Abstract
The Coronavirus disease 2019 (COVID-19) outbreak turned out the greatest pandemic for decades. It challenged enormously the global health system, forcing it to adjust to the new realities. We aimed to analyze articles covering COVID-19 papers in the rheumatological field and outline emerging topics raising within this frame. We applied the bibliometric database Scopus for our literature search and conducted it on the 5th of June using the following keywords: “rheumatic” OR “rheumatology” OR “rheumatoid arthritis” OR “systemic lupus erythematosus” OR “myositis” OR “systemic sclerosis” OR “vasculitis” OR “arthritis” OR “ankylosing spondylitis” AND “COVID-19”. We analyzed all selected articles according to various aspects: type of document, authorship, journal, citations score, rheumatology field, country of origin, language, and keywords. With the help of the software tool VOSviewer version 1.6.15, we have built the visualizing network of authors and keywords co-occurrence. The measurement of the social impact of articles was made using Altmetric data. This study included 1430 retrieved articles with open access mostly. The top five journals in this field were Annals of the Rheumatic Diseases (n = 65), Rheumatology International (n = 51), Clinical Rheumatology (n = 50), Lancet Rheumatology (n = 50), and Frontiers In Immunology (n = 33). Most studies originate from countries with a high incidence of COVID-19 among the general population (the USA—387; Italy—268; UK—184; France—114; Germany—110; India—98 and Spain—96, China—94, Canada—73 Turkey—66). Original Articles (42.1%) were the most common articles’ type, following by Letters (24.4%), Reviews (21.7%), Notes (6%), Editorials (4.8%), Erratum (1%). According to the citations scores, articles dedicated to the clinical course of COVID-19 in patients with rheumatic diseases were of the highest importance for the scientific rheumatologic community. Rheumatoid arthritis (n = 527), systemic lupus erythematosus (n = 393), vasculitis (n = 267), myositis (n = 71), systemic sclerosis (n = 68), and psoriatic arthritis (n = 68) were the most widely discussed rheumatic diseases in the view of COVID-19. The analysis of Altmetric and citations scores revealed a moderate correlation between them. This article provides a comprehensive bibliometric and altmetric analysis of COVID-19 related articles in the rheumatology field and summarizes data about features of rheumatology service in the time of the pandemic.
Keywords: COVID-19, Rheumatology, Bibliometric analysis, Altmetric, Scopus
Introduction
The coronavirus pandemic has become an unprecedented challenge for modern society. The SARS-Cov-2 virus has caused extraordinary global changes in all spheres of human life [1]. The health care system was forced to adapt to the new realities and could not always meet the requirements of the time. Certainly, epidemics and pandemics are not unique to the twenty-first century. They terrified and took millions of lives among past generations too. However, the coronavirus pandemic has radically changed the rules of the game.
One of the features of this pandemic was the rapid spread of large amounts of information, the quality of which did not always meet the standards [2]. In addition to their primary activity, medical workers and scientists constantly had to fight against fakes that quickly filled people's minds. Moreover, even the reputable research opinion had sometimes changed. What we seemed to know a year ago has changed, something has evolved, and something has remained unclear.
The field of rheumatology is not an exception to this common problem. The question was whether patients with autoimmune diseases were at risk for coronavirus infection, whether antirheumatic drugs would effectively combat coronavirus, how to build a rheumatology service in a pandemic, and what vaccination recommendations should be provided. For these almost two years of the pandemic, there were hundreds of articles emphasizing COVID-19 from a rheumatological perspective.
In turn, bibliometric analysis is a valuable tool for navigation in a broad field of scientific data. Its primary purpose is a quantitative and qualitative analysis of publications in a particular research area. With the help of bibliometric analysis, it is possible to outline the core articles and general publishing patterns in a precise scientific topic. The last bibliometric publications in the rheumatological field confirm that this type of study is relevant and is used to analyze various nosologies [3–6]. Although, as far as we know, there was only one attempt to summarize data about COVID-19 in rheumatology [7], although the analysis was performed just within one journal and in the time frame from March 2020 to September 2020.
Herein, with this study, we aimed to summarize and analyze the available scientific data about the influence of COVID-19 on rheumatological practice in general and the interrelation of COVID-19 and different rheumatic pathologies in particular.
Materials and methods
Search strategy
Three primary bibliometric databases are usually used in scientometric studies: Web of Science, Scopus, and Pubmed. Although each of the current databases has its pros and cons, we choose to retrieve the relevant literature data using Scopus bibliometric database. As coverage and accuracy are the main two aspects of scientometry, we have made our choice in favor of this database due to its broadest coverage of peer-reviewed journals (23,500+) [8]. Moreover, Scopus has a friendly interface with comprehensive analytical modalities and extensive operator functions [9]. The literature search was employed on the 5th of June 2021, focusing mainly on English-language articles. For literature retrieval, we used the following MESH keywords: TITLE-ABS-KEY (“rheumatic” OR “rheumatology” OR “rheumatoid arthritis” OR “systemic lupus erythematosus” OR “myositis” OR “systemic sclerosis” OR “vasculitis” OR “arthritis” OR “ankylosing spondylitis” AND “COVID-19”). The inclusion criteria were subsequent:
Accessibility of the abstract;
A study performed as an original article, review, case report or case series, letter, editorial, note, erratum;
Paper reported patients diagnosed with COVID-19 and concomitant rheumatological pathology or explored other links between the rheumatology field and COVID-19.
The book chapters and conference papers were excluded from the study. We analyzed all selected articles comprehensively given various aspects: type of document, authorship, journal, citations score, rheumatology field, country of origin, language, and keywords.
Building bibliometric networks
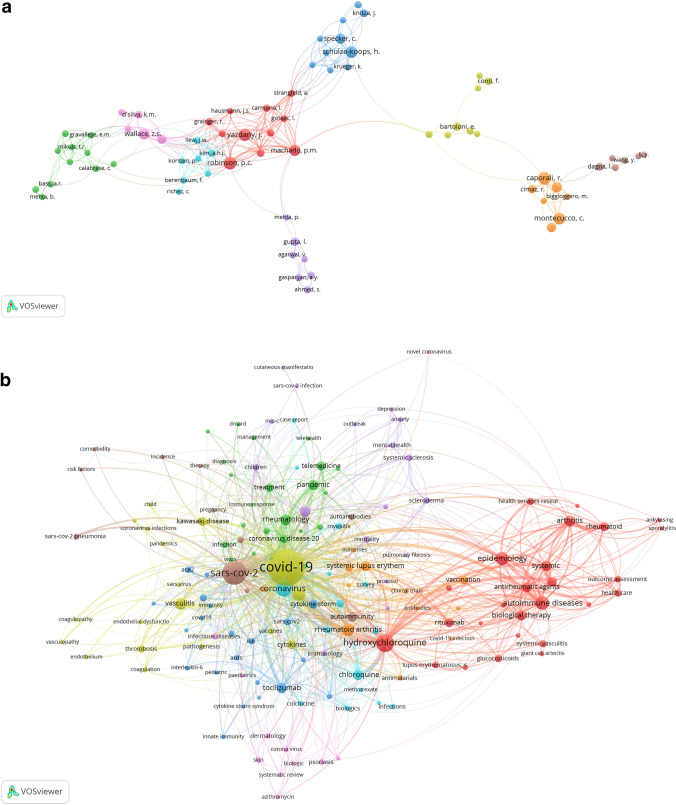
One of the main aspects of bibliometric analysis is exploring the existing networks patterns within the research topic. The best way to present such interactions for readers’ perception is to visualize the data. Using software tool VOSviewer version 1.6.15, we have built the visualizing authors and keywords network (Fig. 1a, b). The visualizing network consists of circles (nodes), which represent the research items (keywords or authors) and lines (edges) indicating connections between the latter [10]. The size of nodes signifies the frequency of occurrence of a specific keyword or number of articles published by the author. The distance between two nodes and line width connecting them reflect the strength of the co-occurrence link between the keywords and the co-authorship link between the authors. For instance, the closer two circles are situated, the thicker line connects them, so the more robust connection between them (keywords are used more frequently within one article/authors work more often in co-authorship). Moreover, with the help of different colors, VOSviewer divides keywords and authors into separate clusters, indicating smaller groups with strong links among the collected data. For building a visualizing network, we limited the number of keywords and authors and used the most high-powered/influential (the minimum threshold for keyword occurrence was set at 6 and for authors occurrence—at 5).
Fig. 1.
a The visualizing authors’ network. b The visualizing keywords network
Analyzing of social attention to the articles
As mass media lively discussed the COVID-19 topic, we analyzed public interest in the selected literature items. The best tool in establishing social attention is the Altmetric Attention Score (AAS). It includes social network visibility (like Twitter and Facebook), mentions on research blogs, bookmarks on reference managers such as Mendeley, appearance in the news, etc. AAS evaluates attention to the article and measures the dissemination of each publication. It could even be considered as an indicator of influence and impact. We aimed to analyze the 50 most cited articles from our list and compare the social impact with scientific attention expressed in the citation index.
Statistical analysis
We have performed our bibliometric and altmetric analyses using the Statistical Package for the Social Sciences version 28.0 software (SPSS Inc., Chicago, IL, USA). Data were presented as numbers (n) and percentages (%). Correlations between AAS, Mendeley reader count, and articles' citation score were established using the Spearman's rho test. We have interpreted rho score < 0.25 as weak, 0.25–0.49 as moderate, 0.50–0.74 as strong, and > 0.75 as a robust correlation. A value of p < 0.05 was regarded as the statistical significance.
Results of bibliographic macro analysis
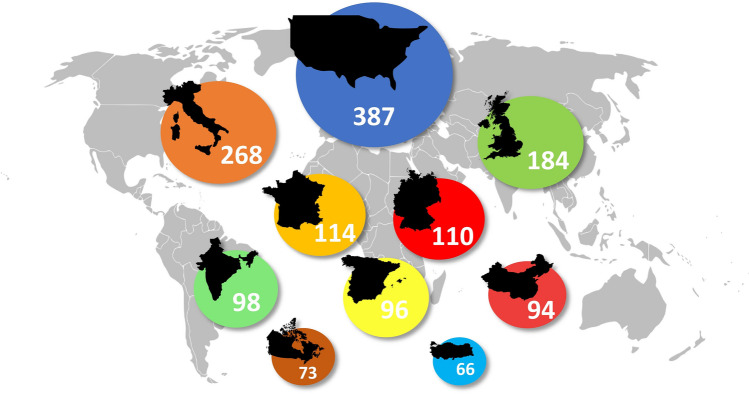
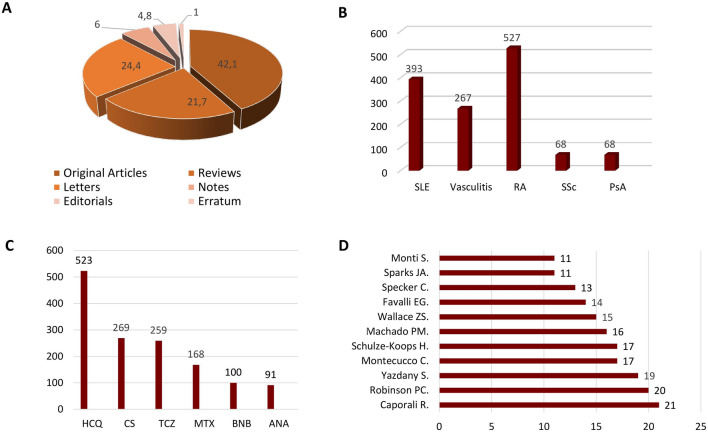
We obtained a total of 1778 scholarly items. After analyzing the title, abstract, and keywords, we excluded 348 records as they did not emphasize the rheumatological perspective on COVID-19. Thus, they were inapplicable for this study. The 1430 retrieved articles were mostly open access publications. Annals Of the Rheumatic Diseases (n = 65), Rheumatology International (n = 51), Clinical Rheumatology (n = 50), Lancet Rheumatology (n = 50), and Frontiers in Immunology (n = 33), Fig. 2 were the top five journals covering this topic. The origin of most studies was from those countries, which belong to the top ten according to the total cases of COVID-19 (the USA—387; Italy—268; UK—184; France—114 Germany—110 India—98 and Spain—96, China—94, Canada—73 Turkey—66), Fig. 3. Most items were written in English, although there were articles in German (n = 25), Spanish (n = 24), Russian (n = 17), French (n = 11), Chinese (n = 3), Portuguese (n = 2), Italian (n = 1), Korean (n = 1). Original Articles were the most common type of scholarly papers (42.1%), the other studies designed as Letters (24.4%), Reviews (21.7%), Notes (6%), Editorials (4.8%), Erratum (1%), Fig. 4A. According to the citations scores, the article that received the most attention was dedicated to the clinical course of COVID-19 in patients with autoimmune disorders [11] (279 Citations, 416 Altmetric score, 417 Tweets, and 458 Mendeley reader count). The other highly cited studies were about the immune mechanism of coagulopathy in COVID-19 [12] (245 Citations, 982 Altmetric scores, 1378 Tweets, and 679 Mendeley reader count) and perspective usage of antirheumatic drugs for severe cases of COVID-19 [13] (118 Citations, 6 Altmetric score, 8 Tweets, and 628 Mendeley reader count). Our analysis of keywords showed that the rheumatic disease of highest interest in the view of COVID-19 was rheumatoid arthritis (RA) (n = 527), followed by systemic lupus erythematosus (SLE) (n = 393), vasculitis (n = 267), myositis (n = 71), systemic sclerosis (SSc) (n = 68), and psoriatic arthritis (PsA) (n = 68), Fig. 4B. The most widely discussed disease-modifying antirheumatic drugs in COVID-19 were hydroxychloroquine (n = 523), corticosteroids (n = 269), tocilizumab (n = 259), methotrexate (n = 168), baricitinib (100) and anakinra (n = 91), Fig. 4C. The list of authors with the most significant number of articles on discussed topic is shown in Fig. 4D.
Fig. 2.
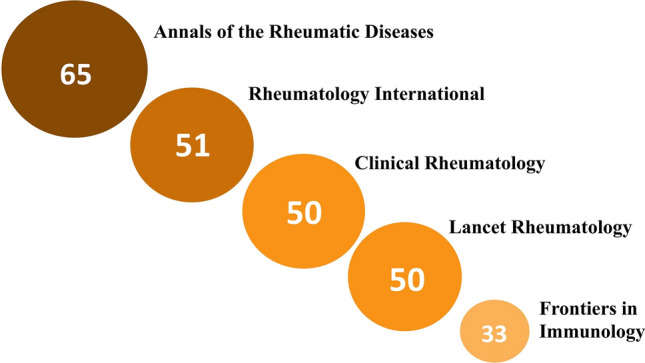
The top five rheumatological journals according to the number of published COVID-19 related articles
Fig. 3.
The top 10 countries of origin of scientific publications on COVID-19 from a rheumatological perspective
Fig. 4.
a Structure of publications by their type. b The 5 most represented rheumatologic disorders considering COVID-19 topic. c The most frequently mentioned rheumatologic drugs in COVID-19 related articles. d The authors with the most significant contribution to the field
Social attention and scientific recognition: do they correlate?
We established a correlation between citation and AAS, citation score and number of tweets, citation score and Mendeley reader count. The strongest correlation was detected between Mendeley reader count and the number of citations (rho = 0.783, p = 0.001). AAS and number of tweets revealed a moderate correlation (rho = 0.364, p = 0.009 and rho = 0.364, p = 0.039, respectively). These results suggest that we cannot entirely rely on social media attention even in the current pandemic's emerging topics. The high impact on social media could not be equalized to scientific one and vice versa. However, active postpublication promotion and AAS increase may positively impact the active dissemination of data among the scientific community. In turn, this can positively affect the level of citation of articles. At the same time, Mendeley reader count proved to indicate the high probability of future article citation.
COVID-19 and rheumatic diseases
Given the rapid spread of the SARS-CoV-2 virus, there was intense debate about the increased risk of developing COVID-19 among patients with autoimmune diseases on immunosuppressive therapy. At the first wave of the pandemic, the data showed that the incidence of COVID-19 among patients with ARDs did not differ from the same in the general population [14–19]. These results tend to be explained by Hooijberg et al. Their study established the stricter isolation measurements taken by patients with ARDs compared to the general population [20]. However, due to more data, Wang et al. proved that the risk of developing COVID-19 in this cohort of patients is higher than among healthy people (OR = 1.53) [21].
This pandemic influenced management strategies across rheumatology services. In a survey study launched by Dejaco et al. [22], most respondents reported difficulties in starting the treatment with biological DMARDs. At the same time, survey participants admitted a shortage of hydroxychloroquine (49%) and tocilizumab (14%) for their needs. Moreover, the number of face-to-face visits was decreased by 52%, while the number of remote consultations has been grown by 129% [23].
To date, data about the course of COVID-19 in patients with rheumatic diseases is full of controversy. On the report of Faye et al. [24], patients with autoimmune diseases or immunosuppressive therapy do not have any increased risks of being admitted to the ICU, getting intubated, or being deceased. The French RMD COVID-19 cohort data claim corticosteroid usage as a risk factor for severe disease regardless of the dose, whereas methotrexate, TNFα, and interleukin-6 (IL-6) inhibitors were safe to use [25]. In turn, another study stated that connective tissue disease was an independent risk factor for severe COVID-19 but not its treatment strategy [26]. Although, the authors present the risks for a severe course of infection among the rheumatic cohort and general population on the level of 31.6% and 28.1%, respectively. As for the risk of death from COVID-19, the undisputed risks were the activity of the underlying autoimmune disease and the use of rituximab and sulfasalazine [27].
COVID-19 and systemic lupus erythematosus
SLE was the most widely discussed rheumatic disease in the focus of the SARS-CoV-2 pandemic. These two diseases share certain similarities in their pathogenesis [28], making SLE the possible base for developing COVID-19 pathogenetic therapy. However, this assumption played a bad joke, causing the mass hysteria around hydroxychloroquine as a possible panacea in the fight against COVID-19.
Although no significant features of the course of SARS-CoV-2 infection among patients with SLE were reported [29], few articles were assuming the direct causative relationship between COVID-19 and SLE [30, 31]. Several case-based reviews analyzed cases with deterioration of clinical course and new-onset SLE during this infective disease [30, 31]. Such cases, however, remain rare, and an exact explanation of their pathogenetic base is still missing. Although the hypothesis about molecular mimicry, viral persistence, and formation of neutrophil extracellular traps [32] are promissable and require further research.
COVID-19 and systemic sclerosis
The authors, across their articles, reasonably emphasized an overlap in lung damage between SSc and COVID-19. Mariano et al. [33] noted the importance of analyzing lung CT data in a dynamic allowing evaluating the pulmonary involvement more precisely. In turn, Ferry admits that the risk of severe SARS-CoV-2 infection is high in patients with SSc [34]. Moreover, SSc was the first rheumatic disease that received guidelines for COVID-19 management strategy from the World Scleroderma Foundation [35].
COVID-19 and vasculitis
The vasculopathy features are one of the numerous manifestations of COVID-19 due to direct systemic endothelial tropism of SARS-CoV-2 [36]. The COVID-19 associated vascular involvement often mimics the typical manifestations of vasculitis, which makes it challenging for differential diagnosis in some cases.
Özdemir et al. [37] described and analyzed series of cases with a tentative differential diagnosis between eosinophilic granulomatosis with polyangiitis (EGPA) and COVID-19. Given the high vigilance about COVID-19, seven patients with EGPA were misdiagnosed even despite negative PCR tests. The main challenges for clinicians were the resemblance of clinical manifestation and instrumental findings between both pathologies. The COVID-19 lung involvement is not specific and shares similarities with various interstitial lung diseases of rheumatic etiology. Another article described the granulomatosis with polyangiitis (GPA) case, diagnosed with delay due to COVID-19 suspicion [38]. It emphasizes a new struggle in the diagnosis of small blood vessel vasculitis caused by the SARS-CoV-2 pandemic.
Long COVID from an autoimmune perspective
Long COVID defines an appearance of post‐COVID‐19 symptoms beyond the initial disease. Although this term has become entrenched in the medical community, there are still some contradictions in the definition of this concept. Several terms are illustrating the long-term persistence of symptoms in the literature: long COVID or postacute COVID (symptoms for more than three weeks) and chronic post‐COVID syndrome (more than 12 weeks) [39]. By now, several hypotheses tend to explain the appearance of this phenomenon from the perspective of postviral fatigue syndrome [40], development of autoimmune reactions [41], and persistence of the SARS-Cov2 virus in the organism [42]. Wang et al. [43] proved the possibility of autoimmune pathology development as a long-term consequence of coronavirus infection. Their results confirmed the molecular autoantigen transformation and post-translational modifications due to the influence of the SARS-CoV-2 virus on host cells. These findings support the reports about the appearance of autoimmune diseases after COVID-19 [44–46] and confirm their nonrandomness. In all cases, the autoimmune pathology occurred after the end of the clinical manifestation of COVID-19. In the study of Toubiana et al. [45], the median interval between COVID-19 and Kawasaki-like pediatric multisystemic inflammatory syndrome onset was 42 days (range 18–79 days), which fits into the long COVID framework. It proves the need for close monitoring of coronavirus convalescents for the appearance of rheumatic diseases in this cohort.
Vaccination and rheumatic pathology
Vaccination is currently the most effective means of combating the spread of COVID-19. Thus, there should be carefully processed and disseminated information regarding regulations and recommendations allowing a community of patients with autoimmune rheumatic diseases (ARDs) to avoid unnecessary access restrictions to COVID-19 vaccines and letting them obtain the highest possible benefit from this prophylactic strategy. Despite the lack of persuasive studies involving this cohort of people, ACR published official guidelines suggesting recommendations and vaccination against COVID-19 strategies for people with ARDs [47]. Over time, local, national guidelines [48–50] and expert opinions [51] appeared. In addition, we identified articles devoted to the vaccination against COVID-19 in patients with ARDs, covering this topic from different perspectives.
It is noticeable that any available vaccine was not contraindicated for use in rheumatic patients by ACR or local recommendations. Although, due to the appearance of safety concerns among FDA and CDC, the ACR task force recommended using mRNA-based instead of adenoviral vector vaccines. However, Tang et al. [52] raised concerns about a possible flare or developing the vaccine-associated enhanced respiratory disease due to mRNA vaccine use in patients with SLE.
Currently, these concerns remain hypothetical as reports about side effects are scarce. The multicenter study conducted by Watad et al. documented only 3 cases of SLE flares. Two of them were mild course, and one included severe hemolysis, ulceration in oral and nasal cavities, arthralgia. The response to treatment (prednisolone 60 mg/daily and rituximab) was slow. Interestingly, the ChAdOx1 vaccine provoked the two flares (including severe ones), and BNT-162b2 caused only one mild flare. This study reported a total of 27 cases of immune-mediated disease concerning COVID-19 vaccination [53]. In 6 patients, it was the first onset of autoimmune disorder; the other cases were the flares of underlying pathologies. Most patients (Twenty-three (85.2%)) received the BNT-162b2, two (7.4%)—mRNA-1273, and two (7.4%)—ChAdOx1 vaccines. Diseases that preceded vaccination were rheumatoid arthritis (4), Behçet’s disease (4), systemic lupus erythematosus(3), gout, dermatomyositis, and polymyalgia rheumatica. The new-onset diseases were Henoch–Schönlein purpura, two cases of chilblain lesions, two arthritis, and one myasthenia gravis case.
In addition, Terracina K.A. reported another flare of RA after BNT-162b2 inoculation [54]. A case of reactive arthritis as a response to the CoronaVac vaccine was described [55]. Flowers et al. [56] reported a case of anti-COVID-19 vaccine-associated septic arthritis, which required six weeks of antibiotics and physical therapy for the patient’s recovery. This case points at the utmost importance of the strict instructions of vaccine administration. It helps to avoid unintentional penetration into the glenohumeral joint.
A feature of patients with rheumatic pathology is the use of drugs that modify the immune response and may affect the effectiveness of vaccination. Rituximab causes a marked reduction of a humoral immune response, and in combination with corticosteroids, the risk of hypogammaglobulinemia development is rising [57]. Thus, ACR recommendations suggest a specific vaccination schedule with a previously created window in RTX administration. Currently, we were able to find only one report about the influence of antirheumatic drugs on mRNA COVID-19 vaccine response [58]. The methotrexate intake can reduce the immunogenicity of BNT162b2 mRNA vaccination up to 62%. [59]. Methotrexate may suppress activation of CD8 + T cell response, creating significant obstacles to effective immunity against SARS-CoV-2 infection. In turn, usage of anti-cytokine or nonmethotrexate oral drugs keeps immunogenicity at the level of healthy control. The current ACR guideline recommends pausing the MTX intake one week after each mRNA vaccine dose or two weeks after a single inoculation. Only further studies can justify if this period is enough for avoiding the negative impact of MTX on vaccine immunogenicity.
Another important aspect is that it has not yet been possible to achieve mass enthusiasm of people to be vaccinated. According to the study from Turkey [60], only 29.2% of respondents with ARDs were ready to undergo vaccination while 19% were against it and 51.8% hesitated. These numbers were comparable with results in the general population 34.6%, 23.3%, and 42.1%, respectively. We suggest that these data may not be consistent for all ARDs patients and may differ among countries with higher vaccination support. To sustain this deduction, we may refer to the VAXICOV international study, where 54.2% of interviewed were ready to get vaccinated against SARS-CoV-2 [61]. In turn 32.2% were uncertain and 13.6% were against vaccination. According to Felten et al., the main patients’ concerns were lack of information regarding mechanisms of action and consequences of revolutionary new technics used in the development of COVID-19 vaccines, the possible deterioration of disease course, and side effects development [61].
Influenza vaccination data were encouraging. As by Fragoulis et al. [62], the numbers of vaccinated individuals with ARDs have risen in the season of 2020/2021 and reached 83%. It could also be beneficial in the view of decrease of COVID-19 mortality [63].
Pregnancy, rheumatic diseases, and COVID-19—a tense triangle
There is currently limited data on rheumatic pathology and COVID-19 comorbidity in pregnant women. We managed to retrieve only two clinical cases describing patients with SLE and concomitant COVID-19 infection [64]. The first woman developed COVID-19 symptoms at 38 + 1 weeks of gestation. Its course was mild, and labor was without any complication. This woman delivered a baby without any congenital malformations and the negative PCR for SARS-CoV-2. During the whole pregnancy, her treatment [azathioprine (25 mg/day), hydroxychloroquine (200 mg/day), prednisone (5 mg/day)] was extant. The second case describes pregnancy in an SLE patient with confirmed COVID-19 on the 19th week of gestation. After a diagnosis of COVID-19, azathioprine and etanercept were withdrawn. Although after the SLE symptoms worsened, treatment was resumed. The pregnancy ended with the birth of a healthy baby.
Given to scares data about the influence of COVID-19 on pregnant ARDs patients, there are no recommendations for this particular cohort. The experts recommend adhering to conventional guidelines for patients with rheumatic diseases during pregnancy [65, 66]. Although recommendations for pregnant women with reproductive failure were established based on the observational studies [67]. Kwak-Kim et al., recommend maintaining low dose prednisone, heparin, tacrolimus, Plaquenil, and IVIg treatment in pregnant women even with mild COVID-19. Although in the case of severe COVID-19, immunotherapy is not welcomed unless essential for COVID-19 treatment.
Of high importance is to preserve an effective screening for rheumatic diseases in early pregnancy. Ramoni and Bellingeri reported their screening method consisting of a questionnaire and further following autoantibody testing [68]. According to their data, this screening method remained efficient and accurate even during the COVID-19 outbreak.
Telemedicine
As a result of the introduction of quarantine measures by most countries, the free travel opportunities of people were restricted. Patients with rheumatic pathology had significantly complicated access to inpatient care and in-person consultation due to the high workload of medical institutions. Thus, the optimal option to continue following up on ARDs patients was implementing telemedicine, which was successfully applied in different countries during the corona outbreak [69, 70]. Although the attitude of patients and rheumatologists to telemedicine as an alternative to full-fledged visits may differ due to technical knowledge and willingness to change established communication habits. The study from Hong Kong have established that only 40% of patients were strongly confident using telemedicine approach, 14.9% denied their confidence in this matter, while the others had neutral response [71]. Kernder et al. [72], instead, reported about 74% among patients and 76% among rheumatologists believing efficiency of digital health applications (DHAs) in rheumatology. 90% of patients and 86% of rheumatologists described themselves as confident users of DHAs. However, most patients (81,3%) were ready to switch to virtual follow-up appointments in a stable disease course. It should be taken into account that studies investigating patients' responses to telemedicine were mainly based on the online surveys, which may influence its results. As people taking part in online surveys tend to be more familiar with digital technologies. The American study reported that 44% of ARDs patients participated in virtual rheumatology appointments, and 73% were satisfied [73].
Interestingly, in the USA, video conferences were more preferred by the patients. However, the main factor in patients' satisfaction with digital consultation is the activity status of their disease [74]. Chevallard et al., have analyzed the outcomes for patients with RA, PsA, and ankylosing spondylitis (AS) used digital medicine during the short period. No substantial differences were found [75]. Moreover, such an approach can also reduce costs spending comparing traditional follow-up [76], which in time of limited budget during a pandemic may facilitate its more efficient distribution.
However, the biggest concern is an inability to evaluate the disease activity, treatment efficacy, and safety using telemedicine. The solution to this problem may be a broad implementation of patient self-sampling [77]. Morf et al. [78] reported that 44% of patients intended to use self-sampling in the future, proving its efficacy.
COVID-19 pandemic challenges the training of future rheumatologists
COVID-19 changed the regular rhythm of life not only for hospitals and practitioners. It significantly affected the educational sphere. Rheumatology trainees could not adhere fully to the traditional formal curriculum, being restricted in traditional face-to-face interactions with patients [79]. However, maintaining the quality of training in rheumatology as a guarantee of supporting the provision of qualified medical care is an essential need. Thus, to adapt to the strict COVID-19 restrictions, educational institutions have switched towards diverse online opportunities and hybrid learning programs [79, 80]. We have indicated a series of articles discussing the challenges and progress of rheumatology training during COVID-19 pandemic. Remote learning allows trainees to acquire the necessary skills in working with telemedicine, use for training social networks, and a lot of visual material [79–81].
Nonetheless, online learning is not free of challenges, in particular lack of mentor–mentee as well as trainee–patient emotional connection and the difficulty in assessing the acquired practical skills [81, 82]. There is a growing risk of emotional failure between future rheumatologists and their patients. During their training, doctors learn to understand the needs and build effective emotional interaction with ARDs patients. The trainees themselves tend to experience anxiety, hopelessness, and helplessness during COVID-19 pandemics [83], which can increase by lacking personal contacts and remote learning. Moreover, rheumatology training programs differ across countries, and not everywhere there were predesigned curricula that provide at least partial online training, which may complicate such a transition in a short time [84]. Nevertheless, the online mode in rheumatology training could be successful by following essential needs established by the EULAR Working Group on Training [85].
At present, we can assume what impact has made a COVID-19 outbreak on rheumatology training only hypothetically. No articles indicate the quality of future rheumatologists' training and the attitude of the trainees to this format of training at the time. Further investigations in this area are needed.
COVID-19 from the ARDs patient’s standpoint
Numerous studies have established the status of patients with rheumatic diseases during the current pandemic [86]. Facing challenges of self-isolation, drug shortage, and controversial press releases, patients with ARDs deserve careful analysis of their needs and fears.
The data about treatment adherence are from rheumatological centers in Greece, Iran, Mexico, and the USA [86–89]. Despite geographical differences, the results of these studies were comparable except for data from Iran, where the treatment adherence was at a higher level. Involving into the study 500, 858, 345, and 530 patients in the forementioned centers, scientists obtained the following percent of self-imposed drug cessation 14.6%, 6.5%, 15%, and 14%, respectively. The most frequent causes of treatment discontinuation were fear of immunosuppression and getting COVID-19 and a shortage of drugs supply. Fragoulis et al., reported no flares associated with treatment discontinuation. Contrariwise, Khabbazi et al., indicated exacerbation in 9.6% of nonadherent patients. The same study revealed seronegative spondyloarthritis as the most common disease among patients with low treatment adherence. Khabbazi et al. assumed that it was due to the frequent use of biologics in this category of patients. These data highlight the significance of building trust between patients and doctors, ensuring good adherence to treatment, and maintaining a stable disease course. Moreover, reporting the information that DMARDs therapy does not affect the risk of developing COVID-19 is essential. It may help to reduce the nonadherent patients' rates and avoid abrupt exacerbations.
The psychosocial wellbeing of ARDs patients was analyzed by Bhatia et al., and Durcan et al., Durcan et al., revealed that children with rheumatic diseases in the age group > 13 years and their parents have higher anxiety and depression levels as compared to the control group [90]. That study also established a straight correlation between parents' and children's anxiety and depression scores. Bhatia et al. [91] draw attention to the vicious circle uniting physical and mental wellbeing. Based on the systemic review data, patients with RA may suffer from aggravation of pain and disease activity in case of depression [92]. To prevent such outcomes, the authors suggested paying more careful attention to mental wellbeing and improve screening for mental health problems in the cohort of rheumatological patients.
Conclusion
The COVID-19 pandemic has brought about changes that make the world “before” and “after.” It will be possible to learn about consequences later, and some phenomena are already an apparent reality. Conduction of bibliometric studies remains crucial for summarizing and analyzing available data in this emerging field. Our study was not devoid of certain limitations. We used the single database Scopus for our analysis. However, this database is one of the most significant leading sources of reliable scientific data, being the consistent option for conducting bibliometric analyses. The other limitation was higher adherence to articles written in English, although we were also able to analyze articles in German and Russian. Despite these abovementioned shortcomings, we managed to conduct the analyses of COVID-19 related studies in the field of rheumatology. We emphasized the patients' perspective as well as the struggles of rheumatologists and trainees. Our study discussed the vaccination issue for patients with ARDs and summarized data about long COVID within its possible connection to rheumatology. Moreover, along with bibliometric analysis was performed an analysis of Altmetric data as well. The correlation between AAS and citation score appeared to be not entirely coherent, making social media attention analytics secondary for the scientific impact.
Author contributions
BD and OZ designed the study. BD, OZ, and RY reviewed the articles and provided the data. BD, IK, and RY analyzed the data. BD wrote the initial manuscript; RY, IK, and OZ reviewed drafts of the paper. BD prepared the figures. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
No human participants/animals were evaluated. These data are public. Therefore, there was no requirement for ethics committee approval.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bohdana Doskaliuk, Email: Doskaliuk_Bo@ifnmu.edu.ua.
Roman Yatsyshyn, Email: yatsyshyn25@gmail.com.
Iryna Klishch, Email: ira181281@ukr.net.
Olena Zimba, Email: zimbaolena@gmail.com.
References
- 1.Pfefferbaum B, North CS. Mental health and the COVID-19 pandemic. N Engl J Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 2.Gupta L, Gasparyan AY, Misra DP, Agarwal V, Zimba O, Yessirkepov M. Information and misinformation on COVID-19: a cross-sectional survey study. J Korean Med Sci. 2020;35(27):e256. doi: 10.3346/jkms.2020.35.e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akyol A, Kocyigit BF. Publication activity in the field of Sjögren’s syndrome: a ten-year Web of Science-based analysis. Rheumatol Int. 2021;41(4):763–769. doi: 10.1007/s00296-020-04714-1. [DOI] [PubMed] [Google Scholar]
- 4.Akyol A, Kocyigit BF. Ankylosing spondylitis rehabilitation publications and the global productivity: a Web of Science-based bibliometric analysis (2000–2019) Rheumatol Int. 2021 doi: 10.1007/s00296-021-04836-0. [DOI] [PubMed] [Google Scholar]
- 5.Ren W, Liao Y. Visualization analysis of traumatic osteoarthritis research hotspots and content based on CiteSpace. Chin J Tissue Eng Res. 2021;25(21):3374. [Google Scholar]
- 6.El Masri D, Alsaayed B, El Masri J, Zreika B, Chanbour H, Salameh P. Contribution of Arab countries to familial mediterranean fever research: a pubmed-based bibliometric analysis. Rheumatol Int. 2021 doi: 10.1007/s00296-021-04852-0. [DOI] [PubMed] [Google Scholar]
- 7.Lauper K, Bijlsma JW, Burmester GR. Trajectories of COVID-19 information in the Annals of the rheumatic diseases: the first months of the pandemic. Ann Rheum Dis. 2021;80(1):26–30. doi: 10.1136/annrheumdis-2020-219217. [DOI] [PubMed] [Google Scholar]
- 8.(2021) https://www.elsevier.com/__data/assets/pdf_file/0017/114533/Scopus_GlobalResearch_Factsheet2019_FINAL_WEB.pdf. Accessed 26 Jul 2021
- 9.AlRyalat SAS, Malkawi LW, Momani SM. Comparing bibliometric analysis using PubMed, Scopus, and Web of Science databases. JoVE. 2019;152:e58494. doi: 10.3791/58494. [DOI] [PubMed] [Google Scholar]
- 10.Perianes-Rodriguez A, Waltman L, Van Eck NJ. Constructing bibliometric networks: a comparison between full and fractional counting. J Informetrics. 2016;10(4):1178–1195. doi: 10.1016/j.joi.2016.10.006. [DOI] [Google Scholar]
- 11.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, Mateus EF. Characteristics associated with hospitalization for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufan A, Güler AA, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho SO, Mak JW, Jacqueline SO, Grace LU, Frankie LU, Jolly LE, Shirley CH, Carmen HO, Chan JM, Shing-Pak KO, Woon-Leung NG. Incidence and clinical course of COVID-19 in patients with rheumatologic diseases: a population-based study. Semin Arthritis Rheum. 2020;50(5):885–889. doi: 10.1016/j.semarthrit.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredi M, Cavazzana I, Moschetti L, et al. COVID-19 in patients with rheumatic diseases in northern Italy: a single-center observational and case-control study. Lancet Rheumatol. 2020;2(9):e549–e556. doi: 10.1016/S2665-9913(20)30169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Gao Y, Zhang Y, Shi S, Chen Y, Tian J. The association between severe or death COVID-19 and autoimmune disease: a systematic review and meta-analysis. J Infect. 2020;81:e93–95. doi: 10.1016/j.jinf.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favalli EG, Ingegnoli F, Cimaz R, Caporali R. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis. 2021;80(2):e18. doi: 10.1136/annrheumdis-2020-217615. [DOI] [PubMed] [Google Scholar]
- 18.Michelena X, Borrell H, López-Corbeto M, et al. Incidence of COVID-19 in a cohort of adult and pediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying antirheumatic drugs. Semin Arthritis Rheum. 2020;50(4):564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quartuccio L, Valent F, Pasut E, Tascini C, De Vita S. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. 2020;87(5):439–443. doi: 10.1016/j.jbspin.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooijberg F, Boekel L, Vogelzang EH, Leeuw M, Boers M, van Vollenhoven R, Lems WF, Nurmohamed MT, Wolbink G. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2(10):e583–e585. doi: 10.1016/S2665-9913(20)30286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Liu J, Shao R, Han X, Su C, Lu W. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: a systematic review and meta-analysis. Rheumatol Int. 2021;41(5):851–861. doi: 10.1007/s00296-021-04803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dejaco C, Alunno A, Bijlsma JW, Boonen A, Combe B, Finckh A, Machado PM, Padjen I, Sivera F, Stamm TA, Buttgereit F. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis. 2021;80(4):518–526. doi: 10.1136/annrheumdis-2020-218697. [DOI] [PubMed] [Google Scholar]
- 23.Ciurea A, Papagiannoulis E, Bürki K, Von Loga I, Micheroli R, Möller B, Rubbert-Roth A, Andor M, Bräm R, Müller A, Dan D. Impact of the COVID-19 pandemic on the disease course of patients with inflammatory rheumatic diseases: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2021;80(2):238–241. doi: 10.1136/annrheumdis-2020-218705. [DOI] [PubMed] [Google Scholar]
- 24.Faye AS, Lee KE, Laszkowska M, Kim J, Blackett JW, McKenney AS, Krigel A, Giles JT, Wang R, Bernstein EJ, Green PH. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York City. J Rheumatol. 2021;48(3):454–462. doi: 10.3899/jrheum.200989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMMEDIATE consortium and contributors Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80(4):527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pablos JL, Galindo M, Carmona L, Lledó A, Retuerto M, Blanco R, Gonzalez-Gay MA, Martinez-Lopez D, Castrejón I, Alvaro-Gracia JM, Fernández DF. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 27.Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, Mateus EF, Richez C, Santos MJ, Schmajuk G, Scirè CA. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020;39:2055–2062. doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez GA, Gerosa M, Beretta L, Bellocchi C, Argolini LM, Moroni L, Della Torre E, Artusi C, Nicolosi S, Caporali R, Bozzolo EP. COVID-19 in systemic lupus erythematosus: data from a survey on 417 patients. Semin Arthritis Rheum. 2020;50(5):1150–1157. doi: 10.1016/j.semarthrit.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardoso EM, Hundal J, Feterman D, Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020;39:2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gracia-Ramos AE, Saavedra-Salinas MÁ. Can the SARS-CoV-2 infection trigger systemic lupus erythematosus? A case-based review. Rheumatol Int. 2021;41:799–809. doi: 10.1007/s00296-021-04794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S, Danda D, Kavadichanda C, Das S, Adarsh MB, Negi VS. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol Int. 2020;40(10):1539–1554. doi: 10.1007/s00296-020-04639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariano RZ, Rio AP, Reis F. Covid-19 overlapping with systemic sclerosis. Rev Soc Bras Med Tro. 2020 doi: 10.1590/0037-8682-0450-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferri C, Giuggioli D, Raimondo V, Dagna L, Riccieri V, Zanatta E, Guiducci S, Tavoni A, Foti R, Cuomo G, De Angelis R. COVID-19 and systemic sclerosis: clinicopathological implications from Italian nationwide survey study. Lancet Rheumatol. 2021;3(3):e166–e168. doi: 10.1016/S2665-9913(21)00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matucci-Cerinic M, Bruni C, Allanore Y, et al. Systemic sclerosis and the COVID-19 pandemic: world scleroderma Foundation preliminary advice for patient management. Ann Rheum. 2020;79:S724–S726. doi: 10.1136/annrheumdis-2020-217407. [DOI] [PubMed] [Google Scholar]
- 36.McGonagle D, Bridgewood C, Ramanan AV, Meaney JF, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3(3):224–233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Özdemir B, Erden A, Güven SC, Armagan B, Apaydin H, Karakas Ö, Akdag AG, Ates İ, Kucuksahin O, Omma A. COVID-19 and eosinophilic granulomatosis with polyangiitis or COVID-19 mimicking eosinophilic granulomatosis with polyangiitis? Rheumatol Int. 2021;41:1515–1521. doi: 10.1007/s00296-021-04896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qurratulain Q, Ahmed A, Jones Q. Lesson of the month: Severe granulomatosis with polyangiitis (GPA): a diagnostic challenge during the COVID-19 pandemic. Clin Med (Lond) 2021;21(1):79–80. doi: 10.7861/clinmed.2020-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halpin S, O'Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242–1243. doi: 10.1002/jmv.26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson C. Concern coronavirus may trigger post-viral fatigue syndromes. New Sci. 2020;246(3278):10–11. doi: 10.1016/S0262-4079(20)30746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs J. Persistent SARS-2 infections contribute to long COVID-19. Med Hypotheses. 2021;149:110538. doi: 10.1016/j.mehy.2021.110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JY, Zhang W, Roehrl MW, Roehrl VB, Roehrl MH. An autoantigen profile of human A549 lung cells reveals viral and host etiologic molecular attributes of autoimmunity in COVID-19. J Autoimmun. 2021;120:102644. doi: 10.1016/j.jaut.2021.102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonometti R, Sacchi M, Stobbione P, et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci. 2020;24(18):9695–9697. doi: 10.26355/eurrev_202009_23060. [DOI] [PubMed] [Google Scholar]
- 45.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selvaraj V, Moustafa A, Dapaah-Afriyie K, Birkenbach MP. COVID-19-induced granulomatosis with polyangiitis. BMJ Case Reports CP. 2021;14(3):e242142. doi: 10.1136/bcr-2021-242142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases—version 1. Arthritis Rheumatol. 2021;73(7):1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JK, Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, Lee MS, Hong SJ, Choi BY, Lee SS, Back HJ. COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: clinical guidance of the Korean College of Rheumatology. J Korean Med Sci. 2021;36(12):e95. doi: 10.3346/jkms.2021.36.e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santosa A, Xu C, Arkachaisri T, Kong KO, Lateef A, Lee TH, Leong KH, Low AHL, Sriranganathan MK, Tan TC, Teng GG. Recommendations for COVID-19 vaccination in people with rheumatic disease: developed by the Singapore Chapter of Rheumatologists. Int J Rheum Dis. 2021;24(6):746–757. doi: 10.1111/1756-185X.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Society, The Greek Rheumatology, and Professional Association of Rheumatologists Vaccination against SARS-CoV-2 in immunosuppressed patients with rheumatic diseases: position statement of the Greek Rheumatology Society. Mediterr J Rheumatol. 2020;31(4):430–432. doi: 10.31138/mjr.31.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti F, Cannatelli R, Benucci M, Carmagnola S, Clementi E, Danelli P, Dilillo D, Fiorina P, Galli M, Gallieni M, Genovese G. How to manage COVID-19 vaccination in immune-mediated inflammatory diseases: an expert opinion by IMIDs Study Group. Front Immunol. 2021;12:1206. doi: 10.3389/fimmu.2021.656362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang W, Askanase AD, Khalili L, Merrill JT. SARS-CoV-2 vaccines in patients with SLE. Lupus Sci Med. 2021;8(1):e000479. doi: 10.1136/lupus-2021-000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watad A, De Marco G, Mahajna H, Druyan A, Entity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, Brodavka M. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021;3(7):e469–e470. doi: 10.1016/S2665-9913(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.An QJ, Qin DA, Pei JX. Reactive arthritis after COVID-19 vaccination. Hum Vaccin Immunother. 2021 doi: 10.1080/21645515.2021.1920274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flowers RC, Rivera Rodriguez B, Corbitt K. Streptococcus Gordonii septic arthritis of the glenohumeral joint following deltoid intramuscular vaccination. BMJ Case Rep. 2021;14(5):e243066. doi: 10.1136/bcr-2021-243066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wade SD, Kyttaris VC. Rituximab-associated hypogammaglobulinemia in autoimmune rheumatic diseases: a single-center retrospective cohort study. Rheumatol Int. 2021;41(6):1115–1124. doi: 10.1007/s00296-021-04847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 59.Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, Koralov S, Atreya R, Tascilar K, Allen J, Castillo R. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yurttas B, Poyraz BC, Sut N, Ozdede A, Oztas M, Uğurlu S, Tabak F, Hamuryudan V, Seyahi E. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: a web-based survey. Rheumatol Int. 2021;41(6):1105–1114. doi: 10.1007/s00296-021-04841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, Costecalde C, Pijnenburg L, Fort J, Chatelus E, Sordet C. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243–e245. doi: 10.1016/S2665-9913(21)00039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fragoulis GE, Grigoropoulos I, Mavrea E, Arida A, Bournia VK, Evangelatos G, Fragiadaki K, Karamanakos A, Kravvariti E, Panopoulos S, Pappa M. Increased influenza vaccination rates in patients with autoimmune rheumatic diseases during the Covid-19 pandemic: a cross-sectional study. Rheumatol Int. 2021;41(5):895–902. doi: 10.1007/s00296-021-04817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zanettini C, Omar M, Dinalankara W, Imada EL, Colantuoni E, Parmigiani G, Marchionni L. Influenza vaccination and covid-19 mortality in the USA: an ecological study. Vaccines. 2021;9(5):427. doi: 10.3390/vaccines9050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smeele HT, Perez-Garcia LF, Grimminck K, Schoenmakers S, Mulders AG, Dolhain RJ. Systemic lupus erythematosus and COVID-19 during pregnancy. Lupus. 2021;30(7):1188–1191. doi: 10.1177/09612033211002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyadzhieva VV, Stoilov NR, Stoilov RM. Coronavirus disease 2019 (COVID-19) during pregnancy in patients with rheumatic diseases. Rheumatol Int. 2020;40(11):1753–1762. doi: 10.1007/s00296-020-04698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scioscia M, Praino E, Scioscia C. Rheumatic diseases during pregnancy and SARS-CoV-2: an appeal for medication adherence. Int J Gynecol Obstet. 2020;150(2):269–270. doi: 10.1002/ijgo.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwak-Kim J, Ota K, Sung N, Huang C, Alsubki L, Lee S, Han JW, Han A, Yang X, Saab W, Derbala Y. COVID-19 and immunomodulation treatment for women with reproductive failures. J Reprod Immunol. 2020;141:103168. doi: 10.1016/j.jri.2020.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramoni VL, Xoxi B, Beneventi F, Bellingeri C, Montecucco C, Spinillo A. Efficacy of a screening strategy for the detection of rheumatic diseases in early pregnancy during COVID-19 pandemic. Lupus. 2020;29(13):1821–1823. doi: 10.1177/0961203320952848. [DOI] [PubMed] [Google Scholar]
- 69.Naveen R, Sundaram TG, Agarwal V, Gupta L. Teleconsultation experience with the idiopathic inflammatory myopathies: a prospective observational cohort study during the COVID-19 pandemic. Rheumatol Int. 2021;41(1):67–76. doi: 10.1007/s00296-020-04737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bos WH, van Tubergen A, Vonkeman HE. Telemedicine for patients with rheumatic and musculoskeletal diseases during the COVID-19 pandemic; a positive experience in the Netherlands. Rheumatol Int. 2021;41(3):565–573. doi: 10.1007/s00296-020-04771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.So H, Szeto CC, Tam LS. Patient acceptance of using telemedicine for follow-up of lupus nephritis in the COVID-19 outbreak. Ann Rheum Dis. 2021;80(6):e97–e97. doi: 10.1136/annrheumdis-2020-218220. [DOI] [PubMed] [Google Scholar]
- 72.Kernder A, Morf H, Klemm P, et al. Digital rheumatology in the era of COVID-19: results of a national patient and physician survey. RMD Open. 2021;7:e001548. doi: 10.1136/rmdopen-2020-001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Howren A, Aviña-Zubieta JA, Rebić N, Dau H, Gastonguay L, Shojania K, Davidson E, De Vera MA. Virtual rheumatology appointments during the COVID-19 pandemic: an international survey of perspectives of patients with rheumatic diseases. Clin Rheumatol. 2020;39(11):3191–3193. doi: 10.1007/s10067-020-05338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.López-Medina C, Escudero A, Collantes-Estevez E. COVID-19 pandemic: an opportunity to assess the utility of telemedicine in patients with rheumatic diseases. Ann Rheum Dis. 2021;80(4):e50–e50. doi: 10.1136/annrheumdis-2020-218008. [DOI] [PubMed] [Google Scholar]
- 75.Chevallard M, Belloli L, Ughi N, Adinolfi A, Casu C, Di Cicco M, Filippini DA, Muscarà M, Schito E, Verduci E, Gentile MG. Use of telemedicine during the COVID-19 pandemic in patients with inflammatory arthritis: a retrospective study on feasibility and impact on patient-reported outcomes in a real-life setting. Rheumatol Int. 2021;41(7):1253–1261. doi: 10.1007/s00296-021-04863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer JL, Siddle HJ, Redmond AC, Alcacer-Pitarch B. Implementation of podiatry telephone appointments for people with rheumatic and musculoskeletal diseases. J Foot Ankle Res. 2021;14:4. doi: 10.1186/s13047-020-00441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morf H, Krusche M, Knitza J. Patient self-sampling: a cornerstone of future rheumatology care? Rheumatol Int. 2021;41(6):1187–1188. doi: 10.1007/s00296-021-04853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu Y, Brady K, Apilado R, et al. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J Pharm Biomed Anal. 2017;140:334–341. doi: 10.1016/j.jpba.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 79.Bilal S, Shanmugam VK. Enhancing rheumatology education during the COVID-19 pandemic. Rheumatol Int. 2021;41:503–508. doi: 10.1007/s00296-020-04769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dua AB, Kilian A, Grainger R, Fantus SA, Wallace ZS, Buttgereit F, Jonas BL. Challenges, collaboration, and innovation in rheumatology education during the COVID-19 pandemic: leveraging new ways to teach. Clin Rheumatol. 2020;39:3535–3541. doi: 10.1007/s10067-020-05449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmed S, Zimba O, Gasparyan AY. Moving towards online rheumatology education in the era of COVID-19. Clin Rheumatol. 2020;39:3215–3222. doi: 10.1007/s10067-020-05405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed S, Zimba O, Gasparyan AY. Diversifying online rheumatology education options in the era of COVID-19. Clin Rheumatol. 2020;39(12):3533–3534. doi: 10.1007/s10067-020-05468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shaw SCK. Hopelessness, helplessness, and resilience: the importance of safeguarding our trainees' mental wellbeing during the COVID-19 pandemic. Nurse Educ Pract. 2020;44:102780–102780. doi: 10.1016/j.nepr.2020.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doskaliuk B, Zimba O, Yatsyshyn R, Kovalenko V. Rheumatology in Ukraine. Rheumatol Int. 2020;40(2):175–182. doi: 10.1007/s00296-019-04504-4. [DOI] [PubMed] [Google Scholar]
- 85.Najm A, Alunno A, Sivera F, Ramiro S, Haines C. Working Group on Training in Rheumatology across Europe. Strategies for the assessment of competences during rheumatology training across Europe: results of a qualitative study. RMD Open. 2020;6(2):e001183. doi: 10.1136/rmdopen-2020-001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fragoulis GE, Evangelatos G, Arida A, et al. Treatment adherence of patients with systemic rheumatic diseases in COVID-19 pandemic. Ann Rheum Dis. 2020;80(4):e60–e60. doi: 10.1136/annrheumdis-2020-217935. [DOI] [PubMed] [Google Scholar]
- 87.Khabbazi A, Kavandi H, Paribanaem R, et al. Adherence to medication in patients with rheumatic diseases during COVID-19 pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218756. [DOI] [PubMed] [Google Scholar]
- 88.Michaud K, Wipfler K, Shaw Y, Simon TA, Cornish A, England BR, Ogdie A, Katz P. Experiences of patients with rheumatic diseases in the United States during early days of the COVID-19 pandemic. ACR Open Rheumatol. 2020;2(6):335–343. doi: 10.1002/acr2.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pineda-Sic RA, Galarza-Delgado DA, Serna-Peña G, Castillo-Torres SA, Flores-Alvarado DE, Esquivel-Valerio JA, Hernández-Galarza IDJ. Treatment adherence behaviours in rheumatic diseases during COVID-19 pandemic: a Latin American experience. Ann Rheum Dis. 2021;80(6):e85–e85. doi: 10.1136/annrheumdis-2020-218198. [DOI] [PubMed] [Google Scholar]
- 90.Durcan G, Barut K, Haslak F, Doktur H, Yildiz M, Adrovic A, Sahin S, Kasapcopur O. Psychosocial and clinical effects of the COVID-19 pandemic in patients with childhood rheumatic diseases and their parents. Rheumatol Int. 2021;41(3):575–583. doi: 10.1007/s00296-021-04790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhatia A, Manish KC, Gupta L. Increased risk of mental health disorders in patients with RA during the COVID-19 pandemic: a possible surge and solutions. Rheumatol Int. 2021;41:843–850. doi: 10.1007/s00296-021-04829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matcham F, Ali S, Irving K, et al. Are depression and anxiety associated with disease activity in rheumatoid arthritis? A prospective study BMC prospective study. BMC Musculoskelet Disord. 2016;17:155. doi: 10.1186/s12891-016-1011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]