Graphical abstract
Keywords: ENVIRONAGE, Placental paraben, BMI z-scores, Obesogenic effect, pre-pregnancy BMI, Gamma-glutamyltransferase
Highlights
-
•
Ethyl paraben (EtP) concentrations above the LOD were found in 88% of the placentas.
-
•
Child BMI z-scores were inversely associated with placental EtP exposure.
-
•
Placental EtP was associated with cord blood γ-glutamyltransferase (GGT) activity.
-
•
Cord blood glucose showed an inverse relationship with placental EtP.
-
•
Placental EtP was associated with methylation of cg08612779 annotated to GGT7.
Abstract
Background
Parabens are used as antimicrobial preservatives in personal care products. Few studies have dealt with adverse health outcomes, transplacental transfer, and obesogenic effects of prenatal exposure to parabens. We examined the association between placental paraben levels and cord blood metabolic biomarkers, considering modulating effects of maternal pre-pregnancy BMI and underlying epigenetic mechanisms, and investigated longitudinal effects of in utero paraben exposure on early childhood trajectories of BMI z-scores.
Methods
Placental concentrations of four parabens [methyl (MeP), ethyl (EtP), propyl (PrP), and butyl (BuP)] were measured by ultra-performance liquid chromatography/tandem mass spectrometry in 229 placentas of the ENVIRONAGE birth cohort. The association with cord blood metabolic biomarkers [glucose, insulin, γ-glutamyltransferase (GGT), high-density and low-density lipoprotein (HDL and LDL)] was analyzed in multiple regression models with two different sets of, a priori selected potential confounders, additionally stratified for different maternal BMI groups and assessed by causal mediation analysis. The association between placental paraben concentration and differential DNA methylation of CpGs annotated to GGT and longitudinal measurements of BMI z-scores were investigated with adjusted linear mixed models.
Results
The geometric means of placental MeP, EtP, PrP, and BuP levels above the limit of detection (LOD) were 4.42, 1.32, 1.51, and 0.35 ng/g respectively, with only EtP showing sufficient (88%) measurements above LOD for further analyses. An interquartile ratio (IQR) increase in placental EtP was associated with an increase of 12.61 % (95% CI: 1.80 24.57) in the geometric mean of cord GGT activity, and with a decrease of −3.64 % (95% CI: −6.80 to −0.39) in the geometric mean of cord glucose. Placental EtP levels were significantly associated with hypermethylation of cg08612779 annotated to GGT7 after correcting for multiple testing (ß = 0.0017, p = 0.049). An interquartile ratio (IQR) increment in placental EtP was associated with a decrease in longitudinal BMI z-score of 0.27 points (95% CI: −0.46 to −0.088).
Conclusion
Prenatal EtP exposure may affect early childhood BMI. The association of placental EtP with cord blood GGT and glucose levels provides a starting point for further research on mechanisms of paraben-related metabolic processes in utero.
1. Introduction
Parabens comprise a group of p-hydroxybenzoic acid esters with alkyl substituents of different lengths, such as methyl paraben (MeP), ethyl paraben (EtP), propyl paraben (PrP), and butyl paraben (BuP) (Aubert et al., 2012). They have been widely used as antimicrobial and antifungal agents in personal care products and pharmaceuticals since the mid-1920s (Elder, 1984). The unrestricted use of parabens is under discussion since the beginning of this millennium, when endocrine disrupting properties of different parabens had been observed in in vitro and in vivo studies (Giulivo et al., 2016, Nowak et al., 2018, Oishi, 2001, Oishi, 2002).
With in vitro and epidemiological studies (Hu et al., 2013, Kim and Chevrier, 2020, Liu et al., 2019) linking parabens to obesogenic changes, adverse metabolic health effects have also become an increasing concern. On the other hand, inverse associations with biomarkers of metabolic disease have been reported as well (Lee et al., 2019, Pazos et al., 2019, Quirós-Alcalá et al., 2018), indicating that effects may differ depending on the type of paraben, the individual's sex and age. Evidence for the transplacental passage of parabens in humans (Kang et al., 2013, Shekhar et al., 2017, Towers et al., 2015, Valle-Sistac et al., 2016, Vela-Soria et al., 2017) raised questions about the possible impact on the fetus. A study investigating urinary MeP, PrP, and BuP concentrations in women before and during pregnancy showed 25–45% lower levels during pregnancy compared with before pregnancy, indicating a shift into the fetal compartment (Smith et al., 2012). According to the “Developmental Origins of Health and Disease” (DOHAD) hypothesis, prenatal exposures can alter the health status later in life through epigenetic programming (Barker, 1995), and in this way could affect overweight and metabolic disease susceptibility later on (Baillie-Hamilton, 2002, Grün and Blumberg, 2009). However, research on potential obesogenic effects of prenatal paraben exposure based on childhood BMI and metabolic biomarkers in newborns is still scarce.
In the prospective mother-child cohort LINA a significant association between maternal urinary iso-butyl paraben levels and BMI trajectories (age 1–8) for 392 children was found (2nd tertile: β = 0.35, 95% CI: 0.08─0.62; 3rd tertile: β = 0.26, 95% CI: 0.02─0.05) (Leppert et al., 2020). Significant positive associations between maternal urinary MeP and PrP levels and BMI z-scores in 309 five-year olds have been reported in a study of the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort (β = 0.08, 95% CI: 0.01─0.16 and β = 0.06, 95% CI: 0.02─0.10) (Berger et al., 2021).
An important biomarker reflecting the metabolic health status is γ-glutamyltransferase (GGT), a liver enzyme located on the external surface of most cells, which catalyses glutathione uptake and is involved in intracellular antioxidant defense (Karp et al., 2001) and pro-oxidant processes (Corti et al., 2009, Enoiu et al., 2000). Historically, high serum GGT activity was regarded as a biological marker of heavy alcohol consumption and hepatobiliary disease, but serum GGT activity is also predictive of metabolic syndrome, cardiovascular disease, and mortality risk (Lee Douglas et al., 2007). Other relevant metabolic biomarkers are cord plasma high-density and low-density lipoprotein (HDL and LDL) concentrations, which are determinants of dyslipidemia (Klop et al., 2013), and insulin and glucose concentration, which serve as indices of insulin resistance (McLaughlin et al., 2003). GGT activity has been shown to correlate with other metabolic markers in previous studies [for glucose (Bijnens et al., 2021), for insulin (Kaushik et al., 2009), for HDL and LDL (Lee et al., 2007b, Lippi et al., 2007)]. In addition, GGT is an important factor in the detoxification of xenobiotics and pollutants (Lee and Jacobs, 2009, Weinmayr, 2019), therefore, seems to occupy a key position in the interface between environmental exposures and metabolic outcomes. The maternal pre-pregnancy body mass index (BMI) may influence the child's metabolic status, as newborn metabolic signatures characteristic of insulin resistance and risk of type 2 diabetes in adults (Lowe et al., 2017, Mocarzel et al., 2020a, Shokry et al., 2019) and changes in the concentration of lipophilic xenobiotics (Erkin-Cakmak et al., 2015, Wolff et al., 2007) have been shown to coincide with a higher maternal pre-pregnancy BMI.
Although parabens are excreted within hours after exposure (Janjua et al., 2008), and individual exposure events may stay unnoticed in maternal spot urine samples, they can still contribute to prenatal exposure. In this context, the placenta as a non-invasive and readily available biomonitoring matrix may be better suited for the assessment of fetal exposure to environmental pollutants. So far, only a small number of studies with an epidemiological design and adequate sample size have investigated paraben levels in human placental tissue (Freire et al., 2020, Vrijens et al., 2020). The present study reports levels of the four most frequently used parabens being MeP, EtP, PrP, and BuP in placental tissue and their link with the cord blood metabolic biomarkers GGT, glucose, insulin, HDL and LDL. We further investigated the association, studying cord blood differential DNA methylation of CpGs annotated to the genes of the metabolic key-enzyme GGT and examining the possibility of a mediating role of parabens in the association between maternal pre-pregnancy BMI with GGT activity and glucose levels. Finally, we tested the relationship between placental EtP levels and early childhood trajectories of BMI z-scores.
2. Material and Methods
2.1. Study population
Eligible mother-newborn pairs with singleton newborns taking part in the ENVIRONAGE birth cohort study (Janssen et al., 2017), were recruited between December 2014 and April 2017 at the East-Limburg Hospital in Genk, Belgium. Mothers who did not have planned caesareans and were able to complete questionnaires in Dutch were enrolled, after providing written consent. The study was conducted according to the principles outlined in the Helsinki Declaration (World Medical Association, 2013) and approved by the Ethical Committee of Hasselt University and the East-Limburg Hospital.
We determined paraben concentrations in 229 placental samples, and biochemical analyses of metabolic biomarkers were carried out in 223 corresponding cord blood samples. For five of the mother-newborn pairs, information on important covariates was unavailable, resulting in a sample size of 218 for statistical analysis. After removing extreme outliers from the measured metabolic biomarkers using the 3IQR method described by Tukey (Tukey, 1977), the association between placental parabens and metabolic biomarkers was analyzed in different biomarker subsets: n = 202 for GGT, glucose, and HDL, n = 193 for insulin, and n = 197 for LDL. For the analysis of differential methylation 109 of the 218 samples with paraben measurements had corresponding information on DNA methylation, and for the analysis of childhood trajectories of BMI z-scores measurements for 63 children were available.
2.2. Study procedure and data collection
Via questionnaires and medical records, we collected detailed information about the participants. Maternal height and weight were measured at the first antenatal visit (weeks 7–9 of gestation). Pre-pregnancy BMI was then calculated by dividing the weight in kilograms by the square of height in meters, and categorized into four groups: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Gestational age was computed from the first day of the mother’s last menstrual period combined with ultrasonographic data. Parity was divided into three categories: mothers having their first, second, or third child or more. In case mothers did not obtain a secondary school diploma, maternal educational level was coded as “low“. If a high school diploma was obtained as the highest diploma, the educational level was classified as ”middle“, and as “high” in the case of a college or university degree. Maternal smoking status was classified as “never smoker”, “former smoker” if the mother used to smoke but quit smoking before pregnancy, and “smoker” if the mother smoked during pregnancy. Alcohol consumption during pregnancy was coded with ”yes“ if the mother reported having consumed any alcohol during her pregnancy. Ethnicity was considered to be European when two or more grandparents of the newborn were European, otherwise, it was non-European. Birth Weight below the 25th percentile was calculated by taking into account parity, sex, and gestational age and coded as a binary variable.
For a subset of 63 children, data on height and weight were collected between the age of zero to 29 months by trained staff of the governmental agency “Kind en Gezin” (K&G – Child and Family), which is responsible for health, child care and wellbeing of young children until school age in Flanders (Van Kerschaver, 2012). During home visits and at regular examination moments at local hospitals and community centers height and weight of the children were measured on average eight times during the trajectory. From these measurements, BMI z-scores were calculated according to the World Health Organization's (WHO) Child Growth Standards based on length/height, weight and age of the child (World Health Organization (WHO) 2006).
2.3. Determination of placental paraben concentrations
After delivery, the placentas were deep-frozen within 10 min at −40 °C and stored until homogenization. For the mass spectrometric analysis of the placental MeP, EtP, PrP, and BuP concentrations, a recently described protocol was followed (Van Overmeire et al., 2019). In detail, the placenta was thawed for sample taking, the chorio-amniotic membrane was removed, and approximately half of the placenta (fixed orientation Supplementary Fig. S1) was homogenized using a Retsch Knife mill Grindomix GM 200 during two pulses of 20 sec at 10,000 rpm. Homogenized samples were kept frozen at −20 °C until analysis. A paraben internal standard mixture consisting of methyl-4-hydroxybenzoate-ring -13C6, ethyl-4-hydroxybenzoate-ring -13C6, propyl-4-hydroxybenzoate-ring _13C6 and butyl-4-hydroxybenzoate-ring -13C6 was purchased from Sigma Aldrich (Overijse, Belgium). A stock solution, containing 5 μg/mL of each paraben internal standard, was prepared in methanol and further diluted with methanol (1/100, v/v) to a working solution which was stored at − 20 °C in the dark until further use. Liquid-liquid extraction was performed by adding 150 µL of the internal standard mixture solution (50 ng/mL) to 1.5 g of the homogenized placenta in a polypropylene tube. After the addition of 6 mL of tert-butyl methyl ether/hexane (50/50, v/v), the sample was shaken for 10 min on a Heidolph multi Reax and centrifuged for 10 min at 3,000 rpm. The organic layer was then transferred to an empty Falcon® tube and evaporated to dryness under a nitrogen stream. Then, 250 μL of a methanol/water solution (50/50, v/v) was added to reconstitute the dry extract and it was filtered across a 0.20 μm PVDF filter (Whatman, Diegem, Belgium). Ultra performance liquid chromatography-tandem mass spectrometric (UPLC-MS/MS) analysis was performed on an Acquity UPLC (Waters, Milford, MA, USA) system combined with a triple quadrupole XevoTQ-S mass spectrophotometer in negative electrospray mode (ESI-). The limits of detection (LOD) were defined as the mean concentration in the blank plus three times the blank's standard deviation, being 0.1 ng/g for MeP and PrP, and 0.2 ng/g for EtP and BuP. Quality control was carried out employing spiked matrices at three different concentration levels.
2.4. Measurement of metabolic biomarkers in cord blood
For assessing the neonate’s metabolic biomarkers (GGT, glucose, insulin, HDL, and LDL) 8 mL of cord blood samples (mixture of arterial and venous blood) were collected immediately after delivery in plastic Lithium Heparin and EDTA BD Vacutainer® Tubes (BD, Franklin Lakes, NJ, USA). The collected cord blood samples were centrifuged (3,200 rpm for 15 min) and the obtained plasma was separated and instantly frozen at −80 °C. Plasma levels of insulin (pmol/L), LDL and HDL (mg/dL) were measured at the clinical laboratory of East-Limburg Hospital with an electrochemiluminescence immunoassay using the Modular E170 automatic analyzer (Roche, Basel, Switzerland). Enzyme activity of GGT (U/L) and concentration of glucose (measured by formation speed of NADPH through enzyme activity of glucose-6-phosphate dehydrogenase) were measured in cord plasma by kinetic photometric determination (bichromatic with 405/660 nm and 340/405 nm respectively), as previously described (Szasz, 1969) and (Neese et al., 1976) using a Cobas 8000 C702 analyzer (Roche, Basel, Switzerland).
2.5. Measurement of DNA Methylation in Cord Blood
To explore underlying epigenetic mechanisms related to GGT activity, we investigated changes in the methylation status of CpGs annotated to GGT promoter regions. Therefore, cord blood samples were collected in BD Vacutainer® Plus Plastic K2EDTA Tubes (BD, Franklin Lakes, NJ, USA) immediately after delivery. The collected cord blood was centrifuged (3,200 rpm for 15 min) to retrieve buffy coat which was instantly frozen at –80 °C. DNA was then extracted and processed at the Epigenetics Group, International Agency for Research on Cancer (IARC). In detail, after thawing and extraction with the QIAamp DNA mini Kit (Qiagen Ltd, Manchester, UK), DNA was quantified (Quant-iT PicoGreen dsDNA Assay Kit, Molecular Probes P7589), and bisulphite-converted using the Zymo EZ DNA methylationTM kit (Zymo, Irvine, CA, USA). The cord blood DNA was then hybridized to Illumina Infinium Human Methylation450K BeadChip arrays (Bibikova et al., 2011) and scanned using the Illumina HiScanSQ system to measure DNA methylation at 485,577 CpGs. The arrays were designed such that sample position and intra- and inter-variability in arrays and chips would not completely confound with biological covariates of interest. After background subtraction using Illumina GenomeStudio, raw intensity files were handled in R using the minfi package (Aryee et al., 2014) to calculate the methylation level at each CpG as the beta-value, the ratio of signal intensity originating from methylated CpGs over the sum of methylated and unmethylated CpGs. The samples were pre-processed, including normalization using the funnorm normalization of the minfi package (Aryee et al., 2014), and underwent quality control using in-house software within the R statistical computing environment. Samples were excluded on the basis of Illumina’s detection p-value of p greater than 0.05 and low bead count (<3 beads). For probes using the Infinium II design additional background subtraction and dye bias correction were performed. Additionally, data were trimmed removing the outliers with values larger than 3 interquartile ranges below the first quartile or above the fourth quartile using the Tukey method. Finally, filtering of potentially sex-mismatching and cross-reactive probes as well as probes known to have common SNPs at the CpG sites was applied for the present analysis.
In a next step CpG sites annotated to genes coding for γ-glutamyltransferase were selected by restriction to CpGs with the expression “GGT” in the corresponding “UCSC_RefGene_Name”, applying the Bioconductor package IlluminaHumanMethylation450kanno.ilmn12.hg19 for the annotation of Illumina’s 450 K methylation arrays (Hansen KD, 2016). In a final step, only probes that were lying within a promoter region transcription start site listed in the annotation file's column “UCSC_RefGene_Group”, were retained. Ninety CpG sites annotated to genes coding for γ-glutamyltransferase were identified in the annotation of Illumina’s 450 K methylation arrays, of which 31 probes were lying within a transcription start site.
2.6. Statistics
As more than 80% of the MeP, PrP, and BuP placental measurements were below the LOD, only the values of placental EtP (88% > LOD) were used for further statistical analysis. For 28 of the 229 placental samples with EtP measurements below the LOD, the EtP values were imputed as recommended (United States Environmental Protection Agency EPA, 2000), replacing them by random imputation in the range between ¼ and ¾ of the LOD, using the R package imputeTS (Moritz and Bartz-Beielstein, 2017). The placental EtP measurements were regressed on the different batches to reduce the influence of technical variation.
In the analysis of associations between EtP and metabolic markers, we applied in a first step multiple linear regression models to establish potential links between prenatal paraben exposure and metabolic changes of the newborn. HDL and LDL values were regressed on the corresponding measurements of total cholesterol to avoid that total cholesterol levels affect the analysis and subsequently the residuals were used in further analyses. To reduce the kurtosis and skewness of the distribution, values of placental EtP and metabolic biomarkers were log-transformed. We constructed a directed acyclic graph (DAG) (Supplementary Fig. S2), which was built with the freely available online tool DAGitty 3.0 (Textor et al., 2016), to select a priori potential confounders as covariates. The DAG describes the association between placental EtP as the exposure and the respective metabolic biomarker as the outcome and includes variables described in the literature which showed a significant direct or indirect association with exposure or outcomes. Based on the DAG, two multiple linear regression models with different sets of covariates were constructed for each of the metabolic biomarkers. Model 1 included a minimal sufficient adjustment set derived from the DAG, comprising newborn sex, parity, maternal age, and alcohol consumption during pregnancy. Model 2 included all identified variables of the DAG, namely: newborn sex, ethnicity, gestational age and birth weight, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI and presence of gestational diabetes mellitus (GDM), maternal alcohol consumption during pregnancy, parity, and in the case of cord GGT as outcome, additionally season of delivery. To interpret the model estimates, we converted the beta (β) coefficients and their corresponding 95% confidence intervals (CI) to the percent change in individual metabolic biomarkers corresponding to an interquartile ratio (IQR) change in placental EtP. To examine the possible effects of GDM on the outcome of the analyses, we performed a sensitivity analysis by excluding samples of neonates born to mothers with the GDM condition. For each of the two models, the relationship between exposure and outcome was also investigated when stratified for the maternal pre-pregnancy BMI categories underweight/normal and overweight/obese. Additionally, in model 2 of the unstratified analysis, also the interaction between placental EtP levels and pre-pregnancy BMI (continuous) was included. The mean log placental EtP values of the underweight/normal weight and overweight/obese women were compared via Student's t-test after verifying the equality of variances using Levene's method. Pearson correlation was applied to investigate the association of maternal pre-pregnancy BMI with placental EtP concentration and metabolic biomarkers. Furthermore, we investigated in 202 samples if EtP had a mediating effect in the relation between dichotomous (underweight/normal and overweight/obese) maternal pre-pregnancy BMI and cord blood GGT and glucose levels by applying causal mediation analyses with the R package “mediation” (Tingley et al., 2014). Mediator and outcome models were run for the covariate sets of model 2. Bias-corrected and accelerated (BCa) confidence intervals were estimated by applying 1000 bootstraps.
In a second step, we further investigated if the association between EtP and GGT activity may be influenced by epigenetic mechanisms modulating the level of GGT. To accomplish this analysis, we applied linear mixed models interrogating the methylation status of CpGs annotated to different GGT genes using EtP measurements as the independent variable. Batch effects caused by the different chips and chip-positions in the methylation analysis were accounted for by treating them as random effects. Additionally, an adjustment for blood cell type composition was applied by using filtered and combined reference datasets available via Bioconductor as “FlowSorted.CordBloodCombined.450 k” (Gervin et al., 2019). Furthermore, the model included newborns’ sex, ethnicity, gestational age, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI and presence of gestational diabetes mellitus (GDM), maternal alcohol consumption during pregnancy, parity and season of delivery as covariates. To account for multiple testing, the Benjamini-Hochberg correction method was applied and individual CpGs sites were considered significant with an adjusted p-value below the 5% significance level.
The association between placental EtP values and trajectories of BMI z-scores based on repeated measurements of weight and height was analyzed in mixed models with age in days (treated as linear and quadratic term) as random intercept, and additionally adjusted for newborn sex, ethnicity, gestational age birth weight below the 25th percentile for gestation, parity, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI and maternal alcohol consumption during pregnancy.
Collinearity of variables was examined by calculating the variance inflation factor with the R package 'faraway' (Faraway, 2005). Statistical significance was defined as p < 0.05. All data analyses were performed in RStudio using R 4.1.
3. Results
3.1. Demographics
The newborns in this study were mostly of European origin (86.6%), had a mean (SD) gestational age of 39.46 (1.14) weeks and a mean (SD) birth weight of 3452 (4 0 2) g (Table 1). The mothers were on average 30.05 (4.39) years old at delivery and had a pre-pregnancy BMI of 25.05 (4.86) kg/m2 (underweight n = 5, normal weight n = 125, overweight n = 56, and obese n = 32). Gestational diabetes mellitus was diagnosed in 8 mothers. The majority of the mothers reported never having smoked (68.3%) and not having consumed alcohol during their pregnancy (84.9%). Most of the births occurred in spring (40.4%) and were primiparous (45.9%). The population characteristics and perinatal factors of the recruited mother-newborn pairs reflect a representative subset of both the ENVIRONAGE birth cohort and the gestational segment of the Flemish population (Supplementary Table S1). The geometric mean and interquartile range [IQR] of EtP in placental tissue was 0.97 [0.48–2.16] ng/g and that of the GGT activity in cord plasma 99.81 [72.0─153.5] U/L. Geometric means [IQR] for cord insulin and glucose were 23.06 [13.4–44.95] pmol/L and 75.96 [66.0–87.0] mg/dL respectively, and for cord HDL and LDL 25.47 [21.0–32.0] mg/dL and 19.15 [15.08─25.14] mg/dL respectively.
Table 1.
Population characteristics and perinatal factors.
| Characteristics | Study population (n = 218) | |
|---|---|---|
| Newborn | ||
| Girls, n | 106 | (48.2%) |
| Birthweight, grams | 3451.73 | ±402.17 |
| Birthweight < 25th percentile for gestation | 45 | (21%) |
| European, n | 191 | (86.6%) |
| Gestational age, weeks | 39.46 | ±1.14 |
| Placental EtP concentration, ng/g (n = 218)* | 0.97 | [0.48–2.16] |
| Cord blood plasma | ||
| GGT activity, U/L (n = 202) | 99.81 | [72.0–153.5] |
| Insulin pmol/L (n = 193) | 23.06 | [13.4–44.95] |
| Glucose mg/dL (n = 202) | 75.96 | [66.0–87.0] |
| HDL mg/dL (n = 202) | 25.47 | [21.0–32.0] |
| LDL mg/dL (n = 197) | 19.15 | [15.08–25.14] |
| Maternal | ||
| Age, years | 30.05 | ± 4.39 |
| Pre-pregnancy BMI, kg/m2 | 25.05 | ± 4.86 |
| underweight (<18.5 kg/m2) | 5 | (2.3%) |
| normal weight (18.5–24.9 kg/m2) | 125 | (57.3%) |
| overweight (25.0–29.9 kg/m2) | 56 | (25.7%) |
| obese (≥30.0 kg/m2) | 32 | (14.7%) |
| Gestational diabetes mellitus, n | 8 | (3.7%) |
| Education | ||
| Low, n | 25 | (11.5%) |
| Middle, n | 79 | (36.2%) |
| High, n | 114 | (52.3%) |
| Smoking, n | ||
| Never smoked | 149 | (68.3%) |
| Former smoker | 47 | (21.6%) |
| Smoking during pregnancy | 22 | (10.1%) |
| Parity (including present birth) | ||
| 1, n | 100 | (45.9%) |
| 2, n | 86 | (39.4%) |
| ≥3, n | 32 | (14.7%) |
| Alcohol during pregnancy, n | 33 | (15.1%) |
| Season of delivery | ||
| Winter | 61 | (28.0%) |
| Spring | 88 | (40.4%) |
| Summer | 21 | (9.6%) |
| Autumn | 48 | (22.0%) |
*Comprises 17 values below the LOD of 0.2 ng/g which were imputed.
The numbers represent counts (percentages) for categorical and mean ± standard deviation (SD) for continuous variables. Placental EtP and cord metabolic biomarker values are reported as geometric mean with its [interquartile range]. EtP = ethyl paraben; GGT = γ-glutamyltransferase; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
3.2. Placental paraben concentrations
Geometric mean (geometric SD) levels of paraben values above the LOD were: for MeP 4.42 (3.19) ng/g (n = 37), for EtP 1.32 (2.40) ng/g (n = 201), for PrP 1.51 (4.72) ng/g (n = 29), and for BuP 0.35 (1.36) ng/g (n = 14). Therefore, for MeP, PrP and BuP, the majority of the measurements were below the LOD (83.84%, 87.34% and 93.89% respectively), while for EtP 87.78% of the measurements were above the LOD (Supplementary Table S2) and were subsequently used for further analysis.
The distribution of the measured EtP values above the LOD (n = 201) was skewed to the right. After imputation of values under the LOD, log transformation and batch correction, the distribution of the processed placental EtP values considered for the statistics of the study population (n = 218) showed less skewness and kurtosis (Fig. 1).
Fig. 1.
Split violin plot with the distribution of ethyl paraben (EtP) values in placental tissue of the ENVIRONAGE birth cohort. Left-hand side in blue, raw EtP values above LOD (n = 201); right-hand side in red, EtP values after imputation of values under the LOD, log transformation and batch correction (n = 218). The black rectangles in the center of the curves represent the interquartile range [IQR], 1st quartile (lower end) and the 3rd quartile (upper end). The horizontal lines in each curve show the median.
3.3. Association between ethyl paraben and metabolic biomarkers
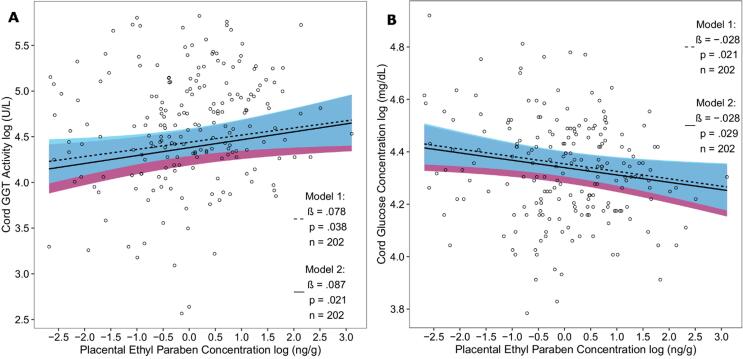
In both models, placental EtP and cord blood GGT activity were significantly positively associated (model 1: β = 0.078, p = 0.038; model 2: β = 0.087, p = 0.021) (Fig. 2 A). In the regression model containing only the variables of the minimal sufficient adjustment set (model 1), an increment in placental EtP (for the subset of n = 202 with GGT measurements) of 3.93 ng/g, equal to its (IQR), was associated with an increase of 11.31 % in the geometric mean of cord blood GGT activity (95% CI: 0.59–23.17). In model 2, an (IQR) increment in placental EtP was associated with an increase of 12.61 % in the geometric mean of cord blood GGT activity (95% CI: 1.80–24.57).
Fig. 2.
Scatterplot of the association between log-transformed placental EtP concentration with A) log-transformed cord blood GGT activity and with B) log-transformed cord glucose levels in two adjusted models (model 1 dashed and model 2 solid regression lines) with 95% confidence intervals (model 1 cyan and model 2 purple areas). The log EtP values were denoised for batch effects. Model 1: adjusted for newborn sex, parity, maternal age, and alcohol consumption during pregnancy. Model 2: adjusted for newborn sex, ethnicity, gestational age and birth weight, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI, gestational diabetes, alcohol consumption during pregnancy, parity, and for GGT additionally for the season of delivery. EtP = ethyl paraben; GGT = γ-glutamyltransferase.
The association between placental EtP and cord glucose levels showed a significantly inverse relationship in both models (model 1: β = −0.028, p = 0.021; model 2: β = −0.028, p = 0.029) (Fig. 2 B). An (IQR) change in placental EtP of 3.76 ng/g (for the subset of n = 202 with glucose measurements) was for model 1 and 2 respectively associated with a decrease of −3.69% (95% CI: −6.70 to −0.58) and −3.64 % (95% CI: −6.80 to −0.39) in the geometric mean of cord glucose.
For the association of placental EtP with cord GGT, the sensitivity analysis showed only a slight change in effect size or significance after excluding the mother-newborn pairs with mothers diagnosed with GDM (model 1: β = 0.080, p = 0.037; model 2: β = 0.087, p = 0.024). Likewise for the association with cord glucose, the exclusion of GDM mother-newborn pairs had no effect on the outcome (model 1: β = -0.030, p = 0.018; model 2: β = -0.029, p = 0.029).
For the other cord blood metabolic biomarkers (insulin, HDL, and LDL), no associations with placental EtP were found in any of the models (Supplementary Fig. S3 A-C).
3.4. Association between ethyl paraben and DNA methylation at promoter-associated CPGs annotated to Gamma-Glutamyltransferase
CpG cg08612779 showed the lowest p-value associated with EtP (n = 109, ß = 0.0017; p = 1.6e-3) and was annotated to Gamma-Glutamyltransferase 7 (GGT7) (Supplementary Table S3). After correcting for multiple testing the association remained significant at a 5% level with an adjusted p-value of 0.049.
3.5. Association between maternal pre-pregnancy BMI, placental EtP, and cord blood metabolic biomarkers
The difference in mean log-transformed placental EtP values between the groups of mothers with underweight/normal pre-pregnancy BMI (0.13 ± 1.08) kg/m2 and overweight/obese pre-pregnancy BMI (-0.15 ± 1.10) kg/m2 was borderline significant (p = 0.062).
Maternal pre-pregnancy BMI showed a trend for an inverse correlation with placental EtP concentration (r = -0.12, p = 0.090) (Fig. 3 A) and was significantly inversely correlated with cord blood GGT activity (r = -0.14, p = 0. 045) (Fig. 3 B). For cord blood glucose, insulin, HDL, and LDL no correlation with maternal pre-pregnancy BMI was observed (Supplementary Fig. S4 A-D).
Fig. 3.
Correlation between maternal pre-pregnancy BMI and A) placental log EtP values and B) log cord blood GGT activity. EtP = ethyl paraben; GGT = γ-glutamyltransferase.
When stratified for two groups of maternal pre-pregnancy BMI (underweight/normal, overweight/obese), an (IQR) increase of placental EtP showed a trend for a stronger effect on cord GGT in the group of overweight/obese mothers in both models (Table 2). For cord glucose, an (IQR) increase in placental EtP showed only in model 2 a trend for a stronger effect in the group of overweight/obese mothers (Table 2). As to the effect of placental EtP on cord insulin, HDL and LDL levels no modification by maternal pre-pregnancy group could be observed. We did not observe any effect on cord GGT activity or glucose concentration by placental EtP interaction with pre-pregnancy BMI (for GGT p = 0.60, for glucose: p = 0.87).
Table 2.
Effect of an (IQR) increase in EtP on cord GGT activity and cord glucose stratified by maternal pre-pregnancy BMI group in both multiple regression models (n = 202).
| Model 1 |
Model 2 |
||||||
|---|---|---|---|---|---|---|---|
| Outcome | BMI group | % Change | 95% CI | p | % Change | 95% CI | p |
| Cord GGT | underweight/normal | 8.13 | −5.62─23.88 | 0.26 | 8.24 | −5.39─23.84 | 0.25 |
| overweight/obese | 11.56 | −2.19─27.25 | 0.10 | 14.91 | −0.63─32.89 | 0.061 | |
| Cord glucose | underweight/normal | −3.94 | −8.42─0.75 | 0.10 | −3.50 | −8.0─1.22 | 0.14 |
| overweight/obese | −2.94 | −6.83─1.11 | 0.15 | −4.04 | −8.13─0.23 | 0.063 | |
EtP = ethyl paraben; GGT = γ-glutamyltransferase; (IQR) = interquartile ratio.
Model 1: adjusted for newborn sex, parity, maternal age, and alcohol consumption during pregnancy.
Model 2: adjusted for newborn sex, ethnicity, gestational age and birth weight, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI, gestational diabetes, alcohol consumption during pregnancy, parity, and for GGT additionally for the season of delivery.
In the mediation model investigating the influence of placental EtP on the relation between maternal pre-pregnancy BMI group and cord blood GGT activity the regression coefficients between maternal pre-pregnancy BMI group and placental EtP, and between placental EtP and cord blood GGT activity were significant (β = −0.35, p = 0.037 and β = 0.087, p = 0.021 respectively), with a significant bootstrapped indirect effect (β = −0.031, 95% CI: −0.086 to −3.0e-3) (Supplementary Fig. S5 A.).
For the association between maternal pre-pregnancy BMI group and cord blood glucose levels the association between maternal pre-pregnancy BMI group and placental EtP, and between placental EtP and cord blood glucose levels were significant (β = −0.39, p = 0.020 and β = −0.30, p = 0.019 respectively) (Supplementary Fig. S5 B.). The effect of maternal pre-pregnancy BMI on cord blood glucose level was fully mediated via the placental EtP levels with a significant bootstrapped indirect effect of β = 0.12 (95% CI: 1.9e-3─0.033).
3.6. Association between ethyl paraben and early childhood trajectories of BMI z-Scores
Placental EtP levels were significantly inversely associated with BMI z-sores based on repeated measurements at the ages 0–2.4 years in a subset of 63 children (β = −0.19, p = 0.013), in a mixed model with age in days as random intercept and adjusted for newborn sex, ethnicity, gestational age and birth weight below the 25th percentile for gestation, parity, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI and maternal alcohol consumption during pregnancy (Fig. 4). An (IQR) increment in placental EtP was associated with a decrease in longitudinal BMI z-score of 0.27 points (95% CI: −0.46 to −0.088).
Fig. 4.
Scatterplot of the association between placental EtP and trajectories of BMI z-scores (490 observations) calculated for 63 children from repeated measurements of height and weight at the ages between 0 and 2.4 years. The grey ribbon shows the 95% confidence interval for the regression line of the linear mixed model, with age in days as (linear and quadratic (squared) term) as random intercept and adjusted for newborn sex, ethnicity, gestational age and birth weight below the 25th percentile for gestation, parity, maternal age, maternal smoking, maternal education, maternal pre-pregnancy BMI and maternal alcohol consumption during pregnancy.
4. Discussion
As key findings in this study of the ENVIRONAGE birth cohort, we found placental EtP levels inversely associated with early childhood trajectories of BMI z-scores and cord blood glucose levels, while being positively associated with cord blood GGT activity. Our results show epigenetic programming for the effect of placental EtP on cord blood GGT activity. In addition, we observed indications that ethyl paraben mediates the associations between pre-pregnancy BMI with GGT and glucose.
4.1. Exposure to parabens
Our study reports concentration levels of different parabens in placental tissue of a Belgian birth cohort. The placenta has been suggested as a non-invasive biomonitoring matrix for the assessment of maternal and prenatal exposure to persistent organic pollutants (Jeong et al., 2018), but previous studies aiming at the optimization of extraction and detection protocols for parabens in placental tissue have employed only very limited sample sizes (Valle-Sistac et al., 2016, Vela-Soria et al., 2017). The examination of placental tissue for the occurrence of nonpersistent chemicals may limit attenuation bias related to high intra-individual variability in spot urine measurements, which has been reported previously in an exposome-wide association study of 1,301 children from six European birth cohorts (Vrijheid et al., 2020). The distribution and concentration of different parabens in placental tissue reported in our study showed some dissimilarities to the findings of studies in other matrices like urine, indicating that fetal paraben exposure could differ from that in adults, and maternal spot urine samples, analyzed for total paraben concentration, may not adequately represent the fetal exposure to (active) free parabens via the placenta. The metabolism of parabens plays an important role in their exposure assessment. Parabens are mainly hydrolyzed to p-hydroxybenzoic acid, a non-specific metabolite of all parabens which is then conjugated with glycine, glucuronide, and sulfate and excreted via urine (Kiwada et al., 1979, Soni et al., 2005). A much smaller percentage of the parent parabens is excreted as free and conjugated species (Dewalque et al., 2014, Moos et al., 2016, Ye et al., 2006), with the parent substance of the xenobiotic as the bioactive form with potentially adverse health effects (van den Berg et al., 2003, Ye et al., 2006). Parabens are largely conjugated by sulfotransferases (SULTs) and UDP-glucuronosyltransferase (UGT) (Scientific Committee on Consumer Safety (SCCS) 2011). As human UGT isoenzymes are only expressed several months after birth (Strassburg et al., 2002) conjugation of parabens in the fetus has been suggested to occur, most likely to a lesser extent, predominantly by SULTs which are expressed in the human liver of all age groups including fetuses (Hines, 2008). Little is known about the metabolic fate of parabens in the human placenta, but several studies have reported higher SULT, and lower UGT activity in the human placenta compared with the liver (Blanco-Castañeda et al., 2020, Coughtrie et al., 1988). The proportion of free paraben in the body is therefore also dependent on the efficiency of the paraben metabolizing enzymes, but little is known about the fetal exposure profile of parabens (Scientific Committee on Consumer Safety (SCCS) 2011). Compared to a 2–5% proportion of free to total MeP and PrP found in adults (Ye et al., 2006) much higher proportions (10–15%) were found in urinary spot samples of hospitalized preterm infants (Calafat et al., 2009), which could indicate a relatively higher exposure of the unborn and newborn to the active compound compared with adults and older children (Scientific Committee on Consumer Safety (SCCS) 2011).
In our study of placental tissue, 88% of the EtP measurements, but only <17% of the MeP measurements, were above the LOD. Similar findings were reported in a study on cord plasma, where relative abundance calculations showed that free EtP values were substantially elevated compared to MeP and PrP (Pycke et al., 2015). In contrast, various spot-urine measurements showed the highest detection rate and concentration for MeP, and the lowest detection rate for EtP (Casas et al., 2011, Philippat et al., 2014, Pycke et al., 2015). This discrepancy may be explained by the dissimilarities in the human pharmacokinetic rate and transplacental transfer of different paraben congeners, as observations in rat offspring, prenatally exposed to maternal percutaneous paraben treatment, indicated different metabolic rates and placental transfer capacities for parabens (Frederiksen et al., 2008). Though a recent study of free paraben levels in 191 placental tissue samples randomly selected from five INMA cohorts (Freire et al., 2020) found lower placental EtP levels in comparison with other parabens, this discrepancy could be explained by the fact that we applied higher LODs which lead to the exclusion of more measurements. Further research is needed to clarify the difference between the fetal paraben exposure measured in our study and the paraben exposures measured in children and adults elsewhere.
4.2. Paraben exposure, GGT, glucose, and other metabolic outcomes
We are the first to describe an association between placental EtP and cord blood GGT. Parabens have previously been suspected to have endocrine-disrupting activity and to act as obesogens (Hu et al., 2013, Kim and Chevrier, 2020, Liu et al., 2019). Our finding that placental EtP is significantly and positively associated with cord blood GGT may partly explain the previously described obesogenic effects of parabens. In adults, serum GGT activity was found associated with fasting insulin (Matsha et al., 2014, Rantala et al., 2000), HDL and LDL (Lippi et al., 2007, Rajarajeswari et al., 2014) levels, and hypertension (Bonnet et al., 2017, Rantala et al., 2000). It may be considered a possible sensitive and early biomarker for developing type 2 diabetes and metabolic syndrome (Kim et al., 2005, Lee et al., 2003, Lee et al., 2007a, Sabanayagam et al., 2009, Zhao et al., 2020). During pregnancy, women with higher GGT levels are at greater risk of developing GDM. A prospective cohort study of 1,512 pregnant women showed a risk ratio (95% CI) of 5.40 (3.36─8.68) for developing GDM when comparing higher versus lower GGT levels (Kong et al., 2018). In a study of 500 women, GGT was identified as an independent risk factor for GDM [adjusted odds ratio, 2.1 (95% CI: 1.2─3.8), p = 0.01] (Tan et al., 2008).
It should be pointed out that research into the association of metabolic endpoints or biomarkers with EtP showed disparate or inconsistent results. We found a significant inverse association between placental EtP and newborn cord blood glucose levels. However, in a recent prospective study of 1,087 Chinese pregnant women, a significant positive association was found between first-trimester urinary EtP and glucose levels only for the highest exposures (fourth exposure quartile: β = 0.237, 95% CI: 0.023─0.450), which was not significant anymore after stratifying by age (Liu et al., 2019). The discrepancy in the direction of association with our results could be explained by the difference in the analyzed matrices, test designs, and timing of paraben exposure characterization. Furthermore, the same Chinese study reported a risk ratio of 1.70 (95% CI: 1.02─2.82) for GDM when comparing the highest with the lowest quartile of urinary EtP exposure (Liu et al., 2019). In contrast, other recent studies claim opposite effects, however, in not pregnant women and men (Kim and Chevrier, 2020, Lee et al., 2019, Ward et al., 2020). Kim and Chevrier found EtP exposure associated with a 63% (95% CI: 2─86) lower prevalence of metabolic syndrome in women (Kim and Chevrier, 2020); a cross-sectional study of 794 adults (≥20 years) from the 2013–2014 National Health and Nutrition Examination Survey (NHANES) showed that urinary EtP levels were positively associated with serum adiponectin levels in women of reproductive age (Lee et al., 2019); the 2005–2014 NHANES survey in adults (n = 8,498) reported an 0.60 adjusted OR (95% CI: 0.51–0.71) for diabetes when comparing the 75th to 25th percentiles of urinary EtP (Ward et al., 2020). The at first sight seemingly protective impact of EtP on metabolic outcomes as suggested by previous research (Kim and Chevrier, 2020, Lee et al., 2019, Ward et al., 2020), may not be operational in pregnant women because the function of drug-metabolizing enzymes and metabolic processes is influenced by pregnancy (Isoherranen and Thummel, 2013). Our study supports the hypothesis that in utero EtP exposure may already influence metabolic pathways in fetal life potentially leading to increased disease risks later in life.
4.3. Paraben exposure and differential methylation of CPGs annotated to the Gamma-Glutamylransferase genes
We investigated the possibility that increased enzymatic activity of GGT may be due to epigenetic changes influencing the expression level of GGT. Human GGT is a multigene family which consists of seven known GGT genes or pseudogenes (Heisterkamp et al., 2008). In a mixed model including multiple covariates and adjusted for technical variation we found that the probe cg08612779, annotated to GGT7 and located at a CpG island was significantly associated with placental EtP levels. GGT7 is a protein-coding gene located at chromosome 20 that encodes enzymes involved in both the metabolism of glutathione and in the transpeptidation of amino acids. The protein is widely expressed in human tissues with the highest percentage in the brains and especially the frontal cortex (Fagerberg et al., 2014). The CpG site was promoter-associated in close proximity to a transcription start site (TSS200) which could indicate gene silencing by hypermethylation in the presence of higher placental EtP levels. Generally, the relationship between methylation status correlates inversely with gene expression in close proximity to the promoter, with CpG islands often being hypomethylated irrespective of gene expression status (Martino and Saffery, 2015, Moore et al., 2013). On the other hand, growing evidence suggests that this assumption may not always hold true, and increasing levels of promoter methylation can also correlate directly with increased gene expression (Rauluseviciute et al., 2020, Smith et al., 2020). The control of GGT expression is the subject of a complex regulatory mechanism (Zhang and Forman, 2009), with every subtype of GGT mRNA being regulated through a distinct promoter and responding differentially to oxidative stress, while promoter activation also depends on the inducing agents and exposure factors (Zhang and Forman, 2009). Even though no clear indication could be found that EtP may directly increase GGT activity through transcriptional upregulation via DNA methylation, a significant alteration on the epigenetic level related to EtP exposure could be observed which may indicate a change in the fetal programming with later-life implications.
4.4. Paraben exposure and pre-pregnancy BMI
We found a trend for an inverse association between maternal pre-pregnancy BMI and placental EtP. This finding is in line with a recent study of 95 pregnant women in Iran describing a negative association between maternal urinary EtP and maternal BMI for the third trimester of pregnancy (r = −0.235, p = 0.022) (Hajizadeh et al., 2020). Other studies of (non-pregnant) women or the general population reported significant associations for other parabens with BMI, but not for EtP (Kang et al., 2016, Kolatorova et al., 2018). Due to their moderatly lipophilic character (El Hussein et al., 2007), it has been suggested that paraben-esters are deposited in the human fat stores explaining negative associations with BMI found in studies on urinary paraben (Kolatorova et al., 2018). Because the human placenta has a fat percentage of only around 3.8% (Pratt et al., 1946), parabens would be expected to be sequestered relatively less in placental tissue of obese women than in their lean counterparts, which could explain the inverse association direction in our study.
4.5. Pre-pregnancy BMI and cord blood GGT and glucose
We found a significant inverse association between maternal pre-pregnancy BMI and cord blood GGT, which has, to our knowledge, not been investigated yet. In studies of children (Cho et al., 2011) and adults (Elshorbagy et al., 2009, Lawlor et al., 2005, Poikolainen and Vartiainen, 1997), the association between BMI and serum GGT activity has repeatedly been reported to be positive instead of negative as in our study. Serum GGT was a positive predictor of BMI (partial r = 0.17, P < 0.001) in a study based on data from 1,550 subjects recruited from nine European countries in the COMAC project (Elshorbagy et al., 2009). GGT was significantly correlated with BMI (r = 0.030, p < 0.0001) in a study of 6,010 healthy individuals in Finland (Poikolainen and Vartiainen, 1997). In a study of 3,789 British postmenopausal women, a standard deviation increase in BMI was associated with a 2.14 U/L (95% CI: 0.99─3.30) increase in GGT (Lawlor et al., 2005). However, all the literature mentioned above investigated the association between BMI and GGT in the same person. Instead, our study examined the BMI/GGT relationship in sets of two different individuals connected by the placenta, which may partly explain the observed disparity in the association direction. There is only sparse literature about fetal GGT which may serve different functions. Cord sera were shown to contain a significantly greater (p < 0.001) proportion of HDL-bound-GGT (68.5 ± 5.5%) than adult sera (59.8 ± 10.2%) (Garcia et al., 1987). Fetal GGT reflects hepatic immaturity, sex of the neonate, gestational age, route of delivery, and perinatal stress (Garcia et al., 1987, Rivera et al., 1990). Different but not yet fully elucidated functions and characteristics of fetal GGT may explain the observed shift in the direction of the association between maternal pre-pregnancy BMI and cord GGT activity.
We did not observe a significant correlation between pre-pregnancy BMI and cord blood glucose levels, yet a significant link has been reported previously for cord glucose levels of neonates born to obese mothers (Mocarzel et al., 2020a). In a cross-sectional case control study investigating offspring from obese mothers (n = 41) and healthy controls (n = 31) it was shown that cord blood glucose levels of newborns of obese mothers [47.8 mg/dL (SD 33.1)] were lower than that in the control group [57.9 mg/dL (SD 12.5)] (Mocarzel et al., 2020b). As paraben exposure has already been associated with the BMI and weight status as well as with metabolic markers including glucose levels, we investigated a potential role of placental EtP in the association between maternal pre-pregnancy BMI and the cord blood metabolic markers GGT and glucose and found significant mediating effects for both relationships. In the case of cord blood GGT, the indirect effect had the same direction as the direct effect of maternal pre-pregnancy BMI on cord blood GGT and therefore led to a higher effect size for the total effect. On the other hand, in the association between pre pregnancy BMI and cord blood glucose the indirect effect via EtP was positive, attenuating the inverse direct effect resulting in roughly half of the effect size for the total effect. While the causal steps approach postulated by Baron and Kenny (Baron and Kenny, 1986) demanded statistical significance of the total effect, this requirement is nowadays not deemed a necessary condition for claiming a mediating (indirect) effect (Hayes, 2009, Rucker et al., 2011).
4.6. Paraben exposure and longitudinal trajectories of BMI z-Scores
In the analysis between placental EtP concentrations and the trajectories of early childhood BMI z-scores we found a significant inverse association not reported before in this constellation. While in two studies a positive relationship between prenatal exposure to other parabens with childhood metabolic outcomes was reported (Berger et al., 2021, Leppert et al., 2020) in one of these studies no significant association between maternal urinary EtP levels and BMI trajectories (age 1–8) for 392 children was found (Leppert et al., 2020). Another study investigated the relationship between postnatal urinary EtP and BMI z-score in 1324 children aged 6–19 years and found a (statistically non-significant) inverse trend (β = −0.08, 95 % CI: −0.24─0.08) (Quirós-Alcalá et al., 2018). In adults, inverse as well as positive relationships were shown for the association between EtP exposure and metabolic outcomes. Urinary ethyl paraben was associated with a 63% (95% CI: 2-86) lower prevalence of metabolic syndrome among 2,564 women participating in Cycle 4 (2014–2015) of the Canadian Health Measures Survey (Kim and Chevrier, 2020). On the other hand, EtP showed significantly increased odds of obesity in the fourth quartile regardless of the dilution adjustment method [for the covariate-adjusted standardization method: OR (95% CI) 1.51 (1.19─1.91)] in an adult population (n = 3782) of the Korean National Environmental Health Survey (KoNEHS) 2015–2017 (Lee et al., 2021).
A direct comparison between the findings of this study with those of cross-sectional studies investigating postnatal exposures may be difficult, as the underlying mechanisms and pathways may differ between the childhood metabolic responses to prenatal and postnatal exposures. Prenatal exposure may initiate alterations in the epigenetic programming according to the DOHAD hypothesis which could lead to other, even contradictory metabolic responses compared to postnatal exposure.
4.7. Strengths and limitations
We acknowledge specific strengths and limitations of our present study. We are the first to investigate the association between cord blood metabolic biomarkers and paraben exposure levels in placental tissue, a matrix that may better reflect fetal instead of maternal exposure. Our analyses for metabolic biomarkers are performed with two different sets of covariates, based on previous results in the literature and evaluated by a DAG. Because our study is nested in an established prospective birth cohort (ENVIRONAGE), representative of the gestational segment of the Flemish population (Janssen et al., 2017), our findings are generalizable. Nevertheless, we also have to recognize potential limitations. The direction of causality between paraben exposure and maternal BMI may be uncertain, as on the one hand paraben exposure could influence the maternal BMI by obesogenic or leptogenic effects, while on the other hand reverse causality is also possible due to retention of parabens in fat stores of obese mothers resulting in lower placental paraben levels for a higher maternal BMI. While the assessment of paraben concentrations in placental tissue allows to investigate integrated exposure during pregnancy, it does not enable the determination of specific exposure windows during in utero development. Because only free paraben concentrations were analyzed in the placental tissue we were not able to compare the proportion of free and total paraben as recommended to check the possibility of external contamination (Moos et al., 2016, Moos et al., 2015), or evaluate differences in the fetal metabolism of parabens. Another limitation could be the fact that the cord blood analyzed in this study was a mixture of arterial and venous blood which hampers the functional distinction of the analyzed metabolic markers as products of placental or fetal metabolism. In the analysis of BMI z-score information, we could not adjust for the child's breastfeeding status, caloric intake and postnatal EtP exposure because this information was not available. Finally, the observational nature of this study does not directly allow to infer causal relationships from the resulting data as it is more challenging to prove the causal impact of a variable on the outcome with non-experimental data (Nichols, 2007). Nevertheless, observational data, especially from prospective cohort studies are valuable in the generation of hypotheses which can then be further investigated within an experimental set-up (Mann, 2003).
5. Conclusion
In a subset of the prospective ENVIRONAGE birth cohort, we could determine EtP concentrations in about 88% of the placental samples. A significant inverse association between placental EtP levels and longitudinal measurements of BMI z-scores for the first years of life was demonstrated. Furthermore, placental EtP levels were significantly associated with cord blood GGT activity in neonates. Because GGT activity is an established marker of metabolic disease in adults, awareness as to potential adverse health effects later in life is necessary. As our findings seem to indicate mediating effects of placental EtP levels in the association between maternal pre-pregnancy BMI and metabolic markers, and modulation of metabolic effects of EtP by pre-pregnancy BMI, the latter could also influence the exposure-outcome associations. The most critical phase of life for exposure to adverse environmental influences is the development in utero (Janesick and Blumberg, 2011). We further showed that prenatal EtP exposure may influence DNA methylation of a gene, encoding a key-metabolic enzyme by fetal programming. While small alterations of metabolic factors in early life are generally not regarded as harmful, especially when they are within the physiological range, they may influence disease risk later in life. In 2015, the European Union lowered the maximum concentration of specific parabens in cosmetic products and banned them in certain other products designed for children under the age of three as a precautionary measure. Even though the use of EtP is still deemed safe (The European Commission, 2014), in light of the findings in this study, further research should reassess the safety of EtP especially in the context of prenatal exposure.
CRediT authorship contribution statement
Brigitte Reimann: Writing – original draft, Methodology, Formal analysis, Visualization. Karen Vrijens: Investigation, Writing – review & editing. Harry A. Roels: Writing – review & editing. Congrong Wang: Software, Writing – review & editing. Charlotte Cosemans: Investigation, Writing – review & editing. Ilse Van Overmeire: Investigation, Resources, Writing – review & editing. Tim S. Nawrot: Project administration, Supervision, Resources, Writing – review & editing. Michelle Plusquin: Conceptualization, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We acknowledge the participating mothers and neonates, as well as the staff of the maternity ward, midwives, and the staff of the clinical laboratory of East-Limburg Hospital in Genk.
Funding
The ENVIRONAGE birth cohort is supported by grants from European Research Council (ERC-2012-StG 310898), the Flemish Research Council (FWO G073315N) and the STOP project (Grant No. 774548-H2020). BR was financially supported by the University Research Fund (Bijzonder Onderzoeksfonds Universiteit Hasselt). Karen Vrijens is a postdoctoral fellow of the FWO (12D7718N).
Handling Editor: Martí Nadal
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106845.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;2014(30):1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert N., Ameller T., Legrand J.-J. Systemic exposure to parabens: Pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem. Toxicol. 2012;50(3-4):445–454. doi: 10.1016/j.fct.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Baillie-Hamilton P.F. Chemical toxins: a hypothesis to explain the global obesity epidemic. J. Alternative Complement. Med. (New York, NY) 2002;8(2):185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P. Fetal origins of coronary heart disease. BMJ (Clinical research ed) 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berger K., Hyland C., Ames J.L., Mora A.M., Huen K., Eskenazi B., Holland N., Harley K.G. Prenatal exposure to mixtures of phthalates, parabens, and other phenols and obesity in Five-Year-Olds in the CHAMACOS Cohort. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18041796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova M., Barnes B., Tsan C., Ho V., Klotzle B., Le J.M., Delano D., Zhang L.u., Schroth G.P., Gunderson K.L., Fan J.-B., Shen R. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98(4):288–295. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Bijnens, E.M., Derom, C., Thiery, E., Martens, D.S., Loos, R.J.F., Weyers, S., Nawrot, T.S., 2021. Serum gamma-glutamyl transferase, a marker of alcohol intake, is associated with telomere length and cardiometabolic risk in young adulthood. Sci. Reports 2021; 11: 12407–12407. [DOI] [PMC free article] [PubMed]
- Blanco-Castañeda R., Galaviz-Hernández C., Souto P.C.S., Lima V.V., Giachini F.R., Escudero C., Damiano A.E., Barragán-Zúñiga L.J., Martínez-Aguilar G., Sosa-Macías M. The role of xenobiotic-metabolizing enzymes in the placenta: a growing research field. Expert Rev. Clin. Pharmacol. 2020;13(3):247–263. doi: 10.1080/17512433.2020.1733412. [DOI] [PubMed] [Google Scholar]
- Bonnet F., Gastaldelli A., Pihan-Le Bars F., Natali A., Roussel R., Petrie J., Tichet J., Marre M., Fromenty B., Balkau B. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies. J. Hypertens. 2017;35:493–500. doi: 10.1097/HJH.0000000000001204. [DOI] [PubMed] [Google Scholar]
- Calafat A.M., Weuve J., Ye X., Jia L.T., Hu H., Ringer S., Huttner K., Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009;117(4):639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L., Fernández M.F., Llop S., Guxens M., Ballester F., Olea N., Irurzun M.B., Rodríguez L.S.M., Riaño I., Tardón A., Vrijheid M., Calafat A.M., Sunyer J. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int. 2011;37(5):858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Cho Y.-G., Park K.-H., Kim C.-W., Hur Y.-I. The Relationship between Serum Gamma-glutamyltransferase Level and Overweight in Korean Urban Children. Korean J. Fam. Med. 2011;32(3):182. doi: 10.4082/kjfm.2011.32.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti A., Duarte T.L., Giommarelli C., De Tata V., Paolicchi A., Jones G.D.D., Pompella A. Membrane gamma-glutamyl transferase activity promotes iron-dependent oxidative DNA damage in melanoma cells. Mutation Res./Fundamental Mol. Mech. Mutagenesis. 2009;669(1-2):112–121. doi: 10.1016/j.mrfmmm.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Coughtrie M.W., Burchell B., Leakey J.E., Hume R. The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol. Pharmacol. 1988;34:729. [PubMed] [Google Scholar]
- Dewalque L., Pirard C., Dubois N., Charlier C. Simultaneous determination of some phthalate metabolites, parabens and benzophenone-3 in urine by ultra high pressure liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2014;949-950:37–47. doi: 10.1016/j.jchromb.2014.01.002. [DOI] [PubMed] [Google Scholar]
- El Hussein S., Muret P., Berard M., Makki S., Humbert P. Assessment of principal parabens used in cosmetics after their passage through human epidermis-dermis layers (ex-vivo study) Exp. Dermatol. 2007;16(10):830–836. doi: 10.1111/j.1600-0625.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- Elder R. 3 Final Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, and Butylparaben. J. Am. College Toxicol. 1984;3:147–209. [Google Scholar]
- Elshorbagy A.K., Refsum H., Smith A.D., Graham I.M. The association of plasma cysteine and gamma-glutamyltransferase with BMI and obesity. Obesity (Silver Spring, Md) 2009;17:1435–1440. doi: 10.1038/oby.2008.671. [DOI] [PubMed] [Google Scholar]
- Enoiu M., Aberkane H., Salazar J.F., Leroy P., Groffen J., Siest G., Wellman M. Evidence for the pro-oxidant effect of gamma-glutamyltranspeptidase-related enzyme. Free Radical Biol. Med. 2000;29:825–833. doi: 10.1016/s0891-5849(00)00370-1. [DOI] [PubMed] [Google Scholar]
- Erkin-Cakmak A., Harley K.G., Chevrier J., Bradman A., Kogut K., Huen K., Eskenazi B. In utero and childhood polybrominated diphenyl ether exposures and body mass at age 7 years: the CHAMACOS study. Environ. Health Perspect. 2015;123(6):636–642. doi: 10.1289/ehp.1408417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., Asplund A., Sjöstedt E., Lundberg E., Szigyarto C.-K., Skogs M., Takanen J.O., Berling H., Tegel H., Mulder J., Nilsson P., Schwenk J.M., Lindskog C., Danielsson F., Mardinoglu A., Sivertsson Å., von Feilitzen K., Forsberg M., Zwahlen M., Olsson IngMarie, Navani S., Huss M., Nielsen J., Ponten F., Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics: MCP. 2014;13(2):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraway J.J. Chapman & Hall/CRC; Boca Raton, Fla: 2005. Linear models with R. [Google Scholar]
- Frederiksen, H., Taxvig, C., Hass, U., Vinggaard, A.M., Nellemann, C., 2008. Higher levels of ethyl paraben and butyl paraben in rat amniotic fluid than in maternal plasma after subcutaneous administration. Toxicol. Sci. 106, 376–383. [DOI] [PubMed]
- Freire C., Vela-Soria F., Beneito A., Lopez-Espinosa M.-J., Ibarluzea J., Barreto F.B., Casas M., Vrijheid M., Fernandez-Tardon G., Riaño-Galan I., Fernandez M.F. Association of placental concentrations of phenolic endocrine disrupting chemicals with cognitive functioning in preschool children from the Environment and Childhood (INMA) Project. Int J Hyg Environ Health. 2020;230:113597. doi: 10.1016/j.ijheh.2020.113597. [DOI] [PubMed] [Google Scholar]
- Garcia M.P., Tutor J.C., Sanjose M.E., Porto J.A., Fraga J.M., Paz J.M., Rodriguez-Segade S. Cord serum gamma glutamyltransferase in newborns. Clin. Biochem. 1987;20(4):269–273. doi: 10.1016/s0009-9120(87)80011-5. [DOI] [PubMed] [Google Scholar]
- Gervin K., Salas L.A., Bakulski K.M., van Zelm M.C., Koestler D.C., Wiencke J.K., Duijts L., Moll H.A., Kelsey K.T., Kobor M.S., Lyle R., Christensen B.C., Felix J.F., Jones M.J. Systematic evaluation and validation of reference and library selection methods for deconvolution of cord blood DNA methylation data. Clin. Epigenetics. 2019;11:125. doi: 10.1186/s13148-019-0717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivo M., Lopez de Alda M., Capri E., Barceló D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Grün F., Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1-2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajizadeh Y., Kiani Feizabadi G., Ebrahimpour K., Shoshtari-Yeganeh B., Fadaei S., Darvishmotevalli M., Karimi H. Urinary paraben concentrations and their implications for human exposure in Iranian pregnant women. Environ. Sci. Pollut. Res. 2020;27(13):14723–14734. doi: 10.1007/s11356-020-07991-2. [DOI] [PubMed] [Google Scholar]
- Hansen, K.D., 2016. IlluminaHumanMethylation450kanno.ilmn12.hg19: Annotation for Illumina's 450k methylation arrays. R package version 0.6.0.
- Hayes A.F. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun. Monographs. 2009;76(4):408–420. [Google Scholar]
- Heisterkamp N., Groffen J., Warburton D., Sneddon T.P. The human gamma-glutamyltransferase gene family. Hum. Genet. 2008;123(4):321–332. doi: 10.1007/s00439-008-0487-7. [DOI] [PubMed] [Google Scholar]
- Hines R.N. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol. Ther. 2008;118(2):250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hu, P., Chen, X., Whitener, R.J., Boder, E.T., Jones, J.O., Porollo, A., Chen, J., Zhao, L., 2013. Effects of parabens on adipocyte differentiation. Toxicol. Sci : Off. J. Soc. Toxicol. 131, 56–70. [DOI] [PMC free article] [PubMed]
- Isoherranen N., Thummel K.E. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos. 2013;41:256–262. doi: 10.1124/dmd.112.050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick A., Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res. Part C, Embryo today : Rev. 2011;93(1):34–50. doi: 10.1002/bdrc.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua N.R., Frederiksen H., Skakkebæk N.E., Wulf H.C., Andersson A.-M. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int. J. Androl. 2008;31(2):118–130. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Janssen B.G., Madlhoum N., Gyselaers W., Bijnens E., Clemente D.B., Cox B., Hogervorst J., Luyten L., Martens D.S., Peusens M., Plusquin M., Provost E.B., Roels H.A., Saenen N.D., Tsamou M., Vriens A., Winckelmans E., Vrijens K., Nawrot T.S. Cohort Profile: The ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int. J. Epidemiol. 2017 doi: 10.1093/ije/dyw269. [DOI] [PubMed] [Google Scholar]
- Jeong Y., Lee S., Kim S., Park J., Kim H.-J., Choi G., Choi S., Kim S., Kim S.Y., Kim S., Choi K., Moon H.-B. Placental transfer of persistent organic pollutants and feasibility using the placenta as a non-invasive biomonitoring matrix. Sci. Total Environ. 2018;612:1498–1505. doi: 10.1016/j.scitotenv.2017.07.054. [DOI] [PubMed] [Google Scholar]
- Kang H.-S., Kyung M.-S., Ko A., Park J.-H., Hwang M.-S., Kwon J.-E., Suh J.-H., Lee H.-S., Moon G.I., Hong J.-H., Hwang I.G. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ. Res. 2016;146:245–251. doi: 10.1016/j.envres.2015.12.032. [DOI] [PubMed] [Google Scholar]
- Kang S., Kim S., Park J., Kim H.-J., Lee J., Choi G., Choi S., Kim S., Kim S.Y., Moon H.-B., Kim S., Kho Y.L., Choi K. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 2013;461-462:214–221. doi: 10.1016/j.scitotenv.2013.04.097. [DOI] [PubMed] [Google Scholar]
- Karp D.R., Shimooku K., Lipsky P.E. Expression of gamma-glutamyl transpeptidase protects ramos B cells from oxidation-induced cell death. J. Biol. Chem. 2001;276:3798–3804. doi: 10.1074/jbc.M008484200. [DOI] [PubMed] [Google Scholar]
- Kaushik G.G., Sharm S., Sharma R., Mittal P. Association between gamma glutamyl transferase and insulin resistance markers in healthy obese children. J. Assoc. Physicians India. 2009;57:695–698. [PubMed] [Google Scholar]
- Kim D.J., Noh J.H., Cho N.H., Lee B.W., Choi Y.H., Jung J.H., Min Y.K., Lee M.S., Lee M.K., Kim K.W. Serum γ-glutamyltransferase within its normal concentration range is related to the presence of diabetes and cardiovascular risk factors. Diabet. Med. 2005;22:1134–1140. doi: 10.1111/j.1464-5491.2005.01581.x. [DOI] [PubMed] [Google Scholar]
- Kim J., Chevrier J. Exposure to parabens and prevalence of obesity and metabolic syndrome: An analysis of the Canadian Health Measures Survey. Sci. Total Environ. 2020;713:135116. doi: 10.1016/j.scitotenv.2019.135116. [DOI] [PubMed] [Google Scholar]
- Kiwada, H., Awazu, S., Hanano, M., 1979. The study on the biological fate of paraben at the dose of practical usage in rat. I. The metabolism and excretion of ethyl p-Hydroxybenzoate (Ethylparaben) and p-Hydroxybenzoic acid. J. Pharmacobio-Dynam. 2, 356–364.
- Klop B., Elte J., Cabezas M. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatorova L., Sramkova M., Vitku J., Vcelak J., Lischkova O., Starka L., Duskova M. Parabens and their relation to obesity. Physiol. Res. 2018;67:S465–S472. doi: 10.33549/physiolres.934004. [DOI] [PubMed] [Google Scholar]
- Kong M., Liu C., Guo Y., Gao Q., Zhong C., Zhou X., Chen R., Xiong G., Yang X., Hao L., Yang N. Higher level of GGT during mid-pregnancy is associated with increased risk of gestational diabetes mellitus. Clin. Endocrinol. 2018;88(5):700–705. doi: 10.1111/cen.13558. [DOI] [PubMed] [Google Scholar]
- Lawlor, D.A., Sattar, N., Smith, G.D., Ebrahim, S., 2005. The Associations of Physical Activity and Adiposity with Alanine Aminotransferase and Gamma-Glutamyltransferase. Am. J. Epidemiol. 161, 1081–1088. [DOI] [PubMed]
- Lee D.-H., Ha M.-H., Kim J.-H., Christiani D.C., Gross M.D., Steffes M., Blomhoff R., Jacobs D.R. Gamma-glutamyltransferase and diabetes—a 4 year follow-up study. Diabetologia. 2003;46(3):359–364. doi: 10.1007/s00125-003-1036-5. [DOI] [PubMed] [Google Scholar]
- Lee D.H., Jacobs D.R. Is serum gamma-glutamyltransferase an exposure marker of xenobiotics? Empirical evidence with polycylic aromatic hydrocarbon. Clin. Chem. Lab. Med. 2009;47:860–862. doi: 10.1515/CCLM.2009.197. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Evans J.C., Robins S.J., Wilson P.W., Albano I., Fox C.S., Wang T.J., Benjamin E.J., D’Agostino R.B., Vasan R.S. Gamma Glutamyl Transferase and Metabolic Syndrome, Cardiovascular Disease, and Mortality Risk. Arterioscler. Thromb. Vasc. Biol. 2007;27(1):127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Evans J.C., Robins S.J., Wilson P.W., Albano I., Fox C.S., Wang T.J., Benjamin E.J., D’Agostino R.B., Vasan R.S. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(1):127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- Lee I., Kim S., Park S., Mok S., Jeong Y., Moon H.-B., Lee J., Kim S., Kim H.-J., Choi G., Choi S., Kim S.Y., Lee A., Park J., Choi K. Association of urinary phthalate metabolites and phenolics with adipokines and insulin resistance related markers among women of reproductive age. Sci. Total Environ. 2019;688:1319–1326. doi: 10.1016/j.scitotenv.2019.06.125. [DOI] [PubMed] [Google Scholar]
- Lee I., Park Y.J., Kim M.J., Kim S., Choi S., Park J., Cho Y.H., Hong S., Yoo J., Park H., Cheon G.J., Choi K., Moon M.K. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Int. 2021;146:106227. doi: 10.1016/j.envint.2020.106227. [DOI] [PubMed] [Google Scholar]
- Leppert B., Strunz S., Seiwert B., Schlittenbauer L., Schlichting R., Pfeiffer C., Röder S., Bauer M., Borte M., Stangl G.I., Schöneberg T., Schulz A., Karkossa I., Rolle-Kampczyk U.E., Thürmann L., von Bergen M., Escher B.I., Junge K.M., Reemtsma T., Lehmann I., Polte T. Maternal paraben exposure triggers childhood overweight development. Nat. Commun. 2020;11:561. doi: 10.1038/s41467-019-14202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Targher G., Montagnana M., Salvagno G.L., Guidi G.C. Relationship between gamma-glutamyltransferase, lipids and lipoprotein(a) in the general population. Clin. Chim. Acta; Int. J. Clin. Chem. 2007;384:163–166. doi: 10.1016/j.cca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhou Y., Li J., Sun X., Liu H., Jiang Y., Peng Y., Zhao H., Xia W., Li Y., Cai Z., Xu S. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environ. Int. 2019;126:468–475. doi: 10.1016/j.envint.2019.02.040. [DOI] [PubMed] [Google Scholar]
- Lowe W.L., Bain J.R., Nodzenski M., Reisetter A.C., Muehlbauer M.J., Stevens R.D., Ilkayeva O.R., Lowe L.P., Metzger B.E., Newgard C.B., Scholtens D.M. Maternal BMI and Glycemia Impact the Fetal Metabolome. Diabetes Care. 2017;40(7):902–910. doi: 10.2337/dc16-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J.: EMJ. 2003;20:54–60. doi: 10.1136/emj.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, D., Saffery, R., 2015. Characteristics of DNA methylation and gene expression in regulatory features on the Infinium 450k Beadchip. bioRxiv.
- Matsha T.E., Macharia M., Yako Y.Y., Erasmus R.T., Hassan M.S., Kengne A.P. Gamma-glutamyltransferase, insulin resistance and cardiometabolic risk profile in a middle-aged African population. Eur. J. Preventive Cardiol. 2014;21(12):1541–1548. doi: 10.1177/2047487313501967. [DOI] [PubMed] [Google Scholar]
- McLaughlin T., Abbasi F., Cheal K., Chu J., Lamendola C., Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann. Intern. Med. 2003;139(10):802. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- Mocarzel C.C., Velarde G.C., Antunes R.A., Moreira de Sá R.A., Kurjak A. Maternal obesity influences the endocrine cord blood profile of their offspring. J. Perinat. Med. 2020;48:242–248. doi: 10.1515/jpm-2019-0387. [DOI] [PubMed] [Google Scholar]
- Mocarzel, C.C., Velarde, G.C., Antunes, R.d.A., Sá, R.A.M.d., Kurjak, A., 2020. Maternal obesity influences the endocrine cord blood profile of their offspring. J. Perinatal Med. 48, 242–248. [DOI] [PubMed]
- Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R.K., Angerer J., Dierkes G., Brüning T., Koch H.M. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch. Toxicol. 2016;90(11):2699–2709. doi: 10.1007/s00204-015-1636-0. [DOI] [PubMed] [Google Scholar]
- Moos R.K., Koch H.M., Angerer J., Apel P., Schröter-Kermani C., Brüning T., Kolossa-Gehring M. Parabens in 24h urine samples of the German Environmental Specimen Bank from 1995 to 2012. Int. J. Hyg. Environ. Health. 2015;218(7):666–674. doi: 10.1016/j.ijheh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Moritz, S., Bartz-Beielstein, T., 2017. imputeTS: Time Series Missing Value Imputation in R. R J. 9.
- Neese J., Duncan P., Bayse D., Robinson M., Cooper T., Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national glucose reference method. Center for Disease Control; Atlanta: 1976. [Google Scholar]
- Nichols A. Causal inference with observational data. Stata J. 2007;7(4):507–541. [Google Scholar]
- Nowak K., Ratajczak–Wrona W., Górska M., Jabłońska E. Parabens and their effects on the endocrine system. Mol. Cell Endocrinol. 2018;474:238–251. doi: 10.1016/j.mce.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Oishi S. Effects of butylparaben on the male reproductive system in rats. Toxicol. Ind. Health. 2001;17(1):31–39. doi: 10.1191/0748233701th093oa. [DOI] [PubMed] [Google Scholar]
- Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol.: Int. J. Published Br. Ind. Biol. Res. Assoc. 2002;40(12):1807–1813. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Pazos, R., Palacios, C., Campa, A., 2019. Urinary Paraben Concentrations and Its Association with Serum Triglyceride Concentrations in 2013–2014 NHANES Participants (P08-120-19). Curr. Develop. Nutrit. 3. [DOI] [PMC free article] [PubMed]
- Philippat C., Botton J., Calafat A.M., Ye X., Charles M.-A., Slama R. Prenatal exposure to phenols and growth in boys. Epidemiology (Cambridge, Mass) 2014;25(5):625–635. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poikolainen K., Vartiainen E. Determinants of gamma-glutamyltransferase: positive interaction with alcohol and body mass index, negative association with coffee. Am. J. Epidemiol. 1997;146:1019–1024. doi: 10.1093/oxfordjournals.aje.a009230. [DOI] [PubMed] [Google Scholar]
- Pratt J.P., Kaucher M., Richards A.J., Williams H.H., Macy I.C. Composition of the Human Placenta: I. Proximate Composition. Am. J. Obstet. Gynecol. 1946;52(3):402–408. doi: 10.1016/s0002-9378(15)30252-0. [DOI] [PubMed] [Google Scholar]
- Pycke B.F.G., Geer L.A., Dalloul M., Abulafia O., Halden R.U. Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ. Int. 2015;84:193–200. doi: 10.1016/j.envint.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros-Alcala, L., Buckley, J.P., Boyle, M., 2018. Parabens and measures of adiposity among adults and children from the U.S. general population: NHANES 2007-2014. Int. J. Hyg. Environ. Health 221, 652–660. [DOI] [PMC free article] [PubMed]
- Rajarajeswari D., Krishna T., Naidu M., Naidu J. Serum gamma glutamyl transferase levels in association with lipids and lipoproteins in type2 diabetes mellitus. Int. J. Res. Med. Sci. 2014;2(3):838. doi: 10.5455/2320-6012.10.5455/2320-6012.ijrms20140819. [DOI] [Google Scholar]
- Rantala A.O., Lilja M., Kauma H., Savolainen M.J., Reunanen A., Kesaniemi Y.A. Gamma-glutamyl transpeptidase and the metabolic syndrome. J. Intern. Med. 2000;248(3):230–238. doi: 10.1046/j.1365-2796.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Rauluseviciute I., Drabløs F., Rye M.B. DNA hypermethylation associated with upregulated gene expression in prostate cancer demonstrates the diversity of epigenetic regulation. BMC Med. Genomics. 2020;13:6. doi: 10.1186/s12920-020-0657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A., Bhatia J., Rassin D. Cord blood gamma glutamyl transferase activity: effect of gestational age, gender, and perinatal events. Am. J. Perinatol. 1990;7(02):110–113. doi: 10.1055/s-2007-999458. [DOI] [PubMed] [Google Scholar]
- Rucker D.D., Preacher K.J., Tormala Z.L., Petty R.E. Mediation analysis in social psychology: Current practices and new recommendations. Soc. Pers. Psychol. Compass. 2011;5:359–371. [Google Scholar]
- Sabanayagam C., Shankar A., Li J., Pollard C., Ducatman A. Serum gamma-glutamyl transferase level and diabetes mellitus among US adults. Eur. J. Epidemiol. 2009;24(7):369–373. doi: 10.1007/s10654-009-9346-7. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Consumer Safety (SCCS). Clarification on Opinion SCCS/1348/10 in the light of the Danish clause of safeguard banning the use of parabens in cosmetic products intended for children under three years of age, 10 October. 2011.
- Shekhar S., Sood S., Showkat S., Lite C., Chandrasekhar A., Vairamani M., Barathi S., Santosh W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017;241:100–107. doi: 10.1016/j.ygcen.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Shokry E., Marchioro L., Uhl O., Bermúdez M.G., García-Santos J.A., Segura M.T., Campoy C., Koletzko B. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: results from the PREOBE cohort study. Acta Diabetol. 2019;56(4):421–430. doi: 10.1007/s00592-019-01291-z. [DOI] [PubMed] [Google Scholar]
- Smith J., Sen S., Weeks R.J., Eccles M.R., Chatterjee A. Promoter DNA Hypermethylation and Paradoxical Gene Activation. Trends Cancer. 2020;6(5):392–406. doi: 10.1016/j.trecan.2020.02.007. [DOI] [PubMed] [Google Scholar]
- Smith K.W., Braun J.M., Williams P.L., Ehrlich S., Correia K.F., Calafat A.M., Ye X., Ford J., Keller M., Meeker J.D., Hauser R. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ. Health Perspect. 2012;120(11):1538–1543. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni M.G., Carabin I.G., Burdock G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem. Toxicol. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Strassburg C.P., Strassburg A., Kneip S., Barut A., Tukey R.H., Rodeck B., Manns M.P. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–265. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin. Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- Tan, P.C., Mubarak, S., Omar, S.Z., 2008. Gamma-glutamyltransferase level in pregnancy is an independent risk factor for gestational diabetes mellitus. J. Obstetr. Gynaecol. Res. 34, 512–517. [DOI] [PubMed]
- Textor J., van der Zander B., Gilthorpe M.S., Liskiewicz M., Ellison G.T. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int. J. Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- The European Commission. Press releases database, Consumers: Commission improves safety of cosmetics, September 26th, 2014, URL: http://europa.eu/rapid/press-release_IP-14-1051_en.htm.
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. mediation : R package for causal mediation analysis Citation.
- Towers C.V., Terry P.D., Lewis D., Howard B., Chambers W., Armistead C., Weitz B., Porter S., Borman C.J., Kennedy R.C.M., Chen J. Transplacental passage of antimicrobial paraben preservatives. J. Eposure Sci. Environ. Epidemiol. 2015;25(6):604–607. doi: 10.1038/jes.2015.27. [DOI] [PubMed] [Google Scholar]
- Tukey J.W. Exploratory data analysis. Addison-Wesley Pub. Co; Reading, Mass: 1977. [Google Scholar]
- United States Environmental Protection Agency EPA, 2000. Guidance for Data Quality Assessment: Practical Methods for Data Analysis: EPA QA/G9: QA00 Update: July 2000 2000.
- Valle-Sistac J., Molins-Delgado D., Díaz M., Ibáñez L., Barceló D., Silvia Díaz-Cruz M. Determination of parabens and benzophenone-type UV filters in human placenta. First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016;88:243–249. doi: 10.1016/j.envint.2015.12.034. [DOI] [PubMed] [Google Scholar]
- van den Berg M., Sanderson T., Kurihara N., Katayama A. Role of metabolism in the endocrine-disrupting effects of chemicals in aquatic and terrestrial systems. Pure Appl. Chem. 2003;75:1917–1932. [Google Scholar]
- Van Kerschaver, E., 2012. An integrated Public Health Programme for the prevention of hearing impairment 10 years of general AABR-screening for newborns in Flanders. Faculty of Medicine and Health Sciences: Antwerp University, https://www.kindengezin.be/img/SCRIPTIEEVKerschaver2012l.pdf.
- Van Overmeire I., Vrijens K., Nawrot T., Van Nieuwenhuyse A.n., Van Loco J., Reyns T. Simultaneous determination of parabens, bisphenols and alkylphenols in human placenta by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2019;1121:96–102. doi: 10.1016/j.jchromb.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Vela-Soria F., Gallardo-Torres M.E., Ballesteros O., Díaz C., Pérez J., Navalón A., Fernández M.F., Olea N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2017;1487:153–161. doi: 10.1016/j.chroma.2017.01.041. [DOI] [PubMed] [Google Scholar]
- Vrijens K., Van Overmeire I., De Cremer K., Neven K.Y., Carollo R.M., Vleminckx C., Van Loco J., Nawrot T.S. Weight and head circumference at birth in function of placental paraben load in Belgium: an ENVIRONAGE birth cohort study. Environ. Health. 2020;19:83. doi: 10.1186/s12940-020-00635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid, M., Fossati, S., Maitre, L., Márquez, S., Roumeliotaki, T., Agier, L., Andrusaityte, S., Cadiou, S., Casas, M., Castro, M.d., Dedele, A., Donaire-Gonzalez, D., Grazuleviciene, R., Haug, L.S., McEachan, R., Meltzer, H.M., Papadopouplou, E., Robinson, O., Sakhi, A.K., Siroux, V., Sunyer, J., Schwarze, P.E., Tamayo-Uria, I., Urquiza, J., Vafeiadi, M., Valentin, A., Warembourg, C., Wright, J., Nieuwenhuijsen, M.J., Thomsen, C., Basagaña, X., Slama, R., Chatzi, L., 2020. Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ. Health Perspect. 128, 067009. [DOI] [PMC free article] [PubMed]
- Ward J.B., Casagrande S.S., Cowie C.C. Urinary phenols and parabens and diabetes among US adults, NHANES 2005–2014. Nutrition, Metabolism, Cardiovasc. Dis.: NMCD. 2020;30(5):768–776. doi: 10.1016/j.numecd.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmayr, G.; J, W.; Peter, R.; M, T.; B, A.; Brunekreef, B.; B, F.; Ulmer, H.; G, N., 2019. Exposure to ambient air pollution and blood levels of gamma-glutamyl transferase (GGT) in a large Austrian cohort. Environ. Epidemiol. 3, 434. [DOI] [PubMed]
- Wolff M.S., Anderson H.A., Britton J.A., Rothman N. Pharmacokinetic variability and modern epidemiology–the example of dichlorodiphenyltrichloroethane, body mass index, and birth cohort. Cancer Epidemiol. Biomarkers Prev. 2007;16(10):1925–1930. doi: 10.1158/1055-9965.EPI-07-0394. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2006. Child Growth Standards based on length/height, weight and age. Acta Paediatrica (Oslo, Norway: 1992) Supplement 2006; 450: 76–85. [DOI] [PubMed]
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Ye X., Bishop A.M., Reidy J.A., Needham L.L., Calafat A.M. Parabens as Urinary Biomarkers of Exposure in Humans. Environ. Health Perspect. 2006;114(12):1843–1846. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Forman H.J. Redox regulation of gamma-glutamyl transpeptidase. Am. J. Respir. Cell Mol. Biol. 2009;41:509–515. doi: 10.1165/rcmb.2009-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Tong J., Liu J., Liu J., Li J., Cao Y. The dose-response relationship between gamma-glutamyl transferase and risk of diabetes mellitus using publicly available data: A longitudinal study in Japan. Int. J. Endocrinol. 2020;2020:1–7. doi: 10.1155/2020/5356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.