Abstract
In 2019, the National Institutes of Health combined the Multicenter AIDS Cohort Study (MACS) and the Women’s Interagency HIV Study (WIHS) into the MACS/WIHS Combined Cohort Study (MWCCS). In this paper, participants who made a study visit during October 2018–September 2019 (targeted for MWCCS enrollment) are described by human immunodeficiency virus (HIV) serostatus and compared with people living with HIV (PLWH) in the United States. Participants include 2,115 women and 1,901 men with a median age of 56 years (interquartile range, 48–63); 62% are PLWH. Study sites encompass the South (18%), the Mid-Atlantic/Northeast (45%), the West Coast (22%), and the Midwest (15%). Participant race/ethnicity approximates that of PLWH throughout the United States. Longitudinal data and specimens collected for 35 years (men) and 25 years (women) were combined. Differences in data collection and coding were reviewed, and key risk factor and comorbidity data were harmonized. For example, recent use of alcohol (62%) and tobacco (28%) are common, as are dyslipidemia (64%), hypertension (56%), obesity (42%), mildly or severely impaired daily activities (31%), depressive symptoms (28%), and diabetes (22%). The MWCCS repository includes serum, plasma, peripheral blood mononuclear cells, cell pellets, urine, cervicovaginal lavage samples, oral samples, B-cell lines, stool, and semen specimens. Demographic differences between the MACS and WIHS can confound analyses by sex. The merged MWCCS is both an ongoing observational cohort study and a valuable resource for harmonized longitudinal data and specimens for HIV-related research.
Keywords: biorepositories, cohort studies, collaborative research, comorbidity, HIV, Multicenter AIDS Cohort Study, MACS/WIHS Combined Cohort Study, Women’s Interagency HIV Study
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- CD4
cluster of differentiation 4
- HIV
human immunodeficiency virus
- IQR
interquartile range
- MACS
Multicenter AIDS Cohort Study
- MSM
men who have sex with men
- MWCCS
MACS/WIHS Combined Cohort Study
- NIH
National Institutes of Health
- PLWH
people living with HIV
- WIHS
Women’s Interagency HIV Study
The Multicenter AIDS Cohort Study (MACS), established in 1983, and the Women’s Interagency HIV Study (WIHS), established in 1993, were prospective observational cohort studies designed to characterize the US human immunodeficiency virus (HIV) epidemic, the natural history of HIV infection, and the pathogenesis of the disease in men and women, respectively (1, 2). Both cohort studies were multicenter studies with a unified protocol, centralized testing, and multiple waves of enrollment. Both included people living with HIV (PLWH) and similar HIV-seronegative participants at risk of HIV infection for comparison.
Because of substantial improvements in the effectiveness of treatment, HIV infection is now a chronic disease. Nearly half of PLWH in the United States are aged 50 years or older (3), and they are increasingly subject to aging-associated non–acquired immunodeficiency syndrome (AIDS)-defining conditions. MACS and WIHS participants have contributed to a rich data set compiled over many years, including detailed information on HIV risk factors, and a large biospecimen repository that includes a diverse set of samples collected during in-person visits at 6-month intervals. These resources can be used to inform research on HIV and the pathogenesis and treatment of health conditions that differentially affect PLWH or that affect all populations.
Purpose of merger
In April 2019 the MACS and WIHS, which were primarily funded by the National Institute of Allergy and Infectious Diseases, were merged to form the MACS/WIHS Combined Cohort Study (MWCCS) (4–6), with primary stewardship by the National Heart, Lung, and Blood Institute and cofunding provided by 13 additional institutes of the National Institutes of Health (NIH). At the time of this merger, they were the longest-running observational studies of PLWH in the United States. The MACS and the WIHS had similar study designs and visit schedules (every 6 months on the same October–March and April–September schedule), and both included HIV-seronegative participants who were demographically similar to participants living with HIV and had collaborated. However, while data collection protocols were similar, they were not identical, which complicated joint analyses and precluded others. Therefore, the NIH recommended combining the cohorts to harmonize protocols and to facilitate the exploration of relevant research questions within the evolving HIV epidemic, including an increased focus on aging and comorbidity. Further, the merger was expected to enable better sex comparisons, increase statistical power for analyses, and streamline the use of the studies’ rich longitudinal data sets.
With the merger, the study’s principal investigators developed a unified scientific agenda to advance understanding of the basic clinical, behavioral, and epidemiologic characteristics of HIV infection. This agenda addresses a wide range of conditions among aging PLWH and seronegative persons, including cardiovascular and pulmonary disease; sleep quality; neurocognitive ability; frailty and physical disability; cancer; HIV pathogenesis; psychosocial and behavioral conditions; metabolic, liver, and kidney dysfunction; and health disparities. It also provides a well-documented, reliable, valid, and standardized structural platform for collaborations with independently funded investigators, including early-stage investigators. Former MACS and WIHS participants described in this paper will be reenrolled in the MWCCS with a harmonized baseline visit from October 2020 through September 2021. In addition, the MWCCS will recruit up to 2,500 new participants to enrich the representation of the epidemic in the South and among Black and Hispanic men.
In this paper, we describe the MACS and WIHS participants who are being targeted for MWCCS enrollment and their sociodemographic, behavioral, and clinical characteristics relative to PLWH in the United States. We review harmonized data that are now available for analysis. Our goal is to characterize the rich retrospective database (now harmonized) and biospecimen collection (now centralized) integrated into the MWCCS; to facilitate use and analysis of the data by all stakeholders, including policy-makers, PLWH, and investigators; and to share lessons learned.
METHODS
Study population
The MACS targeted enrollment of men who have sex with men (MSM), with initial enrollment during 1984–1985 in 4 cities across the United States (2) and additional enrollment waves in 1987–1990 and 2001–2003, and limited replenishment enrollment from 2010 to 2017. In 1995, the NIH administratively censored some seronegative participants to achieve an approximately 1:1 target ratio of PLWH to seronegative men for follow-up. WIHS enrollment targeted women living with HIV and similar seronegative women at a 3:1 ratio. WIHS initial enrollment took place in 1994–1995 in 6 cities across the United States (7), with additional enrollment in 2001–2002 and 2011–2012, the closing of the Los Angeles, California, site in 2013, and the addition of 4 new sites in the southern United States in 2013–2015 (1).
Study design and inclusion criteria
We performed a cross-sectional comparison of men and women living with HIV in the MACS and WIHS, respectively, with seronegative participants in these cohorts. We restricted the analysis to current participants, defined as those who participated in at least 1 of the last 2 MACS or WIHS visits (between October 2018 and September 2019). We excluded from this analysis 18 participants who were known to have died (12 women, 1 man) or withdrawn from the study (5 women) since attending their last MACS or WIHS visit.
Exposures and outcome definitions
Characteristics and behaviors from data collected at the most recent visit were used. Analysis explored sociodemographic variables (e.g., age, race/ethnicity, education), study follow-up, HIV-related variables (cluster of differentiation 4 (CD4) cell count, HIV viral RNA load, antiretroviral therapy (ART)), behavioral variables (sexual activity; alcohol, tobacco, and drug use), and comorbid conditions. Table 1 shows harmonized definitions of comorbidity and the available data used for these outcomes. Biospecimen collection is described in the Results section, and the number of person-visits with samples available for each biospecimen type in the national repository is reported. More details on historical data collection and new MWCCS data collection can be found on the study’s website (https://statepi.jhsph.edu/mwccs/) (6).
Table 1. Harmonized Definitions for Risk Factors, Clinical Characteristics, and Comorbid Conditions Used in the MACS/WIHS Combined Cohort Study.
| Indicator | Summarized Data or Harmonized Definition |
|---|---|
| Summary filesa | |
| dhbasesocdem | Baseline sociodemographic data from participant’s first MACS or WIHS visit, including: visit number and date, enrollment wave, race and ethnicity, education, baseline HCV and HBV status, and lifetime behaviors (tobacco and drug use, sexual behavior) and sexually transmitted infections reported at baseline. Categories harmonized. |
| dhbehavior | Longitudinal data, by visit, on recent behaviors (tobacco, alcohol, and drug use and sexual behavior), income, and lifetime and recent sexually transmitted infections. Categories harmonized. |
| dhchronHlth | Longitudinal data, by visit, on chronic health conditions, including BMI, body measurements, CES-D score, cardiovascular and metabolic measures, diabetes, hypertension, and kidney and liver disease. Definitions and categories harmonized. |
| dhhivhist | Summarized data file (1 record per participant) on key HIV-related variables, including HIV status, CD4 cell count nadir, most recent CD4 cell count, HIV viral RNA load, date of first AIDS diagnosis, first use of ARV agents (ARVs), and dates of most recent study visit, medical events, and death (when applicable). Definitions and categories harmonized. |
| dhhivlabs | Longitudinal data, by visit, on HIV-related laboratory results, including HIV viral RNA load and CD3, CD4, and CD8 cell counts. Harmonized lower limits of quantitation and information on tests used over time. |
| dhvertdatebase | Longitudinal data, by visit, on study visit details, including visit number and date, clinical research site, HIV serostatus at visit, and age at visit. MACS/WIHS Combined Cohort Study site codes and harmonized visit windows based on calendar dates. |
| dhoutcome | Longitudinal data, by outcome, on AIDS, cancer, tuberculosis, neuropsychological status, CVD, and death events with ICD-9, ICD-10, and ICD-O codes and event dates as applicable. Harmonized study-specific event codes, type of event confirmation, and level of confidence in event. |
| dhaidsdrug | Longitudinal data, by visit, on medications used to treat HIV for Food and Drug Administration–approved ARVs, including classification (e.g., protease inhibitor) and use since last interview. Harmonized use of combination and single ARVs, length of use, use since visit (no use, current use, used but not currently), and ARV’s effect on HIV suppression status (HIV viral RNA load undetectable/detectable). |
| Visit filesb | |
| Longitudinal visit files of study participant data across all visits for each study form. Harmonized and standardized, within each cohort, for changes in questions over time. (Not harmonized between cohorts, although some questions asked were the same and other variables differed by cohort or were assessed in one cohort but not the other.) | |
| Comorbid conditions | |
| Hypertension | Having any of the following: 1) SBP ≥140 mg/dL, 2) DBP ≥90 mg/dL, 3) self-reported hypertension, or 4) self-reported use of antihypertensive medication (women only). |
| Diabetes mellitus | Having any of the following: 1) self-reported ever use of antidiabetic medication, 2) fasting glucose level ≥126 mg/dL, 3) hemoglobin A1c concentration ≥6.5%, or 4) self-reported diabetes confirmed by one of the previous 3 conditions. |
| Metabolic syndrome | Having ≥3 of the following: 1) waist circumference ≥102 cm (men) or ≥88 cm (women), 2) fasting triglyceride level ≥150 mg/dL, 3) fasting glucose level ≥100 mg/dL (men) or ≥110 mg/dL (women) or current use of diabetic medication and a history of diabetes, 4) HDL cholesterol level <40 mg/dL (men) or <50 mg/dL (women), or 5) SBP ≥130 mg/dL or DBP ≥85 mg/dL or self-reported use of hypertension medication or history of hypertension. |
| Dyslipidemia | Any of the following diagnostic features: 1) low-density lipoprotein level ≥130 mg/dL, 2) HDL cholesterol level <40 mg/dL, 3) triglyceride level ≥150 mg/dL, 4) self-reported use of lipid-lowering medication (or clinical diagnosis in the past for men), and 5) fasting total cholesterol level ≥200 mg/dL (for men only). |
| CVD risk scores | American College of Cardiology/American Heart Association Pooled Cohort Equations: <5% risk of coronary death or nonfatal MI or fatal or nonfatal stroke within 10 years = low risk; 5.0%–7.5% risk = moderate risk; >7.5% risk = high risk. Framingham Risk Score: <10% estimated risk of MI and coronary death within 10 years = low risk; 10%–20% risk = moderate risk; >20% risk = high risk (49). |
| Cancer diagnosis | Confirmed diagnosis of invasive cancer from cancer registry match or medical record abstraction. Excluded: basal and squamous cell skin cancers, noninvasive tumors, and self-reported or next-of-kin–reported cancer. |
| Kidney function | eGFR ≥60.0 mL/minute/1.73 m2 = normal kidney function; eGFR 15.0–59.9 mL/minute/1.73 m2 = kidney disease; eGFR <15.0 mL/minute/1.73 m2 = kidney failure. |
| IADL | Assessment of IADL occurred at every other visit starting in 2008 for the men and at every visit for the women. Each question evaluated change in function from “best” to “current” score and classified functional status as: severe IADL impairment (major decline for ≥2 questions or major or minor declines in score for ≥4 questions), mild IADL impairment (minor decline for ≥2 questions or major or minor declines in score for 2 or more questions), or no impairment. |
| Aging-related measures | “Slower walker” if 4-m walking speed was ≤0.65 m/s for men with height ≤173 cm and women with height ≤159 cm or ≤0.76 m/s for men with height >173 cm and women with height >159 cm. “Weak” was based on how hard participants squeezed a dynamometer using their dominant hand. Men were defined as weak if grip strength was ≤29 kg (BMI ≤24.0), ≤30 kg (BMI 24.1–26.0), ≤31 kg (BMI 26.1–28.0), or ≤32 kg (if BMI >28.0). Women were defined as weak if grip strength was ≤17 kg (BMI ≤23.0), ≤17.3 kg (BMI 23.1–26.0), ≤18 kg (BMI 26.1–29.0), or ≤21 kg (BMI >29.0). |
| Frailty phenotype | MACS only: Using the 5 Fried frailty phenotype criteria, participants were classified as robust (not frail) if they met 0 criteria, prefrail if they met 1–2 criteria, and frail if they met ≥3 criteria. Fried frailty phenotype criteria are: unintentional weight loss, weakness, exhaustion, slowness, and low physical activity level. |
| Depressive symptoms | CES-D score ≥16. |
| Abnormal liver function | AST or alanine aminotransferase blood test result >40 IU/L. |
| Liver fibrosis | AST:platelet ratio index > 1.5 = significant fibrosis. FIB-4 score > 3.25 = significant fibrosis; FIB-4 score 1.45–3.25 = inconclusive; FIB-4 score < 1.45 = absence of fibrosis. |
| HBV | HBV status was classified as 1) negative (surface antibody-negative and core antibody-negative and surface antigen-negative or vaccinated), 2) resolved infection (surface antibody-positive and core antibody-positive and surface antigen-negative), 3) chronic/active infection (surface antigen-positive), or 4) unknown/inconclusive (missing some measures). HBV serological testing was done using antigen and antibody tests at or shortly after enrollment. For men only, updated HBV status was also measured (though not reported here), with men who remained uninfected during follow-up receiving anti-HBc tests 2 years after their most recent test; those positive for HBsAg received antibody and antigen tests at their next visit, those who seroconverted during follow-up received additional anti-HBc and HBsAg tests to determine when seroconversion occurred, and those with past or recovered HBV infection received HBsAg tests every 5 years to identify reactivation. |
| HCV | HCV status was classified as 1) not infected (antibody-negative, RNA-negative), 2) resolved infection (antibody-positive, RNA-negative), 3) chronic infection (active infection, RNA-positive), or 4) antibody-positive, RNA unknown. HCV serological testing was done using antibody testing at or shortly after enrollment. HCV-seropositive participants received a quantitative HCV RNA test to determine clearance or persistence of infection. Men who were uninfected received HCV antibody tests 2 years after their last negative test; those who seroconverted during follow-up received additional antibody tests to determine seroconversion, those prevalent at study entry received RNA tests once every 5 years to verify ongoing infection, and those who cleared their HCV infection received antibody and RNA tests once every 5 years to identify reinfection or reversions. Select longitudinal HCV testing was done for some women (project-specific). |
Abbreviations: AIDS, acquired immunodeficiency syndrome; anti-HBc, hepatitis B core antibody; ARV, antiretroviral; AST, aspartate aminotransferase; BMI, body mass index; CD3, cluster of differentiation 3; CD4, cluster of differentiation 4; CD8, cluster of differentiation 8; CES-D, Center for Epidemiologic Studies Depression Scale; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis 4; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IADL, instrumental activities of daily living; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision; ICD-O, International Classification of Diseases for Oncology; MACS, Multicenter AIDS Cohort Study; MI, myocardial infarction; SBP, systolic blood pressure; WIHS, Women’s Interagency HIV Study.
a Summary files are analytical data files.
b Visit files are raw data files.
If the value of a variable was missing (e.g., participants who had a telephone interview rather than an in-person visit), the most recent value of that variable was carried forward using data collected up to 2 years prior; otherwise the variable was coded as missing. While data in many domains were collected using similar or identical questions in the 2 cohorts, other domains were assessed differently. For this analysis, we present data on key characteristics for which comparable data were available across the 2 cohorts; some data presented were collected in similar but not identical ways and were harmonized in order to directly compare them (detailed information on criteria used is presented in Table 1). Certain data assessed in only 1 of the cohorts are also presented.
Statistical methods
The prevalences of demographic characteristics, behaviors, and comorbidity were compared between PLWH and seronegative participants. HIV-related characteristics were compared among men and women living with HIV using Wilcoxon 2-sample tests for continuous variables and χ2 tests for categorical variables. Fisher’s exact test was used if any group had fewer than 5 participants.
Trajectories of CD4 cell count (number of cells/mm3), hereafter called CD4, and plasma HIV viral RNA load ([number of copies/mL) over time were explored visually (Figure 1). Each was categorized into a range from the most harmful levels (CD4 < 200 cells/mm3 and HIV viral RNA load > 10,000 copies/mL; colored in red) to the most beneficial (CD4 ≥ 500 cells/mm3 and HIV viral RNA load ≤ 20 copies/mL; colored in blue), and we explored how the proportions in each category changed over time. Distributions of age and race/ethnicity among men and women living with HIV were compared with those in the overall US HIV epidemic using 2018 data from the Centers for Disease Control and Prevention (8).
Figure 1.
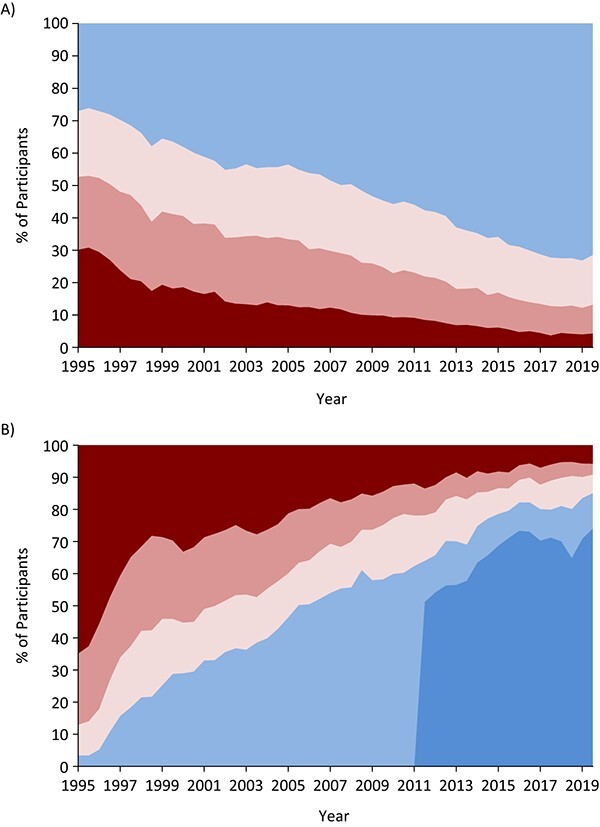
Distributions of cluster of differentiation 4 (CD4) cell counts (A) and plasma human immunodeficiency virus (HIV) viral RNA loads (B) among current Multicenter AIDS Cohort Study and Women’s Interagency HIV Study participants living with HIV, by year, 1995–2019. Data were restricted to current participants, and we used semiannual CD4 cell counts and HIV viral RNA load measures to explore these trends. CD4 cell count categories, from top to bottom (A): blue, ≥500 cells/mm3; lighter pink, 350–499 cells/mm3; darker pink, 200–349 cells/mm3; red, <200 cells/mm3. HIV RNA viral load categories, from top left to bottom right (B): red, >10,000 copies/mL; darker pink, 1,001–10,000 copies/mL; lighter pink, 81–1,000 copies/mL; lighter blue, ≤80 copies/mL; darker blue, ≤20 copies/mL. AIDS, acquired immunodeficiency syndrome.
RESULTS
There are 1,901 male participants, with an approximately 1:1 ratio of men living with HIV to seronegative men, and 2,115 female participants, with an approximately 2.5:1 ratio of women living with HIV to seronegative women (Table 2). The study includes sites throughout the United States, with 22% of participants on the West Coast, 15% in the Midwest, 45% in the Mid-Atlantic/Northeast, and 18% in the South. The 4,016 current participants represent 33% of the 12,340 participants who have ever participated in the studies; 31% of participants have died since enrollment, 14% have been lost to follow-up or have remained in contact but did not complete a study visit in the last year, 2% withdrew, and 20% were administratively censored by the NIH for budgetary reasons. Censored participants included 1,614 seronegative men from the MACS who were censored in 1995 and 282 seronegative women and 569 women living with HIV who were censored from the WIHS, primarily in 2013 (details are shown in Web Figure 1, available online at https://doi.org/10.1093/aje/kwab050).
Table 2.
Demographic Characteristics of Men in the MACS and Women in WIHS at Their 2018–2019 Study Visit, by HIV Status
| Total (n = 4,016) | Prevalence, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Men a | Women b | |||||||
| Characteristic at MostRecent Study Visit | No. | % |
All Men (n = 1,901) |
PLWH (n = 1,004) |
HIV-SN (n = 897) |
All Women (n = 2,115) |
PLWH (n = 1,488) |
HIV-SN (n = 627) |
| Geographic region | ||||||||
| West Coast | 876 | 22 | 32 | 30 | 33 | 13 | 13 | 14 |
| Midwest | 614 | 15 | 19 | 24 | 14 | 12 | 12 | 11 |
| Mid-Atlantic and Northeast | 1,790 | 45 | 49 | 46 | 52 | 41 | 40 | 43 |
| South | 736 | 18 | 0 | 0 | 0 | 35 | 36 | 32 |
| Duration of study follow-up, yearsc | 17 (7–31) | 32 (16–35) | 17 (16–35) | 35 (17–35) | 17 (5–24) | 17 (5–24) | 17 (5–24) | |
| ≤5.00 | 511 | 13 | 3 | 4 | 2 | 21 | 23 | 19 |
| 5.01–15.00 | 701 | 17 | 10 | 17 | 2 | 24 | 25 | 23 |
| 15.01–25.00 | 1,753 | 44 | 32 | 36 | 28 | 54 | 52 | 58 |
| >25.00 | 1,051 | 26 | 55 | 43 | 68 | 0 | 0 | 0 |
| Age, yearsc | 56 (48–63) | 61 (54–68) | 59 (51–65) | 64 (58–71) | 52 (45–58) | 53 (46–59) | 51 (43–57) | |
| Age group, years | ||||||||
| 24–39 | 389 | 10 | 7 | 9 | 6 | 12 | 10 | 16 |
| 40–49 | 738 | 18 | 10 | 12 | 7 | 26 | 25 | 28 |
| 50–59 | 1,370 | 34 | 26 | 33 | 19 | 41 | 43 | 36 |
| 60–69 | 1,088 | 27 | 36 | 35 | 38 | 19 | 19 | 17 |
| 70–94 | 431 | 11 | 20 | 11 | 30 | 2 | 2 | 2 |
| Race/ethnicity | ||||||||
| Black (non-Hispanic) | 1,970 | 49 | 23 | 28 | 16 | 73 | 73 | 73 |
| Hispanic (any race) | 527 | 13 | 12 | 16 | 8 | 14 | 14 | 15 |
| White (non-Hispanic) | 1,402 | 35 | 64 | 54 | 74 | 9 | 10 | 6 |
| Other race | 117 | 3 | 2 | 2 | 2 | 4 | 3 | 5 |
| Education | ||||||||
| Less than high school | 777 | 19 | 5 | 7 | 3 | 32 | 33 | 30 |
| High school graduate/some college | 2,037 | 51 | 41 | 47 | 34 | 60 | 59 | 61 |
| College graduate or higher | 1,200 | 30 | 54 | 46 | 63 | 8 | 8 | 8 |
| Low incomed | 1,738 | 45 | 26 | 33 | 18 | 62 | 62 | 60 |
| Currently employed | 1,790 | 45 | 52 | 51 | 53 | 39 | 37 | 44 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; PLWH, people living with HIV; SN, seronegative; WIHS, Women’s Interagency HIV Study.
a Includes 1,899 cisgender men and 3 transgender women who participated in the MACS.
b Includes 2,114 cisgender women and 1 transgender man who participated in the WIHS.
c Values are expressed as median (interquartile range).
d Low income was defined differently by study among participants with known current income (n = 3,855): as household income ≤$18,000/year in the WIHS and as individual income <$20,000/year in the MACS.
As Table 2 shows, most men (72%) and women (86%) in the MWCCS are between 40 and 69 years of age. However, the median age is about 10 years higher in men than in women (61 years vs. 52 years; P < 0.001). Participants have been followed for a median of 17 years (interquartile range (IQR), 7–31), and 511 (13%), 701 (17%), 1,753 (44%), and 1,051 (26%) participants have had ≤5.00, 5.01–15.00, 15.01–25.00, and >25.00 years of follow-up, respectively (Table 2). Almost half of participants are non-Hispanic Black (n = 1,970; 49%), and over one-third are non-Hispanic White (n = 1,402; 35%), with 13% identifying as Hispanic of any race (n = 527). Significantly fewer men than women are Black (23% vs. 73%; P < 0.001) or Hispanic (12% vs. 14%; P = 0.04). Women are less likely to report having graduated from college (8% vs. 54%; P < 0.001) and more likely to report a low income (62% vs. 26%; P < 0.001) than men.
Sociodemographic characteristics (including age, race/ethnicity, income, and education) are similar between women who are living with HIV and seronegative women (Table 2). By contrast, men living with HIV are younger, less educated, and more likely to be a member of a racial minority group than the seronegative men; this difference is primarily due to high mortality from AIDS among men living with HIV early in the MACS (55% of HIV-seropositive men have died vs. 9% of seronegative men), as well as enriched recruitment of minority men in later waves of MACS enrollment (9–11).
HIV-related characteristics are described in Table 3. The study includes 2,492 PLWH, 2,236 of whom were HIV-seropositive at enrollment and 256 of whom acquired HIV during the study; these latter participants have been followed for a median of 23 years (IQR, 12–30) since seroconversion. One-quarter (24%) of PLWH have a history of clinically defined AIDS, including 11% of men and 33% of women. Most men (96%) and women (92%) report use of ART, with 90% reporting at least 95% adherence to ART. One-quarter (26%) of PLWH had a detectable HIV viral RNA load (≥20 copies/mL) at their last study visit (Table 3).
Table 3.
HIV-Related Characteristics of MACS Men and WIHS Women Living With HIV at Their 2018–2019 Study Visit
| All PLWH (n = 2,492) | Prevalence Among PLWH, % | ||||
|---|---|---|---|---|---|
| HIV Characteristic at Most Recent Study Visit | No. | % |
Men (n = 1,004) |
Women (n = 1,488) |
P Value |
| HIV serostatus | |||||
| HIV-seroprevalent | 2,236 | 90 | 76 | 99 | <0.001 |
| HIV-seroincident (seroconversion since enrollment in study) | 256 | 10 | 24 | 1 | |
| CD4 cell count nadir, cells/mm3 | <0.001 | ||||
| ≥500 | 263 | 11 | 12 | 10 | |
| 350–499 | 427 | 17 | 21 | 15 | |
| 200–349 | 813 | 33 | 35 | 31 | |
| <200 | 989 | 40 | 33 | 44 | |
| Current CD4 cell count, cells/mm3 a | |||||
| <350 | 315 | 13 | 12 | 14 | 0.13 |
| <200 | 107 | 5 | 3 | 5 | 0.01 |
| Current HIV viral RNA loadb | 0.06 | ||||
| Not detected (<20 copies/mL) | 1,765 | 74 | 77 | 73 | |
| Detected (≥20 copies/mL) | 606 | 26 | 23 | 27 | |
| Ever use of ART | 0.98 | ||||
| Never used | 47 | 2 | 2 | 2 | |
| Ever used | 2,445 | 98 | 98 | 98 | |
| Current use of ART | 2,328 | 93 | 96 | 92 | <0.001 |
| ART adherence among those currently receiving treatment | |||||
| 100% adherent | 1,185 | 51 | 49 | 52 | 0.14 |
| ≥95% adherent | 2,088 | 90 | 92 | 88 | 0.003 |
| History of clinical AIDS | 607 | 24 | 11 | 33 | <0.001 |
| Time from estimated HIV seroconversion to most recent visitc, yearsd | 23 (12–30) | 24 (12–30) | 17 (11–20) | 0.02 | |
| CD4 cell count nadir, cells/mm3 d | 241 (125–366) | 273 (159–393) | 223 (106–346) | <0.001 | |
| Current CD4 cell count, cells/mm3 d | 669 (470–903) | 644 (476–850) | 689 (463–930) | 0.01 | |
| HIV viral RNA load, log10 copies/mL (among those detected)d | 2.1 (1.6–3.5) | 1.8 (1.6–2.5) | 2.2 (1.6–3.9) | <0.001 | |
| Years since ART initiation (among current users)d | 16.9 (8.6–23.2) | 20.3 (9.9–27.7) | 13.3 (8.3–22.3) | <0.001 | |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CD4, cluster of differentiation 4; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; PLWH, people living with HIV; WIHS, Women’s Interagency HIV Study.
a Among participants with a known current CD4 cell count (n = 2,372).
b Among participants with a known current HIV viral RNA load (n = 2,371). HIV viral RNA load in the past 2 years was unknown (not measured) among 10% of men (these were primarily men who completed a telephone visit only) and 1% of women.
c Calculated among the 256 participants with known HIV seroconversion (“seroincident”) during study follow-up.
d Values are presented as median (interquartile range).
CD4 cell count at the last visit (in 2018–2019) was high among both men and women living with HIV (median values were 644 cells/mm3 and 689 cells/mm3, respectively); 13% have a current CD4 count less than 350 cells/mm3. Participants have initiated ART at a wide range of CD4 counts. CD4 nadir is less than 200 cells/mm3 in 40% of participants, with 33%, 17%, and 11% having a CD4 nadir of 200–349 cells/mm3, 350–499 cells/mm3, and ≥500 cells/mm3, respectively. Male participants are less likely than women to have a CD4 nadir less than 200 cells/mm3 (33% vs. 44%; P < 0.001); however, the prevalence of a current CD4 count less than 350 cells/mm3 is similar between men and women (12% vs. 14%; P < 0.13).
Figure 1A explores the trajectory of CD4 count between October 1994 and September 2019 among the 2,492 PLWH. The proportion of participants with CD4 <200 cells/mm3 decreased from 30% in 1995 to 10% in 2009 to 4% in 2019, and conversely, the proportion with CD4 ≥500 cells/mm3 increased from 27% to 55% to 71%, respectively (P for trend < 0.001). HIV viral RNA loads also changed dramatically (Figure 1B). The proportion of participants with a viral RNA load greater than 10,000 copies/mL decreased from 65% in 1995 to 14% in 2009 to 6% in 2019 (P for trend < 0.001); the proportion with an undetectable viral RNA load (defined as <80 copies/mL for consistency in assay detection limits across time) increased from 3% to 58% to 85%, respectively.
Comparison with US statistics
Figure 2 shows the geographic distribution of participating research sites against the map of overall US HIV incidence. The geographic representation is broad and overlaps with high-prevalence areas in the Northeast, Southeast, Midwest, and West (12).
Figure 2.
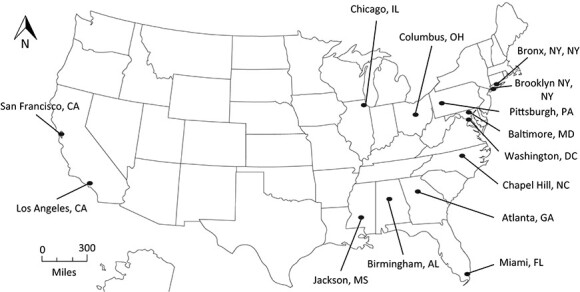
Geographic locations of MACS/WIHS Combined Cohort Study sites across the United States, 2020. AIDS, acquired immunodeficiency syndrome; AL, Alabama; CA, California; DC, District of Columbia; FL, Florida; GA, Georgia; HIV, human immunodeficiency virus; IL, Illinois; MACS, Multicenter AIDS Cohort Study; MD, Maryland; MS, Mississippi; NC, North Carolina; NY, New York; OH, Ohio; PA, Pennsylvania; WIHS, Women’s Interagency HIV Study.
Current MACS and WIHS participants are 24–94 years of age, and 56% are aged 55 years or older, as compared with 32% of current US PLWH (Figure 3A). Another 40% of current participants are 35–54 years of age, as compared with 45% of US PLWH. Only 4% of participants are under 35 years of age, compared with 22% in national data, as expected, given the long study follow-up. The participant age distribution is compared with sex-stratified US data on MSM living with HIV and women living with HIV in Web Figure 2A.
Figure 3.
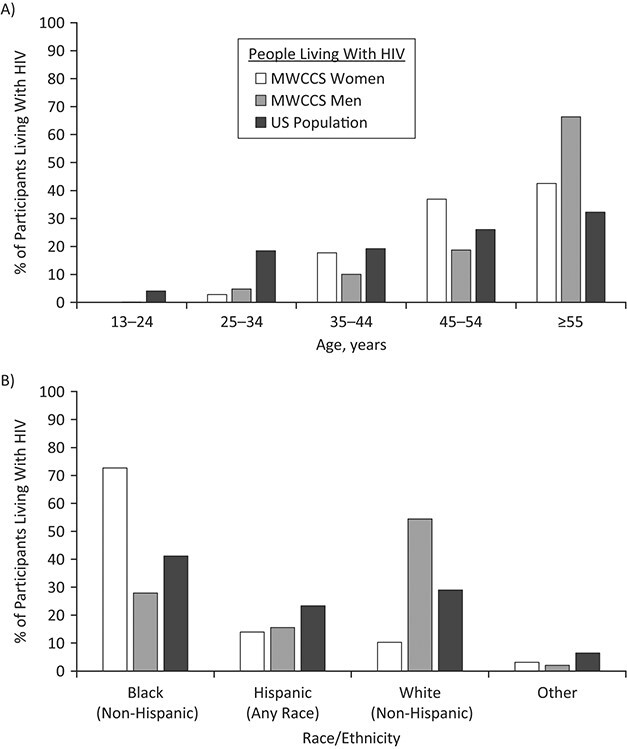
Distributions of current MACS/WIHS Combined Cohort Study (MWCCS) participants living with human immunodeficiency virus (HIV) according to age (A) and race/ethnicity (B), by sex and compared with all people living with HIV in the United States (8), October 2018–September 2019. AIDS, acquired immunodeficiency syndrome; MACS, Multicenter AIDS Cohort Study; WIHS, Women’s Interagency HIV Study. The “Other” category comprises Asian, Native Hawaiian/other Pacific Islander, Native American/Alaska Native, and other races/multiracial.
The overall racial and ethnic distribution (49% Black, 13% Hispanic (all races), and 35% White) is comparable to that of US PLWH (41% Black, 23% Hispanic, and 29% White; Figure 3B). There are differences in race/ethnicity among men and women in the cohort, with a higher proportion of White participants among men living with HIV than among women living with HIV (54% vs. 10%) and a higher proportion of Black participants among women living with HIV than among men living with HIV (73% vs. 28%); these partly reflect differences in the race/ethnicity of MSM and women living with HIV in the United States (Web Figure 2B).
Harmonized data
The merged MWCCS will collect new data under a unified protocol from October 2020 through September 2021, and data from that first MWCCS visit will be cleaned and available for analysis in 2022. The longitudinal data previously collected over 35 years are currently available, and key variables have been harmonized to facilitate utilization and analysis. Table 4 gives the characteristics of harmonized summary files (which utilize longitudinal data to define key risk factors and outcomes) that have recently been created, as well as harmonized comorbidity definitions utilized in this paper, which are all now available for investigator use (upon concept sheet approval—see the investigators’ “Work With Us” section of the website) (6). The data harmonization process involved detailed review of areas of research between the MACS and the WIHS. Study definitions, question wording, and data categorization were compared by the data management team and vetted by scientific collaborators when criteria differed between the studies in order to create combined variables with comparable criteria and maintain clinically or behaviorally appropriate cutoffs by sex.
Table 4.
Behavioral Characteristics of MACS Men and WIHS Women at Their 2018–2019 Study Visit, by Sex and HIV Status
| Characteristic at Most Recent Study Visit |
Total (n = 4,016) |
Prevalence, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Men | Women | |||||||
|
All Men (n = 1,901) |
PLWH (n = 1,004) |
HIV-SN (n = 897) |
P Value |
All Women (n = 2,115) |
PLWH (n = 1,488) |
HIV-SN (n = 627) |
P Value | |||
| Drug use | ||||||||||
| Ever injecting drugs | 571 | 14 | 12 | 16 | 7 | <0.001 | 16 | 16 | 17 | 0.67 |
| Ever using marijuana | 3,024 | 75 | 80 | 80 | 81 | 0.65 | 71 | 68 | 78 | <0.001 |
| Ever using cocaine or heroin | 2,205 | 55 | 55 | 61 | 48 | <0.001 | 55 | 53 | 59 | 0.01 |
| Recent drug use (past 6 months) | ||||||||||
| Marijuana | 1,149 | 29 | 36 | 39 | 32 | 0.002 | 23 | 21 | 28 | 0.001 |
| Medical marijuana | 327 | 8 | 14 | 17 | 10 | <0.001 | 3 | 4 | 2 | 0.03 |
| “Poppers”a | 397 | N/A | 21 | 25 | 18 | <0.001 | N/A | N/A | N/A | N/A |
| Prescription barbiturates | 552 | 14 | 19 | 19 | 18 | 0.81 | 9 | 10 | 7 | 0.04 |
| Opioids (nonprescription or prescription) | 441 | 11 | 7 | 7 | 7 | 0.95 | 15 | 15 | 14 | 0.30 |
| Cocaine or heroin | 300 | 8 | 8 | 10 | 6 | 0.003 | 7 | 6 | 9 | 0.01 |
| PrEP use | ||||||||||
| Ever use of PrEP | 130 | 9 | N/A | N/A | 13 | N/A | N/A | N/A | 2 | N/A |
| Recent use of PrEP | 111 | 7 | N/A | N/A | 12 | N/A | N/A | N/A | 1 | N/A |
| Cigarette smoking | ||||||||||
| Ever having smoked | 2,740 | 68 | 69 | 70 | 67 | 0.19 | 68 | 66 | 73 | 0.002 |
| Current smoker | 1,111 | 28 | 18 | 22 | 14 | <0.001 | 36 | 34 | 41 | 0.004 |
| Pack-years of smoking (among ever smokers)b | 10 (3–23) | 11 (1–30) | 12 (2–29) | 10 (0.3–32) | 0.05 | 10 (4–19) | 9 (4–18) | 10 (3–20) | 0.80 | |
| Alcohol usec | ||||||||||
| Abstainer (no drinks) | 1,508 | 38 | 22 | 23 | 21 | 0.11 | 53 | 56 | 45 | <0.001 |
| Light drinking | 1,953 | 50 | 69 | 69 | 69 | 32 | 31 | 36 | ||
| Moderate, heavy, or binge drinking | 484 | 12 | 9 | 8 | 11 | 15 | 13 | 20 | ||
| Sexual behaviors | ||||||||||
| Ever having a male sexual partner | 3,950 | 98 | 97 | 97 | 96 | 0.22 | 100 | 100 | 100 | 0.30 |
| Ever having a female sexual partner | 1,923 | 48 | 67 | 70 | 63 | 0.005 | 32 | 29 | 39 | <0.001 |
| Recent sex (past 6 months) | ||||||||||
| Any recent male partners | 2,240 | 56 | 61 | 62 | 60 | 0.56 | 53 | 49 | 61 | <0.001 |
| Any recent female partners | 181 | 5 | 7 | 6 | 8 | 0.11 | 3 | 2 | 5 | <0.001 |
| No. of recent sexual partnersd | ||||||||||
| 0 | 1,580 | 40 | 34 | 34 | 34 | 0.34 | 45 | 49 | 35 | <0.001 |
| 1 | 1,495 | 38 | 26 | 25 | 28 | 48 | 46 | 53 | ||
| ≥2 | 871 | 22 | 40 | 41 | 38 | 7 | 4 | 11 | ||
| Sexually transmitted infection (other than HIV) | ||||||||||
| Ever | 2,704 | 67 | 91 | 93 | 88 | <0.001 | 47 | 49 | 41 | <0.001 |
| Past 6 months | 295 | 7 | 14 | 14 | 14 | 0.99 | 2 | 2 | 1 | 0.38 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; N/A, not applicable; PLWH, people living with HIV; PrEP, preexposure prophylaxis; SN, seronegative; WIHS, Women’s Interagency HIV Study.
a Sexual enhancement drugs containing alkyl nitrites.
b Values are expressed as median (interquartile range).
c Alcohol use was categorized differently for men and women, using number of drinks per week. Light alcohol use was defined as 1–7 drinks/week in the WIHS and 1–13 drinks/week in the MACS. Any alcohol use above this level was included in the “moderate, heavy, or binge drinking” group. Binge drinking is reported separately in the text and was defined as consuming ≥4 (women) or ≥5 (men) alcoholic drinks at one sitting at least once per month.
d Among participants with a known number of recent sexual partners (n = 3,945).
Health behavior profile
The behavioral characteristics of current MACS and WIHS participants are presented in Table 4. Because men and women were enrolled within 2 previously independent cohorts, some of their lifestyle characteristics are known to differ, while others are similar. Current use of alcohol (62%) and tobacco (28%) is common. These include binge drinking (7%) and more than 20 pack-years of smoking (20%).
While ever use of drugs and tobacco is similar in MACS and WIHS participants, current use differs significantly. Most participants smoked in the past (68%), and current smoking is more common in women than in men (36% vs. 18%; P < 0.001). Recent (past 6 months) marijuana use (29%) is common, and medical marijuana use is more common in men than in women (14% vs. 3%; P < 0.001). Recent use of “poppers” (sexual enhancement drugs containing alkyl nitrites) is common among male participants (21%). Conversely, recent opioid use is more common among women than among men (15% vs. 7%; P < 0.001), though use of opioids is similar among PLWH and HIV-seronegative persons of each sex (Table 4). More than half (55%) of participants have reported ever using “crack” cocaine, other forms of cocaine, or heroin, and 8% of participants have reported current use of at least 1 of these substances. Current injection drug use is rare (1%), though past use is more common (14%).
Most male participants are MSM (by design at enrollment in the MACS) and report having had sex with another man (97%); however, 67% of men report ever having had sex with a woman (Table 4). Most female participants are heterosexual, with 100% reporting ever having sex with a man but 32% also reporting ever having sex with a woman. Most participants report having had sex in the past 6 months, including with a recent male partner (61% of men, 53% of women), a recent female partner (7% of men, 3% of women), and recent male and female partners (2% of men, 1% of women). The median number of recent sexual partners is the same among men (median, 1; IQR, 0–3) and women (median, 1; IQR, 0–1).
Comorbidity profile
Data on comorbidity and on treatment of some chronic conditions (e.g., lipid-lowering medication, hypertension medication) are routinely collected. Data were compared and harmonized for several key comorbid conditions (Table 1), and prevalence was explored (Table 5). Common comorbid conditions among participants include dyslipidemia (64%), hypertension (56%), obesity (42%), depressive symptoms (28%), diabetes (22%), and severely impaired instrumental activities of daily living (21%). Some participants have kidney disease (11%), frailty (7% of men), and a history of cancer (7%) (Table 5). Participants living with HIV have a higher prevalence than seronegative participants of several comorbid conditions, including diabetes, the metabolic syndrome, kidney disease, liver fibrosis, impaired instrumental activities of daily living, depressive symptoms, and chronic hepatitis B virus and hepatitis C virus infection. The 2 groups have similar prevalences of obesity, hypertension, dyslipidemia, and frailty. Among PLWH, men are less likely than women to be obese (23% vs. 55%; P < 0.001), to have the metabolic syndrome (28% vs. 47%; P < 0.001), or to have chronic hepatitis C virus infection (5% vs. 9%; P < 0.001) and are more likely to have liver fibrosis (4% vs. 3%; P = 0.05) and a history of cancer (10% vs. 6%; P < 0.001); see Web Table 1. These differences may be explained by sex-based differences in age, hepatitis C and other risk factors, and confounders.
Table 5.
Prevalence of Comorbidity Among MACS and WIHS Participants Living With HIV and HIV Seronegative Participants at Their 2018–2019 Study Visit, by HIV Statusa
| Characteristic at Most Recent Study Visit b |
No. of Participants |
Prevalence, % | P Value c | ||
|---|---|---|---|---|---|
|
Total (n = 4,016) |
PLWH (n = 2,492) |
HIV-Seronegative (n = 1,524) |
|||
| Metabolic characteristics | |||||
| Body mass index categoryd | 0.70 | ||||
| Underweight (<18.0) | 62 | 2 | 2 | 2 | |
| Normal-weight (18.0–24.9) | 994 | 25 | 25 | 26 | |
| Overweight (25.0–29.9) | 1,149 | 31 | 31 | 31 | |
| Obese (≥30.0) | 1,588 | 42 | 43 | 41 | |
| Hypertension | 2,164 | 56 | 55 | 56 | 0.83 |
| Diabetes mellitus | 828 | 22 | 23 | 20 | 0.04 |
| Metabolic syndrome | 1,300 | 37 | 40 | 33 | <0.001 |
| Cardiovascular disease | |||||
| Dyslipidemia | 2,435 | 64 | 65 | 63 | 0.33 |
| Use of cholesterol-lowering medication | 1,316 | 33 | 33 | 33 | 0.54 |
| ACC/AHA Pooled Cohort Equations score (risk of coronary death or MI) | <0.001 | ||||
| Low (<5.0%) | 1,470 | 41 | 45 | 33 | |
| Moderate (5.0%–7.4%) | 451 | 12 | 13 | 11 | |
| High (≥7.5%) | 1,704 | 47 | 42 | 56 | |
| Framingham Risk Score (risk of coronary death or MI) | <0.001 | ||||
| Low (<10%) | 2,496 | 67 | 70 | 62 | |
| Moderate (10–20%) | 496 | 13 | 10 | 18 | |
| High (>20%) | 728 | 20 | 20 | 20 | |
| History of cancer | 290 | 7 | 8 | 6 | 0.10 |
| Kidney function | <0.001 | ||||
| Normal (eGFR ≥60.00 mL/minute/1.73 m2) | 3,339 | 88 | 85 | 92 | |
| Kidney disease (eGFR 15.00–59.99 mL/minute/1.73 m2) | 429 | 11 | 14 | 7 | |
| Kidney failure (eGFR <15.00 mL/minute/1.73 m2) | 27 | 0.7 | 0.8 | 0.5 | |
| Instrumental activities of daily living | <0.001 | ||||
| Not impaired (within normal limits) | 2,286 | 70 | 67 | 75 | |
| Mild impairment | 325 | 10 | 11 | 8 | |
| Severe impairment | 676 | 21 | 22 | 18 | |
| Aging | |||||
| “Slow walker” phenotype | 391 | 12 | 11 | 12 | 0.39 |
| “Weak” (grip-strength) phenotype | 768 | 24 | 22 | 26 | 0.005 |
| Fried frailty phenotype (men only) | 0.46 | ||||
| Nonfrail | 909 | 57 | 59 | 56 | |
| Prefrail | 568 | 36 | 35 | 37 | |
| Frail | 111 | 7 | 6 | 8 | |
| CES-D score ≥16 (at risk for depression) | 1,108 | 28 | 30 | 25 | 0.002 |
| Liver function | |||||
| Abnormal AST level (>40 IU/L) | 192 | 5 | 6 | 3 | <0.001 |
| Abnormal ALT level (>40 IU/L) | 241 | 7 | 7 | 6 | 0.07 |
| Significant fibrosis | |||||
| FIB-4 score >3.25 | 103 | 3 | 3 | 2 | 0.005 |
| APRI score >1.50 | 30 | 0.8 | 1.1 | 0.4 | 0.02 |
| HBV statuse | <0.001 | ||||
| Never infected | 2,371 | 59 | 55 | 66 | |
| Resolved | 1,290 | 32 | 34 | 30 | |
| Chronic | 112 | 3 | 3 | 2 | |
| Unknown/inconclusive | 243 | 6 | 8 | 3 | |
| HCV statuse | <0.001 | ||||
| Never infected | 3,506 | 88 | 86 | 91 | |
| Resolved infection (antibody-positive, RNA-negative) | 90 | 2 | 3 | 2 | |
| Chronic infection (active infection, RNA-positive) | 260 | 7 | 8 | 5 | |
| Unknown (antibody-positive, RNA unknown) | 139 | 4 | 4 | 2 | |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; AIDS, acquired immunodeficiency syndrome; ALT, alanine aminotransferase; APRI, AST:platelet ratio index; AST, aspartate aminotransferase; CES-D, Center for Epidemiologic Studies Depression Scale; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis 4; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; MI, myocardial infarction; PLWH, people living with HIV; WIHS, Women’s Interagency HIV Study.
a See Web Table 2 for definitions of these comorbid conditions.
b For some of the measures in this table, no data from the past 2 years were available. The prevalences reported in the table exclude missing data on the following variables: BMI (n = 273; 7%), hypertension (n = 119; 3%), diabetes mellitus (n = 172; 4%), metabolic syndrome (n = 509; 13%, including 3% never measured), dyslipidemia (n = 234; 6%), ACC/AHA Pooled Cohort Equations score (n = 391; 10%), Framingham Risk Score (n = 296; 7%), kidney function (eGFR) (n = 221; 6%), ALT (n = 324; 8%), AST (n = 221;6%), FIB-4 or APRI score (n = 223; 6%), instrumental activities of daily living (n = 729; 5% not measured in the past 5 years, and 13% never measured), “slow walker” phenotype (n = 640; 16%, including 8% never measured), “weak” phenotype (n = 770; 19%, including 8% never measured), and Fried frailty phenotype (n = 313; 16% (men only)). See Web Table 1 for definitions of these conditions.
c P values reported among participants with known values for each variable (i.e., excluding those with missing values).
d Weight (kg)/height (m)2.
e HBV and HCV status were determined for all participants at their baseline (for each wave of participant enrollment). Some additional HCV measurements were made during follow-up among select participants (see Web Table 1). For HBV, additional measurements were done during follow-up in the MACS but not in the WIHS. Therefore, participant status may not reflect current status, since some participants classified as having active infection at baseline have probably since cleared their infections and some persons who were not infected at baseline could have since become infected.
Novel data on cardiac, pulmonary, and sleep-related health from 4 recent substudies funded by the National Heart, Lung, and Blood Institute are available for analysis. These include data from 1) electrocardiograms performed on 3,408 participants (MACS: 2016–2017 (n = 1,473); WIHS: 2017–2019 (n = 1,935)) (13–15) with Zio Patch rhythm monitoring (iRhythm Technologies, Inc., San Francisco, California; among men only); 2) echocardiograms performed on 2,412 participants (MACS: 2017–2018 (n = 1,156); WIHS: 2018–2019 (n = 1,256)); 3) pulmonary function testing on 2,850 participants (MACS: 2017–2018 (n = 1,165); WIHS: 2018–2019 (n = 1,685)), with pre- and postbronchodilator administration spirometry and diffusing capacity (16); and 4) overnight sleep studies performed on 842 participants (MACS only: 2018–2019).
Available specimens
The national repository for the MWCCS (Precision for Medicine, Frederick, Maryland) includes all of the historically collected biospecimens and has the capacity to store prospectively collected materials. The repository includes a wide range of types of samples collected at MACS and WIHS semiannual visits.
In Table 6, the number of person-visits for which samples are available in the repository is listed for each biospecimen type. These include the number of person-visits with samples for current participants (i.e., those active in the study in 2018–2019), and the number of person-visits for all participants who were ever included in the study. Many visits included multiple samples per participant per visit; the number of samples available in the repository is shown in Web Table 2.
Table 6.
Numbers of Participant Visits With Samples in the MACS/WIHS Combined Cohort Study Biorepository as of May 1, 2020, and the Time Period During Which Each Type of Sample Was Collected in the MACS and WIHS
| Specimen Type | No. of Participant Visits | Date Range of Data Collected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Participants (Active, Censored, and Deceased) | Current Participants (n = 4,016) | |||||||||||
| Total | Men | Women | Total | Men | Women | Men (MACS) | Women (WIHS) | |||||
| PLWH | HIV-SN | PLWH | HIV-SN | PLWH | HIV-SN | PLWH | HIV-SN | |||||
| Serum | 224,856 | 68,500 | 63,908 | 68,968 | 23,480 | 120,521 | 33,240 | 33,285 | 39,105 | 14,891 | 1984–2019 | 1994–2019 |
| Plasmaa | 225,277 | 68,665 | 63,769 | 69,379 | 23,464 | 107,922 | 39,122 | 14,839 | 39,122 | 14,839 | 1984–2019 | 1994–2019 |
| Plasma EDTA | 45,764 | 21,215 | 20,562 | 2,824 | 1,163 | 58,010 | 38,043 | 16,021 | 2,798 | 1,148 | 2006–2019 | 2014–2019 |
| Peripheral blood mononuclear cells | 217,167 | 63,825 | 62,509 | 67,741 | 23,092 | 105,938 | 38,352 | 14,617 | 38,352 | 14,617 | 1994–2019 | 1994–2019 |
| Cell pellets | 88,355 | 1,499 | 1,309 | 63,440 | 22,107 | 53,974 | 946 | 856 | 37,691 | 14,481 | 1994–2019 | 1994–2019 |
| Urine (clean void)b | 43,453 | 12,558 | 12,481 | 14,109 | 4,305 | 60,924 | 39,105 | 14,891 | 4,955 | 1,973 | 1984–2014 | 1994–2019 |
| Urine (supernatant)b | 35,039 | N/A | N/A | 25,919 | 9,120 | 21,546 | N/A | N/A | 15,253 | 6,293 | NC | 1994–2019 |
| Cervicovaginal lavage | 91,853 | N/A | N/A | 67,390 | 24,463 | 54,064 | N/A | N/A | 38,043 | 16,021 | NC | 1994–2019 |
| Oral samplec | 7,512 | 669 | 1,000 | 4,773 | 1,070 | 2,165 | 121 | 235 | 1,437 | 372 | 1984–1993 | 1995–2004 |
| B-cell lines | 3,354 | 2,655 | 699 | N/A | N/A | 704 | 509 | 195 | N/A | N/A | N/A | NC |
| Stool | 6,851 | 3,252 | 3,599 | N/A | N/A | 3,946 | 2,798 | 1,148 | N/A | N/A | 1984–1985 | NC |
| Semen | 21,557 | 10,290 | 11,267 | N/A | N/A | 1,809 | 1,437 | 372 | N/A | N/A | 1984–1993 | NC |
Abbreviations: AIDS, acquired immunodeficiency syndrome; EDTA, ethylenediaminetetraacetic acid; HIV, human immunodeficiency virus; MACS, Multicenter AIDS Cohort Study; N/A, not applicable; NC, not collected; PLWH, people living with HIV; SN, seronegative; WIHS, Women’s Interagency HIV Study.
a Plasma samples were stored in cell preparation tubes containing the anticoagulants heparin and sodium citrate for the MACS and WIHS, respectively.
b Oral samples were throat washes in the MACS and saliva samples in the WIHS.
c Urine was collected at every visit during selected periods (men: 1984–1991 and 2009–2014; women: 1994–1998 and annually from 2009 to 2019).
Serum and plasma have been collected at every semiannual in-person visit since the beginning of each study. There are 224,856 person-visits with archived serum and 225,277 person-visits with archived plasma remaining in the biorepository (Table 6), including 1,165,535 and 1,368,395 vials, respectively (Web Table 2). Beginning in 1994, peripheral blood mononuclear cells were collected in both studies from blood samples taken at every visit, and cell pellets were generated when the quantity of material was sufficient. There are 217,167 person-visits with viable cryopreserved peripheral blood mononuclear cells and 88,355 person-visits with cell pellets remaining in the biorepository (Table 6). Urine was collected at every visit during selected periods (men: 1984–1991 and 2009–2014; women: 1994–1998 and annually from 2009 to 2019). Among women, cervicovaginal lavage samples have been collected since 1994. Plasma ethylenediaminetetraacetic acid collection was added for men in 2006 and in 2014 for women. Stool and semen were collected from men and then stopped, as were saliva samples from both men and women (see Table 6 for dates).
DISCUSSION
Participants from the MACS and WIHS provide a rich resource, with detailed, prospectively collected longitudinal data and an array of biospecimens with which to retrospectively examine a wide range of epidemiologic, mechanistic, and clinical research questions related to the progression of HIV. There is an average of 17 years of semiannual collection of comprehensive risk factor information for these participants and similarly collected data (using the same protocol) for HIV-seronegative participants, providing a critical control group for analyses of associations with HIV within each cohort.
We have shown that the study populations of both cohorts are ideal for integration into a single cohort to examine comorbidity among persons living with treated HIV, since most participants report being on ART (with a median of 17 years since ART initiation) and have well-controlled disease (a median current CD4 count of 669 cells/mm3). However, the study also includes participants who started therapy late, participants who are not adherent, and participants with a history of clinical AIDS, allowing analyses of associations with these differences as well. This paper harmonizes these data and presents key characteristics of active participants in the MACS and WIHS, demonstrating the types of comparable data that are available historically in both cohorts and can now be used in joint analyses. Participants largely represent adult PLWH in the United States by age and race/ethnicity (Web Figure 2) and geography (recruitment of Southern men will be a focus of the new recruitment). Because most participants have been treated for many years, they may not represent persons newly initiating therapy; however, 13% of the cohort was recruited in the past 5 years, with additional enrollment of early-stage PLWH newly initiating therapy being planned.
Combining the MACS and WIHS cohorts into the MWCCS was a challenging process and led to several lessons learned (Figure 4). Merging of the scientific operations, cultures, and scientific priorities of 2 large decades-long cohort studies took time, but it was successfully accomplished. This created a study population with several notable strengths, as well as some limitations. First, the data centers for the 2 cohorts have merged and have harmonized core historical exposure and outcome data. Second, there are significant demographic differences in age, race/ethnicity, socioeconomic status, and geography between women from the WIHS and men from the MACS, which can confound analyses by sex. These differences in part represent racial/ethnic (Web Figure 2B) and socioeconomic differences in MSM living with HIV and women living with HIV in the United States. However, they pose a notable limitation of the merger and will need to be accounted for in analyses. Third, integrating operations required agreeing on shared language for data-sharing (Data Use Agreement) and specimen-sharing (Material Transfer Agreement) agreements across the many legal entities involved, which was time-consuming but resulted in a large merged study repository with similar sample types and study intervals in the 2 cohorts. Fourth, going forward, men and women living with HIV and similar seronegative individuals will be followed using identical protocols, facilitating sex-based comparisons.
Figure 4.

Lessons learned from merging the Multicenter AIDS Cohort Study (MACS) and Women’s Interagency HIV Study (WIHS) cohorts into the MACS/WIHS Combined Cohort Study. AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; sIRB, single institutional review board.
MACS and WIHS study investigators have published over 2,900 research articles to date. In 2019, 194 research proposals (called concept sheets) were submitted to the MWCCS for new studies, including 99 from investigators not affiliated with the MWCCS. The MWCCS has 13 scientific working groups in which study investigators collaborate within specific research areas, including aging, clinical outcomes, cardiovascular disease, genomics, human papillomavirus infection, liver disease, malignancy, metabolism, neuropsychology, psychosocial factors, pulmonary function, sleep, and viral immune pathogenesis. Examples of high-impact MACS and WIHS research include: identification of causes of and disparities in mortality among PLWH (10, 17); exploration of the associations of HIV infection with diabetes (18, 19) and cardiovascular disease (20–22); examination of cervical human papillomavirus infection among PLWH (23–26); evaluation of hepatitis C virus–related liver disease as a major cause of morbidity and mortality in PLWH (27, 28); characterization of the impact of HIV on neurocognitive function (29–31) and frailty (32, 33); and increasing understanding of the roles of psychosocial and geographic factors in health-care access, utilization, and outcomes among PLWH (34–36). Examples of impactful findings include research utilizing biospecimens to evaluate prognostic markers for HIV and AIDS and to understand the HIV reservoir (37–40), studies on the associations of HIV infection and HIV therapies with comorbidity (18, 41–43), and studies comparing PLWH and HIV-seronegative persons in the cohorts to understand the associations of HIV infection with comorbidity and mortality (28, 44). In addition, the MACS and WIHS have contributed to many impactful multicohort analyses, such as the examples in references 45–48, each of which has been cited over 950 times (45–48).
With the shift in research focus in recent years from ascertaining the natural history of HIV and responses to ART, to evaluating HIV serostatus-based differences in aging, the merged MWCCS serves as a robust and well-characterized cohort in which to study the myriad factors and circumstances that surround these processes. We encourage interested investigators, especially those at an early career stage, to submit proposals for research. Proposals to use biospecimens or study data are submitted using our online system (see website for details) (6) and reviewed by relevant working groups. External investigators are assigned an MWCCS liaison to facilitate communication and feasibility during concept sheet preparation. Some samples from high-priority visits (for example, visits around HIV seroconversion or comorbidity diagnosis) are considered restricted and require additional committee review and approval before release.
In summary, the MWCCS is a trans-NIH–supported observational cohort study that aims to advance understanding of the effects of HIV on aging, chronic comorbid illnesses, and psychosocial outcomes. It provides access to the rich retrospective data and biospecimens already collected in both the MACS and the WIHS and serves as a unique and valuable resource for investigators not only to study HIV-related research questions but also to increase knowledge of these diseases in HIV-seronegative individuals.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, United States (Gypsyamber D’Souza, Fiona Bhondoekhan, Lorie Benning); Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, United States (Joseph B. Margolick); Department of Epidemiology and Population Health, Albert Einstein College of Medicine, New York, New York, United States (Adebola A. Adedimeji); Department of Epidemiology, Gillings School of Global Public Health, Chapel Hill, North Carolina, United States (Adaora A. Adimora); Department of Medicine, Miller School of Medicine, University of Miami, Miami, Florida, United States (Maria L. Alcaide); Department of Medicine, Stroger Hospital of Cook County Health and Hospitals System, Chicago, Illinois, United States (Mardge H. Cohen); Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California, United States (Roger Detels); Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, United States (M. Reuel Friedman); Department of Medicine, SUNY Downstate Health Sciences University, State University of New York, Brooklyn, New York, New York, United States (Susan Holman); Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, United States (Deborah J. Konkle-Parker); Department of Family Medicine, School of Medicine, Georgetown University, Washington, DC, United States (Daniel Merenstein); Department of Medicine, School of Medicine, Emory University, Atlanta, Georgia, United States (Igho Ofotokun); Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, United States (Frank Palella); Epidemiology Branch, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, Maryland, United States (Sean Altekruse); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland, United States (Todd T. Brown); Division of Endocrinology, Diabetes, and Metabolism, Johns Hopkins Medical Institutions, Baltimore, Maryland, United States (Todd T. Brown); Department of Medicine, School of Medicine, University of California, San Francisco, San Francisco, California, United States (Phyllis C. Tien); and Division of Infectious Disease, San Francisco VA Medical Center, San Francisco, California, United States (Phyllis C. Tien).
This work was supported by the National Institutes of Health (grants U01-HL146241, U01-HL146201, U01-HL146204, U01-HL146202, U01-HL146193, U01-HL146245, U01-HL146240, U01-HL146242, U01-HL146333, U01-HL146205, U01-HL146203, U01-HL146208, U01-HL146192, U01-HL146194, UL1-TR000004, P30-AI-050409, P30-AI-050410, and P30-AI-027767). The MACS/WIHS Combined Cohort Study (MWCCS) is funded primarily by the National Heart, Lung, and Blood Institute, with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, the National Institute of Dental and Craniofacial Research, the National Institute of Allergy and Infectious Diseases, the National Institute of Neurological Disorders and Stroke, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute of Nursing Research, the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute on Minority Health and Health Disparities, and in coordination and alignment with the research priorities of the Office of AIDS Research, National Institutes of Health.
We gratefully acknowledge the contributions of the study participants and the dedication of the staff at the MWCCS study sites. We acknowledge the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that helped support the collection and availability of the cancer registry data. We also thank the following state cancer registries for their help: Alabama, California, Florida, Georgia, Illinois, Maryland, Mississippi, New York, North Carolina, Pennsylvania, and Virginia.
These data were collected by staff of the Multicenter AIDS Cohort Study (MACS) and the Women’s Interagency HIV Study (WIHS), now the MWCCS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood Institute, or the US Department of Health and Human Services. The authors assume full responsibility for all data analysis and interpretation.
The public data set of the MWCCS is available upon request (https://statepi.jhsph.edu/mwccs/). A more tailored data set is available upon concept sheet approval; see the “Work With Us” section of the MWCCS website (https://statepi.jhsph.edu/mwccs/work-with-us/).
A.A. has received consulting fees from Merck & Co., Inc. (Kenilworth, New Jersey), ViiV Healthcare (London, United Kingdom), and Gilead Sciences, Inc. (Foster City, California); Gilead has provided her institution with funding for her research. F.J.P. has received honoraria for speaking for Janssen Pharmaceuticals, Inc. (Titusville, New Jersey), ViiV Healthcare, and Merck. T.T.B. has received consulting fees from Merck, ViiV Healthcare, Janssen, Theratechnologies Inc. (Montreal, Quebec, Canada), and Gilead. P.T. has received an investigator-initiated grant from Merck. M.A. has received honoraria for speaking for Merck and has received funding for research from AbbVie Inc. (North Chicago, Illinois). None of the other authors report any conflicts of interest.
REFERENCES
- 1.Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . HIV and older Americans. https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed August 3, 2020.
- 4.National Heart, Lung, and Blood Institute . MACS/WIHS Combined Cohort Study. https://www.nhlbi.nih.gov/science/macswihs-combined-cohort-study. Accessed June 26, 2019.
- 5.D’Souza G, Golub ET, Gange SJ. The changing science of HIV epidemiology in the United States. Am J Epidemiol. 2019;188(12):2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Data Analysis and Coordination Center (DACC), Johns Hopkins University . MWCCS—MACS/WIHS Combined Cohort Study. https://statepi.jhsph.edu/mwccs/. Accessed January 27, 2021.
- 7.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Diagnoses of HIV Infection in the United States and Dependent Areas, 2018. (HIV Surveillance Reports, 2018 Updated Edition, Vol. 31). Atlanta, GA: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed February 28, 2019. [Google Scholar]
- 9.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada N, Jacobson LP, Cohen M, et al. Cause-specific mortality among HIV-infected individuals, by CD4(+) cell count at HAART initiation, compared with HIV-uninfected individuals. AIDS. 2014;28(2):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider MF, Gange SJ, Williams CM, et al. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS. 2005;19(17):2009–2018. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . HIV in the United States by region. https://www.cdc.gov/hiv/statistics/overview/geographicdistribution.html. Page last reviewed October 26, 2020. Accessed July 24, 2020.
- 13.Heravi AS, Etzkorn LH, Urbanek JK, et al. HIV infection is associated with variability in ventricular repolarization: the Multicenter AIDS Cohort Study (MACS). Circulation. 2020;141(3):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu KC, Zhang L, Haberlen SA, et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart. 2019;105(7):559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu KC, Bhondoekhan F, Haberlen SA, et al. Associations between QT interval subcomponents, HIV serostatus, and inflammation. Ann Noninvasive Electrocardiol. 2020;25(2):e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunisaki KM, Nouraie M, Jensen RL, et al. Lung function in men with and without HIV. AIDS. 2020;34(8):1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessol NA, Kalinowski A, Benning L, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis. 2007;44(2):287–294. [DOI] [PubMed] [Google Scholar]
- 18.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med. 2005;165(10):1179–1184. [DOI] [PubMed] [Google Scholar]
- 19.Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women’s Interagency HIV Study. AIDS. 2007;21(13):1739–1745. [DOI] [PubMed] [Google Scholar]
- 20.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160(7):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna DB, Moon J-Y, Haberlen SA, et al. Carotid artery atherosclerosis is associated with mortality in HIV-positive women and men. AIDS. 2018;32(16):2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis. 2015;61(4):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293(12):1471–1476. [DOI] [PubMed] [Google Scholar]
- 24.Strickler HD, Palefsky JM, Shah KV, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95(14):1062–1071. [DOI] [PubMed] [Google Scholar]
- 25.Robbins HA, Strickler HD, Massad LS, et al. Cervical cancer screening intervals and management for women living with HIV: a risk benchmarking approach. AIDS. 2017;31(7):1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massad LS, Pierce CB, Minkoff H, et al. Long-term cumulative incidence of cervical intraepithelial neoplasia grade 3 or worse after abnormal cytology: impact of HIV infection. Int J Cancer. 2014;134(8):1854–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breskin A, Westreich D, Hurt CB, et al. The effects of hepatitis C treatment eligibility criteria on all-cause mortality among people with human immunodeficiency virus. Clin Infect Dis. 2019;69(9):1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349):1921–1926. [DOI] [PubMed] [Google Scholar]
- 29.Vance DE, Rubin LH, Valcour V, et al. Aging and neurocognitive functioning in HIV-infected women: a review of the literature involving the Women’s Interagency HIV Study. Curr HIV/AIDS Rep. 2016;13(6):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacktor N, Skolasky RL, Seaberg E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki PM, Rubin LH, Valcour V, et al. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology. 2015;84(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69(2):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatukasi TV, Edmonds A, Gustafson DR, et al. Prevalence and 1-year incidence of frailty among women with and without HIV in the Women’s Interagency HIV Study. AIDS. 2019;33(2):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman MR, Stall R, Silvestre AJ, et al. Effects of syndemics on HIV viral load and medication adherence in the Multicentre AIDS Cohort Study. AIDS. 2015;29(9):1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludema C, Edmonds A, Cole SR, et al. Comparing neighborhood and state contexts for women living with and without HIV: understanding the Southern HIV epidemic. AIDS Care. 2018;30(11):1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turan B, Smith W, Cohen MH, et al. Mechanisms for the negative effects of internalized HIV-related stigma on antiretroviral therapy adherence in women: the mediating roles of social isolation and depression. J Acquir Immune Defic Syndr. 2016;72(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322(3):166–172. [DOI] [PubMed] [Google Scholar]
- 38.Mellors JW, Rinaldo CR Jr, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272(5265):1167–1170. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296(12):1498–1506. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358(9293):1593–1601. [DOI] [PubMed] [Google Scholar]
- 41.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289(22):2978–2982. [DOI] [PubMed] [Google Scholar]
- 42.Seaberg EC, Muñoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19(9):953–960. [DOI] [PubMed] [Google Scholar]
- 43.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39(8):1199–1206. [DOI] [PubMed] [Google Scholar]
- 44.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97(8):577–586. [DOI] [PubMed] [Google Scholar]
- 45.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.When to Start Consortium . Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrington M, Nelson GW, Martin MP, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283(5408):1748–1752. [DOI] [PubMed] [Google Scholar]
- 49.Monroe AK, Haberlen SA, Post WS, et al. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. AIDS. 2016;30(13):2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


