Abstract
The constitutive androstane receptor (CAR)-mediated mode of action (MOA) for phenobarbital (PB)-induced rodent liver tumor formation has been established, with increased hepatocyte proliferation, which is a key event in tumor formation. Previous studies have demonstrated that PB and other CAR-activators stimulate proliferation in cultured rodent hepatocytes, but not in cultured human hepatocytes. However, in the genetically humanized CAR and pregnane X receptor (PXR) mouse (hCAR/hPXR mouse, downstream genes are still mouse), PB increased hepatocyte proliferation and tumor production in vivo. In contrast to the hCAR/hPXR mouse, studies with chimeric mice with human hepatocytes (PXB-mouse, both receptor and downstream genes are human) demonstrated that PB did not increase human hepatocyte proliferation in vivo. PB increased hepatocyte proliferation in a chimeric mouse model with rat hepatocytes, indicating that the lack of human hepatocyte proliferation is not due to any functional defect in the chimeric mouse liver environment. Gene expression analysis demonstrated that the downstream genes of CAR/PXR activation were similar in hCAR/hPXR and CD-1 mice, but differed from those observed in chimeric mice with human hepatocytes. These findings strongly support the conclusion that the MOA for CAR-mediated rodent liver tumor formation is qualitatively implausible for humans. Indeed, epidemiological studies have found no causal link between PB and human liver tumors. There are many similarities with respect to hepatic effects and species differences between rodent CAR and peroxisome proliferator-activated receptor α activators. Based on our research, the chimeric mouse with human hepatocytes (PXB-mouse) is reliable for human cancer risk assessment of test chemicals.
Keywords: cell proliferation, constitutive androstane receptor, cultured hepatocytes, mode of action, human relevance, humanized models
Introduction: The History of Chimeric Mouse with Human Hepatocytes (PXB-mouse)
Although the best method for assessing the safety of chemicals and drugs would be by testing them in humans directly, it is unethical or impractical for chemicals, especially pesticides or industrial chemicals. Therefore, the safety evaluation of chemicals is mainly dependent on animal testing. While these animal studies provide valuable information on possible hazards of test chemicals, considering the differences between human and rodent physiology, the predictive value of these rodent studies has limitations1, 2, 3, 4, 5. In vitro study systems, such as human precision-cut liver slices, hepatic microsomes, or primary hepatocytes, have been widely used and potentially provide useful information for predicting actual human in vivo metabolic profiles of test chemicals6. While cultured hepatocytes are considered the gold standard in vitro systems for many applications6, they cannot be employed for long-term studies owing to time-dependent de-differentiation in culture. Arakawa et al. reported that constitutive androstane receptor (CAR) mRNA expression levels in two-dimensional cultured human hepatocytes were unstable between days 2 and 7 of culture period7.
To overcome the weaknesses of the in vitro systems described above or to confirm the findings of in vitro systems, moving towards “humanizing” laboratory animal species came with the use of embryonic stem cells and the technological breakthrough of capability to delete the gene encoding for the animal homologue of a particular gene and transfecting the human homologue into the mouse genome8. Since biological reaction of the in vivo system is complex (i.e., network or crosstalk of cellular signaling), multiple animal gene knock-out and subsequent human gene knock-ins are needed to understand complex biological reactions. Because of technological complexity, genetically humanizing replaces only a few genes (mostly one or two genes), and thus a whole-cell replacement model is strongly preferred.
In order to generate an animal model that closely mirrors human patterns of metabolism and toxicity, a significant replacement of host liver cells with human hepatocytes would clearly be of great advantage8, 9. Consequently, chimeric mouse models with human hepatocytes, in which most mouse hepatocytes were replaced by transferred human hepatocytes, were developed. We used PXB-mouse® constructed by PhoenixBio Co., Ltd. (Higashi-Hiroshima, Japan) based on their described characteristics9, 10. Foster et al. stated that the uPA+/+ severe combined immunodeficient (SCID) mouse system has been the most widely assessed in terms of similarity of drug-exposure in the human condition, and was shown to exhibit a considerably more human-like absorption, distribution, metabolism, and excretion (ADME) profile than their non-chimeric murine controls8.
Characterization and application of chimeric mice with human hepatocytes (PXB-mouse) has recently been summarized in an excellent review by Tateno and Kojima9. Detailed methods and protocols for producing these chimeric mice are shown in a chapter of the book Hepatocyte Transplantation11. Albumin enhancer promoter-driven urokinase plasminogen activator transgenic mice (uPA-Tg mice) were produced in 1990 to investigate the physiological role of uPA in vivo. The mouse liver was damaged by high expression of uPA and could be repopulated by transplanting healthy mouse hepatocytes via spleen. uPA, a serine protease produced in mouse hepatocytes and secreted extracellularly in the uPA-Tg mice, is known to digest the extracellular matrix in the liver and trigger hepatocyte growth after partial hepatectomy, and has a role in activating hepatocyte growth factor. Thus, uPA induces engraftment of transplanted hepatocytes and stimulates the growth of engrafted hepatocytes. The uPA-Tg mice were crossed with immunodeficient mice and transplanted with rat hepatocytes, resulting in successful rat hepatocyte-chimeric mouse production in 199512. Subsequently, human liver chimeric mice were generated using uPA/RAG2−/−, uPA/SCID, Fah−/−/Rag2−/−/Il2rg−/−, and herpes simplex virus type-1 thymidine kinase-NOG (TK-NOG) mice. However, the repopulation index (RI) of these models was 10%–70%. In 2004, Tateno et al. succeeded in producing highly repopulated humanized chimeric mice with an RI of more than 70% stably using uPA/SCID mice (PXB-mouse)13. These highly repopulated chimeric mice can be used as a humanized model for infection studies of hepatitis B virus (HBV) and hepatitis C virus (HCV), or to predict human metabolism and toxicity (reviewed by Tateno and Kojima, 2020)9. Gene expression levels were compared between hepatocytes from uPA/SCID mice and hepatocytes from human liver by microarray analysis, revealing that 82% of transcripts were expressed in both the hepatocytes within a 2-fold range difference14.
However, uPA/SCID mice have four disadvantages: 1) human hepatocyte RI in mouse liver is decreased due to deletion of the uPA transgene by homologous recombination; 2) kidney disorders are likely to develop; 3) body size is small; and 4) hemizygotes cannot be used as hosts as they undergo more frequent homologous recombination than homozygotes. To overcome these disadvantages, Tateno et al. established a novel host strain that has a transgene containing albumin promoter/enhancer-driven urokinase-type plasminogen activator cDNA and has an SCID background (cDNA-uPA/SCID)15. The chimeric hemizygote cDNA-uPA/SCID mice (also known as PXB-mouse) showed a constant increase in body weight and human hepatocyte RI since there was no deletion of uPA genes and no kidney disorders. Furthermore, similar to uPA/SCID chimeric mice, hemizygous cDNA-uPA/SCID chimeric mice were successfully infected with HBV and HCV. Microarray analysis demonstrated that gene expression levels in the liver were similar between hepatocytes from uPA/SCID-chimeric mice and cDNA-uPA/SCID-chimeric mice15. Tateno and Kojima concluded that PXB-mouse livers show nearly normal morphology and express most genes at similar level to those expressed by normal human liver9. The hemizygous cDNA-uPA/SCID mice are useful hosts for producing chimeric mice for use in long-term studies, including hepatitis virus infection analysis or drug toxicity studies15. For transplantation, usually frozen pediatric hepatocytes (6-months-old to 14-years-old) are used as donor cells for chimeric mice because hepatocytes from younger donors have superior growth after transplantation than hepatocytes from older donors16.
The chimeric mouse livers were characterized morphologically (Fig. 1A) and histologically (Fig. 1B) with respect to the extent of chimerism, containing both white and red areas (Fig. 1A). The white areas consisted of human hepatocytes, and were easily distinguishable from the areas of mouse hepatocytes. The red nodules that were distributed sporadically in the livers of chimeric mice represented colonies of transgene-deleted host hepatocytes, as reported previously17.
Fig. 1.
Liver gross pathology and histology in chimeric mice. Photographs present gross (A) and histological (B) appearance of livers of control chimeric mice, with h-heps and m-heps representing human hepatocytes and mouse hepatocytes, respectively. (From Yamada et al., 2014, with permission)19.
In addition to the in vivo chimeric mouse system, Tateno et al. succeeded in isolating 1–2 × 108 hepatocytes using a two-step collagenase perfusion method from a 12-to 20-week old PXB-mouse liver in which human hepatocytes (1–10 × 105 cells) were transplanted into cDNA-uPA/SCID mice between 2 and 4 weeks of age. They refer to hepatocytes as PXB-cells®. Human hepatocytes proliferated up to 2,000-fold in mouse liver from transplantation to isolation. Since fresh human hepatocytes are known to be the most useful cells for in vitro human studies of metabolism and chemical toxicity, PXB-cells made available fresh human hepatocytes from the same donor on demand for at least 5 years9. Since PXB-cells retain high gene expression of cytochrome P450 (CYP), uridine diphosphate glucuronosyltransferase, and transporters18, PXB-cells could be a novel in vitro tool for metabolism and toxicity studies.
Applications of Chimeric Mice with Human Hepatocytes in Studying Xenobiotic Metabolism and Toxicity
According to Tateno and Kojima, total 203 papers utilizing several types of chimeric mice or PXB-cells were published by October 2019, describing efficacy studies on HBV or HCV agents, or drug metabolism and pharmacokinetics studies including ADME, drug-drug interaction, and liver toxicity studies using drugs or chemicals (papers on studies that did not use chemicals or drugs are not included in this number)9. We used chimeric mice with human hepatocytes (PXB-mouse; both uPA/SCID chimeric mice and cDNA-uPA/SCID-chimeric mice) to investigate the hepatic effects of some nongenotoxic constitutive androstane receptor (CAR) activators (i.e., phenobarbital, metofluthrin, and momfluorothrin)19, 20. This was the first challenge in the application of this chimeric model for the evaluation of human relevance of the mode of action (MOA) for rodent liver tumor formation by activators of the CAR. The details are discussed in a later section of this review.
Prior to conducting the study with CAR-activators, the proliferation activity of the transplanted human hepatocytes was examined using a hepatocyte mitogen, human epidermal growth factor (hEGF). The treatment of chimeric mice with hEGF (150 μg/kg four times a day, i.p., for 2 days) significantly increased replicative DNA synthesis (RDS) [determined as 5-bromo-2’-deoxyuridine (BrdU) labeling] assessed with a marker of proliferation, KI-67 (MKI-67) mRNA levels, in human hepatocytes of chimeric mice19. Some BrdU-positive cells were detected in the areas of human hepatocytes in the control animals (Fig. 2B). The BrdU labeling index was only determined in human hepatocytes and not in mouse hepatocytes, in which the rate of RDS was relatively high even in controls (Fig. 2B), making it difficult to compare the control and treatment animals in mouse hepatocytes of the chimeric mice. The cause of the high spontaneous RDS in mouse hepatocytes is unclear but may be related to the induced synthesis of DNA and/or hepatocyte damage by expression of uPA with consequent regeneration20.
Fig. 2.
Liver histology and DNA synthesis in chimeric mice treated with hEGF. Hematoxylin and eosin staining (A) and immunohistochemistory for BrdU (B) of livers of the control chimeric mice, with h-heps and m-heps representing human hepatocytes and mouse hepatocytes, respectively (A and B are serial sections). Immunohistochemistry for BrdU in human hepatocyte area of the control animal (C) and hEGF-treated animal (D). Scale bars are 100 µm. (From Okuda et al., 2017)20.
Furthermore, in two separate experiments, treatment of cultured human hepatocytes from chimeric mice (PXB-cells) with 100 ng/mL hEGF resulted in significant increases in RDS19. These data clearly demonstrated that the transplanted human hepatocytes in the chimeric mice were responsive to hEGF. Based on these findings, we decided to employ this chimeric model to evaluate the human relevance of the MOA for rodent liver tumor formation by CAR-activators.
Evaluation of MOA for Chemical-induced Liver Tumor Formation in Rodents
Since liver is the most common site of tumor formation in rodent carcinogenicity studies of non-genotoxic compounds3, 21, 22, 23, evaluation of the human relevance of chemical-induced liver tumor production in rodents is very important to correctly protect humans from health risks. To avoid misclassifying chemicals as possible human carcinogens due to the limitations of long-term bioassays, it has become imperative to undertake an MOA analysis24. Consequently, MOA studies can help assist regulatory decision-making25. For example, MOA data are now frequently employed to help ascertain the human relevance of tumors produced in rodents by nongenotoxic carcinogens, including liver26, 27, 28, 29 and lung tumors30, 31.
A framework for MOA analysis of rodent tumor and non-tumor toxicity, together with assessment of human relevance, was established by the International Life Sciences Institute (ILSI) (supported by the United States Environmental Protection Agency (US.EPA) and Health Canada) and the International Programme on Chemical Safety (IPCS) of the World Health Organization (WHO) and has been described in a number of publications32, 33, 34, 35, 36, 37, 38, 39, 40.
For carcinogenicity, the first stage is to evaluate whether it is possible to establish an MOA for tumor formation in experimental animals by identifying a series of key and associative events using a weight-of-evidence approach based on the modified Bradford Hill considerations32, 33, 34, 35, 40, 41. A key event is defined as an empirically observable causal precursor step to the adverse outcome, which is a necessary element of the MOA41. Key events are required events for the MOA, but often are not sufficient to induce the adverse outcome in the absence of other key events. Associative events are considered biological processes that are not causal or necessary key events for the MOA, but are reliable indicators or markers for the key events41. Associative events can often be used as surrogate markers for a key event in an MOA evaluation or as indicators of exposure to a xenobiotic that has stimulated the molecular initiating event or a key event. Once a robust MOA is established, the key and associative events are compared, first qualitatively and then quantitatively between effects in experimental animals and humans41.
MOAs have been established for tumor formation by nongenotoxic chemicals in various rodent tissues. For example, a recent analysis of 411 unique agrochemicals that have been evaluated for carcinogenicity by the US.EPA and the European Chemicals Agency (ECHA) identified 170 chemicals as non-genotoxic carcinogens. These chemicals produced 340 cases of treatment-related tumor formation, of which MOAs or MOA networks could be identified in 224 instances3. Further development of innovative test methods and enhanced understanding of carcinogenic processes will permit a better understanding of tumor formation in rodents and an evaluation of the relevance of such rodent tumors to humans2, 3, 4, 42.
A number of MOAs have been established for liver tumor formation, both in humans and in rodent models, which are identified as two major categories, “DNA reactivity” and “Increased cell proliferation”43, 44. For the Increased cell proliferation MOAs, constitutive androstane receptor (CAR) activation, peroxisome proliferator-activated receptor alpha (PPARα) activation, aryl hydrocarbon receptor (AhR) activation, estrogen receptor activation, hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors (Statins), and porphyrias are known as receptor mediated MOAs. In contrast, non-receptor-mediated MOAs include cytotoxicity, infection, metal overload (e.g., iron and copper), and increased apoptosis (e.g., fumonisin B1). Inherited disorders leading to cytotoxicity (e.g., porphyrias, α-1-antitrypsin deficiency, etc.) are also recognized as MOAs for liver tumor formation43, 44.
MOA for Liver Tumor Formation by Phenobarbital and Other CAR-activators
CAR is a nuclear receptor involved in all phases of drug metabolism and disposition, and has recently been implicated in energy metabolism, tumor progression, and cancer therapy45, 46. Phenobarbital (PB) is a non-genotoxic drug known as barbiturate anticonvulsants/hypnotics and is known to activate CAR by a ligand-independent mechanism47. The carcinogenicity of PB and/or its sodium salt (sodium phenobarbital; presented as PB in this review) was investigated by oral administration in multiple studies in mice and several studies in rats48, 49. PB consistently produced hepatocellular adenomas and carcinomas in multiple mouse strains. Hepatocellular adenomas were produced in rats after lifetime exposure in one study50. In contrast to mice and rats, PB did not produce liver tumors in Syrian hamsters48, 49.
In 2001, the International Agency for Research on Cancer (IARC) concluded that PB is “possibly carcinogenic to human (Group 2B)” as there is sufficient evidence in experimental animals for the carcinogenicity of PB49. Because of the extensive therapeutic use of PB in humans as a sedative, hypnotic, and anti-epileptic agent for many years, data from a number of epidemiological studies are available. In contrast to the 2001 IARC conclusion, epidemiological studies, including a more recent analysis (IARC only evaluated epidemiological data up to 1995 and more recent analyses are also available), have found no causal links between PB and human liver tumors47, 48, 49, 51, 52.
Apart from PB, many other chemicals have also been identified as rodent liver tumor producers with CAR-mediated MOA27, 53. According to our more recent critical analysis of available data, at least 21 chemicals have been established for having the CAR activation MOA for mouse and/or rat liver tumor formation5. In such situations, evaluation of the human relevance of the established CAR-mediated MOA for rodent liver tumor formation by PB and other CAR-activators would be very important for risk management of these chemicals.
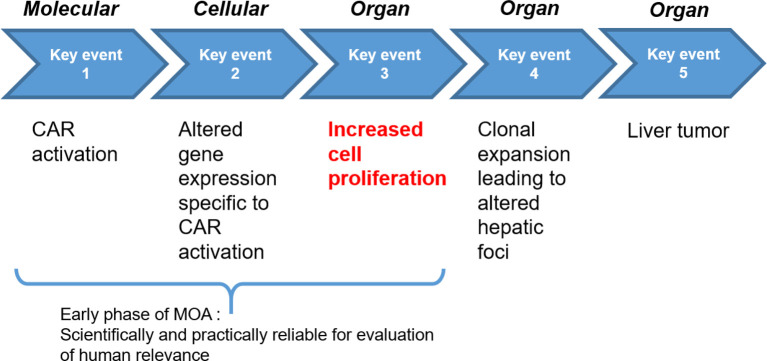
Based on an evaluation of the literature, the key and associative events41 for the CAR-mediated MOA for PB-induced rodent liver tumor formation were established by Elcombe et al47. CAR activation, altered gene expression specific to CAR activation, increased cell proliferation, clonal expansion leading to altered hepatic foci, and ultimately liver tumor formation are considered the key events, as they constitute necessary steps in the MOA (Fig. 3)47. In addition, induction of hepatic CYP2B enzymes and liver hypertrophy (i.e., increase in liver weight and hepatocellular centrilobular or panlobular hypertrophy) are considered associative events and represent reliable markers of CAR activation47.
Fig. 3.
Key events for rodent liver tumor formation by PB and other CAR-activators. Based on an evaluation of literature data, the key events for the CAR-mediated MOA for PB-induced rodent liver tumor formation were established in Elcombe et al. (2014)47. Since increased cell proliferation represents an essential preneoplastic step at an early phase of treatment in carcinogenesis by most nongenotoxic substances26, 54, 55, it is the pivotal endpoint for evaluation of human relevance of the MOA for rodent liver tumor formation.
CAR-dependent Hepatocyte Proliferation
Increased cell proliferation represents an essential preneoplastic step in carcinogenesis by most non-genotoxic substances26, 54, 55. Studies employing mice lacking hepatic CAR (i.e., CAR knockout (KO) mice) have demonstrated the crucial role of hepatic CAR in mouse liver tumor formation for chemicals acting by this MOA. Unlike wild-type mice, the treatment of CAR KO mice with PB did not result in increased liver weight, liver centrilobular hepatocellular hypertrophy, induction of Cyp2b subfamily enzymes, hepatocyte RDS, and following initiation with the genotoxic agent diethylnitrosamine (DEN) did not promote liver tumor formation56, 57, 58, 59.
CAR is present in human liver and can be activated by PB and other drugs and compounds47, 60, 61. To address possible human relevance, we determined the effects of PB and other CAR-activators (e.g., metofluthrin and momfluorothrin, pyrethroid insecticides that produced liver tumors in rats after long-term and high-dose treatment)27, 62, 63, 64 on hepatocyte RDS in three experimental models: in vitro studies with cultured human hepatocytes20, 65, 66, 67, together with in vivo studies with transgenic mice containing human hepatic CAR and PXR68 and/or in chimeric mice with human hepatocytes19, 20. Because the lack of proper ADME properties resulting from cellular disconnection from the circulatory and other organ systems may make the assessment of chemical exposure results difficult with primary hepatocyte cultures6, it is important to conduct in vivo studies to confirm the findings obtained from the in vitro studies. The findings of these three models are summarized below.
Studies in cultured human hepatocytes
As mentioned above, primary cultures of animal and human primary hepatocytes have been extensively used for in vitro testing (e.g., cytotoxicity, CYP enzyme induction, and RDS studies) as they can maintain functional activities for at least 24-72 h6. While PB has been shown to stimulate RDS in cultured mouse and rat hepatocytes, many studies from different laboratories have demonstrated that PB does not increase RDS in cultured human hepatocytes20, 65, 66, 67, 69, 70, 71, 72, 73, 74. In addition to studies with PB, a number of other nongenotoxic rodent CAR-activators, including benfluralin, metazachlor, metofluthrin, momfluorothrin, the natural pyrethrins, nitrapyrin, and sedaxane, and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), have also shown no increased RDS in cultured human hepatocytes20, 65, 66, 71, 72, 73, 74, 75, 76, 77. While the above studies were performed with monolayer cultures (i.e., two-dimensional cultures), a study by Plummer et al. examined the effect of PB on RDS in a three-dimensional culture system and showed no increased RDS in cultured human hepatocytes78. These findings are consistent with those of epidemiological studies showing no increased risk of liver tumors with PB exposure47, 48, 49, 51, 52.
Studies in transgenic mice with human CAR or both CAR and PXR
Treatment of hCAR mice with 500 ppm PB for 1 week resulted in increased hepatocyte proliferation56. Luisier et al. demonstrated that male hCAR/hPXR mice dosed with 0.05% PB in drinking water for 91 days increased relative liver weight, hepatocyte hypertrophy, and induction of Cyp2b10 mRNA levels, together with a transient induction of DNA replication and mitotic genes in hepatocytes79. Recently, Haines et al. treated C57BL/6J wild-type and hCAR/hPXR mice with diets containing 186, 496, 654, and 984 ppm PB for 7 days. In the wild-type mice, a statistically significant dose-dependent increase in hepatocyte RDS was observed at all PB dose levels examined. However, in the hCAR/hPXR mice, 186 ppm PB treatment did not increase hepatocyte RDS significantly, while 496–984 ppm PB did increase hepatocyte RDS statistically significantly. These effects were less marked than those observed in wild-type mice. Finally, in a recent study, the treatment of hCAR/hPXR mice with 1,000 ppm PB in the diet for 7 days resulted in significant increase in relative liver weight, hepatocyte RDS, Cyp2b10, and Cyp3a11 mRNA level, and in mRNA levels of some cell cycling genes, namely Mki67, Mdm2, Pcna, and Gadd45β68. Contrarily, one study involving intraperitoneal injection of 80 mg/kg/d PB for 4 days in hCAR/hPXR mice did not show significant effect on hepatocyte RDS80. This apparent lack of effect is most likely attributable to the treatment time and/or dose levels of PB administered. Overall, these studies demonstrate that the treatment of either hCAR or hCAR/hPXR mice with PB can result in increased hepatocyte RDS, although the effects of PB treatment are less marked in hCAR/hPXR mice than in wild-type mice68, 69.
Braeuning et al. performed an initiation/promotion study in which wild-type and hCAR/hPXR mice were administered with a single dose of DEN followed by treatment with 500 ppm PB in the diet for 40 weeks. While tumor incidence assessed, either multiplicity or tumor volume • fraction was less marked in the hCAR/hPXR mice, and PB promoted DEN-initiated liver tumors in both wild-type and hCAR/hPXR mice81. Based on these findings, these authors suggested that PB-induced liver tumor formation in rodents could be relevant for humans81, 82, 83. Consequently, these findings raised controversy to the conclusion of the 2010 workshop that the MOA for PB-induced rodent liver tumors is not relevant to humans47. However, Bae et al. recently speculated that, although more detailed studies are needed, CAR may function as a tumor suppressor by suppressing liver cancer stem cell (LCSC) activity or hindering de-differentiation of differentiated cells into LCSCs, as well as inhibiting the key markers for LCSCs such as CD13384.
For interpreting findings from the hCAR and hCAR/hPXR mice, it should be noted that in these transgenic mouse models, the human receptor(s) operate in a mouse hepatocyte environment. The downstream genes acted upon by CAR are those of the mouse, not humans5, 27, 29, 85, indicating that the findings from hCAR and hCAR/hPXR mice do not appropriately reflect human responses. Towards better understanding of these controversial findings, it is very important to evaluate the effects of PB on human hepatocyte proliferation in a chimeric mouse model in which human receptor(s) operate in a human hepatocyte environment.
Studies in chimeric mice with human hepatocytes (PXB mouse)
As shown in Table 1, uPA/SCID mice were employed for the evaluation of PB19, and in subsequent investigations, the cDNA-uPA/SCID mouse model has been used in studies with metofluthrin and momfluorothrin20. Metofluthrin and momfluorothrin are pyrethroid insecticides that induced liver tumors in rats with CAR-mediated MOA27, 62, 63, 64. Previous studies with cultured hepatocytes have demonstrated that metofluthrin65, 66 and momfluorothrin20 increased rat hepatocyte RDS, but not in human hepatocytes, which is strongly consistent with the results of PB.
Table 1. Effect of Some Chemicals on Replicative DNA Synthesis in Chimeric Mice with Human Hepatocytes.
Studies with cultured human hepatocytes have demonstrated that while hepatocyte RDS can be increased by treatment with growth factors such as EGF or hepatocyte growth factor (HGF), no chemicals (e.g., drugs or agrochemicals) have been reported to induce RDS in human hepatocytes5. As mentioned in an earlier section, we demonstrated that treatment with EGF enhances RDS in the human hepatocytes of chimeric mice (both uPA/SCID and cDNA-uPA/SCID mouse models), thus demonstrating that the transplanted human hepatocytes in chimeric mice can respond to a hepatocyte mitogen19, 20. In the human hepatocytes of chimeric mice treated with 1500 ppm PB, cytosolic glycogen areas were decreased and the size of the cells was slightly increased in the centrilobular area (Fig. 4A and B). These hepatic changes suggest that human-originated hepatocytes exhibited slight hypertrophic changes after PB treatment, the effect being less marked than that observed in WH rats and CD-1 mice19. Electron microscopic evaluation in chimeric mice treated with 1500 ppm PB revealed that an increase in smooth endoplasmic reticulum was observed in the human-originated hepatocytes (Fig. 4C and D), which supported the light microscopic changes. In contrast to the EGF response, as shown in Table 1, human hepatocytes in the chimeric mouse model did not respond to CAR-activators with increased RDS in rats (PB19, 62, 64, metofluthrin62 and momfluorothrin64)19, 20. Based on data from the chimeric mouse model and cultured human hepatocytes, the ECHA concluded that a classification for carcinogenicity was not justified for metofluthrin and momfluorothrin27.
Fig. 4.
Liver histology in chimeric mice treated with PB. Histopathology (A, B) and ultrastructure (C, D) of human hepatocyte-originated areas of chimeric mice given 0 (A, C) and 1500 ppm (B, D) PB are also presented. Centrilobular hepatocellular hypertrophy (B) and proliferation of the smooth endoplasmic reticulum (D) was observed in PB-treated chimeric mice. (From Yamada et al., 2014, with permission)19.
More recently, we provided data showing that treatment with 1,000 ppm PB significantly increased RDS, together with a small increase in MKI67 mRNA levels, in chimeric rat hepatocyte mice68, which is consistent with a large number of previous in vivo and in vitro studies in rats showing increased RDS. Hence, the chimeric mice with transplanted rat hepatocytes retained the original characteristics of normal rat hepatocytes by increasing RDS in response to stimulation by the CAR-activator PB68. These results strongly support the conclusion that the lack of proliferation of human hepatocytes in the chimeric mouse model is not due to any functional defect in the mouse liver environment, but because human hepatocytes are truly refractory to the mitogenic effects of PB and other rodent CAR-activators, as observed in cultured human hepatocyte studies.
Since increased cell proliferation represents an essential pre-neoplastic step in carcinogenesis by non-genotoxic substances26, 54, 55, the absence of any mitogenic effects of CAR-activators in human hepatocytes strongly suggests that these compounds will not produce liver tumors in humans. This is strongly consistent with the findings from the in vitro cultured human hepatocyte system20, 65, 66, 67, 69, 70, 71, 72, 73, 74 and epidemiological studies showing no increased risk of liver tumors47, 48, 49, 51, 52.
In addition to the effects of rodent CAR-activators, the PPARα-activator fenofibrate was also shown to be a mitogenic agent in mouse hepatocytes but not in human hepatocytes of the chimeric mice86, 87 (Table 1), which is consistent with the previous conclusion that PPARα-activated MOA for rodent liver tumor formation is not relevant to humans28. Furthermore, gene expression analysis in chimeric mice (cDNA-uPA/SCID) treated with fenofibrate suggested that PPARα may have a suppressive effect on DNA synthesis in human hepatocytes88.
In a recent study using the same chimeric human hepatocyte mouse model (PXB-mouse, cDNA-uPA/SCID), treatment with KMTR2 (an anti-human tumor necrosis factor (TNF)-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) monoclonal antibody) was shown to induce hepatotoxicity and apoptosis in human hepatocytes, which is associated with an upregulation of cell cycle-related functions likely representing cellular regeneration89. Ishida et al. reported that the cytotoxicity of aflatoxin B1 was detected by using histological examination and biochemical analysis in chimeric mice with human hepatocytes (PXB-mouse, cDNA-uPA/SCID), although hepatocyte proliferation was not examined90. More recently, Eguchi et al. evaluated the utility of the PXB mouse as an in vivo experimental model to evaluate the key events of the porphyria-mediated cytotoxicity MOA in humans, using 5-aminolevulinic acid, a representative porphyrinogenic compound91. They concluded that the PXB mouse is a useful model for evaluating the key events of porphyria-mediated cytotoxicity MOA in humans91.
Overall, these data demonstrate that functional availability in this chimeric mouse model is not only for receptor-mediated MOA but also for cytotoxicity/regeneration-MOA. Therefore, the chimeric mouse with human hepatocytes (PXB-mouse) is a reliable model for evaluating human relevance of MOA for rodent liver tumor formation of test chemicals.
Comparative Gene Expression Analysis in Humanized Models Treated with PB
Based on the increases in hepatocyte proliferation and liver tumors in the hCAR/hPXR mice, Braeuning et al. suggested that PB-induced liver tumor formation in rodents could be relevant for humans81, 83, 92. As described above, other laboratories also demonstrated similar findings of increased hepatocyte RDS in hCAR and hCAR/hPXR mice. Therefore, these hCAR or hCAR/hPXR mice may provide experimentally correct responses to treatment with CAR-activators; however, this genetically humanized model is of questionable biological significance regarding human relevance. As the hCAR operates in a mouse hepatocyte environment, including downstream genes and their activation85, caution is needed to extrapolate the results of this animal model to humans5, 27, 29. To further investigate the details of CAR-activated signaling in hCAR/hPXR mice compared to chimeric mice, we evaluated global gene expression in the livers of PB-treated chimeric mice and hCAR/hPXR mice68, 93.
Wnt/β-catenin signaling is a useful pathway for evaluating tumorigenicity in humans and rodents. The Wnt/β-catenin signaling pathway regulates key aspects of mammalian cell biology, with aberrant Wnt pathway activation leading to β-catenin stabilization, which can result in tumor formation in the liver and other organs94, 95. Dong et al. demonstrated that activation of β-catenin and CAR in mice resulted in liver tumor formation, with mouse liver tumors having a conserved gene expression signature with those observed in some human hepatocellular carcinomas94. Thus, we focused on the effects of PB on Wnt/β-catenin signaling in chimeric mice with human hepatocytes and hCAR/hPXR mice.
We first conducted comprehensive analyses of DNA methylation, hydroxymethylation, and gene expression using microarrays of hepatic genes in CD-1 mice treated with PB for 1 week, in chimeric human hepatocyte mice treated with PB for 1 week (both models revealed similar serum PB levels after 7-day PB treatment; approximately 70 µg/mL), and in liver adenomas from a DEN/PB initiation/promotion study93. Nine cell proliferation/growth-related genes (Abcc4, Apoa1, Cblb, Ccdc85b, Cdk5r1, Dlg1, Egfr, Prg4 and Tff1) were commonly observed in both the livers of CD-1 mice treated with PB for 7 days and also in the liver adenomas from the DEN/PB study, and thus these genes are considered as candidate genes responsible for early events in PB-induced liver tumor induction; with effects on a large number of genes related to the Wnt/β-catenin signaling pathway being observed. In contrast to the CD-1 mice, chimeric mice with human hepatocytes treated with PB for 7 days had no effect on these nine genes and fewer effects on Wnt/β-catenin signaling pathway genes93.
Recently, we further analyzed the effect of PB on hepatic gene expression pattern in the hCAR/hPXR mice (48 µg/mL), CD-1 mice (43 µg/mL), chimeric mice with human hepatocytes (27 and 75 µg/mL), and liver adenomas from the DEN/PB study (15 µg/mL) (values in parentheses are plasma PB concentrations after treatment)68. The data demonstrate that the gene expression pattern of Wnt/β-catenin signaling in the livers from the hCAR/hPXR mice clustered closely with those of the liver tumor samples from C3H mice. However, the gene expression pattern of Wnt/β-catenin signaling in the chimeric mice with human hepatocytes was clearly different from those of hCAR/hPXR mice, CD-1 mice, and the liver tumor samples, even at higher PB serum concentrations68.
Overall, unlike mouse hepatocytes, exposure of human hepatocytes to nongenotoxic CAR-activators appears to have little effect on the genes associated with the Wnt/β-catenin signaling pathway68, 93. These findings support our consideration that although the hCAR/hPXR genes have been inserted genetically, the downstream genes are still mouse and may be the basis for the increased hepatocyte RDS, Wnt/β-catenin signaling, and tumor production observed in hCAR/hPXR mouse studies.
Conclusion
As described above, data from transgenic mice with either human CAR or human CAR and PXR are not useful for evaluating the human relevance of liver tumorigenesis because the human nuclear receptors function in a mouse hepatocyte environment5, 27, 29, 85. Thus, the data obtained from these models were similar to those obtained from wild-type mice (Fig. 5). In contrast, data from chimeric mice with human hepatocytes are consistent with the findings of in vitro cultured human hepatocytes, where CAR-activators do not stimulate RDS in human hepatocytes, which is distinctly different from rodents. In addition to hepatocyte RDS, global gene expression analysis demonstrated clear differences in the effects of PB on gene expression between chimeric mice and hCAR/hPXR mice. These findings suggest that the chimeric mouse model is reliable for studies investigating the human relevance of the hepatic effects of rodent CAR-activators on liver tumorigenesis, whereas the hCAR/hPXR mouse is not5.
Fig. 5.
Overall summary for effects of phenobarbital on human hepatocyte proliferation in different experimental models and epidemiological studies. The data obtained from transgenic mice with either human CAR or human CAR and PXR (hCAR or hCAR/hPXR mouse) showed increased hepatocyte proliferation, similar to that obtained in wild-type mice or rats. In contrast, data from the chimeric mice with human hepatocytes are consistent with the findings with in vitro cultured human hepatocyte studies where CAR-activators do not increase hepatocyte proliferation, distinctly different from wild-type mice or rats. The data from the in vitro cultured human hepatocyte studies and the chimeric mice with human hepatocytes are consistent with the data from a number of human epidemiological studies showing no increased risk of liver or other tumors. 2D: a two-dimensional culture system. 3D: a three-dimensional culture system.
Current applications of the chimeric mouse model in studies investigating the human relevance of the hepatic effects of test chemicals on liver tumorigenesis are limited to short-term studies as described above19, 20, 86. The ultimate test of carcinogenicity assessment in humans using this chimeric model may be a long-term carcinogenicity study. However, to our knowledge, such a study has not been performed and would be technically very difficult. In addition to the very high cost, several preliminary studies would have to be performed, such as determining the long-term survival rate and suitable dose levels (MTD; maximum tolerated dose). Considering the adverse outcome pathway concept, investigation of the effect on hepatocyte replication (i.e., observable causal precursor step of tumor formation)5, 41 is sufficient for decision making in the safety assessment of test chemicals with CAR-mediated rodent liver tumor production. This is consistent with the suggested carcinogenicity assessment process by Cohen et al., where a transition from the bioassay to a decision-tree matrix that can be applied to a broader range of chemicals, with better predictivity, based on the premise that cancer is the consequence of DNA coding errors that arise either directly from mutagenic events or indirectly from sustained cell proliferation2.
Furthermore, as with PPARα activators, after global acceptance that CAR-mediated rodent liver tumor production is not relevant to humans, it would not be necessary to perform studies in either cultured human hepatocytes or in chimeric with human hepatocytes5.
Overall, the available data demonstrate that the established MOA for rodent liver tumor formation by PB and other CAR-activators is qualitatively not plausible for humans, which is consistent with previous evaluations5, 27, 29, 47. This conclusion is supported by data from several human epidemiological studies showing no increased risk of liver or other tumors in individuals exposed to CAR-activators such as PB5. These findings are similar to the hepatic effects and species differences of PPARα activators5. As pointed out by Lake, based on current knowledge, the 2001 IARC classification of PB (i.e., “possibly carcinogenic to human (Group 2B)”) would appear to be outdated29. Regarding humanized models, some (but not all) humanized models appear to be useful for the prediction of human responses to chemical exposure. For correct prediction, as discussed in this review, we should select suitable humanized model(s) based on their characterization. To my understanding, the accumulated experimental data suggest that the chimeric mouse with human hepatocytes (PXB-mouse) is very reliable for prediction of short-term effects on hepatocyte replication irrespective of mitogenic- or cytotoxicity/regeneration-MOA. Thus, this model would be valuable for the prediction of liver carcinogenicity of test chemicals, especially human-specific metabolites.
Funding Statement
This work was supported by the Sumitomo Chemical Co., Ltd.
Disclosure of Potential Conflicts of Interest
The author declares the following financial interest/personal relationships: Dr. Tomoya Yamada is employed by Sumitomo Chemical Co., Ltd. However, the views presented in this manuscript are those of the author based on many years of research in the respective areas of investigation reported in this document.
Acknowledgments
I am grateful to Prof. Samuel M. Cohen (University of Nebraska Medical Center, Omaha, Nebraska, USA), Prof. Brian G. Lake (University of Surrey, Guildford, Surrey, UK), and Dr. Chise Tateno (PhoenixBio Co., Ltd., Higashi-Hiroshima, Japan) for valuable discussions and review of the manuscript. The author also thanks the other contributors to this research project from Sumitomo Chemical Co., Ltd., Sumitomo Chemical (UK) plc, and Sumika Technoservice Corp. (Takarazuka, Japan).
References
- 1.Doe JE, Boobis AR, Dellarco V, Fenner-Crisp PA, Moretto A, Pastoor TP, Schoeny RS, Seed JG, and Wolf DC. Chemical carcinogenicity revisited 2: Current knowledge of carcinogenesis shows that categorization as a carcinogen or non-carcinogen is not scientifically credible. Regul Toxicol Pharmacol. 103: 124–129. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SM, Boobis AR, Dellarco VL, Doe JE, Fenner-Crisp PA, Moretto A, Pastoor TP, Schoeny RS, Seed JG, and Wolf DC. Chemical carcinogenicity revisited 3: Risk assessment of carcinogenic potential based on the current state of knowledge of carcinogenesis in humans. Regul Toxicol Pharmacol. 103: 100–105. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Heusinkveld H, Braakhuis H, Gommans R, Botham P, Corvaro M, van der Laan JW, Lewis D, Madia F, Manou I, Schorsch F, Wolterink G, Woutersen R, Corvi R, Mehta J, and Luijten M. Towards a mechanism-based approach for the prediction of nongenotoxic carcinogenic potential of agrochemicals. Crit Rev Toxicol. 50: 725–739. 2020. [DOI] [PubMed] [Google Scholar]
- 4.Luijten M, Corvi R, Mehta J, Corvaro M, Delrue N, Felter S, Haas B, Hewitt NJ, Hilton G, Holmes T, Jacobs MN, Jacobs A, Lamplmair F, Lewis D, Madia F, Manou I, Melching-Kollmuss S, Schorsch F, Schütte K, Sewell F, Strupp C, van der Laan JW, Wolf DC, Wolterink G, Woutersen R, Zvonar Z, Heusinkveld H, and Braakhuis H. A comprehensive view on mechanistic approaches for cancer risk assessment of non-genotoxic agrochemicals. Regul Toxicol Pharmacol. 118: 104789. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Cohen SM, and Lake BG. Critical evaluation of the human relevance of the mode of action for rodent liver tumor formation by activators of the constitutive androstane receptor (CAR). Crit Rev Toxicol. 2021. [DOI] [PubMed] [Google Scholar]
- 6.Soldatow VY, Lecluyse EL, Griffith LG, and Rusyn I. In vitro models for liver toxicity testing. Toxicol Res (Camb). 2: 23–39. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arakawa H, Kamioka H, Jomura T, Koyama S, Idota Y, Yano K, Kojima H, and Ogihara T. Preliminary evaluation of three-dimensional primary human hepatocyte culture system for assay of drug-metabolizing enzyme-inducing potential. Biol Pharm Bull. 40: 967–974. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Foster JR, Lund G, Sapelnikova S, Tyrrell DL, and Kneteman NM. Chimeric rodents with humanized liver: bridging the preclinical/clinical trial gap in ADME/toxicity studies. Xenobiotica. 44: 109–122. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Tateno C, and Kojima Y. Characterization and applications of chimeric mice with humanized livers for preclinical drug development. Lab Anim Res. 36: 2. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugahara G, Ishida Y, Sun J, Tateno C, and Saito T. Art of making artificial liver: depicting human liver biology and diseases in mice. Semin Liver Dis. 40: 189–212. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohshita H, and Tateno C. Propagation of human hepatocytes in uPA/SCID mice: Producing chimeric mice with humanized liver. In: Hepatocyte Transplantation: Methods and Protocols, Methods in Molecular Biology. 2016/11/11 ed., vol. 1506, P Stock and B Christ (eds). Humana Press, New York. 91–100. 2017. [DOI] [PubMed] [Google Scholar]
- 12.Rhim JA, Sandgren EP, Palmiter RD, and Brinster RL. Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci USA. 92: 4942–4946. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, Tachibana A, Soeno Y, Asahina K, Hino H, Asahara T, Yokoi T, Furukawa T, and Yoshizato K. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 165: 901–912. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tateno C, Miya F, Wake K, Kataoka M, Ishida Y, Yamasaki C, Yanagi A, Kakuni M, Wisse E, Verheyen F, Inoue K, Sato K, Kudo A, Arii S, Itamoto T, Asahara T, Tsunoda T, and Yoshizato K. Morphological and microarray analyses of human hepatocytes from xenogeneic host livers. Lab Invest. 93: 54–71. 2013. [DOI] [PubMed] [Google Scholar]
- 15.Tateno C, Kawase Y, Tobita Y, Hamamura S, Ohshita H, Yokomichi H, Sanada H, Kakuni M, Shiota A, Kojima Y, Ishida Y, Shitara H, Wada NA, Tateishi H, Sudoh M, Nagatsuka S, Jishage K, and Kohara M. Generation of novel chimeric mice with humanized livers by using hemizygous cDNA-uPA/SCID mice. PLoS One. 10: e0142145. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masumoto N, Tateno C, Tachibana A, Utoh R, Morikawa Y, Shimada T, Momisako H, Itamoto T, Asahara T, and Yoshizato K. GH enhances proliferation of human hepatocytes grafted into immunodeficient mice with damaged liver. J Endocrinol. 194: 529–537. 2007. [DOI] [PubMed] [Google Scholar]
- 17.Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, and Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 66: 245–256. 1991. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki C, Ishida Y, Yanagi A, Yoshizane Y, Kojima Y, Ogawa Y, Kageyama Y, Iwasaki Y, Ishida S, Chayama K, and Tateno C. Culture density contributes to hepatic functions of fresh human hepatocytes isolated from chimeric mice with humanized livers: Novel, long-term, functional two-dimensional in vitro tool for developing new drugs. PLoS One. 15: e0237809. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada T, Okuda Y, Kushida M, Sumida K, Takeuchi H, Nagahori H, Fukuda T, Lake BG, Cohen SM, and Kawamura S. Human hepatocytes support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogen sodium phenobarbital in an in vivo study using a chimeric mouse with humanized liver. Toxicol Sci. 142: 137–157. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Okuda Y, Kushida M, Kikumoto H, Nakamura Y, Higuchi H, Kawamura S, Cohen SM, Lake BG, and Yamada T. Evaluation of the human relevance of the constitutive androstane receptor-mediated mode of action for rat hepatocellular tumor formation by the synthetic pyrethroid momfluorothrin. J Toxicol Sci. 42: 773–788. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, and Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38(Suppl): 5S–81S. 2010. [DOI] [PubMed] [Google Scholar]
- 22.Gold LS, Manley NB, Slone TH, and Ward JM. Compendium of chemical carcinogens by target organ: results of chronic bioassays in rats, mice, hamsters, dogs, and monkeys. Toxicol Pathol. 29: 639–652. 2001. [DOI] [PubMed] [Google Scholar]
- 23.Huff J, Cirvello J, Haseman J, and Bucher J. Chemicals associated with site-specific neoplasia in 1394 long-term carcinogenesis experiments in laboratory rodents. Environ Health Perspect. 93: 247–270. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice JM. On the application of data on mode of action to carcinogenic risk assessment. Toxicol Sci. 78: 175–177. 2004. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael N, Bausen M, Boobis AR, Cohen SM, Embry M, Fruijtier-Pölloth C, Greim H, Lewis R, Bette Meek ME, Mellor H, Vickers C, and Doe J. Using mode of action information to improve regulatory decision-making: an ECETOC/ILSI RF/HESI workshop overview. Crit Rev Toxicol. 41: 175–186. 2011. [DOI] [PubMed] [Google Scholar]
- 26.Wolf DC, Cohen SM, Boobis AR, Dellarco VL, Fenner-Crisp PA, Moretto A, Pastoor TP, Schoeny RS, Seed JG, and Doe JE. Chemical carcinogenicity revisited 1: A unified theory of carcinogenicity based on contemporary knowledge. Regul Toxicol Pharmacol. 103: 86–92. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Yamada T. Case examples of an evaluation of the human relevance of the pyrethroids/pyrethrins-induced liver tumours in rodents based on the mode of action. Toxicol Res (Camb). 7: 681–696. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corton JC, Peters JM, and Klaunig JE. The PPARα-dependent rodent liver tumor response is not relevant to humans: addressing misconceptions. Arch Toxicol. 92: 83–119. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lake BG. Human relevance of rodent liver tumour formation by constitutive androstane receptor (CAR) activators. Toxicol Res (Camb). 7: 697–717. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen SM, Zhongyu Y, and Bus JS. Relevance of mouse lung tumors to human risk assessment. J Toxicol Environ Health B Crit Rev. 23: 214–241. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Yamada T, Kondo M, Miyata K, Ogata K, Kushida M, Sumida K, Kawamura S, Osimitz TG, Lake BG, and Cohen SM. An evaluation of the human relevance of the lung tumors observed in female mice treated with permethrin based on mode of action. Toxicol Sci. 157: 465–486. 2017. [DOI] [PubMed] [Google Scholar]
- 32.Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, Meek E, Rice JM, Younes M, and International Programme on Chemical Safety. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol. 34: 146–152. 2001. [DOI] [PubMed] [Google Scholar]
- 33.Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, and Farland W. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 36: 781–792. 2006. [DOI] [PubMed] [Google Scholar]
- 34.Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, and Patton DE. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 33: 591–653. 2003. [DOI] [PubMed] [Google Scholar]
- 35.Cohen SM, Klaunig J, Meek ME, Hill RN, Pastoor T, Lehman-McKeeman L, Bucher J, Longfellow DG, Seed J, Dellarco V, Fenner-Crisp P, and Patton D. Evaluating the human relevance of chemically induced animal tumors. Toxicol Sci. 78: 181–186. 2004. [DOI] [PubMed] [Google Scholar]
- 36.Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, and Vickers C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38: 87–96. 2008. [DOI] [PubMed] [Google Scholar]
- 37.Seed J, Carney EW, Corley RA, Crofton KM, DeSesso JM, Foster PM, Kavlock R, Kimmel G, Klaunig J, Meek ME, Preston RJ, Slikker W, Jr , Tabacova S, Williams GM, Wiltse J, Zoeller RT, Fenner-Crisp P, and Patton DE. Overview: using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol. 35: 664–672. 2005. [DOI] [PubMed] [Google Scholar]
- 38.Holsapple MP, Pitot HC, Cohen SM, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, and Dragan YP. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 89: 51–56. 2006. [DOI] [PubMed] [Google Scholar]
- 39.Meek ME, Palermo CM, Bachman AN, North CM, and Jeffrey Lewis R. Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 34: 595–606. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, and Vickers C. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34: 1–18. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen ME, Preston RJ, Maier A, Willis AM, and Patterson J. Dose-response approaches for nuclear receptor-mediated modes of action for liver carcinogenicity: Results of a workshop. Crit Rev Toxicol. 44: 50–63. 2014. [DOI] [PubMed] [Google Scholar]
- 42.Rooney J, Hill T, 3rd , Qin C, Sistare FD, and Corton JC. Adverse outcome pathway-driven identification of rat liver tumorigens in short-term assays. Toxicol Appl Pharmacol. 356: 99–113. 2018. [DOI] [PubMed] [Google Scholar]
- 43.Cohen SM. Human carcinogenic risk evaluation: an alternative approach to the two-year rodent bioassay. Toxicol Sci. 80: 225–229. 2004. [DOI] [PubMed] [Google Scholar]
- 44.Cohen SM, and Arnold LL. Critical role of toxicologic pathology in a short-term screen for carcinogenicity. J Toxicol Pathol. 29: 215–227. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch C, Mackowiak B, Huang R, Li L, Heyward S, Sakamuru S, Wang H, and Xia M. Identification of modulators that activate the constitutive androstane receptor from the Tox21 10k compound library. Toxicol Sci. 167: 282–292. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshinari K. Role of nuclear receptors PXR and CAR in xenobiotic-induced hepatocyte proliferation and chemical carcinogenesis. Biol Pharm Bull. 42: 1243–1252. 2019. [DOI] [PubMed] [Google Scholar]
- 47.Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, Goodman JI, Hester S, Jacobs A, Omiecinski CJ, Schoeny R, Xie W, and Lake BG. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol. 44: 64–82. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whysner J, Ross PM, and Williams GM. Phenobarbital mechanistic data and risk assessment: enzyme induction, enhanced cell proliferation, and tumor promotion. Pharmacol Ther. 71: 153–191. 1996. [DOI] [PubMed] [Google Scholar]
- 49.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 79, Some thyrotropic agents: Phenobarbital and its sodium salt. Lyon. 161–288. 2001. [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi L, Ravera M, Repetti G, and Santi L. Long-term administration of DDT or phenobarbital-Na in Wistar rats. Int J Cancer. 19: 179–185. 1977. [DOI] [PubMed] [Google Scholar]
- 51.La Vecchia C, and Negri E. A review of epidemiological data on epilepsy, phenobarbital, and risk of liver cancer. Eur J Cancer Prev. 23: 1–7. 2014. [DOI] [PubMed] [Google Scholar]
- 52.Stritzelberger J, Lang JD, Mueller TM, Reindl C, Westermayer V, Kostev K, and Hamer HM. Anti-seizure medication is not associated with an increased risk to develop cancer in epilepsy patients. J Neurol. 268: 2185–2191. 2021; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lake BG. Species differences in the hepatic effects of inducers of CYP2B and CYP4A subfamily forms: relationship to rodent liver tumour formation. Xenobiotica. 39: 582–596. 2009. [DOI] [PubMed] [Google Scholar]
- 54.Wood CE, Hukkanen RR, Sura R, Jacobson-Kram D, Nolte T, Odin M, and Cohen SM. Scientific and regulatory policy committee (SRPC) review: Interpretation and use of cell proliferation data in cancer risk assessment. Toxicol Pathol. 43: 760–775. 2015. [DOI] [PubMed] [Google Scholar]
- 55.Cohen SM, and Arnold LL. Chemical carcinogenesis. Toxicol Sci. 120(Suppl 1): S76–S92. 2011. [DOI] [PubMed] [Google Scholar]
- 56.Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, and Moore DD. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 19: 1646–1653. 2005. [DOI] [PubMed] [Google Scholar]
- 57.Scheer N, Ross J, Rode A, Zevnik B, Niehaves S, Faust N, and Wolf CR. A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest. 118: 3228–3239. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei P, Zhang J, Egan-Hafley M, Liang S, and Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 407: 920–923. 2000. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, and Maronpot RR. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 64: 7197–7200. 2004. [DOI] [PubMed] [Google Scholar]
- 60.Molnár F, Küblbeck J, Jyrkkärinne J, Prantner V, and Honkakoski P. An update on the constitutive androstane receptor (CAR). Drug Metabol Drug Interact. 28: 79–93. 2013. [DOI] [PubMed] [Google Scholar]
- 61.Moore JT, Moore LB, Maglich JM, and Kliewer SA. Functional and structural comparison of PXR and CAR. Biochim Biophys Acta. 1619: 235–238. 2003. [DOI] [PubMed] [Google Scholar]
- 62.Deguchi Y, Yamada T, Hirose Y, Nagahori H, Kushida M, Sumida K, Sukata T, Tomigahara Y, Nishioka K, Uwagawa S, Kawamura S, and Okuno Y. Mode of action analysis for the synthetic pyrethroid metofluthrin-induced rat liver tumors: evidence for hepatic CYP2B induction and hepatocyte proliferation. Toxicol Sci. 108: 69–80. 2009. [DOI] [PubMed] [Google Scholar]
- 63.Yamada T, Uwagawa S, Okuno Y, Cohen SM, and Kaneko H. Case study: an evaluation of the human relevance of the synthetic pyrethroid metofluthrin-induced liver tumors in rats based on mode of action. Toxicol Sci. 108: 59–68. 2009. [DOI] [PubMed] [Google Scholar]
- 64.Okuda Y, Kushida M, Sumida K, Nagahori H, Nakamura Y, Higuchi H, Kawamura S, Lake BG, Cohen SM, and Yamada T. Editor’s highlight: Mode of action analysis for rat hepatocellular tumors produced by the synthetic pyrethroid momfluorothrin: evidence for activation of the constitutive androstane receptor and mitogenicity in rat hepatocytes. Toxicol Sci. 158: 412–430. 2017. [DOI] [PubMed] [Google Scholar]
- 65.Hirose Y, Nagahori H, Yamada T, Deguchi Y, Tomigahara Y, Nishioka K, Uwagawa S, Kawamura S, Isobe N, Lake BG, and Okuno Y. Comparison of the effects of the synthetic pyrethroid Metofluthrin and phenobarbital on CYP2B form induction and replicative DNA synthesis in cultured rat and human hepatocytes. Toxicology. 258: 64–69. 2009. [DOI] [PubMed] [Google Scholar]
- 66.Yamada T, Kikumoto H, Lake BG, and Kawamura S. Lack of effect of metofluthrin and sodium phenobarbital on replicative DNA synthesis and Ki-67 mRNA expression in cultured human hepatocytes. Toxicol Res (Camb). 4: 901–913. 2015. [Google Scholar]
- 67.Kondo M, Kikumoto H, Osimitz TG, Cohen SM, Lake BG, and Yamada T. An evaluation of the human relevance of the liver tumors observed in female mice treated with permethrin based on mode of action. Toxicol Sci. 175: 50–63. 2020. [DOI] [PubMed] [Google Scholar]
- 68.Yamada T, Ohara A, Ozawa N, Maeda K, Kondo M, Okuda Y, Abe J, Cohen SM, and Lake BG. Comparison of the hepatic effects of phenobarbital in chimeric mice containing either rat or human hepatocytes with humanized constitutive androstane receptor and pregnane X receptor mice. Toxicol Sci. 177: 362–376. 2020. [DOI] [PubMed] [Google Scholar]
- 69.Haines C, Elcombe BM, Chatham LR, Vardy A, Higgins LG, Elcombe CR, and Lake BG. Comparison of the effects of sodium phenobarbital in wild type and humanized constitutive androstane receptor (CAR)/pregnane X receptor (PXR) mice and in cultured mouse, rat and human hepatocytes. Toxicology. 396-397: 23–32. 2018. [DOI] [PubMed] [Google Scholar]
- 70.Parzefall W, Erber E, Sedivy R, and Schulte-Hermann R. Testing for induction of DNA synthesis in human hepatocyte primary cultures by rat liver tumor promoters. Cancer Res. 51: 1143–1147. 1991. [PubMed] [Google Scholar]
- 71.Wiemann C, Goettel M, Vardy A, Elcombe BM, Elcombe CR, Chatham LR, Wang H, Li L, Buesen R, Honarvar N, Treumann S, Marxfeld H, Groeters S, and Lake BG. Metazachlor: Mode of action analysis for rat liver tumour formation and human relevance. Toxicology. 426: 152282. 2019. [DOI] [PubMed] [Google Scholar]
- 72.Lake BG, Price RJ, Scott MP, Chatham LR, Vardy A, and Osimitz TG. Piperonyl butoxide: mode of action analysis for mouse liver tumour formation and human relevance. Toxicology. 439: 152465. 2020. [DOI] [PubMed] [Google Scholar]
- 73.Peffer RC, Cowie DE, Currie RA, and Minnema DJ. Sedaxane-use of nuclear receptor transactivation assays, toxicogenomics, and toxicokinetics as part of a mode of action framework for rodent liver tumors. Toxicol Sci. 162: 582–598. 2018. [DOI] [PubMed] [Google Scholar]
- 74.Strupp C, Quesnot N, Richert L, Moore J, Bomann WH, and Singh P. Weight of evidence and human relevance evaluation of the benfluralin mode of action in rodents (Part I): Liver carcinogenesis. Regul Toxicol Pharmacol. 117: 104758. 2020. [DOI] [PubMed] [Google Scholar]
- 75.Osimitz TG, and Lake BG. Mode-of-action analysis for induction of rat liver tumors by pyrethrins: relevance to human cancer risk. Crit Rev Toxicol. 39: 501–511. 2009. [DOI] [PubMed] [Google Scholar]
- 76.LaRocca JL, Rasoulpour RJ, Gollapudi BB, Eisenbrandt DL, Murphy LA, and LeBaron MJ. Integration of novel approaches demonstrates simultaneous metabolic inactivation and CAR-mediated hepatocarcinogenesis of a nitrification inhibitor. Toxicol Rep. 4: 586–597. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soldatow V, Peffer RC, Trask OJ, Cowie DE, Andersen ME, LeCluyse E, and Deisenroth C. Development of an in vitro high content imaging assay for quantitative assessment of CAR-dependent mouse, rat, and human primary hepatocyte proliferation. Toxicol In Vitro. 36: 224–237. 2016. [DOI] [PubMed] [Google Scholar]
- 78.Plummer S, Beaumont B, Wallace S, Ball G, Wright J, McInnes L, Currie R, Peffer R, and Cowie D. Cross-species comparison of CAR-mediated procarcinogenic key events in a 3D liver microtissue model. Toxicol Rep. 6: 998–1005. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luisier R, Lempiäinen H, Scherbichler N, Braeuning A, Geissler M, Dubost V, Müller A, Scheer N, Chibout SD, Hara H, Picard F, Theil D, Couttet P, Vitobello A, Grenet O, Grasl-Kraupp B, Ellinger-Ziegelbauer H, Thomson JP, Meehan RR, Elcombe CR, Henderson CJ, Wolf CR, Schwarz M, Moulin P, Terranova R, and Moggs JG. Phenobarbital induces cell cycle transcriptional responses in mouse liver humanized for constitutive androstane and pregnane x receptors. Toxicol Sci. 139: 501–511. 2014. [DOI] [PubMed] [Google Scholar]
- 80.Ross J, Plummer SM, Rode A, Scheer N, Bower CC, Vogel O, Henderson CJ, Wolf CR, and Elcombe CR. Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol Sci. 116: 452–466. 2010. [DOI] [PubMed] [Google Scholar]
- 81.Braeuning A, Gavrilov A, Brown S, Wolf CR, Henderson CJ, and Schwarz M. Phenobarbital-mediated tumor promotion in transgenic mice with humanized CAR and PXR. Toxicol Sci. 140: 259–270. 2014. [DOI] [PubMed] [Google Scholar]
- 82.Braeuning A, Henderson CJ, Wolf CR, and Schwarz M. Model systems for understanding mechanisms of nongenotoxic carcinogenesis: response. Toxicol Sci. 147: 299–300. 2015. [PubMed] [Google Scholar]
- 83.Braeuning A, and Schwarz M. Is the question of phenobarbital as potential liver cancer risk factor for humans really resolved? Arch Toxicol. 90: 1525–1526. 2016. [DOI] [PubMed] [Google Scholar]
- 84.Bae SDW, Nguyen R, Qiao L, and George J. Role of the constitutive androstane receptor (CAR) in human liver cancer. Biochim Biophys Acta Rev Cancer. 1875: 188516. 2021. [DOI] [PubMed] [Google Scholar]
- 85.Niu B, Coslo DM, Bataille AR, Albert I, Pugh BF, and Omiecinski CJ. In vivo genome-wide binding interactions of mouse and human constitutive androstane receptors reveal novel gene targets. Nucleic Acids Res. 46: 8385–8403. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tateno C, Yamamoto T, Utoh R, Yamasaki C, Ishida Y, Myoken Y, Oofusa K, Okada M, Tsutsui N, and Yoshizato K. Chimeric mice with hepatocyte-humanized liver as an appropriate model to study human peroxisome proliferator-activated receptor-α. Toxicol Pathol. 43: 233–248. 2015. [DOI] [PubMed] [Google Scholar]
- 87.Kawai M, Jin M, Nishimura J, Dewa Y, Saegusa Y, Matsumoto S, Taniai E, Shibutani M, and Mitsumori K. Hepatocarcinogenic susceptibility of fenofibrate and its possible mechanism of carcinogenicity in a two-stage hepatocarcinogenesis model of rasH2 mice. Toxicol Pathol. 36: 950–957. 2008. [DOI] [PubMed] [Google Scholar]
- 88.de la Rosa Rodriguez MA, Sugahara G, Hooiveld GJEJ, Ishida Y, Tateno C, and Kersten S. The whole transcriptome effects of the PPARα agonist fenofibrate on livers of hepatocyte humanized mice. BMC Genomics. 19: 443. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nihira K, Nan-Ya KI, Kakuni M, Ono Y, Yoshikawa Y, Ota T, Hiura M, and Yoshinari K. Chimeric mice with humanized livers demonstrate human-specific hepatotoxicity caused by a therapeutic antibody against TRAIL-receptor 2/death receptor 5. Toxicol Sci. 167: 190–201. 2019. [DOI] [PubMed] [Google Scholar]
- 90.Ishida Y, Yamasaki C, Iwanari H, Yamashita H, Ogawa Y, Yanagi A, Furukawa S, Kojima Y, Chayama K, Kamiie J, and Tateno C. Detection of acute toxicity of aflatoxin B1 to human hepatocytes in vitro and in vivo using chimeric mice with humanized livers. PLoS One. 15: e0239540. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eguchi A, Fukunaga S, Ogata K, Kushida M, Asano H, Cohen SM, and Sukata T. Chimeric mouse with humanized liver is an appropriate animal model to investigate mode of action for porphyria-mediated hepatocytotoxicity. Toxicol Pathol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braeuning A. Liver cell proliferation and tumor promotion by phenobarbital: relevance for humans? Arch Toxicol. 88: 1771–1772. 2014. [DOI] [PubMed] [Google Scholar]
- 93.Ohara A, Takahashi Y, Kondo M, Okuda Y, Takeda S, Kushida M, Kobayashi K, Sumida K, and Yamada T. Candidate genes responsible for early key events of phenobarbital-promoted mouse hepatocellular tumorigenesis based on differentiation of regulating genes between wild type mice and humanized chimeric mice. Toxicol Res (Camb). 6: 795–813. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong B, Lee JS, Park YY, Yang F, Xu G, Huang W, Finegold MJ, and Moore DD. Activating CAR and β-catenin induces uncontrolled liver growth and tumorigenesis. Nat Commun. 6: 5944. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thompson MD, and Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 45: 1298–1305. 2007. [DOI] [PubMed] [Google Scholar]