Abstract
Tumors escape immune attack by upregulating the surface expression of PD-L1, which interacts with PD-1 on T cells to activate immune inhibitory signaling. Anti-PD-1 treatments can effectively block this inhibitory signaling and activate antitumor immune responses. However, anti-PD-1 treatment also has a tumor suppressive effect in patients whose tumor cells do not express PD-L1. The underlying mechanisms are poorly defined. Here, we report that exosomes from bone marrow-derived cells (BMDCs) in tumor-bearing mice, but not in healthy mice, carry PD-L1. PD-L1 on these exosomes is biofunctional and can inhibit CD8+ T cell proliferation and activation in vitro and in vivo. The transfer of exogenous exosomes from BMDCs and the inhibition of the production of endogenous exosomes by BMDCs promote and suppress tumor growth, respectively. PD-L1+ exosomes from BMDCs can be found in tumor tissues. In addition, exosomes from BMDCs promote tumor metastasis in a PD-L1-dependent manner. Therefore, our results indicate that exosomes from BMDCs play important roles in tumor immunosuppression via PD-L1.
Keywords: Bone marrow-derived cells, Exosomes, PD-L1
Subject terms: Tumour immunology, Cancer
Introduction
The clinical treatment of patients with cancer by immune checkpoint blockade, such as anti-CTLA-4 and anti-PD-1, represents a milestone in tumor immunotherapy. PD-1 is expressed on activated T cells. When binding to its ligand, PD-L1, T cells receive an inhibitory signal that attenuates their activity1. PD-L1 is mainly expressed on tumor cells that have been stimulated with IFN-γ2. However, anti-PD-1 treatment also shows a tumor suppressive effect in patients whose tumor cells do not express PD-L13,4. The mechanism driving this suppression is still poorly understood. Although a recent publication demonstrated that anti-PD-1 treatment probably generates therapeutic effects by activating PD-L1 signaling in NK cells5, the mechanisms driving anti-PD-1 effectiveness when tumor cells do not express PD-L1 are far from being elucidated.
Exosomes are nanoscale extracellular vesicles with lipid bilayer structures. Exosomes are released into the external environment after multivesicular bodies fuse with the plasma membrane6,7. Exosomes play a critical role in communication between cells. Melanocyte exosomes “educate” bone marrow progenitor cells and drive them toward a pro-metastatic phenotype through the transfer of MET8. Exosomes from B cells can inhibit post-chemotherapeutic immune responses through the hydrolysis of ATP by CD39 and CD739. Exosomes from brain astrocytes downregulate PTEN in tumor cells through the transfer of microRNAs that leads to the outgrowth of brain metastatic tumors10. Melanocyte exosomes carry PD-L1 and can suppress the function of CD8+ T cells and facilitate tumor growth via PD-L111. PD-L1 on tumor-derived exosomes has also been reported to suppress systemic antitumor immunity and memory12. Whether PD-L1-containing exosomes from nontumor cells can mediate immunosuppression has yet to be explored.
Bone marrow-derived cells (BMDCs) are involved in tumor progression. VEGFR1+ BMDCs home to tumor-specific premetastatic sites and promote tumor metastasis13. cKit+ BMDCs are reported to be closely associated with the establishment of a premetastatic niche for tumors14. Bone marrow-derived macrophages play a key roles in the liver metastasis of pancreatic cancer15. IBA1+ BMDCs enhance the outgrowth of brain metastatic tumor cells by enhancing their proliferation and inhibiting their apoptosis10. Given the important role of BMDCs in tumor metastasis and growth, we assume that exosomes from BMDCs (BMDC-EXOs) may also contribute to tumor development.
Here, we report that exosomes from the BMDCs of tumor-bearing mice (BMDC-EXOsTu), but not BMDC-EXOs from healthy mice, carry PD-L1. In a PD-L1-dependent manner, BMDC-EXOsTu can inhibit CD8+ T cell proliferation in vitro and can promote tumor growth by suppressing antitumor CD8+ T cell responses. Endogenous BMDC-EXOsTu are able to migrate into tumor tissues and inhibit CD8+ T cell proliferation in a PD-L1-dependent manner. Furthermore, BMDC-EXOsTu are capable of promoting tumor metastasis by impairing the antitumor CD8+ T cell responses at metastatic sites via PD-L1. Altogether, our data reveal that BMDC-EXOsTu have a PD-L1-dependent tumor-promoting effect, which provides a reasonable explanation for why anti-PD-1 treatment is still effective when tumor cells do not express PD-L1.
Materials and methods
Mice and cell lines
Female C57BL/6J and BALB/c (6–8-week-old) mice, thymus-deficient BALB/c nude mice, and CD45.2 mice were purchased from Joint Ventures Sipper BK Experimental Animal Co. NOD Prkdcscid Il2rg−/− (NSG) mice were purchased from Biocytogen (Beijing, China). Mice were housed in a specific pathogen-free facility, and the experimental protocols were approved by the Animal Care and Use Committee of the School of Medicine, Zhejiang University. Murine Lewis lung cancer (LLC) cells, MC38 colon cancer cells, 4T1 breast cancer cells, and HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA, USA).
Reagents
Type I and D collagenase, RNase and DNase I, hyaluronidase, Percoll, phorbol 12-myristate 13-acetate, ionomycin, Histopaque®-1077, recombinant murine IL-2, and a lactate dehydrogenase (LDH) cytotoxicity detection kit were obtained from Sigma-Aldrich (St. Louis, MO, USA). A BCA protein assay kit, permeabilization buffer and intracellular fixation buffer, MagniSortTM streptavidin-positive selection beads, a CD8+ T cell isolation kit, CFSE, and HRP-conjugated secondary antibodies were purchased from Thermo Fisher (Waltham, MA, USA). Polyvinylidene difluoride membranes were purchased from Millipore (Billerica, MA, USA). Anti-CD3, anti-CD28, anti-CD8, anti-PD-L1, and anti-PD-1 antibodies were obtained from Bio X cells (West Lebanon, NH, USA). Lentiviruses expressing a mouse negative control (NC), Smpd3-specific shRNA, and Rab27a-specific shRNA (Lenti-NC shRNA, Lenti-Smpd3 shRNA, and Lenti-Rab27a shRNA, respectively) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-GRP94, anti-Tsg101, anti-Alix, anti-CD63, anti-PD-L1, anti-CD8, and anti-β-Actin antibodies were purchased from Abcam (Cambridge, MA, USA). Anti-IFN-γ, anti-Ki-67, anti-Granzyme B, anti-CD45.2, anti-CD9, anti-CD63, anti-Gr-1, anti-c-Kit, anti-CD11b, anti-PD-L1, anti-PD-L2, anti-CD3, anti-CD19, anti-CD11c, and anti-F4/80 antibodies were purchased from BioLegend (San Diego, CA, USA).
Isolation of exosomes
To isolate BMDC-EXOs and BMDC-EXOsTu, the tibia and femur of healthy and tumor-bearing mice were isolated and placed into PBS. BMDCs in the tibia and femur were removed with the needle of a 1-ml syringe. To prepare tumor tissue suspensions, tumor tissues were detached and dissociated enzymatically for 2 h with 1 mg/ml type I collagenase in the presence of 50 U/ml RNase and DNase I. The BMDC and tumor cell suspensions were filtered using a 0.22-μm filter and were then differentially centrifuged at 300 × g for 10 min, 1200 × g for 20 min, and 10,000 × g for 30 min at 4 °C. The supernatants from the final centrifugation were ultracentrifuged at 100,000 × g for 1 h at 4 °C. After removing the supernatants, the exosomal pellets were washed with a large volume of ice-cold PBS and centrifuged at 100,000 × g for 1 h at 4 °C. The final pellets were resuspended in PBS. The amount of exosomal proteins recovered was measured by a BCA protein assay kit.
Electron microscopy
Exosomes were diluted in 100 μl of PBS, and 20 μl of the suspension was placed onto formvar carbon-coated copper grids at room temperature (RT) for 1 min. The excess suspension was removed using filter paper. Exosomes were stained with 2% phosphotungstic acid at RT for 5 min. The grids were then fixed with 2.5% glutaraldehyde at RT for 5 min, followed by rinsing with PBS for three times. Images were observed with a Philips Tecnai-10 transmission electron microscope (Hanover, MD, USA) operating at 80 kV.
Western blotting
A total of 10 μg of exosomes or crude proteins extracted from cell lysates was separated on a 10% SDS-PAGE gel and transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with 5% BSA in TBST and then incubated with the corresponding primary antibodies overnight at 4 °C. After incubation with HRP-coupled secondary antibodies for 1 h, the membranes were scanned using a Tanon 4500 (Shanghai, China).
Flow cytometry
Exosomes were coated onto 4-μm-diameter aldehyde/sulfate latex beads, as described previously16. To examine IFN-γ+CD8+ T cells in tumor-infiltrating lymphocytes (TILs), TILs were prepared by enzymatic digestion with 1 mg/ml type I collagenase, 0.5 mg/ml DNase I, and 25 μg/ml hyaluronidase at 37 °C for 30 min, followed by Percoll gradient purification. For the intracellular staining of IFN-γ, the TILs were stimulated for 4 h at 37 °C in RPMI 1640 medium containing phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 μg/ml). After staining for CD8, the cells were fixed and permeabilized using permeabilization buffer and intracellular fixation buffer according to the manufacturer’s instructions and were then stained for intracellular IFN-γ, Ki-67, and Granzyme B. The percentage of CD8+ T cells was determined by a FACSCalibur flow cytometer (Franklin, NJ, USA).
Isolation of the CD45.2+ exosome subset from tumor tissues
Exosomes from tumor tissues were incubated with biotinylated anti-CD45.2 (final concentration of 1 mg/ml) for 4 h at RT with gentle shaking and mixed with 40 ml of MagniSortTM streptavidin-positive selection beads overnight at 4 °C with gentle shaking. CD45.2+ exosomes (CD45.2-EXOs) were isolated using a magnet followed by centrifugation at 100,000×g for 1 h. The pellets were resuspended in PBS.
CD8+ T cell proliferation assay
Mouse CD8+ T cells were isolated from splenocytes by negative selection using a mouse CD8+ T cell isolation kit according to the manufacturer’s instructions. The CD8+ T cells were labeled with CFSE and were cultured at a density of 1 × 106/ml in 96-well plates that were precoated with 2 μl of anti-CD3 and anti-CD28 antibodies. Twenty-four hours later, these exosomes (5 μg/ml) or exosomes that were preincubated with 10 μg/ml anti-PD-L1 for 1 h at 37 °C were added to cell cultures. Then, the cells were cultured for another 3 days, harvested and analyzed by flow cytometry.
In vivo tumor growth experiments
A total of 1 × 106 LLC cells or 2 × 106 MC38 cells were injected subcutaneously into C57BL/6 mice or nude mice on day 0. Then, the mice were intravenously injected with 10 μg BMDC-EXOsTu on days 5, 8, 11, and 14. Tumor size was monitored every other day by Vernier calipers. In some experiments, the BMDC-EXOsTu were incubated with 10 μg/ml anti-PD-L1 for 1 h at 37 °C before injection. On day 18, the tumor-bearing mice were euthanized by intraperitoneal injection of 50 mg/kg pentobarbital sodium.
Immunohistochemical assay
After dissection, the tumor tissues were immediately fixed in 10% paraformaldehyde and subjected to immunohistochemical staining. Images of the sections were randomly captured, and the numbers of CD8+ T cells in each field were counted.
Cytotoxic assay of cytotoxic T cells (CTLs)
For the CTL cytotoxic assay, splenic lymphocytes were isolated by Percoll gradient purification on day 18 and cocultured with 2 × 105/ml inactivated MC38 (50 μg/ml mitomycin C for 45 min) at a ratio of 10:1 for 7 days. On the second day of restimulation, 50 U/ml recombinant murine IL-2 was added. Live T cells were recovered by density sedimentation using Histopaque®-1077 and resuspended to 107 cells/ml as CTL effector cells. The syngeneic LLC cells were used as control target cells. The CTL activity was evaluated by an LDH cytotoxicity detection kit. The reaction was measured in an ELISA reader at a wavelength of 490 nm. The percentage of specific LDH release was calculated as 100 × (experimental release − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release)17.
Inhibition of endogenous BMDC-EXOTu production
Lenti-NC shRNA, Lenti-Smpd3 shRNA, or Lenti-Rab27a shRNA was administered by intra-bone marrow (IBM) injection into tumor-bearing mice [1 × 106 infection units of virus (IFU) per mouse] on days 0 and 7. The IBM injections were administered as previously described18.
Reconstitution of mice with chimeric bone marrow
BMDCs were isolated from CD45.2 mice, and 1 × 107 BMDCs were injected intravenously into NSG mice that had received irradiation with 1.5 Gy the day before. The chimeric mice were used for tumor inoculation 6–8 weeks after radiation exposure.
In vivo tumor metastasis experiments
A total of 5 × 105 4T1 cells were injected into the mammary fat pad of female BALB/c mice. Primary tumors were surgically resected on day 16. Then, the mice were intravenously injected with PBS, 10 μg of BMDC-EXO or BMDC-EXOTu on days 17, 19, and 21. The mice were sacrificed to analyze lung metastases with a clonogenic assay on day 25. TILs were also isolated to detect IFN-γ+CD8+ and Ki-67+CD8+ T cells on day 25. In some experiments, the BMDC-EXOsTu were incubated with 10 μg/ml anti-PD-L1 for 1 h at 37 °C before injection. For the depletion of CD8+ T cells, the mice were intraperitoneally injected with 50 μg of anti-CD8 antibody on days 15, 17, 19, 21, and 23.
Clonogenic assay
Lungs were collected and chopped into pieces before being dissociated in DMEM supplemented with 10% fetal calf serum containing 1.5 mg/ml collagenase type D for 30 min in a 37 °C incubator and shaking at 178 rpm. Organs were plated at various dilutions in DMEM supplemented with 10% fetal calf serum and 60 μM 6-thioguanine. Individual colonies representing micrometastases were counted for 5–10 days after plating, as previously described19.
Statistical data analysis
All statistical analyses were performed using Prism 8.0 software (San Diego, CA, USA). The results are expressed as the means ± s.d. Data were analyzed by an unpaired Student’s t-test. Differences were considered significant at p < 0.05.
Results
BMDC-EXOsTu inhibit CD8+ T cell proliferation via PD-L1
We isolated BMDC-EXOs and confirmed that they had a vesicle-like morphology with a lipid bilayer structure (Fig. 1a). The protein components of the BMDCs and BMDC-EXOs were also determined, and the BMDC-EXOs, but not the BMDCs, were negative for the endoplasmic reticulum protein GRP94. However, the BMDC-EXOs were positive for the multivesicular body-related proteins CD63, Tsg101, and Alix (Fig. 1b). We also isolated BMDC-EXOsTu from MC38 tumor-bearing mice and found that these cells had morphology, protein components, and size distribution similar to BMDC-EXOs; however, BMDC-EXOsTu contained more CD63 and Alix than the BMDC-EXOs (Fig. 1a–c). Interestingly, BMDCsTu and BMDC-EXOsTu, but not BMDCs or BMDC-EXOs, contained PD-L1 (Fig. 1b). The PD-L1 expression of the BMDC-EXOsTu was confirmed by immunogold labeling (Fig. 1d). Membranous PD-L1 on BMDC-EXOsTu was also detected (Fig. 1e, f). However, PD-L2 could not be detected on the BMDC-EXOsTu (Fig. 1e, f). Furthermore, low levels of CD11b, a marker of myeloid cells, were also detected on the BMDC-EXOs and BMDC-EXOsTu (Fig. 1e, f). Exosomes from both these cells were positive for the exosomal marker CD9 (Fig. 1e, f). Then, we examined whether BMDC-EXOsTu could inhibit T cell activation via PD-L1. We found that BMDC-EXOsTu, but not BMDC-EXOs, inhibited anti-CD3/CD28-stimulated CD8+ T cell proliferation (Fig. 1g). After preincubation with PD-L1-neutralizing antibodies, the BMDC-EXOsTu could no longer inhibit anti-CD3/CD28-stimulated CD8+ T cell proliferation (Fig. 1g). These results suggest that tumors can confer immunosuppressive ability to BMDC-EXOsTu via PD-L1.
Fig. 1. BMDC-EXOsTu inhibit CD8+ T cell proliferation via PD-L1.
a The morphology of exosomes was detected by electron microscopy. Scale, 100 nm. b Proteins in the BMDCs and BMDCsTu and the BMDC-derived, and BMDCTu-derived exosomes were detected by western blotting. c Size distribution of the exosomes was measured by NTA assay. d Exosomes labeled with immunogold were detected by electron microscopy. Scale, 100 nm. e, f After adsorbing onto latex beads, the proteins on the exosomes were detected by flow cytometry (e) and statistically analyzed (f). MFI mean fluorescent intensity. g CFSE-labeled CD8+ T cells were stimulated with 2 μg/ml anti-CD3 and anti-CD28 antibodies for 24 h. Then, 5 μg/ml exosomes were preincubated with or without 10 μg/ml anti-PD-L1 for 1 h and were added and cocultured for another 3 days. Proliferation of the CD8+ T cells was measured by CFSE dilution. Ctrl, group with PBS treatment. ISO isotype control antibodies. ns not significant, **P < 0.01 and ***P < 0.001 (unpaired Student’s t-test). Representative results from three independent experiments are shown (the means and s.d.) (n = 3).
BMDC-EXOsTu inhibit antitumor immunity in vivo
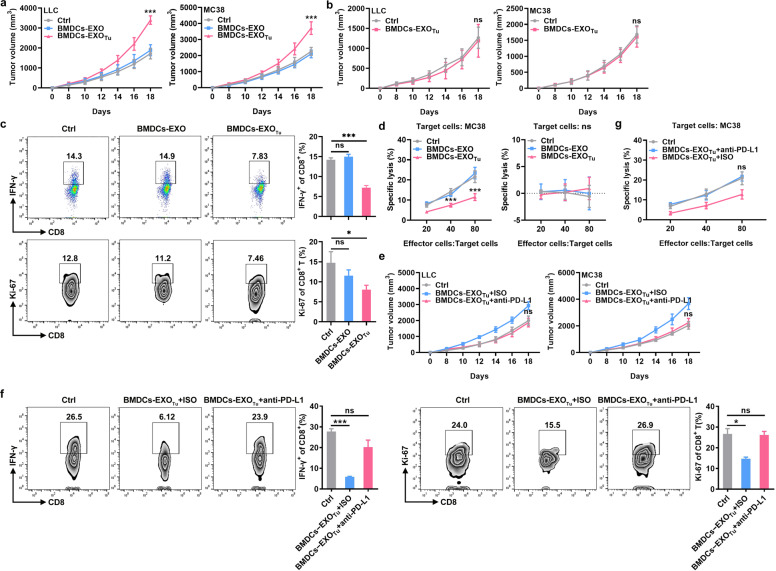
Next, we tested the effect of BMDC-EXOsTu on antitumor immunity. We found that when LLC and MC38 tumor cells were subcutaneously inoculated into wild-type (WT) mice, intravenous treatment with BMDC-EXOsTu, but not with BMDC-EXOs significantly promoted tumor growth in vivo (Fig. 2a). However, BMDC-EXOsTu did not affect tumor growth when LLC or MC38 tumor cells were subcutaneously inoculated into nude mice (Fig. 2b), indicating an essential role of T cells in BMDC-EXOTu-mediated tumor promotion. We found fewer IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of the MC38 tumor tissues after BMDC-EXOTu, but not BMDC-EXOs, treatment (Fig. 2c). To further assess the effect of BMDCs-EXOsTu on the induction of the tumor-specific immune response in vivo, splenic lymphocytes were isolated from MC38-tumor-bearing mice and restimulated in vitro with inactivated MC38 cells to induce CTLs. BMDC-EXOTu, but not BMDC-EXO, treatment led to lower lysis of MC38 cells by CTLs, and the cytotoxicity of the MC38 cells induced by the CTLs was specific, as no cytotoxicity was induced against syngeneic LLC cells (Fig. 2d). To examine the role of PD-L1 in this process, BMDC-EXOsTu were preincubated with PD-L1-neutralizing antibodies before injection. We found that BMDC-EXOsTu no longer accelerated LLC or MC38 tumor growth after cell-surface PD-L1 was blocked (Fig. 2e). Blocking PD-L1BMDC-EXOsTu did not decrease the number of IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of the MC38 tumor tissues (Fig. 2f). Furthermore, BMDC-EXOTu treatment did not decrease the cytotoxicity of the MC38 cells induced by the CTLs (Fig. 2g). These results demonstrate that BMDC-EXOsTu can inhibit antitumor immunity in a PD-L1-dependent manner.
Fig. 2. BMDC-EXOsTu inhibit antitumor immunity in vivo.
a, b A total of 1 × 106 LLC cells or 2 × 106 MC38 cells were injected subcutaneously into C57BL/6 (a) or nude (b) mice on day 0. Then, the mice were intravenously injected with 10 μg of BMDC-EXOs or BMDC-EXOTu on days 5, 8, 11, and 14. Tumor size was monitored every other day. c IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of tumor tissues from MC38-tumor-bearing mice (a) were analyzed by flow cytometry. d Splenic lymphocytes from the MC38-tumor-bearing mice were isolated and restimulated in vitro with inactivated MC38 cells at a ratio of 10:1 for 7 days in the presence of 50 U/ml recombinant murine IL-2. Cytotoxicity against MC38 or LLC cells was determined by LDH release assay. e–g A total of 1 × 106 LLC cells or 2 × 106 MC38 cells were injected subcutaneously into C57BL/6 mice on day 0. After incubation with 10 μg/ml anti-PD-L1 for 1 h, 10 μg of BMDC-EXOTu was intravenously injected into mice on days 5, 8, 11 and 14. Tumor size was monitored every other day (e). IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of tumor tissues from MC38 tumor-bearing mice were analyzed by flow cytometry (f). The cytotoxicity induced by the CTLs in the MC38 cells was determined by LDH release assay (g). Ctrl, group with PBS treatment. ISO isotype control antibodies. ns not significant, *P < 0.05 and ***P < 0.001 (unpaired Student’s t-test). Representative results from three independent experiments are shown (the means and s.d.) (n = 3–5).
Inhibition of endogenous exosome release from BMDCs improves antitumor immunity
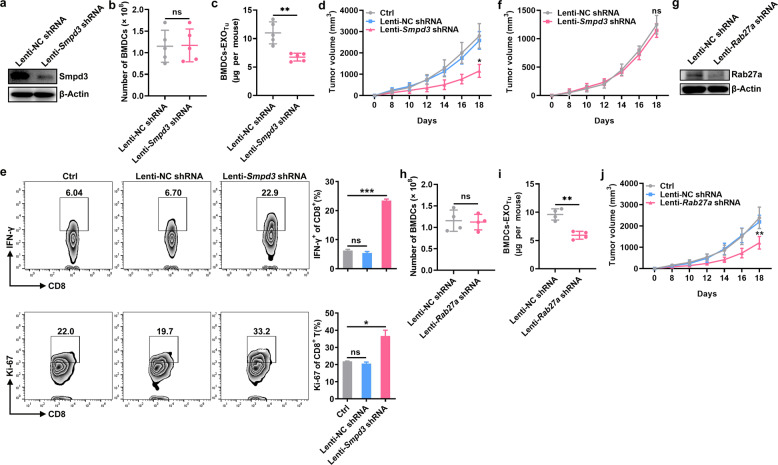
Given that the transfer of exogenous BMDC-EXOsTu inhibited antitumor immunity, we surmised that inhibiting the release of exosomes from BMDCs would improve antitumor immunity. The Smpd3 gene is an effective target for the inhibition of exosome production20. Therefore, we inhibited the exosome release from BMDCs after MC38 tumor establishment by performing IBM injections with Lenti-Smpd3 shRNA. We confirmed that the knockdown of Smpd3 by Lenti-Smpd3 shRNA injection in the BMDCs did not affect the number of BMDCs (Fig. 3a, b). In addition, BMDC-EXOsTu in the MC38-tumor-bearing mice were greatly reduced after Lenti-Smpd3 shRNA injection compared with the those after Lenti-NC shRNA injection (Fig. 3c). Lenti-Smpd3 shRNA, but not Lenti-NC shRNA, treatment significantly inhibited MC38 tumor growth (Fig. 3d). An increase in the number of IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs was observed in the Lenti-Smpd3 shRNA-treated mice compared to the Lenti-NC shRNA-treated mice (Fig. 3e). However, when an immune checkpoint blockade was combined with anti-PD-1 monoclonal antibodies, IBM injections with Lenti-Smpd3 shRNA did not show enhanced tumor suppression (Fig. 3f). Inhibition of exosome generation can also be achieved by knocking down Rab27a21. To exclude the possibility that tumor suppression by the IBM injections with Lenti-Smpd3 shRNA was caused by Smpd3 gene silencing itself, we inhibited the release of exosomes from BMDCs by IBM injections with Lenti-Rab27a shRNA. Silencing Rab27a did not affect the number of BMDCs but greatly reduced the number of BMDC-EXOsTu (Fig. 3g–i). Lenti-Rab27a shRNA also significantly inhibited MC38 tumor growth (Fig. 3j). These results suggest that endogenous exosomes from BMDCs are involved in the inhibition of antitumor immunity via PD-1 signaling.
Fig. 3. Inhibition of endogenous exosome release from the BMDCs improves antitumor immunity.
a–e A total of 2 × 106 MC38 cells were injected subcutaneously into C57BL/6 mice on day 0. The mice also received IBM injections with 1 × 106 IFU of Lenti-NC shRNA or Lenti-Smpd3 shRNA on days 0 and 7. The Smpd3 protein in the BMDCs was detected by western blotting (a), the number of BMDCs was counted (b), and the quantification of BMDC-EXOTu protein was performed by a BCA assay (c) on day 7. Tumor size was monitored every other day (d). IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs in the tumor tissues were detected by flow cytometry (e). f A total of 2 × 106 MC38 cells were injected subcutaneously into C57BL/6 mice on day 0. The mice also received IBM injections with 1 × 106 IFU Lenti-NC shRNA or Lenti-Smpd3 shRNA on days 0 and 7. In addition, the mice were intraperitoneally injected with 80 μg of anti-PD-1 on days 0, 4, 8, 12, and 16. Tumor size was monitored every other day. g–j A total of 2 × 106 MC38 cells were injected subcutaneously into C57BL/6 mice on day 0. The mice also received IBM injections with 1 × 106 IFU of Lenti-NC shRNA or Lenti-Rab27a shRNA on days 0 and 7. The Rab27a protein in the BMDCs was detected by western blotting (g), the number of BMDCs was counted (h), and the quantification of BMDC-EXOTu protein was performed by a BCA assay (i) on day 7. Tumor size was monitored every other day (j). Ctrl, group with PBS treatment. ns not significant, *P < 0.05 and **P < 0.01 (unpaired Student’s t-test). Representative results from two independent experiments are shown (the means and s.d.) (n = 3–5).
PD-L1+ BMDC-EXOsTu can migrate into tumor tissues
To explore whether BMDC-EXOTu can migrate into tumor tissues, we reconstituted irradiated CD45.1 NSG mice with bone marrow from CD45.2 mice and established MC38 tumors in these mice. After isolation from tumor tissues, exosomes were sorted to isolate the CD45.2+ subset. We confirmed that the CD45.2-EXOs were positive for CD45.2, CD9, c-Kit, Gr-1, and CD11b (Fig. 4a). In addition, the CD45.2-EXOs were also positive for PD-L1 (Fig. 4a). However, the CD45.2-EXOs were negative for CD3, CD19, CD11c, and F4/80 (Fig. 4b). These results indicated that the CD45.2-EXOs were mostly derived from immature BMDCs and could enter tumor tissues. To test the bioactivity of PD-L1 on the CD45.2-EXOs, we examined the inhibitory effect of the CD45.2-EXOs on CD8+ T cell proliferation. We found that the CD45.2-EXOs dramatically inhibited CD8+ T cell proliferation and that this effect was abolished when the CD45.2-EXOs were preincubated with PD-L1-neutralizing antibodies (Fig. 4c). These results suggest that BMDC-EXOsTu with bioactive PD-L1 can enter tumor tissues.
Fig. 4. PD-L1+ BMDC-EXOsTu can migrate into tumor tissues.
a, b After adsorbing onto latex beads, CD45.2, CD9, c-Kit, Gr-1, CD11b, PD-L1 (a), CD3, CD19, CD11c, and F4/80 on CD45.2-EXOs were detected by flow cytometry (b). c CFSE-labeled CD8+ T cells were stimulated with 2 μg/ml anti-CD3 and anti-CD28 antibodies for 24 h. Then, 5 μg/ml CD45.2-EXOs preincubated with or without 10 μg/ml anti-PD-L1 for 1 h were added and cocultured for another 3 days. The proliferation of the CD8+ T cells was measured by CFSE dilution. Ctrl, group with PBS treatment. ISO isotype control antibodies. ns not significant and ***P < 0.001 (unpaired Student’s t-test). Representative results from three independent experiments are shown (the means and s.d.) (n = 3).
BMDC-EXOsTu promote tumor metastasis
Myeloid cells play a critical role in tumor metastasis13,22. We examined the role of BMDC-EXOsTu in tumor metastasis. BMDC-EXOsTu substantially increased the lung metastasis of 4T1 tumors (Fig. 5a, b). Additionally, we observed a reduction in the number of IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of lung tumors from the mice that had received the BMDC-EXOTu injections (Fig. 5c). After the depletion of the CD8+ T cells, the BMDC-EXOTu had no effect on the lung metastasis of 4T1 tumors (Fig. 5d, e), which demonstrated that BMDC-EXOTu-mediated tumor metastasis was CD8+ T cell-dependent. To elucidate whether PD-L1 on BMDC-EXOsTu could inhibit CD8+ T cell responses at the metastatic site, we treated mice with BMDC-EXOsTu that had been incubated with PD-L1-neutralizing antibodies and found that the BMDC-EXOTu no longer promoted the lung metastasis of 4T1 tumors (Fig. 5f, g). In addition, similar percentages of IFN-γ+CD8+ and Ki-67+CD8+ T cells were found in the TILs of lung tumors from the mice treated with or without BMDC-EXOsTu (Fig. 5h). These results suggest that BMDC-EXOsTu probably promote tumor metastasis by impairing the antitumor CD8+ T cell responses of metastatic sites via PD-L1.
Fig. 5. BMDC-EXOsTu promote tumor metastasis.
a–e A total of 5 × 105 4T1 cells were injected into the mammary fat pad of female BALB/c mice. Primary tumors were surgically resected on day 16. a–c Then, the mice were intravenously injected with PBS, 10 μg BMDC-EXOs or BMDC-EXOsTu on days 17, 19, and 21. Lung metastases were visualized in hematoxylin and eosin-stained pathological sections (a) or determined by a clonogenic assay on day 25 (b). IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of the tumor tissues were detected by flow cytometry (c). d, e Then, the mice were intraperitoneally injected with 50 μg of anti-CD8 antibodies on days 15, 17, 19, 21, and 23. Lung metastases were visualized in hematoxylin and eosin-stained pathological sections (d) or determined by a clonogenic assay on day 25 (e). f–h After incubation with 10 μg/ml anti-PD-L1 for 1 h, 10 μg of BMDC-EXOsTu were intravenously injected into the mice on days 17, 19, and 21. Lung metastases were visualized in hematoxylin and eosin-stained pathological sections (f) or determined by a clonogenic assay on day 25 (g). IFN-γ+CD8+ and Ki-67+CD8+ T cells in the TILs of the tumor tissues were detected by flow cytometry (h). Ctrl, group with PBS treatment. ns not significant, *P < 0.05 and **P < 0.01 (unpaired Student’s t-test). Scale, 200 μm. Representative results from three independent experiments are shown (the means and s.d.) (n = 3–5).
Discussion
PD-L1 expressed on tumor cells binds to PD-1 on activated T cells to initiate the suppression of antitumor immunity. The anti-PD-1 immune checkpoint blockade, which mainly blocks this process, has striking therapeutic effects. Recently, immunosuppressive signaling mediated by exosomal PD-L1 has also been reported to play a crucial role in inhibiting antitumor immunity. The removal of tumor-derived exosomal PD-L1 inhibits tumor growth, even in models that are resistant to anti-PD-L1 antibodies. Tumor-derived exosomal PD-L1 also contributes to the establishment of the immune memory of the tumor12. An important question is raised from this study: How important is tumor-derived exosomal PD-L1 in the formation of systemic immunosuppression? Tumor cells continue to secrete exosomes, leading to the presence of a large number of physiological exosomes. In addition, because of their size advantage, exosomes probably have more opportunities to interact with T cells. Therefore, the role of exosomal PD-L1 in the formation of systemic immunosuppression is probably far more important than we have previously assumed.
PD-L1 was found on BMDC-EXOsTu, but not on BMDC-EXOs, indicates that the tumor environment determines the presence of PD-L1 on BMDC-EXOsTu. Myeloid-derived suppressor cells (MDSCs) are a group of myeloid cells comprising precursors of macrophages, granulocytes, dendritic cells, and myeloid cells at early stages of differentiation. MDSCs are found to be significantly increased in patients with cancer of all stages23,24. The expression of PD-L1 on MDSCs has been reported to increase under tumor conditions25. We found that BMDC-EXOsTu are positive for CD11b and Gr-1, which are markers for MDSCs. Therefore, PD-L1+ BMDC-EXOsTu may be derived from MDSCs. PD-L1 is also expressed on immunosuppressive B cells in solid tumors26. However, most of the B cells in the BM have not been exposed to antigens. Whether the tumor environment can upregulate PD-L1 on naïve B cells and the exosomes released by these cells is still unknown. In addition, a fraction of blood monocyte-derived myeloid dendritic cells can also express PD-L1, which is further upregulated by tumor environmental factors27. PD-L1 has also been reported to be expressed on dendritic cells and macrophages in the tumor microenvironment28, and PD-L1 expressed on antigen-presenting cells plays an essential role in checkpoint blockade therapy29. Exosomes can faithfully reflect the characteristics of their parent cells. Therefore, PD-L1-positive BMDC-EXOsTu may also be released by these cells. In this study, BMDC-EXOsTu were isolated from total BMDCs; therefore, we cannot confirm the parent cells of the PD-L1+ BMDC-EXOsTu. In addition, how the tumor environment increases PD-L1 on BMDC-EXOsTu requires further study.
The application of anti-PD-L1 to the treatment of clinical cancer patients greatly prolongs the survival of cancer patients. However, it is still difficult to predict which types of tumors or which types of cancer patients will respond to anti-PD-L1 treatment. Initially, it was believed that patients with PD-L1-positive tumors would benefit from anti-PD-L1 treatment. However, many patients with PD-L1-negative tumors have had positive responses to anti-PD-L1 treatment3,4. Although this phenomenon is clinically observed, the underlying mechanisms are still largely unknown. Our study demonstrates that, in addition to tumor cells, BMDC-EXOsTu are also a source of PD-L1 under tumoral conditions and that anti-PD-L1 treatment can abolish the immunosuppression of the BMDC-EXOsTu mediated by PD-L1, resulting in the activation of antitumor immunity. PD-L1-positive BMDC-EXOsTu may reasonably explain the anti-PD-1 tumor suppressive effect on patients whose tumor cells do not express PD-L1.
Altogether, our results demonstrate that the tumor microenvironment can induce BMDC-EXOs to carry PD-L1, which is bioactive and effectively inhibits CD8+ T cell responses in vitro and in vivo. PD-L1 on BMDC-EXOs contributes to tumor immunosuppression and tumor growth. Thus, our study reveals a novel mechanism for tumor immune escape.
Acknowledgements
This work was supported by the Basic Public Welfare Projects of Zhejiang Province (LQ18C080001), the Natural Science Foundation of Zhejiang Province (LY19H100003 and LQ20H160018), and the Hangzhou Health Science and Technology Plan (OO20191170).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yan Sun, Jufeng Guo, Lei Yu
Contributor Information
Xian Wang, Email: Wangx118@zju.edu.cn.
Yinghu Chen, Email: CYH18@zju.edu.cn.
References
- 1.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 5.Dong W, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1 negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9:1422–1437. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mashouri L, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, et al. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol. Immunol. 2020;17:323–334. doi: 10.1038/s41423-020-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, et al. Specific decrease in B-cell-derived extracellular vesicles enhances post-chemotherapeutic CD8(+) T cell responses. Immunity. 2019;50:738–750. doi: 10.1016/j.immuni.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poggio M, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–427 e413. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuonen F, et al. Inhibition of the Kit ligand/c-Kit axis attenuates metastasis in a mouse model mimicking local breast cancer relapse after radiotherapy. Clin. Cancer Res. 2012;18:4365–4374. doi: 10.1158/1078-0432.CCR-11-3028. [DOI] [PubMed] [Google Scholar]
- 15.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Z, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J. Immunol. 2012;188:5954–5961. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 17.Xiu FM, et al. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J. Mol. Med. 2007;85:511–521. doi: 10.1007/s00109-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 18.Kushida T, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–3299. doi: 10.1182/blood.V97.10.3292. [DOI] [PubMed] [Google Scholar]
- 19.Hong X, et al. EBAG9 inducing hyporesponsiveness of T cells promotes tumor growth and metastasis in 4T1 murine mammary carcinoma. Cancer Sci. 2009;100:961–969. doi: 10.1111/j.1349-7006.2009.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 22.Coffelt SB, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol. Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin. Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 25.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Zhang T, Li M, Yin L, Xue J. Immunosuppressive B cells expressing PD-1/PD-L1 in solid tumors: a mini review. QJM. 2019;pii:hcz162. doi: 10.1093/qjmed/hcz162. [DOI] [PubMed] [Google Scholar]
- 27.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Invest. 2018;128:805–815. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang HD, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J. Clin. Invest. 2018;128:580–588. doi: 10.1172/JCI96061. [DOI] [PMC free article] [PubMed] [Google Scholar]