Abstract
Copper (Cu) plays a pivotal role in cancer progression by acting as a co-factor that regulates the activity of many enzymes and structural proteins in cancer cells. Therefore, Cu-based complexes have been investigated as novel anticancer metallodrugs and are considered as a complementary strategy for currently used platinum agents with undesirable general toxicity. Due to the high failure rate and increased cost of new drugs, there is a global drive towards the repositioning of known drugs for cancer treatment in recent years. Disulfiram (DSF) is a first-line antialcoholism drug used in clinics for more than 65 yr. In combination with Cu, it has shown great potential as an anticancer drug by targeting a wide range of cancers. The reaction between DSF and Cu ions forms a copper diethyldithiocarbamate complex (Cu(DDC)2 also known as CuET) which is the active, potent anticancer ingredient through inhibition of NF-κB and ubiquitin-proteasome system as well as alteration of the intracellular reactive oxygen species (ROS). Importantly, DSF/Cu inhibits several molecular targets related to drug resistance, stemness, angiogenesis and metastasis and is thus considered as a novel strategy for overcoming tumour recurrence and relapse in patients. Despite its excellent anticancer efficacy, DSF has proven unsuccessful in several cancer clinical trials. This is likely due to the poor stability, rapid metabolism and/or short plasma half-life of the currently used oral version of DSF and the inability to form Cu(DDC)2 at relevant concentrations in tumour tissues. Here, we summarize the scientific rationale, molecular targets, and mechanisms of action of DSF/Cu in cancer cells and the outcomes of oral DSF ± Cu in cancer clinical trials. We will focus on the novel insights on harnessing the immune system and hypoxic microenvironment using DSF/Cu complex and discuss the emerging delivery strategies that can overcome the shortcomings of DSF-based anticancer therapies and provide opportunities for translation of DSF/Cu or its Cu(DDC)2 complex into cancer therapeutics.
Keywords: disulfiram, diethyldithiocarbamate, copper, disulfiram copper complex, drug repurposing, drug-delivery-system, cancer, cancer stem cells
Introduction
Cancer is a prominent cause of death worldwide which places an increasing burden on health and socioeconomic systems. According to the estimates provided by World Health Organization (WHO) in 2019, cancer is already the first or second cause of death before the age of 70 in 112 of 183 countries and its prevalence is expected to increase (Sung et al., 2021). It is predicted that ageing combined with population growth will result in an increase in the annual number of new cancer cases from 18.1 million in 2018 to 28.4 million in 2040. This increase will be severe in low- and middle-income countries, which are least prepared to the challenge (Wild, 2019; Sung et al., 2021).
In addition to surgery and radiation, chemotherapy which uses broad-spectrum cytotoxic drugs that do not differentiate cancer cells from normal cells, remains as the main arm of cancer treatment, despite its toxicity and significant side effects (Bedard et al., 2020). The discovery of potent metal-based chemotherapeutic drugs such as cisplatin, oxaliplatin and carboplatin paved the way for the development of a new class of inorganic metal-based chemotherapeutic agents (Rottenberg et al., 2021). These metal drugs bind to DNA and block the transcription and replication that initiate the process of apoptosis in cancer cells. Platinum drugs are commonly used alone or in combination with other chemotherapeutic agents to treat various malignant diseases such as testicular, lung, breast, ovarian, colon, head and neck cancer (Ndagi et al., 2017; Rottenberg et al., 2021). However, their use is still limited due to innate or acquired resistance in cancer cells and the undesirable side effects associated with their toxicity (Marine et al., 2020).
By the turn of the millennium, insights into the genomic landscapes of cancers led to the development of drugs that selectively target cancer cells while sparing normal cells, hence having high potency and fewer adverse effects (Druker et al., 2001; Cohen et al., 2021). Over the past two decades, over 40 receptor tyrosine kinase inhibitors (TKIs) have been approved by the US FDA as targeted drugs for cancer treatment (Cohen et al., 2021). Although TKIs such as imatinib have shown promising outcomes in some cancers like chronic myeloid leukaemia, the problems associated with emergence of resistance to treatment, tumour relapse and undesirable side effects and toxicity, remain as the major challenges for their use in cancer patients (Kobayashi et al., 2005; Marine et al., 2020). More recently, it has been identified that even the state-of-the-art immunotherapies, such as immune check-point inhibitors and adoptive T cell therapies which revolutionised the treatment of advanced cancers, are no exception to innate and acquired resistance mechanisms in cancers (Restifo et al., 2016; Hiam-Galvez et al., 2021). Moreover, the current growth in the cost of many targeted therapies for cancer discourages their use in clinical setting in terms of cost benefit ratio evaluation by healthcare agencies (Saluja et al., 2018; Leighl et al., 2021).
Owing to the clinical success of platinum drugs in a wide range of cancers and the requirement for reduction of treatment costs, there has been renewed interest in the development of novel metallodrugs in the field of inorganic drug development (Ndagi et al., 2017; Rottenberg et al., 2021). While the question of how cancer cells develop resistance to various targeted therapies still remains, there has been better understanding of the fundamental roles that several metal ions play in biological processes and their interaction with biomolecules. Recently, several non-platinum metallodrugs such as copper(II) (Cu), gold(I)-, ruthenium(III), gallium(III), cobalt(II)- and nickel(II)-based compounds have been investigated for their anticancer potential (Frezza et al., 2010; Chitambar, 2012; Lee et al., 2020; Yusoh et al., 2020). Each transition metal has unique ligand exchange kinetics and redox potentials and hence the choice of the metal centre and ligand design play a crucial role in the therapeutic effects of the metallo-complex compounds. Among transition metals, Cu has been widely proven to be used in development of therapeutic compounds due to the interesting anticancer properties and ease of handling to reduce side effects (Shanbhag et al., 2021). The nature of Cu ligands and the planar square geometry exhibited by Cu complexes is ideal for causing DNA specific damage very similar to that of platinum compounds (Lelievre et al., 2020). Cu plays crucial roles in many biological processes such as free radical detoxification, mitogenic signalling, immunomodulation, peptide processing, cellular respiration, and proliferation, and hence it is an indispensable micronutrient for all living organisms (Michniewicz et al., 2021; Shanbhag et al., 2021). However, in pathological conditions like cancer, excessive levels of Cu have been associated with enhanced proliferation, angiogenesis, and metastasis of cancer cells leading to cancer progression. Given the important role of Cu in the onset and progression of cancer, targeting Cu homeostasis has emerged as a novel strategy in the treatment of cancer (Li, 2020; Michniewicz et al., 2021).
Considering the reduction of development costs and affordability as a priority, repurposing old metal binding drugs to form complexes with Cu to selectively target cancer cells has been rigorously investigated in the past years (Bertolini et al., 2015; Saluja et al., 2018). One such candidate is Disulfiram (DSF), an old FDA approved anti-alcoholism drug with excellent metal chelation ability and demonstrated anticancer potential in a wide range of cancers in preclinical settings (Lu et al., 2021).
Here, we review the roles for Cu in cancer progression and the potential of harnessing copper homeostasis pathways in cancer cells with the Cu-binding activity of DSF and its derivatives, with a focus on recent developments in the translation of DSF for cancer therapy.
Role of Copper in Cancer Progression
Dietary supplementation and absorption in the stomach, duodenum and upper small intestine is the main source of Cu in humans. Most of the absorbed Cu is stored in the liver and the Cu concentration in serum is approximately 12.0–25.0 μmol/L and varies in tissues between 15.0 and 180.0 μmol/g (Table 1). In physiological conditions the transport of Cu is strictly controlled. Cu is always bound to proteins to prevent excess redox activity and its distribution is precisely regulated by several cellular mechanisms. In mammalian cells, intracellular Cu intake is regulated by copper transport protein 1 (Ctr1) and Cu enters the cells in reduced form [Cu(I)]. Once inside the cell, Cu is bound to Cu chaperons or glutathione (GSH) and trafficked to metallothionein or distributed to specific compartments of the cells. Despite being an essential micronutrient, free Cu can induce toxicity to cells (Wee et al., 2013; Michniewicz et al., 2021).
TABLE 1.
Tissue and serum levels of copper in normal and cancer patients (***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05).
| Cancer type | Number of Samples | Tissue Copper levels (µg/g) (mean ± SD | Reference | ||
|---|---|---|---|---|---|
| Normal tissue | Tumor tissue | Normal tissue | Tumor tissue | ||
| Breast | 25 (matched pair) | 25 (matched pair) | 9.3 ± 2.3 | 21.0 ± 10.7*** | Rizk and Sky-Peck (1984) |
| 68 (matched pair) | 68 (matched pair) | 6.13 ± 4.32 | 11.31 ± 7.83** | Kuo et al. (2002) | |
| Gynaecologic organs | 47 | 53 | 1.26 ± 0.45 | 2.16 ± 0.6** | Margalioth et al. (1983) |
| Ovarian | 0.3 ± 0.1 | 0.7 ± 0.3** | Yaman et al. (2007) | ||
| Lung | 100 | 64 | 5.08 ± 1.09 | 8.23 ± 4.88** | Diez et al. (1989a) |
| Stomach | 47 | 53 | 1.44 ± 0.38 | 2.09 ± 0.52* | Margalioth et al. (1983) |
| 4, 14 (matched pair) | 14 (matched pair) | 1.1 ± 0.4 | 1.7 ± 0.1** | Yaman et al. (2007) | |
| Gallbladder | 30 (sex matched) | 30 | 16.30 ± 7.28 | 21.01 ± 6.67** | Basu et al. (2013) |
| Colorectal cancer | 50 | 50 | 0.12 ± 0.05 | 0.17 ± 0.08 | Sohrabi et al. (2018) |
| Number of Samples | Serum levels of copper (µg/dl) (mean ± SD | ||||
| Normal level | Cancer patients | Normal level | Cancer patients | ||
| Breast | 21 | 8 | 96 ± 11.52 | 152.96 ± 21.76* | Zowczak et al. (2001) |
| 20 (normal) | 56 (carcinoma) 32 (benign) | 101.62 ± 10 | 115.9 ± 14.4** | Feng et al. (2012) | |
| 54 | 54 | 109.56 ± 30.71 | 202.21 ± 89.18*** | Pavithra et al. (2015) | |
| 25 | 68 | 96.5 ± 7.3 | 125.2 ± 15.0** | Kuo et al. (2002) | |
| 30 | 30 | 65.2 ± 15.0 | 95.3 ± 4.9** | Adeoti et al. (2015) | |
| 84 | 88 | 113.2 ± 13.6 | 137.2 ± 36.2*** | Ding et al. (2015) | |
| Leukaemia | 20 | 49 | 86.7 ± 25.3 | 139.9 ± 51.2*** | Zuo et al. (2006) |
| 40 | 42 | 104.7 ± 0.16 | 122.6 ± 0.18*** | Demir et al. (2011) | |
| 40 | 50 | 124.22 ± 10.36 | 146.25 ± 10.47** | Mehdi et al. (2015) | |
| 21 | 12 | 114 ± 29 | 328 ± 74** | Carpentieri et al. (1986) | |
| 13 | 14 | 126.8 ± 21.2 | 256.8 ± 73.8*** | Delves et al. (1973) | |
| Lung | 53 | 109 | 101.1 ± 7.96 | 128.6 ± 6.85* | Oyama et al. (1994) |
| 154 | 154 | 128.5 ± 5.23 | 162.4 ± 8.18** | Jin et al. (2011) | |
| 100 | 20 (benign) 64(malignant) | 112.5 ± 17 | 150.3 ± 33.3*** | (Diez et al. 1989a; Diez et al. 1989b) | |
| Oral | 66 | 60 | 114.20 ± 38.69 | 209.85 ± 160.28*** | Baharvand et al. (2014) |
| 30 | 40 | 105.5 ± 18.81 | 141.99 ± 21.44*** | Tiwari et al. (2016) | |
| Gastrointestinal | 21 | 26 | 96 ± 11.52 | 128.64 ± 20.48* | Zowczak et al. (2001) |
| Thyroid | 20 | 30 | 75.5 ± 8.1 | 85.4 ± 8.7* | Baltaci et al. (2017) |
| Gall bladder | 30 | 30 | 125.97 ± 38.93 | 159.19 ± 24.04*** | Basu et al. (2013) |
| Prostrate | 30 | 22 | 97 ± 22 | 169 ± 31*** | Saleh et al. (2020) |
| Colorectal | 966 (matched pair) | 966 (matched pair) | 135.8 ± 30.5 | 138.6 ± 30.8** | Stepien et al. (2017) |
| Gynaecological | 20 | 100 | 44 ± 0.2 | 179 ± 8.3** | Cetinkaya et al. (1988) |
Many studies have indicated that the levels of Cu in both serum and tumour tissues are remarkably elevated in cancer patients in comparison to healthy individuals as summarised in Table 1. Significantly higher Cu levels in serum have been associated with multiple cancers such as breast, cervical, ovarian, lung, gastric, bladder, thyroid, oral, pancreatic, head, and neck cancer (Li, 2020; Shanbhag et al., 2021). In some malignancies like colorectal cancer and breast cancer, elevated Cu levels are strongly correlated with the stage and progression of the tumours (Gupta et al., 1993; Sharma et al., 1994). On the other hand, in haematological cancers, a reduction in Cu levels was observed during periods of remission in patients, indicating that the distribution of Cu is altered in cancer patients (Kaiafa et al., 2012).
Although the underlying mechanisms alter the Cu levels in the serum of cancer patients remain unclear, the role played by Cu in etiology and progression of tumours has been widely studied in the past two decades. Exposure to high levels of Cu has been linked with pancreatic neuroendocrine tumours and prostate cancer (Ishida et al., 2013; Vella et al., 2017). As a metal able to induce redox reactions, Cu is capable of generating reactive oxygen species (ROS) in the intracellular environment that leads to activation of various pro-oncogenic signalling mechanisms that enhances the proliferation of cancer cells (Prasad et al., 2017). It has been recently shown that Cu is involved in the regulation of MAP kinase pathway and oncogenic BRAF signalling that regulates cell proliferation, differentiation and motility, by interacting with MEK-1 protein, a key penultimate kinase in the MAPK pathway. MEK-1 contains a high affinity Cu binding site, which, upon binding of Cu will stimulates the MEK-1 dependent phosphorylation of ERK1/2, ultimately promoting tumour proliferation (Brady et al., 2014).
Recently it has been shown that enhanced levels of Cu upregulate the expression of programmed death ligand 1 (PD-L1) in tumour cells and modulate the signalling pathways that mediates the PDL-driven immune escape by cancer cells (Voli et al., 2020). It has also been shown in the same study that chelators of Cu enhanced the ubiquitin-mediated degradation of PD-L1 and resulted in the infiltration T lymphocytes and natural killer cells in the tumour site, thereby suppressing tumour growth and improving survival in mouse models (Voli et al., 2020).
Cu has the ability to switch on many pro-angiogenic responses and aid the proliferation and mobility of endothelial cells, which initiates angiogenesis that provides oxygen and essential nutrients to tumours (Finney et al., 2009; Xie and Kang, 2009). Cu is considered as a key angiogenic messenger, because it can stabilise nuclear hypoxia inducible factor-1 (HIF-1), leading to expression and activation of several angiogenic factors including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), tumour necrosis factor alpha (TNF-α), interleukins 6 and 8 (IL-6, IL-8), and fibronectin (Martin et al., 2005; Feng et al., 2009; Rigiracciolo et al., 2015). Cu also promotes the remodelling of extracellular matrix through activation of the Cu-dependent enzyme lysyl oxidase (LOX). Similarly, another Cu-dependent protein known as superoxide dismutase-1 (SOD1) which is a regulator of vasoconstriction and endothelial function, is overexpressed associated with elevated Cu levels in cancers, which eventually stimulates VEGF production and enhances FGF-induced angiogenesis and tumour development, while a decrease in SOD1 activity has been shown to impair angiogenesis (Xiao and Ge, 2012; Shanbhag et al., 2019). In addition to the above, Cu is also shown to enhance the metastatic potential of cancers through activation of metabolic proteins such as (LOX) and lysyl oxidase-like (LOXL) which are involved in remodelling extracellular matrix and creation of pre-metastatic niches that harbours metastatic cancer cells (Salvador et al., 2017). Many recent studies have shown that there are other Cu-dependent or Cu-binding proteins such as mediator Of Cell Motility 1 (MEMO1), copper metabolism MURR1 domain-containing protein 1 (COMMD1), Antioxidant Protein 1 (ATOX1) and secreted protein acidic and rich in cysteine (SPARC) which are involved in the process of cell migration and invasion by modulation of cytoskeleton, extracellular remodelling or by formation of adhesion sites (MacDonald et al., 2014; Nagaraju et al., 2014; Blockhuys et al., 2020).
Copper Chelators and Ionophores in Cancer
Cu homeostasis in cancers is emerging as an attractive target for anti-cancer drug development. There are two main approaches that have been tested in both preclinical and clinical conditions. The first approach involves using Cu chelators which reduce the bioavailability of Cu by directly binding to Cu; the second strategy is to use Cu ionophores that can increase the intracellular levels of Cu and therefore exert antitumor effects through ROS production, proteasome inhibition, and apoptosis induction (Li, 2020). Cu chelators such as D-penicillamine (D-pen), tetrathiomolybdate (TM), and trientine, are being widely investigated for their anticancer activity both in vitro and in vivo as well as in clinical trials (Wadhwa and Mumper, 2013). D-pen is a very strong metal chelator identified in early 1950s. D-Pen has the ability to remove Cu in vivo and the combination of D-pen and Cu could lead to cell death in endothelial cells and lymphocytes possibly as a result of ROS production and LOX and ICAM inhibition, eventually suppressing tumour growth and vascularization (Lipsky and Ziff, 1978; Starkebaum and Root, 1985; Brem et al., 1990; Gupte and Mumper, 2007). TM is another highly specific chelator of Cu and its anticancer activities has been studied well since the 1990s. TM has been demonstrated to inhibit angiogenesis in tumours by targeting multiple pathways including the suppression of NF-κB, HIF1α, LOX and SOD1 activities (Brewer, 2003). More recently, it has been shown that TM can inhibit MEK1/2 kinase activity by reducing the levels of intracellular Cu, and eventually suppressing BRAF-driven tumorigenesis in melanoma, thyroid and colon cancers (Kim et al., 2020; Xuefeng et al., 2020). TM can also enhance the cytotoxicity of BRAF-specific inhibitors like sorafenib. Trientine is another Cu chelator developed in the late 1960s, which has weaker activity than TM and D-Pen but is more tolerable than the other two in patients. The polyamine structure of trientine promotes Cu elimination via urinary excretion. It has been shown that trientine can inhibit endothelial cell proliferation by reducing the levels of IL-8 and CD31 expression (Moriguchi et al., 2002; Yoshiji et al., 2005).
While more research and encouraging clinical trials are ongoing for Cu chelators, Cu ionophores are also being widely studied for their anticancer activities. The mode of action of Cu ionophores is highly dependent on Cu as the compounds on their own have very little anticancer effect. Examples of Cu ionophores include Clioquionol, docosahexaenoic acid, thiosemicarbazone and DSF. Mechanistically these compounds exert their anticancer activities by elevation of extracellular and intracellular ROS, DNA damage and proteasome inhibition (Cater and Haupt, 2011; Yu et al., 2017; Sun et al., 2020). Clioquinol was shown to induce caspase-dependant apoptosis, but its clinical use has been discontinued due to its neurotoxicity (Cater and Haupt, 2011). Preclinical studies using analogues of Cloquionol are ongoing with different routes of administration for better anticancer activity and safety (Wehbe et al., 2018).
Among these compounds, DSF is the most extensively studied compound. DSF is also an attractive compound because of its established safety profile, easy availability, low cost, and less adverse effects than anticancer drugs. DSF can specifically carry Cu ions into tumour tissue, thereby preventing nonspecific interactions (Ekinci et al., 2019). Recently, some studies investigated the action of Cu complexes formed by some Cu-binding compounds such as DSF derivatives and its metabolites. Many clinical trials of DSF and Cu in combination with or without conventional anticancer drugs are under way which will be reviewed and summarised in the later part of this review.
Drug Repurposing and Disulfiram
From boosting immune system to targeting key molecules and developing personalized therapy cancer drug development is at its exciting phase today. New drug development is an expensive process, costing over $1.5 billion and taking approximately 15 yr to complete the process, however, there is a very high failure rate of up to 95% (Bertolini et al., 2015). Costs of this magnitude arrest research and development, forcing pharmaceutical developers to pitch for more money or suspend development altogether. Consequently, some of these new therapies reach price tags of more than $100,000 per patient per year, but still has no “meaningful benefit” (Saluja et al., 2018; Leighl et al., 2021). The prices of cancer drugs have increased 10% every year between 1995 and 2015 due to the escalated expense of R&D in the pharmaceutical industries (Leighl et al., 2021). In recent years there has been an increasing appreciation of the potential of repurposing known drugs like DSF, as an anticancer treatment. The approach is pointed towards developing cheaper alternative over the expensive ineffective drugs and reduce the burden on healthcare systems, while providing effective treatment options for thousands of patients diagnosed every year (Lu et al., 2021).
Disulfiram
DSF or Tetraethylthiuram disulfide was developed in the late 19th century for the industrial production of rubber. The intolerance to alcohol in factory workers exposed to DSF was first observed in the late 1930s and it was not until the late 1940s its potential as an anti-alcoholism medicine was recognised (Suh et al., 2006). Further its potential as a scabicide and vermicide medicine was recognised in the 1947, where DSF was utilised as an effective chelator of iron, copper, and zinc that caused toxicity in the parasites by damaging the copper-containing respiratory enzymes (Gordon and Seaton, 1942). Again, it was observed that upon alcohol intake when using this drug, it produced adverse side effects. In 1948, it was identified that the alcohol-aversive effects of DSF was due to the accumulation of acetaldehyde in patients (Hald and Jacobsen, 1948). Subsequently, DSF was established as a commercially available FDA anti-alcoholism drug in 1951. Overall, DSF has been in use in the clinic for over 70 yr in various fields such as parasitology, infectious diseases, virology and oncology (Suh et al., 2006).
Chemistry and Pharmacokinetics of Disulfiram
Disulfiram is an off-white crystalline powder that is insoluble in water but has variable solubilities in organic solvents like ethanol and ether. DSF is a relatively small molecule with a molecular weight of 296.54, density of 1.30 and a melting point of 70°C−72°C. DSF has oxidative biotransformation properties yielding sulphate, methanethiol and formaldehyde metabolites (PubChem CID3117).
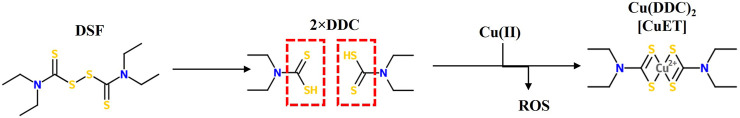
Both DSF and its metabolite diethyldithiocarbamate (DDC) are stable in basic environments, but unstable in acidic conditions. Upon ingestion, more than 99% of DSF is quickly converted to its corresponding thiol metabolite DDC in the acidic environment of the stomach. Both forms are rapidly absorbed through the gastrointestinal mucosa into the portal circulation and enriched in liver where both DSF and DDC are rapidly metabolised and degraded. DDC, due to its hydrophilic polar nature, is easily reduced to diethylamine (DEA) and carbon disulphide (CS2). CS2 is reactive with endogenous nucleophilic groups including thiols, amino acids and proteins and can be oxidized to form carbonyl sulphide (COS). COS can be further oxidised to form carbon dioxide and sulphur radicals, resulting in a permanent inhibition of microsomal mono-oxygenases. Further, CS2 can be involved in re-synthesis of DDC by reacting with DEA in vivo, that might explain the potential intolerance to alcohol for a longer period of time (Eneanya et al., 1981; Johansson, 1992; Petersen, 1992). Both DSF and DDC are strong chelators of divalent transition metal ions and forms stable complexes with heavy metal ions (Figure 1). The metal complex, Cu (DDC)2, is a dark precipitate which is more stable in acidic environments and the neutral and hydrophobic nature of Cu(DDC)2 enables its systemic absorption to take place along the upper GI tract (Johansson, 1992). The intact thiol group in DSF and DDC is essential and indispensable for them to chelate divalent transition metal ions.
FIGURE 1.
The structure of Disulfiram (DSF), its metabolite diethyldithiocarbamate (DDC) and its complex with copper Cu(DDC)2 or (CuET). Intact thiol groups in DS or DDC are indispensable for the chelation of DS or DDC with copper a reaction that results in the generation of reactive oxygen species (ROS).
DSF, after being absorbed into circulation is immediately broken into DDC monomers by the glutathione reductase system in red blood cells. Free DDC quickly binds to thiols of proteins, specifically to albumins, to form disulfides. DDC is also a substrate for Phase II metabolism that is mediated by an S-methyl-transferase, resulting in the formation of S-methyl diethyldithiocarbamate (S-Me-DDC) and S-glucuronide of DDC. Oxidative biotransformation of S-Me-DDC forms S-diethylthiomethylcarbamate (S-Me-DTC), which could be further oxidized to S-Me-DTC-sulphoxide and S-Me-DTC-sulphone metabolites, respectively (Eneanya et al., 1981). All these methylated metabolites are also involved with the inhibition of isozymes of aldehyde dehydrogenases (ALDH) resulting in the disulfiram-ethanol reaction (DER) (Yourick and Faiman, 1989; Hart and Faiman, 1992; Mays et al., 1996; Lipsky et al., 2001). The methylation and glucuronidation reactions of DSF and DDC will block the key reactive thiol group and abolish their metal chelating activity.
Pharmacodynamics of Disulfiram
Ethanol metabolism in the liver predominantly relies on the action of ALDH isozymes and a minor amount of ethanol is metabolised through P450 mono-oxygenases and catalases. Upon intake of alcohol, ethanol oxidation produces acetaldehyde which is further oxidised by ALDH to form acetate. Two important ALDH isoenzymes involved in this process are ALDH1 and ALDH2 (Johansson, 1992; Veverka et al., 1997). Sulphoxide, sulphone and the Me-DTC metabolites of DSF inhibit ALDH1 and ALDH2 through the formation of a covalent adduct, likely with the cysteine residue present at the active site of these enzymes, causing a permanent non-reducible, thiol-resistant reaction, so that this inhibitor is covalently bound into the active site of the enzyme (Hellstrom et al., 1983). This causes a rise in blood acetaldehyde concentrations and results in severe unpleasant side-effects such as hyperventilation, dyspnoea, hypotension, tachycardia, chest pain causing ischemia, vertigo, nausea, vomiting and headaches within 15 min on ingestion known as DER (Hellstrom et al., 1983; Petersen, 1992).
In addition to ALDH, DSF is also known to inhibit other enzymes involved in vital metabolic pathways such as glycolysis, tricarboxylic acid cycle, pentose phosphate shunt, glutathione system, and catecholamine synthesis through S-S linkage or by chelation of copper or zinc from the active sites of the enzymes (Eneanya et al., 1981). DSF can inhibit glyceraldehyde-3-phosphate dehydrogenase, Fructose-l,6-diphosphate dehydrogenase and succinic dehydrogenase which are key enzymes involved in the mitochondrial respiratory metabolism and can also interfere with the normal oxidative phosphorylation by blocking NAD+-dependent mitochondrial oxygen consumption. Both DSF and DDC can inhibit dopamine-β-hydroxylase, an enzyme that converts dopamine to norepinephrine catecholamine synthesis (Marselos et al., 1976; Eneanya et al., 1981).
Anticancer Mechanisms of Disulfiram
The anticancer activity of DSF has been demonstrated in various cancer cells models and is shown to be strongly dependent on formation of Cu(DDC)2 complex with divalent metal ions such as Cu. In in vitro conditions, a mixture of DSF and Cu immediately results in a highly oxidized intermediate form of DDC termed as bis(dialkyliminium)-tetrathiolane dication (Bitt-42+), followed by subsequent spontaneous decomposition of small amount of DSF to its anionic chelate form DDC, which on further redox reaction with Cu2+ forms a stable complex Cu(DDC)2. This redox reaction and the Fenton chemistry involved in the formation of Cu(DDC)2 complex results in the generation of ROS, which induces apoptosis in cancer cells. DSF/Cu, has been shown to be cytotoxic to cancer cells and has the ability to eradicate cancer stem cell (CSC) populations in various cancers with little or no toxicity to normal cells (Liu et al., 2012; Liu et al., 2014; Xu et al., 2017; Yip et al., 2011). DSF/Cu also reverses acquired and hypoxia induced anticancer drug resistance and has been shown to potentiate anticancer drug-induced apoptosis in colon, breast, lung, liver and brain cancers (Ekinci et al., 2019). There are number of underlying mechanisms by which DSF act as an anticancer drug (Figure 2). It can either show cytotoxic effects itself or act as an adjuvant by sensitizing the cancer cells to many first line chemotherapeutic drugs (Wang et al., 2003; Triscott et al., 2012; Liu et al., 2013; Schmidtova et al., 2019; Lu et al., 2021).
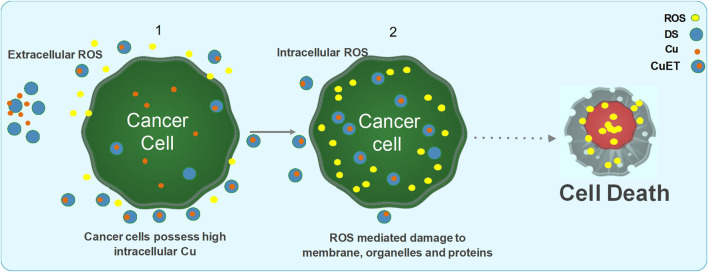
FIGURE 2.
Two phases anticancer model of DS and DDC plus copper induced cancer cell death. 1. DS or DDC and copper contact and react extracellularly. The reaction will generate ROS extracellularly which induce cancer cell apoptosis. Due to the extremely short lifespan of ROS in tissue, the reaction must be taken place adjacent to cancer cells, in order to effectively target cancer cells. 2. The reaction-generated compound, CuDDC2 (CuET), can easily penetrate cancer cells and trigger intracellular ROS generation and induce cancer cell apoptosis.
Disulfiram-induced Cell Death
The anti-cancer mechanism of DSF appears to be Cu-dependent, as this plays a role in redox reactions. As cancer cells contain high amounts of Cu through the trans-membrane Cu transporter Ctr1 transportation, DSF can form a complex with Cu, enabling it to penetrate into cancer cells. This enables DSF to specifically target these cells and not normal healthy cells that express low levels of Cu (Liu et al., 2012). Reaction between DSF, DDC and Cu results in the generation of extracellular ROS, which in turn induce apoptosis in cancer cells (Lewis et al., 2014; Tawari et al., 2015). It is also demonstrated that the metabolite of DSF, DDC and its copper complex Cu(DDC)2 get accumulated in the cancer cells and induce ROS generation leading to apoptosis in cancer cells (Guo et al., 2010; Yip et al., 2011; Tawari et al., 2015; Lu et al., 2020; Majera et al., 2020; Yoshino et al., 2020). Generation of both extracellular and intracellular ROS is completely relied on the intact thiol group which chelates copper (Figure 3).
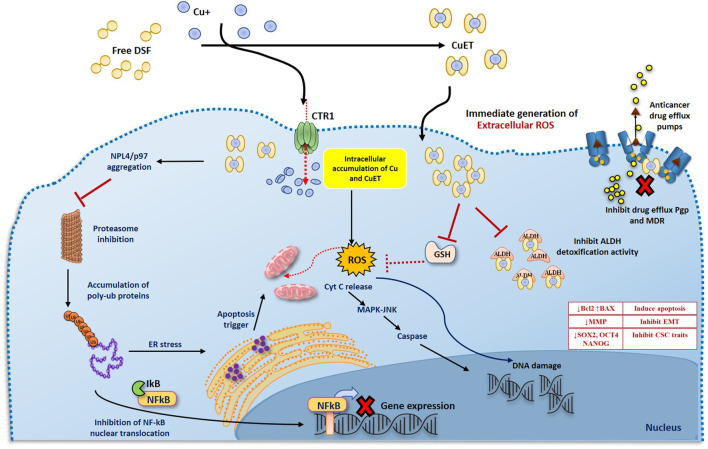
FIGURE 3.
Mechanism of action of DSF/Cu induced anticancer activity. DSF and Cu reaction generates extracellular ROS and damage the membrane proteins. The formation of CuET complex outside the cell and transportation of CuET through lipid bilayer, in addition to increased influx of Cu via the CTR1 transporter, further triggers the intracellular ROS mediated mitochondrial damage and DNA damage leading to apoptosis via the MAPK pathway and JNK activation. CuET also inhibits the proteasome activity via the NPL4/p97 segregase pathway leading to inhibition of NFkB pathway. DSF and CuET further inhibits ALDH mediated ROS detoxification and Pgp mediated drug efflux mechanisms. Collectively, all the above negatively effects the cancer cell survival and maintenance of stemness and resistance thereby sensitizing the cells to ROS and anticancer drug mediated damage, eventually leading to apoptosis.
DSF has been shown to inhibit superoxide dismutase and competes with glutathione reductase causing an imbalance is the ROS scavenging mechanisms of cancer cells. Further, DSF, being a specific inhibitor of ALDH, blocks the ROS scavenging and detoxification mediated by ALDH isozymes. Increased ROS levels and inhibition of ROS scavenging mechanisms render the cells vulnerable to subsequent oxidative stress and further DNA damage which triggers the DNA-damage response mechanisms. DSF associated with Cu is shown to down-regulate the expression of several genes involved in DNA repair pathways. Thus, the ROS generated by DSF/Cu or Cu(DDC)2 is sustained in the cells leading to high levels of DNA damage that induce mitochondrial pore opening and apoptosis via the MAPK trigger (Cen et al., 2004; Morrison et al., 2010; Tawari et al., 2015). Apoptosis induced by ROS is highly dependent on persistent activation of pro-apoptotic MAPK pathway which activates the mitochondrial pro-apoptotic proteins by phosphorylation (Junttila et al., 2008; Xu et al., 2020b). It was found that in breast cancer cells treated by DSF/Cu complex, there was persistent activation of MAPK pathway which were then directed to ROS-induced apoptosis. Also, the cytotoxic effect of DSF/Cu complex was reduced by MAPK pathway inhibitors, indicating the role of MAPK pathway in ROS-induced apoptosis (Yip et al., 2011). Chiba et al. (2014) demonstrated that tumor-initiating hepatocellular carcinoma cells were significantly reduced by DSF-induced apoptosis via activation of MAPK pathway (Chiba et al., 2014). Nasopharyngeal cancer cells were also eradicated by DSF/Cu complex through increasing the ROS levels and activating the apoptosis-related MAPK pathway (Li et al., 2020). Zhang et al. (2019) demonstrated that DSF/Cu complex induced the autophagy-dependent apoptosis in human pancreatic and breast cancer cells through the activation of ER-stress (Zhang et al., 2019). DSF/Cu complex can also induce apoptosis via activation of chloride channel-3 (ClC-3). These ClC-3 channels are highly functional and over-expressed in cancer cells as compared to normal cells, and DSF/Cu complex is only able to trigger activation of over-expressed ClC-3 channels. Therefore, DSF/Cu complex specifically induce apoptosis in the cancer cells, through targeting over-expressed ClC-3 channels, without affecting the normal cells (Xu et al., 2019). In summary, the increased ROS production and subsequent cell death induced by DSF was found to be largely dependent on the presence of Cu at a required level.
Disulfiram and Proteasome Inhibition
A functional proteasome system maintains the synchronized expression of cell cycle-regulatory and apoptosis-control proteins. Hence the proteasome-mediated protein degradation is an important cellular homeostatic system, and in general cancer cells are more sensitive to alterations in this proteasome inhibition than normal cells. Bortezomib was the first clinically approved 20S proteasome inhibitor drug used for its antitumor activity against several types of cancers (Adams, 2004; Papandreou and Logothetis, 2004; Kane et al., 2006). Proteasome inhibition activity of DSF was first reported by Lovborg et al. (2006) by showing its specific inhibition for 26S proteasome (Lovborg et al., 2006). Combination of DSF or metabolite DDC with Cu has been shown to be a potent inhibitor of the functional proteasomes in many cancers (Kona et al., 2011; Triscott et al., 2012; Brüning and Kast, 2014; Yoshino et al., 2020). Notably, DSF/Cu was shown to inhibit the proteasome activity in breast cancer models but not in normal breast cells. The inhibition of proteasome activity by DSF/Cu complex leads to the accumulation of poly-ubiquitinated proteins and cytotoxic protein aggregates of important proteins such as IκB, p27, Kip1 and c-Myc, which results in the inhibition of cell-cycle progression and subsequent apoptosis (Cvek and Dvorak, 2008). Studies from our group (Chen et al., 2006) showed that low concentrations of DSF can partially bind to the 20S proteasome molecule and inhibiting the chymotrypsin-like activity of the proteasome. It is also speculated that DSF inhibits POH1, a protein which plays an important role in deubiquitinating function of the proteasome lid rather than the actual core proteasome (Chen et al., 2006; Cvek and Dvorak, 2007; Gallery et al., 2007; Yu et al., 2007; Cvek et al., 2008; Yoshino et al., 2020).
The protein complex, nuclear factor-kappa B (NF-κB), has a close relationship to cancer cells by acting as a key regulator of multiple pathways necessary for survival, stemness, resistance, angiogenesis and metastasis (Cvek and Dvorak, 2007; Cvek and Dvorak, 2008). NF-κB is a transcriptional regulator of various genes and often expressed in high levels in cancer cells in comparison to normal cells. The activity of proteasomes is very important for activation of NF-κB pathway, where proteasomes are involved in degradation of the inhibitor-KB molecule (IkB), which eventually releases NF-κB p50/p65 heterodimer from the inhibitory complex to translocate into the nucleus and exert its function as transcriptional regulator (Verzella et al., 2020). There is a close relationship between chemoresistance and activation of NF-κB in cancer cells especially in response to DNA damage induced by anticancer drugs (Wang et al., 1999; Wang and Cassidy, 2003). If DSF/Cu blocks the proteasome system, IκB continuously inhibits NF-κB, preventing its nuclear translocation which eventually and favours apoptosis or sensitise cancer cells to anti-cancer drugs. In this regard, DSF played an important role demonstrated by Wang et al. (2003) that DSF when administered along with 5-fluorouracil, it significantly inhibited the activity of NF-κB and enhanced the apoptotic effect of 5-fluorouracil on colorectal cell lines, DLD-1 and RKO (Wang et al., 2003). Reversal of chemo-resistance by DSF through the inhibition of NF-κB was also observed in breast cancer cells and human glioblastoma cell lines (Yip et al., 2011; Liu et al., 2012; Liu et al., 2014). Similarly, DSF-copper complex has also been found quite effective in inhibiting the activity of NF-κB and reversing the chemo-resistance of colon and breast cancer cell lines against the anti-cancer drug gemcitabine (Guo et al., 2010). It has also been found to have cytotoxic effect against the leukaemia stem cells by simultaneously inducing the apoptosis through ROS-JNK pathway and inhibiting the pro-survival NF-κB pathway (Xu et al., 2017). NF-κB induces epithelial–mesenchymal transition EMT by the up-regulation of master-switch transcription factors for EMT (Chua et al., 2007; Julien et al., 2007; Huang et al., 2013) and stabilization of Snail which suppress the expression of adherent junction proteins (Wu et al., 2009; Yang et al., 2013). Therefore, DSF-copper complex can also suppress the EMT event in cancer by inhibiting the activity of NF-κB, clearly demonstrated by Li et al. (2018) in hepatocellular carcinoma (Li et al., 2018).
However, a recent study argued that the DSF/Cu complex (Cu(DDC)2 or CuET) is not a direct inhibitor of proteasomes, instead CuET targeted the p97-NPL4-UFDI pathway upstream to proteasome. It was shown that CuET binds to the NPL4, resulting in aggregation of NPL4 and deactivation of P97 segregase which leads to accumulation of misfolded proteins in the cells and ultimately cell death (Skrott et al., 2017). It was also described those cells treated with the CuET complex shows similar phenotypic characteristics as that of proteasome inhibitors, such as accumulation of poly-ubiquitinated proteins in the cytoplasm (Skrott et al., 2017).
The main target of CuET in p97 segregase pathway is NPL4, a key component of p97 segregase. The interaction of NPL4 and CuET results in the formation of protein aggregates and immobilization of otherwise very mobile NPL4-p97 complex which triggers the cellular heat shock response and endoplasmic reticulum (ER) stress/unfolded protein response, eventually leading to cell death (Ding and Zhu, 2018; Skrott et al., 2019). CuET can also induce replication stress via NPL4 targeting. Skrott et al. (2017) reported that in addition to NPL4/p97, the CuET-induced aggregates consist of several proteotoxic stress-related proteins, including HSP70, SUMO2/3, polyubiquitin chains, and TDP-43. In their further studies they found that ATR kinase, a key factor required for proper cellular response to replication stress, is also trapped and sequestered in the NPL4 aggregates (Skrott et al., 2017). It is known that ATR kinase dysfunction triggers the replication stress, therefore, one could say that ATR aggregation is the primary and/or major cause of the CuET-induced replication stress (Majera et al., 2020). CuET has high affinity for binding to NPL4 because NPL4 contains two zinc-finger domains: C-terminal NZF (NPL4-zinc-finger) and putative zf-NPL4, respectively, which bind bi-valent metals and metal complexes. It was observed that it is putative zf-NPL4 which binds with CuET as it might chemically resembles the metal complexes, which binds to zf-NPL4 (Skrott et al., 2017). Recently, a more detailed description about the interaction of DSF/Cu complex with NPL4 has come into light. It has been shown that NPL4 goes through three conformational states while in complex with p97. These conformational changes seem like a seesaw motion. This motion of NPL4 takes place due to its zinc finger motifs interaction with N domain of p97 and it is essential for the unfolding activity of p97 (Pan et al., 2021). It has been observed that DSF/Cu complex bypasses the copper transporter system of the cell membrane and release the cupric ions under oxidative conditions. These cupric ions interact with the zinc fingers of NPL4 and lock the conformational switch of NPL4/p97 complex essential for the unfolding activity of the p97, hence, inhibits the function of p97 (Pan et al., 2021).
The same group also discovered that cells lacking BRCA1 and BRCA2 were particularly sensitive to DSF/Cu or CuET treatment. CuET induced replication stress-associated DNA damage in several cancer cell lines and increased γH2AX, as an indicator for DNA double-strand breaks in these cells (DSB). Homologous recombination (HR), a DSB repair mechanism, requires BRCA1 and BRCA2 for its activity. Interestingly, a co-localization of ATR with immobilized NLP4 aggregates was found after CuET treatment. Moreover, CuET interfered with the activation of the RPA-ATRIP-ATR-CHK1 repair pathway, by suppressing the ATR kinase despite of high replication stress, induced by ssDNA aggregates (Majera et al., 2020).
Inhibition of Cancer Stem Cells (CSC)
Cancer stem cells (CSC) are highly resistant to several conventional chemotherapies and therefore, these cells are responsible for the cancer recurrence (Al-Hajj et al., 2003; Singh et al., 2004; Ginestier et al., 2007; Morgan et al., 2009; Fernando et al., 2015; Liu et al., 2021). ALDH belongs to an enzyme superfamily that catalyses aldehydes, the toxic metabolite of alcohol. It is crucial for the synthesis of molecules that play important role in cell proliferation, differentiation, and survival (Jackson et al., 2011; Pors and Moreb, 2014). Over a decade, the association between high ALDH activity and the cancer stem cell (CSC) phenotype has inspired scientists to develop specific ALDH inhibitors with greater clinical potential to effectively suppress CSCs and tumour progression (Jackson et al., 2011). The ability of DSF to induce apoptosis in breast CSCs is due to the inhibition of ALDH1 activity (Yip et al., 2011). DSF was found to enhance the cytotoxic effect of cisplatin by inhibiting the stemness of CSCs derived from breast cancer cell lines through the inhibition of expression of stemness-related transcription factors such as Sox, Nanog, and Oct and also by inhibiting the activity of ALDH in ALDH + stem-like cells (Liu et al., 2013; Yang et al., 2019). Guo et al. (2019) also demonstrated that DSF sensitized the ovarian cisplatin-resistant ALDH+ stem-like population to cisplatin treatment by suppressing the ALDH activity and inducing the apoptosis (Guo et al., 2019). DSF also reverses the resistance of testicular germ cell tumours towards cisplatin by inhibiting the ALDH activity (Schmidtova et al., 2019). It was found that supplementation of Cu with DSF augments its activity in reversing the chemoresistance of drug-resistant cancer cells (Liu et al., 2013). DSF-Cu complex supressed the CSCs in breast cancer by inhibiting the ALDH1 activity, supressing the CD44+/CD24− and CD49f+/CD24+ subpopulations and downregulating the phospho-STAT3, cyclin D1 and surviving of STAT3 signalling pathway (Kim et al., 2017). DSF-Cu complex also reversed the microtubule inhibitors resistance in A549/Taxol (Taxol-resistant cells) and KB/VCR (vincristine-resistant cells) cells by decreasing the activity of ALDH2 and inhibiting the expression of P-gp (glycoprotein P) which is highly expressed in microtubule inhibitors resistant cancer cells (Wang et al., 2018). DSF-Cu complex was found to be more efficient as compared to DSF alone in eliminating the multiple myeloma stem cells by inhibiting the ALDH activity and suppressing the stemness-related transcription factors such as Nanog, and Oct4 (Jin et al., 2018).
Limitations of Disulfiram
Although DSF shows high in vitro toxicity in cancer cells, there was almost no positive clinical data published in cancer patients https://clinicaltrials.gov/ct2/results?term=disulfiram+AND+cancer&Search=Search). A summary of all the clinical trials using oral DSF is given in Table 2.
TABLE 2.
Summary of clinical trials using disulfiram for cancer treatment.
| Study Title | Tumour Type | Drugs | Dose | Status | Outcome | Identifier |
|---|---|---|---|---|---|---|
| Phase II Trial of Disulfiram With Copper in Metastatic Breast Cancer | Breast cancer (Metastatic) | Disulfiram/Copper Supplement | DSF: 400 mg p.o./d Cu: 2 mg p.o./d | Phase II, Recruiting | N/A | NCT03323346 |
| Vinorelbine, Cisplatin, Disulfiram and Copper in CTC_EMT Positive Refractory Metastatic Breast Cancer | Breast Cancer (Refractory, HER2-VE) | Vinorelbine, Cisplatin, Disulfiram, Copper | DSF: 400 mg p.o./d Cu: 2 mg p.o./d | Phase II, Recruiting | N/A | NCT04265274 |
| Disulfiram and Cisplatin in Refractory TGCTs | Germ cell tumour | Disulfiram, Cisplatin | DSF: 400 mg p.o./d | Phase II, Recruiting | N/A | NCT03950830 |
| Disulfiram in Treating Patients With Glioblastoma Multiforme After Radiation Therapy With Temozolomide | Glioblastoma | Disulfiram/Copper, Gluconate, Temozolomide | DSF: 500 mg p.o./d; Cu: 6 mg p.o./d | Early Phase I, Completed | Max. tolerated dose = 500 mg/day | NCT01907165 |
| Disulfiram and Copper Gluconate With Temozolomide in Unmethylated Glioblastoma Multiforme | Glioblastoma | Disulfiram/Copper Gluconate Temozolomide | DSF 125 mg p.o./twice daily; Cu: 2 mg p.o./twice daily | Phase II, Recruiting | N/A | NCT03363659 |
| Disulfiram/Copper Combination In The Treatment of Newly Diagnosed Glioblastoma Multiform | Glioblastoma | Disulfiram/Copper Temozolomide | N/A | Phase II, Not Yet Recruiting | N/A | NCT01777919 |
| Disulfiram in Recurrent Glioblastoma | Glioblastoma | Disulfiram/Copper Temozolomide | DSF 400 mg p.o./d; Cu: 2 mg p.o./d | Phase II/III, Completed | N/A | NCT02678975 |
| Bioavailability of Disulfiram and Metformin in Glioblastomas | Glioblastoma | Disulfiram, Metformin | DSF: 200 mg p.o./twice/d Cu: 2.5 mg p.o./d | Early Phase I, Terminated | N/A | NCT03151772 |
| Disulfiram/Copper With Concurrent Radiation Therapy and Temozolomide in Patients With Newly Diagnosed Glioblastoma | Glioblastoma | Disulfiram/Copper Gluconate | DSF: 125 mg, 250 mg, 375 mg or 500 mg p.p./d | Phase I/II, Recruiting | Low toxicity at 250 mg/day. Median follow-up of 12.3 mo, 1-yr PFS: 57%; 1-yr OS: 69% | NCT02715609 |
| Cu: 2 mg p.o./thrice daily | ||||||
| Safety, Tolerability and Efficacy of Disulfiram and Copper Gluconate in Recurrent Glioblastoma | Glioblastoma (recurrent) | Disulfiram/Copper, Temozolamide | DSF/Cu: 80 mg p.o./thrice daily | Phase II, Completed | Low toxicity (1/23 with grade 3 elevated ALT); ORR 0/23; 14% with clinical benefit | NCT03034135 |
| A Proof-of-concept Clinical Trial Assessing the Safety of the Coordinated Undermining of Survival Paths by nine Repurposed Drugs Combined With Metronomic Temozolomide (CUSP9v3 Treatment Protocol) for Recurrent Glioblastoma | Glioblastoma (recurrent) | Disulfiram Metronomic Temozolamide | 250 mg p.o./d; 250 mg p.o. twice daily | Phase I, Active Not Recruiting | N/A | NCT02770378 |
| Disulfiram and Chelated Zinc for the Rx of Disseminated Mets Mel That Has Failed First Line Therapy | Melanoma | Disulfiram and Zinc | N/A | Phase II, Completed | 1 grade 3+ toxicity (confusional episode); ORR 0/12; 1 target lesion –27% | NCT02101008 |
| Disulfiram Plus Arsenic Trioxide In Patients With Metastatic Melanoma and at Least One Prior Systemic Therapy | Melanoma (Metastatic) | Disulfiram | 250 mg p.o. twice daily | Phase I, Terminated | N/A | NCT00571116 |
| Disulfiram in Patients With Metastatic Melanoma | Melanoma (Stage IV) | Disulfiram | 250 mg p.o. twice daily, escalated until maximum tolerated dose | Phase I/II, Completed | N/A | NCT00256230 |
| Study of Disulfiram and Copper Gluconate in Patients With Treatment-Refractory Multiple Myeloma | Multiple Myeloma (Refractory) | Disulfiram, Copper Gluconate | N/A | Phase 1, Recruiting | N/A | NCT04521335 |
| Initial Assessment of the Effect of the Addition of Disulfiram (Antabuse) to Standard Chemotherapy in Lung Cancer | Non-Small Cell lung Cancer | Disulfiram, Chemotherapy | N/A | PhaseII/III, completed | Benefit in PFS (5.9 vs 4.9 mo) and OS (10.0 vs 7.1 mo) | NCT00312819 |
| Disulfiram-Copper Gluconate in Met Pancreas Cancer w Rising CA19-9 on Abraxane-Gemzar, FOLFIRINOX or Gemcitabine | Pancreatic Cancer (Metastatic) | Disulfiram/Copper Gluconate | N/A | Phase II, Recruiting | N/A | NCT03714555 |
| Disulfiram and Chemotherapy in Treating Patients With Refractory Solid Tumors or Metastatic Pancreatic Cancer | Pancreatic Cancer (Metastatic, Recurrent) | Disulfiram, Gemcitabine | 250 mg p.o./d, 28 days | Phase I, Recruiting | N/A | NCT02671890 |
| A Phase Ib Study of Intravenous Copper Loading With Oral Disulfiram in Metastatic, Castration Resistant Prostate Cancer | Prostate Cancer (Metastatic Castrate-resistant) | Disulfiram/Copper Gluconate | DSF: 80 mg p.o./three times daily; Cu: 1, 3, 5, or 7 mg on cycle 1 days 1, 8, and 15 | Phase I, Terminated | No grade > 3 toxicities; no effect on PSA; 64Cu-PET shows Cu-uptake in some metastases | NCT02963051 |
| Study of Recurrent Prostate Cancer With Rising Prostate Specific Antigen (PSA) | Prostate Cancer (Recurrent) | Disulfiram | 250 mg p.o./d, 28 days; 500 mg p.o./d, 28 days | Completed | Moderate tolerability (6/19 with grade 3); 5/19 (26%) patients with change in 5-methyl-cytosine; no effect on PSA levels | NCT01118741 |
| Phase I Study of Disulfiram and Copper Gluconate for the Treatment of Refractory Solid Tumors Involving the Liver | Solid Cancer (Refractory, liver) | Disulfiram/Copper Gluconate | DSF: 250 mg p.o./d Cu: 2 mg p.o./d | Phase I, Completed | Well tolerated; no dose limiting toxicity; one stable disease | NCT00742911 |
This inefficiency of DSF could be attributed to the very short half-life of the currently available oral version of DSF in the blood stream which is approximately 2–4 min (Marselos et al., 1976; Eneanya et al., 1981; Johansson, 1992). Our previous findings indicated that the cytotoxic effect of DSF on cancer cells is executed in two phases: 1) The instant ROS mediate damages induced by the reaction between DSF and Cu; 2) A delayed killing induced by the end product, Cu-DDC (Butcher et al., 2018). After ingestion of the current oral version, DSF is rapidly reduced into two DDC molecules via the disulphide bond breakage in the gastrointestinal system and the bloodstream of the portal vein (Johansson, 1992). The DDC which is accumulated in the liver is still a highly reactive chelator of divalent metal ions, specifically Cu and Zn. But in the liver DDC is rapidly degraded into carbonyl disulphide and dimethylamine or undergoes methylation enzymatically to form S-methyl-DDC (Johansson, 1992; Butcher et al., 2018). As a result, DDC and all metabolites of DSF lose their active functional thiol groups and their ability to chelate with Cu or other metal ions is compromised. Surprisingly this process does not inhibit the anti-alcohol dependency activity of DSF as the metabolization of DSF takes place in the liver, which is the site of ethanol breakdown and hence it is able to successfully inhibit ALDH activity (Marselos et al., 1976; Eneanya et al., 1981; Petersen, 1992; Lewis et al., 2014). Whereas in the case of cancer, the reaction of DSF and Cu must take place inside or adjacent to cancer cell but mostly the target tissue where tumour is located are at a distant site. Hence the active sulfhydryl groups in DDC are indispensable for the chelation reaction between DDC and Cu and formation of Cu-DDC complex (Liu et al., 2014; Butcher et al., 2018).
Therefore, we hypothesised that using nano-drug-delivery system to protect the sulfhydryl groups in DDC will overcome the discrepancy between the anticancer activity of DSF in laboratory and clinic and pave the path between “bench and bed.” Based on this hypothesis, our group first encapsulated DSF into liposomes and demonstrated significantly improved anticancer efficacy in mouse models (Liu et al., 2014). Development of novel formulations of DSF using nano-drug delivery strategies will be of significant clinical importance in translation of DSF into cancer treatment (Figure 4).
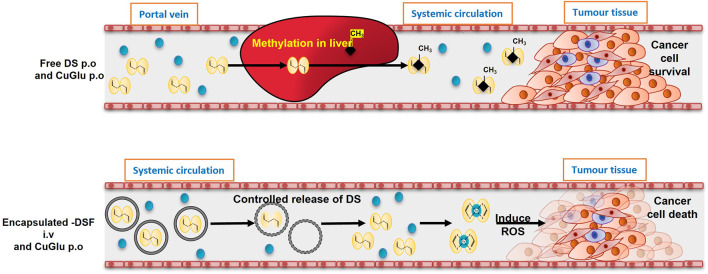
FIGURE 4.
The models of oral and intravenous administration of DS. A. After oral administration, DS will be delivered via portal vein and enriched in liver in which it is instantly methylated. The methylated DS inhibits ALDH and remains its antialcoholism function. Because the thiol group is blocked, its anticancer activity is completely abolished. B. In the new intravenous formulation of DS, the thiol groups in DS are protected. After delivered to cancer tissues, DS chelates copper to generate ROS inducing apoptosis. The end product, DDC-Cu, can penetrate into cancer cells and trigger apoptosis, as well (Tawari et al. Toxicol Res 2015; 4:1439). This hypothesis explained the discrepancy of anticancer activity of DS in the lab and in clinic and the need to develop intravenously applicable nano-encapsulated DS, e.g. liposome and PLGA encapsulated DS.
Nanotechnology-based Formulation Strategies for Delivery of Disulfiram
The use of nanotechnology, a booming field in the last decade, could provide us with a cutting-edge drug delivery system for translating DSF into cancer treatment. There are various methods such as adjusting the formulation, structural modification, or encapsulation techniques, all of which aims at increasing the bioavailability of the interested compound. Nanoencapsulation involves the use of biodegradable compounds such as liposomes, polymers, polymeric micelles or protein (albumin) particles to encapsulate the drug of interest (Shi et al., 2017). Several FDA-approved formulations of nano encapsulated conventional anticancer drugs such as albumin nanoparticle Nab-paclitaxel (Abraxane™) and liposome encapsulated doxorubicin are currently used in the clinics. A summary of various strategies developed by different groups for delivering DSF is given in Table 3.
TABLE 3.
Formulation strategies used for delivery of disulfiram for cancer treatment.
| Formulation | Components and technique used | Cancer indication | Cell lines/models | Study outcome | Reference |
|---|---|---|---|---|---|
| Polymers | |||||
| DSF encapsulated mixed nanoparticles | mPEG5000-PCL5000, PCL5000, MCT and DSF – High pressure homogenization method | Mouse hepatocellular adenocarcinoma | H22 xenograft tumor model | • Increased Drug loading and stability | Zhuo et al. (2018) |
| • Higher degree of tumor necrosis and tumor inhibition in animal models | |||||
| DSF encapsulated PLGA nanoparticles | PVA, PLGA, DSF Nanoprecipitation method | Breast Cancer | MCF7 cell lines | • Significantly enhanced stability when compared to free drug in serum solution (10% FBS) | Fasehee et al. (2017) |
| • Enhanced cytotoxicity compared to free drug | |||||
| Folate receptor targeted PLGA-PEG nanoparticles encapsulated with DSF | PLGA, (PEG)-bis amine, PVA, Folate, DSF Nanoprecipitation method | Breast cancer | MCF7 and 4T1 cell lines, BALB/c mice models | • Showed a higher degree of cytotoxicity compared to free drug and non-folate formulations | Fasehee et al. (2016) |
| • Significant decrease of tumor size in mouse models | |||||
| Combination of polyethylene glycol-cisplatin complex and DSF encapsulated nanoparcticles | mPEG-PLGA/PCL, PGA, DSF, Cisplatin | Lung cancer | A549 and A549DDP cell lines. BALB/c mouse model | • synergistically enhanced the cytotoxic effect of cisplatin in vitro | Song et al. (2016) |
| • The combination effectively inhibited the tumor growth and displayed excellent safety in mouse models | |||||
| PLGA encapsulated DSF Nanoparticles | PVA, PLGA, DSF. emulsion-solvent evaporation method | Lung cancer | A549 cell lines | • enhanced half-life in serum | Najlah et al. (2017) |
| • Higher cytotoxicity compared to free DSF in vitro | |||||
| Brain tumor-penetrating disulfiram nanoparticles | mPEG, PLGA, DSF. emulsion-solvent evaporation method | Brain cancer | T98G and DAOY cell lines and Female CD-1 mice, female athymic nude (nu/nu) mice, and the triple immune-deficient NCG mice models | • Enhanced delivery of DSF to the brain tumor site and increased stability | Madala et al. (2018) |
| • Potent cytotoxic and anti-clonogenic activities | |||||
| Lipid based nanoparticles | |||||
| Liposome encapsulated formulation of DSF | PC, CHOL, PG Thin film hydration to form multilamellar vesicles subject to size reduction -100–300 nm | Breast Cancer | MCF7, MDA-MB-231, T47D S180 mouse sarcoma models - BALB/c mice. MDA breast cancer models -CD1 Nu/Nu mice | • Selective targeting of cancer cells, induction of apoptosis | (EP2648709B1, 2011; Liu et al., 2014) |
| • Enhanced efficacy of conventional anticancer drugs | |||||
| • Target cancer stem cells- reduction in CSC markers and clonogenicity | |||||
| • Protection of thiol group in vivo, react with Cu to exert anticancer activity in mouse models of sarcoma and breast cancer | |||||
| Co-encapsulated liposomal formulation of Doxorubicin and DSF | DSPC, CHOL, mPEG2000-DSPE. Thin film hydration method | Breast cancer | MCF7, MDA-MB-231 | • Decrease in Pgp expression and reversal of Pgp mediated drug resistance | Rolle et al. (2020) |
| • Enhanced efficacy of DOX | |||||
| DSF entrapped vitamin E-TPGS-modified PEGylated nanostructured lipid carriers | Lecithin, Precirol® ATO, Labrafac Lipophile WL134, DSF. Emulsification ultrasonication method | Breast cancer | MCF7, 4T1, BALB/C mouse models | • Enhanced long term stability | Banerjee et al. (2019) |
| • Higher toxicity compared to free drug in vitro | |||||
| • Significant increase in tumor inhibition with no toxicity in vivo | |||||
| PEGylated Liposome Encapsulating DSF | HSPC, DPPC, CHOL, DSPE-PEG2000, DSF. Ethanol-based proliposome technology | Colorectal cancer | H630-WT and H630-R10 | • Significantly improved serum stability | Najlah et al. (2019) |
| • Cytotoxic to colorectal cancer cell lines in presence of copper in vitro | |||||
| Micelle based nanoparticles | |||||
| pH triggered polymeric micelles for the co-delivery of paclitaxel/disulfiram | Methoxy PEG-b-PLL, DMA, PTX and DSF. Nanoprecipitation method | Breast Cancer | MCF7 and MAF7/ADR | • Enhanced internalization of nanoparticles into tumor cells | Huo et al. (2017) |
| • Inhibition of Pgp transport function | |||||
| DSF encapsulated micelles | mPEG5k-b-PLGA2k/PCL3.4 k/MCT, DSF solvent diffusion method | Liver cancer | H22 xenograft mouse model | • Increased stability and bioavailability in plasma | Miao et al. (2018) |
| • Enhanced tumor inhibition in mouse model | |||||
| DSF loaded redox-sensitive shell crosslinked micelles | SMA micelles were crosslinked by adding cystamine dihydrochloride and sodium bicarbonate | Breast cancer | 4T1 | • Significant tumour inhibition | Duan et al. (2014) |
| • Increased Stability | |||||
| Other Nanoparticle delivery systems | |||||
| DSF- loaded magnetic mesoporous silica nanoparticles | PEI-FA, Fe3O4@mSiO2 Np’s, DSF Thermal decomposition and Sol gel reaction method | Breast Cancer | MCF7 | • Exhibited selective toxicity to tumor cells | Solak et al. (2021) |
| • Highly cytotoxic in the presence of copper | |||||
| Cu(DDC)2 and regorafenib encapsulated BSA nanoparticles | Denaturing of BSA by urea to induce nanoparticle formulation | Glioma | H22 | • Improved stability and increased efficacy against resistant xenografted tumors | Zhao et al. (2018) |
| DSF loaded gold nanorods | Seed solution preparation and surface PEG modification | Breast Cancer | MCF-7 | • Improved circulation time, increased tumor accumulation, tumor shrinkage in vivo | Xu et al. (2020a) |
| Cyclodextrin Cu(DDC)2 | SBE-CD and HP-CD were the cyclodextrins used | Breast cancer | MDA-MB231 | • Stability for 28 days, retained anti-cancer properties of Cu(DDC)2 | Said Suliman et al. (2021) |
Polymeric nanoparticles are simple in design and encompass extensive structural diversity, outstanding bio-compatibility and stability (El-Say and El-Sawy, 2017). One of the best examples of polymeric nano encapsulation is PLGA. It was demonstrated that PLGA encapsulation shielded DS from breakdown in the bloodstream and transported the intact DSF to cancer tissues (Wang et al., 2017b). The efficacy of PLGA-based DSF nanoparticles was demonstrated in a wide range of cancers using xenograft mouse models of hepatocellular carcinoma, lung cancer, ovarian cancer, and breast cancer (McConville et al., 2015; Fasehee et al., 2016; Wang et al., 2017a; Fasehee et al., 2017; Najlah et al., 2017). In addition, polymeric nanoparticles can be modified extensively to add functional groups that can be targeted for delivery to a tissue of interest. For example, a folate receptor targeted PEG-PLGA was shown to deliver and enrich DSF specifically to breast cancer tissues while avoiding degradation of DSF in the bloodstream (Fasehee et al., 2016). Our group developed DSF loaded PLGA nanoparticles through emulsion-solvent evaporation method with excellent entrapment efficiency and better drug loading. The nanoparticles were in the size range of ∼136 nm and had significantly extended the half-life from 2 min to 7 h in serum. The encapsulated DSF also demonstrated a significant synergistic cytotoxicity in combination with 5-FU or sorafenib and inhibited the growth of cancer stem cell populations in liver cancer models and reduced the metastatic activity and recurrence in mouse HCC xenograft and metastatic models (Wang et al., 2017b).
Liposomes which are artificial spherical vesicles prepared from naturally derived phospholipids are the most widely investigated and well-established drug delivery carriers used in clinics to treat various cancers due to their biocompatibility, specifically being non-immunogenic, non-toxic, and biodegradable. In addition, liposomes serve as sustained release systems providing protection against degradation and increased the time in circulation (Moosavian et al., 2021; van der Koog et al., 2021). In this sense, our group recently demonstrated that liposome encapsulated DSF a relatively extended half-life in serum and a significantly increased anticancer efficacy in in vitro and in vivo breast cancer mouse models (Liu et al., 2014).
Considering the hydrophobic nature of DSF, liposome-based delivery system would be an exciting prospect. Our group has developed liposome encapsulated DSF which shows significantly improved anticancer efficacy in breast cancer and sarcoma mouse models (EP2648709B1, 2011; Liu et al., 2014). Recently, we also encapsulated DSF into a PEGylated liposomal formulation of DSF using ethanol-based proliposome technique followed by high-pressure homogenization with significantly improvement in the stability of DS in serum and effective cytotoxicity to colorectal cancer cell lines in the presence of Cu in vitro (Najlah et al., 2019). Another group has developed an encapsulated formulation of DSF and doxorubicin which reduced the expression of P-gp and increased the efficacy of doxorubicin in breast cancer cells (Rolle et al., 2020). Banerjee et al. (2019) has shown that a formulation of nanostructured lipid carriers (NLC) encapsulated DSF modified with d-α-tocopheryl polyethylene glycol 1000 succinate (vitamin E-TPGS) showed an enhanced uptake in breast cancer cell lines and significantly reduced the tumour size and enhanced tumour suppression in xenografted mouse models (Banerjee et al., 2019).
Micelles have unique properties such as low molecular weight, better entrapment and enhanced delivery in tumour sites and are very suitable for a hydrophobic drug like DSF. They can easily penetrate the tumour tissue in comparison to other nanoparticles (Deshmukh et al., 2017). Our group developed a Pluronic micelle formulation to deliver DSF for triple negative breast cancer treatment. However, the main drawback of using micelles for DSF is their stability. Duan et al. developed a micelle cross-linked with redox sensitive shells that delivers DSF in reduced environments of the tumours (Duan et al., 2014). Another modified version of micelles using PEG and poly-lysine copolymers blocks were also used for delivery of DSF and paclitaxel together for tackling multidrug resistance and P-gp inhibition in breast cancer models and resulted in enhanced uptake of paclitaxel by breast cancer cells (Huo et al., 2017).
There are also more unique methods of delivering DSF; for example, magnetic mesoporous silica nanoparticles were used to specifically deliver DSF to breast cancer cells (MCAF) in vitro (Solak et al., 2021). DSF has also been loaded onto gold nanorods with a reported a drug loading content of up to 23.2% (Xu et al., 2020a). The Au-DSF product was able to effectively increase ROS and induce apoptosis in breast cancer cells in vitro.
Delivery of DDC Complexes with Macromolecules
Albumin is a promising macromolecular drug carrier as it is biocompatible, biodegradable, non-immunogenic and soluble in water to allow for injection. In addition to this, it is possible to modify the surface of albumin nanoparticles for targeting. Previously, nanoparticle albumin bound technology has been used to solubilize the hydrophobic cancer drug paclitaxel (Abraxane) which is now a first line drug for PDAC (Stinchcombe, 2007). Since the complexes Cu(DDC)2 and Zn(DDC)2 are hydrophobic; nanoparticle albumin bound technology may be the key to develop an injectable, long circulating formulation.
Cu(DDC)2 and Regorafenib (Rego) were successfully encapsulated in bovine serum albumin (BSA) nanoparticles via denaturation of BSA by urea (Zhao et al., 2017). The formulation targeted secreted protein acidic and rich in cysteine (SPARC) receptors. Furthermore, mannose receptors were targeted by modifying the surface of the BSA nanoparticles. It had enhanced anti-cancer efficacy in an animal model bearing resistant tumours via apoptosis, upregulation of intracellular ROS, anti-angiogenesis and tumor-associated macrophage repolarization. These nanoparticles could also effectively cross the blood brain barrier to deliver Cu(DDC)2 and Rego to treat gliomas in mice (Zhao et al., 2018). The option to selectively target cancer cells by modifying the surface of the BSA nanoparticles could be vital when delivering DDC already complexed with Cu compared with DSF alone. DSF targets cancer cells due to their increased levels of Cu. On the other hand, a Cu(DDC)2 complex may have increased toxicities on normal cells. Another suitable macromolecule carrier for DSF is Cyclodextrans (CDs) which are cyclic oligosaccharides that can form truncated-cone-shaped macrocyclic molecules that possess hydrophobic cavities. These cavities can solubilize hydrophobic drugs and Suliman et al. (2021) developed a CD formulation of Cu(DDC)2 and demonstrated stability for 28 days with enhanced cytotoxicity against resistant breast cancer cell lines (Said Suliman et al., 2021).
Together, all the above evidence has indicated that using nanotechnology-based formulation strategies can significantly improve the half-life, stability, bioavailability and it is possible to achieve delivery of DSF at therapeutic doses in cancer patients.
Conclusion
The success of platinum drugs has led to the search for metal complex-based anticancer therapeutics. The search for novel metallodrugs or metal complexes that target cancers is still a field of high interest in the pharmaceutical sector. The fundamental role of Cu in aiding cancer progression and the dysregulation of Cu homeostasis in cancers has been well documented in both preclinical and clinical settings. With Cu as an emerging novel target, there has been major advancements in the development of Cu coordination complexes as anti-cancer therapeutics. Use of Cu chelators and Cu ionophores have yielded encouraging anticancer activities in many preclinical models. Particularly many drugs that are used for other indications have been identified and repurposed as Cu coordination drugs, not only to achieve better antitumour efficacy, but also to enhance the efficacy of first line anticancer drugs such as platins.
Among many repurposed metal chelator drugs, DSF has a great promise and potential to be used as an anticancer agent. With its anti-angiogenic potential, and the ability to target CSCs and synergistically potentiate many first line conventional drugs, DSF could provide new opportunities to develop effective combination therapeutic strategies. DSF also has a great potential be an effective immunomodulatory drug by regulating NF-kB signalling or by reducing PDL-1 expression by Cu chelation. In this review we have summarised the effectiveness of DSF as an anticancer agent, which applies to a wide range of cancers. Despite several clinical trials, no reliable efficacy of DSF as an anticancer drug for tumours has been observed due to the lack of biological availability and short half-life of the currently available oral DSF. Therefore, the development of an appropriate formulation is critical for the successful translation of DSF into cancer indication. Here we summarized different delivery strategies that have been developed for DSF-based cancer therapy. Most of the identified or developed drug delivery systems for DSF are at the proof-of-concept stage either in in vitro studies or in vivo pre-clinical animal models. Although many of these delivery strategies demonstrated great potentials for DSF as an anticancer agent, these delivery systems must be extensively evaluated with further studies to gather complete preclinical information such as safety, toxicology, pharmacokinetic and optimal physicochemical properties which are crucial to facilitate clinical translation.
Although many different delivery strategies using novel materials indicated additional benefits, the use of FDA approved excipients could minimize regulatory hurdles and considerably reduce the preclinical development time. Moreover, the development of encapsulation strategies should be suitable for scale-up production for manufacturing and commercialization. In addition to these technical issues, there are many regulatory and marketing hurdles for commercialization of DSF based anticancer product, predominantly due to the lack of patent protection on DSF.
Therefore, pharma companies do not show much interest to invest in clinical trials for DSF-based anticancer therapeutics. The development of novel formulations of DSF together with appropriate strategies for exploitation of regulatory guidelines, would lead not only to the development of intellectual properties and enhanced anticancer efficacy, but also will lead to the successful development and clinical translation of DSF as an anticancer product for commercialization.
Author Contributions
VK, WW, and QPD conceived the idea. VK played main role in liaising the collaborative team, collecting data, writing up and reviewing the whole manuscript. WW and QPD critically reviewed and revised the manuscript. MA, BS, GR, SE, and HT contributed to manuscript writing. BS and GR played key roles in providing data in formulation of DSF using nano-drug-delivery system. All the authors read and approved the final version of the manuscript.
Conflict of Interest
VK is the Science Projects Manager and WW is the Director and CSO of Disulfican Ltd, an early-stage University spin-out company, with patents and a pre-clinical data package for a proprietary formulation of encapsulated disulfiram for cancer therapy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adams J. (2004). The Proteasome: A Suitable Antineoplastic Target. Nat. Rev. Cancer 4, 349–360. 10.1038/nrc1361 [DOI] [PubMed] [Google Scholar]
- Adeoti M., Oguntola A., Akanni E., Agodirin O., Oyeyemi G. (2015). Trace Elements; Copper, Zinc and Selenium, in Breast Cancer Afflicted Female Patients in LAUTECH Osogbo, Nigeria. Indian J. Cancer 52, 106–109. 10.4103/0019-509x.175573 [DOI] [PubMed] [Google Scholar]
- al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J., Clarke M. F. (2003). Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. 100, 3983–3988. 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharvand M., Manifar S., Akkafan R., Mortazavi H., Sabour S. (2014). Serum Levels of Ferritin, Copper, and Zinc in Patients with Oral Cancer. Biomed. J. 37, 331–336. 10.4103/2319-4170.132888 [DOI] [PubMed] [Google Scholar]
- Baltaci A. K., Dundar T. K., Aksoy F., Mogulkoc R. (2017). Changes in the Serum Levels of Trace Elements Before and After the Operation in Thyroid Cancer Patients. Biol. Trace Elem. Res. 175, 57–64. 10.1007/s12011-016-0768-2 [DOI] [PubMed] [Google Scholar]
- Banerjee P., Geng T., Mahanty A., Li T., Zong L., Wang B. (2019). Integrating the Drug, Disulfiram into the Vitamin E-TPGS-Modified PEGylated Nanostructured Lipid Carriers to Synergize its Repurposing for Anti-cancer Therapy of Solid Tumors. Int. J. Pharmaceutics 557, 374–389. 10.1016/j.ijpharm.2018.12.051 [DOI] [PubMed] [Google Scholar]
- Basu S., Singh M. K., Singh T. B., Bhartiya S. K., Singh S. P., Shukla V. K. (2013). Heavy and Trace Metals in Carcinoma of the Gallbladder. World J. Surg. 37, 2641–2646. 10.1007/s00268-013-2164-9 [DOI] [PubMed] [Google Scholar]
- Bedard P. L., Hyman D. M., Davids M. S., Siu L. L. (2020). Small Molecules, Big Impact: 20 Years of Targeted Therapy in Oncology. The Lancet 395, 1078–1088. 10.1016/s0140-6736(20)30164-1 [DOI] [PubMed] [Google Scholar]
- Bertolini F., Sukhatme V. P., Bouche G. (2015). Drug Repurposing in Oncology-Patient and Health Systems Opportunities. Nat. Rev. Clin. Oncol. 12, 732–742. 10.1038/nrclinonc.2015.169 [DOI] [PubMed] [Google Scholar]
- Blockhuys S., Zhang X., Wittung-Stafshede P. (2020). Single-cell Tracking Demonstrates Copper Chaperone Atox1 to Be Required for Breast Cancer Cell Migration. Proc. Natl. Acad. Sci. USA 117, 2014–2019. 10.1073/pnas.1910722117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady D. C., Crowe M. S., Turski M. L., Hobbs G. A., Yao X., Chaikuad A., et al. (2014). Copper Is Required for Oncogenic BRAF Signalling and Tumorigenesis. Nature 509, 492–496. 10.1038/nature13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S. S., Zagzag D., Tsanaclis A. M., Gately S., Elkouby M. P., Brien S. E. (1990). Inhibition of Angiogenesis and Tumor Growth in the Brain. Suppression of Endothelial Cell Turnover by Penicillamine and the Depletion of Copper, an Angiogenic Cofactor. Am. J. Pathol. 137, 1121–1142. [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J. (2003). Tetrathiomolybdate Anticopper Therapy for Wilson's Disease Inhibits Angiogenesis, Fibrosis and Inflammation. J. Cell. Mol. Med. 7, 11–20. 10.1111/j.1582-4934.2003.tb00198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning A., Kast R. E. (2014). Oxidizing to Death. Cell Cycle 13, 1513–1514. 10.4161/cc.28959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher K., Kannappan V., Kilari R. S., Morris M. R., Mcconville C., Armesilla A. L., et al. (2018). Investigation of the Key Chemical Structures Involved in the Anticancer Activity of Disulfiram in A549 Non-small Cell Lung Cancer Cell Line. BMC Cancer 18, 753. 10.1186/s12885-018-4617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentieri U., Myers J., Thorpe L., Daeschner C. W., Haggard M. E. (1986). Copper, Zinc, and Iron in Normal and Leukemic Lymphocytes from Children. Cancer Res. 46, 981–984. [PubMed] [Google Scholar]
- Cater M. A., Haupt Y. (2011). Clioquinol Induces Cytoplasmic Clearance of the X-Linked Inhibitor of Apoptosis Protein (XIAP): Therapeutic Indication for Prostate Cancer. Biochem. J. 436, 481–491. 10.1042/bj20110123 [DOI] [PubMed] [Google Scholar]
- Cen D., Brayton D., Shahandeh B., Meyskens F. L., JR., Farmer P. J. (2004). Disulfiram Facilitates Intracellular Cu Uptake and Induces Apoptosis in Human Melanoma Cells. J. Med. Chem. 47, 6914–6920. 10.1021/jm049568z [DOI] [PubMed] [Google Scholar]
- Chen D., Cui Q. C., Yang H., Dou Q. P. (2006). Disulfiram, A Clinically Used Anti-alcoholism Drug and Copper-Binding Agent, Induces Apoptotic Cell Death in Breast Cancer Cultures and Xenografts via Inhibition of the Proteasome Activity. Cancer Res. 66, 10425–10433. 10.1158/0008-5472.can-06-2126 [DOI] [PubMed] [Google Scholar]
- Chiba T., Suzuki E., Yuki K., Zen Y., Oshima M., Miyagi S., et al. (2014). Disulfiram Eradicates Tumor-Initiating Hepatocellular Carcinoma Cells in ROS-P38 MAPK Pathway-dependent and -independent Manners. PLoS One 9, e84807. 10.1371/journal.pone.0084807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar C. R. (2012). Gallium-containing Anticancer Compounds. Future Med. Chem. 4, 1257–1272. 10.4155/fmc.12.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua H. L., Bhat-Nakshatri P., Clare S. E., Morimiya A., Badve S., Nakshatri H. (2007). NF-κB Represses E-Cadherin Expression and Enhances Epithelial to Mesenchymal Transition of Mammary Epithelial Cells: Potential Involvement of ZEB-1 and ZEB-2. Oncogene 26, 711–724. 10.1038/sj.onc.1209808 [DOI] [PubMed] [Google Scholar]
- Cohen P., Cross D., Jänne P. A. (2021). Kinase Drug Discovery 20 Years after Imatinib: Progress and Future Directions. Nat. Rev. Drug Discov. 20, 551–569. 10.1038/s41573-021-00195-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvek B., Dvorak Z. (2007). Targeting of Nuclear Factor-Κb and Proteasome by Dithiocarbamate Complexes with Metals. Cpd 13, 3155–3167. 10.2174/138161207782110390 [DOI] [PubMed] [Google Scholar]
- Cvek B., Dvorak Z. (2008). The Value of Proteasome Inhibition in Cancer. Drug Discov. Today 13, 716–722. 10.1016/j.drudis.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Cvek B., Milacic V., Taraba J., Dou Q. P. (2008). Ni(II), Cu(II), and Zn(II) Diethyldithiocarbamate Complexes Show Various Activities Against the Proteasome in Breast Cancer Cells. J. Med. Chem. 51, 6256–6258. 10.1021/jm8007807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves H. T., Alexander F. W., Lay H. (1973). Copper and Zinc Concentration in the Plasma of Leukaemic Children. Br. J. Haematol. 24, 525–531. 10.1111/j.1365-2141.1973.tb01678.x [DOI] [PubMed] [Google Scholar]
- Demir C., Demir H., Esen R., Sehitogullari A., Atmaca M., Alay M. (2011). Altered Serum Levels of Elements in Acute Leukemia Cases in Turkey. Asian Pac. J. Cancer Prev. 12, 3471–3474. [PubMed] [Google Scholar]
- Deshmukh A. S., Chauhan P. N., Noolvi M. N., Chaturvedi K., Ganguly K., Shukla S. S., et al. (2017). Polymeric Micelles: Basic Research to Clinical Practice. Int. J. Pharmaceutics 532, 249–268. 10.1016/j.ijpharm.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Díez M., Arroyo M., Cerdàn F. J., Muñoz M., Martin M. A., Balibrea J. L. (1989a). Serum and Tissue Trace Metal Levels in Lung Cancer. Oncology 46, 230–234. 10.1159/000226722 [DOI] [PubMed] [Google Scholar]
- Díez M., Cerdà F. J., Arroyo M., Balibrea J. L. (1989b). Use of the Copper/zinc Ratio in the Diagnosis of Lung Cancer. Cancer 63, 726–730. [DOI] [PubMed] [Google Scholar]
- Ding N., Zhu Q. (2018). Disulfiram Combats Cancer via Crippling Valosin-Containing Protein/p97 Segregase Adaptor NPL4. Transl. Cancer Res. 7, S495–S499. 10.21037/tcr.2018.03.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Jiang M., Jing H., Sheng W., Wang X., Han J., et al. (2015). Analysis of Serum Levels of 15 Trace Elements in Breast Cancer Patients in Shandong, China. Environ. Sci. Pollut. Res. 22, 7930–7935. 10.1007/s11356-014-3970-9 [DOI] [PubMed] [Google Scholar]
- Druker B. J., Sawyers C. L., Kantarjian H., Resta D. J., Reese S. F., Ford J. M., et al. (2001). Activity of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in the Blast Crisis of Chronic Myeloid Leukemia and Acute Lymphoblastic Leukemia with the Philadelphia Chromosome. N. Engl. J. Med. 344, 1038–1042. 10.1056/nejm200104053441402 [DOI] [PubMed] [Google Scholar]
- Duan X., Xiao J., Yin Q., Zhang Z., Yu H., Mao S., et al. (2014). Multi-targeted Inhibition of Tumor Growth and Lung Metastasis by Redox-Sensitive Shell Crosslinked Micelles Loading Disulfiram. Nanotechnology 25, 125102. 10.1088/0957-4484/25/12/125102 [DOI] [PubMed] [Google Scholar]
- Ekinci E., Rohondia S., Khan R., Dou Q. P. (2019). Repurposing Disulfiram as an Anti-cancer Agent: Updated Review on Literature and Patents. Pra 14, 113–132. 10.2174/1574892814666190514104035 [DOI] [PubMed] [Google Scholar]
- El-Say K. M., El-Sawy H. S. (2017). Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharmaceutics 528, 675–691. 10.1016/j.ijpharm.2017.06.052 [DOI] [PubMed] [Google Scholar]
- Eneanya D. I., Bianchine J. R., Duran D. O., Andresen B. D. (1981). The Actions and Metabolic Fate of Disulfiram. Annu. Rev. Pharmacol. Toxicol. 21, 575–596. 10.1146/annurev.pa.21.040181.003043 [DOI] [PubMed] [Google Scholar]
- EP2648709B1 (2011). DISULFIRAM FORMULATION AND USES THEREOF. EU patent application EP2648709 B1. [Google Scholar]
- Fasehee H., Ghavamzadeh A., Alimoghaddam K., Ghaffari S. H., Faghihi S. (2017). A Comparative Cytotoxic Evaluation of Disulfiram Encapsulated PLGA Nanoparticles on MCF-7 Cells. Int. J. Hematol. Oncol. Stem Cel Res 11, 102–107. [PMC free article] [PubMed] [Google Scholar]
- Fasehee H., Dinarvand R., Ghavamzadeh A., Esfandyari-Manesh M., Moradian H., Faghihi S., et al. (2016). Delivery of Disulfiram into Breast Cancer Cells Using Folate-Receptor-Targeted PLGA-PEG Nanoparticles: In Vitro and In Vivo Investigations. J. Nanobiotechnol 14, 32. 10.1186/s12951-016-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.-F., Lu L., Zeng P., Yang Y.-H., Luo J., Yang Y.-W., et al. (2012). Serum Total Oxidant/antioxidant Status and Trace Element Levels in Breast Cancer Patients. Int. J. Clin. Oncol. 17, 575–583. 10.1007/s10147-011-0327-y [DOI] [PubMed] [Google Scholar]
- Feng W., Ye F., Xue W., Zhou Z., Kang Y. J. (2009). Copper Regulation of Hypoxia-Inducible Factor-1 Activity. Mol. Pharmacol. 75, 174–182. 10.1124/mol.108.051516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando J., Malfettone A., Cepeda E. B., Vilarrasa-Blasi R., Bertran E., Raimondi G., et al. (2015). A Mesenchymal-like Phenotype and Expression of CD44 Predict Lack of Apoptotic Response to Sorafenib in Liver Tumor Cells. Int. J. Cancer 136, E161–E172. 10.1002/ijc.29097 [DOI] [PubMed] [Google Scholar]
- Finney L., Vogt S., Fukai T., Glesne D. (2009). Copper and Angiogenesis: Unravelling a Relationship Key to Cancer Progression. Clin. Exp. Pharmacol. Physiol. 36, 88–94. 10.1111/j.1440-1681.2008.04969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza M., Hindo S., Chen D., Davenport A., Schmitt S., Tomco D., et al. (2010). Novel Metals and Metal Complexes as Platforms for Cancer Therapy. Cpd 16, 1813–1825. 10.2174/138161210791209009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallery M., Blank J. L., Lin Y., Gutierrez J. A., Pulido J. C., Rappoli D., et al. (2007). The JAMM Motif of Human Deubiquitinase Poh1 Is Essential for Cell Viability. Mol. Cancer Ther. 6, 262–268. 10.1158/1535-7163.mct-06-0542 [DOI] [PubMed] [Google Scholar]
- Ginestier C., Hur M. H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., et al. (2007). ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 1, 555–567. 10.1016/j.stem.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. M., Seaton D. R. (1942). Treatment of Scabies. Bmj 1, 685–687. 10.1136/bmj.1.4248.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Yang Z., Kulbe H., Albers A. E., Sehouli J., Kaufmann A. M. (2019). Inhibitory Effect on Ovarian Cancer ALDH+ Stem-like Cells by Disulfiram and Copper Treatment Through ALDH and ROS Modulation. Biomed. Pharmacother. 118, 109371. 10.1016/j.biopha.2019.109371 [DOI] [PubMed] [Google Scholar]
- Guo X., Xu B., Pandey S., Goessl E., Brown J., Armesilla A. L., et al. (2010). Disulfiram/copper Complex Inhibiting NFκB Activity and Potentiating Cytotoxic Effect of Gemcitabine on Colon and Breast Cancer Cell Lines. Cancer Lett. 290, 104–113. 10.1016/j.canlet.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Shukla V. K., Vaidya M. P., Roy S. K., Gupta S. (1993). Serum and Tissue Trace Elements in Colorectal Cancer. J. Surg. Oncol. 52, 172–175. 10.1002/jso.2930520311 [DOI] [PubMed] [Google Scholar]
- Gupte A., Mumper R. J. (2007). An Investigation into Copper Catalyzed D-Penicillamine Oxidation and Subsequent Hydrogen Peroxide Generation. J. Inorg. Biochem. 101, 594–602. 10.1016/j.jinorgbio.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Hald J., Jacobsen E. (1948). A Drug Sensitising the Organism to Ethyl Alcohol. The Lancet 252, 1001–1004. 10.1016/s0140-6736(48)91514-1 [DOI] [PubMed] [Google Scholar]
- Hart B. W., Faiman M. D. (1992). In Vitro and In Vivo Inhibition of Rat Liver Aldehyde Dehydrogenase by S-Methyl N, N-Diethylthiolcarbamate Sulfoxide, A New Metabolite of Disulfiram. Biochem. Pharmacol. 43, 403–406. 10.1016/0006-2952(92)90555-w [DOI] [PubMed] [Google Scholar]
- Hellstrom E., Tottmar O., Widerlöv E. (1983). Effects of Oral Administration or Implantation of Disulfiram on Aldehyde Dehydrogenase Activity in Human Blood. Alcohol. Clin. Exp. Res. 7, 231–236. 10.1111/j.1530-0277.1983.tb05448.x [DOI] [PubMed] [Google Scholar]
- Hiam-Galvez K. J., Allen B. M., Spitzer M. H. (2021). Systemic Immunity in Cancer. Nat. Rev. Cancer 21, 345–359. 10.1038/s41568-021-00347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Chen Z., Fang L. (2013). Curcumin Inhibits LPS-Induced EMT through Downregulation of NF-Κb-Snail Signaling in Breast Cancer Cells. Oncol. Rep. 29, 117–124. 10.3892/or.2012.2080 [DOI] [PubMed] [Google Scholar]
- Huo Q., Zhu J., Niu Y., Shi H., Gong Y., Li Y., et al. (2017). pH-Triggered Surface Charge-Switchable Polymer Micelles for the Co-delivery of Paclitaxel/disulfiram and Overcoming Multidrug Resistance in Cancer. Ijn 12, 8631–8647. 10.2147/ijn.s144452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S., Andreux P., Poitry-Yamate C., Auwerx J., Hanahan D. (2013). Bioavailable Copper Modulates Oxidative Phosphorylation and Growth of Tumors. Proc. Natl. Acad. Sci. 110, 19507–19512. 10.1073/pnas.1318431110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B., Brocker C., Thompson D. C., Black W., Vasiliou K., Nebert D. W., et al. (2011). Update on the Aldehyde Dehydrogenase Gene (ALDH) Superfamily. Hum. Genomics 5, 283–303. 10.1186/1479-7364-5-4-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N., Zhu X., Cheng F., Zhang L. (2018). Disulfiram/copper Targets Stem Cell‐like ALDH + Population of Multiple Myeloma by Inhibition of ALDH1A1 and Hedgehog Pathway. J. Cel. Biochem. 119, 6882–6893. 10.1002/jcb.26885 [DOI] [PubMed] [Google Scholar]
- Jin Y., Zhang C., Xu H., Xue S., Wang Y., Hou Y., et al. (2011). Combined Effects of Serum Trace Metals and Polymorphisms of CYP1A1 or GSTM1 on Non-small Cell Lung Cancer: A Hospital Based Case-Control Study in China. Cancer Epidemiol. 35, 182–187. 10.1016/j.canep.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Johansson B. (1992). A Review of the Pharmacokinetics and Pharmacodynamics of Disulfiram and its Metabolites. Acta Psychiatr. Scand. 86, 15–26. 10.1111/j.1600-0447.1992.tb03310.x [DOI] [PubMed] [Google Scholar]
- Julien S., Puig I., Caretti E., Bonaventure J., Nelles L., Van Roy F., et al. (2007). Activation of NF-Κb by Akt Upregulates Snail Expression and Induces Epithelium Mesenchyme Transition. Oncogene 26, 7445–7456. 10.1038/sj.onc.1210546 [DOI] [PubMed] [Google Scholar]
- Junttila M. R., Li S. P., Westermarck J. (2008). Phosphatase‐mediated Crosstalk Between MAPK Signaling Pathways in the Regulation of Cell Survival. FASEB j. 22, 954–965. 10.1096/fj.06-7859rev [DOI] [PubMed] [Google Scholar]
- Kaiafa G. D., Saouli Z., Diamantidis M. D., Kontoninas Z., Voulgaridou V., Raptaki M., et al. (2012). Copper Levels in Patients with Hematological Malignancies. Eur. J. Intern. Med. 23, 738–741. 10.1016/j.ejim.2012.07.009 [DOI] [PubMed] [Google Scholar]
- Kane R. C., Farrell A. T., Sridhara R., Pazdur R. (2006). United States Food and Drug Administration Approval Summary: Bortezomib for the Treatment of Progressive Multiple Myeloma After One Prior Therapy. Clin. Cancer Res. 12, 2955–2960. 10.1158/1078-0432.ccr-06-0170 [DOI] [PubMed] [Google Scholar]
- Kim Y.-J., Kim J. Y., Lee N., Oh E., Sung D., Cho T.-M., et al. (2017). Disulfiram Suppresses Cancer Stem-like Properties and STAT3 Signaling in Triple-Negative Breast Cancer Cells. Biochem. Biophysical Res. Commun. 486, 1069–1076. 10.1016/j.bbrc.2017.03.164 [DOI] [PubMed] [Google Scholar]
- Kim Y.-J., Tsang T., Anderson G. R., Posimo J. M., Brady D. C. (2020). Inhibition of BCL2 Family Members Increases the Efficacy of Copper Chelation in BRAFV600E-Driven Melanoma. Cancer Res. 80, 1387–1400. 10.1158/0008-5472.can-19-1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Boggon T. J., Dayaram T., Jänne P. A., Kocher O., Meyerson M., et al. (2005). EGFRMutation and Resistance of Non-small-cell Lung Cancer to Gefitinib. N. Engl. J. Med. 352, 786–792. 10.1056/nejmoa044238 [DOI] [PubMed] [Google Scholar]
- Kona F. R., Buac D., Burger A. M. (2011). Disulfiram, and Disulfiram Derivatives as Novel Potential Anticancer Drugs Targeting the Ubiquitin-Proteasome System in Both Preclinical and Clinical Studies. Curr. Cancer Drug Targets 11, 338–346. 10.2174/156800911794519798 [DOI] [PubMed] [Google Scholar]
- Kuo H. W., Chen S. F., Wu C. C., Chen D. R., Lee J. H. (2002). Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Elem. Res. 89, 1–11. 10.1385/bter:89:1:1 [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Kim C. Y., Nam T. G. (2020). Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Devel Ther. 14, 5375–5392. 10.2147/dddt.s275007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl N. B., Nirmalakumar S., Ezeife D. A., Gyawali B. (2021). An Arm and A Leg: The Rising Cost of Cancer Drugs and Impact on Access. Am. Soc. Clin. Oncol. Educ. Book 41, 1–12. 10.1200/EDBK_100028 [DOI] [PubMed] [Google Scholar]
- Lelievre P., Sancey L., Coll J. L., Deniaud A., Busser B. (2020). The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers (Basel) 12 (12), 3594. 10.3390/cancers12123594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. J., Deshmukh P., Tedstone A. A., Tuna F., O'Brien P. (2014). On the Interaction of Copper(II) with Disulfiram. Chem. Commun. (Camb) 50, 13334–13337. 10.1039/c4cc04767b [DOI] [PubMed] [Google Scholar]
- Li Y., Chen F., Chen J., Chan S., He Y., Liu W., et al. (2020). Disulfiram/Copper Induces Antitumor Activity Against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways. Cancers (Basel) 12. 10.3390/cancers12010138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. (2020). Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 72, 1900–1908. 10.1002/iub.2341 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang L. H., Zhang H. T., Wang Y. T., Liu S., Zhou W. L., et al. (2018). Disulfiram Combined with Copper Inhibits Metastasis and Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Through the NF-kappaB and TGF-Beta Pathways. J. Cel Mol Med 22, 439–451. 10.1111/jcmm.13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky J. J., Shen M. L., Naylor S. (2001). In Vivo Inhibition of Aldehyde Dehydrogenase by Disulfiram. Chem. Biol. Interact 130-132, 93–102. 10.1016/s0009-2797(00)00225-8 [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. (1978). The Effect of D-Penicillamine on Mitogen-Induced Human Lymphocyte Proliferation: Synergistic Inhibition by D-Penicillamine and Copper Salts. J. Immunol. 120, 1006–1013. [PubMed] [Google Scholar]
- Liu P., Brown S., Goktug T., Channathodiyil P., Kannappan V., Hugnot J. P., et al. (2012). Cytotoxic Effect of Disulfiram/copper on Human Glioblastoma Cell Lines and ALDH-Positive Cancer-stem-like Cells. Br. J. Cancer 107, 1488–1497. 10.1038/bjc.2012.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Kumar I. S., Brown S., Kannappan V., Tawari P. E., Tang J. Z., et al. (2013). Disulfiram Targets Cancer Stem-like Cells and Reverses Resistance and Cross-Resistance in Acquired Paclitaxel-Resistant Triple-Negative Breast Cancer Cells. Br. J. Cancer 109, 1876–1885. 10.1038/bjc.2013.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Wang Z., Brown S., Kannappan V., Tawari P. E., Jiang W., et al. (2014). Liposome Encapsulated Disulfiram Inhibits NFkappaB Pathway and Targets Breast Cancer Stem Cells In Vitro and In Vivo. Oncotarget 5, 7471–7485. 10.18632/oncotarget.2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., He M., Li L., Wang X., Han S., Zhao J., et al. (2021). EMT and Cancer Cell Stemness Associated with Chemotherapeutic Resistance in Esophageal Cancer. Front. Oncol. 11, 672222. 10.3389/fonc.2021.672222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovborg H., Oberg F., Rickardson L., Gullbo J., Nygren P., Larsson R. (2006). Inhibition of Proteasome Activity, Nuclear Factor-KappaB Translocation and Cell Survival by the Antialcoholism Drug Disulfiram. Int. J. Cancer 118, 1577–1580. 10.1002/ijc.21534 [DOI] [PubMed] [Google Scholar]
- Lu C., Li X., Ren Y., Zhang X. (2021). Disulfiram: A Novel Repurposed Drug for Cancer Therapy. Cancer Chemother. Pharmacol. 87, 159–172. 10.1007/s00280-020-04216-8 [DOI] [PubMed] [Google Scholar]
- Lu X., Lin B., Xu N., Huang H., Wang Y., Lin J. M. (2020). Evaluation of the Accumulation of Disulfiram and its Copper Complex in A549 Cells Using Mass Spectrometry. Talanta 211, 120732. 10.1016/j.talanta.2020.120732 [DOI] [PubMed] [Google Scholar]
- Macdonald G., Nalvarte I., Smirnova T., Vecchi M., Aceto N., Dolemeyer A., et al. (2014). Memo Is a Copper-dependent Redox Protein with an Essential Role in Migration and Metastasis. Sci. Signal. 7, ra56. 10.1126/scisignal.2004870 [DOI] [PubMed] [Google Scholar]
- Madala H. R., Punganuru S. R., Ali-Osman F., Zhang R., Srivenugopal K. S. (2018). Brain- and Brain Tumor-Penetrating Disulfiram Nanoparticles: Sequence of Cytotoxic Events and Efficacy in Human Glioma Cell Lines and Intracranial Xenografts. Oncotarget 9, 3459–3482. 10.18632/oncotarget.23320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majera D., Skrott Z., Chroma K., Merchut-Maya J. M., Mistrik M., Bartek J. (2020). Targeting the NPL4 Adaptor of P97/VCP Segregase by Disulfiram as an Emerging Cancer Vulnerability Evokes Replication Stress and DNA Damage While Silencing the ATR Pathway. Cells 9 (2), 469. 10.3390/cells9020469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalioth E. J., Schenker J. G., Chevion M. (1983). Copper and Zinc Levels in Normal and Malignant Tissues. Cancer 52, 868–872. [DOI] [PubMed] [Google Scholar]
- Marine J. C., Dawson S. J., Dawson M. A. (2020). Non-genetic Mechanisms of Therapeutic Resistance in Cancer. Nat. Rev. Cancer 20, 743–756. 10.1038/s41568-020-00302-4 [DOI] [PubMed] [Google Scholar]
- Marselos M., Lang M., Torronen R. (1976). Modifications of Drug Metabolism by Disulfiram and Diethyldithiocarbamate. II. D-Glucuronic Acid Pathway. Chem. Biol. Interact 15, 277–287. 10.1016/0009-2797(76)90153-8 [DOI] [PubMed] [Google Scholar]
- Martin F., Linden T., Katschinski D. M., Oehme F., Flamme I., Mukhopadhyay C. K., et al. (2005). Copper-dependent Activation of Hypoxia-Inducible Factor (HIF)-1: Implications for Ceruloplasmin Regulation. Blood 105, 4613–4619. 10.1182/blood-2004-10-3980 [DOI] [PubMed] [Google Scholar]
- Mays D. C., Nelson A. N., Lam-Holt J., Fauq A. H., Lipsky J. J. (1996). S-methyl-N,N-diethylthiocarbamate Sulfoxide and S-Methyl-N,n-Diethylthiocarbamate Sulfone, Two Candidates for the Active Metabolite of Disulfiram. Alcohol. Clin. Exp. Res. 20, 595–600. 10.1111/j.1530-0277.1996.tb01099.x [DOI] [PubMed] [Google Scholar]
- Mcconville C., Tawari P., Wang W. (2015). Hot Melt Extruded and Injection Moulded Disulfiram-Loaded PLGA Millirods for the Treatment of Glioblastoma Multiforme via Stereotactic Injection. Int. J. Pharm. 494, 73–82. 10.1016/j.ijpharm.2015.07.072 [DOI] [PubMed] [Google Scholar]
- Mehdi W. A., Yusof F., Mehde A. A., Zainulabdeen J. A., Raus R. A., Abdulbari A. S. (2015). Effects of Acute Lymphoblastic Leukemia on Ceruloplasmin Oxidase, Copper and Several Markers of Oxidative Damage, in Children. Asian Pac. J. Cancer Prev. 16, 5205–5210. 10.7314/apjcp.2015.16.13.5205 [DOI] [PubMed] [Google Scholar]
- Miao L., Su J., Zhuo X., Luo L., Kong Y., Gou J., et al. (2018). mPEG5k- b-PLGA2k/PCL3.4k/MCT Mixed Micelles as Carriers of Disulfiram for Improving Plasma Stability and Antitumor Effect In Vivo. Mol. Pharm. 15, 1556–1564. 10.1021/acs.molpharmaceut.7b01094 [DOI] [PubMed] [Google Scholar]
- Michniewicz F., Saletta F., Rouaen J. R. C., Hewavisenti R. V., Mercatelli D., Cirillo G., et al. (2021). Copper: An Intracellular Achilles' Heel Allowing the Targeting of Epigenetics, Kinase Pathways, and Cell Metabolism in Cancer Therapeutics. ChemMedChem 16 (15), 2315–2329. 10.1002/cmdc.202100172 [DOI] [PubMed] [Google Scholar]
- Moosavian S. A., Bianconi V., Pirro M., Sahebkar A. (2021). Challenges and Pitfalls in the Development of Liposomal Delivery Systems for Cancer Therapy. Semin. Cancer Biol. 69, 337–348. 10.1016/j.semcancer.2019.09.025 [DOI] [PubMed] [Google Scholar]
- Morgan T. M., Lange P. H., Porter M. P., Lin D. W., Ellis W. J., Gallaher I. S., et al. (2009). Disseminated Tumor Cells in Prostate Cancer Patients after Radical Prostatectomy and without Evidence of Disease Predicts Biochemical Recurrence. Clin. Cancer Res. 15, 677–683. 10.1158/1078-0432.ccr-08-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi M., Nakajima T., Kimura H., Watanabe T., Takashima H., Mitsumoto Y., et al. (2002). The Copper Chelator Trientine Has an Antiangiogenic Effect Against Hepatocellular Carcinoma, Possibly Through Inhibition of Interleukin-8 Production. Int. J. Cancer 102, 445–452. 10.1002/ijc.10740 [DOI] [PubMed] [Google Scholar]
- Morrison B. W., Doudican N. A., Patel K. R., Orlow S. J. (2010). Disulfiram Induces Copper-dependent Stimulation of Reactive Oxygen Species and Activation of the Extrinsic Apoptotic Pathway in Melanoma. Melanoma Res. 20, 11–20. 10.1097/cmr.0b013e328334131d [DOI] [PubMed] [Google Scholar]
- Nagaraju G. P., Dontula R., El-Rayes B. F., Lakka S. S. (2014). Molecular Mechanisms Underlying the Divergent Roles of SPARC in Human Carcinogenesis. Carcinogenesis 35, 967–973. 10.1093/carcin/bgu072 [DOI] [PubMed] [Google Scholar]
- Najlah M., Ahmed Z., Iqbal M., Wang Z., Tawari P., Wang W., et al. (2017). Development and Characterisation of Disulfiram-Loaded PLGA Nanoparticles for the Treatment of Non-small Cell Lung Cancer. Eur. J. Pharm. Biopharm. 112, 224–233. 10.1016/j.ejpb.2016.11.032 [DOI] [PubMed] [Google Scholar]
- Najlah M., Said Suliman A., Tolaymat I., Kurusamy S., Kannappan V., Elhissi A. M. A., et al. (2019). Development of Injectable PEGylated Liposome Encapsulating Disulfiram for Colorectal Cancer Treatment. Pharmaceutics 11 (11), 610. 10.3390/pharmaceutics11110610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndagi U., Mhlongo N., Soliman M. E. (2017). Metal Complexes in Cancer Therapy - An Update from Drug Design Perspective. Drug Des. Devel Ther. 11, 599–616. 10.2147/dddt.s119488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T., Matsuno K., Kawamoto T., Mitsudomi T., Shirakusa T., Kodama Y. (1994). Efficiency of Serum Copper/zinc Ratio for Differential Diagnosis of Patients with and without Lung Cancer. Biol. Trace Elem. Res. 42, 115–127. 10.1007/bf02785383 [DOI] [PubMed] [Google Scholar]
- Pan M., Zheng Q., Yu Y., Ai H., Xie Y., Zeng X., et al. (2021). Seesaw Conformations of Npl4 in the Human P97 Complex and the Inhibitory Mechanism of a Disulfiram Derivative. Nat. Commun. 12, 121. 10.1038/s41467-020-20359-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou C. N., Logothetis C. J. (2004). Bortezomib as a Potential Treatment for Prostate Cancer. Cancer Res. 64, 5036–5043. 10.1158/0008-5472.can-03-2707 [DOI] [PubMed] [Google Scholar]
- Pavithra V., Sathisha T. G., Kasturi K., Mallika D. S., Amos S. J., Ragunatha S. (2015). Serum Levels of Metal Ions in Female Patients with Breast Cancer. J. Clin. Diagn. Res. 9, BC25–c27. 10.7860/JCDR/2015/11627.5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E. N. (1992). The Pharmacology and Toxicology of Disulfiram and its Metabolites. Acta Psychiatr. Scand. Suppl. 369, 7–13. 10.1111/j.1600-0447.1992.tb03309.x [DOI] [PubMed] [Google Scholar]
- Pors K., Moreb J. S. (2014). Aldehyde Dehydrogenases in Cancer: An Opportunity for Biomarker and Drug Development? Drug Discov. Today 19, 1953–1963. 10.1016/j.drudis.2014.09.009 [DOI] [PubMed] [Google Scholar]
- Prasad S., Gupta S. C., Tyagi A. K. (2017). Reactive Oxygen Species (ROS) and Cancer: Role of Antioxidative Nutraceuticals. Cancer Lett. 387, 95–105. 10.1016/j.canlet.2016.03.042 [DOI] [PubMed] [Google Scholar]
- Restifo N. P., Smyth M. J., Snyder A. (2016). Acquired Resistance to Immunotherapy and Future Challenges. Nat. Rev. Cancer 16, 121–126. 10.1038/nrc.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigiracciolo D. C., Scarpelli A., Lappano R., Pisano A., Santolla M. F., de Marco P., et al. (2015). Copper Activates HIF-1alpha/GPER/VEGF Signalling in Cancer Cells. Oncotarget 6, 34158–34177. 10.18632/oncotarget.5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk S. L., Sky-Peck H. H. (1984). Comparison Between Concentrations of Trace Elements in Normal and Neoplastic Human Breast Tissue. Cancer Res. 44, 5390–5394. [PubMed] [Google Scholar]
- Rolle F., Bincoletto V., Gazzano E., Rolando B., Lollo G., Stella B., et al. (2020). Coencapsulation of Disulfiram and Doxorubicin in Liposomes Strongly Reverses Multidrug Resistance in Breast Cancer Cells. Int. J. Pharm. 580, 119191. 10.1016/j.ijpharm.2020.119191 [DOI] [PubMed] [Google Scholar]
- Rottenberg S., Disler C., Perego P. (2021). The Rediscovery of Platinum-Based Cancer Therapy. Nat. Rev. Cancer 21, 37–50. 10.1038/s41568-020-00308-y [DOI] [PubMed] [Google Scholar]
- Said Suliman A., Khoder M., Tolaymat I., Webster M., Alany R. G., Wang W., et al. (2021). Cyclodextrin Diethyldithiocarbamate Copper II Inclusion Complexes: A Promising Chemotherapeutic Delivery System Against Chemoresistant Triple Negative Breast Cancer Cell Lines. Pharmaceutics 13 (1), 84. 10.3390/pharmaceutics13010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh S. A. K., Adly H. M., Abdelkhaliq A. A., Nassir A. M. (2020). Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 14, 44–49. 10.1159/000499261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja R., Arciero V. S., Cheng S., Mcdonald E., Wong W. W. L., Cheung M. C., et al. (2018). Examining Trends in Cost and Clinical Benefit of Novel Anticancer Drugs over Time. J. Oncol. Pract. 14, e280–e294. 10.1200/jop.17.00058 [DOI] [PubMed] [Google Scholar]
- Salvador F., Martin A., Lopez-Menendez C., Moreno-Bueno G., Santos V., Vazquez-Naharro A., et al. (2017). Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 77, 5846–5859. 10.1158/0008-5472.can-16-3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtova S., Kalavska K., Gercakova K., Cierna Z., Miklikova S., Smolkova B., et al. (2019). Disulfiram Overcomes Cisplatin Resistance in Human Embryonal Carcinoma Cells. Cancers (Basel). 11, 1224. 10.3390/cancers11091224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag V. C., Gudekar N., Jasmer K., Papageorgiou C., Singh K., Petris M. J. (2021). Copper Metabolism as a Unique Vulnerability in Cancer. Biochim. Biophys. Acta Mol. Cel Res 1868, 118893. 10.1016/j.bbamcr.2020.118893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag V., Jasmer-Mcdonald K., Zhu S., Martin A. L., Gudekar N., Khan A., et al. (2019). ATP7A Delivers Copper to the Lysyl Oxidase Family of Enzymes and Promotes Tumorigenesis and Metastasis. Proc. Natl. Acad. Sci. U S A. 116, 6836–6841. 10.1073/pnas.1817473116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Mittal D. K., Kesarwani R. C., Kamboj V. P., Chowdhery (1994). Diagnostic and Prognostic Significance of Serum and Tissue Trace Elements in Breast Malignancy. Indian J. Med. Sci. , 48, 227–232. [PubMed] [Google Scholar]
- Shi J., Kantoff P. W., Wooster R., Farokhzad O. C. (2017). Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 17, 20–37. 10.1038/nrc.2016.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K., Hawkins C., Clarke I. D., Squire J. A., Bayani J., Hide T., et al. (2004). Identification of Human Brain Tumour Initiating Cells. Nature 432, 396–401. 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- Skrott Z., Majera D., Gursky J., Buchtova T., Hajduch M., Mistrik M., et al. (2019). Disulfiram's Anti-cancer Activity Reflects Targeting NPL4, Not Inhibition of Aldehyde Dehydrogenase. Oncogene 38, 6711–6722. 10.1038/s41388-019-0915-2 [DOI] [PubMed] [Google Scholar]
- Skrott Z., Mistrik M., Andersen K. K., Friis S., Majera D., Gursky J., et al. (2017). Alcohol-abuse Drug Disulfiram Targets Cancer via P97 Segregase Adaptor NPL4. Nature 552, 194–199. 10.1038/nature25016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi M., Gholami A., Azar M. H., Yaghoobi M., Shahi M. M., Shirmardi S., et al. (2018). Trace Element and Heavy Metal Levels in Colorectal Cancer: Comparison Between Cancerous and Non-cancerous Tissues. Biol. Trace Elem. Res. 183, 1–8. 10.1007/s12011-017-1099-7 [DOI] [PubMed] [Google Scholar]
- Solak K., Mavi A., Yilmaz B. (2021). Disulfiram-loaded Functionalized Magnetic Nanoparticles Combined with Copper and Sodium Nitroprusside in Breast Cancer Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 119, 111452. 10.1016/j.msec.2020.111452 [DOI] [PubMed] [Google Scholar]
- Song W., Tang Z., Lei T., Wen X., Wang G., Zhang D., et al. (2016). Stable Loading and Delivery of Disulfiram with mPEG-PLGA/PCL Mixed Nanoparticles for Tumor Therapy. Nanomedicine 12, 377–386. 10.1016/j.nano.2015.10.022 [DOI] [PubMed] [Google Scholar]
- Starkebaum G., Root R. K. (1985). D-penicillamine: Analysis of the Mechanism of Copper-Catalyzed Hydrogen Peroxide Generation. J. Immunol. 134, 3371–3378. [PubMed] [Google Scholar]
- Stepien M., Jenab M., Freisling H., Becker N. P., Czuban M., Tjonneland A., et al. (2017). Pre-diagnostic Copper and Zinc Biomarkers and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Carcinogenesis 38, 699–707. 10.1093/carcin/bgx051 [DOI] [PubMed] [Google Scholar]
- Suh J. J., Pettinati H. M., Kampman K. M., O'Brien C. P. (2006). The Status of Disulfiram: A Half of a Century Later. J. Clin. Psychopharmacol. 26, 290–302. 10.1097/01.jcp.0000222512.25649.08 [DOI] [PubMed] [Google Scholar]
- Sun D. L., Poddar S., Pan R. D., Rosser E. W., Abt E. R., Van Valkenburgh J., et al. (2020). Isoquinoline Thiosemicarbazone Displays Potent Anticancer Activity with In Vivo Efficacy Against Aggressive Leukemias. RSC Med. Chem. 11, 392–410. 10.1039/c9md00594c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Tawari P. E., Wang Z., Najlah M., Tsang C. W., Kannappan V., Liu P., et al. (2015). The Cytotoxic Mechanisms of Disulfiram and Copper(ii) in Cancer Cells. Toxicol. Res. (Camb) 4, 1439–1442. 10.1039/c5tx00210a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R., David C. M., Mahesh D. R., Sambargi U., Rashmi K. J., Benakanal P. (2016). Assessment of Serum Copper, Iron and Immune Complexes in Potentially Malignant Disorders and Oral Cancer. Braz. Oral Res. 30, e101. 10.1590/1807-3107BOR-2016.vol30.0101 [DOI] [PubMed] [Google Scholar]
- Triscott J., Lee C., Hu K., Fotovati A., Berns R., Pambid M., et al. (2012). Disulfiram, A Drug Widely Used to Control Alcoholism, Suppresses the Self-Renewal of Glioblastoma and Over-rides Resistance to Temozolomide. Oncotarget 3, 1112–1123. 10.18632/oncotarget.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Koog L., Gandek T. B., Nagelkerke A. (2021). Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. [Epub ahead of print]. 10.1002/adhm.202100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella V., Malaguarnera R., Lappano R., Maggiolini M., Belfiore A. (2017). Recent Views of Heavy Metals as Possible Risk Factors and Potential Preventive and Therapeutic Agents in Prostate Cancer. Mol. Cel Endocrinol 457, 57–72. 10.1016/j.mce.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Verzella D., Pescatore A., Capece D., Vecchiotti D., Ursini M. V., Franzoso G., et al. (2020). Life, Death, and Autophagy in Cancer: NF-kappaB Turns up Everywhere. Cell Death Dis 11, 210. 10.1038/s41419-020-2399-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veverka K. A., Johnson K. L., Mays D. C., Lipsky J. J., Naylor S. (1997). Inhibition of Aldehyde Dehydrogenase by Disulfiram and its Metabolite Methyl Diethylthiocarbamoyl-Sulfoxide. Biochem. Pharmacol. 53, 511–518. 10.1016/s0006-2952(96)00767-8 [DOI] [PubMed] [Google Scholar]
- Voli F., Valli E., Lerra L., Kimpton K., Saletta F., Giorgi F. M., et al. (2020). Intratumoral Copper Modulates PD-L1 Expression and Influences Tumor Immune Evasion. Cancer Res. 80, 4129–4144. 10.1158/0008-5472.can-20-0471 [DOI] [PubMed] [Google Scholar]
- Wadhwa S., Mumper R. J. (2013). D-penicillamine and Other Low Molecular Weight Thiols: Review of Anticancer Effects and Related Mechanisms. Cancer Lett. 337, 8–21. 10.1016/j.canlet.2013.05.027 [DOI] [PubMed] [Google Scholar]
- Wang C., Yang J., Han H., Chen J., Wang Y., Li Q., et al. (2017a). Disulfiram-loaded Porous PLGA Microparticle for Inhibiting the Proliferation and Migration of Non-small-cell Lung Cancer. Int. J. Nanomedicine 12, 827–837. 10.2147/ijn.s121948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Cusack J. C., JR., Liu R., Baldwin A. S., JR. (1999). Control of Inducible Chemoresistance: Enhanced Anti-tumor Therapy Through Increased Apoptosis by Inhibition of NF-kappaB. Nat. Med. 5, 412–417. 10.1038/7410 [DOI] [PubMed] [Google Scholar]
- Wang N. N., Wang L. H., Li Y., Fu S. Y., Xue X., Jia L. N., et al. (2018). Targeting ALDH2 with Disulfiram/copper Reverses the Resistance of Cancer Cells to Microtubule Inhibitors. Exp. Cel Res 362, 72–82. 10.1016/j.yexcr.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Wang W., Cassidy J. (2003). Constitutive Nuclear Factor-Kappa B mRNA, Protein Overexpression and Enhanced DNA-Binding Activity in Thymidylate Synthase Inhibitor-Resistant Tumour Cells. Br. J. Cancer 88, 624–629. 10.1038/sj.bjc.6600753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Mcleod H. L., Cassidy J. (2003). Disulfiram-mediated Inhibition of NF-kappaB Activity Enhances Cytotoxicity of 5-fluorouracil in Human Colorectal Cancer Cell Lines. Int. J. Cancer 104, 504–511. 10.1002/ijc.10972 [DOI] [PubMed] [Google Scholar]
- Wang Z., Tan J., Mcconville C., Kannappan V., Tawari P. E., Brown J., et al. (2017b). Poly Lactic-Co-Glycolic Acid Controlled Delivery of Disulfiram to Target Liver Cancer Stem-like Cells. Nanomedicine 13, 641–657. 10.1016/j.nano.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee N. K., Weinstein D. C., Fraser S. T., Assinder S. J. (2013). The Mammalian Copper Transporters CTR1 and CTR2 and Their Roles in Development and Disease. Int. J. Biochem. Cel Biol 45, 960–963. 10.1016/j.biocel.2013.01.018 [DOI] [PubMed] [Google Scholar]
- Wehbe M., Malhotra A. K., Anantha M., Lo C., Dragowska W. H., Dos Santos N., et al. (2018). Development of a Copper-Clioquinol Formulation Suitable for Intravenous Use. Drug Deliv. Transl Res. 8, 239–251. 10.1007/s13346-017-0455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C. P. (2019). The Global Cancer Burden: Necessity Is the Mother of Prevention. Nat. Rev. Cancer 19, 123–124. 10.1038/s41568-019-0110-3 [DOI] [PubMed] [Google Scholar]
- Wu Y., Deng J., Rychahou P. G., Qiu S., Evers B. M., Zhou B. P. (2009). Stabilization of Snail by NF-kappaB Is Required for Inflammation-Induced Cell Migration and Invasion. Cancer Cell 15, 416–428. 10.1016/j.ccr.2009.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q., Ge G. (2012). Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron 5, 261–273. 10.1007/s12307-012-0105-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Kang Y. J. (2009). Role of Copper in Angiogenesis and its Medicinal Implications. Curr. Med. Chem. 16, 1304–1314. 10.2174/092986709787846622 [DOI] [PubMed] [Google Scholar]
- Xu B., Wang S., Li R., Chen K., He L., Deng M., et al. (2017). Disulfiram/copper Selectively Eradicates AML Leukemia Stem Cells In Vitro and In Vivo by Simultaneous Induction of ROS-JNK and Inhibition of NF-kappaB and Nrf2. Cel Death Dis 8, e2797. 10.1038/cddis.2017.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Zhang T., Lu G., Chen K., Tao J., Zhang Y., et al. (2020a). Disulfiram-gold-nanorod Integrate for Effective Tumor Targeting and Photothermal-Chemical Synergistic Therapy. Biomater. Sci. 8, 3310–3319. 10.1039/d0bm00062k [DOI] [PubMed] [Google Scholar]
- Xu X., Xu J., Zhao C., Hou X., Li M., Wang L., et al. (2019). Antitumor Effects of Disulfiram/copper Complex in the Poorly-Differentiated Nasopharyngeal Carcinoma Cells via Activating ClC-3 Chloride Channel. Biomed. Pharmacother. 120, 109529. 10.1016/j.biopha.2019.109529 [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhou Q., Feng X., Dai Y., Jiang Y., Jiang W., et al. (2020b). Disulfiram/copper Markedly Induced Myeloma Cell Apoptosis through Activation of JNK and Intrinsic and Extrinsic Apoptosis Pathways. Biomed. Pharmacother. 126, 110048. 10.1016/j.biopha.2020.110048 [DOI] [PubMed] [Google Scholar]
- Xuefeng X., Hou M. X., Yang Z. W., Agudamu A., Wang F., Su X. L., et al. (2020). Epithelial-mesenchymal Transition and Metastasis of Colon Cancer Cells Induced by the FAK Pathway in Cancer-Associated Fibroblasts. J. Int. Med. Res. 48, 300060520931242. 10.1177/0300060520931242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman M., Kaya G., Yekeler H. (2007). Distribution of Trace Metal Concentrations in Paired Cancerous and Non-cancerous Human Stomach Tissues. World J. Gastroenterol. 13, 612–618. 10.3748/wjg.v13.i4.612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Li Y., Wang K., Wang Y., Yin W., Li L. (2013). P38/NF-kappaB/snail Pathway Is Involved in Caffeic Acid-Induced Inhibition of Cancer Stem Cells-like Properties and Migratory Capacity in Malignant Human Keratinocyte. PLoS One 8, e58915. 10.1371/journal.pone.0058915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Guo F., Albers A. E., Sehouli J., Kaufmann A. M. (2019). Disulfiram Modulates ROS Accumulation and Overcomes Synergistically Cisplatin Resistance in Breast Cancer Cell Lines. Biomed. Pharmacother. 113, 108727. 10.1016/j.biopha.2019.108727 [DOI] [PubMed] [Google Scholar]
- Yip N. C., Fombon I. S., Liu P., Brown S., Kannappan V., Armesilla A. L., et al. (2011). Disulfiram Modulated ROS-MAPK and NFkappaB Pathways and Targeted Breast Cancer Cells with Cancer Stem Cell-like Properties. Br. J. Cancer 104, 1564–1574. 10.1038/bjc.2011.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiji H., Yoshii J., Kuriyama S., Ikenaka Y., Noguchi R., Yanase K., et al. (2005). Combination of Copper-Chelating Agent, Trientine, and Methotrexate Attenuates Colorectal Carcinoma Development and Angiogenesis in Mice. Oncol. Rep. 14, 213–218. 10.3892/or.14.1.213 [DOI] [PubMed] [Google Scholar]
- Yoshino H., Yamada Y., Enokida H., Osako Y., Tsuruda M., Kuroshima K., et al. (2020). Targeting NPL4 via Drug Repositioning Using Disulfiram for the Treatment of Clear Cell Renal Cell Carcinoma. PLoS One 15, e0236119. 10.1371/journal.pone.0236119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourick J. J., Faiman M. D. (1989). Comparative Aspects of Disulfiram and its Metabolites in the Disulfiram-Ethanol Reaction in the Rat. Biochem. Pharmacol. 38, 413–421. 10.1016/0006-2952(89)90380-8 [DOI] [PubMed] [Google Scholar]
- Yu N., Zhu H., Yang Y., Tao Y., Tan F., Pei Q., et al. (2017). Combination of Fe/Cu -chelators and Docosahexaenoic Acid: An Exploration for the Treatment of Colorectal Cancer. Oncotarget 8, 51478–51491. 10.18632/oncotarget.17807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Wang F., Milacic V., Li X., Cui Q. C., Zhang B., et al. (2007). Evaluation of Copper-dependent Proteasome-Inhibitory and Apoptosis-Inducing Activities of Novel Pyrrolidine Dithiocarbamate Analogues. Int. J. Mol. Med. 20, 919–925. 10.3892/ijmm.20.6.919 [DOI] [PubMed] [Google Scholar]
- Yusoh N. A., Ahmad H., Gill M. R. (2020). Combining PARP Inhibition with Platinum, Ruthenium or Gold Complexes for Cancer Therapy. ChemMedChem 15, 2121–2135. 10.1002/cmdc.202000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Hu P., Ding S. Y., Sun T., Liu L., Han S., et al. (2019). Induction of Autophagy-dependent Apoptosis in Cancer Cells through Activation of ER Stress: An Uncovered Anti-cancer Mechanism by Anti-alcoholism Drug Disulfiram. Am. J. Cancer Res. 9, 1266–1281. [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Wang Y., Kang X., Wu A., Yin W., Tang Y., et al. (2018). Dual-targeting Biomimetic Delivery for Anti-glioma Activity via Remodeling the Tumor Microenvironment and Directing Macrophage-Mediated Immunotherapy. Chem. Sci. 9, 2674–2689. 10.1039/c7sc04853j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo X., Lei T., Miao L., Chu W., Li X., Luo L., et al. (2018). Disulfiram-loaded Mixed Nanoparticles with High Drug-Loading and Plasma Stability by Reducing the Core Crystallinity for Intravenous Delivery. J. Colloid Interf. Sci 529, 34–43. 10.1016/j.jcis.2018.05.057 [DOI] [PubMed] [Google Scholar]
- Zowczak M., Iskra M., Torlinski L., Cofta S. (2001). Analysis of Serum Copper and Zinc Concentrations in Cancer Patients. Biol. Trace Elem. Res. 82, 1–8. 10.1385/BTER:82:1-3:001 [DOI] [PubMed] [Google Scholar]
- Zuo X. L., Chen J. M., Zhou X., Li X. Z., Mei G. Y. (2006). Levels of Selenium, Zinc, Copper, and Antioxidant Enzyme Activity in Patients with Leukemia. Biol. Trace Elem. Res. 114, 41–53. 10.1385/bter:114:1:41 [DOI] [PubMed] [Google Scholar]
- çetinkaya N., çetinkaya D., Yüce M. (1988). Serum Copper, Zinc Levels, and Copper. Biol. Trace Elem. Res. 18, 29–38. 10.1007/bf02917486 [DOI] [PubMed] [Google Scholar]