Abstract
The study aimed to evaluate a commercial blend of functional oils based on liquid from the cashew nutshell and castor oil as a growth promoter in newly weaned piglets. A total of 225 piglets, castrated males and females with 28 days of age were randomly distributed in pens with 15 animals composing three treatments and five repetitions. The treatments were: control (without the inclusion of additives), probiotics, or functional oils. The performance was evaluated. At 50 days of age, a pool of fresh feces from 3 animals/repetition was collected to perform the sequencing of microbiota using the Illumina MiSeq platform. Supplementation with functional oils improved the piglets' daily weight gain and feed conversion ratio (P < 0.05) in the first weeks of the experiment, which resulted in higher final live weight (P < 0.05) in the phase when compared to the control treatment (24.34 kg and 21.55 kg, respectively). The animals that received probiotics showed an intermediate performance (23.66 kg final live weight) at the end of the 38 experimental days. Both additives were effective in increasing groups essential for intestinal health, such as Ruminococcaceae and Lachnospiraceae. The functional oils were more effective in reducing pathogenic bacteria, such as Campylobacter and Escherichia coli. In conclusion, the use of functional oils optimized performance and effectively modulated the microbiota of newly weaned piglets.
Subject terms: Microbiology, Microbial communities
Introduction
Several stressors occur during the weaning of piglets, such as separation from the mother and siblings, transport and handling, or reformulation of the social hierarchy due to the mixing of different groups of piglets. These stressors lead to oxidative stress, as well as to inflammation and dysbiosis, which, may consequently result in diarrhea, decreased growth and increased mortality rate1. Diarrhea is an important factor with negative economic impact in the nursery, and its main pathogenic agent is enterotoxigenic Escherichia coli (ETEC) K882.
For decades, antibiotics have been fed at low dosages to nursery pigs to minimize the negative impact of weaning3. However, most countries have been implementing policies and regulations to reduce or ban the use of antimicrobials in animal production. These changes are motivated by the overuse of antibiotics, which results in the appearance of super resistant bacteria, compromising their effectiveness in human and/or animal health4.
The regulatory restrictions in the use of antibiotics coupled with the reduced number of compounds with similar productive potential has resulted in reductions in performance and higher pig mortalities, especially during the nursery phase5. Therefore, new compounds are needed to replace the antibiotics in the diet of weaned piglets.
Probiotics are live microorganisms that modulate the host's intestinal microbiota. Their beneficial effects are associated with their adherence to the epithelium, the inhibition of the growth and the reduction of toxins produced by pathogenic bacteria6. For example, supplementation with Lactobacillus spp. has been shown to reduce the fecal counts of Salmonella serovar Typhimurium KCTC 2515 and Escherichia coli KCTC 2571 in weaned piglets, increasing the average daily gain and average daily feed intake7.
Functional oils are defined as oils that have an action beyond their nutritional value8, and are being disseminated in the pig industry due to their antimicrobial action and the modulation of intestinal microbiota1.
The liquid from the cashew nut shell is a renewable resource, with cardol in its composition, which has an antimicrobial potential, mainly against gram-positive bacteria such as Streptococcus mutans, Bacillus subtilis and Staphylococcus aureus9. Additionally, ricinoleic acid, the main component of castor oil, acts by denaturing and coagulating proteins in bacterial cell wall10.
The commercial mixture containing functional oils from cashew nut liquid and castor oil has already demonstrated positive effects on performance, modulation of intestinal microbiota and immune system of broilers challenged by coccidiosis11–14. However, there are no published studies analyzing the effects of this product on the performance and intestinal microbiota of swine.
Our hypothesis is that the use of functional oils or probiotics will provide piglets with better performance and an intestinal microbiota beneficial to the animal due to the modulation caused by the additives. Thus, the objective of this study was to evaluate the effects of supplementing a probiotic or a commercial blend of cashew nutshell liquid and castor oil, on the performance, blood parameters and intestinal microbial composition of weaned piglets.
Results
Performance and frequency of diarrhea
The effects of additives on the performance are shown in Table 1. The DWG in phases 1, 2 and the general average was higher for the group receiving the blend of functional oils in the diet when compared to the control group, the probiotic group showed an intermediate result (P < 0.05). Similar results were observed for the live weights at 57 and 66 days of life. Pigs supplemented with the blend of functional oils ate less than the other groups (p < 0.05), which also resulted in a better average FCR (p < 0.05).
Table 1.
Effect of different feed additives on the performance of weaned pigs in phase 1 (28–43 days old), phase 2 (43–57 days old), phase 3 (57–66 days old), and on average (28–66 days old).
| Item | Additives | p-value | |||
|---|---|---|---|---|---|
| Control1 | Oils2 | Probiotic3 | SEM4 | ||
| Live weight, kg | |||||
| Day 28 | 8.66 | 8.77 | 8.83 | 0.3261 | 0.6897 |
| Day 43 | 11.25 | 12.21 | 11.5 | 0.4370 | 0.2936 |
| Day 57 | 17.90b | 19.84a | 18.68ab | 0.6206 | 0.0427 |
| Day 66 | 21.55b | 24.34a | 23.66ab | 0.8046 | 0.0498 |
| Average daily gain, kg | |||||
| Phase 1 | 0.182b | 0.228a | 0.211ab | 0.0172 | 0.0147 |
| Phase 2 | 0.522b | 0.662a | 0.587ab | 0.0181 | 0.0031 |
| Phase 3 | 0.431 | 0,491 | 0.494 | 0.0265 | 0.2845 |
| Average | 0.313 b | 0.429a | 0.395ab | 0.0201 | 0.0165 |
| Daily feed intake, kg | |||||
| Phase 1 | 0.325 | 0.328 | 0.320 | 0.0100 | 0.2026 |
| Phase 2 | 0.879 | 0.770 | 0.814 | 0.0480 | 0.3368 |
| Phase 3 | 0.943 | 0.986 | 1.005 | 0.0320 | 0.3564 |
| Average | 0.725a | 0.688b | 0.709a | 0.0210 | 0.0350 |
| Feed conversion ratio, kg kg | |||||
| Phase 1 | 1.771a | 1.482b | 1.538ab | 0.2925 | 0.0371 |
| Phase 2 | 1.726a | 1.350b | 1.387ab | 0.0512 | 0.0385 |
| Phase 3 | 2.174 | 1.932 | 2.050 | 0.3401 | 0.1689 |
| Average | 2.275a | 1.732b | 1.853a | 0.0951 | 0.0470 |
Least squares: based on observations of 5 stalls per diet.
1Control: without the inclusion of zootechnical additives; 2Probiotics: inclusion of 0.4% probiotic. Probiotic composition: Bacillus subtilis, Enterococcus faecium, Lactobacillus acidophilus, Bifidobacterium bifidum, Saccharomyces cerevisiae; 3Functional oils with inclusion of 0.2% Essential + 0.15% Integrity; 4SEM: standard error of the mean.
abAverages within the same row with different overwrites are statistically different (P < 0.05).
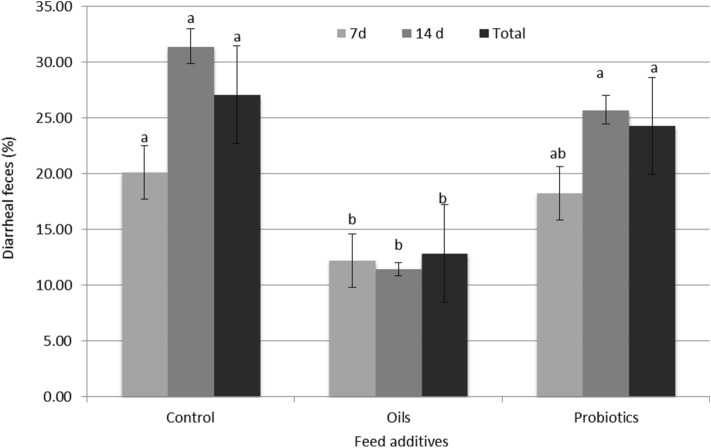
The percentage of diarrheal feces was lower for the pigs that received the functional oils (p < 0.05) (Fig. 1).
Figure 1.
Frequency of diarrheal feces over 2 weeks and in the total period of rearing piglets submitted to different experimental diets in the nursery phase. Control: without the inclusion of zootechnical additives; oils: functional oils with inclusion of 0.2% Essential + 0.15% Integrity; 2Probiotics: inclusion of 0.4% probiotic. Probiotic composition: Bacillus subtilis, Enterococcus faecium, Lactobacillus acidophilus, Bifidobacterium bifidum, Saccharomyces cerevisiae; abAverages within the same row with different overwrites are statistically different (P < 0.05).
Intestinal microbiota and leukogram
The study sequenced a total of 15 fecal pool samples, being three treatments (control, oils, and probiotics) with 5 replicates collected at 50 days of trial. Illumina sequencing analysis of the V3-V4 region of the 16S rDNA gene of 15 fecal pool samples generated a total of 967,431 trimmed quality sequences with an average number of reads per sample of 64,495.4 ± 18,795.6 (Table S1). One sample (181113520761-1-1-1) of the functional oils treatment was disregarded due to the low number of sequences compared to the other samples. The reads were processed and classified into 1690 amplicon sequence variants (ASVs).
The rarefaction curves generated from ASVs (Fig. S1) showed high sequencing coverage in all samples. The rarefaction curves tended to reach the saturation plateau, this result demonstrates that the microbiota of the 14 samples was deep enough to estimate the phenotypic richness and the diversity of the microbial community.
Variation in alpha and beta diversity of the microbiota.
Alpha diversity
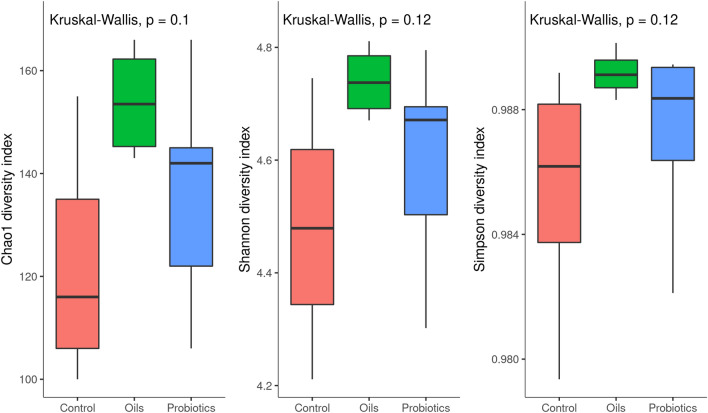
The Chao 1 index was based on the richness amplicon sequence variant (ASVs) present in the sample. The Shannon index considered uniformity in taxa abundance, and the Simpson index was based on the taxa abundance dominance (Fig. 2). The Chao, Shannon and Simpson indices showed no significant difference among the three treatments (p > 0.05). However, there is a tendency to increase the indices and the uniformity in the functional oils group followed by the probiotics, in comparison to control.
Figure 2.
Alpha diversity of fecal pool samples of weaned piglets at 50 days of trial. The study consisted of three feed additives in the nursery phase: basal diet (control), Functional Oils or Probiotics.
Beta diversity
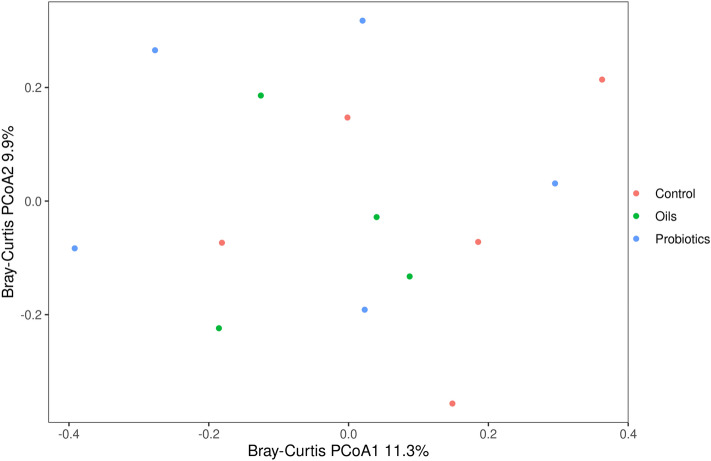
The Bray–Curtis dissimilarity index (BC) was used to analyze inter-individual differences. Based on the PCoA graph (Fig. 3), it was possible to verify that the microbial populations of the animals of the three treatments showed homogeneous dispersion (p > 0.05). The index showed that the treatments had a similar microbial composition (Adonis with 999 permutations, p = 0.337).
Figure 3.
Principal coordinate analysis (PCoA) of beta diversity based on Bray–Curtis dissimilarity of fecal pool samples of weaned piglets at 50 days of trial. The study consisted of three feed additives in the nursery phase: basal diet (control), Functional Oils or Probiotics. Comparison among Oils, Probiotics and Control treatments (Adonis with 999 permutations, p = 0.337).
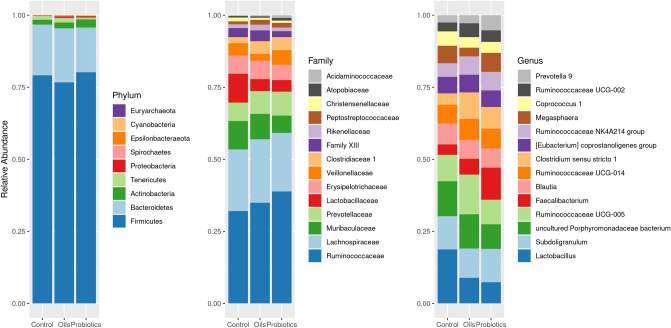
All sequences were classified into nine phyla, although four phyla were more common (> 1%): Firmicutes, Bacteroidetes, Actinobacteria and Tenericutes. Firmicutes was the most abundant phylum in all treatments (> 76%) (Fig. 4). A complete list of the identified sequences (relative abundance) per treatment is provided in Supplementary Table 3. Bacteroidetes and Tenericutes were more abundant for the blend and less abundant for probiotics treatment. Actinobacteria was more abundant in the blend of functional oils and in the group supplemented with probiotics when compared to the control group.
Figure 4.
The relative abundance of the fecal microbiota of weaned piglets at 50 day of age receiving three treatments: basal diet (Control), Functional oils (Oils) or Probiotics. The 14 most abundant taxa at Family and Genus level.
Thirty-five families (35) were identified, of which fourteen (14) had relative abundance > 1% (Table S2 and Fig. 4). The Ruminococcaceae family was predominant in all groups (> 30%) and showed an increase in animals supplemented with probiotics with a log2 FoldChange of 0.6266 (padj < 0.001) (Fig. 4). The Lachnospiraceae family was the second most abundant (> 19%). Compared to the control, Lactobacillaceae were less abundant for both additives (~ 9% vs. ~ 3%). Similar behavior was observed for Muribaculaceae. Clostridiaceae 1 was more abundant for both additives.
Piglets fed the control diet showed a higher concentration of E.coli when compared to the treatment with the blend of functional oils, whereas the treatment with probiotic was not different from any of the other two treatments (P < 0.05; Fig. S2).
The leukogram (Table 2) showed that the concentration of lymphocytes in the control and probiotic groups was higher when compared to treatment with functional oils (p < 0.05). The other parameters, leukocytes, neutrophils, eosinophils and monocytes did not differ statistically among treatments (P > 0.05). Mortality tended to be lower in pigs supplemented with either of the two additives.
Table 2.
Blood analysis of piglets at 50 days of age receiving different feed additives.
| Item | Additives | p-value | |||
|---|---|---|---|---|---|
| Control1 | Oils2 | Probiotic3 | SEM | ||
| Mortality % | 10.00 | 2.65 | 4.85 | 2.228 | 0.0504 |
| Leukocytes, mm3 | 21,080 | 17,060 | 20,020 | 0.485 | 0.5218 |
| Neutrophils, mm3 | 13,297 | 11,740 | 13,003 | 0.558 | 0.8490 |
| Lymphocytes, mm3 | 6046a | 3363b | 5418a | 0.437 | 0.0056 |
| Eosinophils, mm3 | 926 | 606 | 649 | 1.271 | 0.7989 |
| Monocytes, mm3 | 810 | 1262 | 948 | 0.755 | 0.2979 |
Least squares means based on 5 pen observations per diet.
1Control: without the inclusion of zootechnical additives; 2Probiotics: inclusion of 0.6% probiotic. Probiotic composition: Bacillus subtilis, Enterococcus faecium, Lactobacillus acidophilus, Bifidobacterium bifidum, Saccharomyces cerevisiae; 3Blend of functional oils with the inclusion of 0.2% Essential + 0.15% Integrity.
abAverages within the same line with different overwrites are statistically different (P < 0.05).
Discussion
The first week after weaning is the most the critical phase of weaning, when the diet changes from highly digestible (breast milk) to a more complex digestible solid food. This change directly affects the physiology of the piglets' gastrointestinal tract that is not fully adapted15, causing intestinal and immune system dysfunction and result in less health, growth, and feed intake16. The use of functional oils resulted in greater average daily weight gain (DWG) and feed conversion (FCR) in piglets during the first and second periods, which also resulted in heavier pigs at the end of the experiment, when compared to the control treatment.
Although it is not clear how the supplementation of functional oils improved pig growth, several mechanisms have been proposed, including antimicrobial activity, such as reducing pathogenic stress or increasing the abundance of beneficial microorganisms in the intestine, such as Lactobacillus spp.1; protecting intestinal villi and regulating enzyme activity17; also, modulating the intestinal microbiota and increasing the absorption of nutrients18.
Previous studies have shown that the supplementation of a blend of essentials oils (cinnamaldehyde and thymol) in the diet of weaned piglets positively influenced characteristics of zootechnical interest, such as higher DWG and lower FCR (P < 0.05)19,20, similarly to the results of the present study. Evaluating the supplementation of L. acidophilus in weaned piglets21, observed an improvement in DWG and FCR (P < 0.05) compared to the control group, and the same result was observed in the group receiving probiotics in the present study.
Probiotics act by modulating the microbiota, mainly by adhesion and competitive exclusion of pathogens at binding sites in the intestinal epithelium6. The blend of functional oils used in this study acts by modulating the immune system and the intestinal microbiota with antimicrobial action, mainly against gram-positive bacteria11,13. Both additives provided better performance and modulation of the microbiota in the face of the weaning challenge due to different mechanisms of action. In this study, there was no difference in microbial diversity between the additives, estimated by the Chao, Shannon and Simpson indices. Similar results were found by22 and23, who evaluated the supplementation of essential oils and probiotics in weaned piglets, respectively. Although without a statistical difference, there was a tendency to increased diversity for the group supplemented with functional oils. The increase in microbial richness and diversity can be seen as a predictor of the stability of the microbial ecosystem24.
It is important to highlight that for the microbiome analysis, the low number of replicates and the collection were conducted in just one period. The collection was performed at 50 days of age (22 days after weaning). The piglets' microbiota is more stabilized at this age6. However, evaluating the role of additives in the intestinal environment, even in the period when the microbiota is most stabilized, is extremely important to understand their role in animal performance. In this way, relative abundance was discussed as an exploratory analysis.
The phyla Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria, in decreasing order concerning relative abundance, are predominant in swine gastrointestinal tracts6. These results are in line with the findings in this study.
The ratio presented between Firmicutes/Bacteroidetes was 4.50, 5.51 and 5.17 respectively for the control treatments, functional oils and probiotics. It has been shown that heavier pigs tend to have a higher Firmicutes vs. Bacteroidetes ratio than lighter animals25. In this study there was no statistical difference, only a greater numerical relationship for the treatments with functional oils and probiotics. Functional oils modify the composition of intestinal microbiota, increasing the relative abundance of Firmicutes in the intestine, as demonstrated in broilers in vivo studies by26 and in pigs by22. It is necessary to highlight that the increase in the Firmicutes. Bacteroidetes ratio is a natural trend found in the healthy intestinal microbiota of matured piglets. However, in dysbiotic situations, such as those caused by weaning, it can result in a decrease in Firmicutes and an increase in Bacteroidetes. These changes provide a favorable environment for the proliferation of some pathogenic genera of this second phylum and, consequently, a reduction in the feeding efficiency of the animals27.
Also, a large reduction in Bacteroidetes can cause damage to the host. Bacteroidetes, despite encompassing some pathogenic species, are known to have a large number of genes that encode active carbohydrate enzymes and can readily switch between different energy sources, in addition to being an important source of propionate28,29. Additionally, Firmicutes has members nutritionally more specialized in the degradation of complex substrates, such as plant cell walls, starch particles and mucin30. Therefore, a stable relationship between Firmicutes and Bacteroidetes can result in better utilization of the diet by animals. In the present study, both additives were effective in maintaining the Firmicutes. Bacteroidetes ratio, a fact that may have contributed to the better performance of the animals in both supplemented groups.
Tenericutes also seem to be involved in improving the use of nutrients by the host. In a study with piglets31, found a positive correlation of this phylum with a better apparent digestibility of crude fiber. However, data on the relationship of this phylum with animal performance are still scarce. Proteobacteria are known to harbor numerous opportunistic pathogens in animals and humans, including Escherichia coli, Escherichia Shigella, Salmonella, Brucella, Rickettsiaos spp. Thus, it is associated with several intestinal disorders and infectious diseases32,33. In this study, the use of functional oils increased Tenericutes and inhibited Proteobacteria.
Although only the Ruminococcaceae family showed different relative abundance between treatments. The supplementation with both additives kept the relative abundance of Lachnospiraceae stable, increased the Ruminococcaceae and Prevotellaceae abundances, and reduced Lactobacillaceae. These four families are known to be part of a group fundamental to the microbial activity in the piglets' intestines34.
Ruminococcaceae, for example, are associated with fiber degradation and higher concentrations of butyrate in piglets35. Butyrate contributes to a better absorption of nutrients stimulating the growth of intestinal mucosa cells, improving the retention of calcium and phosphorus in the diet, mitigating the challenge of weaning36 and inducing secretion of mucin, a glycoprotein, which forms a protective layer in enterocytes37. The genus Faecalibacterium, from Ruminococcaceae, has been negatively associated with feed efficiency in pigs. In the present study, this genus was present in a higher percentage in the probiotics group when compared to the other treatments.
Prevotella and Lachnospiraceae are positively correlated with gene functions associated with the metabolism of amino acids, energy, cofactors and vitamins, indispensable to the host38. Prevotella has also been positively associated with higher luminous IgA concentrations and body weight in weaned piglets, showing its importance to the health of piglets38.
Lactobacillus is prevalent in the fecal microbiota of piglets in early life and tends to decrease during the weaning transition39. Several species of Lactobacillus are associated with beneficial characteristics for the host. It has been shown that the swine microbial population differs between more efficient and less efficient animals. More efficient animals have a higher number of Lactobacillus spp. than less efficient animals40.
Interestingly, the opposite behavior was observed in the present study, where the blend and probiotics groups showed numerically a less relative abundance of this genus (~ 3%), but better performance, while the control group, greater relative abundance (~ 9%) and less performance. The higher concentration of Lactobacillus in the control group may be associated with the activation of the immune system of animals in this group in the face of a greater challenge (as evidenced by the higher rate of diarrheal incidence and E. coli count in feces of these animals). Many species of Lactobacilli act in the innate and acquired system stimulating immune cells to release pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ) and interleukin-12 (IL-12)41. It is possible to conclude that the lower concentration of Lactobacillus in the functional oils and probiotics groups did not result in losses to the animals' performance.
The Clostridiaceae family is known to have different species, including C. pectinovor one, Clostridium butyricum, Clostridium perfringens. Clostridium butyricum, for example, acts in the production of short-chain fatty acids and has been studied as a probiotic in other animal species, such as broilers, where resulted in the improvement of the function of the intestinal barrier and the inhibition of pathogens42. The groups supplemented with Functional Oils and Probiotics presented an average of 4% and the control 2% of relative abundance of this family.
Curiously, the supplementation with probiotics (Lactobacillus spp., Bifidobacterium and Saccharomyces cerevisiae) did not result in an increase of these genera, other than for Bifidobacterium, in the fecal microbiota of piglets. Two factors may explain this result. On one hand, the technology and conditions involved in the preservation of these probiotics, which can negatively influence the viability of the strains used until they reach the small intestine of piglets. On the other hand, it is known that different species of bacteria are subject to adverse conditions in the gastrointestinal tract, and when a exogenous microorganism is fed as a probiotic and enters the gastrointestinal tract, it needs to compete with the existing microbiota ecosystem43. Thus, in some cases, unfavorable circumstances may end up hindering the proliferation of the microorganism used as a probiotic44.
The blend of functional oils kept the Muribaculaceae family (phylum Bacteroidetes) stable when compared to the other treatments. Bacteria in this group have been positively related to the regulation of genes for carbohydrate metabolism in mice45. Muribaculaceae members may be involved in the fermentation of starch into propionate, and its composition is an important predictor of higher concentrations of short-chain fatty acids in healthy intestinal microbiota of the animals46.
In contrast, the Probiotic provided a greater relative abundance of Veillonellaceae (4%) compared to the functional oils group (2%), but not compared to the control (5%). This family is directly involved in metabolic functions related to proteins and enzymes essential to the host47.
Shigella spp. and Escherichia coli are closely related and, although they have some differences, they are considered unique genome species. Shigella spp. are among the most important enteric pathogens that cause bacillary dysentery worldwide, especially in humans48. As observed in Enterobacteriaceae, it was very low, 0.07% for the control, 0.03% and 0.04% for the functional and probiotic oils. This may have occurred due to the age of sample collection or due to the limitation of the technique. In this same sense, using the E. coli culture technique, it significantly reduced (P < 0.05) E. coli in the feces of piglets treated with the blend, a result that agrees with those observed by22. These authors showed that supplementation of 100 ppm of functional oils based on thymol and cinnamaldehyde to the control diet reduced the E. coli count in the feces of weaned piglets.
The reason for these effects may be associated with the antimicrobial activity of phytogenics, demonstrated in vitro by42. The authors evaluated a mixture based on thymol and cinnamaldehyde and observed its ability to damage the cell membrane and alter the morphology of E. coli and S. aureus pathogenic cells. Similarly49, evaluated the supplementation of six essential oils in vivo (including thymol, carvacrol, and eugenol) in piglets challenged by enteropathogenic species (E. coli, Salmonella spp. and C. perfringens), reporting antimicrobial activity of these essential oils against at least one of these species. The intermediate results of the Probiotic group agree with other studies21,44, which also reported the ability of the Probiotic supplementation (L. acidophilus, Pediococcus acidilactici) to reduce fecal E. coli in weaned piglets.
The genus Campylobacter was found in 0.03% of the microbiota when supplemented with functional oils and 0.05% and 0.08%, respectively for the control and probiotic group. Campylobacter is the predominant bacterial agent in diarrheal piglets, reducing the relative abundance of bacterial species of the classes Bacteroidia and Clostridia. Both ferment the non-digestible carbohydrate. This reduction results in less production of short-chain fatty acids, which are the main metabolites of the intestinal microbiota, and which could promote barrier function and maintain a healthy and slightly acidic environment in the colon42.
The erythrocyte, hematocrit, hemoglobin and platelet values of all treatments varied within the reference intervals for young piglets, as recommended by50. This indicates that the animals were, in general, in good health and not anemic. Similarly47, found no significant effect of supplementing 40 ppm of functional Oregano oils on the hematological status of weaned piglets.
The difference in leukocytes circulating in pigs may be associated with two specific factors: inflammatory state or stress state, caused during the weaning period51. In a study with rats under stress, the levels of circulating inflammatory leukocytes increased by directly stimulating the proliferation of hematopoietic stem cells52. Similarly, it has been reported that some types of stressors have increased the total leukocyte count and the proliferation of T cells in pigs53.
In another study, evaluating hematological parameters in piglets challenged by Salmonella54, found no significant differences in Salmonella concentrations in the animals' feces, and suggested that variations in hematological parameters, in that study, were more related to the state of stress than to actual infection by this pathogen. On the other hand, the increase in the percentage of lymphocytes in the blood in piglets challenged by enterotoxigenic Escherichia coli17 was associated with a change in the inflammatory state of these animals, due to the challenge. Opposite results weres observed in this study for the blend of functional oil group, suggesting that it can mitigate E. coli infection.
Research that reports the impact of additives during the microbiota transition period, that is, before weaning and in the first 14 days of weaning are still needed to elucidate the effect of additives on piglet intestinal health.
The commercial blend of functional oils based on cashew nut shell liquid and castor oil improved the performance of piglets weaned during the nursery period. The animals that received probiotics presented intermediate performance and the piglets that did not receive either additive performed worse. The use of functional oils reduced the concentration of Escherichia coli in piglet feces at 50 days of age, demonstrating a modulating effect on the intestinal microbiota of newly weaned piglets.
Material and methods
The described study was performed according to protocol nº 3665110718 and approved by the Ethics Committee on the Use of Animals at the Universidade Federal de Santa Catarina, performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals, and reported according to the ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines). This experiment was performed in a group of piglets that were reared in a commercial pig farm, located in the municipality of Jaguaruna—Santa Catarina/Brazil.
Animals, facilities and diets
A total of 225 piglets descended from commercial lines of F1 females (Landrace × Large White) with tricross males (Hampshire × Duroc × Pietrain), weaned at 28 days of age, females and castrated males, weighing 8.54 ± 0.622 kg were randomly distributed in 15 pens, with a density of 0.3 piglets/m3, hollow wooden floor and equipped with automatic feeder and drinker.
Water and feed were offered ad libitum throughout the experimental period. The study was divided into the three phases according to their age. Phase I: from 28 to 43 days; phase II: from 43 to 57 days; and phase III: from 57 to 66 days. All diets were formulated to meet the nutritional requirements of piglets55. The only difference among diets was the additive used, as described in Supplementary Table 3. The treatments were: (1) control group—without the inclusion of zootechnical additives; (2) probiotic group—inclusion of 0.6%; (3) blend group of functional oils with inclusion of 0.35% (0.2% of Essential + 0.15% of Integrity). All feed additives were included in the diets by replacing inert (kaolin) in the basal diet in all phases. Additive doses were used according to the manufacturer's recommendation. The commercial product Integrity is basically composed of cardanol (75 g/kg) and cardol (15 g/kg) and Essential by cardanol (200 g/kg), ricinoleic acid (90 g/kg) and cardol (40 g/kg). The composition of the probiotic used was Bacillus subtilis (3.66 × 107 cfu/kg), Enterococcus faecium (3.5 × 106 cfu/kg), Lactobacillus acidophilus (3.5 × 107 cfu/kg), Bifidobacterium bifidum (3.5 × 107 cfu/kg) and Saccharomyces cerevisiae (2 × 109 cfu/kg).
Experimental procedures and collections
Animals were weighed at the beginning and the end of each phase to determine average daily weight gain (DWG), average daily feed consumption (DFI), and to calculate the feed conversion (FCR). The left-overs were collected daily, weighed and subtracted from the quantity supplied to the animals.
During the first 14 days of the experiment, the occurrence of diarrhea was monitored daily by visual observation, always by the same observer. Fecal consistency was assessed according to the following scores: 1—normal feces; 2—pasty feces; and 3—liquid feces. Feces assigned with scores 1 and 2 were considered normal and feces with score 3 were considered diarrhea. The frequency of diarrhea was calculated based on the number of observation days. The frequency of fecal scores 1, 2 and 3 was the percentage of days that piglets presented these fecal scores in each pen. The calculation was performed as follows: Frequency of feces scores 1 or 2 or 3 (%) = [[(P1 × D) + (P2 × D) + (Pn × D)]/n/TD × 100], where P (1, 2 … N) = represents each piglet inside the pen (n); D = number of days that each piglet showed fecal scores 1, 2 or 3 within a pen; TD = total number of days on which the diarrhea scores were monitored56.
At 50 days of age, 2 mL of blood were collected to perform a blood count. The collection was performed through the jugular vein of one piglet per repetition—animal weighing closest to the average weight of the group in each pen. An automatic cell counter (Vet Scan HM 5; Abaxis) was used to evaluate hemoglobin, hematocrit, erythrocytes and leukocytes, and the ratio between neutrophils to lymphocytes was calculated.
At 50 days of age, a pool of fresh feces from 3 animals per repetition was swabbed to isolate Escherichia coli. These swabs were striated in Petri dishes containing MacConkey agar (Merck), incubated at 37 °C for 24 h to count the colony forming units. From this same pool, 2 g of feces were used for the sequencing of the microbiota by Illumina MiSeq. The samples were identified and frozen at − 20 °C for further analysis.
DNA extraction, PCR amplification and sequencing
The feces pool samples were placed in a sterile 1.5 mL tube and sent to Neoprospecta Microbiome Technologies (Florianópolis-SC, Brazil). All procedures were performed according to the methodology previously described57. Sample preparation and sequencing were performed by Neoprospecta Microbiome Technologies. For total DNA extraction, the commercial QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) was used according to the manufacturer's instructions. It consisted of the V3/V4 regions of the 16S rRNA gene, which were amplified using primers 341F (5′-CCTACGGGRSGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), with Illumina adapters, necessary for sequencing. The amplification was performed in 35 cycles at 50 °C of the annealing temperature, which was tripled for each sample. The sequencing was performed by Illumina MiSeq using V2 kits, with runs of 300 single-ended nucleotides.
Sequence analysis
Read quality was assessed using the FastQC software (version 0.11.5) (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Low quality reads and adapters were removed using the Trimmomatic program. The following steps were implemented using QIIME2 software (v. 2020.2)58 (https://qiime2.org/). The reads were subjected to a Denoising approach for low quality sequence removal, sequencing error correction, chimera removal and identification of amplicon sequence variants (ASVs) using the DADA2 method with default parameters, and 290 truncated read length. Taxonomy was attributed to ASVs using the SILVA database (v. 132), with 97% correspondence. Rare ASVs below a frequency of 0.1% in the samples were removed.
Statistical analysis
The experimental design was completely randomized with three treatments (without additives, probiotics and a blend of functional oils), five repetitions per treatment (pens) and 15 piglets per repetition. The variables of performance, frequency of diarrhea, blood and E. coli quantification were subjected to an analysis of variance with 5% significance level, and means were tested by Tukey, using the SAS statistical program. Relative abundance, alpha rarefaction, alpha (Chao-1, Shannon and Simpson) and beta diversity indices were performed using the R program (v. 3.6.1) (https://www.R-project.org/) and plyr (v. 1.8.4)59, reshape2 (v. 1.4.3)59 and phyloseq packages (v. 1.14.0)60. Beta diversity was estimated after normalization by centered log-ratio using the DESeq2 R package (v. 1.26.0). After normalization, a principal coordinate analysis (PCoA) was performed using the Bray–Curtis dissimilarity index by vegan (v. 2.4.1)61 and heatmaps (v. 1.8.0) packages61. Alpha diversity and relative abundance were tested using the Kruskal–Wallis test. The Permutational Multivariate Analysis of Variance (Adonis at 999 permutations) was performed based on beta diversity, the assumption of homogeneity of variances was checked (p > 0.05) using the R vegan package. Differential abundances statistical was calculated using the DESeq2 R package62, aggregating the data at Family level.
Supplementary Information
Acknowledgements
TATS was supported by a PhD scholarship provided by FAPESC. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
A.M.V., T.A.T.S., L.H., P.d.O.M.—these authors contributed equally to all stages of this work. A.P.S., P.G.d.S.P., K.M.C., G.W., A.L.F.L.—these authors contributed to the analyzes presented in this work. P.G.d.S.P., K.M.C., A.L.F.L., F.D., D.P.N.—these authors contributed by reviewing the technical-scientific.
Data availability
The raw sequences related to this article have been deposited at the Sequence Read Archive (SRA) under the BioProject ID PRJNA752610.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98549-w.
References
- 1.Omonijo FA, et al. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan L, et al. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 2017;95:2627–2639. doi: 10.2527/jas.2016.1243. [DOI] [PubMed] [Google Scholar]
- 3.Cairo PLG, et al. Effects of dietary supplementation of red pepper (Schinusterebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J. Sci. Food Agric. 2018;98:541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- 4.Yi HB, et al. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 2016;6:12. doi: 10.1038/srep25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langemeier A, Morton J, Scotten S, Thayer M, Nelssen J. Effects of a combination of essential Oils (Victus LIV), increased zinc oxide and copper sulfate, or their combination in nursery diets on pig performance. Kansas Agric. Exp. Stn. Res. Rep. 2017;3:27. [Google Scholar]
- 6.Hu S, Wang L, Jiang Z. Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept. Lett. 2017;24:382–387. doi: 10.2174/0929866524666170223143615. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed ST, Hoon J, Mun H-S, Yang C-J. Evaluation of Lactobacillus and Bacillus-based probiotics as alternatives to antibiotics in enteric microbial challenged weaned piglets. Afr. J. Microbiol. Res. 2014;8:96–104. doi: 10.5897/AJMR2013.6355. [DOI] [Google Scholar]
- 8.Bess F, Favero A, Vieira S, Torrent J. The effects of functional oils on broiler diets of varying energy levels. J. Appl. Poultry Res. 2012;21:567–578. doi: 10.3382/japr.2011-00481. [DOI] [Google Scholar]
- 9.Himejima M, Kubo I. Antibacterial agents from the cashew Anacardiumoccidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991;39:418–421. doi: 10.1021/jf00002a039. [DOI] [Google Scholar]
- 10.Guimarães DO, Momesso LDS, Pupo MT. Antibióticos: importância terapêutica e perspectivas para a descoberta e desenvolvimento de novos agentes. Quim. Nova. 2010;33:667–679. doi: 10.1590/S0100-40422010000300035. [DOI] [Google Scholar]
- 11.Moraes PO, et al. Comparison between a commercial blend of functional oils and monensin on the performance and microbiota of coccidiosis-challenged broilers. Poult. Sci. 2019;98:5456–5464. doi: 10.3382/ps/pez345. [DOI] [PubMed] [Google Scholar]
- 12.Moraes P, et al. Effect of functional oils on the immune response of broilers challenged with Eimeria spp. Animal. 2019;13:2190–2198. doi: 10.1017/S1751731119000600. [DOI] [PubMed] [Google Scholar]
- 13.Vieira AM, et al. Modulation of the intestinal microbiota of broilers supplemented with monensin or functional oils in response to challenge by Eimeria spp. PLoS ONE. 2020;15:15. doi: 10.1371/journal.pone.0237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami AE, Eyng C, Torrent J. Effects of functional oils on coccidiosis and apparent metabolizable energy in broiler chickens. Asian Australas. J. Anim. Sci. 2014;27:981–989. doi: 10.5713/ajas.2013.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalles J-P, Bosi P, Smidt H, Stokes CR. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JM, Crenshaw JD, Polo J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013;4:1–4. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian QY, Piao XS. Essential oil blend could decrease diarrhea prevalence by improving antioxidative capability for weaned pigs. Animals. 2019;9:12. doi: 10.3390/ani9100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alagawany M, et al. The usefulness of oregano and its derivatives in poultry nutrition. Worlds Poult. Sci. J. 2018;74:463–474. doi: 10.1017/S0043933918000454. [DOI] [Google Scholar]
- 19.Zeng Z, et al. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015;86:279–285. doi: 10.1111/asj.12277. [DOI] [PubMed] [Google Scholar]
- 20.Su G, et al. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018;17:1–10. doi: 10.1186/s12944-017-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan R, Koo J, Kim I. Effects of Lactobacillus acidophilus supplementation in different energy and nutrient density diets on growth performance, nutrient digestibility, blood characteristics, fecal microbiota shedding, and fecal noxious gas emission in weaning pigs. Anim. Feed Sci. Technol. 2016;219:181–188. doi: 10.1016/j.anifeedsci.2016.06.018. [DOI] [Google Scholar]
- 22.Li Y, et al. Intestinal microbiome-metabolome responses to essential oils in piglets. Front. Microbiol. 2018;9:1988. doi: 10.3389/fmicb.2018.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luise D, et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019;10:1–16. doi: 10.1186/s40104-018-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mes TH. Microbial diversity–insights from population genetics. Environ. Microbiol. 2008;10:251–264. doi: 10.1111/j.1462-2920.2007.01449.x. [DOI] [PubMed] [Google Scholar]
- 25.Han GG, et al. Tracing of the fecal microbiota of commercial pigs at five growth stages from birth to shipment. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-24508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaheen S, Kim SW, Haley BJ, Van Kessel JAS, Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 2017;8:11. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betancourt L, et al. Effects of Colombian oregano essential oil (Lippiaoriganoides Kunth) and Eimeria species on broiler production and cecal microbiota. Poult. Sci. 2019;98:4777–4786. doi: 10.3382/ps/pez193. [DOI] [PubMed] [Google Scholar]
- 28.Lapébie P, Lombard V, Drula E, Terrapon N, Henrissat B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun. 2019;10:1–7. doi: 10.1038/s41467-019-10068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley D, Hughes RJ, Moore RJ. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan PO, et al. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb. Genomics. 2016;2:16. doi: 10.1099/mgen.0.000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu Q, et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015 doi: 10.1038/srep09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A common factor in human diseases. Biomed. Res. Int. 2017;2017:7. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, et al. Identification of the core bacteria in rectums of diarrheic and non-diarrheic piglets. Sci. Rep. 2019;9:10. doi: 10.1038/s41598-019-55328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. Fecal microbiota succession of piglets from birth to post-weaning by 454 pyrosequencing analysis. Trans. Tianjin Univ. 2017;23:211–220. doi: 10.1007/s12209-017-0045-2. [DOI] [Google Scholar]
- 35.Zhong X, et al. Microbial-driven butyrate regulates jejunal homeostasis in piglets during the weaning stage. Front. Microbiol. 2019;9:3335. doi: 10.3389/fmicb.2018.03335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng W, et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell. Physiol. Biochem. 2018;47:1617–1629. doi: 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- 37.Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mach N, et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 2015;7:554–569. doi: 10.1111/1758-2229.12285. [DOI] [PubMed] [Google Scholar]
- 39.Gresse R, et al. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Fouhse J, Zijlstra R, Willing B. The role of gut microbiota in the health and disease of pigs. Anim. Front. 2016;6:30–36. doi: 10.2527/af.2016-0031. [DOI] [Google Scholar]
- 41.Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014;54:938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- 42.Yang CM, et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012;91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- 43.Verdenelli MC, et al. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009;48:355–363. doi: 10.1007/s00394-009-0021-2. [DOI] [PubMed] [Google Scholar]
- 44.Dowarah R, Verma A, Agarwal N, Patel B, Singh P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017;195:74–79. doi: 10.1016/j.livsci.2016.11.006. [DOI] [Google Scholar]
- 45.Chung YW, Gwak H-J, Moon S, Rho M, Ryu J-H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE. 2020;15:e0227886. doi: 10.1371/journal.pone.0227886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, B. J. et al. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol.19, 130 (2018). [DOI] [PMC free article] [PubMed]
- 47.Zhang L, Wu W, Lee Y-K, Xie J, Zhang H. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front. Microbiol. 2018;9:48. doi: 10.3389/fmicb.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragupathi ND, Sethuvel DM, Inbanathan F, Veeraraghavan B. Accurate differentiation of Escherichia coli and Shigella serogroups: Challenges and strategies. New Microbes New Infect. 2018;21:58–62. doi: 10.1016/j.nmni.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez-García M, et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porcine Health Manag. 2019;5:1–8. doi: 10.1186/s40813-019-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perri AM, O'Sullivan TL, Harding JCS, Wood RD, Friendship RM. Hematology and biochemistry reference intervals for Ontario commercial nursing pigs close to the time of weaning. Can. Vet. J. 2017;58:371–376. [PMC free article] [PubMed] [Google Scholar]
- 51.Gimsa U, Tuchscherer M, Kanitz E. Psychosocial stress and immunity—what can we learn from pig studies? Front. Behav. Neurosci. 2018;12:64. doi: 10.3389/fnbeh.2018.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heidt T, et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salak-Johnson JL, Webb SR. Pig social status and chronic cold or crowd stressors differentially impacted immune response. Open J. Anim. Sci. 2018;8:280. doi: 10.4236/ojas.2018.83021. [DOI] [Google Scholar]
- 54.Burdick Sanchez NC, Carroll JA, Corley JR, Broadway PR, Callaway TR. Changes in the hematological variables in pigs supplemented with yeast cell wall in response to a Salmonella challenge in weaned pigs. Front. Vet. Sci. 2019;6:246. doi: 10.3389/fvets.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rostagno, H. S. et al. Brazilian tables for poultry and swine: Composition of feedstuffs and nutritional requirements (4th ed.), (UFV, Viçosa, Minas Gerais, Brazil, 2017)
- 56.Milani C, et al. Dietary zinc oxide nanoparticles as growth promoter for weanling pigs. Anim. Feed Sci. Technol. 2017;227:13–23. doi: 10.1016/j.anifeedsci.2017.03.001. [DOI] [Google Scholar]
- 57.Christoff, A. et al. Bacterial identification through accurate library preparation and high-throughput sequencing. Neoprospecta Microbiome Technologies, 25 (2017).
- 58.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickham H. Reshaping data with the reshape package. J. Stat. Softw. 2007;21:1–20. doi: 10.18637/jss.v021.i12. [DOI] [Google Scholar]
- 60.McMurdie PJ, Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perry, M. Flexible Heatmaps for Functional Genomics and Sequence Feature S. R Package Version 1.11. 0 (2016).
- 62.Duşa A. QCA with R: A Comprehensive Resource. Springer; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequences related to this article have been deposited at the Sequence Read Archive (SRA) under the BioProject ID PRJNA752610.