Abstract
Objectives.
To quantify the secondary impacts of the coronavirus disease 2019 (Covid-19) pandemic disruptions to cervical cancer (CC) screening, stratified by step in the screening process and primary test modality, on CC burden.
Setting.
United States
Methods.
We conducted a comparative model-based analysis using three independent NCI Cancer Intervention and Surveillance Modeling Network (CISNET)-cervical models to quantify the impact of eight alternative Covid-19-related screening disruption scenarios compared to a scenario of no disruptions. Scenarios varied by the duration of the disruption (6 or 24 months), steps in the screening process being disrupted (primary screening, surveillance, colposcopy, excisional treatment), and primary screening modality (cytology alone or cytology plus HPV “cotesting”).
Results.
The models consistently showed that Covid-19-related disruptions yield small net increases in CC cases by 2027, which are greater for women previously screened with cytology compared with cotesting. When disruptions affected all four steps in the screening process under cytology-based screening, there were an additional 5–7 and 38–45 cases per 1 million screened for 6- and 24-month disruptions, respectively. In contrast, under cotesting, there were additional 4–5 and 35–45 cases per 1 million screened for 6- and 24-month disruptions, respectively. The majority (58–79%) of the projected increases in cases under cotesting were due to disruptions to surveillance, colposcopies or excisional treatment, rather than to primary screening.
Conclusions.
Women in need of surveillance, colposcopies or excisional treatment, or whose last primary screen did not involve HPV testing, may comprise priority groups for reintroductions.
In the U.S., an estimated 13,800 women will develop cervical cancer (CC) and over 4,290 will die from CC in 2020 (1). The natural history of CC is amendable to screening, which aims to detect the presence of high-grade precancers that can be treated before progressing to cancer or to detect invasive cancer earlier. Secondary impacts of the COVID-19 pandemic on preventive health care, such as CC screening, are largely unknown. Certain provider networks in the United States have reported 80% reductions in screening rates during periods of disruption (2), which is similar to other areas of the US (3). Sustained disruptions may contribute to poor outcomes for individuals unable to access usual care. COVID-19-related disruptions to the screening process may not only delay an individual’s primary screening test, but also interrupt the downstream processes for individuals who have already been identified as moderate or high-risk and in need of surveillance, diagnostic testing, or even treatment to remove precancerous lesions. Even before empirical data become available, models can quantify health consequences of alternative screening disruption scenarios and isolate complex interactions of the screening pathway. Using three CC models from the Cancer Intervention and Surveillance Modeling Network (CISNET) consortium (https://cisnet.cancer.gov/), we quantified potential CC screening-related impacts of alternative COVID-19-related care disruptions, stratified by primary screening modality, duration of disruption, and by step in the screening process (i.e., primary screening, surveillance, colposcopy, precancer treatment).
We used three CISNET-Cervical microsimulation models to project changes in expected number of CC cases between 2020 and 2027 (inclusive) under eight alternative COVID-19-related screening disruption scenarios, compared to a scenario of no COVID-19-related disruptions (Supplementary Table 1). As the primary screening modality varies in the U.S., we explored the impact of screening delays in the context of primary cytology (i.e., Pap smear only) with and without switching to primary cytology and high-risk human papillomavirus (HPV) “cotesting” at age 30 years. Model outcomes included the short-term number of CC cases (2019–2027) per one million women aged 21–84 years-old screened. CCs were age-standardized using United Nations US female population projections for 2019–2027 (4). The Harvard, MISCAN-Cervix and Policy1-Cervix CISNET models, which have been described in multiple comparative modeling analyses (5, 6), differ with respect to the type and number of health states, HPV genotype categorizations, histological cancer types, model cycle length and data sources used to parameterize the model prior to fitting to the U.S. setting. Common U.S. model inputs include hysterectomy rates, all-cause mortality, and CC survival (5). The models were calibrated to HPV and cervical disease outcomes in the U.S., achieving good-fit to empirical targets (5, 6).
We compared a scenario without COVID-19-related disruptions to eight alternative COVID-19-related disruption scenarios that varied by the duration of the disruption and step in the screening process being disrupted: primary screening only; primary screening and surveillance; primary screening, surveillance and colposcopy visits; or primary screening, surveillance, colposcopy visits and excisional treatments (Supplementary Table 1). We assumed all delays experienced a 100% temporary loss in attendance, but the screening process resumed following the delay. Women aged 21–65 years were screened using either cytology alone or switched to cotesting at age 30 years. Screen-positive women were managed according to guidelines (7). Scenarios were simulated in the context of historical HPV vaccination (6) coverage and Kaiser Permanente Northern California screening practice patterns (Supplementary Table 1) (8).
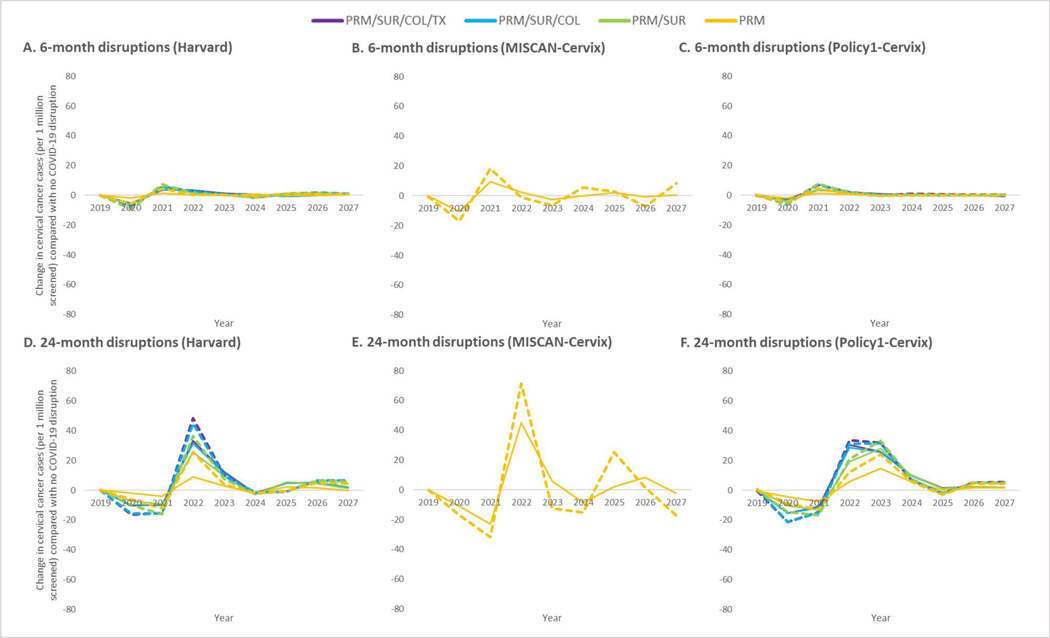
Compared with a scenario without COVID-19 disruptions, all three models projected consistent decreases in the number of detected CC cases during the estimated 6-month or 24-month COVID-19-disruption period, and an increase in the number of CC cases following the disruption (Figure 1). Projected disruptions yielded greater net increases in cases for cytology-based compared with cotesting-based screening, for 24-month disruptions compared with 6-month disruptions, and when all screening process steps were disrupted compared with only primary screening. By 2027, disruptions for all screening process steps under cytology-based screening were projected to increase the number of CC cases by 5–7 and 38–45 cases per 1 million screened-women under 6- and 24-month disruptions, respectively (Supplementary Figure 1). Compared with cytology, delays in cotesting resulted in fewer additional cases, particularly those cases stemming from disruptions to the primary screening step rather than downstream screening steps. Most (58–79%) of the projected increases in CC cases under cotesting were due to disruptions to ongoing surveillance, colposcopies or excisional treatment, although these additional cases stemmed from approximately 25–30% of women being disrupted (Supplementary Table 2).
Figure 1. Projected impact of COVID-19-related disruptions to different steps in the cervical cancer screening process on the number of cervical cancer cases per 1 million screened women aged 21–84 years over time in the context of primary cytology-based screening (stapled lines) or cotest-based screening (solid lines) for three CISNET-Cervical disease simulation models.
COVID-19-related disruptions varied by the duration of the disruption, (6 months (top panels) or 24 months (bottom panels)) and step(s) in the screening process the disruption(s) impacted: i) primary screening only (PRM), ii) primary screening and surveillance (PRM/SUR), iii) primary screening, surveillance and colposcopy visits (PRM/SUR/COL), or iv) primary screening, surveillance, colposcopy visits and excisional treatments (PRM/SUR/COL/TX). Women aged 21–65 years were screened using cytology with or without an option to switch to cytology and HPV “cotesting” starting at age 30 years. See Supplementary Table 1 for alternative COVID-19-related scenarios and assumptions. Projected 2020 baseline rates per 1 million women in the absence of COVID-19 for Harvard: 79 (cytology) and 26 (cotesting); Policy1-Cervix: 43 (cytology) and 28 (cotesting); MISCAN-Cervix: 48 (cytology) and 37 (cotesting). Note: MISCAN-Cervix simulated disruptions to the primary screening only scenario, while the Harvard and Policy1-Cervix models projected outcomes for disruptions across the multi-step screening process.
We consistently found that temporary suspensions in the CC screening pathway may potentially result in temporal shifts in cancer detection, and yield small net increases in cancer burden between 2020 and 2027. Our results suggest that prioritizing reintroduction of services for women needing surveillance, colposcopy or excisional treatment - a relatively small number of women compared with women in need of primary screening - could avert a large proportion of the additional cases. Risk-based prioritization also offers the most efficient reintroduction strategy in a care setting with constrained capacity. We also showed that the increases in cancer risk are impacted less by disruptions to primary cotesting compared with cytology alone. Due to the high negative predictive value of a single HPV test (9), this finding likely extends to screening with primary HPV testing alone; thus, prioritizing women whose last screen did not involve HPV testing may also help mitigate impacts. This comparative model-based analysis is, to our knowledge, the first to demonstrate the potential secondary COVID-19 impacts on cervical cancer, a slow-growing and preventable cancer, in the United States; although there are recent model-based projections for COVID-19 disruptions on breast and colorectal cancers (10).
Although each model provided a single-projection point estimate, by using three independent models, we captured a range of parameter and structural uncertainties. Differences across model projections are primarily due to differences in natural history assumptions (5),and several structural assumptions that consolidate the multi-step screening process. For example, the variations by year for MISCAN-Cervix are more pronounced after a disruption period due to model structural assumptions about when a cervical cancer is counted. However, despite these differences, cumulatively over time, the net results between the three models are similar (results not shown).
Importantly, our analysis did not quantify the number of women (and family members) who might acquire COVID-19 as a result of screening-related visits, so we are not able to capture the tradeoffs between CC and COVID-19 risk. However, it is important to understand the impacts of different durations of COVID-19 disruptions and the relevant groups to prioritize once reintroductions begin. We also assumed that all screening services resumed immediately following the disruption period. While this assumption was not realistic, we are able to more precisely quantify the health consequences of the period of delay without conflating the uncertainties of more nuanced reintroductions of services. The timing and scope of reintroductions may vary by U.S. state/local mandates, policies, and capacity based on COVID-19-related protocols. For women who lost insurance and are unable to access care, or for women who may avoid care due to fear of COVID-19, the disruptions may continue. Our projections provide benchmarks for setting-specific calculations. The ‘COVID-19 and Cancer Global Modelling Consortium’ (https://ccgmc.org) aims to aid more nuanced cancer screening decision-making due to the COVID-19 pandemic, including use of real-world data on the magnitude and length of disruptions when those data become available.
Our independent models consistently predicted that prioritizing reintroduction of services for women in need of surveillance, colposcopies or excisional treatment, as well as women whose last primary screen did not involve HPV testing, may mitigate the potential small secondary impacts of COVID-19 on CC.
Supplementary Material
Acknowledgements
Funding. U.S. National Cancer Institute (U01CA199334and 1UM1CA221940).
KC is the co-PI of an investigator-initiated trial of cervical cancer screening, Compass, run by the VCS Foundation, which is a government-funded not-for-profit charity. Neither KC nor her institution have received funding from industry for this or any other research project.
Support
This study was supported by funding from the National Cancer Institute (U01CA199334and 1UM1CA221940). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. Emily A Burger receives salary support from the Norwegian Cancer Society (#198073), and Megan A Smith receives salary support from the National Health and Medical Research Council, Australia (APP1159491) and Cancer Institute NSW (ECF181561). Nicole G Campos receives other salary support from the National Cancer Institute through an Interagency Personnel Agreement.
Footnotes
Data Availability
Supporting Information contained in the Supplementary Material of References 5 and 6 provide details on microsimulation model inputs, calibration to epidemiologic data and calibration approach in line with good modeling practice. Access to the raw results data will be made available upon reasonable request.
Disclosures
All other authors declare no conflicts.
REFERENCES
- 1.American Cancer Socity. “Key Statistics for Cervical Cancer” Available at: https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html (accessed August 19, 2020).
- 2.Miller MJ. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System—Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR. Morbidity and Mortality Weekly Report. 2021;70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mast C, Munoz del Rio A. Delayed cancer screenings—a second look. Verona, WI: Epic Health Research Network; 2020. https://ehrn.org/articles/delayed-cancer-screenings-a-second-lookexternalAccessed February 5, 2021. [Google Scholar]
- 4.United Nations Population Division. World Population Prospects: The 2017 Revision.[Online] Accessed 18 December 2017. Available at: https://esa.un.org/unpd/wpp/.
- 5.Burger EA, de Kok I, Groene E, Killen J, Canfell K, Kulasingam S, et al. Estimating the Natural History of Cervical Carcinogenesis Using Simulation Models: A CISNET Comparative Analysis. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger EA, Smith MA, Killen J, Sy S, Simms KT, Canfell K, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. The Lancet Public Health. 2020;5(4):e213–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Jama. 2018;320(7):674–86. [DOI] [PubMed] [Google Scholar]
- 8.Rendle KA, Schiffman M, Cheung LC, Kinney WK, Fetterman B, Poitras NE, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Preventive medicine. 2018;109:44–50. [DOI] [PubMed] [Google Scholar]
- 9.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. Journal of the National Cancer Institute. 2011;103(5):368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290-. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.