Abstract
Shoulder pain has been reported as a common side-effect after COVID-19 vaccination particularly after administration of mRNA vaccines. Although it is usually mild and self-limiting, occasionally it can become more extensive causing severe pain and marked limited range of motion. Shoulder injury related to vaccine administration has been reported following injection of other routine vaccines. In this case report, we describe 2 cases of shoulder injury related to vaccine administration due to subacromial-subdeltoid bursitis after administration of mRNA COVID-19 vaccines.
Keywords: SIRVA, Subacromial-subdeltoid bursitis, COVID-19, Vaccine
Introduction
As the widespread use of COVID-19 vaccination is ongoing worldwide, radiologists have become more aware of its related imaging findings. Incidental axillary lymphadenopathy has been among the initially recognized and widely reported post vaccination findings in different imaging modalities [[1], [2], [3], [4]]. On the other hand, post-vaccination arm/shoulder pain, particularly at the site of injection, has been reported as one of the most common vaccine related side effects though usually self-limiting and resolving within a few days with or without conservative treatment for which imaging examination is rarely needed. Shoulder injury related to vaccine administration (SIRVA) is a well-known entity described previously and before the COVID-19 era with other types of vaccinations, particularly influenza vaccine [[5], [6], [7]]. SIRVA includes a variety of conditions such as adhesive capsulitis, tendinitis, and subacromial-subdeltoid bursitis. A recent case report described a case of SIRVA, specifically subacromial-subdeltoid bursitis, after administration of Oxford-AstraZeneca vaccine (Serum Institute of India) [8]. In this paper, we report 2 cases of SIRVA, namely subacromial-subdeltoid bursitis, after administration of mRNA COVID-19 vaccines.
Case 1
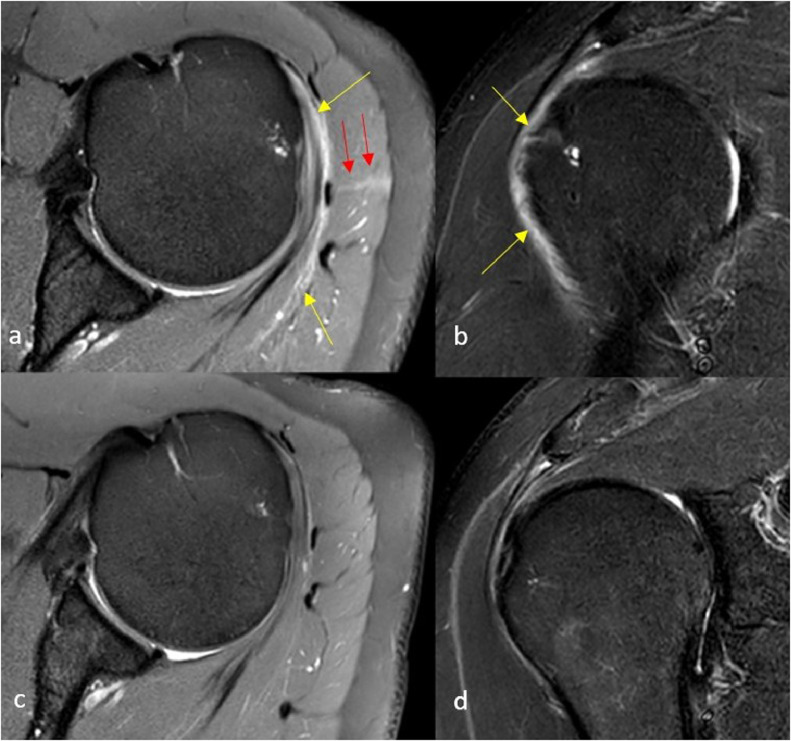
A 42-year-old male right-handed healthcare worker with no significant past medical history presented with severe left shoulder pain (numerical pain rating scale of 10/10) and severe limited range of motion. The symptoms developed within 2 days after receiving the first dose of Moderna's mRNA 1273 vaccine. A high injection site was reported by the patient at the time of vaccine administration. MRI of the left shoulder was performed in which subacromial-subdeltoid bursitis was detected (Figs. 1A and B). Due to severe pain, 1 course of oral Prednisolone was administered after which the pain subsided in few days. Follow-up MRI of the left shoulder 2 months later demonstrated resolution of the bursitis (Figs. 1C and D).
Fig. 1.
Baseline and follow up MRI of the vaccinated shoulder: Axial PD (A) and Coronal STIR (B) images of the left shoulder after receiving the vaccine demonstrate mild to moderate amount of effusion in subacromial-subdeltoid bursa suggesting bursitis (yellow arrows). Note the linear hyperintensity traversing the deltoid muscle and contacting the bursa in keeping with the trajectory of the needle (red arrows). Follow up Axial PD (C) and Coronal STIR (D) images of the same shoulder approximately 2 months later demonstrate near complete resolution of bursitis.
Case 2
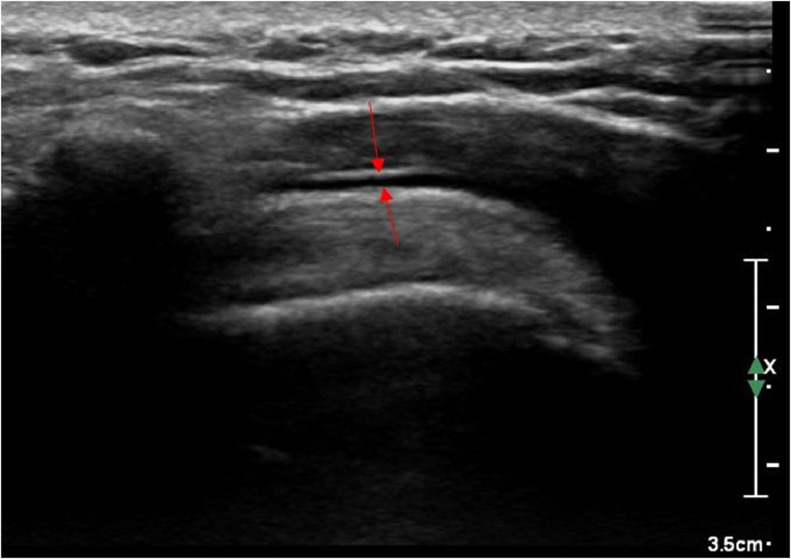
A 38-year-old male right-handed healthcare worker with no significant past medical history presented with non-traumatic severe left shoulder pain (numerical pain rating scale of 10/10). The symptoms developed 2 weeks after receiving the second dose of Pfizer-BioNTech COVID19 vaccine (BNT162b2) leading to severe limited range of motion. The patient did not report any significant vaccine related symptoms with the first dose of vaccine except for moderate arm pain around the injection site. There was no report of deep or high injection site at the time of vaccine administration. Subsequently, ultrasound of the left shoulder was performed in which subacromial-subdeltoid bursitis was detected (Fig. 2). The symptoms almost completely resolved by conservative management in less than a week.
Fig. 2.
Grayscale ultrasound image of the left shoulder: red arrows demonstrate effusion within the subacromial-subdeltoid bursa suggesting bursitis.
Discussion
Data available from United States clinical trials suggest that mRNA COVID-19 vaccines, Pfizer-BioNTech and Moderna, can be highly immunogenic which can affect their reactogenicity and subsequently their side-effect profiles. Given the short time frame since the introduction of mRNA COVID-19 vaccines, the exact pathophysiology of their side-effects has not been fully understood; however, it has been postulated that it may be related to lipid nanoparticle and formulation components or the sequence selection for the vaccine RNA [9]. Reactogenicity of vaccines has been previously contributed to adjuvants routinely utilized to enhance immunogenicity of the vaccines via increasing the antigen-specific response. On the other hand, adjuvants can simultaneously increase the probability of autoimmune reactions which has been defined and described as autoimmune/inflammatory syndrome induced by adjuvants (ASIA) or Shoenfeld's syndrome. Specifically, ASIA is characterized by the appearance of myalgia, myositis, muscle weakness, arthralgia, arthritis, chronic fatigue, sleep disturbances, cognitive impairment/memory loss, and possible emergence of a demyelinating autoimmune disease caused by systemic exposure after administration of vaccines and adjuvants [10]. Nevertheless, majority of the population receiving mRNA COVID-19 vaccines demonstrate at least 1 or more local or systemic symptoms [11]. This may have some impact on radiological findings of recently vaccinated population being referred to imaging centers.
One of the most common side effects of the COVID-19 vaccines has been reported as pain, redness, and swelling in the arm where the vaccine is administered followed by other common systemic side effects such as headache, fever, tiredness, myalgia, fatigue [11]. Arm pain due to vaccine injection usually resolves within a few days with or without conservative treatment. However, in some cases, the severity of the arm pain is out of proportion and may extend to other surrounding structures resulting in SIRVA. As mentioned before, SIRVA has been reported after administration of other routine vaccines and can include variety of conditions such as adhesive capsulitis, tendinitis, and subacromial-subdeltoid bursitis. When clinical symptoms become out of proportion that they cannot be contributed to injection induced muscle pain only, imaging can help to investigate the presence of SIRVA.
Both patients who experienced subacromial-subdeltoid bursitis in our case report routinely received the influenza vaccine annually without development of SIRVA. Although clinical symptoms were similar, the time frame of symptom development was different between our reported cases suggesting different pathophysiologies for development of subacromial-subdeltoid bursitis. The patient in our first case reported a high injection site and acute pain shortly after administration of the vaccine. This favored probable direct and local effect of the vaccine likely due to high level or deep intrabursal injection as suggested on the MR image (Fig. 1A). This was similar to the reported case of SIRVA due to Oxford-AstraZeneca vaccine in whom the symptoms of subacromial-subdeltoid bursitis developed immediately after administration of the first dose followed by persistent pain and worsening of range of motion [8]. Similarly, Atanasoff et al described 13 cases of SIRVA which developed rapidly (immediate to 4 days) after receiving different types of vaccines. They proposed unintentional injection of the antigenic material into synovial tissue and subsequent immune-mediated inflammatory reaction as the possible underlying mechanism [12]. Intrabursal injection can be contributed to either deep or high-level of injection and as such can be avoided by proper injection techniques [8]. Anatomical variation can also contribute to intrabursal injection. Bodor et al previously described 2 SIRVA cases in whom the bursa was extended beyond the lateral border of the acromion locating just below the skin surface where a vaccine needle is easily accessible [13].
Our second case, however, had a subacute course with subacromial-subdeltoid bursitis that developed 2 weeks after administration of the vaccine. In the absence of any prior shoulder condition or triggering injury, developing subacromial-subdeltoid bursitis was clinically contributed to the vaccination as well. However, the 2-week time frame between injection and developing bursitis favored a pathophysiology other than intrabursal injection. One possible mechanism could be delayed inflammatory response to injection or ASIA. Alternatively, although rare, cases of myositis leading to bursitis were reported in literature [14].
Conclusion
To our knowledge our cases are the first reported cases of imaging and clinically proven SIRVA due to mRNA COVID-19 vaccines. Given the widespread COVID-19 vaccination clinicians and radiologists should be familiar with potential musculoskeletal side effects of these vaccines.
Patient consent
Written informed consents for publication of the cases were obtained from the patients and will be available upon request.
Footnotes
Competing interests: Authors have no conflict of interest, grants or other funding resources to disclose.
References
- 1.Mortazavi S. Coronavirus Disease (COVID-19) vaccination associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- 2.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of Coronavirus Disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25604. Epub ahead of print. PMID: 33543649. [DOI] [PubMed] [Google Scholar]
- 3.Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021 doi: 10.1148/radiol.2021210275. Epub ahead of print. PMID: 33625300; PMCID: PMC7909072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. Epub ahead of print. PMID: 33486146; PMCID: PMC7817408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright A, Patel R, Motamedi D. Influenza vaccine-related subacromial/subdeltoid bursitis: a case report. J Radiol Case Rep. 2019;13(6):24–31. doi: 10.3941/jrcr.v13i6.3656. PMID: 31558960; PMCID: PMC6742453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins M, Rupp D, Goebel LJ. Post-influenza vaccine subdeltoid bursitis. Cureus. 2020;12(10):e10764. doi: 10.7759/cureus.10764. PMID: 33154837; PMCID: PMC7606224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook IF. Subdeltoid/subacromial bursitis associated with influenza vaccination. Hum Vaccin Immunother. 2014;10(3):605–606. doi: 10.4161/hv.27232. Epub 2013 Nov 27. PMID: 24284281; PMCID: PMC4130290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantarelli Rodrigues T, Hidalgo PF, Skaf AY, Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA) Skeletal Radiol. 2021:1–5. doi: 10.1007/s00256-021-03803-x. Epub ahead of print. PMID: 33944967; PMCID: PMC8094125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. PMID: 33630816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perricone C, Colafrancesco S, Mazor RD, Soriano A, Agmon-Levin N, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1–16. doi: 10.1016/j.jaut.2013.10.004. Epub 2013 Nov 13. PMID: 24238833. [DOI] [PubMed] [Google Scholar]
- 11.Walsh EE, Frenck RW, Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. Epub 2020 Oct 14. PMID: 33053279; PMCID: PMC7583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA) Vaccine. 2010;28(51):8049–8052. doi: 10.1016/j.vaccine.2010.10.005. Epub 2010 Oct 16. PMID: 20955829. [DOI] [PubMed] [Google Scholar]
- 13.Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585–587. doi: 10.1016/j.vaccine.2006.08.034. Epub 2006 Sep 8. PMID: 17064824. [DOI] [PubMed] [Google Scholar]
- 14.Kim KR, Konig MF, Park JK. Subscapular bursitis as a rare manifestation of dermatomyositis: a case report. Eur J Rheumatol. 2015;2(2):80–82. doi: 10.5152/eurjrheum.2015.0083. Epub 2015 Mar 31. PMID: 27708933; PMCID: PMC5047269. [DOI] [PMC free article] [PubMed] [Google Scholar]