Abstract
Tuning the facet exposure of Cu could promote the multi-carbon (C2+) products formation in electrocatalytic CO2 reduction. Here we report the design and realization of a dynamic deposition-etch-bombardment method for Cu(100) facets control without using capping agents and polymer binders. The synthesized Cu(100)-rich films lead to a high Faradaic efficiency of 86.5% and a full-cell electricity conversion efficiency of 36.5% towards C2+ products in a flow cell. By further scaling up the electrode into a 25 cm2 membrane electrode assembly system, the overall current can ramp up to 12 A while achieving a single-pass yield of 13.2% for C2+ products. An insight into the influence of Cu facets exposure on intermediates is provided by in situ spectroscopic methods supported by theoretical calculations. The collected information will enable the precise design of CO2 reduction reactions to obtain desired products, a step towards future industrial CO2 refineries.
Subject terms: Electrocatalysis, Solar fuels, Electrochemistry
Regulation of Cu facets to promote electrocatalytic CO2 reduction is interesting and challenging. Here the authors describe a deposition-etch-bombardment synthetic approach to prepare Cu(100)-rich thin film electrodes for CO2 electroreduction with over 50% ethylene Faradaic efficiency at a total current of 12 A.
Introduction
The renewable electricity-powered CO2 reduction has been considered as one promising route to the product of chemical feedstocks, which might close the carbon loop1,2. Multi-carbon (C2+) products such as ethylene, ethanol, n-propanol, etc. are important raw materials in the chemical industry or can be used directly as fuels, thus the efficient CO2 reduction to C2+ products system is essential to the production of high-value commodity chemicals with a net negative carbon emissions footprint. Among various CO2 reduction catalyst materials, Cu has been studied extensively as it can produce C2+ products with appreciable selectivity3. Among numerous ways to promote C2+ formation4–8, adjusting the facet of Cu-based catalysts is an effective method since the dimerization reaction is facet sensitive9. According to the theoretical calculations, the Cu(100) facet can significantly lower the dimerization energy barrier10. Thus, it is an effective approach to improve the selectivity toward C2+ by designing Cu catalysts with Cu(100) as the dominant exposed facet10. Colloidal chemistry is an inexpensive, simple, and widely used method for facet exposure control. It produces catalysts with preferential exposure of specific facets by using capping agents to manipulate surface energies, which changes the growth rates of different facets to alter the shape of nanocatalysts11,12. Recently, colloidally synthesized Cu nanocubes rich in Cu(100) facets were shown to achieve a Faradaic efficiency (F.E.) of ~57% towards ethylene13. However, the corresponding electricity conversion efficiency (E.C.E.) and single-pass yield have been rarely reported. Some studies also propose that the intermediates along the CO2 reduction pathways can control the formation of specific facets, where the adsorption of the intermediates plays a role analogous to that of capping agents14,15. Nonetheless, these approaches require specific chemicals, such as capping agents, to be selectively adsorbed on particular facets to reduce the surface energy11,12. However, the effectiveness of the colloidal method might compromise to some extent since low-index facets of fcc transition metals often possess similar surface energies (1.25 J cm−2 for Cu(111) and 1.43 J cm−2 for Cu(100))15,16. Besides, residual capping agents left on the catalyst surface could block catalytically active sites17,18. Moreover, the colloidally synthesized catalyst needs to be dispersed in a solution containing polymer binders (such as Nafion) to form a well-mixed ink before being drop-casted on the conductive support to form an electrode, which is not always compatible with the catalytic electrode system due to agglomeration and the peeling-off of catalysts especially when scaling up the electrodes19,20. At the same time, due to the existence of the cross-linked network formed by polymer binders21, the contact between the colloidally synthesized catalyst and the conductive substrate is weak22,23, and plenty of active sites are further encapsulated20, which will lead to slow electron transfer and low E.C.E. Thus, it is highly desirable to develop a novel approach to replace colloidal synthesis to prepare Cu electrodes with dominant (100) facets in one-step without using capping agents and polymer binders to achieve a high E.C.E. and a single-pass yield.
In this work, we describe the design and realization of a dynamic deposition-etch-bombardment process to produce Cu(100)-rich films as the CO2 reduction electrode in one-step, which could break the limitation of using capping agents while avoiding the issue of catalyst loading faced by conventional methods. This Cu(100)-rich film yields a full-cell electricity conversion efficiency of 40.5% towards C2+ products in the 4 cm2-membrane electrode assemble (MEA) system. This facile one-step method also makes it easier for scaling up electrodes, with a 25 cm2 electrode exhibiting a 13.2% single-pass yield of C2+ product at a total current of 12 A in the MEA system. Thanks to the controllable structure, the influence of facets on the adsorption and activation of intermediates is further revealed by in situ spectroscopy and density functional theory (DFT) calculation.
Results
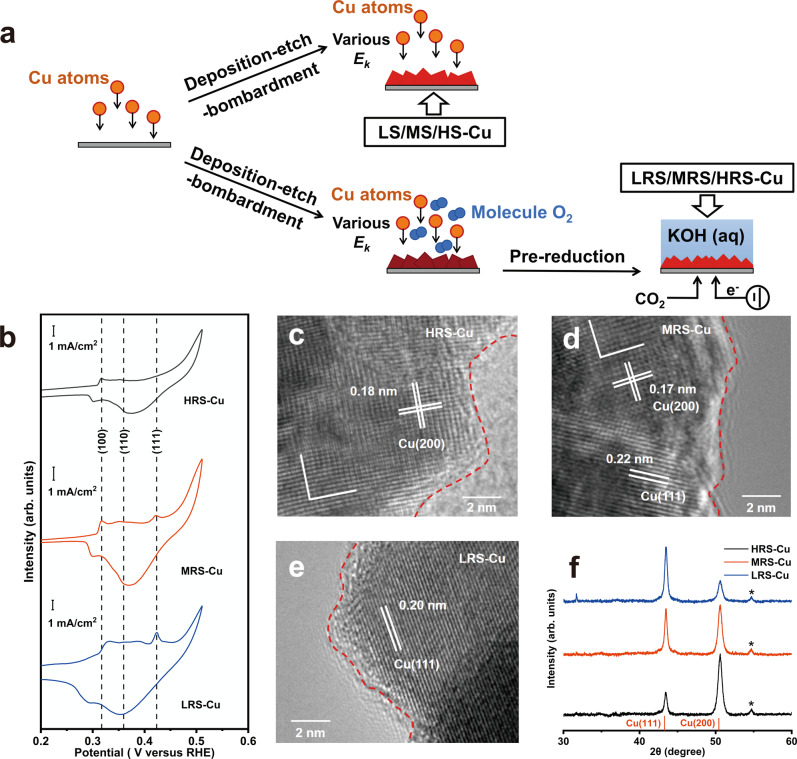
To amplify, when Cu atoms with high kinetic energy bombard the deposited Cu film, the high local temperature causes dynamic recrystallization24,25. Facets with the relatively loose atomic arrangement, like Cu(100) (Supplementary Fig. 1c), would receive less damage and remain at lower temperatures thereby acting as recrystallization centers26,27. As a result, the Cu(100) will preferentially grow, replacing the more densely packed Cu(111) facet (Supplementary Fig. 1b). Therefore, by controlling the kinetic energy of bombarding copper atoms, the exposure ratio of Cu(100) facets could be adjusted.
To realize the simultaneous deposition, etching, and bombardment, high-energy radio frequency (RF) sputtering was adopted to prepare Cu(100)-rich films (details in the “Methods”), which enables the direct deposition of catalysts on carbon-based gas diffusion layers (GDLs) as electrodes in one-step (Supplementary Fig. 1a). Three typical types of sputtered Cu films with different Cu(100) proportions were obtained by adjusting the RF power to control the kinetic energy (Ek) of the bombarding Cu atoms (Fig. 1a, details in “Methods”), resulting in low-power, medium-power, and high-power sputtered Cu films (denoted as LS-Cu, MS-Cu, HS-Cu, respectively). The obtained HS-Cu film is prone to expose the Cu(100) facet, as evidenced by transmission electron microscopy (TEM, Supplementary Fig. 2a) and X-ray diffraction (XRD, Supplementary Fig. 3a), while LS-Cu tends to form Cu(111) facet (Supplementary Figs. 2c and 3a). The energy of Cu atoms generated by thermal evaporation2 or low-rate sputtering process4,28 is usually not high enough, rendering Cu films that favor the Cu(111) facet exposure similar to LS-Cu. As different Cu facets feature distinctive OH− electrochemical adsorption behaviors29,30, surface structures of these samples were probed by using the OH− electroadsorption technique (the features were labeled by comparing the CVs to those of single crystals shown in Supplementary Fig. 4)31. The cyclic voltammograms (CVs) of OHads peaks (Supplementary Fig. 3b) reveal that the presence of Cu(111) is suppressed in the HS-Cu film. Therefore, this deposition-etch-bombardment process successfully realizes the replacement of Cu(100) to Cu(111) facets, adjusting the facet exposure of Cu.
Fig. 1. characterization of various Cu films.
a A schematic illustration of the synthesis process for LS/MS/HS-Cu and LRS/MRS/HRS-Cu. b Voltammograms of resulting electrodes collected immediately after pre-reduction. Typical TEM images of c HRS-Cu, d MRS-Cu, and e LRS-Cu. f Typical XRD patterns of resulting electrodes. As visual aids, the red dash line indicates the surfaces of the resulting electrodes. The precatalysts of HRS/MRS/LRS-Cu were pre-reduced under −0.5 V versus the RHE for 1 h, and the other conditions of prereduction are the same as that of the CO2 reduction. The labeling of Cu(200) is used for easier comparison with XRD, in which only (200), the second-order diffraction of (100) could be detected. The peaks marked with an asterisk in XRD patterns originate from carbon-based GDL substrate.
However, these obtained Cu films (i.e. LS-Cu, MS-Cu, HS-Cu) lack a large electrochemically active surface area (ECSA, Supplementary Fig. 26 and Supplementary Table 1) to realize high-rate CO2 electrolysis32. To increase the ECSA, an oxidation−reduction procedure was included, during which the preferential exposure of Cu(100) could be maintained. Specifically, molecular O2 (with a constant partial pressure of 0.67 Pa) was introduced during this deposition-etch-bombardment process to obtain Cu2O as precatalysts, which could be further reduced during the pre-reduction process (−0.5 V vs. the RHE) under the same CO2 reduction conditions to metallic Cu with the Cu(100) facets retained (Fig. 1a, details in “Methods”). The same crystallographic relationship exists between the oxidation and reduction processes for Cu(100)/(111) and Cu2O(100)/(111)10,33, since oxygen atoms could pack into the interlayer sites of different planes of Cu, causing a lattice expansion while retaining the underlying fcc crystal structure with the inclusion of oxygen33. Therefore, the introduction of an oxidation-reduction step will be able to increase the ECSA while retaining the same Cu(100) exposure. Correspondingly, samples obtained by reactive sputtering followed by reduction are referred to as low-power, medium-power, and high-power reactively sputtered Cu films followed by reduction (denoted as LRS-Cu, MRS-Cu, and HRS-Cu). Typical XRD patterns (Supplementary Fig. 5a) show that all the obtained precatalysts are mainly Cu2O, which is confirmed by X-ray photoelectron spectroscopy (XPS), Auger spectroscopy (Supplementary Fig. 5b, c), and Raman spectroscopy (Supplementary Fig. 4d). The same type of Cu facets distribution could be maintained for these electrodes regardless of the oxidation-reduction process according to TEM (Fig. 1c–e, more images of different samples are also provided in Supplementary Figs. 7–9) and XRD (Fig. 1f), consistent with previous reports10,34. The different proportions of Cu(100) facet on these samples (i.e. HRS-Cu, MRS-Cu, and LRS-Cu) are also evidenced by the electrochemical OH− adsorption peaks on Cu(100) and Cu(111) at potentials of ~0.33 and 0.43 V versus the RHE (Fig. 1b). Meanwhile, large ECSA is obtained for these samples (Supplementary Table 1). Thus, this one-step surfactant-free route indeed leads to nanostructured Cu films with preferred Cu(100) exposure and high surface area that could be further used as CO2 reduction electrodes.
To further explore the importance of the high-energy atom bombardment proposed above, another control electrode was prepared. Cu2O precatalyst (Supplementary Fig. 10) was obtained through wet chemistry without using capping agents (denoted as W-Cu). Then this control Cu2O precatalyst was airbrushed onto GDEs and pre-reduced to Cu under CO2 reduction conditions to form a control electrode (details in “Methods”). The typical TEM image shows that the lattice fringes corresponding to Cu(111) are widely distributed in this control electrode (Supplementary Fig. 11a)32. These observations are in good agreement with the XRD pattern (Supplementary Fig. 11b) and CV of OHads peaks (Supplementary Fig. 11c). It is also worth noting that LRS-Cu, MRS-Cu, HRS-Cu, and W-Cu all consist of metallic Cu only without residual oxides after the pre-reduction, as confirmed by in-situ Raman spectroscopy (Supplementary Fig. 12, details in “Methods”). Thus, this control experiment reveals that the lack of high-energy atom bombardment during the synthesis process would result in the dominant exposure of Cu(111) facets, similar to the scenario of lacking capping agents that are widely used in the synthesis of Cu nanocubes34,35.
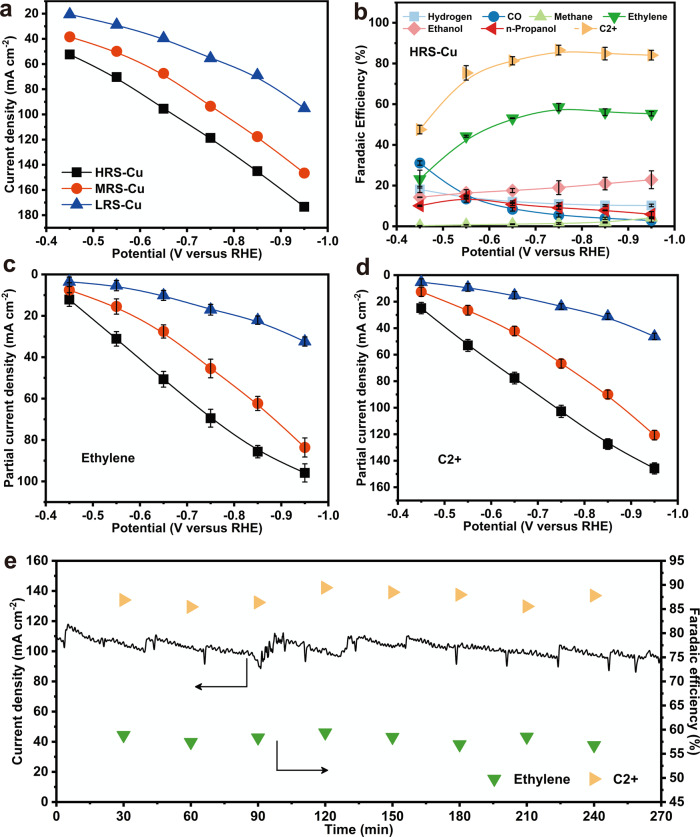
Before performance testing, it was confirmed that different samples (LRS-Cu, MRS-Cu, HRS-Cu, and W-Cu) possess similar mass loadings (Supplementary Table 2), catalyst layer thickness (Supplementary Fig. 13) and morphology (Supplementary Fig. 14) of Cu, which ensures a fair comparison to explore the activity difference among various samples36–39. The activities were evaluated at different potentials using 2 M KOH (aq.) as the electrolyte (Fig. 2a and Supplementary Figs. 16–19, details in “Methods”) in a flow cell electrolyzer (Supplementary Fig. 15) for CO2 reduction with an effective electrode geometric area of ~0.64 cm2 for both cathode and anode. As for HRS-Cu, the products detected in significant quantities were ethylene, ethanol, n-propanol, and CO. At more negative potentials, a small amount of methane was produced, and the remaining charge was attributed to the competing hydrogen evolution reaction (Fig. 2b). Compared with the control samples, the HRS-Cu exhibits a maximum F.E. of 58.6% for ethylene, 86.6% for C2+ products (containing ethylene). Consistently, the HRS-Cu sample also exhibits the largest ethylene and C2+ products partial current densities (Fig. 2c, d and Supplementary Fig. 17c, d) among the four samples at all applied potentials. Moreover, at the applied potential of −0.85 V versus the RHE, the C2+-to-C1 ratio of the HRS-Cu electrode reaches about 15.2, which largely outperforms that of its counterparts (Supplementary Fig. 18). Due to the close contact between the catalytic Cu film and the GDL substrate, the reaction system could exhibit higher E.C.E. The corresponding full-cell E.C.E. towards ethylene and C2+ products of this HRS-Cu-based reaction system reaches 24.8 and 36.5%, respectively, exceeding the efficiency of many other reaction systems reported so far (see below). The stability of the HRS-Cu was also examined under a constant applied potential of −0.75 V versus the RHE for 4.5 h (270 min), where ethylene and C2+ F.E.s remained stable over the test duration (Fig. 2e and Supplementary Fig. 19). Although the surface of the Cu-based catalysts may undergo reconstruction during the reaction to obtain Cu(100)40, the HRS-Cu, MRS-Cu, and LRS-Cu samples after use show similar surface exposures as before the reaction (Supplementary Figs. 20–22), indicating that the reconstruction process could not significantly change the main facet exposure, and thus surface reconstruction alone cannot guarantee electrodes similar to the HRS-Cu (showing a predominant Cu(100) exposure without undergoing reconstruction). The mass of the HRS-Cu sample after the reaction is also almost the same as that before the reaction (Supplementary Table 3), which further indicates that the firm conjunction between the substrate and the active composition. If the hydrophobicity of GDLs can be further improved2 and the reaction rate of carbonate formation can be reduced41, the stability of the sample in the flow cell will be longer.
Fig. 2. Catalytic performance of various Cu films.
a Current−voltage (I–V) curves on various electrodes measured in CO2-flowed 2 M KOH electrolytes. b Faradaic efficiencies of CO2 reduction products on the HRS-Cu sample as a function of different applied potentials. c Ethylene and d C2+ products partial current densities obtained on various samples at different applied potentials. e Stability test over a span of 4.5 h (270 min) of CO2− electrolysis in 2 M KOH (aq.) at −0.75 V versus the RHE. The oscillation of the current density is due to the repeated release of O2 bubbles in the anode side of the flow cell. C2+ products include ethylene, ethanol, and n-propanol. Error bars represent the standard deviation from at least three independent measurements.
To examine the influence of Cu(100) exposure over C2+ products formation in CO2 reduction, Tafel analyses (Supplementary Fig. 23) were conducted on various samples. It is clear that C2+ products formation depends strongly on the surface structure. With the increasing exposure of Cu(100) facets, a lower change of the Tafel slope can be observed, which indicates that the Cu(100) surface is beneficial to the formation of C2+ products. However, the Tafel slope of all samples locates at approximately 120−140 mV dec−1, implying that they might share the same rate-determining step (details in “Methods”), which is further validated by the results of CO partial pressure dependence experiments (Supplementary Figs. 24, 25). To further compare the reaction rates, the performances are normalized to the ECSA (details in “Methods”). Obviously, the current densities of specific ethylene and C2+ products of HRS-Cu are larger than other electrodes (Supplementary Fig. 27), which indicates the highest intrinsic activities of HRS-Cu.
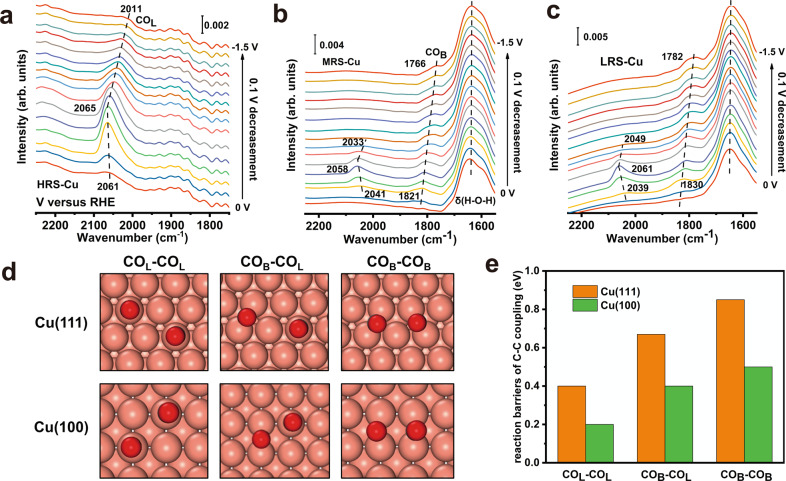
Understanding the behaviors of adsorbates is essential to the inquiry into the nature of the catalytic activity. Thus, in-situ attenuated total reflectance (ATR)-surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS, details in “Methods”) was applied to investigate the effect of facets exposure on the adsorption of intermediates. When the potential is swept from −0.1 to −1.5 V versus the RHE, a positive band centered at ~2050 cm−1 which corresponds to the linear-bond CO (COL, a reactive adsorbed species) is observed on all electrodes (Fig. 3a–c and Supplementary Fig. 28)42–44. Meanwhile, a small positive band centered at ~1800 cm−1, which corresponds to the bridge-bond CO (COB, an unreactive adsorbed species)44 is also observed on the surface of other samples except the HRS-Cu. These results of the ATR-SEIRAS are also corroborated by the corresponding Raman spectra, where the HRS-Cu surface was covered by abundant COL45,46, while the LRS-Cu surface exhibited a pronounced appearance of COB (Supplementary Fig. 30). The appearance of COB might result from the interconversion of COL. According to the literature, when the *CO (*denotes the adsorbed species) coverage of the electrode decreases, a fraction of COL would convert to COB44. Therefore, the increase of COB ratio reveals the reduction of surface *CO coverage. By calculating the COB to COL ratio of different samples, the *CO coverage might decrease as the exposure of Cu(100) facets decreases (Supplementary Fig. 29). Normally, *H and *CO occupy most of the surface sites, so they are in direct competition with each other for surface sites. Meanwhile, the surface coverage of *CO eventually influences the distribution of products derived from CO2 reduction. It has been previously speculated that when the surface cannot maintain a high *CO coverage, the corresponding *H coverage will increase, inhibiting the dimerization of *CO to produce C2+ products, thereby shifting the selectivity to C1 products and H247–49, which is also demonstrated by the results of our CO partial pressure dependence experiments (Supplementary Figs. 24, 25). Meanwhile, the results of CO2 reduction performance support the above speculation. The LRS-Cu and the W-Cu with a lower exposure of Cu(100) facets exhibit the highest F.E. of methane, meanwhile, the methane F.E. increases rapidly as the potential becomes negative (Supplementary Fig. 31). Thus, these ATR-SEIRAS results prove that Cu(100) facet might be a type of strong *CO adsorption site, and a higher exposure of Cu(100) facets is beneficial to the increase of strong adsorption sites on the surface that maintains a higher *CO coverage on the surface, thereby leading to the catalyst with a higher C−C coupling performance, which is also supported by other experimental and computational studies50–52.
Fig. 3. Spectroscopic investigations of various Cu films and DFT calculations on different Cu facets.
In situ ATR-SEIRAS spectra of a HRS-Cu; b MRS-Cu; c LRS-Cu; d adsorption geometry for different C−C coupling precursors (i.e., COL−COL, COB−COL, COB−COB), where solvent molecules are not presented to show the adsorbate configurations; e reaction barriers for C−C coupling from different precursors on Cu(100) and Cu(111) facets.
Since the ATR-SEIRAS cannot directly provide the adsorption energy and activation barrier of the intermediate, DFT calculations on Cu(111) and Cu(100) (3×3) models were performed to further understand the facet exposure effect (Supplementary Fig. 32, details in “Methods”). Based on the Tafel analysis and CO partial pressure dependence study (Supplementary Figs. 23, 24), the energetics of *OCCO formation is chosen as the main consideration. Previous work has found that the solvent and cation effects can stabilize *COCO, hence an appropriate electrochemical interface was built up to explore the mechanism of this dimerization procedure (Fig. 3d and Supplementary Figs. 34–36)53.
From the DFT calculation results, the binding of *CO is the weakest on the Cu(111) facet (Supplementary Fig. 33), which is not suitable to build up a sufficient coverage of *CO on the surface to promote the kinetics of C−C coupling. Therefore, LRS-Cu and W-Cu would shorten the *CO stay and reduce the *CO coverage as compared to HRS-Cu, leading to a higher CO F.E. (Supplementary Fig. 16). In addition, the barriers of various C−C coupling processes are always higher on the Cu(111) facet than on Cu(100) facet, implying that *CO dimerization is the most sluggish step on the Cu(111) facet (Fig. 3e). Thus, LRS-Cu and W-Cu are less active in catalyzing the reduction of CO2 to C2+ products. These theoretical calculations are in good agreement with our ATR-SEIRAS and performance test results.
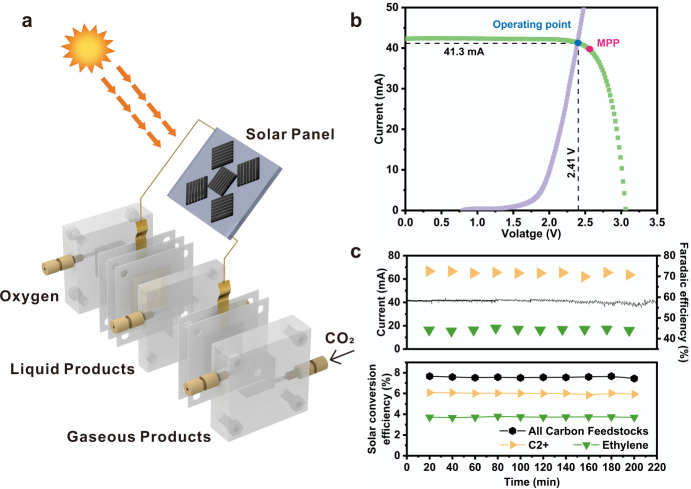
Due to the impressive E.C.E of this HRS-Cu-based reaction system, a PV-EC system (Fig. 4a) was further constructed to demonstrate the photosynthesis of C2+ products. Using the same test conditions (electrolyzer, cathode, anode, and electrolyte, details in “Methods”) for measuring the CO2 reduction performance on the cathode side (as in Fig. 2), it is found that an overall cell voltage of about 2.5 V is required (Fig. 4b) to obtain an operating current density varying from 60 to 70 mA cm−2 (Fig. 2a), which could be translated to a cathodic potential of ~−0.55 V versus the RHE that yields ~45 and ~72% F.E. for ethylene and C2+ products (containing ethylene), respectively (Fig. 2b). The deviation of these F.E.s from the maximized F.E.s for ethylene and C2+ products is because of the matching between electrolyzer and the solar panel towards the maximum power point (MPP) of the solar panel. Considering that the effective area of the HRS-Cu sample is ~0.64 cm2, the operating current varies from 38.4 to 44.8 mA. The widely available p−n+ solar cells, with an open circuit potential of ~0.6 V and a short-circuit current density of ~36 mA cm−2, were selected and cut into ~1.14 cm2 pieces. By connecting five of them in series, the solar panel would provide the suitable voltage and current for the CO2 reduction electrolyzer near the MPP of the solar panel (i.e., the PV system). I−V curve of the obtained solar panel was measured under simulated AM 1.5G 1-sun illumination (Fig. 4b). This curve crosses the I−V curve of the electrolyzer at the point (i.e., the operating point) where its cathodic current and voltage are ~41.3 mA and ~2.41 V, respectively, which matches well with the MPP output metrics of the solar panel (39.8 mA at 2.53 V), indicating the optimum solar-to-electricity conversion process in our integrated device.
Fig. 4. Solar-powered CO2 reduction.
a Schematic of the PV-EC system. b Photovoltaic and electrocatalytic I-V behaviors. The photovoltaic performance is shown under light (green) with the MPP marked by a red dot. The measured operating current of the CO2 electrolysis system (cathode, anode, and anion exchange membrane) at different voltages has been marked by the purple curve. The observed long-term operating point is marked by a blue dot, with the black dashed lines showing the corresponding current and voltage. c Faradaic efficiency towards ethylene and C2+ products, solar current, and solar conversion efficiency as a function of reaction time. Carbon feedstocks include CO, methane, ethylene, ethanol, and n-propanol. C2+ products include ethylene, ethanol, and n-propanol.
During ~3.7 h (220 min) of electrolysis powered by 1-sun solar illumination, the cathodic current of the electrolyzer was stable at ~41.3 mA and the F.E. of ethylene and C2+ products stabilized at ~45 and ~72%, respectively (Fig. 4c and Supplementary Figs. 37–39). Although this selectivity is already deviated from the maximum F.E.s owing to MPP matching, such a PV-EC system still yields a solar-to-ethylene efficiency of ~4.0% and a solar-to-C2+ products efficiency of ~6.0% (Fig. 4c) under simulated 1 Sun illumination, which exceeds the efficiency of general natural photosynthesis for producing carbohydrates (3−6%)54. Such efficiency is also able to match a recent state-of-the-art perovskite solar cells-powered Cu−Ag bimetallic reaction system55, while a longer stability and larger CO2 reduction current are obtained in this system. The performance of this work also exceeds that of most previously reported copper-based PV-EC systems (Supplementary Table 4), which provides a benchmark for solar conversion efficiency while using feasibly available Si-based solar cells and earth-abundant low-cost electrode materials.
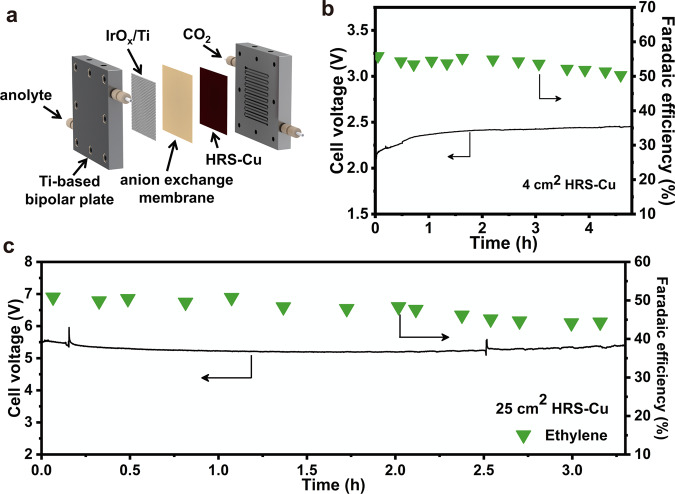
The membrane electrode assemble system (MEA) was further adopted to scale up the CO2 reduction system (Supplementary Fig. 40, details in “Methods”). On the cathode side, humidified CO2 gas was supplied (Fig. 5a), which reduces the direct contact between the catalyst and the aqueous electrolyte, while reducing the ohmic resistance of the electrolyte56,57. At the same time, the one-step deposition-etch-bombardment process proposed in this work is based on a widely used vacuum deposition process similar to the photovoltaic industries, thus its marginal cost for producing large-area electrodes can be largely reduced with mass production. In addition, this method does not require additional catalyst loading steps and does not use additional chemicals, accelerating the continuous preparation of large-area electrodes and avoiding potential electrode contamination. Therefore, the combination of a MEA reaction system and this preparation method is particularly suitable for the scale-up of CO2 reduction.
Fig. 5. Scaling up the CO2 reduction system.
a Schematic of the MEA system. The gaskets are not shown. b Stability test over a span of ~4.5 h of CO2-electrolysis in a 4 cm2-MEA system at the total current density of 120 mA cm−2. c Stability test over a span of ~3.5 h of CO2-electrolysis in a 25 cm2-MEA system at the total current of 12 A. The decrease in ethylene F.E. and the increase in cell voltage may be attributed to the formation of carbonate on the cathode side and the cathode water flooding.
The electrode area was enlarged to 4 cm2, and the CO2 reduction performance test was performed in the MEA system (the area of cathodic flow fields is 4 cm2, referred as to the 4 cm2-MEA) at a current density of 120 mA cm−2 corresponding to the maximum ethylene F.E. (58.6%) in the flow cell system (Fig. 5b). The optimal ethylene F.E. reaches 55.8%, while the corresponding full-cell E.C.E of ethylene and C2+ products increase to 26.4 and 40.2%, respectively. After ~4.5 h of operation (Supplementary Fig. 41), the ethylene selectivity (50.4%) still remains above 90% of the initial value (55.8%). Although many works have obtained impressive CO2 reduction selectivity, the single-pass yield for C2+ products is generally low (mostly below 3%, Supplementary Table 5). In order to improve the single-pass yield of C2+ products, it is vital to further increase the current density and electrode area without affecting the transport of CO2 (details in the “Methods”). Using the one-step method proposed in this work, a HRS-Cu electrode with 25 cm2 activity area could be easily fabricated with the deposition-etch-bombardment process (Supplementary Fig. 42). In a 25 cm2 MEA system (i.e., the area of cathodic flow fields is 25 cm2), the optimal ethylene F.E. of HRS-Cu reached 50.9% and maintained above 45% (Fig. 5c and Supplementary Fig. 43) after ~3.5 h of operation at a total current of 12 A (i.e., 480 mA cm−2). The corresponding single-pass yield of CO2 towards C2+ products increased to and 13.2%, with ethylene increased to 12.0%. However, a corresponding decrease in E.C.E. was observed (Supplementary Table 5). Therefore, using more efficient anode materials, while controlling the operating conditions of the device such as temperature and pressure, or designing a new flow field structure to enhance CO2 mass transfer may be effective ways to further improve the E.C.E.
Discussion
In conclusion, this work demonstrates a deposition-etch-bombardment process that feasibly increases the exposure of Cu(100) facets in low-cost nanostructured Cu films. This strategy obviates the use of capping agents, achieving precise control of surface structures in a one-step approach. The obtained Cu(100)-rich film functions as a high-performance electrode for CO2 reduction towards ethylene and C2+ products. In flow cell, it realized ethylene and C2+ (containing ethylene) F.E.s of 58.6 and 86.6%, respectively. In addition, this deposition-etch-bombardment method bypasses the electrode assembly process, avoids the use of polymer binders, enhances the contact between the catalytic film and the substrate. Due to the above advantages, in flow cell, a corresponding full-cell E.C.E. of 24.8 and 36.5% for ethylene and C2+ products were obtained, respectively, which is a notable advance over existing single metallic Cu-based systems. Moreover, this preparation method is flexible and easy to achieve electrode scale-up. When a 4 cm2 electrode was applied to the MEA system, the corresponding full-cell E.C.E. could be increased to 40.5 and 26.8%, respectively, without compromising product selectivity. When the electrode area of the MEA system is increased to 25 cm2, the single-pass yield of C2+ products can be further increased to 13.2%, with an ethylene yield of 12.0%. In situ ATR-SEIRAS studies and theoretical calculations provide insights into the role of Cu(100) facets for increasing *CO coverage and reducing the energy barrier of C−C coupling, vital for ethylene and C2+ products formation. The potential of using this Cu(100)-rich film for photosynthesis was also demonstrated using renewable electricity generated by Si solar panels, achieving a solar-to-C2+ products efficiency of ~6.0% under simulated 1 Sun illumination. Future research endeavors may focus on the generation of energetic particles (atoms, molecules, ions, etc.) in other environments such as solutions in ambient conditions, which may further reduce the cost of one-step facet control by this vacuum-based deposition-etch-bombardment method.
Methods
Synthesis of LS-Cu, MS-Cu, HS-Cu
The deposition-etch-bombardment process was conducted in a custom-designed radio frequency (RF, 13.56 MHz) magnetron sputtering system (Supplementary Fig. 1a). For LS-Cu, Ar was delivered into the system. The deposition chamber was pumped down by a mechanical pump and a molecular pump that resulted in a base pressure of 2.0 × 10−4 Pa. The flow rate of Ar was set as 20 standard cubic centimeters per minute (sccm). During the deposition, the RF power was 40 W, and the working pressure was 4 Pa. The deposition time was 15 min. The target to substrate distance was set to 7 cm. Commercial GDLs were cut into squares (5 × 5 cm) for use as substrates. For MS-Cu, the RF power was set as 100 W and the deposition time was 7.5 min, other conditions were the same as LS-Cu. For HS-Cu, the RF power was set as 200 W and the deposition time was 3.5 min, other conditions were the same as LS-Cu.
Synthesis of LRS-Cu, MRS-Cu, and HRS-Cu
During the synthesis process of precatalysts of LRS-Cu, MRS-Cu, and HRS-Cu, O2 was also introduced to the Ar atmosphere. The flow rates of Ar and O2 were 20 and 4 sccm, respectively, to achieve a partial pressure of O2 of 0.67 Pa. Other conditions were the same as those of LS-Cu, MS-Cu, and HS-Cu. The obtained precatalysts were pre-reduced at −0.5 V versus the RHE for 1 h under the same conditions as CO2 electroreduction. Pre-reduction was carried out with a potentiostat (CompactStat.e20250, IVIUM). After pre-reduction, the final samples (i.e., LRS-Cu, MRS-Cu, and HRS-Cu) were obtained.
Synthesis of W-Cu
The W-Cu was fabricated through the electrochemical pre-reduction of the Cu2O nanorods at −0.5 V versus the RHE for 1 hour under the same conditions as CO2 electroreduction. After pre-reduction, the electrode was directly used for CO2 reduction. The Cu2O nanorods were prepared by annealing the Cu(OH)2 nanorods in the Ar atmosphere at 500 °C for 2 h with a heating rate of 10 °C min−1. The Cu(OH)2 nanorods were prepared by a previous method with some modifications32. The obtained Cu2O nanorods were airbrushed onto the commercial GDLs at an approximate loading of 0.9 mg/cm2, measured through weighing GDLs before and after airbrushing. The catalyst ink was prepared by dispersing 200 mg of Cu2O nanorods and 50 μL of Nafion Solution (Sigma-Aldrich) in 750 μL isopropyl alcohol and 250 μL of ultra-purity water (18.2 MΩ cm) and sonicated for 1 h before airbrushing (H-SET, Paasche). Pre-reduction was carried out with a potentiostat (CompactStat.e20250, IVIUM).
Characterizations
Field-emission scanning electron microscopy (FESEM) (Hitachi S-4800, 3 kV) was used to characterize the morphology and microstructure of the samples. Transmission electron microscopy (TEM), High-resolution TEM (HRTEM) images were obtained at 200 kV (JEOL JEM-2100F). The crystal structure was determined by X-ray Diffractometer (XRD, Bruker D8 Focus) with Cu Kα radiation (λ = 1.54056 Å) at 40 kV and 40 mA. XRD spectra were collected over a 2θ range of 30−60° at a scanning speed of 8°/min. XPS analyses of precatalysts were carried out on a Physical Electronics PHI 1600 ESCA system with an Al Kα X-ray source (1486.6 eV). The binding energy was calibrated using the C 1s photoelectron peak at 284.6 eV as the reference.
In-situ Raman spectroscopy measurements
In-situ Raman spectroscopy was carried out in a custom-designed flow cell (Supplementary Fig. 12a), which was manufactured by Gaossunion Co., Ltd., Tianjin. The electrode was encased in a PEEK fitting, with an exposed circular geometric surface area of ~1 cm2. A platinum wire and an Ag/AgCl electrode (saturated KCl, Gaossunion Co., Ltd., Tianjin) were used as the counter and the reference electrode, respectively. The counter electrode is separated from the working electrode by an anion exchange membrane (FAA-3-PK-75, Fumatech) to avoid cross-contamination. In situ Raman spectroscopy was performed with a Raman microscopy system (LabRAM HR Evolution, Horiba Jobin Yvon). A He−Ne laser (λ = 532 nm) served as the excitation source. All spectra were collected at a constant potential (−0.5 V versus the RHE). Electrochemical measurements were carried out with a potentiostat (CompactStat.e20250, IVIUM).
In-situ ATR-SEIRAS measurements
In-situ ATR-SEIRAS was performed with an ATR configuration. Au nanofilms were deposited directly on the reflecting plane of a Si prism using a modified electroless chemical deposition method outlined by Xu et al.58. The spectroelectrochemical cell was based on the design of Xu et al.59 and manufactured by Gaossunion Co., Ltd., Tianjin. In order to reduce the corrosion of Si crystal, 0.1 M KOH was used as the electrolyte. The counter electrode (a graphite rod) was separated from the working and reference electrodes, i.e., the catalyst film and a saturated Ag/AgCl electrode (saturated KCl, Gaossunion Co., Ltd., Tianjin), respectively, with a piece of anion exchange membrane (AEM, FAA-3-PK-75, Fumatech). This cell is integrated into the FTIR (is50, Nicolet) spectrometer with a modified accessory at a 60° incident angle (VeeMax III, PIKE Technology). All spectra were collected with a 4 cm−1 resolution. Spectra are presented in absorbance, with positive and negative peaks showing an increase and decrease in signal, respectively. As for LRS-Cu, MRS-Cu and HRS-Cu, they were deposited on the Au nanofilm coated-Si prisms like the process described above, while W-Cu was drop-casted onto the Au nanofilm coated-Si prisms. The background was taken at +0.1 V versus the RHE in Ar saturated electrolyte for each electrode. Electrochemical measurements are carried out with a potentiostat (CompactStat.e20250, IVIUM).
OH− electroadsorption measurements
In-situ OHads studies were conducted by flowing Ar in the flow cell (Supplementary Fig. 15). First, CO2 electrolysis was conducted at a constant potential of −0.5 V versus the RHE for 1 h by switching the gas feed to CO2 and flowing the electrolyte. Immediately after electrolysis, the electrolyte (1 M NaOH (a.q.)) flow rate was stopped to minimize the fluctuation in the voltammogram, and the gas feed was switched to Ar, the electrolyte flow rate was stopped, and then cyclic voltammetry (20 mV/s) was performed. Electrochemical measurements are carried out with a potentiostat (Autolab PGSTAT204, Metrohm).
ECSA measurements
The ECSA was determined by measuring the double-layer capacitance (CDL) of various electrodes in Ar-purged 2 M KOH (aq.) in the flow cell (Supplementary Fig. 15) and the ECSA was measured after CO2 electrolysis at a constant potential of −0.5 V versus the RHE for 1 h. Immediately after electrolysis, the gas feed was switched to Ar, and then the electrolyte flow rate was stopped to minimize the fluctuation in the voltammogram. The scan rate was varied from 25 to 125 mV s−1 in the non-faradaic potential region and the observed current was plotted as a function of scan rate to obtain the CDL. ECSA was determined by normalizing the CDL to that of a Cu foil. Electrochemical measurements are carried out with a potentiostat (CHI 660E, CH Instruments Inc.).
Electrochemical reduction of CO2 in a flow cell
CO2 reduction was conducted in a custom-designed three-chamber flow cell manufactured by Gaossunion Co., Ltd. (Supplementary Fig. 15), where the CO2 gas was supplied directly to the catalyst layer (cathode, working electrode). The CO2 gas flow rate was controlled using a mass flow controller (MC-Series, Alicat Scientific) and set to 10 sccm. However, it is well known that OH− can react with CO2 to form HCO3−or CO32−. Therefore, the calculation based on the inlet CO2 flow rate will result in overestimated F.E. results60,61. For this reason, we used another flowmeter (M-Series, Alicat Scientific) to detect the CO2 flow rate at the outlet of the reactor and used this number as the basis for calculating F.E. Aqueous KOH solution (2 M) was used as both the catholyte and the anolyte. Activated Ni foam was used as the anode (counter electrode). Peristaltic pumps (EC200-01, Gaossunion Co., Ltd.) were used to control the flow rate of the electrolytes at ~10 ml min−1. An AEM (FAA-3-PK-75, Fumatech) was used to separate the cathode and anode chambers. Electrolysis experiments were conducted using chronoamperometry with a potentiostat (CompactStat.e20250, IVIUM). The cathode potentials were measured against a Hg/HgO reference electrode (1 M KOH, Gaossunion Co., Ltd., Tianjin). For each measurement, products were quantified after the amount of electron flowing through the cathode achieved 50 C and at least three replicates were conducted to obtain an average value with the standard deviation. It should be noted that iR correction was not performed.
Electrochemical reduction of CO2 in the MEA system
The MEA cell (manufactured by Gaossunion Co., Ltd.) consists of a titanium anode (cathode) bipolar plate with serpentine flow field, associated nuts, bolts, and insulating kit. The geometric area of each flow field is 4 or 25 cm2 (Supplementary Fig. 40). An AEM membrane (FAA-3-PK-75, Fumatech) was activated in 0.1 M KOH for 24 h, washed with ultra-purity water prior to use. The anode consisted of iridium oxide supported on titanium mesh (IrOx/Ti mesh) was prepared by a dip-coating and thermal decomposition method62. The MEA was assembled in a way as illustrated in Fig. 5a. A direct current power supply (UTP1300, UNI-T Group Co., Ltd) was used to apply current to the MEA. A Corrtest CS350M in a galvanostatic mode was used to measure the cell voltage. No iR compensation was applied. Aqueous KHCO3 electrolyte (0.1 M) was used as the anolyte and was circulated using a peristaltic pump (EC200-01, Gaossunion Co., Ltd.). The electrolyte flow rate was kept at 10 mL min–1. As the current density and electrode area increase, the CO2 flow rate should be adjusted upwards to avoid mass transfer limitations of CO2 while maintaining optimum selectivity. The flow rate of the CO2 gas flowing into the cathode flow field was kept at 20 or 60 sccm by a mass flow controller (MC-Series, Alicat Scientific) for different geometries of the flow field. CO2 was flowed through a homemade humidifier (7/8 full of Milli-Q water, room temperature) prior to the MEA. The flow rate of the CO2 gas flowing out the cathode flow field was also measured by a flowmeter (M-Series, Alicat Scientific). The liquid products carried by CO2 gas are absorbed by low-temperature ultra-purity water obtained from an ice salt bath
Analysis of CO2 reduction products
During electrolysis, gas products were quantified using an on-line gas chromatography system (GC7890B, Agilent Technologies, Inc.). The thermal conductivity detector (TCD) connected to a MolSieve 5A packed column (Agilent Technologies, Inc.) to detect H2, O2, and N2 and a back flame ionization detector (FID) connected to a Porapak Q packed column (Agilent Technologies, Inc.) to detect CO. A methanizer was installed to enable the back FID to detect CO with 1000 times higher sensitivity. A front FID connected to an HP-PLOT Al2O3 capillary column (Agilent Technologies, Inc.) to detect hydrocarbons (C1~C3). Ar was used as the carrier gas. After passing through the reactor, the gas was allowed to flow directly into the gas sampling loop of the gas chromatography for online gaseous product analysis.
In the performance test using flow cell and the MEA system, the liquid products were collected from the cathode and anode chambers60. The liquid products were analyzed by headspace gas chromatography (HS-GC) and 1H-NMR. HS-GC measurements were carried out using a BCHP HS-2 Headspace Sampler with GC2060 gas chromatography (Shanghai Ruimin Instrument Co., Ltd.). Typically, 10 mL vials were filled with 3 mL of the liquid sample and sealed. They were heated to 70 °C over 20 min in the headspace sampler and 1 mL of the headspace gas composition was automatically injected into the GC. The sample loop (110 °C) and transfer line (110 °C) were both heated to avoid condensation. Ar was used as the carrier gas. An HP-INNOWax capillary column (Length: 60 m; ID: 0.32 mm; Film: 0.5 μm, Agilent) was used to separate the compounds in the sample. 1H-NMR was performed using AVANCE IIITM HD 400 MHz NanoBAY. The water suppression method was used. DMSO (10 mM) and phenol (50 mM) were added as internal standards. For CO2 reduction products showing multiple sets of NMR peaks, the set of peaks with the highest intensity were chosen for calibration and quantification.
Construction of the photovoltaic-electrolyzer (PV-EC) system
The simulated solar illumination was obtained from a 300 W Xenon arc lamp (Microsolar 300 UV, Beijing Perfectlight Technology Co. Ltd.) equipped with an air mass 1.5 global (AM 1.5G) filter, and the power intensity of the incident light was calibrated to 100 mW/cm2 using a Si photodiode (FDS100, Thorlabs). The solar panel was based on five p−n+ Si solar cells connected in series (effective illuminated area of ~5.7 cm2). A Source Measure Unit (2450, Keithely) was wired in series with 0 V applied to monitor the current. The electrolyzer is the flow cell.
Computational methods
Vienna ab initio simulation package (VASP) was used to carry out calculations with the PBE exchange-correlation functional63,64. Van der Waals interactions were accounted for by using the DFT-D3 method65. The cut-off energy is 400 eV. The interactions between the atomic cores and electrons were described by the projector augmented wave (PAW) method66. All structures were optimized until the force on each atom has been less than 0.02 eV/Å. The transition state search was conducted with the climbing image nudged elastic band (CI-NEB) method, followed by the dimer method to converge the saddle point within 0.05 eV/Å. We access CO dimerization on the three models. A four-layer Cu(111)-(3×3) slab with a (3×3×1) k-point grid and a four-layer Cu(100)-(3×3) slab with a (3×3×1) k-point grid were used as models for DFT calculations. The bottom two layers are fixed while the upper two layers were relaxed during optimization. One layer of water with a simple hydronium ion was chosen to simulate the electrochemical interface67–69.
Supplementary information
Acknowledgements
We acknowledge the National Key R&D Program of China (2016YFB0600901), the National Natural Science Foundation of China (22038009, 21722608, 51861125104, 2212100031), the Natural Science Foundation of Tianjin City (18JCJQJC47500), and the Program of Introducing Talents of Discipline to Universities (B06006) for financial support.
Author contributions
J.L.G. supervised the project. J.L.G., T.W., G.Z., and Z.J.Z. conceptualized the project. G.Z. H.M.L., and H.G synthesized Cu-based catalysts. G.Z., H.Y.W., J. Y., and J.Y.G. conducted the catalytic tests and the related data processing. Q.Z.W. fabricated the solar panel. Z.J.Z. and D.F.C. carried out the theoretical calculations. All the authors participate in the writing of the paper.
Data availability
The authors declare that all data supporting the results of this study are available within the paper and its supplementary information files or from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Wei An, Antonio José Martín, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gong Zhang, Zhi-Jian Zhao.
Change history
7/13/2023
In this article several affiliation details were incomplete: the affiliation ‘School of Chemical Engineering and Technology, Tianjin University, Tianjin, China’ should have been ‘School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China’; ‘Key Laboratory for Green Chemical Technology of Ministry of Education, Tianjin University, Tianjin, China’ should have been ‘Key Laboratory for Green Chemical Technology of Ministry of Education, Tianjin University, Tianjin 300072, China’; ‘Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin, China’ should have been ‘Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China’; ‘Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou, China’ should have been ‘Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou 350207, China’. The original article has been corrected.
Contributor Information
Tuo Wang, Email: wangtuo@tju.edu.cn.
Jinlong Gong, Email: jlgong@tju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-26053-w.
References
- 1.Kattel S, Ramirez PJ, Chen JG, Rodriguez JA, Liu P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science. 2017;355:1296–1299. doi: 10.1126/science.aal3573. [DOI] [PubMed] [Google Scholar]
- 2.Dinh CT, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science. 2018;360:783–787. doi: 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- 3.Nitopi S, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019;119:7610–7672. doi: 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- 4.Li F, et al. Molecular tuning of CO2-to-ethylene conversion. Nature. 2020;577:509–513. doi: 10.1038/s41586-019-1782-2. [DOI] [PubMed] [Google Scholar]
- 5.Yang PP, et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ fuels. J. Am. Chem. Soc. 2020;142:6400–6408. doi: 10.1021/jacs.0c01699. [DOI] [PubMed] [Google Scholar]
- 6.Zhong M, et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature. 2020;581:178–183. doi: 10.1038/s41586-020-2242-8. [DOI] [PubMed] [Google Scholar]
- 7.Ma WC, et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C−C coupling over fluorine-modified copper. Nat. Catal. 2020;3:478–487. doi: 10.1038/s41929-020-0450-0. [DOI] [Google Scholar]
- 8.Jung H, et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C−C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 2019;141:4624–4633. doi: 10.1021/jacs.8b11237. [DOI] [PubMed] [Google Scholar]
- 9.Durand WJ, Peterson AA, Studt F, Abild-Pedersen F, Norskov JK. Structure effects on the energetics of the electrochemical reduction of CO2 by copper surfaces. Surf. Sci. 2011;605:1354–1359. doi: 10.1016/j.susc.2011.04.028. [DOI] [Google Scholar]
- 10.Jiang K, et al. Metal ion cycling of Cu foil for selective C−C coupling in electrochemical CO2 reduction. Nat. Catal. 2018;1:111–119. doi: 10.1038/s41929-017-0009-x. [DOI] [Google Scholar]
- 11.Xia Y, Xiong Y, Lim B, Skrabalak SE. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009;48:60–103. doi: 10.1002/anie.200802248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Yang G, Zhang Z, Jin M, Yin Y. Selectivity on etching: creation of high-energy facets on copper nanocrystals for CO2 electrochemical reduction. ACS Nano. 2016;10:4559–4564. doi: 10.1021/acsnano.6b00602. [DOI] [PubMed] [Google Scholar]
- 13.De Gregorio GL, et al. Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities. ACS Catal. 2020;10:4854–4862. doi: 10.1021/acscatal.0c00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HX, et al. Self-selective catalyst synthesis for CO2 reduction. Joule. 2019;3:1927–1936. doi: 10.1016/j.joule.2019.05.023. [DOI] [Google Scholar]
- 15.Wang Y, et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 2019;3:98–106. doi: 10.1038/s41929-019-0397-1. [DOI] [Google Scholar]
- 16.Tao AR, Habas S, Yang PD. Shape control of colloidal metal nanocrystals. Small. 2008;4:310–325. doi: 10.1002/smll.200701295. [DOI] [Google Scholar]
- 17.Niu Z, Li Y. Removal and utilization of capping agents in nanocatalysis. Chem. Mater. 2013;26:72–83. doi: 10.1021/cm4022479. [DOI] [Google Scholar]
- 18.Hou YH, et al. Photonic curing: activation and stabilization of metal membrane catalysts (MMCs) for the electrochemical reduction of CO2. ACS Catal. 2019;9:9518–9529. doi: 10.1021/acscatal.9b03664. [DOI] [Google Scholar]
- 19.Nwabara UO, Cofell ER, Verma S, Negro E, Kenis PJA. Durable cathodes and electrolyzers for the efficient aqueous electrochemical reduction of CO2. ChemSusChem. 2020;13:855–875. doi: 10.1002/cssc.201902933. [DOI] [PubMed] [Google Scholar]
- 20.Yang H., et al. Recent progress in self-supported catalysts for CO2 electrochemical reduction. Small Methods4, 1900826 (2020).
- 21.Ott S, et al. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020;19:77–85. doi: 10.1038/s41563-019-0487-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y-G, Kang Y, Huang J. Fluidized electrocatalysis. CCS Chem. 2020;2:31–41. doi: 10.31635/ccschem.020.201900065. [DOI] [Google Scholar]
- 23.Wei X, Li Y, Chen L, Shi J. Formic acid electro-synthesis by concurrent cathodic CO2 reduction and anodic CH3OH oxidation. Angew. Chem. Int. Ed. 2021;60:3148–3155. doi: 10.1002/anie.202012066. [DOI] [PubMed] [Google Scholar]
- 24.Dobrev D. Ion-beam-induced texture formation in vacuum-condensed thin metal films. Thin Solid Films. 1982;92:41–53. doi: 10.1016/0040-6090(82)90186-9. [DOI] [Google Scholar]
- 25.Abadias G. Stress and preferred orientation in nNitride-based PVD coatings. Surf. Coat. Technol. 2008;202:2223–2235. doi: 10.1016/j.surfcoat.2007.08.029. [DOI] [Google Scholar]
- 26.Lv YH, et al. Influence of substrate bias voltage on structure and properties of the CrAlN films deposited by unbalanced magnetron sputtering. Appl. Surf. Sci. 2012;258:3864–3870. doi: 10.1016/j.apsusc.2011.12.048. [DOI] [Google Scholar]
- 27.Zheng JY, Bao SH, Guo Y, Jin P. Anatase TiO2 films with dominant {001} facets fabricated by direct-current reactive magnetron sputtering at room temperature: oxygen defects and enhanced visible-light photocatalytic behaviors. ACS Appl. Mater. Interfaces. 2014;6:5940–5946. doi: 10.1021/am500979j. [DOI] [PubMed] [Google Scholar]
- 28.Li F, et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 2019;3:75–82. doi: 10.1038/s41929-019-0383-7. [DOI] [Google Scholar]
- 29.Cao L, et al. Mechanistic insights for low-overpotential electroreduction of CO2 to CO on copper nanowires. ACS Catal. 2017;7:8578–8587. doi: 10.1021/acscatal.7b03107. [DOI] [Google Scholar]
- 30.Droog JMM, Schlenter B. Oxygen electrosorption on copper single crystal electrodes in sodium hydroxide solution. J. Electroanal. Chem. Interfacial Electrochem. 1980;112:387–390. doi: 10.1016/S0022-0728(80)80421-9. [DOI] [Google Scholar]
- 31.Schouten KJ, Qin Z, Perez Gallent E, Koper MT. Two pathways for the formation of ethylene in CO reduction on single-crystal copper electrodes. J. Am. Chem. Soc. 2012;134:9864–9867. doi: 10.1021/ja302668n. [DOI] [PubMed] [Google Scholar]
- 32.Lv JJ, et al. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 2018;30:e1803111. doi: 10.1002/adma.201803111. [DOI] [PubMed] [Google Scholar]
- 33.LaGrow AP, Ward MR, Lloyd DC, Gai PL, Boyes ED. Visualizing the Cu/Cu2O interface transition in nanoparticles with environmental scanning transmission electron microscopy. J. Am. Chem. Soc. 2017;139:179–185. doi: 10.1021/jacs.6b08842. [DOI] [PubMed] [Google Scholar]
- 34.Feng X, Jiang K, Fan S, Kanan MW. Grain-boundary-dependent CO2 electroreduction activity. J. Am. Chem. Soc. 2015;137:4606–4609. doi: 10.1021/ja5130513. [DOI] [PubMed] [Google Scholar]
- 35.Jin M, et al. Shape-controlled synthesis of copper nanocrystals in an aqueous solution with glucose as a reducing agent and hexadecylamine as a capping agent. Angew. Chem. Int. Ed. 2011;50:10560–10564. doi: 10.1002/anie.201105539. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Varela AS, Bergmann A, Kuhl S, Strasser P. Catalyst particle density controls hydrocarbon product selectivity in CO2 electroreduction on CuOx. ChemSusChem. 2017;10:4642–4649. doi: 10.1002/cssc.201701179. [DOI] [PubMed] [Google Scholar]
- 37.Kas RK, et al. Electrochemical CO2 reduction on nanostructured metal electrodes: fact or defect? Chem. Sci. 2020;11:1738–1749. doi: 10.1039/C9SC05375A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reske R, et al. Controlling catalytic selectivities during CO2 electroreduction on thin Cu metal overlayers. J. Phy. Chem. Lett. 2013;4:2410–2413. doi: 10.1021/jz401087q. [DOI] [Google Scholar]
- 39.Tan YC, Lee KB, Song H, Oh J. Modulating local CO2 concentration as a general strategy for enhancing C−C coupling in CO2 electroreduction. Joule. 2020;4:1104–1120. doi: 10.1016/j.joule.2020.03.013. [DOI] [Google Scholar]
- 40.Lee SH, et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ x-ray characterization. J. Am. Chem. Soc. 2021;143:588–592. doi: 10.1021/jacs.0c10017. [DOI] [PubMed] [Google Scholar]
- 41.Jouny M, Luc W, Jiao F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 2018;1:748–755. doi: 10.1038/s41929-018-0133-2. [DOI] [Google Scholar]
- 42.Heyes J, Dunwell M, Xu B. CO2 reduction on Cu at low overpotentials with surface-enhanced in situ spectroscopy. J. Phy. Chem. C. 2016;120:17334–17341. doi: 10.1021/acs.jpcc.6b03065. [DOI] [Google Scholar]
- 43.Sartin MM, et al. Effect of particle shape and electrolyte cation on CO adsorption to copper oxide nanoparticle electrocatalysts. J. Phys. Chem. C. 2018;122:26489–26498. doi: 10.1021/acs.jpcc.8b08541. [DOI] [Google Scholar]
- 44.Gunathunge CM, Ovalle VJ, Li YW, Janik MJ, Waegele MM. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 2018;8:7507–7516. doi: 10.1021/acscatal.8b01552. [DOI] [Google Scholar]
- 45.Ren D, Fong J, Yeo BS. The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction. Nat. Commun. 2018;9:925. doi: 10.1038/s41467-018-03286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren D, Gao J, Zakeeruddin SM, Grätzel M. New insights into the interface of electrochemical flow cells for carbon dioxide reduction to ethylene. J. Phys. Chem. Lett. 2021;12:7583–7589. doi: 10.1021/acs.jpclett.1c02043. [DOI] [PubMed] [Google Scholar]
- 47.Goodpaster JD, Bell AT, Head-Gordon M. Identification of possible pathways for C−C bond formation during electrochemical reduction of CO2: new theoretical insights from an improved electrochemical model. J. Phys. Chem. Lett. 2016;7:1471–1477. doi: 10.1021/acs.jpclett.6b00358. [DOI] [PubMed] [Google Scholar]
- 48.Schreier M, Yoon Y, Jackson MN, Surendranath Y. Competition between H and CO for active sites governs copper-mediated electrosynthesis of hydrocarbon fuels. Angew. Chem. Int. Ed. 2018;57:10221–10225. doi: 10.1002/anie.201806051. [DOI] [PubMed] [Google Scholar]
- 49.Clark EL, Hahn C, Jaramillo TF, Bell AT. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 2017;139:15848–15857. doi: 10.1021/jacs.7b08607. [DOI] [PubMed] [Google Scholar]
- 50.Li J, et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2019;2:1124–1131. doi: 10.1038/s41929-019-0380-x. [DOI] [Google Scholar]
- 51.Malkani AS, Dunwell M, Xu B. Operando spectroscopic investigations of copper and oxide-derived copper catalysts for electrochemical CO reduction. ACS Catal. 2018;9:474–478. doi: 10.1021/acscatal.8b04269. [DOI] [Google Scholar]
- 52.Sandberg RB, Montoya JH, Chan K, Norskov JK. CO−CO coupling on Cu facets: coverage, strain, and field effects. Surf. Sci. 2016;654:56–62. doi: 10.1016/j.susc.2016.08.006. [DOI] [Google Scholar]
- 53.Resasco J, et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 2017;139:11277–11287. doi: 10.1021/jacs.7b06765. [DOI] [PubMed] [Google Scholar]
- 54.Ren D, Loo NWX, Gong L, Yeo BS. Continuous production of ethylene from carbon dioxide and water using intermittent sunlight. ACS Sustain. Chem. Eng. 2017;5:9191–9199. doi: 10.1021/acssuschemeng.7b02110. [DOI] [Google Scholar]
- 55.Gao J, et al. Selective C−C coupling in carbon dioxide electroreduction via efficient spillover of intermediates as supported by operando Raman pectroscopy. J. Am. Chem. Soc. 2019;141:18704–18714. doi: 10.1021/jacs.9b07415. [DOI] [PubMed] [Google Scholar]
- 56.Weng LC, Bell AT, Weber AZ. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 2018;20:16973–16984. doi: 10.1039/C8CP01319E. [DOI] [PubMed] [Google Scholar]
- 57.Burdyny T, Smith WA. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019;12:1442–1453. doi: 10.1039/C8EE03134G. [DOI] [Google Scholar]
- 58.Dunwell M, et al. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold. J. Am. Chem. Soc. 2017;139:3774–3783. doi: 10.1021/jacs.6b13287. [DOI] [PubMed] [Google Scholar]
- 59.Li J, et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 2021;12:3264. doi: 10.1038/s41467-021-23582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Luo W, Zuttel A. Crossover of liquid products from electrochemical CO2 reduction through gas diffusion electrode and anion exchange membrane. J. Catal. 2020;385:140–145. doi: 10.1016/j.jcat.2020.03.013. [DOI] [Google Scholar]
- 61.Ma M, et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 2020;13:977–985. doi: 10.1039/D0EE00047G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luc W, Rosen J, Jiao F. An Ir-based anode for a practical CO2 electrolyzer. Catal. Today. 2017;288:79–84. doi: 10.1016/j.cattod.2016.06.011. [DOI] [Google Scholar]
- 63.Kresse G, Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B Condens. Matter. 1994;49:14251–14269. doi: 10.1103/PhysRevB.49.14251. [DOI] [PubMed] [Google Scholar]
- 64.Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010;132:154104. doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- 65.Liu S, Zong J, Zhao Z-J, Gong J. Exploring the initial oxidation of Pt, Pt3Ni, Pt3Au (111) surfaces: a genetic algorithm based global optimization with density functional theory. Green. Chem. Eng. 2020;1:56–62. doi: 10.1016/j.gce.2020.09.006. [DOI] [Google Scholar]
- 66.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 67.Gauthier JA, Dickens CF, Chen LD, Doyle AD, Norskov JK. Solvation effects for oxygen evolution reaction catalysis on IrO2(110) J. Phys. Chem. C. 2017;121:11455–11463. doi: 10.1021/acs.jpcc.7b02383. [DOI] [Google Scholar]
- 68.Montoya JH, Shi C, Chan K, Norskov JK. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 2015;6:2032–2037. doi: 10.1021/acs.jpclett.5b00722. [DOI] [PubMed] [Google Scholar]
- 69.Cheng D, et al. The nature of active sites for carbon dioxide electroreduction over oxide-derived copper catalysts. Nat. Commun. 2021;12:395. doi: 10.1038/s41467-020-20615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the results of this study are available within the paper and its supplementary information files or from the corresponding authors upon reasonable request.