M, Seol YJ, Chae WB, Choi KJ, Park BS, Yu HJ, Mun JH. Elucidating the triplicated ancestral genome structure of radish based on chromosome-level comparison with the Brassica genomes. Theor Appl Genet. 2016;129(7):1357–1372. doi: 10.1007/s00122-016-2708-0. [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J. The Role of Annexin 1 in Drought Stress in Arabidopsis. Plant Physiol. 2009;150(3):1394–1410. doi: 10.1104/pp.109.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Hofmann A. Structure, function and membrane interactions of plant annexins: an update. Plant Sci. 2011;3:230–241. doi: 10.1016/j.plantsci.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Ayaydin F, Oberschall A, Ipacs I, Toth EC. Immunolocalization of a novel annexin-like protein encoded by a stress and abscisic acid responsive gene in alfalfa. Plant J. 1998;15(2):185–197. doi: 10.1046/j.1365-313X.1998.00194.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Davies JM. Annexins. New Phytol. 2011;189(1):40–53. doi: 10.1111/j.1469-8137.2010.03533.x. [DOI] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:302–305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichocka M, Rymaszewski W, Morgiewicz K, Filoniuk IB, Chlebowski A, Sobczak M, Samuel MA, Schmelzer E, Krzymowska M, Hennig J. Nucleus- and plastid-targeted annexin 5 promotes reproductive development in Arabidopsis and is essential for pollen and embryo formation. Bmc Plant Biol. 2018;18(1):183. doi: 10.1186/s12870-018-1405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lohani N, Singh MB, Bhalla PL. High temperature susceptibility of sexual reproduction in crop plants. J Exp Bot. 2020;71(2):555–568. doi: 10.1093/jxb/erz426. [DOI] [PubMed] [Google Scholar]
- Lu Y, Ouyang B, Zhang J, Wang T, Lu C, Han Q, Zhao S, Ye Z, Li H. Genomic organization, phylogenetic comparison and expression profiles of annexin gene family in tomato (Solanum lycopersicum) Gene. 2012;499(1):14–24. doi: 10.1016/j.gene.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Mahesh K, Balaraju P, Ramakrishna B, Rao SSR. Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Am J Plant Sci. 2013;4(12):2305–2313. doi: 10.4236/ajps.2013.412285. [DOI] [Google Scholar]
- Mitsui Y, Shimomura M, Komatsu K, Namiki N, Hatta MS, Ima M, Katayose Y, Mukai Y, Kanamori H, Kurita K, Kagami T, Wakatsuki A, Ohyanagi H, Ikawa H, Minaka N, Nakagawa K, Shiwa Y, Sasaki T. The radish genome and comprehensive gene expression profile of tuberous root formation and development. Sci Rep. 2015;5(1):10835. doi: 10.1038/srep10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoi J, Kanazawa N, Kidokoro S, Takahashi F, Qin F, Morimoto K, Shinozaki K, Yamaguchi-Shinozaki K. Heat-induced inhibition of phosphorylation of the stress-protective transcription factor DREB2A promotes thermotolerance of Arabidopsis thaliana. J Biol Chem. 2019;294:902–917. doi: 10.1074/jbc.RA118.002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM. Annexins: multifunctional components of growth and adaptation. J Exp Bot. 2008;59(3):533–544. doi: 10.1093/jxb/erm344. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Chapman BA. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci USA. 2004;101(26):9903–9908. doi: 10.1073/pnas.0307901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao B, Zhang Q, Liu D, Wang H, Zhu Z. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J Exp Bot. 2015;66(19):5853–5866. doi: 10.1093/jxb/erv294. [DOI] [PubMed] [Google Scholar]
- Richards SL, Laohavisit A, Mortimer JC, Shabala L, Swarbreck SM, Shabala S, Davies JM. Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J. 2014;77(1):136–145. doi: 10.1111/tpj.12372. [DOI] [PubMed] [Google Scholar]
- Rombauts S, Déhais P, Van Montagu M, Rouzé P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999;27(1):295–296. doi: 10.1093/nar/27.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18(5):1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Upadhyay S, Bhattacharya S, Singh A. Abiotic stress-responsive miRNA and transcription factor-mediated gene regulatory network in Oryza sativa: construction and structural measure study. Front Genet. 2021;12:618089. doi: 10.3389/fgene.2021.618089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Que Z, Xia Y, Tang N, Li D, He RH, Cao ML. Knock out of the annexin gene OsAnn3, via CRISPR/Cas9-mediated genome editing decreased cold tolerance in rice. J Plant Biol. 2017;60(6):539–547. doi: 10.1007/s12374-016-0400-1. [DOI] [Google Scholar]
- Szalonek M, Sierpien B, Rymaszewski W, Gieczewska K, Garstka M, Lichocka M, Sass L, Paul K, Vass I, Vankova R, Dobrev P, Szczesny P, Marczewski W, Krusiewicz D, Strzelczyk-Zyta D, Hennig J, Konopka-Postupolska D. Potato Annexin STANN1 promotes drought tolerance and mitigates light stress in transgenic Solanum tuberosum L. Plants Plos one. 2015;10(7):e0132683. doi: 10.1371/journal.pone.0132683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar T, Gorecka KM, de Carvalho-Niebel F, Downie JA, Cullimore J, Pikula S. Annexins-calcium-and membrane-binding proteins in the plant kingdom: potential role in nodulation and mycorrhization in Medicago truncatula. Acta Biochim Pol. 2009;56(2):199–210. doi: 10.4067/S0718-16202010000300011. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu L, Zhu X, Zhai L, Wang Y, Yu R, Gong Y, Limera C, Liu L. Transcriptome-Wide Characterization of Novel and Heat-Stress-Responsive microRNAs in Radish (Raphanus Sativus L.) Using Next-Generation Sequencing. Plant Molecular Biology Reporter. 2015;33:867–880. doi: 10.1007/s11105-014-0786-1. [DOI] [Google Scholar]
- Wang R, Mei Y, Xu L, Zhu X, Wang Y, Guo J, Liu L. Genome-wide characterization of differentially expressed genes provides insights into regulatory network of heat stress response in radish (Raphanus sativus L.) Funct Integr Genomics. 2018;18(2):225–239. doi: 10.1007/s10142-017-0587-3. [DOI] [PubMed] [Google Scholar]
- Wang R, Mei Y, Xu L, Zhu X, Wang Y, Guo J, Liu L. Differential proteomic analysis reveals sequential heat stress-responsive regulatory network in radish (Raphanus sativus L.) taproot. Planta. 2018;247(5):1109–1122. doi: 10.1007/s00425-018-2846-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ying J, Zhang Y, Xu L, Zhang W, Ni M, Zhu Y, Liu L. Genome-wide identification and functional characterization of the cation proton antiporter (CPA) family related to salt stress response in radish (Raphanus sativus L.) Int J Mol Sci. 2020;421(21):8262. doi: 10.3390/ijms21218262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Liu Y, Shi Y, Han D, Wu Y, Ye W, Yang H, Li G, Cui F, Wan S, Lai J, Yang C. SUMOylation stabilizes the transcription factor DREB2A to improve plant thermotolerance. Plant Physiol. 2020;183(1):41–50. doi: 10.1104/pp.20.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ying J, Tang M, Wang Y, Xu L, Liu M, Liu L. Genome-wide identification of AUX/IAA in radish and functional characterization of RsIAA33 gene during taproot thickening. Gene. 2021;795:145782. doi: 10.1016/j.gene.2021.145782. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang Y, Xu Y, Wang L, Zhai L, Zhu X, Gong Y, Ye S, Liu L. Identification and characterization of novel and conserved microRNAs in radish (Raphanus sativus L.) using high-throughput sequencing. Plant Sci. 2013;201(260):108–114. doi: 10.1016/j.plantsci.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Xu L, Tang Y, Gao S, Su S, Hong L, Wang W, Fang Z, Li X, Ma J, Quan W, Sun H, Li X, Wang Y, Liao X, Gao J, Zhang F, Li L, Zhao C. Comprehensive analyses of the annexin gene family in wheat. BMC Genomics. 2016;17(1):415. doi: 10.1186/s12864-016-2750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li W, Su X, Ge P, Zhou Y, Hao Y, Shu H, Gao C, Cheng S, Zhu G, Wang Z. Early response of radish to heat stress by strand-specific transcriptome and miRNA analysis. Int J Mol Sci. 2019;20(13):3321. doi: 10.3390/ijms20133321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Geng X, Liu K, Wang F, Liu Z, Zhang L, Zhao Y, Tian X, Hu Z, Yao Y, Ni Z, Xin M, Sun Q, Peng H. Ectopic expression of TaOEP16-2-5B, a wheat plastid outer envelope protein gene, enhances heat and drought stress tolerance in transgenic Arabidopsis plants. Plant Sci. 2017;258:1–11. doi: 10.1016/j.plantsci.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu L, Zhu X, Gong Y, Xiang F, Sun X, Liu L. Proteomic analysis of heat stress response in leaves of radish (Raphanus sativus L.) Plant Mol Biol Report. 2013;31(1):195–203. doi: 10.1007/s11105-012-0486-7. [DOI] [Google Scholar]
- Zhang Z, Li X, Han M, Wu Z. Genome-wide analysis and functional identification of the annexin gene family in maize (Zea mays L.) Plant Omics. 2015;8(5):420–428. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.