Abstract
Rapid commercialization, industrialization and the use of nanotechnology has led to an increase in the distribution of nanoparticles (NPs) in the environment. The most common metal oxide NPs which is present within products is Titanium dioxide (TiO2). TiO2 NPs have photocatalytic nature and can affect plant growth. The current study investigated the morphological, anatomical and biochemical features of Baby sun rose (Aptenia cordifolia) after exposure to different concentrations of TiO2 nanoparticles (0, 1, 5, 10 and 20 mg L−1). Treatment with TiO2 NPs showed changes in the morphological features and increased photosynthetic pigmentation within the plant. An increase in the level of phenolics (12%) and flavonoid compounds (13%) was observed when plants were treated with moderate levels of TiO2 NPs. A reduction in the diameter of the vascular bundles and increased thickening of the transverse wall were observed in several samples. The number of scattered vascular bundles in the stems increased. The morphological, biochemical, and anatomical responses of Baby sun rose indicates that plants can adapt to environments contaminated with up to 20 mg L−1 TiO2 NPs. The cultivation of Baby sun rose plants in environments polluted with TiO2 NPs is recommended. This study enhances the knowledge of the effect of TiO2 NPs on the growth of Baby sun rose which is an ornamental plant, widely cultivated in different regions of Iran. The results of this study suggest that contaminated environments up to 20 mg L−1 TiO2 NPs can be managed by phytoremediation. Further studies are needed to investigate this plant's tolerance strategies against stress caused by TiO2 NPs and bulk TiO2 as well as the effect of other nanoparticles on plant.
Keywords: Anatomy, Baby sun rose, Phenolic content, Chlorophyll content, TiO2
Introduction
Rapid commercialization, industrial development and the use of nanotechnology has led to increased distribution of nanoparticles (NPs) in the environment which will inevitably impact living organisms (Rajput et al. 2019). NPs are atomic or molecular assemblies with dimensions of 1 to 100 nm that show different physicochemical properties depending on their constituent elements (Khan et al. 2020). Decreasing the size of these materials and consequently increasing their surface-to-volume ratio, changes their physical properties (size, shape, electrostatic charge) and chemistry (surface coating, specific element or compound type and solubility). In order to protect humans and the environment from the effects of a wide range of NPs, many studies have focused on assessing their toxicity (Yang and Watts 2005). Currently there is widespread debate about the dangers and benefits of many NPs in the environment (USEPA 2007). Titanium dioxide (TiO2) NPs have photocatalytic nature and can affect plant growth. Also, TiO2 NPs have three crystalline forms. Its anatase form has more photocatalytic activity than brookite and rutile (Ziental et al. 2020). TiO2 NPs are among the most abundant being produced annually around the world. Out of the list of nanotechnology products used, TiO2 is the most common metal oxide NPs utilized (Piccinno et al. 2012). Applications include reducing air pollution, removal of cadmium and 4-chlorophenol from water and the removal of toxic carbon monoxide from the air (Shi et al. 2013). Therapeutic applications include dental cultures, anticancer drugs (Macwan et al. 2011), surgical instruments (Manchikanti and Bandopadhyay 2010). Other applications of titanium include self-cleaning coatings, electrodes for color-sensitive solar cells, solar panels, light-emitting diodes, disinfectant sprays, sporting goods, sunscreen (Marcone et al. 2012) and vehicle anti-fog mirrors.
NPs cause a variety of physiological and morphological changes in plants that vary depending on the type of NPs and plant type. In one study, the treatment of tomato plants with TiO2 NPs in a hydroponic system at low concentrations of 0.5 and 2 g L−1 showed increased photosynthetic parameters and total chlorophyll content. The study revealed a negative impact at high concentrations (Tiwari et al. 2017). Hu et al. (2020) reported that TiO2 NPs with a concentration of 50 mg L−1 significantly increased root and shoot fresh biomass in coriander (Coriandrum sativum L.) in a hydroponic system, but at a concentration of 400 mg L−1 significantly reduced plant root fresh biomass, and caused wrinkling of root cell membranes. In yet another study TiO2 NPs at a concentration of 100 mg L−1 reduced the negative effects on all salinity levels in the Dracocephalum moldavica plant (Gohari et al. 2020). Application of TiO2 NPs in hydroponic cultures at concentrations of 0, 5, 10, 20 and 40 mg L−1 did not have a statistically significant effect on wheat (Triticum vulgare L.) growth except 5 mg L−1 (Dağhan et al. 2020). Wu et al. (2017) showed the impact of TiO2 NPs on rice (Oryza sativa L.) plants which significantly reduced plant biomass and destroyed the plant's antioxidant defense system. Plant response to environmental stress is highly variable. The ability of plants to adapt to environmental stress also depends on the type, severity, stress duration, the stage of growth, and the plant species (Kolahi et al., 2021). The atmospheric absorption of metal NPs by leaves has been proven which affects morphological features such as crack density, presence or absence of the hypodermis, and the number and condition of stomata (Christie and Zhang 2019). Any level of NPs that makes a significant difference in plant adaptation is considered stress (Amini and Ehsanpour 2004). TiO2-NPs is photoinducible, redox active. They cause the release of reactive oxygen species (ROS) (Li et al. 2020). The main members of the reactive oxygen species include free radicals such as superoxide (O2) and hydroxyl (OH) and non-radicals such as hydrogen peroxide (H2O2) and singlet oxygen (O2) (Sgherri et al. 2018). These ROS can damage living cell membranes and DNA. TiO2 NPs therefore possess antimicrobial properties (El-Shafai et al. 2019).
The defense mechanism of plants against nanomaterial toxicity is not completely understood. The uptake and transport of metal oxide NPs in different parts of the plant depends on its bioavailability, concentration, solubility and exposure time (Siddiqi and Husen 2017). Plants have complex cellular mechanisms that may be involved in detoxifying metals thereby creating resistance to metals in plants (Hall 2002). These mechanisms include reducing metal uptake, formation of inactive metal compounds from plant peptides, or metal sequestration within vacuoles. Some resistant plants can store metals in the walls of epidermal cells by forming protein and silicate bonds (Li et al. 2019).
Baby sun rose, Aptenia cordifolia, belongs to family Mesembryanthemaceae (Aizoaceae) and is a perennial plant with Crassulacean acid metabolism (CAM) (Gaffney 2006). The plant is ornamental and exhibits medicinal properties. Previous study has also shown that the mucilage of A. cordifolia can be used to remove anion dyes from aqueous solutions (Mozafarjalali et al. 2020). Baby sun rose is a popular plant for growth in green spaces and gardens and is cultivated in most parts of Iran and around the world. This plant also has relatively good resistance to salinity (Karakas et al. 2020) and drought stress (Pintó-Marijuan et al. 2017). Considering the resistance and adaptation of this plant to various stress conditions, the effect of TiO2 NPs on the growth, morphological, anatomical and biochemical parameters of Baby sun rose plants in a hydroponic culture was evaluated.
Materials and method
Plant material and experimental conditions
Aptenia cordifolia seeds (variety: Red Apple) were supplied by Pakan-Bazr Institute, Isfahan. The seeds were surface-sterilized with 2% sodium hypochlorite for 10 min followed by washing with distilled water. Coco-peat and perlite cultivation medium was used for seed germination in the dark in a growth chamber in a laboratory at the Department of Plant Sciences, University of Tabriz, Iran (38.0560°N, 46.3254°E) in October 2016. Initial plant germination was conducted for 7 days. The seedlings were exposed to a photoperiod of 16 h of light and 8 h of darkness at a temperature of 25 °C with a relative humidity of 65% and at a photon flux density of about 350 µmol m−2 s−1. Plants with four leaves were transferred to a medium containing Hoagland nutrient solution (KNO3 6.0 mM, Ca(NO3)2.4H2O 4.0 mM, NH4H2PO4 2.0 mM, MgSO4·7H2O 1.0 mM); KCl 50.0 μM, H3BO3 25.0 μM, MnSO4·H2O 2.0 μM, ZnSO4·7H2O 2.0 μM, CuSO4·5H2O 0.5 μM, H2MoO4 0.5 μM, FeEDTA 20 μM pH 5.8) (Hoagland and Arnon 1950). After two days of adaptation, plants were treated with different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) in 400 ml pots. It is the same condition as above mention. Each pot has 4 holes (3 holes for plant and one for air) and was covered with slightly large size cap. Pots were covered with aluminum foil to prevent light from entering. The nutrient solution was ventilated with an air pump. The solutions were changed every third day. After four weeks of treatment, plants were harvested.
Characterization and treatment of TiO2 NPs utilized
TiO2 NPs were purchased from Neutrino Trading Company, Tehran, Iran. In order to investigate the reaction mechanism, Fourier transform infrared spectroscopy (FTIR; TENSOR 27, Bruker Company, German) analysis was used (Fig. 1). The characteristics of TiO2NPs were also determined as shown in Table 1. First, the mother suspension containing TiO2 NPs with a concentration of 2 mg mL1 was prepared using an ultrasonic bath (Sonica, 2200 EP S3, Italy) to disperse the NPs in distilled water for an hour. Different concentrations of NPs (0, 1, 5, 10 and 20 mg L−1) were immediately removed from the mother suspension and added to nutrient solutions (Hoagland) in specified proportions.
Fig. 1.
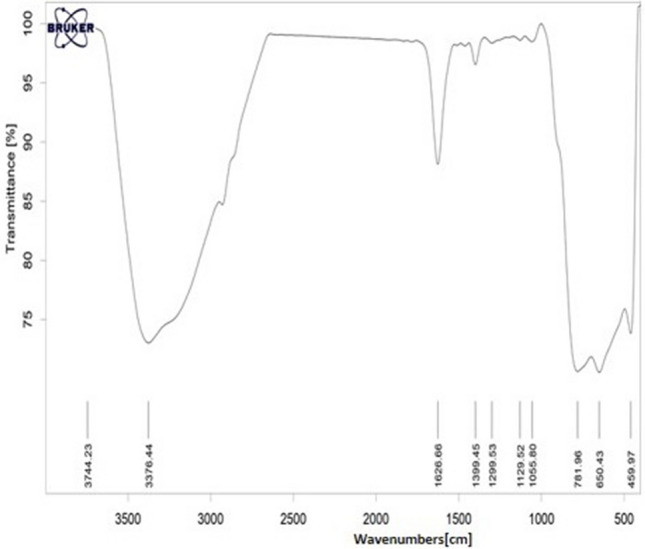
FTIR pattern of TiO2 NPs
Table 1.
Properties of TiO2 NPs
| Appearance of TiO2 NPs | Surface area of total porosity volume (m2 g−1) | Purity (cm3 g−1) | (Average particle size) (nm) |
|---|---|---|---|
| White powder | > 200 | 99/9% | 30 nm |
Measurement of growth parameters
In this study, the roots were harvested and washed with distilled water and separated from the aerial parts. The fresh weight (FW) of the roots (g) and shoots (g) of the plants was measured. Then dry weight (DW) was measured by placing the samples in paper bags separately in the oven at 60° C for 48 h. The dry weight of shoots and roots were measured utilizing a scale with a sensitivity of 0.001 g. To measure leaf area (mm2), the leaf was drawn on a 1 mm square graph paper and the number of grid squares inside the leaf outlines counted. Root length (cm) and stem length (cm) as well as internode length (cm) were measured using a ruler (Hajiboland et al. 2014).
Measurement of total phenol and flavonoid content
Methanolic extracts of the plants were prepared according to Elufioye et al. (2019). The leaves were extracted with 80% methanol, kept in the dark for an hour, centrifuged at 1000 g for 10 min and the supernatant was removed to a new tube for determining total phenolics using Folin-Ciocalteu method (Bastola et al. 2017). The absorption of the solutions along with control was measured at 720 nm. Gallic acid was used as the standard. Total phenolic content was expressed as gallic acid equivalent per plant fresh weight (FW) (µg g−1) (Elufioye et al. 2019). Whereas, spectrophotometric assay was used for determining total flavonoids after added with aluminum chloride and potassium acetate. The absorption of the solutions was measured at 415 nm. Quercetin was used as the standard. The data was expressed as quercetin equivalent per plant fresh weight (µg g−1) (Ogidi et al. 2018).
Chlorophyll and total carotenoid content
Measurement of chlorophyll and carotenoid content was performed according to Etemadian et al. (2017). Fresh leaf material (0.05 g) was homogenized in methanol before keeping in the dark at 4 °C for 24 h. After centrifugation at 10,000 g for 5 min, the absorption of the solution was measured at wavelengths of 653, 666 and 470 nm. Chlorophyll concentration was calculated according to the following formulas and were reported as µg g−1 FW. Carotenoid content for each extract was calculated using the formulas (Etemadian et al. 2017):
Anatomical studies
Cross-sections of the stems and roots were examined to study of the vegetative organs structure. In this method, both fresh and living tissues were utilized. The tissue was cut manually, stained with carmin and methyl green and stabilized on slides (Słomka et al. 2020). In order to analyze the leaves, enumeration of lower and upper stomata on the leaves was performed using colorless varnish and counting the stomata with a microscope (Olympus BX-51, Japan) (Paul et al. 2017).
Statistical analysis
All experiments were performed in triplicate and repeated three times. Statistical data analysis was performed based on a completely randomized design using SPSS ver.16 software and Duncan's test (Permanasari et al. 2010) at the statistical probability level of p < 0.05.
Results
Effect of TiO2 NPs on growth parameters
There was a significant difference in the growth of the treated plants as compared to the control (Fig. 2 and Table 2). Generally, root length in the treated plant (10 mg L−1 TiO2 NPs) was reduced to about 33% compared to the control plant. But it was increased at the highest concentration of TiO2. Maximum plant length and root length were observed in control plants and plants treated with 20 mg L−1 TiO2 NPs. The lowest plant length was observed in plants treated with 10 mg L−1 TiO2 NPs. The lowest fresh weights and dry weights of roots and aerial organs were recorded in plants treated with 10 mg L−1 TiO2 NPs which decreased about 40% relative to the control. A significant difference of leaf area was observed with the lowest mean values of the 5 mg L−1 NPs (244 mm2) third leaf which decreased by about 60% compared to the control plant. With respect to the fourth leaf surface, the highest mean was observed in the control and plants treated with 1 mg of NPs, and the lowest average in plants treated with 5 and 10 mg L−1 NPs. For the internodes, highest averages were observed in the control plant (Table 2).
Fig. 2.
Baby sun rose (Aptenia cordifolia) plantlets treated with different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) for 4 weeks
Table 2.
Growth and morphometric parameters of Baby sun rose (Aptenia cordifolia) plantlets treated with different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) for 4 weeks
| Parameters | Treatment | |||||
|---|---|---|---|---|---|---|
| Control | 1 mg L−1 TiO2 | 5 mg L−1 TiO2 | 10 mg L−1 TiO2 | 20 mg L−1 TiO2 | CV (%) | |
| Plant length (cm) | 29.9 ± 0.635a | 23.5 ± 0.981bc | 21.3 ± 1.674 cd | 18.35 ± 1.88d | 27.4 ± 0.230ab | 8.9 |
| Shoot length (cm) | 6.5 ± 0.288a | 6.05 ± 0.375a | 5.95 ± 0.202a | 4.1 ± 0.0577b | 6 ± 0.5773a | 10.50 |
| Root length (cm) | 23.65 ± 0.952a | 18.2 ± 1.039a | 16.5 ± 1.443b | 14.5 ± 0.866c | 22 ± 0.577b | 9.27 |
| Shoot fresh weight (g) | 4.069 ± 0.120a | 4.062 ± 0.114a | 3.282 ± 0.114b | 2.409 ± 0.197c | 3.267 ± 0.1281b | 7.04 |
| Root fresh weight (g) | 0.220 ± 0.003a | 0.114 ± 0.0083a | 0.094 ± 0.0075b | 0.046 ± 0.009c | 0.061 ± 0.0060c | 11.6 |
| Shoot dry weight (g) | 3.930 ± 0.159a | 3.902 ± 0.0678a | 3.220 ± 0.0845b | 2.388 ± 0.185c | 3.213 ± 0.1203b | 6.8 |
| Root dry weight (g) | 0.184 ± 0.006a | 0.065 ± 0.002b | 0.041 ± 0.004c | 0.026 ± 0.0020d | 0.033 ± 0.0029 cd | 9.9 |
| 3rd Leaf area (mm2) | 556.5 ± 11.83b | 628 ± 12.12a | 244 ± 12.12d | 356.5 ± 26.269c | 413.5 ± 30.310c | 7.96 |
| 4th Leaf area (mm2) | 870.5 ± 9.526a | 813.5 ± 10.1036ab | 558 ± 30.022c | 587 ± 51.384c | 728.5 ± 56.86b | 9.08 |
| 3rd Internode length (cm) | 1.35 ± 0.028a | 1.1 ± 0.057b | 1.1 ± 0.115c | 0.9 ± 0.057c | 1.05 ± 0.028ab | 8.41 |
| 4th Internode length (cm) | 1.05 ± 0.0288a | 0.9 ± 0.057b | 0.7 ± 0.057b | 0.6 ± 0.0001b | 0.95 ± 0.028b | 10.36 |
Bars represent the means of three replicates (± SE). Different letters indicated statistically significant difference at p < 0.05
Effect of TiO2 NPs on biochemical properties
Phenols and flavonoid content
In the third leaf, highest level of phenolic was observed in control plants and in plants treated with 10 mg L−1 TiO2 NPs with an average value of 393 and 400 µg g −1 FW, respectively. In the fourth leaf, highest levels of phenolic were observed for treatments of 5, 10 and 20 mg L−1 with values of 426, 393 and 409 µg g−1 FW, which increased by about 12, 6 and 9% relative to the control, respectively (Fig. 3A). A comparison of the effect of different levels of TiO2 NPs on the mean index of flavonoids showed a significant difference between the control and plants treated with TiO2 NPs for the third and fourth leaves. In the third leaf, highest contents of flavonoids (80.24 μg g-1 FW) were observed when treated with 10 mg L-1 of TiO2 NPs. The lowest values were observed in treatments with 1 mg L−1 TiO2 NPs (74.25 µg g−1 FW, Fig. 3B). For the fourth leaf, treatments with 5 and 20 mg L−1 of TiO2 NPs resulted in 85.4–81.8 µg g−1 FW of flavonoids, which increased by about 9 and 13% relative to the control, respectively.
Fig. 3.
Changes in A Total phenolics (µg gallic acid in g FW), B Flavonoid content (µg quercetin in g FW) in Baby sun rose (Aptenia cordifolia) affected by different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) for four weeks. Bars represent the means of three replicates (± SE). Different letters indicated statistically significant differences at p < 0.05
Chlorophyll and carotenoid content
The chlorophyll a and b content of the third leaf, in plants treated with TiO2 NPs trended to increase when compared to the control. Highest values were observed in treatments with 20 mg L−1 TiO2 NPs (chlorophyll a, 49.3 µg g−1 FW and chlorophyll b, 35.2 µg g−1 FW) which increased by about 62 and 63% relative to the control (Fig. 4A). A comparison of the average level of chlorophyll a in the fourth leaves showed that highest levels were observed in plants treated with 1 and 5 mg L−1 of TiO2 NPs (29.5 and 24.9 µg g−1 FW, respectively) and lowest levels were observed in plants treated with 10 mg L−1 of TiO2 NPs (17.3 µg g−1 FW). With respect to chlorophyll b for the fourth leaf, highest values were observed with treatments of 5 and 20 mg L−1 of TiO2 NPs (25.4 and 26.2 µg g−1 FW, respectively) which increased by about 50 and 56%, relative to the control (Fig. 4B). Carotenoid content in the third leaf, was highest in treatments of 1 mg L−1 of TiO2 NPs and lowest in plants treated with 10 mg L−1 of TiO2. For the fourth leaf, the highest level of carotenoids was observed in the treated plants (Fig. 4C).
Fig. 4.
Changes in A Chlorophyll a (Chla) (µg g−1 FW), B Chlorophyll b (Chlb) (µg g−1 FW), C Carotenoids (µg g−1 FW) in Baby sun rose (Aptenia cordifolia) affected by different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) for 4 weeks. Bars represent the means of three replicates (± SE). Different letters indicated statistically significant difference at p < 0.05
Effect of TiO2 NPs on the anatomy of Baby sun rose plant
Anatomical changes in the roots and stems
Histological observation of the anatomical structure of the roots and stems of the plants were evaluated. In the hydroponic environment, NPs covered the root surfaces. They are therefore expected to be able to penetrate the plant through the apoplast and symplast pathways. This can be verified from the cross-sectional images of the root cortex area. Treated plants had irregular cells that became larger towards the center. Root diameter was reduced in treatments of 20 mg L−1 of NPs (Fig. 5A–C). The number and diameter of vascular elements in the roots of the control plants was higher than that of treated plants (Fig. 5G–I). Cross-sections of the root of plants treated with 20 mg L−1 of TiO2 NPs revealed the presence of space within the apoplast (Fig. 5M–O). Anatomical changes were also observed in the stems of Baby sun rose with treatments of 5 and 20 mg L−1 TiO2 NPs. The number of scattered vascular bundles in the cortex area in the control plant had an average of 12, and in treatments of 5 and 20 mg L−1 of NPs, the average was approximately 14 (Fig. 5D–F). A decrease in the diameter of the vascular apertures in treatments with 5 mg L−1 of TiO2 was evident as wall thickness increased due to lignin deposition (Fig. 5J–L). The shape of the stem of the control plant was hexagonal, while in the treated plants it was cylindrical (Fig. 5D–F).
Fig. 5.
Effect of TiO2 NPs on root, stem and leaf anatomy of Baby sun rose (Aptenia cordifolia) for four weeks. A Cross-sections of root of control plant, B Cross-sections of root of plant exposed to 5 mg L−1 TiO2 NPs, C Cross-sections of root of plant exposed to 20 mg L−1 TiO2 NPs, D Cross-sections of stem of control plant, E Cross-sections of stem of plant exposed to 5 mg L−1 TiO2 NPs, F Cross-sections of root of plant exposed to 20 mg L−1 TiO2 NPs (Scale bars, 1000 µm in A-F), G Xylem and phloem in root of control plant, H Xylem and phloem in root of plant exposed to 5 mg L−1 TiO2 NPs, I Xylem and phloem in root of plant exposed to 20 mg L−1 TiO2 NPs, J Xylem and phloem in stem of control plant, K Xylem and Phloem in stem of plant exposed to 5 mg L−1 TiO2 NPs, L Xylem and phloem in stem of plant exposed to 20 mg L−1 TiO2 NPs, M Control plant root cortex, N Root cortex of plant exposed to 5 mg L−1 TiO2 NPs, O Root cortex of plant exposed to 20 mg L−1 TiO2 NPs (Scale bars, 100 µm in G-O), P Epidermal cells of leaf in control plant,magnification 10×, Q Epidermal cells of leaf in plant exposed to 5 mg L−1 TiO2 NPs, magnification 10×, R Epidermal cells of leaf in plant exposed to 20 mg L−1 TiO2 NPs, magnification 10x. Labels: CO, Cortex cells, xl, Xylem, wc, Water storage cells stained with carmine and methylene blue
Changes in stomata density and the number of epidermal cells
The stomata of Baby sun rose leaves are of the anisocytic type, with higher numbers on the lower surface of the leaf (Fig. 5P–R). Treatment with TiO2 NPs did not alter the number of epidermal cells on either leaf surface. The number of upper and lower surface stomata did not change with the concentration of TiO2 NPs (Fig. 6). However, in the fourth leaf found an increasing in the stomata on the lower surface of plants treated with TiO2 NPs. (Fig. 6B). The effect of TiO2 NPs on water storage cells of the third leaf was significant. The highest number of water storage cells in the lower surface of the third leaf was in relation to plants treated with 5 mg L−1 TiO2 NPs which increased approximately 20% compared to the control plant. On the upper surface, highest levels of water storage cells were related to plants treated with 5 and 10 mg L−1 TiO2 NPs, which increased approximately 45% and 50%, respectively, compared to the lowest amount (plants treated with 20 mg L−1 TiO2 NPs) (Fig. 7A). The effect of TiO2 NPs on water storage cells in the fourth leaf was not significant. On the lower leaf surface, the number of water storage cells in plants treated with 10 and 20 mg L−1 increased approximately 20 and 15% compared to control plants. On the upper surface of the leaf, the number of water storage cells in plants treated with 10 mg L−1 TiO2 NPs increased by 15% relative to the control (Fig. 7B).
Fig. 6.
Changes in stomata density of Baby sun rose (Aptenia cordifolia) affected by different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) for four weeks. Bars represent the means of three replicates (± SE). Different letters indicate statistically significant differences at p < 0.05
Fig. 7.
Changes in the number of epidermal water storage cells in the leaves of Baby sun rose (Aptenia cordifolia) treated with different concentrations of TiO2 NPs (0, 1, 5, 10, 20 mg L−1) for four weeks. Bars represent the means of three replicates (± SE). Different letters indicate statistically significant differences at p < 0.05
Discussion
There was a decline in growth parameters, inclusive of root length, fresh weight of root, and dry weight of aerial parts and leaf area, in treatments of 10 mg L−1 NPs. Raliya et al. (2015) showed that the growth rate of tomato plants changes when exposed to TiO2 NPs. Similarly, Dağhan (2018) reported that root dry weight in maize (Zea mays L.) decreased at 10 mg L−1 TiO2 NPs, but it increased at 20 mg L−1 TiO2 NPs. TiO2 NPs applied at low concentrations led to significant decrease in barley root length, whereas other treatments had no effect (Mattiello and Marchiol 2017). Laware and Raskar (2014) believe that roots directly exposed to TiO2 experienced increased ROS. In present study, the difference in root length at high concentrations was due to the levels of ROS production. High concentrations of NPs may produce sufficient quantities of ROS (Gill and Tuteja 2010). TiO2 reduced root biomass in Linum usitatissimum at low concentrations (10 mg L−1). According to previous studies, root length of Nicotiana tabacum was negatively affected by TiO2 (Frazier et al. 2014). Root growth inhibition varies based on the actual NPs, the plant species and NPs concentration (Farooqui et al. 2016). Other factors leading to a decline in growth indicators include early differentiation and lignified cell walls in the cell's longitudinal growth zone, which can be attributed to a decrease in water absorption and impaired cell division (Fusconi et al. 2007). Research on soybeans showed that titanium moves slowly and is more effective in the body being treated.
The results of this study revealed that TiO2 NPs had a great impact on the roots than the aerial regions of the plant. Decreased aerial length was observed in treatments with 10 mg L−1 TiO2 NPs. Experiments conducted by Raliya et al. (2015) showed that tomato plant height increased when treated with TiO2 up to a concentration of 250 mg kg−1. A decrease in root length was observed at all concentrations. Larue et al. (2012a, b) explained that high surface reactivity of TiO2 NPs can increase root absorption or increase water flow in roots by creating new vascular elements. In a study of leaf surface parameters, a significant decrease in leaf area in the third and fourth leaves of 5 mg L−1 treated plant was observed. TiO2 NPs filling the space between cellulosic microfibrils in the cell wall, have a negative effect on leaf growth, hydroxy enzyme activity of the root and transpiration in corn seedlings (Asli and Newmann 2009, Feizi et al. 2011). Rapid inhibition of leaf growth and transpiration were observed in Zea mays L. TiO2 NPs increased fresh weight and dry weight of spinach plants as compared to the control plant. This may be due to increased absorption of minerals and increased metabolism of spinach by this nano-compound (Yang et al. 2006). The use of TiO2 NPs in Azolla filliculoides resulted in increasing toxicity and other growth disorders (Mary et al. 2015).
The phenolic and flavonoid contents in the third leaf were highest in plants treated with 10 mg L−1 TiO2 NPs and control, respectively; while in the fourth leaf, the highest amounts were obtained with 5, 20, and 10 mg L−1 TiO2 NPs, respectively. The results of this study suggest that flavonoids play a critical protective role against active oxygen radicals (Agati et al. 2007) as they can control the production of free radicals and remove them (Kumar et al. 2013).
The decline in phenolic and flavonoid content in the third leaf of plants exposed to NPs as compared to the control indicates that these compounds are part of the defense mechanisms in the plant. Results from Chehrgani et al. (2016) showed that in treated bean plants the level of catalase was reduced compared to the control. Accumulation of NPs at high concentrations leads to increased ROS content. ROS may act as a stimulant in the production of secondary metabolites. Results from research conducted by Khanjanzadeh et al. (2017) showed that carotenoid, flavonoid, and anthocyanin content in cumin increased with increasing concentrations of TiO2 NPs.
A study of the impact of iron oxide NPs on grass (Lolium Perenne L.) and squash (Cucurbita maxima) revealed more oxidative stress and a high induction of antioxidant systems (Gaharwar et al. 2017). One of the reasons for oxidative stress in these two plants is the adsorption of iron oxide NPs on the root surface. Treatment with TiO2 NPs increased the levels of chlorophyll and carotenoids in the plants. According to Anderson et al. (2004), increased chlorophyll levels are due to cells being active and increasing their sugar production. It has been reported that some NPs, such as TiO2, can impact the absorption of light by chloroplasts and can play a decisive role in increasing the photosynthetic efficiency of plants (Zheng et al. 2007). Qi et al. (2013) reported that after the application of titanium NPs, the rate of photosynthesis, and transpiration rate in tomato leaves increased. The effect of titanium nanofluid on spinach chloroplast under light showed that titanium nanodioxide could protect chloroplast against aging over a long period. The highest concentration of TiO2 NPs inhibited root growth in barley (Hordeum vulgare L.) but did not alter chlorophyll a and b level. (Kořenková et al. 2017). In present work, despite reduced root length, chlorophyll content increased in some of the treated plants. Baby sun rose plant utilizes several strategies against stress so that plants under drought stress had higher photosystem II activity (Cela et al. 2009). Due to the special characteristics of this plant, as well as possessing CAM metabolism, an increase in chlorophyll content was observed in the treated plants.
Anatomical examination of the root of the plant exposed to stress showed a decrease in root diameter as compared to the control plant. In the vascular cylinder, there was a decrease in the number, and diameter of vessels within the root. The number of scattered vascular bundles increased in the stem of plants exposed to NPs. The decrease in the diameter of vessels was evident in treatment with TiO2. Wall thickness increased due to lignin deposition. The effect of CuO NPs on the lignification of cucumber seedlings has been previously reported (Nair and Chung 2015). Li et al. (2016) reported that NPs (g-Fe2O3) entered the root epidermis passing through the apoplastic pathway via the endoderm accumulating in the vacuole. In some studies, heavy metals stimulate premature and lignification differentiation of cell walls in the root growth zone and prevents growth (Tangahu et al. 2011). It appears that the interaction of NPs and plants causes the release of the element in relation to the NPs, which interacts with plant cells and increases the toxicity of NPs. NPs penetrate in to the plant wall through the cell wall and the rhizodermal layer of the root and enter the stem and leaf. Studies have shown that environmental changes can lead to anatomical changes in cells and plant tissues (Ma et al. 2010). In a study by Mahajan et al. (2011), a high concentration of nano-ZnO particles resulted in turbulence in bean root tissue. It also led to a spread of the vacuole system in the parenchymal cells of the cortex and compression of the vascular cylinder (Mahajan et al. 2011). The results showed that NPs can affect the path of the genetic mechanism of growth hormones which in turn can affect the formation of vessels. Some researchers have also observed that the presence of NPs on the cell wall has resulted in cell deformation (Aslani et al. 2014). Another study reported that TiO2-NPs placed inside wheat roots, were transferred to the leaves without altering their crystal structure (Larue et al. 2012a, b). They also reported on the entry of TiO2 NPs into rapeseed and wheat in hydroponic conditions, with most of the titanium being found in the root epidermis and less titanium being observed in the parenchyma. Titanium was also observed inside the vascular cylinder. Zarafshar et al. (2015) observed the presence and adhesion of silica NPs in pear (Pyrus biosseriana) root epidermis.
Current findings suggest that the identification of NPs-resistant plants is a very promising approach in combatting environmental pollution. This is especially important for non-edible plants with vast growth potential in urban environments. The results of this study showed that the A. cordifolia plant can absorb heavy metals from contaminated soil (Zaimoglu et al. 2009), and that Baby sun rose plants can tolerate adverse conditions such as TiO2 contamination.
Conclusion
TiO2 NPs are one of the most widely produced NPs in the world. Treatment of plants with TiO2 NPs 10 mg L−1 reduced the height, leaf area, fresh and dry root weight, and aerial parts of Baby sun rose. An increase in the concentrations of chlorophyll a, and b, as well as carotenoids was also observed. The highest levels of phenol and flavonoids in the third and fourth leaves, was observed after treatment with 5, 20, and 10 mg L−1 of TiO2. TiO2 NPs resulted in several changes in anatomical features of the plant. The roots showed most significant change as they are directly subjected to stress. Microscopic observations showed that the treatment of Baby sun rose plants with TiO2 NPs reduced the diameter of the vessels in the internodes and roots and increased the thickness of the transverse walls in vessels in some treatments. Morphological, anatomical and biochemical changes in Baby sun rose revealed the ability of this plant to grow in different concentrations of TiO2 NPs. It suggests that the plant may be utilized in TiO2 NPs contaminated environments. Baby sun rose appears to have a variety of morphometric, anatomic and biochemical responses to deal with TiO2 NPs. Their ease of growth, non-food use and their attractive ornamental characteristics are all attributes that make these plants suitable for more study in response to polluted and industrial environments. Measurement of NPs accumulation in the plant and how to eliminate the dry biomass of the plant are some issues that would need further investigation.
Declarations
Conflict of interest
The authors report no declarations of interest and they are responsible for the content and writing of the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hanieh Mohajjel Shoja, Email: mohajelh@yahoo.com.
Maryam Kolahi, Email: m.kolahi@scu.ac.ir.
References
- Agati G, Mattini P, Goti A, Tattini M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007;174:77–89. doi: 10.1111/j.1469-8137.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Amini F, Ehsanpour AA. Selection of salt tolerant cell lines from cell suspension cultures of alfalfa (Medicago sativa) Iran Int J Sci. 2004;5(2):145–150. [Google Scholar]
- Andersen L, Williams MH, Serek M. Reduced water availability improves drought tolerance of potted miniature roses: Is the ethylene pathway involved? J Hortic Sci Biotechnol. 2004;79(1):1–13. doi: 10.1080/14620316.2004.11511719. [DOI] [Google Scholar]
- Aslani F, Bagheri S, Muhd Julkapli N, Juraimi AS, Hashemi FS, Baghdadi A. Effects of engineered nanomaterials on plants growth: an overview. Sci World J. 2014;2014:641759. doi: 10.1155/2014/641759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asli S, Neumann PM. Colloidal suspensions of clay or TiO2 nanoparticles s can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009;32(5):577–584. doi: 10.1111/j.1365-3040.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Bastola KP, Guragain YN, Bhadriraju V, Vadlani PV. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am J Anal Chem. 2017;8(06):416. doi: 10.4236/ajac.2017.86032. [DOI] [Google Scholar]
- Cela J, Arrom L, Munné-Bosch S. Diurnal changes in photosystem II photochemistry, photoprotective compounds and stress-related phytohormones in the CAM plant, Aptenia Cordifolia. Plant Sci. 2009;177(5):404–410. doi: 10.1016/j.plantsci.2009.07.001. [DOI] [Google Scholar]
- Dağhan H. Effects of TiO2 nanoparticles on maize (Zea mays L) growth, chlorophyll content and nutrient uptake. Appl Ecol Environ Res. 2018;16(5):6873–6883. [Google Scholar]
- Dağhan H, Gülmezoğlu N, Köleli N, Karakaya B. Impact of titanium dioxide nanoparticles (TiO2-NPs) on growth and mineral nutrient uptake of wheat (Triticum vulgare L.) Biotech Stud. 2020;29(2):69–76. doi: 10.38042/biost.2020.29.02.03. [DOI] [Google Scholar]
- El-Shafai N, El-Khouly ME, El-Kemary M, Ramadan M, Eldesoukey I, Masoud M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide NPs: fabrication, characterization, DNA interaction, and antibacterial activity. R Soc Chem Adv. 2019;9(7):3704–3714. doi: 10.1039/c8ra09788g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elufioye TO, Olusola DM, Oyedeji AO. Correlation of total phenolic, flavonoid and tannin content of Bryophyllum pinnatum (Lam.) (crassulaceae) extract with the antioxidant and anticholinesterase activities. Pharmacogn J. 2019;11(5):1003. doi: 10.5530/pj.2019.11.158. [DOI] [Google Scholar]
- Etemadian Y, Shabanpour B, Ghaemi V, Kordjazi M. Compare the chlorophyll amount in three brown algae species of the Persian Gulf by using three solvents and applying two formulas. Int J Biochem Biophys Mol Biol. 2017;2(6):77. [Google Scholar]
- Farooqui A, Tabassum H, Ahmad A, Mabood A, Ahmad AD, Ahmad IZ. Role of NPs in growth and development of plants: A review. Int J Pharm Bio Sci. 2016;7(4):22–37. doi: 10.22376/ijpbs.2016.7.4.p22-37. [DOI] [Google Scholar]
- Feizi H, Rezvani Moghad P, Fotovat A, Shah Tahmasbi N (2011) Reaction of wheat seed to different concentrations of TiO2 nanoparticles in comparison with non- NPs. Proc. of 2nd Congress on Science and Technology Seed. Nov. 4–5, Mashhad, Iran. pp 565–569.
- Frazier TP, Burklew CE, Zhang BH. TiO2 nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum) Funct Integr Genomics. 2014;14(1):75–83. doi: 10.1007/s10142-013-0341-4. [DOI] [PubMed] [Google Scholar]
- Fusconi A, Gallo C, Camusso W. Effect of cadmium on root apical meristems of Pisum sativum L.: Cell viability, cell proliferation and microtubule pattern as suitable markers for assessment of stress pollution. Mutat Res, Genet Toxicol Environ Mutagen. 2007;632:9–19. doi: 10.1016/j.mrgentox.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gaffney C D (2006) A study of Mesembryanthemaceae alkaloids (Doctoral dissertation, University of Johannesburg)
- Gaharwar US, Meena R, Rajamani P. Iron oxide NPs induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J Appl Toxicol. 2017;37(10):1232–1244. doi: 10.1002/jat.3485. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gohari G, Mohammadi A, Akbari A, Panahirad S, Dadpour MR, Fotopoulos V, Kimura S. TiO2 nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci Rep. 2020;10(1):912. doi: 10.1038/s41598-020-57794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiboland R, Norouzi F, Poschenrieder C. Growth, physiological, biochemical and ionic responses of pistachio seedlings to mild and high salinity. Trees. 2014;28(4):1065–1078. doi: 10.1007/s00468-014-1018-x. [DOI] [Google Scholar]
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347 (2nd Edit).
- Hu J, Wu X, Wu F, Chen W, White JC, Yang Y, Wang X. Potential application of titanium dioxide nanoparticles to improve the nutritional quality of coriander (Coriandrum sativum L.) J Hazard Mater. 2020;389:121837. doi: 10.1016/j.jhazmat.2019.121837. [DOI] [PubMed] [Google Scholar]
- Karakas S, Dikilitas M, Almaca A, Tipirdamaz R. Physiological and biochemical responses of (Aptenia cordifolia) to salt stress and its remediative effect on saline soils. Appl Ecol Environ Res. 2020;18(1):1329–1345. doi: 10.15666/aeer/1801_13291345. [DOI] [Google Scholar]
- Khan MA, Wallace WT, Samb J, Rogers DT, Littleton JM, Rankin SE, Knutson BL. Nanoharvesting of bioactive materials from living plant cultures using engineered silica NPs. Mater Sci Eng. 2020;C106:110190. doi: 10.1016/j.msec.2019.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanjanzadeh H, Morteza T, Morteza E (2017) Study the effects of nano TiO2 on non-enzymatic mech anisms of cumin. ARPN Journal of Agricultural and Biological Science ISSN 1990–6145.
- Kolahi M, Faghani E, Kazemian M, Goldson-Barnaby A, Dodangi S. Changes in secondary metabolites and fiber quality of cotton (Gossypium hirsutum) seed under consecutive water stress and in silico analysis of cellulose synthase and xyloglucan endotransglucosylase. Physiol Mol Biol Plants. 2021;27:1837–1857. doi: 10.1007/s12298-021-01033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kořenková L, Šebesta M, Urík M, Kolenčík M, Kratošová G, Bujdoš M, Dobročka E. Physiological response of culture media-grown barley (Hordeum vulgare L.) to titanium oxide NPs. Acta Agric Scand Sect B Soil Plant Sci. 2017;67(4):285–291. [Google Scholar]
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013:1. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank AM, Brisset F, Carrière M. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ. 2012;431:197–208. doi: 10.1016/j.scitotenv.2012.04.073. [DOI] [PubMed] [Google Scholar]
- Larue C, Veronesi G, Flank AM, Surble S, Herlin-Boime N, Carriere M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J Toxicol Environ Health Part A. 2012;75:722–734. doi: 10.1080/15287394.2012.689800. [DOI] [PubMed] [Google Scholar]
- Laware SL, Raskar S. Effect of TiO2 nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int J Curr Microbiol App Sci. 2014;3(7):749–760. [Google Scholar]
- Li J, Hu J, Ma C, Wang Y, Wu C, Huang J. Uptake, translocation and physiological effects of magnetic iron oxide (g-Fe2O3) NPs in corn (Zea mays L.) Chemosphere. 2016;159:326–334. doi: 10.1016/j.chemosphere.2016.05.083. [DOI] [PubMed] [Google Scholar]
- Li J, Jia Y, Dong R, Huang R, Liu P, Li X, Chen Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int J Mol Sci. 2019;20(20):5096. doi: 10.3390/ijms20205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Qian J, Wang P, Wang C, Lu B, Jin W, Gao P. Responses of freshwater biofilm formation processes (from colonization to maturity) to anatase and rutile TiO2 nanoparticles: effects of NPs aging and transformation. Water Res. 2020;182:115953. doi: 10.1016/j.watres.2020.115953. [DOI] [PubMed] [Google Scholar]
- Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408(16):3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Macwan DP, Dave PN, Chaturvedi S. A review on nano-TiO2 sol–gel type syntheses and its applications. J Mater Sci. 2011;46:3669–3686. doi: 10.1007/s10853-011-5378-y. [DOI] [Google Scholar]
- Mahajan P, Dhoke SK, Khanna AS. Effect of Nano-ZnO particle suspension on growth of mungbean (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J Nanotechnol. 2011;2011:696535. doi: 10.1155/2011/696535. [DOI] [Google Scholar]
- Manchikanti P, Bandopadhyay TK. Nanomaterials and effects on biological systems: development of effective regulatory norms. NanoEthics. 2010;4:77–83. doi: 10.1007/s11569-010-0084-9. [DOI] [Google Scholar]
- Mary X, Saji M, Kumar SA, Mathuran T. Toxic effects of titanium dioxide (TiO2) NPs on growth of aquatic plant Azolla filliculoides. Int Res J Nat Appl Sci. 2015;2(12):113–121. [Google Scholar]
- Mattiello A, Marchiol L. Application of nanotechnology in agriculture: assessment of TiO2 nanoparticle effects on barley. Application of Titanium Dioxide. London: InTech; 2017. pp. 23–39. [Google Scholar]
- Mozafarjalali M, Hajiani M, Haji A. Efficiency of Aptenia cordifolia mucilage in removal of anion dyes from aqueous solution. Int J New Chem. 2020;7(2):111–124. [Google Scholar]
- Nair PMG, Chung IM. Biochemical, anatomical and molecular level changes in cucumber (Cucumis sativus) seedlings exposed to copper oxide NPs. Biologia. 2015;70(12):1575–1585. doi: 10.1515/biolog-2015-0193. [DOI] [Google Scholar]
- Ogidi CO, Oyetayo VO, Akinyele BJ. Estimation of total phenolic, flavonoid contents and free radical scavenging activity of a wild macrofungus, Lenzites quercina (L.) P. Karsten. Curr Res Environ Appl Mycol. 2018;8(4):425–437. doi: 10.5943/cream/8/4/2. [DOI] [Google Scholar]
- Paul V, Sharma L, Pande R, Meena R C (2017) Measurements of stomatal density and stomatal index on leaf/plant surfaces. In: Manual of ICAR Sponsored Training Programme for Technical Staff of ICAR Institutes on—Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants 27.
- Permanasari AE, Rambli DRA, Dominic PDD (2010) Forecasting method selection using ANOVA and Duncan multiple range tests on time series dataset. In: 2010 international symposium on information technology, IEEE, vol 2: pp. 941–945
- Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res. 2012;14(9):1–11. doi: 10.1007/s11051-012-1109-9. [DOI] [Google Scholar]
- Pintó-Marijuan M, Cotado A, Fleta-Soriano E, Munné-Bosch S. Drought stress memory in the photosynthetic mechanisms of an invasive CAM species, Aptenia Cordifolia. Photosynth Res. 2017;131(3):241–253. doi: 10.1007/s11120-016-0313-3. [DOI] [PubMed] [Google Scholar]
- Qi M, Liu Y, Li T. Nano-TiO2 improves the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res. 2013;156:323–328. doi: 10.1007/s12011-013-9833-2. [DOI] [PubMed] [Google Scholar]
- Rajput VD, Minkina T, Sushkova S, Mandzhieva S, Fedorenko A, Lysenko V, Chaplygin V (2019) Structural and ultrastructural changes in nanoparticle exposed plants. In: Nanoscience for Sustainable Agriculture, Springer, Cham, pp 281–295
- Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P. Mechanistic evaluation of translocation and physiological impact of TiO2 and zinc oxide NPs on the tomato (Solanum lycopersicum L.) plant. Metallomics. 2015;7(12):1584–1594. doi: 10.1039/C5MT00168D. [DOI] [PubMed] [Google Scholar]
- Sgherri C, Pinzino C, Quartacci M F (2018) Reactive oxygen species and photosynthetic functioning: past and present. Reactive Oxygen Species in Plants: Boon or Bane–Revisiting the Role of ROS 137–155.
- Shi H, Magaye R, Castranova V, Zhao J. TiO2 nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:1–33. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi KS, Husen A. Plant response to engineered metal oxide NPs. Nanoscale Res Lett. 2017;12:92. doi: 10.1186/s11671-017-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słomka A, Gubernat M, Pliszko A, Bothe H. The unusual property of the sand violet, Viola rupestris, to cope with heavy metal toxicity. Flora. 2020;271:151663. doi: 10.1016/j.flora.2020.151663. [DOI] [Google Scholar]
- Tangahu BV, Sheikh ASR, Basri H, Idris M, Anuar N, Mukhlisin M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int J Chem Eng. 2011;2011:939161. doi: 10.1155/2011/939161. [DOI] [Google Scholar]
- Tiwari M, Sharma NC, Fleischmann P, Burbage J, Venkatachalam P, Sahi SV. Nanotitania exposure causes alterations in physiological, nutritional and stress responses in tomato (Solanum lycopersicum) Front Plant Sci. 2017;8:633. doi: 10.3389/fpls.2017.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (2007) Document Number EPA 100, B-07001 1 Febrary 2007. www.epa.gov/osa.
- Wu B, Zhu L, Le XC. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.) Environ Pollut. 2017;230:302–310. doi: 10.1016/j.envpol.2017.06.062. [DOI] [PubMed] [Google Scholar]
- Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P. Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res. 2006;110(2):179–190. doi: 10.1385/BTER:110:2:179. [DOI] [PubMed] [Google Scholar]
- Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina NPs. Toxicol Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Zaimoglu Z, Erdogan R, Kekec S, Sucu MY, Budak F. Heavy metal uptake by Aptenia cordifolia as utility for sewage sludge compost recuperation using leachate. Asian J Chem. 2009;21(2):1081. [Google Scholar]
- Zarafshar M, Akbarinia M, Askari H, Hosseini SM, Rahaie M, Struve D. Toxicity assessment of SiO2 NPs to pear seedlings. Int J Nanosci Nanotechnol. 2015;11(1):13–22. [Google Scholar]
- Zheng L, Mingyu S, Chao L, Liang C, Huang H, Xiao W, Xiaoqing L, Yang F, Gao F, Hong F. Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol Trace Elem Res. 2007;119:68–76. doi: 10.1007/s12011-007-0047-3. [DOI] [PubMed] [Google Scholar]
- Ziental D, Czarczynska-Goslinska B, Mlynarczyk T, Glowacka-Sobotta A, Stanisz B, Goslinski T, Sobotta L. Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials. 2020;10(2):387. doi: 10.3390/nano10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]








