Highlights
-
•
AGRICOH analyzed cancer incidence in >248,000 agricultural workers from 6 countries.
-
•
Cancer overall occurred less in agricultural workers than in the general population.
-
•
An excess was found for multiple myeloma, melanoma of the skin, and prostate cancer.
-
•
Direction of risk was largely consistent across cohorts with a few deviations.
-
•
A large deficit of larynx and lung cancers was observed in nearly all cohorts.
Keywords: Agriculture, Farmers, Occupational health, Cohort, Consortium, Cancer incidence
Abbreviations: AGRICOH, International consortium of agricultural cohort studies; AGRICAN, French Agriculture and Cancer cohort study; AHS, Agricultural Health Study; CI, confidence interval; CLL, chronic lymphocytic leukaemia; CNAP, Cancer in the Norwegian Agricultural Population; DDT, Dichlorodiphenyltrichloroethane; ICD, International Classification of Diseases; KMCC, Korean Multi-Center Cancer; MESA, Marshfield Epidemiologic Study Area Farm; NHL, non-Hodgkin lymphoma; SEER, the Surveillance Epidemiology and End Results; SIR, standardized incidence ratio; SLL, small lymphocytic lymphoma; SUS, Danish Sund Stald Study; US, the United States of America; UVR, ultraviolet radiation
Abstract
Background
Agricultural work can expose workers to potentially hazardous agents including known and suspected carcinogens. This study aimed to evaluate cancer incidence in male and female agricultural workers in an international consortium, AGRICOH, relative to their respective general populations.
Methods
The analysis included eight cohorts that were linked to their respective cancer registries: France (AGRICAN: n = 128,101), the US (AHS: n = 51,165, MESA: n = 2,177), Norway (CNAP: n = 43,834), Australia (2 cohorts combined, Australian Pesticide Exposed Workers: n = 12,215 and Victorian Grain Farmers: n = 919), Republic of Korea (KMCC: n = 8,432), and Denmark (SUS: n = 1,899). For various cancer sites and all cancers combined, standardized incidence ratios (SIR) and 95% confidence intervals (CIs) were calculated for each cohort using national or regional rates as reference rates and were combined by random-effects meta-analysis.
Results
During nearly 2,800,000 person-years, a total of 23,188 cancers were observed. Elevated risks were observed for melanoma of the skin (number of cohorts = 3, meta-SIR = 1.18, CI: 1.01–1.38) and multiple myeloma (n = 4, meta-SIR = 1.27, CI: 1.04–1.54) in women and prostate cancer (n = 6, meta-SIR = 1.06, CI: 1.01–1.12), compared to the general population. In contrast, a deficit was observed for the incidence of several cancers, including cancers of the bladder, breast (female), colorectum, esophagus, larynx, lung, and pancreas and all cancers combined (n = 7, meta-SIR for all cancers combined = 0.83, 95% CI: 0.77–0.90). The direction of risk was largely consistent across cohorts although we observed large between-cohort variations in SIR for cancers of the liver and lung in men and women, and stomach, colorectum, and skin in men.
Conclusion
The results suggest that agricultural workers have a lower risk of various cancers and an elevated risk of prostate cancer, multiple myeloma (female), and melanoma of skin (female) compared to the general population. Those differences and the between-cohort variations may be due to underlying differences in risk factors and warrant further investigation of agricultural exposures.
1. Introduction
Worldwide, an estimated 880 million people were employed in the agricultural sector in 2019 (ILO, 2020). Farmers and farm workers contribute to a large part of the world economy and food production while facing various occupational hazards, including outside work with prolonged exposure to ultraviolet radiation (UVR) and heat, dangerous machinery, and exposure to potentially hazardous agents, such as zoonotic pathogens, pesticides, endotoxin, solvents, metals, welding fumes, crystalline silica and diesel exhaust fumes (Nieuwenhuijsen et al., 1999, ILO, 2007, Coble et al., 2002, Radon, 2006). Some of these agents, such as UVR (IARC, 2012), arsenic, including arsenical insecticides (IARC, 2012), lindane (IARC, 2018), crystalline silica (IARC, 2012), and diesel exhaust (IARC, 2014) have been established as human carcinogens.
Individual studies comparing cancer incidence or mortality in agricultural workers with the general population have consistently shown a deficit for total cancer and some specific cancer types, such as esophagus, colon/colon and rectum, lung, female breast, and bladder cancers (Blair et al., 1992, Acquavella et al., 1998, Wang et al., 2002, Frost et al., 2011, Kachuri et al., 2017, Wiklund and Dich, 1994, Pukkala et al., 2009). Conversely, some studies have found an excess for cancers of the lip, prostate, and brain, leukaemia, Hodgkin disease, multiple myeloma, and melanoma of the skin (Blair et al., 1992, Acquavella et al., 1998, Frost et al., 2011, Kachuri et al., 2017, Pukkala et al., 2009). While these excesses could suggest a potential role of agricultural exposures in the development of these diseases, the presence or magnitude of the excess risk has been somewhat inconsistent across studies.
The reasons for the observed inconsistencies are not entirely clear but may partially reflect differences in exposure to various etiologic agents. Agricultural work is very heterogeneous, and tasks vary by the overall system of farming and employment practices, which may have an impact on individual exposures. Furthermore, exposure to pesticides, for instance, depends on several factors, including the type of agro-production, environmental conditions present, application methods, use of machinery and personal protective equipment, and local legislation. Additionally, differences in lifestyle-related cancer risk factors in the study population, disease classification, referent population applied, and follow-up periods examined may explain some of the differences observed across studies.
AGRICOH is an international consortium of prospective cohort studies of agricultural workers (https://agricoh.iarc.fr/) (Leon et al., 2011). One of the goals in its initiation was to facilitate the pooling of data for the purpose of exploring hypotheses with greater power than any one study, and to compare results across different exposure scenarios to help identify etiologic agents. The present study aimed to evaluate the overall patterns of cancer incidence in eight agricultural cohorts participating in AGRICOH, relative to their respective general populations.
2. Materials and methods
2.1. Study cohorts
The present analysis included the following eight AGRICOH cohort studies where cancer incidence data were collected through periodic linkage to cancer registries:
-
·
the French Agriculture and Cancer cohort study (AGRICAN (Leveque-Morlais et al., 2015),
-
·
the United States Agricultural Health Study (AHS (Alavanja et al., 1996),
-
·
the Cancer in the Norwegian Agricultural Population cohort (CNAP (Kristensen et al., 1996),
-
·
the Korean Multi-Center Cancer cohort (KMCC (Yoo et al., 2002),
-
·
the United States Marshfield Epidemiologic Study Area Farm cohort (MESA (Greenlee et al., 2005),
-
·
the Danish Sund Stald Study (SUS (Elholm et al., 2010),
-
·
the Australian Pesticide Exposed Workers cohort (MacFarlane et al., 2010), and
-
·
the Victorian Grain Farmers cohort (MacFarlane et al., 2008).
The analysis included male and female participants aged ≥ 15 years at enrolment who worked in agriculture, including active or retired farmers and farm workers, farm holders, plant nursery workers, student farmers, and licenced pesticide applicators. For the KMCC cohort, a general population cohort, and for the AGRICAN cohort, which includes non-agricultural workers, the present analysis was restricted to agricultural workers. The Australian Pesticide Exposed Workers cohort included farmers and other types of workers, such as ornamental gardeners and sporting grounds maintenance workers (MacFarlane et al., 2010), but we were unable to exclude non-farmers from the Pesticide Exposed Workers cohort due to lack of access to the individual-level data at the time of meta-analysis. The eligibility criteria for each study and inclusion/exclusion criteria applied for the analysis are presented in more detail in Table 1.
Table 1.
Characteristics of AGRICOH cohorts included in the analysis.
| AGRICAN | AHS | CNAP | Australian cohorts1 | KMCC | MESA | SUS | ||
|---|---|---|---|---|---|---|---|---|
| Number of subjects | 128,101 | 51,165 | 43,834 | 13,134 | 8,432 | 2,177 | 1,899 | |
| Geography | Doubs, Gironde, Isère, Loire Atlantique, Manche, Bas Rhin, Haut Rhin, Somme, Tarn, Vendée, Cote d’Or, France | Iowa & North Carolina, USA | Norway | New South Wales & Victoria, Australia | Haman County & Uljin County, Kyungnam Province; Chungjui City & Pohang City, Chungbuk Province, South Korea | Central & North Wisconsin, USA | Denmark | |
| Enrolment | 2005–2007 | 1993–1997 | 1969, 1979, 1989 | 1960 s-1980 s (Pesticide Exposed Workers) 1996–1998 (Victorian Grain Farmers) |
1993–2005 | 1991–2010 | 1992–1994 | |
| Cancer follow-up | enrolment to 2012 | enrolment to 2012 | 1991–2011 | 1983 to 2002 | enrolment to 2012 | enrolment to 2009 | enrolment to 2014 | |
| Gender, % (n) | Males | 56.2 (71,944) | 97 (49,829) | 94.01 (41,208) | 91.7 (12,050) | 44.2 (3,727) | 88.0 (1,915) | 88.2 (1,675) |
| Females | 43.8 (56,157) | 2.6 (1,336) | 6.09 (2,626) | 8.3 (1,084) | 55.8 (4,705) | 12.0 (262) | 11.8 (224) | |
| No. person-years | Males | 390,455.13 | 779,725.70 | 823,114 | 227,532.5 | 40,682 | 29,288 | 34,290 |
| Females | 315,012.01 | 20,939.13 | 53,041 | NA | 56,952 | 3,212 | 4,545 | |
| Age at enrolment, mean | 64.4 | 46.8 | 43.5 | 33.3 | 58 | 38.2 | 19.2 | |
| Age at enrolment, range | 20–105 years | 12–93 years | NA | NA | 19–91 years | 15–99 years | 17–49 years | |
| Mean duration of follow-up in years | 5.5 | 15.7 | 20 | 18.9 | 11.6 | 15.6 | 20.4 | |
| Mean year of entry | 2006 | 1995 | 1991 | 1983 | 1999 | 1992 | 1993 | |
| Eligibility criteria | Member of MSA2 aged ≥ 18 years, agricultural worker for more than 3 years & living in defined regions in 2005 | All pesticide applicators requesting pesticide licenses | All Norwegian farm holders in at least one of the Agricultural Censuses in 1969–1989 | Pesticide exposed workers who were employed during 1960 s-1980 s and had participated in a biomonitoring and occupational health surveillance program run by the New South Wales and Victoria State governments Members of the Victorian Farmers' Federation from randomly selected branches |
Volunteer for national cancer screening survey from 1993, aged > 35 years in defined area | Farmers identified in selected listings in the state | All farming school attendants from February 1992 until February 1994 at level 1a (i.e., 2nd stay at a farming school) | |
| Inclusion/exclusion for the present analysis | Inclusion: Members of MSA who worked for more than 3 years in a farm (active & retired professional farmers, co-farmers, agricultural workers) Exclusion: Cote d’Or region3, known history of cancer |
Inclusion: Private pesticide applicators Exclusion: known history of cancer |
Inclusion: Farm holders who are still active according to the 1999 or 2010 Census & with >0.5 ha of agricultural land or 0.1 ha of horticultural acreage Exclusion: known history of cancer |
Same as above | Inclusion: KMCC participants who responded yes to “Have you ever been employed in agriculture or livestock” Exclusion: known history of cancer |
Inclusion: A place from which ≥ $1,000 of agricultural products were produced & sold, or normally would have been sold, during the census year | Inclusion: Farming school attendants who completed compulsory farming training program | |
| Type of productions, % (n) | Livestock only | 13.2 (16,937) | 2 (998) | NA | NA | 0 (0) | ||
| Crops only | 21.2 (27,216) | 32 (16,584) | 4.27 (81) | |||||
| Both | 61.4 (78,673) | 56 (28,552) | 92.8 (1,763) | |||||
| At least crops | 100 (919)5 | 97.1 (1,844) | ||||||
| At least livestock | 74 (101,987)4 | 92.8 (1,763) | ||||||
| Missing | 4.1 (5,275) | 10 (5,061) | 0 (4) | |||||
| Education, % (n) | No formal education | 21.3 (27,355) | 0 (0) | NA | 0 (0) | 24.8 (2,090) | NA | 0 (0) |
| Primary school; 1–6 years | 30.5 (39,058) | 4.4 (2,248) | 0.8 (7)5,6 | 63.9 (5,391) | 0 (0) | |||
| Secondary school; 7–11 years | 35.3 (45,242) | 51.4 (26,273) | 74.2 (682)5 | 4.5 (376) | 0 (0) | |||
| Tertiary education; >11 years | 6.5 (8,274) | 39.7 (20,307) | 24.9 (229)5 | 6.3 (533) | 100 (1,899) | |||
| Missing | 6.4 (8,172) | 4.6 (2,337) | 0.1 (1)5 | 0.5 (42) | 0 (0) | |||
| Smoking status at enrolment, % (n) | Current | 8.7 (11,114) | 15.8 (8,093) | NA | 11.8 (108)5 | 29.3(2,474) | NA | 29.7 (563) |
| Former | 24.2 (31,012) | 30.2 (15,447) | 31.2 (287)5 | 11.0 (923) | 4.5 (86) | |||
| Never | 60.6 (77,591) | 51.6 (26,377) | 56.7 (521)5 | 59.5 (5,018) | 65.8 (1,250) | |||
| Missing | 6.5 (8,384) | 2.4 (1,248) | 0.3 (3)5 | 0.2 (17) | 0 (0) | |||
| Alcohol consumption at enrolment, % (n) | Current | 74.1 (94,936)7 | 61.2 (31,323) | NA | 67.1 (617)5 | 36.2 (3,053) | NA | NA |
| Former | NA | 6.5 (60)5 | 5.6 (474) | |||||
| Never | 18.7 (23,930) | 31.9 (16,339) | 26.0 (239)5 | 57.8 (4,869) | ||||
| Missing | 7.2 (9,235) | 6.9 (3,503) | 0.3 (3)5 | 0.4 (36) | ||||
Abbreviation. n: number, NA: not available, AGRICAN: the French Agriculture and Cancer cohort study, AHS: Agricultural Health Study, CI: confidence interval, CNAP: Cancer in the Norwegian Agricultural Population, KMCC: Korean Multi-Center Cancer, MESA: Marshfield Epidemiologic Study Area Farm, SIR: standardized incidence ratio, SUS: Danish Sund Stald Study.
Pesticide Exposed Workers cohort and Victorian Farmers cohort combined.
MSA is a specialized health insurance program for agricultural activities in France.
The Cote d’Or area was excluded because the registry only captured haematological, gastrointestinal, and gynaecological cancers.
Based on the total cohort of 137,821 subjects (30).
Information from the Victorian Grain Farmers cohort only.
No formal education and primary school combined.
Ever alcohol users.
2.2. Cohort follow-up
In all cohorts except for CNAP and the Australian Pesticide Exposed Workers cohort, the subjects were followed from the time of enrolment, and follow-up duration (person-years) for each participant was calculated as the time between enrolment into the study and censoring, which was the first of the following events: diagnoses of primary cancer (incidence), death, migration, loss to follow-up, or end of the follow-up period. Number of person-years was calculated by sex, age, and calendar year (5-year interval). In CNAP, although the enrolment began in 1969, cancer follow-up for the present analysis was set to start from 1 January 1991 which is closer to the years when follow-up began in most other cohorts. In the Australian Pesticide Exposed Workers cohort, the follow-up ended in 2002 because more recent cancer registration data was not available for the present analysis. For the AHS, AGRICAN, CNAP and KMCC cohorts, individuals diagnosed with a cancer prior to the start of follow-up were excluded.
In each study cohort, primary incident invasive cancers (excluding non-melanoma skin cancer) were identified by linkage to cancer registries. Benign and in situ neoplasms were excluded from consideration in the present analysis. An exception was made for bladder cancer, for which carcinoma in situ also contributed to the incidence rates in all cohorts except KMCC and the Australian cohorts because carcinoma in situ was routinely recorded, registered and counted along with invasive tumours and contributed to cancer statistics. The 10th revision of the International Classification of Diseases (ICD-10) was used to categorise cancers as in the Cancer Incidence in Five Continents (CI5) (Bray et al., 2017) (Supplementary Table 1).
Data in a specified aggregated format were transferred from the AGRICAN, CNAP, KMCC, and MESA cohorts to the IARC. Individual-level data without personal identifiable information were transferred from AHS to the IARC for the present analysis. For the SUS and the combined Australian cohorts, the analyses were conducted on-site. The data from the two Australian cohorts combined have been published elsewhere (MacFarlane et al., 2010) and were included in the meta-analysis.
2.3. Statistical analysis
Standardised incidence ratios (SIR) were estimated for each cancer type (Supplementary Table 1) with at least three cases and for all cancers combined for males and females, separately, and combined. To calculate SIRs, expected numbers of cancer cases were calculated by multiplying the age- (5-year interval), calendar year- (5-year interval), and sex-specific person-years of the individual cohort by the respective incidence rates from the reference population. For the AHS cohort which included race information, we applied race-specific rates from 9 registries from the Surveillance, Epidemiology, and End Results (SEER) program which were available from the CI5 Time Trends (CI5plus) database (Bray et al., 2017). For the MESA cohort, the rates of the US white population from CI5plus were used as reference. For the AGRICAN and KMCC cohorts, the average of the regional rates from the IARC CI5plus was used as reference because the national rates were not available in CI5plus. For the CNAP and SUS cohorts, the national rates were applied. SIRs were then calculated as the number of observed cases divided by expected number of cases. Corresponding 95% confidence intervals (CIs) were estimated using Fisher’s exact method when the number of observed cases was less than 15 (Dean and May, 2020, Breslow and Day, 1987) and Vandenbroucke method (Boyle and Parkin, 1991) otherwise. We pooled the cohort-specific adjusted SIRs for cancer sites with at least three cases in at least two cohorts using random-effects models which included cohort-specific log-SIRs and corresponding 95% CIs (STATA command “metan”). Meta-SIRs were estimated by sex and overall (additionally adjusted for sex) with a null hypothesis being a meta-SIR of one. I2 was calculated as a measure of heterogeneity across cohorts. P values less than 0.05 were considered as statistically significant.
Sensitivity analyses were conducted by excluding the Australian cohort which started earlier (1983) and halted earlier (2002) compared to the other cohorts (start: 1991–2005, end: 2009–2014) and included non-agricultural workers and by excluding the KMCC cohort which showed somewhat different patterns compared to the other cohorts.
The analyses were performed with Stata version 14.0 (StataCorp LP, College Station, TX).
3. Results
3.1. Cohort characteristic
A total of 182,348 males and 66,394 females were included in the analysis (Table 1). The cohorts predominantly consisted of men except for KMCC and AGRICAN, where female farmers and agricultural workers constituted a sizeable fraction of the cohort (KMCC: 56%, AGRICAN: 44%). The cohorts differed in age composition with extremes represented by the SUS cohort, which consisted of student farmers (mean age at enrolment: 19 years), and the AGRICAN cohort where about half of the members were retirees at enrolment (mean age at enrolment: 64 years). >80% of the farmers were engaged in crop farming in the AGRICAN, AHS, SUS, and the Victorian Grain Farmers cohorts. Livestock farming was also common in AGRICAN (75%), AHS (58%), CNAP (74%, based on the whole cohort) (El-Zaemey et al., 2019) and SUS (71%). For the KMCC, MESA and Pesticide Exposed Workers cohorts, information on the types of farming was unavailable. The SUS and AHS cohorts had a higher proportion of workers who completed tertiary education (100% and 40%, respectively) than other cohorts. Of the cohorts with information on smoking status (AGRICAN, AHS, KMCC, Victorian Grain Farmers, SUS), the prevalence of ever smokers was higher in the AHS (46%) and the Victorian Grain Farmers (43%) cohorts, and the prevalence of current smokers was higher in the KMCC (29%) and SUS (30%) cohorts compared to the other cohorts. Among the cohorts with information on alcohol consumption, the proportion of farmers who reported ever alcohol drinking was lowest in the KMCC cohort (42%) and highest in the AGRICAN (74%) and the Victorian Grain Farmers (74%) cohorts.
3.2. Standardized incidence ratios (SIRs)
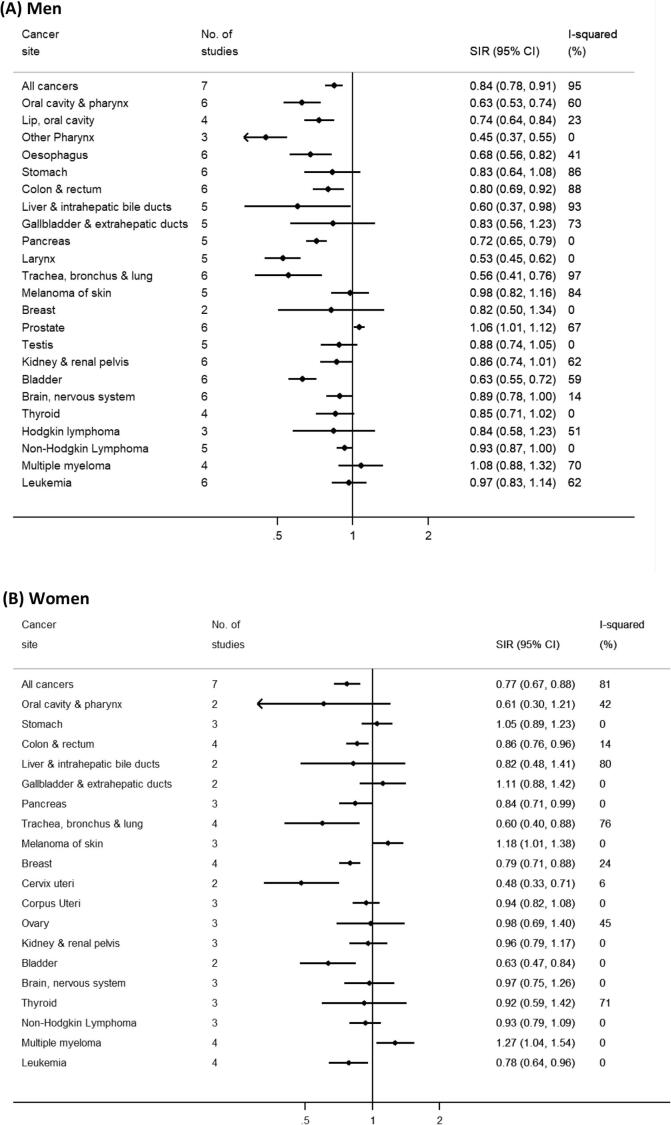
Overall. During nearly 2,800,000 person-years, a total of 23,188 cancers (excluding non-melanoma skin cancer) were newly diagnosed. The meta-SIRs for overall cancer incidence based on eight cohorts of men and women were 0.84 (95% CI = 0.78–0.91, I2 = 95%) and 0.77 (95% CI = 0.67–0.88, I2 = 81%), respectively (Fig. 1) and 0.83 (95% CI = 0.77–0.90, I2 = 95%) for both sexes combined (Supplementary Fig. 1). The estimated SIR for each cohort was lower than 1.0 in all cohorts of men and women except for KMCC men (SIR = 1.04, 95% CI = 0.96–1.12) and SUS women (SIR = 2.17, 95% CI = 0.70–5.06) (Table 2, Table 3).
Fig. 1.
Forest plot of meta-standardized incidence ratios (SIRs) in men and in women, Legend: The number of studies, meta-SIRs, corresponding 95% confidence intervals, and I2 for all cancers combined and each cancer type are displayed for men (A) and women (B), separately.
Table 2.
Overall and cancer site-specific incidence ratios (SIRs) in males.
| AGRICAN |
AHS |
CNAP |
Australian cohorts |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer site | N | SIR | (95% CI) | N | SIR | (95% CI) | N | SIR | (95% CI) | N | SIR | (95% CI) |
| All cancers | 5938 | 0.86 | (0.84–0.88) | 6940 | 0.90 | (0.88–0.92) | 4914 | 0.75 | (0.73–0.77) | 819 | 0.81 | (0.76–0.87) |
| Oral cavity & pharynx1 | 164 | 0.60 | (0.51–0.69) | 168 | 0.68 | (0.58–0.79) | 84 | 0.47 | (0.38–0.58) | 50 | 0.83 | (0.63–1.09) |
| Lip, oral cavity | 99 | 0.77 | (0.63–0.93) | 128 | 0.80 | (0.67–0.95) | 69 | 0.61 | (0.47–0.76) | |||
| Nasopharynx | 2 | 7 | 0.83 | (0.33–1.70) | ||||||||
| Other Pharynx | 63 | 0.45 | (0.34–0.57) | 33 | 0.42 | (0.29–0.58) | ||||||
| Oesophagus | 113 | 0.76 | (0.63–0.91) | 88 | 0.72 | (0.58–0.88) | 37 | 0.48 | (0.34–0.65) | 7 | 0.44 | (0.18–0.91) |
| Stomach | 161 | 0.87 | (0.74–1.01) | 100 | 0.77 | (0.63–0.93) | 107 | 0.68 | (0.56–0.81) | 19 | 0.60 | (0.38–0.94) |
| Colorectum | 726 | 0.86 | (0.79–0.92) | 715 | 0.99 | (0.92–1.06) | 594 | 0.70 | (0.65–0.76) | 124 | 0.79 | (0.66–0.94) |
| Liver & intrahepatic bile ducts | 175 | 0.68 | (0.58–0.78) | 50 | 0.38 | (0.28–0.49) | 16 | 0.39 | (0.22–0.60) | 6 | 0.54 | (0.20–1.18) |
| Gallbladder & extrahepatic ducts | 34 | 0.70 | (0.48–0.96) | 26 | 0.73 | (0.48–1.04) | 20 | 0.68 | (0.41–1.01) | 3 | 0.50 | (0.10–1.46) |
| Pancreas | 133 | 0.71 | (0.59–0.83) | 140 | 0.74 | (0.62–0.87) | 102 | 0.66 | (0.54–0.80) | 18 | 0.86 | (0.54–1.36) |
| Larynx | 40 | 0.45 | (0.32–0.60) | 57 | 0.58 | (0.44–0.75) | 30 | 0.46 | (0.31–0.63) | 11 | 0.66 | (0.33–1.18) |
| Trachea, bronchus & lung2 | 453 | 0.48 | (0.44–0.53) | 670 | 0.63 | (0.58–0.68) | 317 | 0.41 | (0.37–0.46) | 73 | 0.52 | (0.41–0.65) |
| Melanoma of skin | 145 | 0.89 | (0.75–1.04) | 304 | 0.78 | (0.69–0.87) | 313 | 0.93 | (0.83–1.03) | 122 | 0.97 | (0.81–1.15) |
| Breast | 14 | 0.92 | (0.50–1.54) | 5 | 0.58 | (0.19–1.35) | ||||||
| Prostate | 2225 | 1.05 | (1.01–1.09) | 2790 | 1.11 | (1.07–1.15) | 1906 | 1.05 | (1.00–1.09) | 189 | 1.00 | (0.87–1.15) |
| Testis | 12 | 0.66 | (0.34–1.15) | 42 | 0.90 | (0.65–1.19) | 69 | 0.95 | (0.74–1.19) | 10 | 0.59 | (0.28–1.09) |
| Kidney & renal pelvis3 | 220 | 0.97 | (0.84–1.10) | 248 | 0.96 | (0.84–1.08) | 163 | 0.71 | (0.61–0.83) | 27 | 0.86 | (0.59–1.25) |
| Ureter | 8 | 0.50 | (0.21–0.91) | |||||||||
| Bladder4 | 208 | 0.56 | (0.48–0.64) | 336 | 0.66 | (0.59–0.73) | 255 | 0.61 | (0.54–0.69) | 18 | 0.48 | (0.30–0.77) |
| Brain, nervous system | 81 | 1.10 | (0.87–1.35) | 79 | 0.77 | (0.61–0.95) | 174 | 0.86 | (0.73–0.99) | 16 | 0.80 | (0.49–1.31) |
| Thyroid | 26 | 0.76 | (0.50–1.08) | 70 | 0.92 | (0.71–1.14) | 27 | 0.83 | (0.55–1.18) | |||
| Hodgkin lymphoma | 19 | 1.23 | (0.74–1.85) | 22 | 0.71 | (0.44–1.04) | 16 | 0.68 | (0.39–1.05) | |||
| Non-Hodgkin Lymphoma | 216 | 0.95 | (0.83–1.09) | 310 | 0.93 | (0.83–1.03) | 211 | 0.92 | (0.80–1.05) | 37 | 0.89 | (0.65–1.23) |
| Multiple myeloma | 155 | 1.33 | (1.13–1.55) | 121 | 1.08 | (0.90–1.28) | 95 | 0.89 | (0.72–1.08) | 11 | 0.92 | (0.46–1.65) |
| Leukemia | 193 | 0.99 | (0.85–1.13) | 225 | 0.99 | (0.86–1.12) | 146 | 0.79 | (0.67–0.92) | 25 | 0.88 | (0.60–1.31) |
| KMCC |
MESA |
SUS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer site | N | SIR | (95% CI) | N | SIR | (95% CI) | N | SIR | (95% CI) |
| All cancers | 631 | 1.04 | (0.96–1.12) | 190 | 0.78 | (0.67–0.89) | 17 | 0.51 | (0.3–0.79) |
| Oral cavity & pharynx1 | 8 | 0.73 | (0.31–1.43) | 4 | 0.53 | (0.15–1.37) | |||
| Lip, oral cavity | 2 | 3 | 0.60 | (0.12–1.76) | |||||
| Nasopharynx | 2 | ||||||||
| Other Pharynx | 4 | 0.93 | (0.25–2.37) | ||||||
| Oesophagus | 15 | 0.93 | (0.52–1.46) | 3 | 0.83 | (0.17–2.41) | |||
| Stomach | 162 | 1.30 | (1.11–1.51) | 3 | 0.73 | (0.15–2.13) | |||
| Colorectum | 64 | 0.75 | (0.58–0.95) | 12 | 0.49 | (0.25–0.85) | |||
| Liver & intrahepatic bile ducts | 93 | 1.29 | (1.04–1.57) | 2 | |||||
| Gallbladder & extrahepatic ducts | 32 | 1.57 | (1.07–2.16) | 1 | |||||
| Pancreas | 16 | 0.85 | (0.48–1.32) | 2 | 1 | ||||
| Larynx | 7 | 0.77 | (0.31–1.60) | ||||||
| Trachea, bronchus & lung | 148 | 1.29 | (1.09–1.50) | 7 | 0.20 | (0.08–0.42) | |||
| Melanoma of skin | 25 | 2.09 | (1.35–2.99) | 1 | |||||
| Breast | |||||||||
| Prostate | 34 | 0.70 | (0.48–0.95) | 97 | 1.25 | (1.02–1.52) | |||
| Testis | 5 | 1.01 | (0.33–2.35) | ||||||
| Kidney & renal pelvis | 6 | 0.46 | (0.17–1.01) | 8 | 1.01 | (0.44–1.99) | |||
| Ureter | 2 | ||||||||
| Bladder | 25 | 1.07 | (0.69–1.54) | 11 | 0.67 | (0.34–1.21) | |||
| Brain, nervous system | 4 | 1.11 | (0.30–2.84) | 3 | 0.87 | (0.18–2.54) | 1 | ||
| Thyroid | 4 | 0.50 | (0.14–1.27) | 2 | 1 | ||||
| Hodgkin lymphoma | 2 | ||||||||
| Non-Hodgkin Lymphoma | 7 | 0.69 | (0.28–1.42) | 2 | 2 | ||||
| Multiple myeloma | 1 | 1 | |||||||
| Leukemia | 12 | 2.21 | (1.14–3.86) | 8 | 1.12 | (0.48–2.21) | |||
Abbreviations: AGRICAN: the French Agriculture and Cancer cohort study, AHS: Agricultural Health Study, CI: confidence interval, CNAP: Cancer in the Norwegian Agricultural Population, KMCC: Korean Multi-Center Cancer, MESA: Marshfield Epidemiologic Study Area Farm, SIR: standardized incidence ratio, SUS: Danish Sund Stald Study.
Cancers of oral cavity and pharynx were combined (C00-C14).
Cancer of trachea is not included for the AHS cohort.
Cancer of renal pelvis is not included for the AHS cohort.
Bladder includes bladder and Ureter for the CNAP cohort.
Table 3.
Overall and cancer site-specific incidence ratios (SIRs) in females.
| AGRICAN |
AHS |
CNAP |
KMCC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer site | N | SIR | (95% CI) | N | SIR | (95% CI) | N | SIR | (95% CI) | N | SIR | (95% CI) |
| All cancers | 2968 | 0.88 | (0.85–0.91) | 147 | 0.87 | (0.74–1.02) | 228 | 0.66 | (0.58–0.75) | 328 | 0.79 | (0.71–0.88) |
| Oral cavity & pharynx1 | 28 | 0.49 | (0.33–0.69) | 1 | 2 | 4 | 1.08 | (0.29–2.77) | ||||
| Lip, oral cavity | 25 | 0.62 | (0.40–0.89) | 2 | 2 | |||||||
| Oesophagus | 21 | 0.75 | (0.46–1.11) | 1 | 2 | 1 | ||||||
| Stomach | 85 | 1.04 | (0.83–1.27) | 4 | 0.83 | (0.23–2.12) | 66 | 1.07 | (0.83–1.34) | |||
| Colorectum | 439 | 0.85 | (0.78–0.94) | 19 | 1.21 | (0.73–1.82) | 29 | 0.69 | (0.46–0.97) | 54 | 0.86 | (0.64–1.10) |
| Liver & intrahepatic bile ducts | 34 | 0.62 | (0.43–0.85) | 1 | 34 | 1.08 | (0.75–1.47) | |||||
| Gallbladder & extrahepatic ducts | 43 | 1.03 | (0.75–1.36) | 2 | 1 | 25 | 1.28 | (0.82–1.82) | ||||
| Pancreas | 124 | 0.86 | (0.72–1.02) | 2 | 3 | 0.41 | (0.09–1.21) | 10 | 0.65 | (0.31–1.19) | ||
| Trachea, bronchus & lung2 | 104 | 0.52 | (0.42–0.62) | 14 | 0.60 | (0.33–1.02) | 11 | 0.36 | (0.18–0.65) | 38 | 0.95 | (0.67–1.28) |
| Melanoma of skin | 135 | 1.21 | (1.02–1.42) | 8 | 1.18 | (0.51–2.32) | 19 | 0.96 | (0.58–1.44) | 1 | ||
| Breast | 790 | 0.81 | (0.76–0.87) | 48 | 0.89 | (0.66–1.17) | 74 | 0.75 | (0.59–0.93) | 23 | 0.56 | (0.36–0.81) |
| Cervix uteri | 24 | 0.53 | (0.34–0.77) | 2 | 7 | 0.33 | (0.13–0.68) | |||||
| Corpus Uteri | 170 | 0.93 | (0.80–1.08) | 23 | 1.05 | (0.66–1.52) | 3 | 0.49 | (0.10–1.43) | |||
| Ovary | 102 | 0.88 | (0.72–1.06) | 10 | 1.71 | (0.82–3.14) | 13 | 0.80 | (0.43–1.37) | 2 | ||
| Kidney & renal pelvis3 | 93 | 0.96 | (0.78–1.17) | 4 | 1.08 | (0.29–2.77) | 1 | 5 | 0.78 | (0.25–1.82) | ||
| Bladder4 | 45 | 0.65 | (0.47–0.85) | 3 | 0.40 | (0.08–1.15) | 1 | |||||
| Brain, nervous system | 40 | 0.95 | (0.68–1.27) | 1 | 16 | 1.06 | (0.61–1.65) | 3 | 0.76 | (0.16–2.23) | ||
| Thyroid | 72 | 1.20 | (0.94–1.49) | 4 | 0.81 | (0.22–2.08) | 1 | 36 | 0.70 | (0.49–0.95) | ||
| Hodgkin lymphoma | 8 | 1.06 | (0.45–1.92) | |||||||||
| Non-Hodgkin lymphoma | 133 | 0.92 | (0.77–1.09) | 6 | 0.89 | (0.32–1.93) | 2 | 9 | 1.07 | (0.49–2.03) | ||
| Multiple myeloma | 93 | 1.27 | (1.02–1.54) | 4 | 1.89 | (0.51–4.83) | 3 | 0.72 | (0.15–2.11) | 4 | 1.24 | (0.34–3.17) |
| Leukaemia | 82 | 0.75 | (0.60–0.93) | 3 | 0.77 | (0.16–2.26) | 5 | 0.68 | (0.22–1.59) | 7 | 1.46 | (0.58–3.00) |
| Australian cohorts |
MESA |
SUS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | SIR | (95% CI) | N | SIR | (95% CI) | N | SIR | (95% CI) | |
| All cancers | 53 | 0.62 | (0.48–0.82) | 10 | 0.46 | (0.22–0.84) | 5 | 2.17 | (0.70–5.06) |
Abbreviations: AGRICAN: the French Agriculture and Cancer cohort study, AHS: Agricultural Health Study, CI: confidence interval, CNAP: Cancer in the Norwegian Agricultural Population, KMCC: Korean Multi-Center Cancer, SIR: standardized incidence ratio, SUS: Danish Sund Stald Study.
Cancers of oral cavity and pharynx were combined (C00-C14).
Cancer of trachea is not included for the AHS cohort.
Cancer of renal pelvis is not included for the AHS cohort.
Bladder includes bladder and Ureter in CNAP.
Digestive organs. An incidence deficit was consistently observed for cancers of the esophagus in men (number of studies = 6; meta-SIR = 0.68, 95% CI = 0.56–0.82, I2 = 41%, Fig. 1A) and pancreas in both men and women (n = 5; meta-SIR for men and women combined = 0.74, 95% CI = 0.68–0.81, I2 = 0%, Supplementary Fig. 1). Although the SIRs varied across cohorts, none of the SIRs for colorectal cancer exceeded 1.0 in men (n = 6; meta-SIR = 0.80, 95% CI = 0.69–0.92, I2 = 88%). In men, the meta-SIRs were also lower than one for cancers of the liver (n = 5; meta-SIR = 0.60, 95% CI = 0.37–0.98, I2 = 93%), stomach (n = 6; meta-SIR = 0.83, 95% CI = 0.64–1.08, I2 = 86%) and gallbladder (n = 5; meta-SIR = 0.83, 95% CI = 0.56–1.23, I2 = 73%), but an excess incidence for these cancers was observed in KMCC (Table 2). Hence, these meta-SIRs became lower after excluding the men of KMCC (Supplementary Fig. 2B).
Respiratory system. The incidence of larynx cancer in men was substantially lower than that in the general population (n = 5; meta-SIR = 0.53, 95% CI = 0.45–0.62, I2 = 0%, Fig. 1A). The lung cancer incidence overall was also strikingly lower than in the general population for both men (n = 6; meta-SIR = 0.56, 95% CI = 0.41–0.76) and women (n = 4; meta-SIR = 0.60, 95% CI = 0.40–0.88) with large between-cohort variations (men: I2 = 97%, women: I2 = 76%) (Fig. 1). The I2 statistic reduced to 0% (Supplementary Fig. 2B) in women after excluding the women of KMCC with an SIR close to the null (SIR = 0.95, 95% CI = 0.67–1.28, Table 3) whereas in men it reduced only slightly (I2 = 92%, Supplementary Fig. 2B) after excluding the men of KMCC with an SIR of 1.29 (95% CI = 1.09–1.50, Table 2).
Female and male reproductive systems and breast. There was an excess of prostate cancer on the basis of six cohorts (n = 6, meta-SIR = 1.06, 95% CI = 1.01–1.12, I2 = 67%, Fig. 1A). The SIR did not exceed 1.0 in KMCC (SIR = 0.70, 95% CI = 0.48–0.95) and the combined Australian cohort (SIR = 1.00, 95% CI = 0.87–1.15) (Table 2). In women, a deficit was observed for breast cancer (n = 4; meta-SIR = 0.79, 95% CI = 0.71–0.88, I2 = 24%) and cervical cancer (n = 2; meta-SIR = 0.48, 95% CI: 0.33–0.71, I2 = 6%), but no significant deficit was observed for cancers of corpus uteri (n = 3; meta-SIR = 0.94, 95% CI = 0.82–1.08, I2 = 0%) and ovary (n = 3; meta-SIR = 0.98, 95% CI = 0.69–1.40, I2 = 45%). The SIR for ovarian cancer was highest in AHS based on 10 cases (SIR = 1.71, 95% CI: 0.82–3.14, Table 3).
Urinary system and others. The risk of kidney cancer in men was lower than in the general population (n = 6; meta-SIR = 0.86, 95% CI = 0.74–1.01, I2 = 62%) (Fig. 1A). In women the meta-SIR based on three cohorts was close to 1.0 (n = 3: meta-SIR = 0.96, 95% CI = 0.79–1.17, I2 = 0%) (Fig. 1B). A deficit of bladder cancer incidence was observed in both men and women (n = 6; meta-SIR for men and women combined = 0.62, 95% CI = 0.56–0.68, I2 = 35%, Supplementary Fig. 1).
Melanoma of the skin occurred at a higher rate among women in the cohorts relative to the general population (n = 3; meta-SIR = 1.18, 95% CI = 1.01–1.38, I2 = 0%), particularly in AGRICAN (SIR = 1.21, 95% CI = 1.02–1.42, Table 3). No excess risk was observed among men (n = 5; meta-SIR = 0.98, 95% CI = 0.82–1.16, I2 = 84%) with the exception of MESA (SIR = 2.09, 95% CI = 1.35–2.99, Table 2).
Lymphohematopoietic malignancies. The meta-SIR for multiple myeloma was significantly greater than 1.0 in women (n = 4; meta-SIR = 1.27, 95% CI = 1.04–1.54, I2 = 0%), but not in men (n = 4; meta-SIR = 1.08, 95% CI = 0.88–1.32, I2 = 70%) (Fig. 1). In AGRICAN, the incidence of multiple myeloma exceeded that expected in the general population for both men (SIR = 1.33, 95% CI = 1.13–1.55) and women (SIR = 1.27, 95% CI = 1.02–1.54) (Table 2, Table 3). No excess or deficit was observed for leukemia in men overall, while some heterogeneity was observed across cohorts (n = 6; meta-SIR = 0.97, 95% CI = 0.83–1.14, I2 = 62%), with an elevated SIR observed among the men in KMCC (SIR = 2.21, 95% CI = 1.14–3.86) and the men in MESA (SIR = 1.12, 95% CI = 0.48–2.21). Conversely, a deficit of leukaemia was observed in women (n = 4; meta-SIR = 0.78, 95% CI = 0.64–0.96, I2 = 0%). The meta-SIR for non-Hodgkin lymphoma (NHL) was lower than 1.0 in men and women combined (n = 5; meta-SIR = 0.92, 95% CI = 0.86–0.98, I2 = 0%, Supplementary Fig. 1).
Excluding the combined Australian cohort had little impact on the results (Supplementary Fig. 2A).
4. Discussion
The data from eight AGRICOH cohorts of agricultural workers from six countries enabled us to characterize patterns of cancer incidence in agricultural populations by investigating cancer incidence in a variety of agricultural settings relative to the respective general populations. The combined data showed an excess incidence for prostate cancer in male agricultural workers and melanoma of the skin and multiple myeloma in female agricultural workers and a deficit incidence of the majority of cancer sites examined compared to the general population. The direction of risk was largely consistent across the cohorts, with a few deviations.
The observed deficits and excesses could suggest the presence of protective and risk factors for cancers in agricultural settings or populations. The small excess of prostate cancer in the present analysis may be partly attributable to exposure to pesticides as suggested by previous studies where an elevated risk was observed among workers who apply pesticides (Lemarchand et al., 2016, Meyer et al., 2007, Krstev and Knutsson, 2019, Koutros et al., 2013), such as organochlorines (Krstev and Knutsson, 2019) and certain organophosphates (Koutros et al., 2013). In contrast, we observed a deficit of prostate cancer in the men of KMCC. The reasons for the deficit are unknown, but it may be partly due to greater uptake of opportunistic prostate cancer screening among higher socioeconomic occupation workers compared to lower socioeconomic occupation workers in Korea (Lee et al., 2020) or due to some protective factor present in this cohort. Further analyses are warranted to elucidate the factors underlying the observed higher incidence of prostate cancer in agricultural workers.
A link between farming and multiple myeloma has been suggested over the past decades (Perrotta et al., 2008), however, the causes remain unknown. The elevated risk of multiple myeloma found in our analysis of women has also been observed in Canadian female crop farmers and farm workers (Kachuri et al., 2017) and British female pesticide users (Frost et al., 2011), but not in female farmers in New York (Wang et al., 2002) or Hispanic farm workers in California (Mills and Kwong, 2001). Although the number of multiple myeloma cases in women was small in most of the existing studies, the data overall are suggestive of an excess of multiple myeloma in female agricultural workers, particularly farmers. An excess incidence of multiple myeloma has also been observed in men (Frost et al., 2011). Our analysis showed a significant excess only in the AGRICAN cohort for both men and women, which could indicate higher levels of exposure to certain risk factors for multiple myeloma in the French agricultural setting. Epidemiological studies examining risk factors for multiple myeloma have further suggested an aetiological role of exposure to certain pesticides, e.g. exposures to permethrin (Alavanja et al., 2014), chlordane (Louis et al., 2017), carbaryl, captan and DDT (Presutti et al., 2016). Further analyses accounting for multiple occupational exposures among farmers and other possible risk factors, such as obesity are warranted.
In addition, we observed an excess of melanoma of the skin based on three cohorts of women combined, which is consistent with the study conducted in Canada (Kachuri et al., 2017) where an excess of skin melanoma was observed in female crop farmers and farm workers. The excess may partially be explained by some outdoor farming tasks that are commonly performed by women including harvesting and re-entry tasks which can increase their exposure of skin to UVR and pesticides (Baldi et al., 2014) and possibly elevate the risk of melanoma of the skin (Narayanan et al., 2010, Dennis et al., 2010). However, other previous studies of female agricultural workers (Wang et al., 2002, Frost et al., 2011, Wiklund and Dich, 1994) did not show an excess. The findings in men have also been inconsistent, and controversies remain with regards to the role of occupational sun exposure (Vuong et al., 2014). In the analysis of melanoma of the skin in men, we observed an excess only in the MESA cohort. The aforementioned Canadian study also observed an excess risk of melanoma of the skin in male farmers and managers, but not in male manual labourers (Kachuri et al., 2017). To clarify the existent inconsistencies, future studies should consider sun protection behaviours, climatic conditions, continuous vs intermittent sun exposure, histologic sub-types, genetic susceptibility, and other potential occupational risk factors for melanoma of the skin, such as pesticides.
Overall, our results showed lower incidence for all cancers combined compared to the respective general populations, which has been speculated to be due to lower rates of smoking, and to a lesser extent more occupational physical activity and the healthy worker effect (Pukkala et al., 2009, Blair and Freeman, 2009). This is supported by the prevalence of smoking in most of the cohorts (with smoking information) being lower than that of the general population (Supplementary Table 3). Furthermore, the cancer sites for which a deficit of incidence was observed in our analysis are consistent with those that are known to be associated with tobacco smoking, including cancers of the lung, oral cavity, nasopharynx, pharynx, esophagus, stomach, colon, rectum, bladder, liver, pancreas, larynx, and kidney and renal pelvis (Wild et al., 2020), as well as those inversely associated with physical activity, such as breast and colon cancers (Rezende et al., 2018). Other factors beyond lower smoking rates and more occupational physical activity could possibly explain the reduced risk, such as a putative anti-carcinogenic effect of exposure to endotoxin for cancer of the lung (Lerro et al., 2019, Lenters et al., 2010).
The present analysis displayed substantial heterogeneity in SIR estimates for all cancers combined and several cancer sites including liver and lung in men and women, and stomach, colorectum, and skin in men (I2 > 75%). The observed heterogeneity may have resulted from some differing underlying prevalence of agricultural or non-agricultural exposures associated with cancer risks in cohorts or the referent populations. Notably, the between-study heterogeneity for some cancers was predominantly driven by unique patterns in KMCC, e.g. stomach and gallbladder cancers in men and lung cancer in women (Supplementary Fig. 2B). The profile of the KMCC cohort was somewhat different from the others, e.g., the prevalence of smoking appeared to be more similar to that of the general population than for the other cohorts (Supplementary Table 3). The positive finding for stomach cancer is in line with the data from a study in the Republic of Korea where they compared cancer mortality between agricultural workers and the general population (Lee et al., 2010) and may be explained by over-representation of people from rural areas/lower socioeconomic positions where the underlying prevalence of risk factors, such as consumption of salt and Helicobacter pylori infection (Lim et al., 2013) and tobacco smoking (Chang et al., 2019) is higher. Another study of an agricultural community in Republic of Korea (Sull et al., 2002) showed an association between pesticide exposure and an increased risk of several cancers, including stomach, gallbladder, and liver cancers, which remained after adjusting for lifestyle factors, such as smoking and alcohol drinking habits. Further studies of agricultural exposures in Republic of Korea are warranted to elucidate the drivers of the observed excesses in gastrointestinal cancers.
Cancer incidence in the AGRICAN, AHS, and CNAP cohorts has been analysed individually and published elsewhere. The results from the current analyses are mostly consistent with the previous results with some differences that are likely explained by the differences in methodology. The previous analysis of the AGRICAN study included multiple myeloma in NHL overall (Lemarchand et al., 2017) whereas in the present study, multiple myeloma was separated from NHL to allow comparability with the CI5 data used as reference rates. Similarly, chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) was classified as NHL in the previous analyses whereas the present analysis included CLL/SLL as a leukaemia. The previous AHS analysis used state-specific reference rates whereas the present analysis relied on the rates based on the SEER 9 registries. The incidence rates from the SEER 9 registries were higher than the state specific rates, particularly from North Carolina, for testis, thyroid, cutaneous melanoma and Hodgkin lymphoma, resulting in lower SIRs in the present analysis than in previously published analyses from the AHS (Lerro et al., 2019, Koutros et al., 2010). The previous analysis of CNAP cohort (Kristensen et al., 1996) differed from the present analysis in terms of inclusion criteria, e.g. definition of farmers, and follow up period (1969–1991 vs 1991–2011), which may partially explain the lower SIR we observed for Hodgkin lymphoma and leukemia in the present analysis. Differences in the methodology including inclusion criteria, follow-up period, adjustment, classification of cancers, and reference rates should be considered carefully when interpreting the results.
The strengths of the present analysis include the pooling of cohort studies which allowed for an analysis with a large sample size, including female agricultural workers for whom the evidence remains limited. Also, the present pooled analysis has the largest geographical variation thus far although only one cohort represents Asia, and none represent Africa or South America. Another strength is that the present analysis harmonized methods across cohorts including disease classification, statistical methods, and reference rates for the most part, allowing comparability across cohorts. However, despite the harmonization of the cancer coding, we cannot exclude the possibility of influence by deviations in local reporting. Potential limitations of the present analysis include that subtype-specific analyses were not conducted, possibly masking some subtype-specific excesses if present, and that the number of cases/studies was small for some of the cancer sites, especially in women. Furthermore, the Pesticide Exposed Workers cohort included non-agricultural workers, which could have reduced the generalizability of our findings to the agricultural population; however, the sensitivity analysis excluding the Australian cohorts did not change the results substantially.
Lastly, it is important to note that when reference rates are derived from a general population, any modest effect of some carcinogenic exposures among agricultural workers can be masked due to underlying lower prevalence of risk factors for cancer, e.g. tobacco smoking, alcohol drinking, obesity, in agricultural workers compared to the general population. Therefore, the absence of excess incidence for certain cancers in the present analysis should by no means discourage further investigations of certain agricultural exposures in relation to cancer risks, especially when carcinogenic effect is supported by different lines of scientific evidence. From AGRICOH, associations between haematological lymphoid malignancies and specific exposures, such as animal farming and pesticides have been reported (El-Zaemey et al., 2019, Leon et al., 2019). Further analyses of specific agricultural exposures in relation to cancer risks are underway or planned to investigate further the excess risks and heterogeneity observed in the present analysis.
5. Conclusions
The present analysis of eight AGRICOH cohorts provides an overview of cancer incidence patterns in agricultural workers, suggesting lower incidence of cancer overall, including cancers of the bladder, breast (female), colorectum, esophagus, larynx, lung, and pancreas and higher incidence of prostate cancer among men and multiple myeloma and melanoma of skin among women compared to the general population. Despite the large variations in geographical locations and agricultural practices, the findings were largely consistent across cohorts, with a few deviations, suggesting the presence of some common underlying risk or protective factors among agricultural workers of both men and women. This warrants further analyses to investigate specific agricultural exposures while accounting for lifestyle and other potential confounders. Given the large size of the agricultural population worldwide and the presence of various potential hazards in its working environment, such epidemiological data are important in improving occupational health measures and ensuring better workers’ health.
CRediT authorship contribution statement
Kayo Togawa: Methodology, Formal analysis, Writing – original draft. Maria E. Leon: Methodology, Writing – original draft. Pierre Lebailly: Investigation, Writing – review & editing. Laura E Beane Freeman: Funding acquisition, Conceptualization, Writing – review & editing, Investigation. Karl-Christian Nordby: Investigation, Writing – review & editing. Isabelle Baldi: Investigation, Writing – review & editing. Ewan MacFarlane: Investigation, Writing – review & editing. Aesun Shin: Investigation, Writing – review & editing. Sue Park: Investigation, Writing – review & editing. Robert T Greenlee: Investigation, Writing – review & editing. Torben Sigsgaard: Investigation, Writing – review & editing. Ioannis Basinas: Formal analysis, Writing – review & editing. Jonathan N. Hofmann: Writing – review & editing. Kristina Kjaerheim: Investigation, Writing – review & editing. Jeroen Douwes: Writing – review & editing. Rachel Denholm: Formal analysis, Writing – review & editing. Gilles Ferro: Writing – review & editing. Malcolm R. Sim: Investigation, Writing – review & editing. Hans Kromhout: Writing – review & editing. Joachim Schüz: Funding acquisition, Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Ms. Sooyoung Cho for preparation of aggregated cancer incidence data for KMCC cohort. We also thank Prof Vivi Schlünssen who was part of the preparatory work for the data from Danish cohort.
Funds
The creation of the AGRICOH consortium was supported in part by the International Agency for Research on Cancer and the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
The AGRICAN cohort was supported by the Ligue Contre le Cancer, the Mutualité Sociale Agricole, the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES), the Office national de l’eau et des milieux aquatiques, the Office Français pour la Biodiversité, the Institut National de Médecine Agricole, the Centre François Baclesse, the Ministère de l’Enseignement Supérieur et de la Recherche. The Agricultural Health Study is funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (Z01 CP 010119). The Australian cohorts were supported by the Australian National Health and Medical Research Council (34093) and the Cancer Council Victoria (V-52). The Korean Multi-Center Cancer (KMCC) cohort was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C1127). The MESA cohort was supported in part by the Clinical and Translational Science Award (CTSA) program through the U.S. National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The SUS cohort was supported by the Danish Agency for Science Technology and Innovation, the Danish Medical Research Council, The Danish Agricultural Research Council, Helsefonden, the P.C. Petersen Foundation, the Danish Working Environment Research Fund, the Danish Research Council Aarhus University and the Danish Lung Association.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the institutions with which they are affiliated.
Handling Editor: Mark Nieuwenhuijsen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106825.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Acquavella J., Olsen G., Cole P., Ireland B., Kaneene J., Schuman S. Cancer among farmers: A meta-analysis. Ann. Epidemiol. 1998;8(1):64–74. doi: 10.1016/S1047-2797(97)00120-8. [DOI] [PubMed] [Google Scholar]
- Alavanja M.C., Hofmann J.N., Lynch C.F., Hines C.J., Barry K.H., Barker J. Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavanja M.C.R., Sandler D.P., McMaster S.B., Zahm S.H., McDonnell C.J., Lynch C.F. The agricultural health study. Environ. Health Persp. 1996;104(4):362–369. doi: 10.1289/ehp.96104362. Doi 10.2307/3432672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi I., Lebailly P., Bouvier G., Rondeau V., Kientz-Bouchart V., Canal-Raffin M. Levels and determinants of pesticide exposure in re-entry workers in vineyards: Results of the PESTEXPO study. Environ. Res. 2014;132:360–369. doi: 10.1016/j.envres.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Blair A., Freeman L.B. Epidemiologic studies in agricultural populations: observations and future directions. J. Agromed. 2009;14(2):125–131. doi: 10.1080/10599240902779436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A., Zahm S.H., Pearce N.E., Heineman E.F., Fraumeni J.F. Clues to Cancer Etiology from Studies of Farmers. Scand. J. Work Env. Hea. 1992;18(4):209–215. doi: 10.5271/sjweh.1578. [DOI] [PubMed] [Google Scholar]
- Boyle P., Parkin D.M. Cancer registration: principles and methods. Statistical methods for registries. IARC Sci. Publ. 1991;95:126–158. [PubMed] [Google Scholar]
- Bray, F.C.M., Mery, L., Piñeros, M., Znaor, A., Zanetti, R., Ferlay, J. (Eds.)., 2017. Cancer Incidence in Five Continents, Vol. XI (electronic version). International Agency for Research on Cancer <http://ci5.iarc.fr> (accessed 19 September, 2019).
- Breslow N.E., Day N.E. Statistical methods in cancer research. Volume II–The design and analysis of cohort studies. IARC Sci. Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- Chang Y., Kang H.Y., Lim D., Cho H.J., Khang Y.H. Long-term trends in smoking prevalence and its socioeconomic inequalities in Korea, 1992–2016. Int. J. Equity Health. 2019;18(1):148. doi: 10.1186/s12939-019-1051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble J., Hoppin J.A., Engel L., Elci O.C., Dosemeci M., Lynch C.F. Prevalence of exposure to solvents, metals, grain dust, and other hazards among farmers in the Agricultural Health Study. J. Expo. Anal. Environ. Epidemiol. 2002;12(6):418–426. doi: 10.1038/sj.jea.7500248. [DOI] [PubMed] [Google Scholar]
- Dean, A.G.S.K., Soe, M.M., May 13, 2020. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. <www.OpenEpi.com>. May 13, 2020.
- Dennis L.K., Lynch C.F., Sandler D.P., Alavanja M.C.R. Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ. Health Persp. 2010;118(6):812–817. doi: 10.1289/ehp.0901518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elholm G., Omland O., Schlunssen V., Hjort C., Basinas I., Sigsgaard T. The cohort of young Danish farmers - A longitudinal study of the health effects of farming exposure. Clin. Epidemiol. 2010;2:45–50. doi: 10.2147/clep.s9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zaemey S., Schinasi L., Ferro G., Tual S., Lebailly P., Baldi I. Animal farming and the risk of lymphohaematopoietic cancers: a meta-analysis of three cohort studies within the AGRICOH consortium. Occup. Environ. Med. 2019 doi: 10.1136/oemed-2018-105655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G., Brown T., Harding A.H. Mortality and cancer incidence among British agricultural pesticide users. Occupat. Med. (Oxford, England) 2011;61(5):303–310. doi: 10.1093/occmed/kqr067. [DOI] [PubMed] [Google Scholar]
- Greenlee R.T., Zentner J., Kieke B., Jr., Elliott J., Marlenga B. Farm health surveillance in the Marshfield Epidemiologic Study Area: a pilot study. J. Agric. Saf. Health. 2005;11(2):211–218. doi: 10.13031/2013.18188. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2012. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres and Dusts. Volume 100C. International Agency for Research on Cancer, Lyon, France. [PMC free article] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2012. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Radiation. Volume 100D. International Agency for Research on Cancer, Lyon, France.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2014. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Volume 105. International Agency for Research on Cancer, Lyon (FR). [PMC free article] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2018. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. DDT, Lindane, and 2,4-D. Volume 113. International Agency for Research on Cancer, Lyon, France. [PubMed]
- International Labour Organization, 2007. Agricultural workers and their contribution to sustainable agriculture and rural development. Switzerland.
- International Labour Organization, 2020. Employment distribution by economic activity (by sex) — ILO modelled estimates, Nov. 2020. ILOSTAT database.
- Kachuri L., Harris M.A., MacLeod J.S., Tjepkema M., Peters P.A., Demers P.A. Cancer risks in a population-based study of 70,570 agricultural workers: results from the Canadian census health and Environment cohort (CanCHEC) BMC cancer. 2017;17(1):343. doi: 10.1186/s12885-017-3346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutros S., Alavanja M.C., Lubin J.H., Sandler D.P., Hoppin J.A., Lynch C.F. An update of cancer incidence in the Agricultural Health Study. J. Occup. Environ. Med. 2010;52(11):1098–1105. doi: 10.1097/JOM.0b013e3181f72b7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutros S., Beane Freeman L.E., Lubin J.H., Heltshe S.L., Andreotti G., Barry K.H. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am. J. Epidemiol. 2013;177(1):59–74. doi: 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen P., Andersen A., Irgens L.M., Laake P., Bye A.S. Incidence and risk factors of cancer among men and women in Norwegian agriculture. Scand. J. Work Environ. Health. 1996;22(1):14–26. doi: 10.5271/sjweh.104. [DOI] [PubMed] [Google Scholar]
- Krstev S., Knutsson A. Occupational Risk Factors for Prostate Cancer: A Meta-analysis. J Cancer Prev. 2019;24(2):91–111. doi: 10.15430/JCP.2019.24.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.J., Cha E.S., Moon E.K. Disease Prevalence and Mortality among Agricultural Workers in Korea. J. Korean Med. Sci. 2010;25:S112–S118. doi: 10.3346/jkms.2010.25.S.S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-E., Zaitsu M., Kim E.-A., Kawachi I. Cancer Incidence by Occupation in Korea: Longitudinal Analysis of a Nationwide Cohort. Saf. Health Work. 2020;11(1):41–49. doi: 10.1016/j.shaw.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarchand C., Tual S., Boulanger M., Leveque-Morlais N., Perrier S., Clin B. Prostate cancer risk among French farmers in the AGRICAN cohort. Scand. J. Work Environ. Health. 2016;42(2):144–152. doi: 10.5271/sjweh.3552. [DOI] [PubMed] [Google Scholar]
- Lemarchand C., Tual S., Leveque-Morlais N., Perrier S., Belot A., Velten M. Cancer incidence in the AGRICAN cohort study (2005–2011) Cancer Epidemiol. 2017;49:175–185. doi: 10.1016/j.canep.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters V., Basinas I., Beane-Freeman L., Boffetta P., Checkoway H., Coggon D. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Caus. Control : CCC. 2010;21(4):523–555. doi: 10.1007/s10552-009-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M.E., Beane Freeman L.E., Douwes J., Hoppin J.A., Kromhout H., Lebailly P. AGRICOH: a consortium of agricultural cohorts. Int. J. Environ. Res. Public Health. 2011;8(5):1341–1357. doi: 10.3390/ijerph8051341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M.E., Schinasi L.H., Lebailly P., Beane Freeman L.E., Nordby K.C., Ferro G. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int. J. Epidemiol. 2019 doi: 10.1093/ije/dyz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerro C.C., Koutros S., Andreotti G., Sandler D.P., Lynch C.F., Louis L.M. Cancer incidence in the Agricultural Health Study after 20 years of follow-up. Cancer Caus. Control : CCC. 2019;30(4):311–322. doi: 10.1007/s10552-019-01140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque-Morlais N., Tual S., Clin B., Adjemian A., Baldi I., Lebailly P. The AGRIculture and CANcer (AGRICAN) cohort study: enrollment and causes of death for the 2005–2009 period. Int. Arch. Occup. Environ. Health. 2015;88(1):61–73. doi: 10.1007/s00420-014-0933-x. [DOI] [PubMed] [Google Scholar]
- Lim S.H., Kwon J.W., Kim N., Kim G.H., Kang J.M., Park M.J. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. doi: 10.1186/1471-230x-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis L.M., Lerro C.C., Friesen M.C., Andreotti G., Koutros S., Sandler D.P. A prospective study of cancer risk among Agricultural Health Study farm spouses associated with personal use of organochlorine insecticides. Environ. Health. 2017;16(1):95. doi: 10.1186/s12940-017-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane E., Chapman A., Benke G., Meaklim J., Sim M., McNeil J. Training and other predictors of personal protective equipment use in Australian grain farmers using pesticides. Occup. Environ. Med. 2008;65(2):141–146. doi: 10.1136/oem.2007.034843. [DOI] [PubMed] [Google Scholar]
- MacFarlane E., Benke G., Del Monaco A., Sim M.R. Causes of death and incidence of cancer in a cohort of Australian pesticide-exposed workers. Ann. Epidemiol. 2010;20(4):273–280. doi: 10.1016/j.annepidem.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Meyer T.E., Coker A.L., Sanderson M., Symanski E. A case–control study of farming and prostate cancer in African-American and Caucasian men. Occup. Environ. Med. 2007;64(3):155. doi: 10.1136/oem.2006.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills P.K., Kwong S. Cancer incidence in the United Farmworkers of America (UFW), 1987–1997. Am. J. Ind. Med. 2001;40(5):596–603. doi: 10.1002/ajim.1125. [DOI] [PubMed] [Google Scholar]
- Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010;49(9):978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen M.J., Noderer K.S., Schenker M.B., Vallyathan V., Olenchock S. Personal exposure to dust, endotoxin and crystalline silica in California agriculture. Ann. Occup. Hyg. 1999;43(1):35–42. [PubMed] [Google Scholar]
- Perrotta C., Staines A., Cocco P. Multiple myeloma and farming. A systematic review of 30 years of research. Where next? J. Occup. Med. Toxicol. 2008;3:27. doi: 10.1186/1745-6673-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presutti R., Harris S.A., Kachuri L., Spinelli J.J., Pahwa M., Blair A. Pesticide exposures and the risk of multiple myeloma in men: An analysis of the North American Pooled Project. Int. J. Cancer. 2016;139(8):1703–1714. doi: 10.1002/ijc.30218. [DOI] [PubMed] [Google Scholar]
- Pukkala E., Martinsen J.I., Lynge E., Gunnarsdottir H.K., Sparen P., Tryggvadottir L. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol. (Stockholm, Sweden) 2009;48(5):646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- Radon K. The two sides of the “endotoxin coin”. Occup. Environ. Med. 2006;63(1):73–110. doi: 10.1136/oem.2004.017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende L.F.M., Sá T.H., Markozannes G., Rey-López J.P., Lee I.-M., Tsilidis K.K. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br. J. Sports Med. 2018;52(13):826–833. doi: 10.1136/bjsports-2017-098391. [DOI] [PubMed] [Google Scholar]
- Sull J.W., Yi S.W., Sohn T.Y., Jee S.H., Nam C.M., Ohrr H. Pesticides and Cancer Incidence: The Kangwha Cohort Study. Korean J. Prev. Med. 2002;35(1):24–32. [Google Scholar]
- Vuong K., McGeechan K., Armstrong B.K., Cust A.E. Occupational sun exposure and risk of melanoma according to anatomical site. Int. J. Cancer. 2014;134(11):2735–2741. doi: 10.1002/ijc.28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lewis-Michl E.L., Hwang S.A., Fitzgerald E.F., Stark A.D. Cancer incidence among a cohort of female farm residents in New York State. Arch. Environ. Health. 2002;57(6):561–567. doi: 10.1080/00039890209602089. [DOI] [PubMed] [Google Scholar]
- Wiklund K., Dich J. Cancer risks among female farmers in Sweden. Cancer Causes Control. 1994;5(5):449–457. doi: 10.1007/BF01694759. [DOI] [PubMed] [Google Scholar]
- Wild C., Weiderpass E., Stewart B., editors. World Cancer Report: Cancer Research for Cancer Prevention. International Agency for Research on Cancer; Lyon, France: 2020. [Google Scholar]
- Yoo K.Y., Shin H.R., Chang S.H., Lee K.S., Park S.K., Kang D. Korean Multi-center Cancer Cohort Study including a Biological Materials Bank (KMCC-I) Asian Pac. J. Cancer Prev. 2002;3(1):85–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.