Abstract
A case of invasive pulmonary aspergillosis in an allogeneic bone marrow transplant recipient caused by Aspergillus ustus is presented. A. ustus was also recovered from the hospital environment, which may indicate that the infection was nosocomially acquired. A literature review revealed seven cases of invasive infections caused by A. ustus, and three of these were primarily cutaneous infections. In vitro susceptibility testing of 12 A. ustus isolates showed that amphotericin B and terbinafine had fungicidal activity and that itraconazole and voriconazole had fungistatic activity.
Invasive pulmonary aspergillosis is an important cause of morbidity and mortality in immunocompromised patients, especially those who receive hematopoetic stem cell transplants (25). Besides Aspergillus fumigatus, at least 20 species of Aspergillus have been reported to cause invasive infections, including A. terreus (8), A. nidulans (21), and A. niger (10). Here we report on a case of invasive pulmonary infection caused by A. ustus in a patient with chronic myeloid leukemia during treatment for graft-versus-host disease following allogeneic bone marrow transplantation (BMT). Furthermore, we report on a review of the literature for descriptions of cases of invasive aspergillosis caused by A. ustus and the in vitro activities of antifungal agents against A. ustus isolates.
Case report.
A 38-year-old male received T-cell-depleted bone marrow from a matched unrelated donor as therapy for chronic myeloid leukemia which had been diagnosed 5 years previously. The conditioning regimen consisted of cyclophosphamide and total-body irradiation, and he received cyclosporine A for immunoprophylaxis. Antifungal prophylaxis included amphotericin B suspension and aerosol spray. Two days after BMT the patient developed a high fever, and treatment with ceftazidime was begun. A chest X ray showed no infiltrates, and blood cultures remained sterile. Acute graft-versus-host disease of the skin, liver, and intestine (grade IV) became apparent, but this responded to methylprednisolone sodium succinate (Solu-Medrol) at 1 g/day, and the dose was gradually reduced. Marrow engraftment was rapid, and the neutrophil count exceeded 1.0 × 109/liter on day 15. A second febrile episode developed 17 days after BMT, but pulmonary infiltrates were not evident on a chest X ray. Acute graft-versus-host disease relapsed on day 23 and the dosage of methylprednisolone was increased. The patient had seizures, but computed tomography of the brain showed no abnormalities. The patient was seropositive for toxoplasma, and magnetic resonance imaging of the brain on day 33 showed multiple hyperdense lesions. These lesions were suspected to result from toxoplasmosis or cerebral aspergillosis. At that time a pulmonary infiltrate had developed in the right upper lobe of the lung. A mucus plug was obtained during bronchoscopy on day 35, and culture yielded non-A. fumigatus Aspergillus species. Treatment with amphotericin B at a dosage of 1 mg/kg of body weight/day was begun, but the clinical condition of the patient deteriorated. The patient died 51 days after BMT from massive bleeding from the gastrointestinal tract.
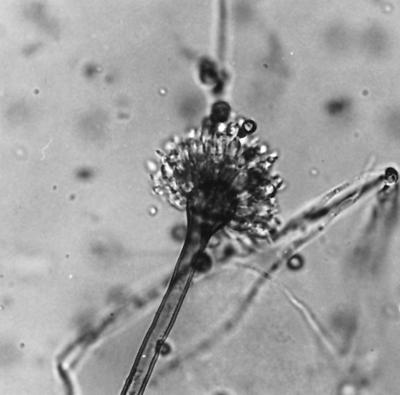
At autopsy, a large infiltrate was found in the upper lobe of the right lung. Mycelia with dichotomous branching were seen in the tissues of the right lung, and an olive-gray filamentous fungus was recovered by culture on Sabouraud agar containing 10% chloramphenicol after 2 days of incubation. The reverse showed the production of a yellow diffusing pigment. Microscopic examination revealed biseriate conidiogenous cells bearing very rough walled dark-yellow to brown conidia (Fig. 1). Irregular to elongate Hülle cells are characteristic for this fungus but are formed only by a minority of isolates. The isolate was identified as A. ustus by the Centraalbureau voor Schimmelcultures (CBS; Baarn, The Netherlands). There was no evidence of the dissemination of the Aspergillus infection. Microscopic examination of the brain showed several hypoxia-induced lesions but no evidence of toxoplasmosis or cerebral aspergillosis.
FIG. 1.
Brown and smooth-walled conidiophore of A. ustus. The vesicle bears a double series of sterigmata. The conidia are rough and greenish brown to dark yellow-brown.
Air sampling with a Casella sampler is performed systematically in selected areas of our hospital with high-risk patients. The patient rooms and corridors in the hematology ward are sampled every month. All Aspergillus isolates recovered from the environment are identified to the species level and stored. The database was searched for Aspergillus species which had been cultured from patients and the hospital environment between January 1991 and August 1998.
Antigen detection.
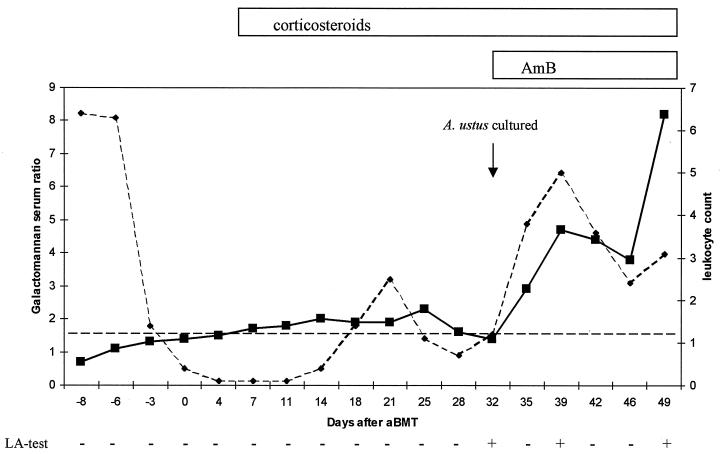
The presence of the Aspergillus antigen galactomannan was determined in the serum by the latex agglutination (LA) test (Pastorex Aspergillus) (24) and by a sandwich enzyme-linked immunosorbent assay (ELISA; Platelia Aspergillus; Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France) (19). Both kits use the same monoclonal antibody (monoclonal antibody EB-A2) and are available commercially outside the United States. The LA test and ELISA were performed according to the manufacturers’ instructions. A titer was obtained for the LA test by testing serially diluted serum samples. For the ELISA, a ratio was calculated by dividing the optical density of the serum sample by that of a threshold control sample which contained 1 ng of galactomannan per ml. Galactomannan was detected by the LA test in 3 of 18 serum samples, while the sandwich ELISA was positive for 13 samples (Fig. 2). The first serum sample with ELISA reactivity was obtained 1 week after BMT (day 7) and 28 days before A. ustus was cultured from a mucus plug and treatment with amphotericin B was begun. The course of the antigen titer is shown in Fig. 2.
FIG. 2.
Results of antigen detection by sandwich ELISA and LA test with serum from the patient infected with A. ustus. ■, ratio for galactomannan in serum; ⧫, leukocyte count (109/liter); –––, cutoff for a positive ELISA result. The LA test result is given at the bottom. The different treatments are indicated at the top.
In vitro susceptibility testing.
The in vitro activities of amphotericin B (Bristol Myers-Squibb, Woerden, The Netherlands), itraconazole (Janssen-Cilag B.V., Tilburg, The Netherlands), voriconazole (Pfizer Central Research, Sandwich, United Kingdom), and terbinafine (Novartis, Basel, Switzerland) were determined by a broth microdilution method according to the standardized procedure for antifungal susceptibility testing proposed by the National Committee for Clinical Laboratory Standards (NCCLS) Subcommittee on Antifungal Susceptibility Testing (12). That procedure was modified for the testing of filamentous fungi. An itraconazole-susceptible A. fumigatus control isolate (isolate AF71), an itraconazole-resistant A. fumigatus control isolate (isolate AF90), and Paecilomyces variotii ATCC 22319 were included in each test. For amphotericin B and terbinafine, the MIC endpoint was defined as complete inhibition of visible growth, and for the azoles, 75% growth inhibition compared with the growth of the controls. Wells without visible growth were subcultured onto Sabouraud glucose agar and were incubated at 35°C for 48 h. Subcultures from the wells with the lowest drug concentrations showing a 99.9% reduction from the initial inoculum size were judged to contain the minimal fungicidal concentration (MFC). Both terbinafine and amphotericin B showed fungicidal activity against A. ustus, but the MICs and MFCs of terbinafine were approximately threefold lower than those of amphotericin B (Table 1). The azoles itraconazole and voriconazole were less active in vitro, and for most isolates fungicidal activity was not achieved at concentrations of ≤32 μg/ml.
TABLE 1.
Characteristics of 12 A. ustus isolates and in vitro susceptibility to amphotericin B, itraconazole, voriconazole, and terbinafine
| Isolate no.a | Date of isolation (day/mo/yr) | Originb | Aspergillus infection | MIC (μg/ml)/MFC (μg/ml)c
|
|||
|---|---|---|---|---|---|---|---|
| AmB | ITZ | VCZ | TBF | ||||
| AZN677 | 9/12/1992 | BAL, case patient | Proven | 2/2 | 2/16 | 8/16 | 0.25/0.25 |
| AZN678 | 15/12/1992 | Sputum, case patient | Proven | 2/2 | 2/32 | 4/32 | 0.25/0.25 |
| AZN682 | 25/12/1992 | Autopsy, lung, case | Proven | 2/2 | 4/16 | 8/8 | 0.5/0.5 |
| AZN741 | 26/1/1993 | Hematology ward, room | 2/2 | 2/32 | 8/16 | 0.5/0.5 | |
| AZN924 | 7/5/1993 | Hematology ward, room | 2/2 | 2/32 | 4/8 | 0.5/0.5 | |
| AZN943 | 14/7/1993 | Laboratory contaminant | 2/2 | 2/32 | 4/16 | 0.5/0.5 | |
| AZN2725 | 23/1/1995 | Sputum, patient in ENT department | No infection | 2/2 | 8/32 | 4/16 | 0.5/0.5 |
| AZN3297 | 15/6/1995 | Feces, patient in neurology department | No infection | 2/2 | 2/32 | 4/4 | 0.5/0.5 |
| AZN6989 | 22/9/1997 | Ascites, HIV+ patient | No infection | 2/2 | 2/32 | 2/8 | 0.5/0.5 |
| AZN7134 | 15/12/1997 | Hematology ward, room | 2/2 | 2/32 | 4/32 | 0.5/0.5 | |
| CBS239.90 | 1990 | Biopsy of brain abscess | Proven | 2/2 | 1/1 | 0.25/0.25 | 0.06/0.06 |
| A252 | 1/2/1994 | Needle biopsy of lung | Proven | 2/2 | 2/32 | 4/8 | 0.5/0.5 |
AZN numbers refer to isolates cultured at the University Hospital Nijmegen, Nijmegen, The Netherlands; isolate CBS239.90 was cultured at the University Hospital Utrecht, Utrecht, The Netherlands; isolate A252 was cultured at Hôpital Henri Mondor, Créteil, France.
BAL, bronchoalveolar lavage specimen; ENT, ear-nose-throat; HIV+, human immunodeficiency virus positive.
AmB, amphotericin B; ITZ, itraconazole; VCZ, voriconazole; TBF, terbinafine.
Literature review.
The literature for the years 1960 to 1998 was reviewed with the use of MEDLINE for case reports of invasive infections caused by A. ustus. Seven cases of invasive A. ustus infections have been described (Table 2).
TABLE 2.
Summary of published cases of invasive A. ustus infection
| Patient no., sex/age (reference)a | Underlying disease or conditionb | Diagnosis, infected site | Therapy | Clinical response, outcome | Autopsy findings |
|---|---|---|---|---|---|
| 1, M/50 (2) | Prosthetic valve | Proven, aortic valve prosthesis and mitral valve | Amphotericin B (40 mg/day for 6 weeks); flucytosine (8 g/day) for 10 weeks; valve replacement | Cure, survival | ND |
| 2, M/57 (16) | Excessive skin burns | Probable, skin | Amphotericin B cream | Failure, death | No evidence of disseminated infection |
| 3, M/72 (26) | Cardiac surgery, diabetes mellitus, chronic renal failure | Proven, lung | No antifungal treatment | Failure, death | Multiple infiltrations in lungs, myocardium, kidney, thyroid, and peritoneum |
| 4, F/62 (18) | Liver transplant for end-stage hepatitis C-induced cirrhosis | Proven, skin | Amphotericin B (25 mg every other day); terbinafine cream | Cure, death | No invasive aspergillosis |
| 5, M/9 (1) | AML, aBMT, GvHD | Proven, lung | Amphotericin B (1.5 mg/kg/day) | Failure, death | ND |
| 6, F/64 (15) | COPD, prednisone (60 mg/day) | Probable, skin | Itraconazole (400 mg/day for 2 weeks) | Failure, death | No evidence of disseminated infection |
| 7, F/46 (9) | MDS, aPSCT | Proven, lung | Itraconazole (400 mg/day for 53 days); liposomal amphotericin B (5 mg/kg/day for 12 days) | Failure, death | Multiple infiltrations in lung, myocardium, thyroid, and skin |
| 8, M/38 (PR) | CML, aBMT, GvHD | Proven, lung | Amphotericin B (1 mg/kg/day for 18 days) | Failure, death | Bronchopneumonia; no evidence of disseminated infection |
M, male; F, female; PR, present report.
AML, acute myeloid leukemia; aBMT, allogeneic BMT; aPSCT, allogeneic peripheral stem cell transplantation; GvHD, graft-versus-host disease; COPD, chronic obstructive pulmonary disease; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome.
ND, not done.
Invasive infections caused by A. ustus are uncommon, and including the present case, only eight cases have been documented in the literature since 1960. Information from the reported cases show that A. ustus may cause a variable spectrum of disease. Among the case patients, three patients were described as having primarily cutaneous infections. In patient 4 (Table 2) cutaneous lesions developed on an arm and leg which had been stabilized. Both devices had occluded the skin and had caused local irritation (18). In patient 6 the infection started as erosion of the skin from a plastic identification bracelet (15). Cutaneous infections caused by opportunistic fungi related to occlusion of the skin have been described previously (11). Occlusion of the skin creates a warm and humid environment that allows fungal spores, which may be present in nonsterile material or the environment, to germinate and invade the skin, especially when it is disrupted. The third case of cutaneous infection occurred in a patient (patient 2) with excessive burns to the skin (16). Although the skin was not occluded, application of a prednisone-containing emulsion and the presence of excessive burns could have predisposed the patient to infection. These cases suggest that A. ustus infection may be of nosocomial origin. We succeeded in culturing A. ustus from the hospital environment, but over an 8-year period of systematic sampling, A. ustus was recovered only three times in the hematology ward. The mold was cultured on two different occasions shortly after the case patient died (Table 1). Very little is known about the diversity of Aspergillus species within the hospital environment since most studies fail to identify Aspergillus species other than A. fumigatus and A. flavus (7). However in one hospital, A. ustus represented 5% of all Aspergillus isolates cultured from the air (13).
A commercial sandwich ELISA which detects Aspergillus galactomannan has been evaluated in several institutes outside the United States (1, 23). This assay allows the detection of low levels of galactomannan in body fluids and has a higher sensitivity than the LA test (1, 23). Galactomannan is produced by Aspergillus species involved in human disease (20) and was detected in the serum of patients infected with A. fumigatus (23), A. flavus (1), A. niger (1), and A. nidulans (21) and one patient infected with A. ustus (1). The finding of galactomannan in serum samples from our patient confirms the reactivity of the assay for patients infected with A. ustus. Twice-weekly collection of serum samples allowed the detection of galactomannan at an early stage of infection. The antigen titer increased continuously, despite treatment with amphotericin B, and the titer increase corresponded to the clinical failure of therapy.
For the treatment of invasive aspergillosis, voriconazole is a promising antifungal azole that is fungicidal against A. fumigatus (6). Patients with invasive aspergillosis, including those infected with non-A. fumigatus Aspergillus isolates (21), have been reported to show a favorable response to treatment with voriconazole (3, 5). Voriconazole has been shown to be active in vitro against several non-A. fumigatus Aspergillus species including A. flavus, A. nidulans, A. versicolor, and A. niger (14). Our results suggest that the drug is less active in vitro against A. ustus than it is against A. fumigatus. The effect appears to be fungistatic and similar to that of itraconazole. Terbinafine is active in vitro against several Aspergillus species (17), and the drug is under evaluation for the treatment of invasive aspergillosis. Among the drugs tested, terbinafine proved to be the most active in vitro and was also the most fungicidal. These results and previous in vitro susceptibility data (17) indicate that terbinafine may be a promising agent for the treatment of invasive aspergillosis, including those infections caused by non-A. fumigatus Aspergillus species. The results of in vitro susceptibility testing add to the evidence that different Aspergillus species have variable susceptibilities to antifungal agents (4, 22), which underscores the importance of susceptibility testing of clinically significant isolates.
Acknowledgments
We thank Stephane Bretagne, Laboratoire de Parasitologie-Mycologie, Hôpital Henri Mondor, Créteil, France, for providing clinical data and A. ustus A252 and Ton Rijs for excellent technical assistance.
REFERENCES
- 1.Bretagne S, Marmorat-Khuong A, Kuentz M, Latgé J P, Bart-Delabesse E, Cordonnier C. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: practical use in neutropenic patients. J Infect. 1997;35:7–15. doi: 10.1016/s0163-4453(97)90833-1. [DOI] [PubMed] [Google Scholar]
- 2.Carrizosa J, Levison M E, Lawrence T, Kaye D. Cure of Aspergillus ustus endocarditis on a prosthetic valve. Arch Intern Med. 1974;133:486–490. [PubMed] [Google Scholar]
- 3.Denning D W, del Favero A, Gluckman E, Norfolk D, Rhunke M, Yonren S, Troke P, Sarantis N. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. UK-109,496, a novel, wide-spectrum triazole derivative for the treatment of fungal infections: clinical efficacy in acute invasive aspergillosis, abstr. F80; p. 126. [Google Scholar]
- 4.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Nanning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Sévaux R G L, Kullberg B J, Verweij P E, Van De Nes J A P, Festen J, Meis J F G M, Van Der Meer J W M. Microgranulomatous aspergillosis in a patient with chronic granulomatous disease: cure with voriconazole. Clin Infect Dis. 1998;26:996–997. doi: 10.1086/517646. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson R P, Bell A S, Hitchcock C A, Narayanaswami S, Ray S J, Richardson K, Troke P F. Novel antifungal 2-aryl-1-(1H-1,2,4-triazol-1-YL)butan-2-OL derivates with high activity against Aspergillus fumigatus. Bioorg Med Chem Lett. 1996;16:2031–2036. [Google Scholar]
- 7.Hospenthal D R, Kwon-Chung K J, Bennett J E. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol. 1998;36:165–168. [PubMed] [Google Scholar]
- 8.Iwen P C, Rupp M E, Langnas A N, Reed R C, Hinrichs S H. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of the literature. Clin Infect Dis. 1998;26:1092–1097. doi: 10.1086/520297. [DOI] [PubMed] [Google Scholar]
- 9.Iwen P C, Rupp M E, Bishop M R, Rinaldi M G, Sutton D A, Tarantolo S, Hinrichs S H. Disseminated aspergillosis caused by Aspergillus ustus in a patient following allogeneic peripheral stem cell transplantation. J Clin Microbiol. 1998;36:3713–3717. doi: 10.1128/jcm.36.12.3713-3717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luce J M, Ostenson R C, Springmeyer S C, Hudson L D. Invasive aspergillosis presenting as pericarditis and cardiac tamponade. Chest. 1979;76:703–705. doi: 10.1378/chest.76.6.703. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell S J, Gray J, Morgan M E, Hocking M D, Durbin G M. Nosocomial infection with Rhizopus microsporus in preterm infants: association with wooden tongue depressors. Lancet. 1996;348:441–443. doi: 10.1016/s0140-6736(96)05059-3. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Nolard, N. Personal communication.
- 14.Oakley K L, Moore C B, Denning D W. In-vitro activity of voriconazole against Aspergillus spp. and comparison with itraconazole and amphotericin B. J Antimicrob Chemother. 1998;42:91–94. doi: 10.1093/jac/42.1.91. [DOI] [PubMed] [Google Scholar]
- 15.Ricci R M, Evans J S, Meffert J J, Kaufman L, Sadkowski L C. Primary cutaneous Aspergillus ustus infection: second reported case. J Am Acad Dermatol. 1998;38:797–798. doi: 10.1016/s0190-9622(98)70460-8. [DOI] [PubMed] [Google Scholar]
- 16.Sandner V K, Schönborn C. Schimmelpilzinfektion der Haut bei ausgedektner Verbrennung. Deutsch Ges-Wesen. 1973;28:125–128. [PubMed] [Google Scholar]
- 17.Schmitt H J, Bernard E M, Andrade J, Edwards F, Schmitt B, Armstrong D. MIC and fungicidal activity of terbinafine against clinical isolates of Aspergillus spp. Antimicrob Agents Chemother. 1988;32:780–781. doi: 10.1128/aac.32.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiller M J, Teperman L, Rosenthal S A, Riordan A, Potter J, Shupack J L, Gordon M A. Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. J Am Acad Dermatol. 1994;31:344–347. doi: 10.1016/s0190-9622(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 19.Stynen D, Goris A, Sarfati J, Latgé J P. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi: 10.1128/jcm.33.2.497-500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanink C M A, Meis J F G M, Rijs A J M M, Donnelly J P, Verweij P E. Specificity of an enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J Clin Microbiol. 1997;35:257–260. doi: 10.1128/jcm.35.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van ’t Hek L G, Verweij P E, Weemaes C M, Van Dalen R, Yntema J B, Meis J F G M. Successful treatment with voriconazole of invasive aspergillosis in chronic granulomatous disease. Am J Respir Crit Care Med. 1998;157:1694–1696. doi: 10.1164/ajrccm.157.5.9709068. [DOI] [PubMed] [Google Scholar]
- 22.Verweij P E, Oakley K L, Morrisey J, Morrisey G, Denning D W. In vivo activity of LY 303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij P E, Poulain D, Obayashi T, Patterson T F, Denning D W, Ponton J. Current trends in the detection of antigenemia, metabolites and cell wall markers for the diagnosis and therapeutic monitoring of fungal infections. Med Mycol. 1998;36(Suppl. I):146–155. [PubMed] [Google Scholar]
- 24.Verweij P E, Rijs A J M M, De Pauw B E, Horrevorts A M, Hoogkamp-Korstanje J A A, Meis J F G M. Clinical evaluation and reproducibility of the Pastorex Aspergillus antigen latex agglutination test for diagnosing invasive aspergillosis. J Clin Pathol. 1995;48:474–476. doi: 10.1136/jcp.48.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wald A, Leisenring W, van Burik J A, Bowden R A. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 26.Weiss L M, Thiemke W A. Disseminated Aspergillus ustus infection following cardiac surgery. Am J Clin Pathol. 1983;80:408–411. doi: 10.1093/ajcp/80.3.408. [DOI] [PubMed] [Google Scholar]