Abstract
CCNE1 amplification is an oncogenic driver for many gynecologic cancers and is associated with poor patient outcomes. In this issue, Xu et al.1 identify a combination therapy that is responsive to high CCNE1-copy number ovarian and endometrial cancers using PDX models.
CCNE1 amplification is an oncogenic driver for many gynecologic cancers and is associated with poor patient outcomes. In this issue, Xu et al.1 identify a combination therapy that is responsive to high CCNE1-copy number ovarian and endometrial cancers using PDX models.
Main text
Endometrial cancers (EMCAs), including uterine serous cancer and uterine carcinosarcomas, are the most common gynecologic malignancies in the US, and high-grade serous ovarian carcinomas (HGSOCs) are the deadliest subset of ovarian cancers.2 Because these cancers tend to present with nonspecific symptoms at a late stage and have a high risk of recurrence, there is an imperative need for predictive diagnostic tools and targeted therapeutics.
Many HGSOCs and EMCAs are TP53-mutated and a significant portion are associated with CCNE1 oncogene amplification, leading to overexpression of the Cyclin E1 oncoprotein. High Cyclin E1 levels limit G1 arrest, causing premature S-phase entry, impaired DNA damage response, and replication stress (RS). Therefore, Cyclin E1-high cells rely on the G2/M checkpoint for attenuation of catastrophic levels of DNA damage and survival. This involves upregulation of the ATR axis, which facilitates G2 arrest and repair of RS-induced DNA damage through an ATR/Chk1/WEE1 phosphorylation cascade. Indeed, cell line models with overexpressed Cyclin E1 have been shown to upregulate ATR signaling and phosphorylation of its downstream effector proteins,3 but these interactions have yet to be studied in depth in clinically relevant gynecologic cancer models.
G2/M checkpoint inhibitors are promising therapeutics for CCNE1-high cancers.3, 4, 5 Inhibition of the G2 checkpoint is synthetically lethal to G1 checkpoint-deficient cells, sparing normal cells with intact G1 checkpoint function. In triple-negative breast cancer (TNBC) cell models, ATR inhibition (ATRi) and WEE1 inhibition (WEE1i) have been shown to destabilize mitotic genome integrity and promote selective cytotoxicity in a Cyclin E1-dependent manner.3,4 There are currently four ATRi and one WEE1i small molecules in phase I and II clinical trials for the treatment of various gynecologic malignancies and other solid tumors,5 but many of these chemotherapy regimens come with severe and sometimes life-threatening adverse effects, namely hematologic toxicity. However, compared to monotherapy, combination therapies can synergistically target cancer cells, allowing for lower dosing and the opportunity to curb intra-tumor heterogeneity and potential resistance. For example, the Simpkins group previously found that combination PARP inhibitor (PARPi) and ATRi treatment decreased colony formation more than either monotherapy alone in BRCA-mutant cancer cells, as well as significantly decreased tumor load and prolonged survival of mice harboring PARPi-resistant and platinum-resistant patient-derived tumor xenografts6 (PDX models, Figure 1A). These are important findings considering a large proportion of all HGSOCs are either BRCA1/2-mutated or CCNE1-amplified, and many ovarian cancer patients are likely to encounter PARPi- or platinum-resistance at some point during their treatment course. Therefore, there is a critical need to similarly study combination therapies in CCNE1-amplified gynecologic malignancies, as CCNE1 amplification is linked to a poor primary therapy response and increased risk of resistance.
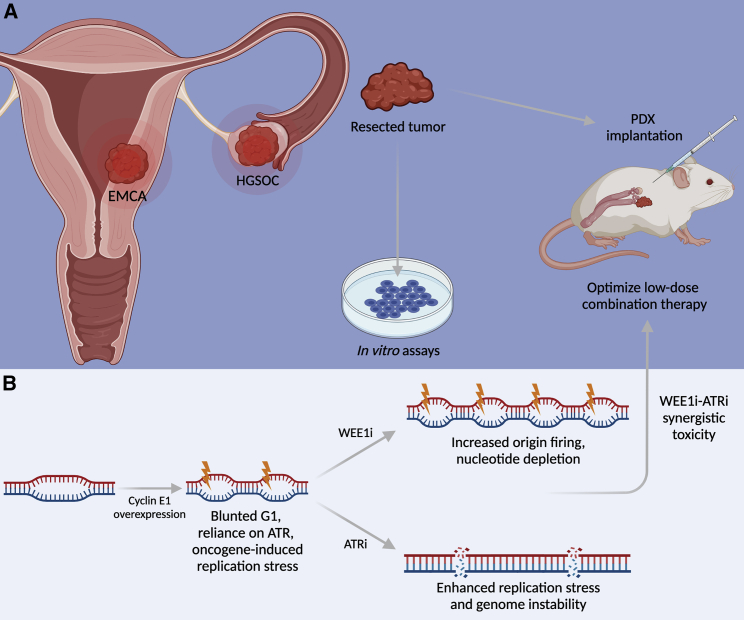
Figure 1.
CCNE1-driven gynecologic cancer models and mechanisms of combination treatment
(A) Patient-derived xenograft (PDX) models of endometrial cancers (EMCAs) and high grade serous ovarian cancers (HGSOCs). Resected tumors are implanted into immunocompromised mice for translational assays.
(B) Combination WEE1 and ATR inhibition treatment is synergistically toxic in HGSOC and EMCA PDX models through distinct molecular mechanisms.
In this issue of Cell Reports Medicine, Xu and colleagues1 explore the mechanisms by which combination WEE1i-ATRi therapy is selectively toxic in a CCNE1 copy number (CN)-dependent manner. Similar to what others have observed in breast cancer models,3,4 the authors find that Cyclin E1 overexpression increases sensitivity to both ATRi and WEE1i in multiple gynecologic cancer cell lines. They identify a relatively low dose of WEE1i-ATRi combination therapy with minimal effect on Cyclin E1-normal cells and a maximal effect on Cyclin E1-overexpressed cells. Combination WEE1i-ATRi treatment dramatically decreases cell viability and tumor volume, while improving survival in ovarian and endometrial cancer PDX models compared to either WEE1i or ATRi monotherapy. Furthermore, this response to combination therapy was more pronounced in PDX models with a high CCNE1 CN, with fewer or no significant effects in models with normal CCNE1 CN. A major strength of this study is the quantitative association established between CCNE1 CN and sensitivity to checkpoint inhibition across multiple cell lines, thereby uncovering CCNE1 CN as a powerful new biomarker for the treatment of a subset of HGSOCs and EMCAs. The Simpkins group has therefore identified a low-dose WEE1i-ATRi combination therapy suitable for the treatment of CCNE1-high gynecologic malignancies in preclinical studies.
Mechanistically, Xu and colleagues ultimately show that combination WEE1i-ATRi induces RS-associated DNA damage and apoptosis most dramatically in Cyclin E1-overexpressed cells when compared to either monotherapy alone or Cyclin E1-normal cells. Consistent with a previous study that linked WEE1i-mediated CDK activity to nucleotide depletion caused by aberrant origin firing,7 the authors find that WEE1i alone significantly lowers inhibitory phospho-CDK levels and decreases nucleotide incorporation during S phase. Therefore, the authors propose a mechanism of synergy between WEE1i and ATRi, whereby WEE1i induces RS through increased origin firing and ATRi further decreases genome integrity through improper fork recovery and failed repair of RS-induced DNA damage (Figure 1B). This synergy is likely CCNE1 CN-dependent due to the upregulation of inhibitory phospho-CDK that is associated with high Cyclin E1 expression observed throughout this study.
Cyclin E1 overexpression alone is associated with oncogene-induced RS,8 but the specific mechanisms of Cyclin E1-induced RS (increased origin firing, nucleotide depletion, impact on transcription machinery) are not well studied in gynecologic cancer models. A deeper understanding of how WEE1i stimulates or exacerbates Cyclin E1-induced RS and Cyclin E1-independent mechanisms of WEE1i-induced RS could uncover new biomarkers or targets to be exploited in treating malignancies independent of CCNE1 CN status. Intriguingly, two TP53-mutated, CCNE1 CN-normal cell lines (OVKATE and SNU685) exhibit moderate signs of S-phase genomic instability and apoptosis in response to WEE1i and combination therapy in this study.1 WEE1 is a potent cell cycle regulator, and others have observed that WEE1i induces replication stress, DNA damage, and cytotoxicity in Cyclin E1-normal TNBC models.9 Could TP53 mutations alone sufficiently sensitize CCNE1 CN-normal gynecologic cancers to various checkpoint combination therapies? Understanding WEE1i-induced genomic instability mechanisms in CCNE1 CN-normal models could also open the door to treatment of these gynecologic cancers in earlier stages (i.e., before significant CCNE1 CN amplification), as TP53 mutations are likely an earlier tumorigenic event than CCNE1-CN gain.10
The impact of genetic mutations on HGSOC and EMCA treatment can be further explored in streamlined PDX models (Figure 1A), such as those optimized by the Simpkins group,1,6 who have employed these models to study major HGSOC and EMCA subtypes in diverse genetic backgrounds. The use of PDX models that allow concurrent analysis of both translational and biochemical assays are critical for studying future combination therapy regimens that will allow faster times to clinical trials. These pipelines are powerful tools that will need to continue to be made more efficient as a step toward individualized treatment.
Acknowledgments
T.T.H is supported by the National Institutes of Health grants GM139610 and ES031658, and the V Foundation BRCA Research collaborative grant. L.G. is supported by the MSTP T32GM136573 through NYU School of Medicine.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Xu H., George E., Kinose Y., Kim H., Shah J.B., Peake J., Ferman B., Medvedev S., Murtha T., Barger C.J. CCNE1 copy-number is a biomarker for response to combination WEE1-ATR inhibition in ovarian and endometrial cancer models. Cell Rep. Med. 2021;2:100394-1–100394-17. doi: 10.1016/j.xcrm.2021.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer D.W. The epidemiology of endometrial and ovarian cancer. Hematol. Oncol. Clin. North Am. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kok Y.P., Guerrero Llobet S., Schoonen P.M., Everts M., Bhattacharya A., Fehrmann R.S.N., van den Tempel N., van Vugt M.A.T.M. Overexpression of Cyclin E1 or Cdc25A leads to replication stress, mitotic aberrancies, and increased sensitivity to replication checkpoint inhibitors. Oncogenesis. 2020;9:88. doi: 10.1038/s41389-020-00270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrero Llobet S., van der Vegt B., Jongeneel E., Bense R.D., Zwager M.C., Schröder C.P., Everts M., Fehrmann R.S.N., de Bock G.H., van Vugt M.A.T.M. Cyclin E expression is associated with high levels of replication stress in triple-negative breast cancer. NPJ Breast Cancer. 2020;6:40. doi: 10.1038/s41523-020-00181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorecki L., Andrs M., Korabecny J. Clinical Candidates Targeting the ATR-CHK1-WEE1 Axis in Cancer. Cancers (Basel) 2021;13:795. doi: 10.3390/cancers13040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H., Xu H., George E., Hallberg D., Kumar S., Jagannathan V., Medvedev S., Kinose Y., Devins K., Verma P. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020;11:3726. doi: 10.1038/s41467-020-17127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck H., Nähse-Kumpf V., Larsen M.S.Y., O’Hanlon K.A., Patzke S., Holmberg C., Mejlvang J., Groth A., Nielsen O., Syljuåsen R.G., Sørensen C.S. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol. Cell. Biol. 2012;32:4226–4236. doi: 10.1128/MCB.00412-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotsantis P., Petermann E., Boulton S.J. Mechanisms of Oncogene-Induced Replication Stress: Jigsaw Falling into Place. Cancer Discov. 2018;8:537–555. doi: 10.1158/2159-8290.CD-17-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Low K.H., Alexander A., Jiang Y., Karakas C., Hess K.R., Carey J.P.W., Bui T.N., Vijayaraghavan S., Evans K.W. Cyclin E overexpression sensitizes triple negative breast cancer to Wee1 kinase Inhibition. Clin. Cancer Res. 2018;24:6594–6610. doi: 10.1158/1078-0432.CCR-18-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karst A.M., Jones P.M., Vena N., Ligon A.H., Liu J.F., Hirsch M.S., Etemadmoghadam D., Bowtell D.D., Drapkin R. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014;74:1141–1152. doi: 10.1158/0008-5472.CAN-13-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]