Abstract
As countries work towards malaria elimination, it is important to monitor imported cases to prevent reestablishment of local transmission. The Plasmodium falciparum Pfs47 gene has strong geographic population structure, because only those parasites with Pfs47 haplotypes compatible with the mosquito vector species in a given continent are efficiently transmitted. Analysis of 4,971 world-wide Pfs47 sequences identified two SNPs (at 707 and 725 bp) as sufficient to establish the likely continent of origin of P. falciparum isolates. Pfs47 sequences from Africa, Asia, and the New World presented more that 99% frequency of distinct combinations of the SNPs 707 and 725 genotypes. Interestingly, Papua New Guinea Pfs47 sequences have the highest diversity in SNPs 707 and 725. Accurate and reproducible High-Resolution Melting (HRM) assays were developed to genotype Pfs47 SNPs 707 and 725 in laboratory and field samples, to assess the geographic origin and risk of local transmission of imported P. falciparum malaria cases.
Subject terms: Population genetics, Genotyping and haplotyping
Molina-Cruz et al. analyzed 4,971 sequences of the Plasmodium falciparum Pfs47 gene and identified a combination of two SNP haplotypes highly correlated with geographic origin and transmission potential by mosquito vectors. A simple High-Resolution Melting RT-PCR assay was developed to genotype these SNPs and establish the likely continent of origin and transmission risk of P. falciparum isolates.
Introduction
Malaria in humans is caused by Plasmodium parasites and, as a result of global elimination efforts, the disease burden has decreased considerably in the last 20 years, with a decrease of 29% in the incidence of malaria and of 60% in the mortality rate. However, despite these recent advances, malaria still infects more than 220 million people every year, leading to an estimated 409,000 deaths in 20191, the majority caused by Plasmodium falciparum in sub-Saharan Africa. The WHO Global Malaria Program coordinates international efforts to control and eliminate malaria and has already certified several countries as malaria-free1. Once local elimination is achieved, preventing the reintroduction of the disease becomes the primary focus of national malaria control programs2. Since the local mosquito vectors of malaria are still present after the parasite is eliminated3, imported malaria cases have the potential to give rise to new outbreaks, particularly as immunity to the parasite in the population decreases over time4. Given extensive and ever-growing international human mobility, constant monitoring for potential resurgence of malaria infections and assessing the risk of local transmission are critical measures to guide a cost-effective response to imported malaria cases. Thus, there is need for practical assays with informative Plasmodium genetic markers to predict the transmission risk of an imported malaria case in a given region.
Malaria is transmitted by mosquitoes of the genus Anopheles, and different anopheline species tend to be restricted to distinct biogeographic regions or continents3. The globalization of P. falciparum malaria out of Africa5, involved the adaptation of the parasite to more than 70 different anopheline vector species6. Several lines of evidence indicate that adaptation of P. falciparum to evolutionary distant vectors resulted in strong natural selection on Pfs477, a gene mediating the parasite’s ability to evade the mosquito immune system, and therefore critical for the parasite’s survival and transmission8. Consistent with natural selection by mosquito vectors, Pfs47 has a strong geographic population structure, with different haplotypes predominating in continents harboring distinct vector species7,9,10. Controlled experimental infections have directly shown that P. falciparum from a given continent infects sympatric vectors more efficiently when compared to vectors from a different continent7. Furthermore, the compatibility of a P. falciparum line with a given mosquito species can be modified by exchanging the Pfs47 haplotype the parasite carries7. We have shown that Pfs47 interacts with a specific mosquito midgut receptor, and the in vitro binding affinity of different P. falciparum Pfs47 haplotypes, with the receptor in a given mosquito species, is consistent with the parasite/vector compatibility observed in vivo7,11. Therefore, the interaction of Pfs47 haplotypes with different vector species follows a “lock-and-key” model. This model proposes that those parasites expressing a Pfs47 haplotype compatible with the Pfs47 receptor of a given vector can avoid activating an immune response, survive, complete their development in the mosquito, and be transmitted to a new human host7.
Here, we analyzed a large number of Pfs47 sequences collected around the world, to identify combinations of Pfs47 gene polymorphisms unique to a given continent, which could be used as molecular markers to establish the geographic origin and transmission potential of malaria cases of unknown origin. Simple and low-cost PCR-based high-resolution melting (HRM) assays were developed to facilitate the genotyping of the two most informative Pfs47 single nucleotide polymorphisms (SNPs). We provide direct evidence that the combination of these two Pfs47 SNPs can be easily determined with these assays and makes it possible to establish the geographic origin of P. falciparum isolates, to assess the risk of local transmission of imported malaria cases.
Results
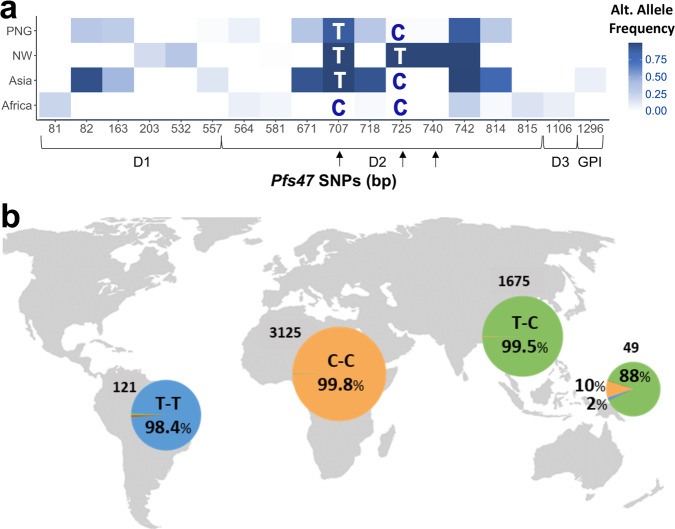
A detailed analysis of 4,971 Pfs47 DNA sequences from countries in different geographic regions (Africa, Asia, New World (NW), and Papua New Guinea (PNG)) (Supplementary Data 1) was conducted to determine the geographic population structure of Pfs47. A total of 84 Pfs47 SNPs were identified (Supplementary Data 1), with 18 SNPs having an alternate allele frequency higher than 0.05 (Fig. 1a). Pfs47 has three domains, with the central domain (Domain 2) being the most polymorphic, harboring 10 out of the 18 frequent SNPs present (Fig. 1a). The SNPs located at positions 707, 725, and 740 bp in Domain 2 have the most drastic differences in frequency between continents (Fig. 1a, Supplementary Data 1), and the genotype of SNP 740 appears linked to that of SNP 725 (Fig. 1a, Supplementary Data 1). The degree of population differentiation of Pfs47 SNPs was established by determining Wright’s fixation index (FST). Among all Pfs47 polymorphisms, Pfs47 SNP 707 presents the highest FST between populations from Africa and other continents (0.89–0.99), while Pfs47 SNPs 725 and 740 present the highest FST between the New World and other continents (0.97–0.99) (Supplementary Data 2). Indeed, the combined genotype of SNPs 707 and 725 is sufficient to differentiate Pfs47 haplotypes originating from African (C-C), Asian (T-C), or New World (T-T) origin with a probability of 99% (Table S1, Fig. 1a, b). Although the predominant genotype in Asia of SNPs 707 and 725 (T-C) is also the most frequent (0.88) in PNG, the most prevalent genotype from Africa (C-C) and the New World (T-T) are also present in PNG at lower frequencies, 0.10 and 0.02, respectively. While both SNPs 707 and 725 result in amino acid substitutions, it is interesting that the “C-T” combination for SNPs 707 and 725 was not detected in any continent.
Fig. 1. Alternate allele of Pfs47 polymorphisms by geographic region.
a Heat map of the frequency of 18 Pfs47 SNPs with alternate allele frequencies >0.05 in Africa, Asia, the New World (NW), and Papua New Guinea (PNG)). The most common African Allele (GB4) was used as reference. Pfs47 polymorphisms with highest differences between continents are indicated with arrows (SNPs at 707, 725, and 740 bp). The letters in the boxes indicate the most frequent Pfs47 genotype combinations for SNPs 707 and 725 in each geographic region. Coding regions of predicted Pfs47 Domain 1 (D1, 82–546 bp), Domain 2 (D2, 547–846 bp), Domain 3 (D3, 847–1239 bp), and GPI anchoring region (GPI, 1240–1317 bp) are indicated. b Frequency of the most common Pfs47 genotype combinations for SNPs 707 and 725 by geographic region. The number of sequences analyzed per continent is indicated in the map. The dominant genotype(s) in each region is indicated by different colors, Africa (orange), Asia (green), New World (Blue). Papua New Guinea presents genotypes from different geographic regions.
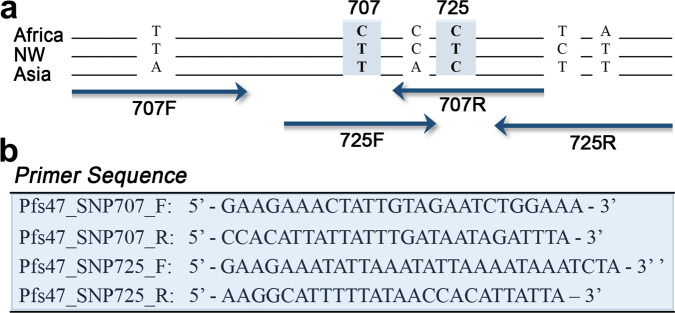
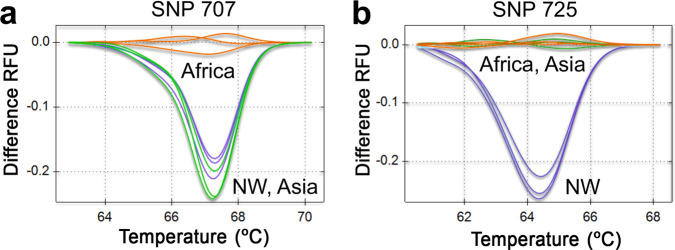
HRM assays were developed to facilitate genotyping of Pfs47 SNPs 707 and 725. HRM analysis has been previously implemented and validated to genotype Plasmodium for molecular barcoding and detection of drug resistance12–15. The HRM assay consists of a PCR amplification, followed by careful melting curve analysis of the PCR products. This allows for fast genotyping of specific SNPs without the need for DNA sequencing16. Separate HRMs were necessary for each SNP, due to the presence of other non-informative polymorphisms in close proximity (Fig. 2a). Multiple potential primer sets were designed and tested, until two suitable primer sets (one pair for each SNP) for accurate HRM SNP assays were identified and optimized (Fig. 2b). The HRM assays were tested using two independent P. falciparum lines from each continent (Africa, Asia, and the New World). The HRM assay for Pfs47 SNP 707 distinguished the predominant African allele from Asian and New World alleles with more than 96.6–99.8% clustering confidence (Figs. 3a, S1a, S2a, Table S2). Conversely, the HRM for Pfs47 SNP 725 distinguished the main Pfs47 New World allele from the African and Asian alleles with more than 97.2–99.6% clustering confidence (Figs. 3b, S1b, S2b, Table S3). The most frequent Asian Pfs47 allele could be identified by analyzing the results from both HRM assays, since it clustered with the predominant New World allele in Pfs47 SNP 707, and with the predominant African allele in Pfs47 SNP 725 (Figs. 3a, b; S1a, b, S2a, b; Tables S2, S3). Both HRM assays were reproducible at template concentrations of 2, 0.2, and 0.02 ng/l DNA (Figs. 3a, b; S1a, b; Tables S1, S2).
Fig. 2. Pfs47 polymorphisms near SNPs 707 and 725 and High-Resolution Melting assay (HRM) primer design.
a Schematic representation of Pfs47 polymorphisms in representative haplotypes of P. falciparum isolates from Africa (GB4), New World (7G8), and Asia (Asia 33). The diagram illustrates the approximate location of the forward and reverse primers for HRM assays to genotype SNPs at 707 and 725. b Primer sequences used in HRM assays.
Fig. 3. High-Resolution Melting (HRM) analysis of Pfs47 SNPs 707 and 725.
HRM profile in P. falciparum DNA samples from Africa (GB4, orange), Asia (Thai 17, green), and New World (7G8, blue) at three DNA concentrations (2, 0.2, 0.02 ng/l). a HRM difference curves for Pfs47 SNP 707. b HRM difference curves for Pfs47 SNP 725.
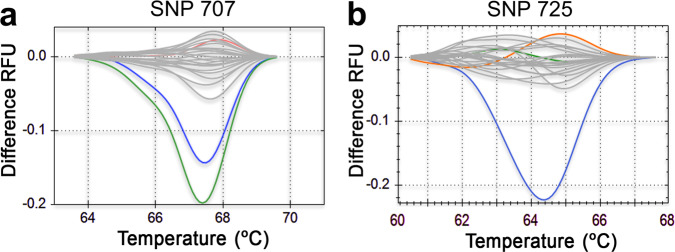
The robustness of the Pfs47 HRM SNP assays to genotype clinical samples was established by testing dried blood spots collected from individuals presenting with acute febrile malaria in a cohort study in Kalifabougou, Mali that has been described previously17. Parasite densities in these blood samples (n = 20) ranged from 80,700 to 312,300 P. falciparum asexual parasites/μl of blood (Table S4). We were able to genotype Pfs47 SNPs 707 and 725 in all samples using our HRM assays, with cycle threshold (CT) values ranging from 22.4 to 27.8 (Table S4) during the PCR amplifications. The HRM assays accurately identified in the Malian samples both Pfs47 SNPs 707 and 725 from African origin, with more than 95% clustering confidence (Figs. 4a, b, S3, Table S5). The limit of detection of the Pfs47 SNP 707 and 725 HRM assays was estimated by analyzing serial dilutions from three field blood samples with high (Sample 599), medium (Sample 309), or lower parasitemia (Sample 614). The Pfs47 SNP 707 HRM was within the method recommended CT value of 30 with accurate clustering up to an estimated parasitemia of 2,875 +/− 98 parasites/μl (Fig. S4a–d; Table S6), while the 725 HRM was within the method limit of detection up to 4,911+/− 136 parasites/ml (Fig. S5a–d; Table S7). It is worth noting that the detection limit of the Pfs47 SNP HRM assays developed, could in principle be increased by analyzing a larger dried blood sample.
Fig. 4. High-Resolution Melting (HRM) analysis of Pfs47 SNPs 707 and 725 in field samples from Africa (gray).
a HRM difference curves for Pfs47 SNP 707 in 20 dry blood spots from Kalifabougou, Mali, Africa. b HRM difference curves for Pfs47 SNP 725 in 20 dry blood spots from Kalifabougou, Mali, Africa. Reference sequences from Africa (GB4 orange), Asia (Thai 17, green), and New World (7G8 blue) are also included.
Discussion
In countries with ongoing malaria elimination programs, monitoring and managing imported cases is critical to prevent the re-establishment of local disease transmission. For example, the Chinese national malaria surveillance and control system currently deploys costly vector control measures and extensive screening for Plasmodium infection in local residents in response to imported malaria cases2. Assessing the risk of local malaria transmission more accurately, by determining whether the imported parasite is compatible with the local mosquito vector population, would thus be helpful to determine the appropriate response.
Here, we validate the use of Pfs47 SNPs as markers for assessing the geographic origin and transmission potential of a P. falciparum malaria case. Our analysis of an extensive number of Pfs47 gene sequences (4,971) confirmed the strong population structure of this gene according to the continental origin of parasites7. In fact, Pfs47 is one of the genes with the highest geographic population structure detected in the P. falciparum genome10, with Pfs47 SNPs at 707, 725, and 740 bp having marked differences in allele frequency at the continent level (Fig. 1, Supplementary Data 1). We have previously shown that these SNPs are major determinants of the compatibility of different Pfs47 haplotypes with distinct mosquito species from different continents18,19. This would suggest that the amino acids encoded by these Pfs47 SNPs in Domain 2 are critical for the interaction of Pfs47 with its mosquito midgut receptor11.
The presence of multiple Pfs47 haplotypes typical of diverse geographic origins in PNG could be due to more diversity of local mosquito vectors, which allows for transmission of multiple Pfs47 haplotypes. Alternatively, some anophelines in that region may be particularly permissive to malaria parasites with different Pfs47 haplotypes. Additional information is necessary to understand the diverse Pfs47 haplotypes in PNG. This includes more detailed information on the compatibility between parasites containing different Pfs47 haplotypes with local vectors, as well as information on the distribution of Pfs47 haplotypes circulating in these vectors across PNG. The multiple Pfs47 haplotypes present in PNG could also complicate the identification of the geographic origin of malaria cases exported from this region. When available, travel information on the affected individual would establish if the case originated in PNG. Nevertheless, local anopheline mosquitoes from other continents presumably would also limit transmission of incompatible Pfs47 haplotypes from PNG, which can be determined using the proposed HRM analysis.
In practical terms, if the Pfs47 SNPs 707 and 725 genotype of a putative imported malaria case coincides with local genotype(s) of a pre-elimination malaria region, this would predict high potential for local transmission. Conversely, if the genotype of Pfs47 SNPs 707 and 725 of a putative imported case differs from the local genotype(s), this would predict likely low potential for local transmission. In the latter case, Pfs47 genotyping would also confirm that the malaria case is indeed imported and would provide its possible continental origin. The Pfs47 HRM analysis presented here is not reliable for individuals co-infected with parasites from different continents (e.g., African and Asian Pfs47 haplotypes combined). Boundary regions between continents, such as Northeast Africa and the Arabian Peninsula, could also be problematic if Asian vectors, such as Anopheles stephensi, co-exist with African anophelines.
If the Pfs47 haplotypes in a patient sample do not cluster with the standards used in the HRM analysis we developed, it would be necessary to amplify and sequence the Pfs47 Domain 2 region containing the most informative SNPs (707, 725, and 740 bp) to determine the geographic origin of the sample. Interestingly, the “C-T” combination for SNPs 707 and 725 was not detected in any continent, suggesting that it may code for an amino acid combination in Domain 2 of Pfs47 that is deleterious to the parasite and is thus under strong negative selection.
Plasmodium vivax Pvs47, the orthologue of Pfs47, also presents a marked geographic population structure20, suggesting that Pvs47 may also be used as a maker to assess geographic origin of P. vivax. However, the compatibility of Pvs47 haplotypes with specific vectors remains to be determined. Thus, it is unclear if polymorphisms in Pvs47 can be used to determine the risk of P. vivax importation and transmission.
Other genotyping assays have been used to determine the geographic origin of P. falciparum, but they typically require analyzing a large number of SNPs21–23. For example, an assay that identifies the continent of origin of malaria cases with 92% accuracy uses 23 mitochondrial and apicoplast SNPs23. However, genotyping only 2 SNPs using the Pfs47 HRM assays is a much simpler strategy. In addition, previous SNP-based assays to determine the geographic origin of P. falciparum are limited in that their markers were not biologically linked to transmission risk. The relatively simple and low-cost HRM Pfs47 genotyping assays developed here, provide a practical epidemiological tool to establish the putative geographic origin of imported P. falciparum malaria cases and to assess transmission risk in a particular region.
Methods
Pfs47 sequences and statistical analysis
Pfs47 gene sequences were obtained from the literature7,24 and recovered from P. falciparum samples in the publicly available databases of the Malaria Genomic Epidemiology Network25 (MalariaGEN). A total of 4,971 Pfs47 haplotype sequences were used, originating from Africa (3,126), New World (121), Asia (1,675), and Papua New Guinea (PNG, 49). The New World was the continent least represented in the analysis of Pfs47 sequences. This publication uses data from the MalariaGEN P. falciparum Community Project, PfCP (www.malariagen.net/projects/p-falciparum-community-project) and the Pf3K project (2016) pilot data release 5 (www.malariagen.net/data/pf3k-5). Genome sequencing was performed by the Wellcome Trust Sanger Institute and the Community Projects is coordinated by the MalariaGEN Resource Centre with funding from the Wellcome Trust (098051, 090770). The Pfs47 sequences from Pf3K polygenomic samples were obtained after deconvolution using DEploid as described26. In the case of the Pfs47 sequences in samples from other sources, only the major allele was analyzed as described24. Single nucleotide polymorphisms (SNPs) in the recovered Pfs47 sequences were analyzed using dnaSP version 627 according to their geographic allele occurrence. The most frequent African allele (GB4) was used as a reference sequence.
Plasmodium falciparum samples
Laboratory maintained P. falciparum lines from Africa (NF54, GB4), New World (7G8, HB3), and Asia (Thai 17, P95-15) were used. P. falciparum-infected dried blood spots (13-mm diameter) were collected in 2013 with informed consent from subjects (n = 20) enrolled in a cohort study in Kalifabougou, Mali, who presented with acute febrile malaria. The cohort has been described in detail previously17. Blood spots were collected from finger prick samples onto Whatman® protein saver cards, dried, and stored at room temperature in individual sealed bags until their use. From the same finger prick blood sample, thick blood smears were stained with Giemsa and counted against 300 leukocytes, and P. falciparum densities were recorded as the number of asexual parasites per µL of whole blood based on a mean leukocyte count of 7500 cells per µl. Each smear was evaluated separately by at least 2 expert microscopists.
DNA extractions
DNA was extracted from P. falciparum cultures using the QIAamp® DNA Mini Kit (Qiagen). Briefly, saponin pellets from P. falciparum cultures were used to extract DNA. In the case of the dried blood spots, one-third of a 13-mm spot was used for DNA extraction. During optimization of the HRM, it was determined that the elution of DNA should be done in AE buffer (Qiagen) diluted 1 to 10 in nuclease-free water, since higher concentrations of the AE buffer affected the reproducibility of the HRM assay. The DNA samples used for geographic origin standards in the HRM assay were extracted from dried blood spots, using the same filter paper as the field-collected samples. Once all DNA had been extracted from standards, each was brought to a concentration of 2 ng/µl in 1:10 Buffer AE. Ten-fold serial dilutions were done in 1:10 Buffer AE, with final concentrations at 2, 0.2, and 0.02 ng/µl.
SNP assay primer design, qPCR, and HRM assay
Several sets of primers were designed due to the highly polymorphic nature of Pfs47 Domain 2, where both SNPs 707 and 725 are located (Fig. 2a). These primers were tested with all samples (GB4, NF54, 7G8, HB3, Thai17, P95-15, and Malian Kali), and ultimately primers were chosen based on their efficiency and reproducibility with all samples, as reflected by the lowest Ct values, and single peak in the Melting Curves and accurate HRM analysis. The primers used for SNP 707 bp were Pfs47_SNP707_F: GAAGAAACTATTGTAGAATCTGGAAA, Pfs47_SNP707_R: CCACATTATTATTTGATAATAGATTTA, and the primers for SNP 725 bp were Pfs47SNP725_F: GAAGAAATATTAAATATTAAAATAAATCTA, and Pfs47SNP725_R: AAGGCATTTTTATAACCACATTATTA (Fig. 2b). SNP 707 and 725 bp reactions were run separately. Primers were diluted first in TE to 100 μM, and then in nuclease-free water to 8 µM. Each reaction was 20 µL total volume: 10 µL of Precision Melt Supermix (Bio-Rad), 4 µl nuclease-free water, 1 µL of each 8 µM primer (forward and reverse), and 4 µL of template DNA. qPCR Conditions were (1) 95 °C for 5 min, (2) 95 °C for 10 s, (3) 50 °C for 30 s, (4) 60 °C for 30 s + plate read, (5) go back to step 2 and repeat 39 more times, (6) 95 °C for 30 s, (7) 55 °C for 1 min, (8) Melt Curve 60–90 °C at 0.2 °C increments for 10 s including a plate read. All samples were run in duplicates. Analysis of the HRM assay was done using Precision Melt Analysis Software version 1.3 (Bio-Rad).
Pfs47 SNP 707 and 725 HRM detection limit
The detection limit was defined as the lowest parasitemia in the blood samples that would generate reproducible HRM signal. According to the HRM reagent kit used (Bio-Rad), reliable results are obtained with samples that reach the signal threshold before 30 PCR cycles (30Ct).
Statistics and reproducibility
The reproducibility of each HRM genotype was assessed running each sample at least in duplicate. The reproducibility of HRM genotypes corresponding to a given geographical area was assessed by running at least two biological samples from a given region.
Ethics statement
The ethics committee of the Faculty of Medicine, Pharmacy and Dentistry at the University of Sciences, Techniques and Technology of Bamako, and the Institutional Review Board of NIAID, NIH approved the Mali study (NIH protocol number 11-I-N126; ClinicalTrials.gov NCT01322581). Written, informed consent was obtained from the parents or guardians of participating children or from adult participants.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We would like to thank Jacob Almagro for his help with Pfs47 sequence data gathering and deconvolution. Jianbing Mu generously provided P. falciparum DNA of Asian strains. This work was supported by the Intramural Research Program of the Division of Intramural Research, NIAID/NIH Z01AI000947. The cohort study in Mali was also supported by the Intramural Research Program of the Division of Intramural Research, NIAID/NIH.
Author contributions
A.M.-C. and C.B.-M. designed research; P.D.C. and B.T. designed field research in Mali, Africa; A.M.-C., N.R., R.W., and A.D. performed research; A.M.-C., A.D., G.C., and J.C.S. analyzed data; and A.M.-C. and C.B.-M. wrote the paper.
Data availability
Pfs47 gene sequences were retrieved from the literature7,24 and from the publicly available databases of the Malaria Genomic Epidemiology Network25 (MalariaGEN) P. falciparum Community Project, PfCP (www.malariagen.net/projects/p-falciparum-community-project), and the Pf3K project (2016) pilot data release 5 (www.malariagen.net/data/pf3k-5). Sample ID, geographic origin, source, study, and accession numbers are indicated in Supplementary Data 3.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationCommunications Biology thanks Rachel Daniels and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Caitlin Karniski.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alvaro Molina-Cruz, Email: amolina-cruz@niaid.nih.gov.
Carolina Barillas-Mury, Email: cbarillas@niaid.nih.gov.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02667-0.
References
- 1.WHO. World Malaria Report 2020 (World Health Organization, Geneva, 2020).
- 2.Yu T, et al. Epidemiological characteristics of imported malaria in Shandong Province, China, from 2012 to 2017. Sci. Rep. 2020;10:7568. doi: 10.1038/s41598-020-64593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinka ME, et al. A global map of dominant malaria vectors. Parasit. Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatem AJ, et al. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect. Dis. 2017;17:98–107. doi: 10.1016/S1473-3099(16)30326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loy DE, et al. Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. Int. J. Parasitol. 2017;47:87–97. doi: 10.1016/j.ijpara.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina-Cruz A, Zilversmit MM, Neafsey DE, Hartl DL, Barillas-Mury C. Mosquito Vectors and the Globalization of Plasmodium falciparum Malaria. Annu. Rev. Genet. 2016;50:447–465. doi: 10.1146/annurev-genet-120215-035211. [DOI] [PubMed] [Google Scholar]
- 7.Molina-Cruz A, et al. Plasmodium evasion of mosquito immunity and global malaria transmission: the lock-and-key theory. Proc. Natl Acad. Sci. USA. 2015;112:15178–15183. doi: 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina-Cruz A, et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthony TG, Polley SD, Vogler AP, Conway DJ. Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Mol. Biochem. Parasitol. 2007;156:117–123. doi: 10.1016/j.molbiopara.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Manske M, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina-Cruz A, et al. Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc. Natl Acad. Sci. USA. 2020;117:2597–2605. doi: 10.1073/pnas.1917042117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels R, et al. Rapid, field-deployable method for genotyping and discovery of single-nucleotide polymorphisms associated with drug resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 2012;56:2976–2986. doi: 10.1128/AAC.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slomka, M., Sobalska-Kwapis, M., Wachulec, M., Bartosz, G. & Strapagiel, D. High resolution melting (HRM) for high-throughput genotyping—limitations and caveats in practical case studies. Int. J. Mol. Sci.18, 2316 (2017). [DOI] [PMC free article] [PubMed]
- 14.Bankole BE, et al. Characterization of Plasmodium falciparum structure in Nigeria with malaria SNPs barcode. Malar. J. 2018;17:472. doi: 10.1186/s12936-018-2623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murillo E, Muskus C, Agudelo LA, Vélez ID, Ruiz-Lopez F. A new high-resolution melting analysis for the detection and identification of Plasmodium in human and Anopheles vectors of malaria. Sci. Rep. 2019;9:1674. doi: 10.1038/s41598-018-36515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammad Rahimi H, Pourhosseingholi MA, Yadegar A, Mirjalali H, Zali MR. High-resolution melt curve analysis: a real-time based multipurpose approach for diagnosis and epidemiological investigations of parasitic infections. Comp. Immunol., Microbiol. Infect. Dis. 2019;67:101364. doi: 10.1016/j.cimid.2019.101364. [DOI] [PubMed] [Google Scholar]
- 17.Tran TM, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin. Infect. Dis. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canepa GE, Molina-Cruz A, Barillas-Mury C. Molecular analysis of Pfs47-mediated plasmodium evasion of mosquito immunity. PLoS ONE. 2016;11:e0168279. doi: 10.1371/journal.pone.0168279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium P47: a key gene for malaria transmission by mosquito vectors. Curr. Opin. Microbiol. 2017;40:168–174. doi: 10.1016/j.mib.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hupalo DN, et al. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat. Genet. 2016;48:953–958. doi: 10.1038/ng.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels R, et al. A general SNP-based molecular barcode for Plasmodium falciparum identification and tracking. Malar. J. 2008;7:223. doi: 10.1186/1475-2875-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campino S, et al. Population genetic analysis of Plasmodium falciparum parasites using a customized Illumina GoldenGate genotyping assay. PLoS ONE. 2011;6:e20251. doi: 10.1371/journal.pone.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston MD, et al. A barcode of organellar genome polymorphisms identifies the geographic origin of Plasmodium falciparum strains. Nat. Commun. 2014;5:4052. doi: 10.1038/ncomms5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser KA, et al. Strains used in whole organism Plasmodium falciparum vaccine trials differ in genome structure, sequence, and immunogenic potential. Genome Med. 2020;12:6. doi: 10.1186/s13073-019-0708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Network TME. A global network for investigating the genomic epidemiology of malaria. Nature. 2008;456:732–737. doi: 10.1038/nature07632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu SJ, Almagro-Garcia J, McVean G. Deconvolution of multiple infections in Plasmodium falciparum from high throughput sequencing data. Bioinformatics. 2017;34:9–15. doi: 10.1093/bioinformatics/btx530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Pfs47 gene sequences were retrieved from the literature7,24 and from the publicly available databases of the Malaria Genomic Epidemiology Network25 (MalariaGEN) P. falciparum Community Project, PfCP (www.malariagen.net/projects/p-falciparum-community-project), and the Pf3K project (2016) pilot data release 5 (www.malariagen.net/data/pf3k-5). Sample ID, geographic origin, source, study, and accession numbers are indicated in Supplementary Data 3.