Abstract
Adherence to a healthy lifestyle—characterized by abstaining from smoking, being physically and cognitively active, having a high-quality diet, and limiting alcohol use—is associated with slower cognitive decline in older adults, but whether this relationship extends to persons with a genetic predisposition (e.g., carriers of the ε4 allele of the apolipoprotein E gene (APOE*E4)) remains uncertain. Using data from a population-based study, the Chicago Health and Aging Project (Chicago, Illinois), we followed 3,886 individuals who underwent regular clinical and cognitive assessments from 1993 to 2012. Of 3,886 older adults, 1,269 (32.7%) were APOE*E4 carriers. Compared with noncarriers, APOE*E4 carriers had faster cognitive decline (β = −0.027 units/year, 95% confidence interval (CI): –0.032, −0.023). In contrast, persons with 2–3 and 4–5 healthy lifestyle factors had slower cognitive decline (β = 0.008 units/year (95% CI: 0.002, 0.014) and β = 0.019 units/year (95% CI: 0.011, 0.026), respectively) compared with those with 0–1 factor. In analyses stratified by APOE*E4 status, adherence to a healthy lifestyle (e.g., 4–5 factors vs. 0–1 factors) was associated with a slower rate of cognitive decline in both APOE*E4 carriers (β = 0.029, 95% CI: 0.013, 0.045) and noncarriers (β = 0.013, 95% CI: 0.005, 0.022). These results underscore the impact of a healthy lifestyle on cognition, particularly among persons with a genetic predisposition, who are more vulnerable to cognitive decline as they age.
Keywords: Alzheimer dementia, APOE ε4 allele, apolipoprotein E, apolipoprotein E gene, cognitive dysfunction, diet, exercise, health behavior
Abbreviations
- AD
Alzheimer dementia
- APOE
apolipoprotein E gene
- APOE*E4
ε4 allele of the apolipoprotein E gene
- CHAP
Chicago Health and Aging Project
- CI
confidence interval
- MIND
Mediterranean-DASH Diet Intervention for Neurodegenerative Delay
One in 10 Americans aged 65 years or more has Alzheimer dementia (AD) (1, 2). AD is characterized by cognitive and functional impairment, and it is devastating for patients, families, and societies because of the unavailability of treatment (3). Therefore, the primary prevention of AD has become a public health priority (4–6).
The etiology of AD is multifactorial, involving genetic and environmental factors. Prior studies have shown that the presence of the ε4 allele of the apolipoprotein E gene (APOE*E4) contributes to the development of AD (7, 8). In parallel, further studies have identified several lifestyle factors, including a high-quality diet (9, 10), physical activity (11, 12), nonsmoking (13), moderate alcohol use (14), and cognitive activities (15), that reduce the risk of AD. In our earlier work, we have shown that adherence to these healthy lifestyle behaviors simultaneously is associated with a substantially reduced risk of AD (16). A critical challenge of these studies is to quantify the impact of genetic risk on the association between lifestyle factors and cognition and to determine whether lifestyle modifications could become a “fit-for-all” strategy to prevent AD in the general population. It is challenging, because in a recently published study, Licher et al. (17) reported that modifiable lifestyle factors were associated with a decrease in dementia risk only among people with low genetic risk (i.e., no APOE*E4 allele). These findings lead to a pessimistic attitude toward the possibility of preventing AD in people who are genetically predisposed to the disease. However, these findings originated from a single-center study in Europe (17); therefore, more research is required to evaluate the role of lifestyle factors in persons with and without the APOE*E4 allele.
In this study, we prospectively examined the role of the APOE*E4 allele in the relationship between adherence to a healthy lifestyle and cognitive decline in a population-based study, the Chicago Health and Aging Project (CHAP).
METHODS
Study design, settings, and population
CHAP is a longitudinal, population-based epidemiologic study of AD and other health conditions among adults aged 65 years or older in 3 Chicago, Illinois, neighborhoods (18).
For the present study, we selected CHAP participants who had been genotyped, had baseline data on diet and lifestyle factors, and had undergone at least 2 neuropsychological assessments and were clinically determined to not have AD at baseline. DNA samples were randomly collected from 5,807 (53.8%) participants. Of these 5,807 individuals, 4,089 (70.4%) had baseline data on diet and lifestyle factors, and 3,886 (95%) had undergone 2 or more neuropsychological assessments during follow-up. The CHAP participants who were included in the study (n = 3,886) were approximately 3 years younger than those who were excluded, had better global cognitive test scores at baseline (P < 0.001), were less likely to be African-American (60% vs. 64%), and had more years of education (12.8 years vs. 11.9 years).
The Rush University Medical Center Institutional Review Board approved this study, and all participants provided informed consent.
APOE genotype and diet and lifestyle factors
The Broad Institute of MIT and Harvard (Cambridge, Massachusetts) conducted genotyping of the apolipoprotein E gene (APOE) single nucleotide polymorphisms rs7412 and rs429358 in each participant using the homogeneous Mass Extend MassARRAY platform (Sequenom, Inc., San Diego, California) (19). The 3 common allelic variants were based on the combinations of TT, CT, and CC alleles. We designated the participants as APOE*E4 carriers if they had at least 1 ε4 allele (*E2/*E4, *E3/*E4, or *E4/*E4) and as noncarriers if they had no ε4 alleles (*E2/*E2, *E3/*E3, or *E2/*E3).
Dietary intake was assessed by means of a 144-item food frequency questionnaire that was validated for use among older Chicago residents (20). The participants were asked how often, on average, they had consumed specific foods and beverages with prespecified portion sizes over the past year. To assess overall diet quality, we calculated the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) diet score, which summarizes information on 10 brain-healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, and wine) and 5 unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food) (10). The participants reported their average frequency of intake of wine and other alcoholic beverages through the food frequency questionnaires. Because we evaluated alcohol intake separately, we did not include wine in the MIND diet score calculation. Physical activity was assessed by means of a validated questionnaire from the 1985 National Health Interview Survey that was adapted for use among older adults (21). The participants reported the amounts of time they had spent in any of 5 moderate or vigorous physical activities (i.e., walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, and swimming) within the past 2 weeks. Information on the participants’ smoking status was obtained through the interview, in which they specified whether they were current, former, or never smokers (13). Participation in cognitively stimulating activities was assessed with a structured questionnaire that gathers information on the usual amount of time spent in the past year on 7 cognitive activities: overall reading; visiting the library; reading newspapers; reading magazines; reading books; writing letters; and playing games, like checkers or other board games. A composite measure of the frequency of participation in cognitively stimulating activities was calculated (22).
Classification of healthy lifestyle categories
We selected 5 modifiable lifestyle factors based on evidence that these factors have been associated with dementia (16, 23). We then categorized each factor as healthy or unhealthy, identifying a behavior as “healthy” if it met the following criterion: 1) MIND diet score (without alcohol) in the top 40% of the cohort distribution; 2) cognitive activities in the top 40% of the cohort distribution; 3) not being a current smoker; 4) moderate or vigorous exercise for at least 150 minutes per week; and 5) light-to-moderate alcohol consumption (1–15 g/day for women and 1–30 g/day for men—that is, up to approximately 1 drink per day for women and 2 drinks per day for men).
In the absence of a threshold for a healthy diet and cognitive activities, we imposed an upper 40% cutoff based on the cohort distribution, in line with the method used in previous studies (24–27).
For each healthy lifestyle factor, participants received a score of 1 if they met the above “healthy” criterion; otherwise, they received a score of 0. The sum of these 5 scores yielded a final score within the range of 0–5, with higher scores indicating a healthier lifestyle.
Demographic and other health measures
Race/ethnicity was defined using questions from the 1990 US Census. Education was measured as the number of years of formal schooling completed. Body mass index (weight (kg)/height (m)2) was computed from measured weight and height. Symptoms of depression were measured using a modified version of the Center for Epidemiologic Studies Depression Scale. A history of heart disease, stroke, cancer, hypertension, or diabetes was determined by self-report questions from the Established Populations for the Epidemiologic Study of the Elderly.
Cognitive function
A battery of 4 tests was used to evaluate the cognitive function of all CHAP participants during the in-home interviews by trained technicians (28). These included 2 tests of episodic memory (immediate and delayed story recall) derived from the East Boston Memory Test (29, 30), a test of perceptual speed (the Symbol Digit Modalities Test) (31), and a test of general orientation and global cognition (the Mini-Mental State Examination) (32). Each of the 4 test scores was then standardized individually, and the scores were combined into a composite test score by averaging the 4 test scores together—that is, the global cognitive score (33). The standardized score ranges from negative values (indicating poor cognitive function) to 0 (indicating average cognitive function) to positive values (indicating higher cognitive function).
Statistical analysis
The baseline characteristics of the study population are presented as mean values with standard deviations, percentages of participants, and median values with interquartile ranges. Differences between participants according to APOE*E4 carrier status were compared using the χ2 test, Student’s t test, and the Mann-Whitney U test, as appropriate.
Linear mixed-effects regression models were used to examine the associations of healthy lifestyle score, APOE*E4 carrier status, and cognitive decline during follow-up. The participants were grouped into 3 categories of the lifestyle factor score (0 or 1 factor, 2 or 3 factors, or 4 or 5 factors) based on their adherence to a healthy lifestyle. We also evaluated the rate of cognitive decline per 1-point increase in a healthy lifestyle score. All models adjusted for age (measured in years and centered at 75 years); sex (male vs. female); race/ethnicity (African-American vs. non-Hispanic White); education (measured in number of years of completed schooling and centered at 12 years); the presence of cardiovascular disease, including heart disease and stroke (yes vs. no); and the interactions of these factors with time. Time indicates the mean annual change in the cognitive score. The interaction with time is the mean difference in the annual rate of cognitive change associated with the variable of interest (e.g., healthy lifestyle score). In addition, to account for potential practice effects due to repeated cognitive testing, which may influence change in cognitive function during follow-up, we adjusted the results of our multivariable models by the square root of the number of prior assessments, as previously described (34). The maximum follow-up time was 17 years, and only the participants with 2 or more cognitive assessments were included in the analyses.
In our primary analyses, we assessed the independent associations of lifestyle factors and APOE*E4 status with cognitive decline by including the lifestyle score and APOE*E4 status in the same multivariable model. We also evaluated the impact of healthy lifestyle factors on cognitive decline among people with and without the *E4 allele, by conducting a stratified analysis according to APOE*E4 status. To test whether the associations of lifestyle factors with cognitive decline differed by the presence of the *E4 allele, we evaluated a term for interaction between lifestyle score and APOE*E4 status in association with cognitive decline.
We conducted a series of sensitivity analyses to evaluate the robustness of our findings. First, we additionally adjusted for body mass index, hypertension, and diabetes in our multivariable model to estimate the impact of cardiovascular risk factors in our associations. Second, to account for comorbid conditions such as cancer and depression, we additionally adjusted for the prevalence of cancer and Center for Epidemiologic Studies Depression Scale score at baseline. Third, we excluded persons with a poor cognitive score (lowest 10% of the distribution) at baseline to explore the possibility of reverse causality or recall bias. Fourth, we removed the cognitive activities from the lifestyle score, because cognitive activities could be influenced by subtle cognitive deterioration. Fifth, to address potential concern regarding light-to-moderate alcohol use, we create an additional lifestyle score based on 4 healthy factors. In this analysis, we adjusted for alcohol intake in multivariable models. Sixth, because our focus was on modifiable lifestyle factors, we included former and never smokers in the healthy category to encourage current smokers to quit. To address the adverse effect of former smoking, we created another score that was based only on never smoking as a healthy lifestyle factor. Seventh, to allow our study’s findings to be compared with previously published data, we limited our analysis to only non-Hispanic White participants. Eighth, we excluded persons with 2 copies of the APOE*E4 allele to ensure that our findings were not explained by this subgroup of persons at high risk of cognitive decline. Ninth, to address the differences in sample size and baseline characteristics between *E4 carriers and noncarriers, we conducted a propensity score analysis using nearest-neighbor matching (details on the method are provided in the Web Appendix, available online at https://doi.org/10.1093/aje/kwab033). We also assessed the robustness of our associations by computing the E value, which provides a measure of the minimum strength of the association that an unmeasured confounder would need to have with both exposure and outcome to completely explain away observed associations (35, 36). For our longitudinal study, we calculated the E value using the standardized mean difference.
All analyses were performed using R software (CRAN, version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria) (37) with accompanying packages (e.g., nlme (38) and MatchIt (39)).
RESULTS
The study involved 1,269 (32.7%) APOE*E4 carriers (with 1 or 2 ε4 alleles) and 2,617 (67.3%) noncarriers (without any ε4 alleles). The APOE*E4 carriers were younger (71.4 years vs. 72.3 years), comprised more African Americans (68.2% vs. 57.1%), and had a lower cognitive score at the baseline (0.37 vs. 0.43). Other sociodemographic and clinical characteristics, as well as lifestyle factors, did not significantly differ between the groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Study Population According to the Presence of the ε4 Allele of the Apolipoprotein E Gene, Chicago Health and Aging Project, 1993–2012
| APOE*E4 Carrier Status | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | *E4 Noncarriers (n = 2,617) | *E4 Carriers (n = 1,269) | P Value a | ||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | ||
| Age, years | 72.3 (6.2) | 71.4 (5.6) | <0.001 | ||||
| Male sex | 948 | 36.2 | 494 | 38.9 | 0.109 | ||
| African-American race/ethnicity | 1,493 | 57.1 | 866 | 68.2 | <0.001 | ||
| Education, years | 12.8 (3.5) | 12.8 (3.4) | 0.738 | ||||
| Body mass indexb | 27.9 (5.7) | 28.0 (5.7) | 0.481 | ||||
| Hypertension | 1,281 | 49.0 | 627 | 49.5 | 0.796 | ||
| Diabetes | 508 | 19.4 | 248 | 19.5 | 0.957 | ||
| Heart disease | 345 | 13.2 | 157 | 12.4 | 0.515 | ||
| Stroke | 223 | 8.5 | 111 | 8.7 | 0.861 | ||
| Cancer | 524 | 20.0 | 269 | 21.2 | 0.418 | ||
| CES-D score (depression) | 1.4 (1.8) | 1.4 (1.9) | 0.356 | ||||
| MIND diet score | 6.9 (1.6) | 7.0 (1.7) | 0.056 | ||||
| Late-life cognitive activity score | 3.2 (0.7) | 3.2 (0.7) | 0.977 | ||||
| Physical activityc | 90 (0–255) | 90 (0–245) | 0.946 | ||||
| Smoking status | 0.969 | ||||||
| Never smoker | 1,275 | 48.7 | 613 | 48.3 | |||
| Former smoker | 1,060 | 40.5 | 519 | 40.9 | |||
| Current smoker | 282 | 10.8 | 137 | 10.8 | |||
| Alcohol intake, g/day | 4.3 (10.9) | 3.9 (10.5) | 0.329 | ||||
| Global cognition, z score | 0.43 (0.61) | 0.37 (0.64) | 0.010 | ||||
Abbreviations: APOE, apolipoprotein E gene; APOE*E4, ε4 allele of the apolipoprotein E gene; CES-D, Center for Epidemiologic Studies Depression Scale; MIND, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay; SD, standard deviation.
a P values were based on the t test for continuous measures and the χ2 test for categorical measures.
b Weight (kg)/height (m)2.
c Values are expressed as median (interquartile range).
The presence of APOE*E4 alleles and adherence to healthy lifestyle factors were both independently associated with cognitive decline in the multivariable-adjusted model. In the APOE*E4 carriers, cognitive decline was faster, at 0.027 units/year (β = –0.027, 95% confidence interval (CI): –0.032, −0.023) compared with participants who had no copies of the *E4 allele. Among persons with 2–3 and 4–5 healthy lifestyle factors, cognitive decline was slower, at 0.008 units/year (95% CI: 0.002, 0.014) and 0.019 units/year (95% CI: 0.011, 0.026), respectively, in comparison with those who had 0–1 healthy lifestyle factors (Table 2).
Table 2.
Independent Associations of APOE*E4 Status and Adherence to Healthy Lifestyle Factorsa With Cognitive Decline, Chicago Health and Aging Project, 1993–2012b
| APOE*E4 Status and Healthy Lifestyle Score c |
No. of Participants |
% | β | 95% CI |
|---|---|---|---|---|
| APOE*E4 status | ||||
| Noncarrier | 2,617 | 67.3 | 0 | Referent |
| Carrier | 1,269 | 32.7 | −0.027 | −0.032, −0.023 |
| Healthy lifestyle score | ||||
| Continuousd | 3,886 | 100.0 | 0.006 | 0.004, 0.008 |
| Categorical | ||||
| 0–1 factor | 922 | 23.7 | 0 | Referent |
| 2–3 factors | 2,153 | 55.4 | 0.008 | 0.002, 0.014 |
| 4–5 factors | 811 | 20.9 | 0.019 | 0.011, 0.026 |
Abbreviations: APOE, apolipoprotein E gene; APOE*E4, ε4 allele of the apolipoprotein E gene; CI, confidence interval; MIND, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay.
a A behavior was classified as “healthy” if it met the following criterion: 1) MIND diet score (without alcohol) in the top 40% of the cohort distribution; 2) cognitive activities in the top 40% of the cohort distribution; 3) not being a current smoker; 4) moderate or vigorous exercise for ≥150 minutes/week; and 5) light-to-moderate alcohol consumption (1–15 g/day for women and 1–30 g/day for men).
b Adjusted for age, sex, education, race, prevalence of cardiovascular disease (heart disease and stroke), time, the interactions of these factors with time, and practice effects.
c APOE*E4 status and healthy lifestyle score were included simultaneously in the same multivariable-adjusted model.
d “Continuous” refers to a 1-point increase in healthy lifestyle score.
In the stratified analyses, adherence to a healthy lifestyle was associated with slower cognitive decline among both APOE*E4 carriers and noncarriers (Table 3). In the strata of APOE*E4 noncarriers, participants with 2–3 and 4–5 healthy lifestyle factors had slower cognitive decline than those with 0–1 factors, by 0.007 (95% CI: 0.001, 0.013) and 0.013 (95% CI: 0.005, 0.022) units/year, respectively. In the strata of APOE*E4 carriers, these estimates were 0.008 units/year (95% CI: –0.004, 0.020) for persons with 2–3 healthy lifestyle factors and 0.029 units/year (95% CI: 0.013, 0.045) for those with 4–5 factors. The estimates of cognitive decline per 1 additional healthy behavior in the score were 0.004 units/year (95% CI: 0.002, 0.006) in *E4 noncarriers and 0.009 units/year (95% CI: 0.004, 0.013) in *E4 carriers. There were no significant interaction effects between healthy lifestyle score and APOE*E4 status in cognitive decline (P = 0.261), suggesting no differential association of lifestyle factors with cognitive decline in APOE*E4 carriers and noncarriers.
Table 3.
Association Between Adherence to Healthy Lifestyle Factorsa and Cognitive Decline According to APOE*E4 Status, Chicago Health and Aging Project, 1993–2012b
| APOE*E4 Status and Healthy Lifestyle Score c |
No. of Participants |
% | β | 95% CI |
|---|---|---|---|---|
| APOE*E4 noncarrier | ||||
| Continuousc HLS | 2,617 | 100.0 | 0.004 | 0.002, 0.006 |
| Categorical HLS | ||||
| 0–1 factor | 622 | 23.8 | 0 | Referent |
| 2–3 factors | 1,469 | 56.1 | 0.007 | 0.001, 0.013 |
| 4–5 factors | 526 | 20.1 | 0.013 | 0.005, 0.022 |
| APOE*E4 carrier | ||||
| Continuousc HLS | 1,269 | 100.0 | 0.009 | 0.004, 0.013 |
| Categorical HLS | ||||
| 0–1 factor | 300 | 23.6 | 0 | Referent |
| 2–3 factors | 684 | 53.9 | 0.008 | −0.004, 0.020 |
| 4–5 factors | 285 | 22.5 | 0.029 | 0.013, 0.045 |
Abbreviations: APOE, apolipoprotein E gene; APOE*E4, ε4 allele of the apolipoprotein E gene; CI, confidence interval; HLS, healthy lifestyle score; MIND, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay.
a A behavior was classified as “healthy” if it met the following criterion: 1) MIND diet score (without alcohol) in the top 40% of the cohort distribution; 2) cognitive activities in the top 40% of the cohort distribution; 3) not being a current smoker; 4) moderate or vigorous exercise for ≥150 minutes/week; and 5) light-to-moderate alcohol consumption (1–15 g/day for women and 1–30 g/day for men).
b Adjusted for age, sex, education, race/ethnicity, prevalence of cardiovascular disease (heart disease and stroke), time, the interactions of these factors with time, and practice effects.
c “Continuous” refers to a 1-point increase in HLS.
The associations between lifestyle and cognitive decline were not different in APOE*E4 carriers and noncarriers when we included body mass index, hypertension, and diabetes (Web Table 1) or adjusted for the presence of cancer or depressive symptoms (Web Table 2). Excluding persons with the lowest cognitive scores at baseline resulted in a slightly stronger association of lifestyle with cognitive decline in APOE*E4 carriers (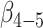 = 0.032 vs.
= 0.032 vs.  = 0.029 in the entire sample) but did not change estimates in noncarriers (Web Table 3). In the lifestyle score without cognitive activities, the estimates of cognitive decline per 1-factor increase in healthy lifestyle factors were 0.004 units/year (95% CI: 0.001, 0.006) for APOE*E4 noncarriers and 0.008 units/year (95% CI 0.003, 0.013) for APOE*E4 carriers (Web Table 4). When we used a healthy behavior score without light-to-moderate alcohol intake, the estimates of cognitive decline per 1–healthy-factor increase were 0.003 units/year (95% CI: 0.001, 0.006) for *E4 noncarriers and 0.009 units/year (95% CI: 0.005, 0.014) for *E4 carriers (Web Table 5). Results similar to those of the primary analysis were also found when we used never smoking as a healthy lifestyle factor in the score (Web Table 6).
= 0.029 in the entire sample) but did not change estimates in noncarriers (Web Table 3). In the lifestyle score without cognitive activities, the estimates of cognitive decline per 1-factor increase in healthy lifestyle factors were 0.004 units/year (95% CI: 0.001, 0.006) for APOE*E4 noncarriers and 0.008 units/year (95% CI 0.003, 0.013) for APOE*E4 carriers (Web Table 4). When we used a healthy behavior score without light-to-moderate alcohol intake, the estimates of cognitive decline per 1–healthy-factor increase were 0.003 units/year (95% CI: 0.001, 0.006) for *E4 noncarriers and 0.009 units/year (95% CI: 0.005, 0.014) for *E4 carriers (Web Table 5). Results similar to those of the primary analysis were also found when we used never smoking as a healthy lifestyle factor in the score (Web Table 6).
Restricting our analysis to non-Hispanic White participants resulted in a stronger association between lifestyle factors and cognitive decline. The β estimates per 1–healthy-factor increase were 0.006 units/year (95% CI: 0.002, 0.010) for *E4 noncarriers and 0.013 units/year (95% CI: 0.005, 0.022) for *E4 carriers (Web Table 7). We found results similar to those of the primary analysis when we excluded persons with 2 copies of the ε4 allele, lending confidence that the high-risk subgroup did not explain our main findings (Web Table 8), or when we propensity-matched APOE*E4 carriers to noncarriers to ensure that differences in estimates were not explained by differences in sample size or baseline characteristics (Web Tables 9–11). On the basis of coefficients and residual standard deviation estimates of our linear mixed-effect models for the association of lifestyle score (per 1–standard-deviation increase) with cognitive decline, E values were 1.105 in APOE*E4 carriers and 1.072 in noncarriers. These estimates were comparable to E values for the association of age (per 1–standard-deviation increase) with cognitive decline in APOE*E4 carriers (E = 1.099) and noncarriers (E = 1.064).
Figure 1 shows rates of change in global cognitive score over 17 years according to adherence to healthy lifestyle factors and APOE4*E4 status. APOE*E4 carriers with 0–1 healthy lifestyle factors had a rate of cognitive change of −0.116 units/year, and those with 4–5 healthy factors had a rate of −0.087 units/year. APOE*E4 noncarriers with 0–1 healthy lifestyle factors had a cognitive change rate of −0.071 units/year, and those with 4–5 healthy factors had a rate of −0.058 units/year. Among APOE*E4 carriers, persons with 4–5 healthy lifestyle factors were 6.2 years younger cognitively than persons with 0–1 healthy factors.
Figure 1.

Rate of change in global cognitive score over 17 years among carriers of the ε4 allele of the apolipoprotein E gene (APOE*E4) (A) and APOE*E4 noncarriers (B) according to their adherence to healthy lifestyle factors, Chicago Health and Aging Project, 1993–2012. A behavior was classified as “healthy” if it met the following criterion: 1) MIND diet score (without alcohol) in the top 40% of the cohort distribution; 2) cognitive activities in the top 40% of the cohort distribution; 3) not being a current smoker; 4) moderate or vigorous exercise for ≥150 minutes/week; and 5) light-to-moderate alcohol consumption (1–15 g/day for women and 1–30 g/day for men). APOE*E4 carriers with 0–1 healthy lifestyle factors had a rate of cognitive change of −0.116 units/year, and those with 4–5 healthy factors had a rate of −0.087 units/year. APOE*E4 noncarriers with 0–1 healthy lifestyle factors had a rate of cognitive change of −0.071 units/year, and those with 4–5 healthy factors had a rate of −0.058 units/year. MIND, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay.
DISCUSSION
In this population-based study, we studied the potential impact of the APOE*E4 allele on the association between lifestyle factors and cognitive decline. While APOE*E4 carriers were at higher risk of faster cognitive decline, adherence to a healthy lifestyle was associated with slower cognitive decline during follow-up. In APOE*E4 carriers and noncarriers, a healthy lifestyle was associated with slower cognitive decline.
Epidemiologic studies have previously investigated the moderation of genetic associations by lifestyle factors to study whether a healthy lifestyle can modify the genetic risk of dementia (23, 40–42). In a recent population-based study using data from 196,383 persons in the UK Biobank, Lourida et al. (23) reported that lifestyle factors were associated with dementia risk regardless of genetic risk. Similar to the UK Biobank study, our study examined several modifiable lifestyle factors, such as no current smoking, regular physical activity, a healthy diet, and moderate alcohol consumption. In addition, we included cognitive activity in the score because of its strong relationship with dementia risk (15). However, we defined genetic risk differently. While the UK Biobank study determined the genetic risk based on a polygenic score, which requires assessing several different genes, for generalizability and practicality we focused only on a single gene (e.g., APOE*E4). In line with these results, in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), Ngandu et al. (43) concluded that multidomain lifestyle interventions contributed to improved cognitive performance among older persons, similarly in APOE*E4 carriers and noncarriers after 2 years of follow-up.
Our study’s findings contrast with those of the Rotterdam Study, another population-based study that included 6,352 persons aged 55 years and older, in which Licher et al. (17) reported that during 15 years of follow-up, a healthy lifestyle was associated with a lower risk of dementia only in people without an APOE*E4 allele. There are several differences between the studies that could explain these conflicting findings. In the Rotterdam Study, Licher et al. defined a healthy lifestyle as a composite score of modifiable (abstinence from smoking, regular physical activity, avoiding social isolation, and a healthy diet) and nonmodifiable (not having diabetes or depression) risk factors (17), while in our study we focused only on modifiable lifestyle factors. The outcome was also different; in the Rotterdam Study, the authors determined the risk of dementia, whereas, in our study, we studied annual cognitive decline. The competing risk of death could be a critical issue when investigating dementia risk, particularly among APOE*E4 carriers, because the *E4 allele increases the risk of all-cause, cardiovascular, and cancer mortality (44, 45). While Licher et al. accounted for competing risk in their analysis (17) using the Fine and Gray method (46), investigating annual change in cognition could be more sensitive when attempting to detect small variations in cognition. Another difference is our study population: While the Rotterdam Study included exclusively Caucasians living in a middle- and high-income area, our population comprised African Americans and non-Hispanic Whites living in the South Side of Chicago. However, our findings in non-Hispanic White participants were similar to those in our entire sample.
While our study demonstrated that a healthy lifestyle is associated with slower cognitive decline in both APOE*E4 carriers and noncarriers, we noted some differences in β estimates by allele status, suggesting that lifestyle factors may be more protective in APOE*E4 carriers (40). Nevertheless, a healthy lifestyle has the potential to slow cognitive decline, regardless of *E4 status. It has been shown previously that adherence to a healthy lifestyle contributes to a lower risk of AD, as well as lower risks of cardiovascular disease, type 2 diabetes, and cancer (16, 24–26).
Our study had several limitations. First, lifestyle factors were assessed at baseline, and changes over time were not considered. Second, collections of cognitive data occurred 3 years apart, making short-term changes in cognitive function less detectable. However, cognitive decline associated with AD typically occurs slowly, over many years. Third, our results could be due to reverse causality because of the long prodromal phase of AD. Fourth, as in all population-based studies, survival bias was present in our study. Persons with APOE*E4 alleles and an unhealthy lifestyle profile have an increased risk of premature mortality (44, 45). Fifth, our study was limited to a single gene variant and selected modifiable lifestyle factors. Inclusion of a polygenic risk score or additional environmental factors might provide additional information on the interplay between genetic and lifestyle factors in cognitive decline. Future studies should also consider the social context when studying the role of lifestyle in cognition. Sixth, given the nature of our study design, we could not access whether 1) lifestyle had already begun to influence cognition by the time of enrollment in the study; 2) earlier-life cognition had influenced lifestyle choices; or 3) underlying disease progression had influenced both lifestyle and cognition. Therefore, a life-course approach is warranted to understand the role of lifestyle in the disease pathogenesis and to elucidate a critical age window during which lifestyle factors may exert most of their favorable effects. Finally, as with any observational study, our study was prone to bias due to unmeasured confounding. We computed E values to determine the effect of the unmeasured confounding, and we noted smaller estimates, suggesting that unmeasured confounders could easily explain our associations.
Significant strengths of this study include the objective assessment of cognition by trained technicians, use of numerous neuropsychological tests, long-term follow-up of participants, and generalizability of the findings across different racial/ethnic groups (e.g., African Americans and Whites).
In conclusion, our study suggests that a healthy lifestyle is associated with slower cognitive decline in older adults, including persons with a genetic predisposition to AD.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, Illinois, United States (Klodian Dhana, Kumar B. Rajan, Denis A. Evans, Martha C. Morris); Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois, United States (Klodian Dhana, Kumar B. Rajan, Denis A. Evans, Martha C. Morris); Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, United States (Neelum T. Aggarwal, Lisa L. Barnes); Department of Neurology, Rush University Medical Center, Chicago, Illinois, United States (Neelum T. Aggarwal, Lisa L. Barnes); and Department of Public Health Sciences, School of Medicine, University of California, Davis, Davis, California, United States (Kumar B. Rajan).
This study was supported by the National Institute on Aging (grants R21AG070287, RF1AG057532, R01AG051635, R01AG058679, and R01AG11101).
This paper is dedicated to the memory of the late Dr. Martha C. Morris—a scientist, mentor, and friend.
Conflict of interest: none declared.
REFERENCES
- 1.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaugler J, James B, Johnson T, et al. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387. [Google Scholar]
- 3.Sugino H, Watanabe A, Amada N, et al. Global trends in Alzheimer disease clinical development: increasing the probability of success. Clin Ther. 2015;37(8):1632–1642. [DOI] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Disease International; World Health Organization . Dementia: A Public Health Priority. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 6.Global Action Against Dementia . G8 Dementia Summit Declaration. https://www.mendeley.com/research-papers/g8-dementia-summit-declaration/. Published 2013. Accessed June 17, 2020.
- 7.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 8.Liu C-C, Liu C-C, Kanekiyo T, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175(7):1094–1103. [DOI] [PubMed] [Google Scholar]
- 10.Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovio S, Kåreholt I, Helkala E-L, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–711. [DOI] [PubMed] [Google Scholar]
- 12.Stephen R, Hongisto K, Solomon A, et al. Physical activity and Alzheimer’s disease: a systematic review. J Gerontol A Biol Sci Med Sci. 2017;72(6):733–739. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident Alzheimer’s disease in a biracial urban community population. Neuroepidemiology. 2006;26(3):140–146. [DOI] [PubMed] [Google Scholar]
- 14.Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson RS, Segawa E, Boyle PA, et al. Influence of late-life cognitive activity on cognitive health. Neurology. 2012;78(15):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhana K, Evans DA, Rajan KB, et al. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology. 2020;95(4):e374–e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25(9):1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185–189. [DOI] [PubMed] [Google Scholar]
- 19.Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337(8750):1158–1159. [DOI] [PubMed] [Google Scholar]
- 20.Morris MC, Tangney CC, Bienias JL, et al. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213–1217. [DOI] [PubMed] [Google Scholar]
- 21.McPhillips JB, Pellettera KM, Barrett-Connor E, et al. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5(2):65–72. [PubMed] [Google Scholar]
- 22.Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911–1920. [DOI] [PubMed] [Google Scholar]
- 23.Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138(4):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhana K, Haines J, Liu G, et al. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: results from two prospective cohort studies of mother-child pairs in the United States. BMJ. 2018;362:k2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bienias JL, Beckett LA, Bennett DA, et al. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5(5):349–355. [DOI] [PubMed] [Google Scholar]
- 29.Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57(3-4):167–178. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910–1914. [DOI] [PubMed] [Google Scholar]
- 31.Smith A. Symbol Digit Modalities Test (SDMT) Manual (Revised). Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):P155–P160. [DOI] [PubMed] [Google Scholar]
- 34.Vivot A, Power MC, Glymour MM, et al. Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol. 2016;183(4):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 36.Ding P, TJ VW. Sensitivity analysis without assumptions. Epidemiology. 2016;27(3):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 38.Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and nonlinear mixed effects models. https://cran.r-project.org/web/packages/nlme/index.html. (Version 3.1-152). Published February 4, 2021. Accessed May 11, 2021.
- 39.Ho DE, Imai K, King G, et al. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 40.Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E ε4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6B):2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samieri C, Perier M-C, Gaye B, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320(7):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajan KB, Barnes LL, Wilson RS, et al. Apolipoprotein E genotypes, age, race, and cognitive decline in a population sample. J Am Geriatr Soc. 2019;67(4):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, et al. Plasma levels of apolipoprotein E, APOE genotype, and all-cause and cause-specific mortality in 105 949 individuals from a white general population cohort. Eur Heart J. 2019;40(33):2813–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajan KB, Barnes LL, Wilson RS, et al. Racial differences in the association between apolipoprotein E risk alleles and overall and total cardiovascular mortality over 18 years. J Am Geriatr Soc. 2017;65(11):2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


