Abstract
Vitamin K refers to a group of structurally similar vitamins that are essential for proper blood coagulation, as well as bone and cardiovascular health. Previous studies have indicated that vitamin K may also have anti-inflammatory properties, although the underlying mechanisms of its anti-inflammatory effects remain unclear. The NLRP3 inflammasome is a multiprotein complex, and its activation leads to IL-1β and IL-18 secretion and contributes to the pathogenesis of various human inflammatory diseases. Here, we show that synthetic vitamins K3 and K4 are selective, potent inhibitors of the NLRP3 inflammasome and specifically block the interaction between NLRP3 and ASC, thereby inhibiting NLRP3 inflammasome assembly. Moreover, we show that treatment with vitamin K3 or K4 attenuates the severity of inflammation in a mouse model of peritonitis. Our results demonstrate that vitamins K3 and K4 exert their anti-inflammatory effects by inhibiting NLRP3 inflammasome activation and indicate that vitamin K supplementation may be a treatment option for NLRP3-associated inflammatory diseases.
Keywords: Synthetic vitamin K, Anti-inflammation, NLRP3 inflammasome
Subject terms: Inflammation, Immunological disorders
Introduction
Vitamin K refers to a group of vitamins that share a similar structure, with a 2-methyl-1,4 naphthoquinone ring and a variable aliphatic chain.1 There are several forms of vitamin K, such as vitamins K1, K2, K3, and K4. Fat-soluble vitamin K1 (also known as phylloquinone) and vitamin K2 (also known as menaquinone) are the two main types of natural vitamin K. Vitamin K1 is synthesized by plants and is enriched in leafy green vegetables, while vitamin K2 is primarily produced by gut bacteria and can also be found in meats and fermented foods.2 Originally, vitamin K3 (also known as menadione) was considered to be a synthetic water-soluble form of vitamin K. Research that began in the 1990s demonstrated that vitamin K3 was a catabolic product of oral vitamin K1 produced in the intestine and could be delivered to tissues and subsequently converted to vitamin K2.3–5 Vitamin K4 (also known as menadiol) is a synthetic water-soluble derivative that can be converted to vitamin K3 in the body.6 Vitamin K exerts its well-known beneficial effects by acting as the coenzyme for γ-glutamate carboxylase (GGCX), which promotes the posttranslational γ-glutamyl carboxylation of various vitamin K-dependent proteins.1 Several vitamin K-dependent proteins, such as prothrombin, factor VII and protein C, play crucial roles in normal blood clotting through either procoagulant or anticoagulant mechanisms. In addition, certain vitamin K-dependent proteins, such as matrix Gla protein (MGP) and osteocalcin (OC), regulate bone metabolism and are important for maintaining bone health.7 Several studies have shown that vitamin K deficiency, in addition to being associated with coagulopathy and osteoporosis, is associated with multiple chronic inflammatory diseases, such as type 2 diabetes (T2D), inflammatory bowel disease and chronic kidney disease.8–10 Moreover, it has also been shown that vitamin K supplementation can reduce lipopolysaccharide (LPS)-induced inflammation both in vitro and in vivo.11,12 Although several studies have demonstrated the anti-inflammatory effect of vitamin K, the mechanism is still not clear.
NLR family pyrin domain containing 3 (NLRP3) is an intracellular pattern-recognition receptor (PRR) that can recruit the adaptor protein ASC and the cysteine protease caspase-1 to form a complex termed the NLRP3 inflammasome. A two-signal model has been generally accepted to explain the priming and activation of the NLRP3 inflammasome. Multiple microbial molecules (e.g., LPS) can act as signal 1 (also known as the priming signal) to promote the expression of NLRP3 and pro-IL-1β through the activation of the TLR-dependent NF-κB pathway.13 Numerous pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated molecular patterns (DAMPs), such as bacterial pore-forming toxins, microbial RNA, ATP, monosodium urate (MSU), and cholesterol crystals, can act as signal 2 (also known as the activation signal) to induce the assembly and activation of the NLRP3 inflammasome.13 The assembly of the inflammasome leads to the activation of caspase-1, which promotes the secretion of the proinflammatory cytokines IL-1β and IL-18 and induces a specific type of inflammatory cell death termed pyroptosis.14 Aberrant NLRP3 inflammasome activation has been implicated in the pathogenesis of various human inflammatory diseases, such as type 2 diabetes, atherosclerosis, Alzheimer’s disease, and gout.15 In addition, gain-of-function mutations in the NLRP3 gene cause cryopyrin-associated autoinflammatory syndromes (CAPS), which are a group of rare inherited autoinflammatory diseases.16 In the past, several NLRP3 inflammasome inhibitors, including synthetic small-molecule compounds, clinical drugs, and traditional Chinese medicine ingredients, have been reported to inhibit the activation of the NLRP3 inflammasome and exert beneficial effects in NLRP3-related diseases in animal models.17–19 However, no NLRP3-targeting inhibitors have been used for the clinical treatment of NLRP3-related diseases in humans.
In this study, we aimed to determine whether different types of vitamin K can exert their anti-inflammatory effects by inhibiting the activation of the NLRP3 inflammasome. Our data showed that pretreatment of LPS-primed mouse bone marrow-derived macrophages (BMDMs) with vitamins K3 and K4 but K1 and K2 could indeed inhibit multiple agonist-induced NLRP3 inflammasome activation. Vitamins K3 and K4 prevented NLRP3 inflammasome assembly by blocking the interaction between NLRP3 and ASC. Moreover, vitamin K3 treatment inhibited NLRP3-dependent IL-1β secretion and neutrophil recruitment in a mouse model of peritonitis. Our results demonstrate that synthetic vitamin K3 or K4 can inhibit the NLRP3 inflammasome both in vitro and in vivo, indicating that vitamin K supplementation may be a potential strategy for the treatment of NLRP3-associated inflammatory diseases in humans.
Materials and methods
Mice
Specific pathogen-free (SPF) C57BL/6J mice were obtained and maintained in the Model Animal Research Center of Nanjing University before being transferred to the Laboratory Animal Center of the University of Science and Technology of China. All animal procedures were approved by the Ethics Committee of the University of Science and Technology of China.
Reagents
Vitamin K3 (S1949) was supplied by Selleck. Ultrapure LPS, MitoSOX, MitoTracker, and Pam3CSK4 were supplied by Invitrogen. Nigericin, monosodium urate crystal, ATP, poly(dA:dT), vitamin K1 (95271), vitamin K2 (V9378), menadione (vitamin K3) sodium bisulfite (M5750), and vitamin K4 (SMB00580) were supplied by Sigma-Aldrich. Anti-DYKDDDDK-Tag mouse mAbs (agarose-conjugated) and β-actin pAbs (P30002) were supplied by Abmart. Protein G agarose (Fast Flow) was supplied by Millipore. Anti-IL-1β (mouse) (AF-401-NA) antibodies were supplied by R&D Systems. Caspase-1 (p20) (mouse) (AG-20B-0042) and NLRP3 (mouse or human) antibodies (AG-20B-0014) were supplied by Adipogen. ASC (N-15) (SC-22514-R) and Nek7 antibodies (N-20) (SC-50756) were supplied by Santa Cruz. Monoclonal FLAG(R) (F2555) and VSV-G antibodies (V4888) were supplied by Sigma.
Cell culture and stimulation
Bone marrow-derived macrophages (BMDMs) were derived from precursors, which were collected from C57BL/6 mouse bone marrow and cultured for 4 days in 10% L929 supernatant in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. HEK-293T and L929 cells, originally obtained from ATCC, were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. BMDMs were stimulated to induce inflammasome activation as previously described.20 In brief, 5 × 105/ml BMDMs were seeded in 12-well plates. After 8–12 h, the medium was replaced with Opti-MEM supplemented with 50 ng/ml LPS for 3 h. Different doses of vitamin K (1–5 μM) or DMSO (1:1000) were then added to the BMDMs for 30 min before the cells were stimulated with different inflammasome activators. To stimulate the NLRP3 or NLRC4 inflammasome, BMDMs were stimulated with 150 μg/ml MSU (4 h), 2.5 mM ATP (30 min), 3 μM nigericin (30 min), or S. typhimurium (multiplicity of infection (MOI): 10) (4 h). To stimulate the AIM2 inflammasome, BMDMs were transfected with 0.5 μg/ml poly(dA:dT) (4 h) using Lipofectamine 2000 (Invitrogen). To stimulate the pyrin inflammasome, 0.5 μg/ml purified recombinant TcdB was added to the cell culture medium for 2–3 h. To induce activation of the noncanonical NLRP3 inflammasome, BMDMs were stimulated as previously described.21 In brief, BMDMs were primed with Opti-MEM containing 400 ng/ml Pam3CSK4 for 3 h. Then, the cells were transfected with 500 ng/ml LPS (4 h) using Lipofectamine 2000 (Invitrogen). Supernatants and cell lysates were collected for ELISA and immunoblot analyses.
ELISA
Mouse IL-1β (DY401) and mouse TNF-α (DY410) were supplied by R&D systems and used to measure the IL-1β and TNF-α concentrations in cell culture supernatants or mouse peritoneal fluids according to the manufacturer’s instructions.
Confocal microscopy
BMDMs (2 × 105/ml) were seeded in 12-well plates containing clean coverslips for 8–12 h, and subsequently, the medium was replaced with Opti-MEM supplement with 50 ng/ml LPS for 3 h. The cells were then treated with DMSO (1:1000) or different doses of vitamin K for 30 min. After that, nigericin (3 μM) and MitoTracker Red (50 nM) or MitoSOX (5 μM) were added into the medium for 30 min. The medium was then removed, and ice-cold PBS was used to wash the cells three times. The cells were fixed with 4% PFA in PBS for 15 min and subsequently washed with PBST three times. A Zeiss LSM 700 confocal microscope was used to scan and record the stained cells.
Intracellular potassium measurement
To detect the concentration of intracellular potassium, BMDMs were stimulated as described above. Then, the medium was removed, and the cells were washed three times using ice-cold potassium-free PBS and lysed with 3% ultrapure HNO3. The cell lysates were then transferred to a canning jar and boiled for 30 min at 100 °C. The precipitate was collected in 5 ml of ultrapure water. Inductively coupled plasma optical emission spectrometry with a PerkinElmer Optima 7300 DV spectrometer was used to measure the potassium concentration.
ASC oligomerization assay
BMDMs were stimulated as described above. Then, the supernatants were collected for immunoblot analyses, and the cells were washed with ice-cold PBS and lysed with NP-40 at 4 °C for 30 min. The lysates were centrifuged at 330 g for 10 min at 4 °C to obtain pellets. After being washed with 1 ml of ice-cold PBS, the pellets were resuspended in 500 μl of ice-cold PBS. Two millimolar disuccinimidyl suberate (DSS) was added to the resuspension solution, which was then incubated at room temperature with rotation for 30 min. The mixture was then centrifuged at 4 °C and 330 g for 10 min. Sample buffer (30 μl) was used to lyse the cross-linked pellets, and immunoblotting was performed.
Immunoprecipitation
For the endogenous interaction assay, BMDMs were stimulated as described above. Then, the cells were lysed with NP-40 lysis buffer supplemented with a protease inhibitor. The cell lysates were coincubated with the primary antibodies and Protein G Mag Sepharose (GE Healthcare) for 6 h at 4 °C. For the exogenous interaction assay, HEK-293T cells were transfected with plasmids via polyethylenimine in six-well plates and cultured for 24 h. Then, 293 T cells were lysed with NP-40 lysis buffer supplemented with a protease inhibitor. The cell lysates were coincubated with the anti-DYKDDDDK-Tag mouse mAb (agarose conjugated) for 6 h at 4 °C. Immunoblotting was then used to analyze the mixture.
Quantitative real-time PCR
Total RNA was collected from BMDMs by extraction with TRIzol reagent (Takara). RNA (800 ng) from each sample was used for reverse transcription with Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Quantitative PCR was performed using SYBR Green premix (Takara Bio) with a Roche LightCycler 96. Gapdh was used as a reference gene. The sequences of the gene-specific primers used were as follows: mouse Ggcx forward, GTTGCTCCCGCCTCAGATAAA; mouse Ggcx reverse, TAAGCAGGGTCACGACACTCT; mouse Gapdh forward, GGTGAAGGTCGGTGTGAACG; and mouse Gapdh reverse, CTCGCTCCTGGAAGATGGTG.
MSU-induced peritonitis
PBS (0.5 ml) containing 1.5 mg of MSU crystals was injected into the peritoneal cavity of 8- to 10-week-old C57BL/6 mice after pretreatment with 4 mg/kg menadione sodium bisulfite (dissolved in ultrapure water) nu injection for 30 min. After 6 h, the mice were killed, and the peritoneal cavities were washed with 10 ml of ice-cold PBS. ELISA was performed to measure the amount of IL-1β in the peritoneal lavage fluid. Flow cytometry was used to determine the number of neutrophils (labeled with Ly6G and CD11b) in mouse peritoneal fluids.
Statistical analyses
All data are expressed as the mean ± SEM. The unpaired t-test (two-tailed) was used to analyze statistical significance. P values < 0.05 were deemed significant.
Results
Water-soluble vitamin K inhibits NLRP3 inflammasome activation
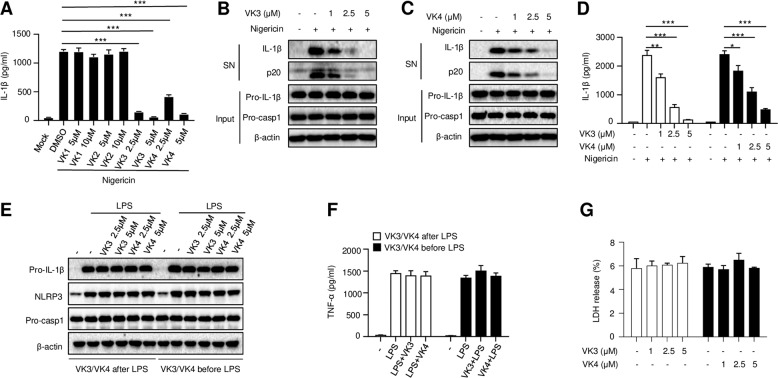
To assess whether vitamin K can prevent NLRP3 inflammasome activation, lipopolysaccharide (LPS)-primed mouse bone marrow-derived macrophages (BMDMs) were pretreated with different types of vitamin K for 30 min before stimulation with the NLRP3 activator nigericin. We observed that two water-soluble vitamins, vitamins K3 and K4, dramatically reduced the amount of bioactive IL-1β in the supernatant, while the fat-soluble vitamins K1 and K2 had no effects on IL-1β secretion (Fig. 1a). Further studies confirmed that vitamins K3 and K4 inhibited nigericin-induced caspase-1 cleavage (p20) and IL-1β secretion in a dose-dependent manner (Fig. 1b–d). We then examined whether vitamins K3 and K4 affect LPS-induced priming of inflammasome activation. As shown in Fig. 1e, f, treatment with vitamin K3 or K4 before or after LPS stimulation had no effect on LPS-induced NLRP3 or pro-IL-1β expression or tumor necrosis factor-a (TNF-α) production. These results suggest that the water-soluble vitamins K3 and K4 inhibit nigericin-induced NLRP3 activation but have no effect on LPS-induced priming of NLRP3 activation. To determine whether vitamins K3 and K4 affect the viability of BMDMs, the release of LDH in the supernatant after treatment with vitamin K3 or K4 for 12 h was measured. As shown in Fig. 1g, vitamin K3 or K4 treatment did not induce LDH release, indicating that the concentration of vitamin K used in this study had no effect on the viability of BMDMs.
Fig. 1.
Vitamins K3 and K4 inhibit nigericin-induced NLRP3 inflammasome activation. a–d BMDMs were primed with LPS and then pretreated with or without different doses of various forms of vitamin K before stimulation with nigericin. (a) ELISA analysis of mature IL-1β in the culture supernatant of BMDMs. b, c Western blot analysis of cleaved caspase-1 and mature IL-1β in the culture supernatant (SN), as well as pro-IL-1β, pro-caspase-1 and β-actin levels in the cell lysates (Input). d ELISA analysis of mature IL-1β in the culture supernatant of BMDMs. e, f BMDMs were primed with LPS before or after treatment with different doses of vitamin K3 or K4. Western blot analysis of pro-IL-1β, pro-caspase-1, NLRP3 and β-actin levels in the cell lysates. e ELISA analysis of TNF-α in the culture supernatant of BMDMs (f). LDH release in the culture supernatant of BMDMs after vitamin K3 or K4 treatment (g). All data are from no fewer than three independent experiments. The data shown in a, d, and f are the mean ± SEM, n = 6. Unpaired t-tests (two-tailed) were applied to analyze statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001
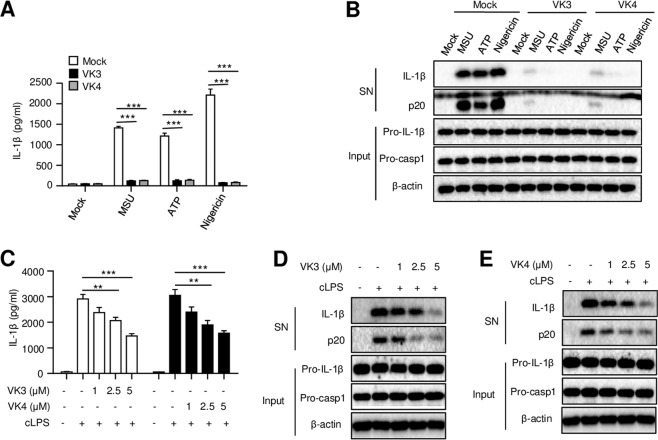
To determine whether vitamin K only prevents nigericin-induced NLRP3 activation, the effects of vitamins K3 and K4 on NLRP3 activation induced by other NLRP3 stimuli were also examined. We found that pretreatment with vitamin K3 or K4, similar to the effects on nigericin-induced changes, also inhibited the cleavage of caspase-1 and the maturation of IL-1β triggered by MSU or ATP (Fig. 2a, b). These results suggest that vitamins K3 and K4 exert robust inhibitory effects on the activation of the NLRP3 inflammasome in response to multiple agonists. In addition to facilitating the priming of the canonical NLRP3 inflammasome through TLR4 signaling, intracellular LPS produced by gram-negative bacteria can also be sensed by caspase-11 and induce caspase-11-dependent noncanonical NLRP3 inflammasome activation.22 To ascertain whether vitamin K also controls activation of the noncanonical NLRP3 inflammasome, we pretreated BMDMs with vitamin K3 or K4 before intracellular LPS transfection. Our results showed that intracellular LPS-induced IL-1β secretion and caspase-1 cleavage were also inhibited by vitamins K3 and K4 (Fig. 2c–e). Taken together, these results suggest that vitamins K3 and K4 can inhibit both canonical and noncanonical NLRP3 inflammasome activation.
Fig. 2.
Vitamins K3 and K4 inhibit both canonical and noncanonical NLRP3 inflammasome activation. a, b BMDMs were primed with LPS and then pretreated with or without vitamin K3 or K4 (5 μM) before stimulation with MSU, ATP, or nigericin. a ELISA analysis of mature IL-1β in the culture supernatant of BMDMs. b Western blot analysis of cleaved caspase-1 and mature IL-1β in the culture supernatant (SN), as well as pro-IL-1β, pro-caspase-1, and β-actin levels in the cell lysates (Input). c–e BMDMs were primed with Pam3CSK4 and then pretreated with or without different doses of vitamin K3 or K4 before intracellular LPS transfection. (c) ELISA analysis of mature IL-1β in the culture supernatant of BMDMs. d, e Western blot analysis of cleaved caspase-1 and mature IL-1β in the culture supernatant (SN), as well as pro-IL-1β, pro-caspase-1, and β-actin levels in the cell lysates (Input). All data are from no fewer than three independent experiments. The data shown in A and C are the mean ± SEM, n = 6. Unpaired t-tests (two-tailed) were applied to analyze statistical significance: **P < 0.01, ***P < 0.001
Vitamins K3 and K4 have no effects on the activation of the AIM2, NLRC4, or Pyrin inflammasomes
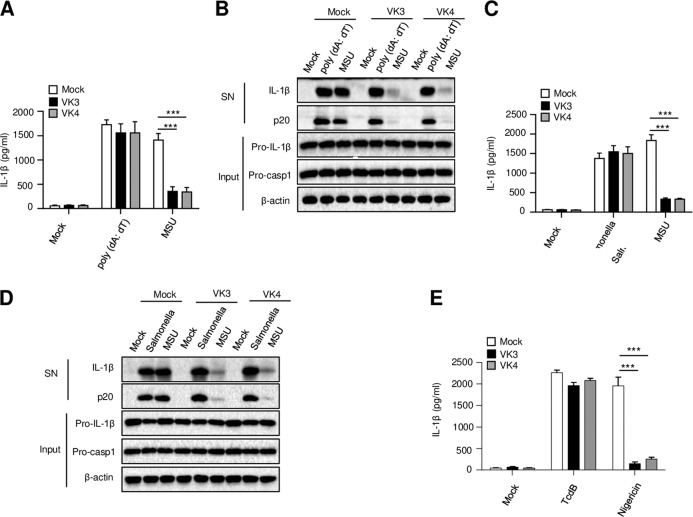
In addition to NLRP3, other PRRs can also induce the assembly of inflammasomes. The AIM2, NLRC4, and Pyrin inflammasomes are three well-known inflammasomes, and the activation of these inflammasomes plays an important role in host defense against infection.14,23,24 To explore whether vitamin K can also inhibit AIM2 and NLRC4 inflammasome activation, the AIM2 or NLRC4 inflammasomes were activated by transfection with the dsDNA analog poly(dA:dT) or infection with S. Typhimurium, respectively. We found that the maturation of IL-1β and the cleavage of caspase-1 triggered by AIM2 and NLRC4 activation were unaffected by pretreatment with vitamin K3 or K4 (Fig. 3a–d). In addition, vitamin K3 or K4 pretreatment also had no effect on TcdB toxin-induced Pyrin inflammasome activation and IL-1β secretion (Fig. 3e). Thus, these results indicate that vitamins K3 and K4 can inhibit the NLRP3 inflammasome but have no effect on the activation of the AIM2, NLRC4, and Pyrin inflammasomes.
Fig. 3.
Vitamins K3 and K4 have no effect on the activation of other inflammasomes. BMDMs were primed with LPS and then pretreated with or without vitamin K3 or K4 (5 μM) before MSU, nigericin, or TcdB stimulation, poly(dA:dT) transfection or S. typhimurium infection. a, c, e ELISA analysis of mature IL-1β in the culture supernatant of BMDMs. b, d Western blot analysis of cleaved caspase-1 and mature IL-1β in the culture supernatant (SN), as well as pro-IL-1β, pro-caspase-1 and β-actin levels in the cell lysates of BMDMs (Input). All data are from no fewer than three independent experiments. The data shown in A and C are the mean ± SEM, n = 6. Unpaired t-tests (two-tailed) were applied to analyze statistical significance: ***P < 0.001
Vitamins K3 and K4 act downstream of K+ efflux and mitochondrial dysfunction to inhibit the NLRP3 inflammasome independent their coenzyme activities
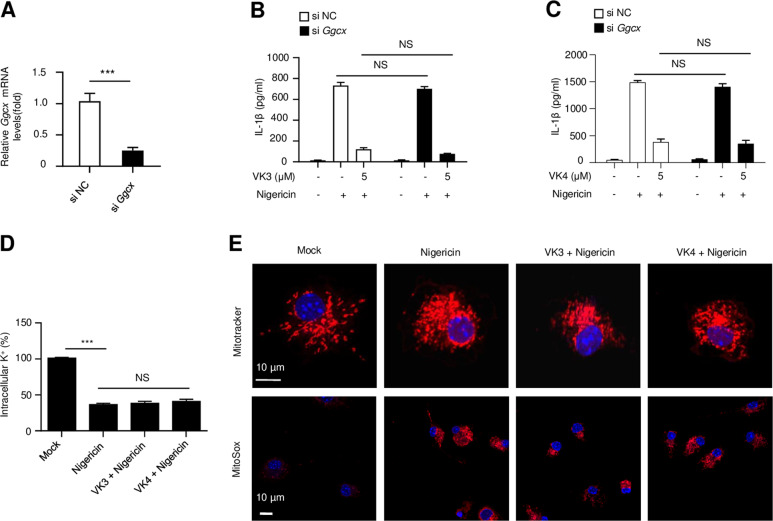
Next, we studied the mechanism by which vitamins K3 and K4 block NLRP3 inflammasome activation. As described above, vitamin K exerts its well-known beneficial effects by acting as the coenzyme for GGCX, which promotes posttranslational γ-glutamyl carboxylation of various vitamin K-dependent proteins.1 To examine the role of GGCX in vitamin K3-induced inhibition of NLRP3 inflammasome activation, GGCX expression in BMDMs was silenced by siRNA (Fig. 4a). Our results showed that GGCX knockdown had no effect on either nigericin-induced NLRP3 activation or vitamin K3/K4-induced inhibition of NLRP3 inflammasome activation (Fig. 4b, c). These results indicate that the GGCX-dependent γ-glutamyl carboxylation of vitamin K-dependent proteins is not required for NLRP3 activation and that vitamin K inhibits the NLRP3 inflammasome independent of its coenzyme activity.
Fig. 4.
Vitamins K3 and K4 act downstream of K+ efflux and mitochondrial dysfunction to inhibit the NLRP3 inflammasome independent of their coenzyme activities. a Ggcx mRNA in BMDMs transfected with control siRNA or Ggcx-specific siRNA. b–e BMDMs were primed with LPS and then pretreated with or without vitamin K3 or K4 (5 μM) before stimulation with nigericin. b, c ELISA analysis of mature IL-1β in the culture supernatant of BMDMs transfected with control siRNA or Ggcx-specific siRNA. d Potassium efflux in BMDMs was quantified by inductively coupled plasma optical emission spectrometry (n = 4, mean ± SEM). e Confocal microscopy analysis of BMDMs stained with MitoTracker Red or MitoSOX. DAPI was used to stain the nuclei. Scale bars, 10 µm. All data are from no fewer than three independent experiments. The data shown in A are the mean ± SEM, n = 4. Unpaired t-tests (two-tailed) were applied to analyze statistical significance: ***P < 0.001. ns not significant
Potassium (K+) efflux, which is considered an upstream signaling event that promotes NLRP3 inflammasome activation, is often detected during NLRP3 inflammasome activation in response to different stimuli.13 To examine whether intracellular potassium efflux can be affected by vitamin K3 or K4, we investigated the intracellular potassium concentration in BMDMs that were pretreated with or without vitamin K before nigericin stimulation. We found that the level of intracellular potassium was dramatically reduced by nigericin stimulation and that treatment with vitamin K3 or K4 did not mitigate the nigericin-induced intracellular potassium reduction (Fig. 4d). Mitochondrial dysfunction, as indicated by mitochondrial fission, clustering, and ROS production, is also considered another important upstream signaling pathway of NLRP3 inflammasome activation.13,25 Vitamin K3 also had no effect on nigericin-induced mitochondrial fission, clustering, or ROS production (Fig. 4e). These results suggest that vitamin K acts downstream of potassium efflux and mitochondrial dysfunction to inhibit NLRP3 inflammasome assembly and activation.
Vitamins K3 and K4 inhibit NLRP3 inflammasome assembly by preventing the NLRP3-ASC interaction
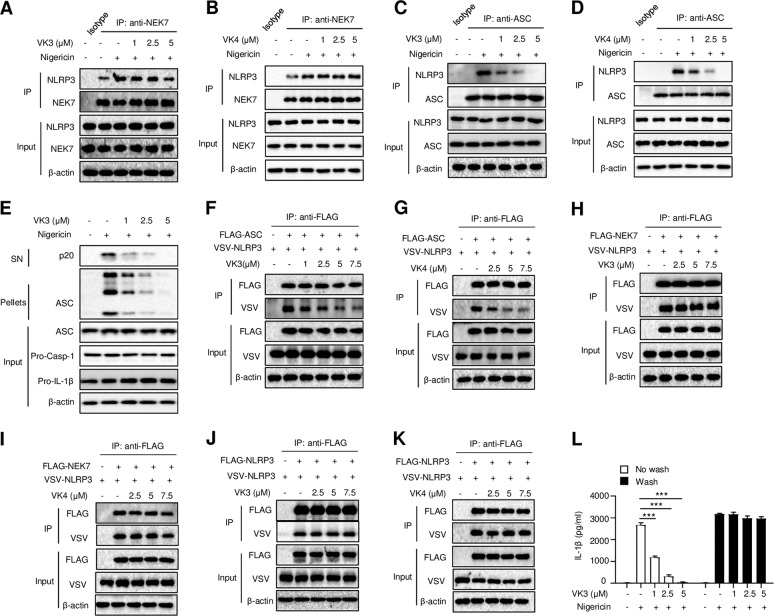
We next investigated how vitamin K inhibits NLRP3 inflammasome activation downstream of potassium efflux and mitochondrial dysfunction. NEK7 has been suggested to act downstream of potassium efflux to promote NLRP3 oligomerization and inflammasome assembly through its interaction with NLRP3.26,27 Indeed, we found that nigericin induced the endogenous NEK7-NLRP3 interaction (Fig. 5a). However, the nigericin-induced NEK7-NLRP3 interaction was not suppressed by treatment with vitamin K3 or K4 (Fig. 5a, b), indicating that vitamin K acts downstream of the NEK7-NLRP3 interaction to inhibit NLRP3 inflammasome activation. Another critical step in NLRP3 inflammasome assembly is the recruitment of ASC to NLRP3, which is crucial for ASC oligomerization and subsequent caspase-1 activation.28 We next determined whether vitamin K3 or K4 could prevent the interaction between NLRP3 and ASC. As shown in Fig. 5c, d, the nigericin-induced endogenous NLRP3-ASC interaction was dramatically inhibited by vitamin K3 or K4. Moreover, nigericin-induced ASC oligomerization was suppressed by vitamin K3 in a dose-dependent manner (Fig. 5e). These results indicate that vitamins K3 and K4 block NLRP3-ASC complex formation and subsequent ASC oligomerization to inhibit NLRP3 inflammasome activation. Additionally, these results were confirmed by the overexpression of NLRP3, NEK7, or ASC in 293 T cells. The direct interaction between NLRP3 and ASC was inhibited by treatment with vitamin K3 or K4 (Fig. 5f, g), while neither the NEK7-NLRP3 nor NLRP3-NLRP3 interaction was impacted by treatment with vitamin K3 or K4 (Fig. 5h–k). To further investigate the reversibility of vitamin K-induced inhibition of the NLRP3 inflammasome, LPS-primed BMDMs were incubated with vitamin K for 15 min and washed three times over a 15 min period to remove unbound vitamin K before nigericin stimulation. The results showed that vitamin K could not inhibit nigericin-induced IL-1β production after being washed away, which indicates that the inhibitory effects of vitamin K are reversible (Fig. 5l). Taken together, these results demonstrate that vitamin K inhibits NLRP3 inflammasome assembly and activation by directly and reversibly preventing the NLRP3-ASC interaction.
Fig. 5.
Vitamins K3 and K4 inhibit the interaction between NLRP3 and ASC. a, b Western blot analysis of the endogenous interaction between NEK7 and NLRP3 in BMDMs primed with LPS and then pretreated with different doses of vitamin K3 or K4 before stimulation with nigericin. c, d Western blot analysis of the endogenous interaction between NLRP3 and ASC in BMDMs primed with LPS and then pretreated with different doses of vitamin K3 or K4 before stimulation with nigericin. e Western blot analysis of cross-linked ASC in the NP-40-insoluble pellet of BMDMs primed with LPS and then pretreated with different doses of vitamin K3 before stimulation with nigericin. f, g Immunoprecipitation (IP) and immunoblot analysis of the interaction between Flag-ASC and VSV-NLRP3 in lysates from HEK-293T cells stimulated with different doses of vitamin K3 or K4. h, i Immunoprecipitation (IP) and immunoblot analysis of the interaction between Flag-NEK7 and VSV-NLRP3 in lysates from HEK-293T cells stimulated with different doses of vitamin K3. j, k Immunoprecipitation (IP) and immunoblot analysis of the interaction between Flag-NLRP3 and VSV-NLRP3 in lysates from HEK-293T cells stimulated with different doses of vitamin K3. Input, cell extract without immunoprecipitation. All data are from no fewer than three independent experiments. g ELISA analysis of mature IL-1β in the culture supernatant of BMDMs treated with different doses of vitamin K3 for 15 min, washed three times, and then stimulated with nigericin
Vitamin K3 and K4 suppress NLRP3 activation in vivo
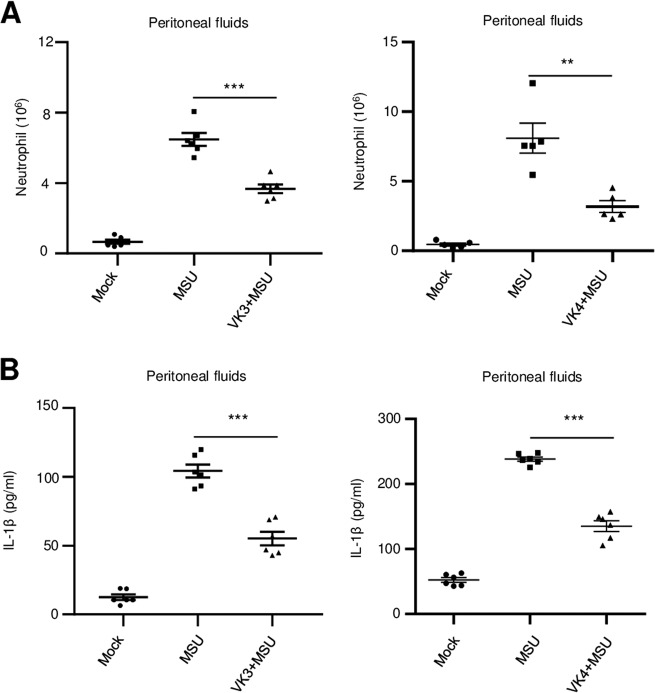
Since vitamin K3 and K4 inhibit NLRP3 inflammasome activation and IL-1β secretion in vitro, we next determined whether vitamin K could also exert anti-inflammasome effects in vivo. Gout and pseudogout, which are associated with bioactive IL-1β-mediated recruitment of neutrophils into the intraarticular and periarticular spaces, result from MSU-induced NLRP3 inflammasome activation.29 Intraperitoneal injection of MSU, which has been used as a mouse model to easily examine the extent of NLRP3 inflammasome activation in vivo, induces massive neutrophil influx and peritonitis. Using this well-established model, the inhibitory effect of vitamin K on NLRP3 inflammasome activation was investigated in vivo. We measured the production of IL-1β and counted the number of neutrophils in the peritoneal cavities of C57BL/6J mice that were pretreated with vitamin K and then intraperitoneally injected with MSU. Our results showed that both IL-1β production and the number of neutrophils were significantly decreased in the peritoneal cavities of C57BL/6J mice treated with vitamin K3 or K4 (Fig. 6a, b). Thus, vitamin K can indeed inhibit NLRP3 inflammasome activation in vivo.
Fig. 6.
Vitamins K3 and K4 suppress NLRP3-dependent peritonitis in vivo. a ELISA analysis of IL-1β in the peritoneal cavities of C57BL/6J mice that were pretreated with vitamin K3 or vitamin K4 before intraperitoneal injection of MSU. b FACS analysis of neutrophil numbers in the peritoneal cavities of C57BL/6J mice that were pretreated with vitamin K3 or vitamin K4 before intraperitoneal injection of MSU. All data are from no fewer than three independent experiments. The data shown in a and b are the mean ± SEM, n = 6 or 5. Unpaired t-tests (two-tailed) were applied to analyze statistical significance: ***P < 0.001
Discussion
Vitamin K refers to a group of structurally similar vitamins that play crucial roles in blood coagulation.2 Previous studies have indicated that vitamin K may also have anti-inflammatory effects, although little is known about the underlying mechanisms. In this study, we found that two water-soluble forms of vitamin K, vitamins K3 and K4, could specifically inhibit NLRP3 inflammasome activation both in vitro and in vivo. Mechanistically, vitamin K can inhibit NLRP3 inflammasome assembly and ASC oligomerization by blocking the interaction between NLRP3 and ASC. Our study indicates that vitamin K3 supplementation may be a potential strategy for the treatment of NLRP3-associated inflammatory diseases.
Our results show that water-soluble vitamins K3 and K4 target the NLRP3 inflammasome to exert their anti-inflammatory effects. Although a previous study indicated that vitamin K3 suppresses LPS-induced NF-κB activation and TNF-α production,30 our data suggest that vitamins K3 and K4 cannot inhibit LPS-induced TNF-α production or pro-IL-1β expression. This contradiction may be due to the different concentrations of vitamin K3 used. In previous studies, macrophages were treated with 50 μΜ vitamin K3, which is much higher than the concentrations used in our study (1–5 μM).
Our data indicate that vitamin K3 or K4 can inhibit NLRP3 inflammasome assembly by reversibly blocking the interaction between NLRP3 and ASC. However, how vitamin K prevents the NLRP3-ASC interaction remains unclear. One possible mechanism is that vitamin K inhibits the NLRP3 inflammasome by directly targeting NLRP3. Another possibility is that vitamin K can target other proteins that regulate the NLRP3-ASC interaction. The detailed mechanisms by which vitamin K inhibits the NLRP3-ASC interaction and whether vitamin K targets NLRP3 or other inflammasome regulators need to be further investigated.
Although dietary vitamin K deficiency is extremely rare in healthy adult individuals, it often occurs in infants and elderly individuals.31–33 In addition, a reduction in vitamin K can occur under certain circumstances. For example, vitamin K production has been shown to be reduced by nearly 74% in the gut in patients who take broad-spectrum antibiotics to treat infection, and this reduction may be caused by intestinal microbiota killing.34 In patients with inflammatory bowel disease, intestinal damage results in the malabsorption of vitamin K, resulting in vitamin K deficiency.35 In addition, vitamin K deficiency is associated with multiple chronic inflammatory diseases, such as type 2 diabetes (T2D), inflammatory bowel disease, osteoarthritis, and chronic kidney diseases.8–10,36 Both vitamins K3 and K4 have been considered to be synthetic water-soluble forms of vitamin K. Research that began in the 1990s demonstrated that vitamin K3 is also a catabolic product of oral vitamin K1 produced in the intestine that can be delivered to tissues and subsequently converted to vitamin K2.3–5 Researchers can indeed detect vitamin K3 in the serum following oral ingestion of vitamin K1.4 Although the measured concentration of vitamin K3 in serum is much lower than the concentration we used in our study, the physiological concentration of vitamin K3 in tissues has not been determined. Whether physiological vitamin K3 can also inhibit NLRP3-related inflammation in vivo needs to be further investigated. Moreover, since anti-inflammatory vitamin K3 can also be converted into vitamin K2, which has no effect on the activation of the NLRP3 inflammasome, whether the vitamin K1-K3-K2 axis acts as an additional signaling cascade to maintain immune homeostasis also needs to be further considered.
As described above, vitamin K refers to a group of structurally similar vitamins that share a similar structure, with a 2-methyl-1,4 naphthoquinone ring and a variable aliphatic chain.1 Both vitamin K1 and K2 have a much longer aliphatic chain than vitamins K3 and K4. Our data showed that unlike vitamins K3 and K4, dietary vitamin K1 and K2 could not inhibit NLRP3 inflammasome activation. Although further studies are needed to investigate the different effects of vitamin K on NLRP3 activation, the longer aliphatic chain of vitamin K1 and K2 may abolish the inhibitory effect of vitamin K on NLRP3 inflammasome activation.
High-dose vitamin K3 has been shown to cause potential toxicity, such as liver damage and hemolytic anemia, and it is only allowed as an additive for pet feed in several countries.37 However, low-dose vitamin K3 is still used to treat vitamin K deficiency in humans in less developed countries. One of the reasons is that vitamin K3 can be synthesized very easily, making it much cheaper than other natural forms of vitamin K. In addition, unlike natural vitamin K, which can be rapidly degraded by light, vitamin K3 is very stable.38,39 Given the safety of low-dose vitamin K3 supplementation, whether patients with these inflammatory diseases benefit greatly from vitamin K3 treatment needs to be further evaluated.
Acknowledgements
This research was supported by the National Key Research and Development Program of China (grant number 2019YFA0508503), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB29030102), the National Natural Science Foundation of China (grant numbers 81701549, 91742202, 81525013, 81722022, and 81821001), the Young Talent Support Program and Fundamental Research Funds for the Central Universities and the University Synergy Innovation Program of Anhui Province (GXXT-2019-026).
Author contributions
C.Z., Y.H., B.H., Y.C., and X.W. performed the experiments. R.Z., X.W., T.G., and W.J. designed the research. T.G., W.J., and R.Z. wrote the manuscript. W.J. supervised the project.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xicui Zheng, Yingting Hou
Contributor Information
Xiaqiong Wang, Email: wxq1989@ustc.edu.cn.
Tao Gong, Email: gtao@ustc.edu.cn.
Wei Jiang, Email: ustcjw@ustc.edu.cn.
References
- 1.Ivanova D, et al. Vitamin K: Redox-modulation, prevention of mitochondrial dysfunction and anticancer effect. Redox Biol. 2018;16:352–358. doi: 10.1016/j.redox.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiNicolantonio JJ, Bhutani J, O’Keefe JH. The health benefits of vitamin K. Open Heart. 2015;2:e000300. doi: 10.1136/openhrt-2015-000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 2006;95:260–266. doi: 10.1079/BJN20051630. [DOI] [PubMed] [Google Scholar]
- 4.Hirota Y, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J. Biol. Chem. 2013;288:33071–33080. doi: 10.1074/jbc.M113.477356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thijssen HH, Drittij-Reijnders MJ. Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 1994;72:415–425. doi: 10.1079/BJN19940043. [DOI] [PubMed] [Google Scholar]
- 6.Rosati M. Saunders handbook of veterinary drugs: small and large animals. Can. Vet. J. 2017;58:728. [Google Scholar]
- 7.Wen L, Chen J, Duan L, Li S. Vitamin Kdependent proteins involved in bone and cardiovascular health (Review) Mol. Med. Rep. 2018;18:3–15. doi: 10.3892/mmr.2018.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Chen JP, Duan L, Li S. Effect of vitamin K2 on type 2 diabetes mellitus: a review. Diabetes Res. Clin. Pract. 2018;136:39–51. doi: 10.1016/j.diabres.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Nowak JK, et al. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci. Rep. 2014;4:4768. doi: 10.1038/srep04768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cozzolino M, et al. Vitamin K in chronic kidney disease. Nutrients. 2019;11:168. doi: 10.3390/nu11010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohsaki Y, et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation. J. Nutr. Biochem. 2010;21:1120–1126. doi: 10.1016/j.jnutbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Ohsaki Y, et al. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci., Biotechnol., Biochem. 2006;70:926–932. doi: 10.1271/bbb.70.926. [DOI] [PubMed] [Google Scholar]
- 13.Gong T, Yang Y, Jin T, Jiang W, Zhou R. Orchestration of NLRP3 inflammasome activation by ion fluxes. Trends Immunol. 2018;39:393–406. doi: 10.1016/j.it.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Gong T, Jiang W, Zhou R. Control of inflammasome activation by phosphorylation. Trends Biochem Sci. 2018;43:685–699. doi: 10.1016/j.tibs.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Tang T, Gong T, Jiang W, Zhou R. GPCRs in NLRP3 inflammasome activation, regulation, and therapeutics. Trends Pharm. Sci. 2018;39:798–811. doi: 10.1016/j.tips.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Mortimer L, Moreau F, MacDonald JA. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat. Immunol. 2016;17:1176–1186. doi: 10.1038/ni.3538. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017;214:3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018;10:e8689. doi: 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He H, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018;9:2550. doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 22.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 23.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 25.Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol. Med. 2015;21:193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi H, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanson KV, Deng M. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017;13:639–647. doi: 10.1038/nrrheum.2017.155. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S, et al. Vitamin K3 attenuates lipopolysaccharide-induced acute lung injury through inhibition of nuclear factor-kappaB activation. Clin. Exp. Immunol. 2010;160:283–292. doi: 10.1111/j.1365-2249.2009.04083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr. Nutr. Rep. 2016;5:90–98. doi: 10.1007/s13668-016-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodges SJ, Pilkington MJ, Shearer MJ, Bitensky L, Chayen J. Age-related changes in the circulating levels of congeners of vitamin K2, menaquinone-7 and menaquinone-8. Clin. Sci. (Lond., Engl.: 1979). 1990;78:63–66. doi: 10.1042/cs0780063. [DOI] [PubMed] [Google Scholar]
- 33.Shearer MJ. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev. 2009;23:49–59. doi: 10.1016/j.blre.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Conly J, Stein K. Reduction of vitamin K2 concentrations in human liver associated with the use of broad spectrum antimicrobials. Clin. Invest. Med. Med. Clin. et. Exp. 1994;17:531–539. [PubMed] [Google Scholar]
- 35.Kuwabara A, et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos. Int. 2009;20:935–942. doi: 10.1007/s00198-008-0764-2. [DOI] [PubMed] [Google Scholar]
- 36.Misra D, et al. Vitamin K deficiency is associated with incident knee osteoarthritis. Am. J. Med. 2013;126:243–248. doi: 10.1016/j.amjmed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf P, Hupfeld C, Dorrestein G, Kamphues J. Investigations in pet birds (Agapornis spp.) fed different vitamin K contents in the diet. J. Anim. Physiol. Anim. Nutr. 2005;89:222–228. doi: 10.1111/j.1439-0396.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 38.Ho PC, Wong VK. Influence of DL methionine and sodium metabisulphite on the photostability of vitamin k1. PDA J. Pharm. Sci. Technol. 1998;52:129–133. [PubMed] [Google Scholar]
- 39.Hassan G. S. Menadione. Profiles of Drug Substances, Excipients and Related Methodology. 227–313, Vol. 38 (Elsevier; 2013). [DOI] [PubMed]