Abstract
Background
It is of paramount importance that clinical trials are designed with adequate health equity considerations to prevent disproportionate analyses of specific demographics.
Objective
In this study, we investigated the representation of sex, race, and ethnicity in pivotal clinical trials for drugs with dermatological disease indications approved by the U.S. Food and Drug Administration between 1995 and 2019.
Methods
Thirty-six novel drugs with indications to treat dermatological diseases, approved by the U.S. Food and Drug Administration between January 1995 and December 2019 were abstracted from Drugs@FDA. The drug approval label, statistical review, official record, and trial publication were reviewed for data on disease indication, approval year, pathway, number of participants, participant demographics (sex, race, and ethnicity), location, and sponsor type.
Results
The overall female representation was 45.6% (n = 17,492 of 38,320). Adequate female representation was noted for five of six disease indications. Caucasians were predominantly overrepresented (80.4%; n = 28,065 of 34,890); Blacks (9.8%; n = 3242 of 33,240) and Asians (5.5%; n = 1535 of 27,696) were consistently underrepresented. Across sponsor types, there was a significant difference in the distribution of women (χ2 = 6.332; p = .042), as well as Caucasians (χ2 = 12.813; p = .002), Blacks (χ2 = 13.002; p = .002), and Hispanics/Latinos (χ2 = 7.747; p = .021).
Conclusion
Persistence of disparities disproportionately affect the quality of data behind therapies for certain demographics; as such, enrollment practices must continue to address the issue of underrepresentation. Efforts to facilitate demographic equity among clinical trial participants must be supported to ensure that safety and efficacy conclusions are drawn from representative population samples.
Keywords: Dermatological drugs, Clinical trials, Minorities, Disparity, Sex, Representation
Introduction
The U.S. Food and Drug Administration (FDA) serves as the regulatory body that ensures the safety and efficacy of all approved drugs (FDA, 2018). Data from clinical trials are comprehensively and prudently scrutinized by the FDA to critically judge prospective drugs. It is of paramount importance that clinical trials are designed with adequate health equity considerations to prevent disproportionate analyses of a specific sex, race, and/or ethnicity. Those who lead clinical trials for new drugs have a social responsibility, not only to the majority demographics but also to minority groups within the population. Seemingly innocuous oversights in the methodology of a clinical trial, such as enrolling a nonrepresentative proportion of participants from a certain demographic, may negatively affect public health due to misinformed or incomplete safety and efficacy conclusions (Pinn, 1994).
Differences in sex, race, and ethnicity cannot be discounted when evaluating the pharmacodynamics and pharmacokinetics of a drug (Anderson, 2005, Chen, 2006, Gandhi et al., 2004). With respect to dermatological diseases specifically, understanding sex and ethnicity differences facilitates the treatment of skin diseases (Chen et al., 2010, Rahrovan et al., 2018). Nevertheless, the underrepresentation of women and minorities in pivotal clinical trials has been a pervasive issue (Clark et al., 2019, Poon et al., 2013). In 1977, the FDA published the General Considerations for the Clinical Evaluation of Drugs, aimed at protecting women of childbearing potential from the uncertainties of early clinical trials (FDA, 1977). However, this guidance document inadvertently catalyzed the normalization of predominantly enrolling men in clinical studies (Poon et al., 2013). The repercussions were observed even decades later when it was reported that 80% of drugs withdrawn by the FDA between January 1997 and December 2000 exhibited a higher incidence of adverse effects in women (Heinrich, 2001). Increasing the diversity of clinical trial participants is a complex issue with no all-encompassing solution, given that practical aspects must also be considered. For instance, the potential teratogenicity of new drugs remains a valid concern (i.e., women may not want to enroll in trials, and similarly, researchers may be less likely to recruit them). In addition, trials are often time consuming, which further disinclines some people from participating.
In an attempt to mediate the demographic disparity in clinical trial participation, the FDA released updated guidelines in 1993 that called for a greater representation of women (FDA, 1993). In recent years, the FDA has drafted additional guidelines with the intention of encouraging increased diversity in clinical trial demographics not limited to sex (U.S. Food and Drug Administration, 2016, U.S. Food and Drug Administration, 2019). Recent studies have reported on the racial and ethnic disparities in clinical trial enrollment for drugs that treat specific dermatological conditions (Price et al., 2019, Price et al., 2020). The purpose of this study was to evaluate the representation of sex, race, and ethnicity in pivotal clinical trials for drugs with dermatological disease indications approved by the FDA between 1995 and 2019.
Methods
Statement of ethics
This study was exempt from institutional review board approval because the data were extracted entirely from publicly available resources.
Data collection
Drugs with dermatological disease indications approved for marketing by the FDA between January 1995 and December 2019 were abstracted for this study. Operating under the FDA, the Center for Drug Evaluation and Research (CDER) regulates the approval of new molecular entities (NMEs) and maintains a publicly accessible dataset that contains product information and disease indication for all those approved between 1985 and 2019 (FDA, 2020). To prevent unintentional omission of relevant NMEs, the CDER dataset was comprehensively reviewed to generate a preliminary list of drugs with dermatological disease indications. Subsequently, all shortlisted drugs were searched in the FDA database, Drugs@FDA. NMEs approved under the classification “Type 1: New Molecular Entity” were accepted for analysis, and those with a different or missing classification were excluded.
The drug approval label was reviewed to collect primary data on drug name, disease indication, approval year, and approval pathway. Section 14 of the label, Clinical Studies, was inspected to acquire information on pivotal clinical studies that led to the approval of each NME and its disease indication. The identifying information was then used to find the record of each pivotal clinical study archived on clinicalstudies.gov. This allowed for the collection of data on the total number of participants, enrollment by participant demographic (sex, race, and ethnicity), study location, and sponsor type. For all NMEs, participant demographic data extracted from clinicaltrials.gov was corroborated with the statistical analysis review (available on Drugs@FDA). In cases of ambiguity concerning the label, clinicalstudy.gov, or statistical review, the clinical trial publication was searched on PubMed and reviewed for additional information. The data acquisition procedure was adapted from a previously validated methodology (Khan et al., 2020).
Sex was recorded as either male or female. Racial demographics were documented as Caucasian, Black, Asian, or other. Ethnic demographics were captured as either Hispanic/Latino or non-Hispanic/Latino. Study location was classified as North America exclusively, Europe exclusively, or multicontinental. Approval pathway was noted as standard or priority. Lastly, sponsor type was classified as government, U.S.-based industry, non-U.S.-based industry, or collaboration.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics, version 25 (IBM Corporation, Armonk, NY) and Microsoft Excel (Microsoft Corp, Redmond, WA). Categorical data were reported as a fraction of the whole with a corresponding percentage. Percentages were adjusted to reflect the proportion among trials that reported data on the demographic, and trial populations with missing or incomplete demographic data were subtracted from the corresponding total population. Continuous data were presented as a mean with a 95% confidence interval (CI).
An alpha of 0.05 was used as the cutoff for significance. Due to the lack of data normality as determined by the Shapiro–Wilk test, the distribution differences between groups were assessed by nonparametric evaluations. Mann–Whitney U and Kruskal–Wallis H tests were performed to analyze the demographic distribution differences between the two (approval pathway) and three (location and sponsor type) groups, respectively. Where applicable, post hoc analyses with Bonferroni corrections for multiple comparisons were conducted to single out the pairs that were significantly different.
To evaluate adequate female representation in the participant enrollment of pivotal clinical trials based on dermatological disease indication, female participation in each trial was calculated as a percentage and grouped by disease indication. Subsequently, the percentage of women among the global disease population for each indication was abstracted from the Global Burden of Disease database, which is one of the most comprehensive and authoritative epidemiologic datasets developed in collaboration with the World Health Organization (World Health Organization, 2017). The database was accessed in July 2020 (Global Burden of Disease Collaborative Network, 2018). The participation-to-prevalence ratio (PPR) was obtained by dividing the percentage of female trial participants by that of the global disease population (Khan et al., 2020, Poon et al., 2013). Adequate female representation was represented by a PPR between 0.8 and 1.2, and any ratio below or above indicated under- or overrepresentation of trial female participants compared with the disease population, respectively (Eshera et al., 2015, Poon et al., 2013). Dermatological diseases not available in the Global Burden of Disease database were excluded from the PPR analysis.
The pervasiveness of Caucasian participant predominance in pivotal clinical trials was compared to Black and Asian racial minority participation. For each year between 1995 and 2019 with complete racial demographic data, the Caucasian–Black and Caucasian–Asian participant ratios were calculated by dividing the adjusted proportion of Caucasian participants by that of the corresponding racial minority. These two ratios were compared to gauge the relative predominance of Caucasian participants and to identify the more underrepresented racial minority for each year.
Results
General characteristics
The characteristics of the NMEs are presented in Table 1. A total of 36 drugs with dermatological disease indications approved by the FDA between January 1995 and December 2019 were analyzed. Collectively, there were 75 pivotal clinical trials, and the median number of trials per approval for a disease indication was two. The demographic breakdown by sex was available for all 38,320 trial participants. Complete racial demographic information (i.e., specified number of Caucasian, Black, Asian, and other participants) was reported for 55 trials (73.3%). Incomplete racial demographic information (i.e., specified number of Caucasian participants, but the number of Black and/or Asian participants was missing) was extracted for 11 trials (14.7%). Demographic information on ethnicity (Hispanic/Latino or non-Hispanic/non-Latino) was only reported for 27 trials (36.0%). Data on approval pathway, location, and sponsor type were available for all 75 pivotal clinical trials.
Table 1.
Characteristics of new molecular entities with dermatological disease indications approved by the U.S. Food and Drug Administration between 1995 and 2019.
| Drug name | Approval year | Approval pathway | Disease indication | Total population, N | Female, n (%) | Trials, N |
|---|---|---|---|---|---|---|
| Azelaic acid | 1995 | Standard | Acne vulgaris | 545 | 267 (48.99) | 2 |
| Butenafine | 1996 | Standard | Tinea pedis | 185 | 52 (28.11) | 2 |
| Penciclovir | 1996 | Standard | Herpes labialis | 3773 | 2790 (73.95) | 2 |
| Tazarotene a | 1997 | Standard | Plaque psoriasis | 1575 | 608 (38.60) | 5 |
| Acne vulgaris | 773 | 396 (51.23) | 2 | |||
| Linezolid | 2000 | Priority | ABSSSI | 1382 | 742 (53.69) | 2 |
| Docosanol | 2000 | Standard | Herpes labialis | 737 | 524 (71.10) | 2 |
| Pimecrolimus | 2001 | Standard | Atopic dermatitis | 589 | 285 (48.39) | 3 |
| Ertapenem | 2001 | Standard | ABSSSI | 338 | 114 (33.73) | 1 |
| Daptomycin | 2003 | Priority | ABSSSI | 1092 | 491 (44.96) | 2 |
| Sertaconazole | 2003 | Standard | Tinea pedis | 598 | 152 (25.42) | 2 |
| Tigecycline | 2005 | Priority | ABSSSI | 1116 | 418 (37.46) | 2 |
| Retapamulin | 2007 | Standard | Impetigo | 210 | 107 (50.95) | 1 |
| Telavancin | 2009 | Standard | ABSSSI | 1794 | 764 (42.59) | 2 |
| Icatibant | 2011 | Priority | Hereditary angioedema | 267 | 173 (64.79) | 3 |
| Ceftaroline fosamil | 2011 | Standard | ABSSSI | 1144 | 422 (36.89) | 2 |
| Vemurafenib | 2011 | Priority | MM | 675 | 294 (43.56) | 1 |
| Spinosad | 2011 | Standard | Pediculosis | 1038 | 856 (82.47) | 2 |
| Vismodegib | 2012 | Priority | BCC | 104 | 41 (39.42) | 1 |
| Ingenol mebutate | 2012 | Standard | Actinic keratosis | 1005 | 254 (25.27) | 4 |
| Luliconazole | 2013 | Standard | Tinea pedis | 679 | 122 (17.97) | 3 |
| Trametinib | 2013 | Standard | MM | 322 | 149 (46.27) | 1 |
| Dabrafenib | 2013 | Standard | MM | 250 | 101 (40.40) | 1 |
| Dalbavancin | 2014 | Priority | ABSSSI | 1312 | 545 (41.54) | 2 |
| Tavaborole | 2014 | Standard | Onychomycosis | 1194 | 215 (18.01) | 2 |
| Oritavancin | 2014 | Priority | ABSSSI | 1959 | 676 (34.51) | 2 |
| Tedizolid phosphate | 2014 | Priority | ABSSSI | 1333 | 492 (36.91) | 2 |
| Efinaconazole | 2014 | Standard | Onychomycosis | 1651 | 376 (22.77) | 2 |
| Cobimetinib | 2015 | Priority | MM | 495 | 209 (42.22) | 1 |
| Sonidegib | 2015 | Standard | BCC | 230 | 86 (37.39) | 1 |
| Crisaborole | 2016 | Standard | Atopic dermatitis | 1522 | 847 (55.65) | 2 |
| Delafloxacin | 2017 | Priority | ABSSSI | 1510 | 557 (36.89) | 2 |
| Ozenoxacin | 2017 | Standard | Impetigo | 877 | 380 (43.33) | 2 |
| Sarecycline | 2018 | Standard | Acne vulgaris | 2002 | 1146 (57.24) | 2 |
| Omadacycline | 2018 | Priority | ABSSSI | 1380 | 502 (36.38) | 2 |
| Trifarotene | 2019 | Standard | Acne vulgaris | 2420 | 1324 (54.71) | 2 |
| Afamelanotide | 2019 | Priority | Erythropoietic protoporphyria b | 244 | 115 (47.13) | 3 |
ABSSSI, acute bacterial skin and skin structure infections; BCC, basal cell carcinoma; MM, metastatic melanoma.
Two dermatologic disease indications were approved based on independent sets of pivotal clinical trials.
Indicated to increase pain-free light exposure for patients with erythropoietic protoporphyria who experience phototoxic reactions.
Table 2 presents a breakdown of sex, race, and ethnicity of trial participants by year of approval. The overall female representation was 45.6% (n = 17,492 of 38,320). Altogether, Caucasian was the predominant racial identity (80.4%; n = 28,065 of 34,890), followed by Black (9.8%; n = 3242 of 33,240) and Asian (5.5%; n = 1535 of 27,696). Of the 13,860 participants who had their ethnicity ascertained, 2614 (18.9%) identified as Hispanic/Latino. Table 3 presents the representation of sex, race, and ethnicity in pivotal clinical trials by location, sponsor type, and approval pathway. There was a significant difference in the distribution of female (χ2 = 6.332; p = .042), Caucasian (χ2 = 12.813; p = .002), Black (χ2 = 13.002; p = .002), and Hispanic/Latino (χ2 = 7.747; p = .021) participants across sponsor types. Post hoc testing revealed that, except for the female distribution, which showed no significant pairwise comparisons, the significant differences for the Caucasian (p = .001), Black (p = .002), and Hispanic/Latino (p = .041) distributions were exclusively between U.S. and non-U.S. pharmaceuticals.
Table 2.
Representation of sex, race, and ethnicity in pivotal clinical trials for new molecular entities approved by the U.S. Food and Drug Administration between 1995 and 2019.
| Approval year | Trials, N | Total population, N | Female, n/N (%)a | Caucasian, n/N (%)b | Black, n/N (%)b | Asian, n/N (%)b | Hispanic/Latino, n/N (%)b |
|---|---|---|---|---|---|---|---|
| 1995 | 2 | 545 | 267/545 (48.99) | 496/545 (91.01) | 26/545 (4.77) | 5/545 (0.92) | 16/545 (2.94) |
| 1996 | 4 | 3958 | 2842/3958 (71.80) | 3730/3958 (94.24) | 49/3958 (1.24) | 5/185 (2.70) | 50/185 (27.03) |
| 1997 | 7 | 2348 | 1004/2348 (42.76) | 2038/2348 (86.80) | 103/2348 (4.39) | 27/2348 (1.15) | NR |
| 2000 | 4 | 2119 | 1266/2119 (59.75) | NR | NR | NR | NR |
| 2001 | 4 | 927 | 399/927 (43.04) | 507/927 (54.69) | 162/927 (17.48) | 31/927 (3.34) | 71/338 (21.01) |
| 2003 | 4 | 1690 | 643/1690 (38.05) | 998/1690 (59.05) | 413/1690 (24.44) | 11/598 (1.84) | 85/598 (14.21) |
| 2005 | 2 | 1116 | 418/1116 (37.46) | 753/1116 (67.47) | 91/1116 (8.15) | 44/1116 (3.94) | 107/573 (18.67) |
| 2007 | 1 | 210 | 107/210 (50.95) | 77/210 (36.67) | 5/210 (2.38) | 89/210 (42.38) | 62/210 (29.52) |
| 2009 | 2 | 1794 | 764/1794 (42.59) | 1381/1794 (76.98) | 254/1794 (14.16) | 98/1794 (5.46) | NR |
| 2011 | 8 | 3124 | 1745/3124 (55.86) | 1828/1917 (95.36) | 35/1242 (2.82) | 30/1242 (2.42) | 2/675 (0.30) |
| 2012 | 5 | 1109 | 295/1109 (26.60) | 1005/1005 (100.00) | 0/1005 (0.00) | 0/1005 (0.00) | 2/104 (1.92) |
| 2013 | 5 | 1251 | 372/1251 (29.74) | 939/1251 (75.06) | 259/1001 (25.87) | 0/322 (0.00) | NR |
| 2014 | 10 | 7449 | 2304/7449 (30.93) | 5811/7449 (78.01) | 495/7449 (6.65) | 915/7449 (12.28) | 1091/5490 (19.87) |
| 2015 | 2 | 725 | 295/725 (40.69) | 678/725 (93.52) | NR | NR | NR |
| 2016 | 2 | 1522 | 847/1522 (55.65) | 923/1522 (60.64) | 424/1522 (27.86) | 79/1522 (5.19) | NR |
| 2017 | 4 | 2387 | 937/2387 (39.25) | 1736/2387 (72.73) | 401/2387 (16.80) | 62/2387 (2.60) | 435/1510 (28.81) |
| 2018 | 4 | 3382 | 1648/3382 (48.73) | 2814/3382 (83.21) | 360/3382 (10.64) | 75/3382 (2.22) | 287/968 (29.65) |
| 2019 | 5 | 2664 | 1439/2664 (54.02) | 2351/2664 (88.25) | 165/2664 (6.19) | 64/2664 (2.40) | 406/2664 (15.24) |
| Overall | 75 | 38,320 | 17,592/38,320 (45.91) | 28,065/34,890 (80.44) | 3242/33,240 (9.75) | 1535/27,696 (5.54) | 2614/13,860 (18.86) |
NR, not reported.
Data on sex were available for all trials; therefore, percentages were calculated using the corresponding total population.
Percentages were adjusted to reflect the proportion among trials that reported data on the demographic, and trial populations with missing or incomplete demographic data were subtracted from the corresponding total population.
Table 3.
Representation of sex, race, and ethnicity in pivotal clinical trials by location, sponsor type, and approval pathway.
| Trials, N |
Total Population, N |
Female, n/N (%)a |
Caucasian, n/N (%)b |
Black, n/N (%)b |
Asian, n/N (%)b |
Hispanic/Latino, n/N (%)b |
|
|---|---|---|---|---|---|---|---|
| Location | |||||||
| North America | 35 | 16,545 | 8224/16,545 (49.71%) |
10,557/13,304 (79.35%) |
1429/13,304 (10.74%) |
297/11,132 (2.67%) | 954/5014 (19.03%) |
| Europe | 1 | 278 | 128/278 (46.04%) |
268/278 (96.40%) |
1/278 (0.36%) |
9/278 (3.24%) |
NR |
| Multicontinental | 39 | 21,497 | 9240/21,497 (42.98%) |
17,240/21,308 (80.91%) |
1812/19,658 (9.22%) |
1229/16,286 (7.55%) |
1660/8846 (18.77%) |
| p-value | 0.339 | 0.228 | 0.339 | 0.511 | 0.577 | ||
| Sponsor Type | |||||||
| US pharmaceutical | 42 | 22,335 | 9081/22,335 (40.66%) |
14,242/18,905 (75.33%) |
2186/18,905 (11.56%) |
1221/17,134 (7.13%) |
1841/8798 (20.93%) |
| Non-US pharmaceutical | 31 | 13,983 | 7365/13,983 (52.67%) |
12,271/13,983 (87.76%) |
755/12,333 (6.12%) |
250/8560 (2.92%) |
486/4094 (11.87%) |
| Collaboration | 2 | 2002 | 1146/2002 (57.24%) |
1552/2002 (77.52%) |
301/2002 (15.03%) |
64/2002 (3.20%) |
287/968 (29.65%) |
| p-value | 0.042 | 0.002 | 0.002 | 0.102 | 0.021 | ||
| Approval Pathway | |||||||
| Standard | 50 | 25,451 | 12,337/25,451 (48.47%) |
19,106/23,676 (80.70%) |
2385/23,196 (10.28%) |
820/18,744 (4.37%) |
1486/8109 (18.33%) |
| Priority | 25 | 12,869 | 5255/12,869 (40.83%) |
8959/11,214 (79.89%) |
857/10,044 (8.53%) |
715/8952 (7.99%) |
1128/5751 (19.61%) |
| p-value | 0.753 | 0.398 | 0.336 | 0.696 | 0.300 | ||
NR, not reported.
p-value indicates the significance of the difference between the distributions of the demographic proportions of the categories.
Data on sex were available for all trials; therefore, percentages were calculated using the corresponding total population.
Percentages were adjusted to reflect the proportion among trials that reported data on the demographic, and trial populations with missing or incomplete demographic data were subtracted from the corresponding total population.
Participant-to-prevalence ratio
Six dermatological disease indications (45 trials) were included for the PPR analysis. The mean PPR values and their 95% CIs are illustrated in Fig. 1. The global female prevalence values used to calculate PPR are provided in Appendix Table A1. The mean PPR of five disease indications fell within the range of adequate female trial representation (0.8–1.2). The entire 95% CI of only two diseases (acne vulgaris and atopic dermatitis) were fully within the 0.8 to 1.2 range.
Fig. 1.
Participation-to-prevalence ratio of pivotal clinical trials grouped by dermatological disease indication. The mean participation-to-prevalence ratios and 95% confidence intervals are inscribed within the bars. Ratios were calculated by dividing the percentage of women among trial participants by that of the disease population. The number of clinical trials included for each disease indication is presented within parentheses. ABSSSI, acute bacterial skin and skin structure infections; BCC, basal cell carcinoma.
Minority representation
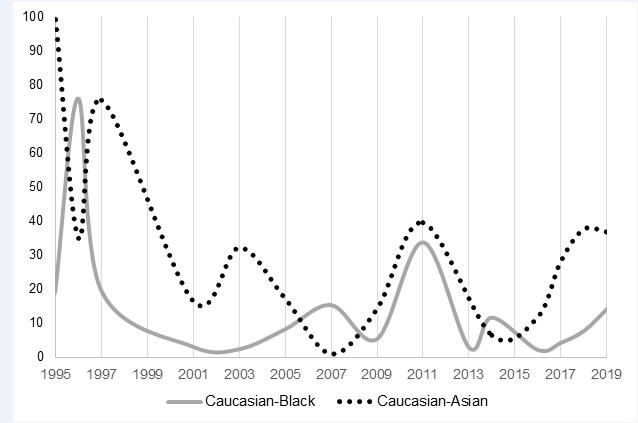
Fig. 2 presents a comparison between Caucasian–Black and Caucasian–Asian participation ratios by approval year. Black participants were more underrepresented than their Asian counterparts in 1996, 2007, and 2014. In all remaining years between 1995 and 2019, Asians received less representation compared with Blacks in pivotal clinical trials.
Fig. 2.
Comparison of Caucasian–Black versus Caucasian–Asian participation ratios from 1995 to 2019. Ratios were calculated by dividing the adjusted proportions of Caucasian participants by that of the corresponding racial minority for each year. The Caucasian–Black and Caucasian–Asian ratios are depicted by the gray solid and black dotted lines, respectively. For each year, the larger ratio represents the more underrepresented racial minority.
Discussion
After systematically reviewing 36 NMEs approved for marketing by the FDA for dermatological disease indications, we identified key patterns pertaining to the representation of sex, race, and ethnicity in the participation of pivotal clinical trials. Across all 75 trials, 46% of participants were female, which was a positive indication because the number fell within the 40% to 60% gender-balance zone of adequate representation (Kuhlmann et al., 2017). However, the overall female proportion can be deceiving trials with extremely high or low female representation are masked. As shown in Table 2, the female proportion ranged from a low of 27% to a high of 72%. Excluding the years without approval of relevant drugs, the female proportions in seven independent years failed to meet the 40% to 60% gender-balance zone. Notably, 1996 was the only year that missed the gender-balance zone due to an overrepresentation of women. The FDA has made repeated attempts over the years to encourage more-equitable representation (U.S. Food and Drug Administration, 1993, U.S. Food and Drug Administration, 2019), but the issue of sex disparity in clinical trial participation has not been resolved and cannot be regarded as an issue of the past.
Despite a seemingly frequent underrepresentation of women in pivotal clinical trials, an analysis based on approval year is not the most informative. Drugs approved in different years were for distinct disease indications, which warranted variation in female representation according to their respective prevalence among the global population. Recruitment of clinical trial participants depends on disease epidemiology (e.g., a predominantly male group of participants is reasonable for trials of antiandrogens for male pattern baldness). The results presented in Fig. 1 showed that five of six dermatological disease indications that underwent a mean PPR analysis had adequate female representation. However, the mean PPR of all five indications was situated on the low end (i.e., 0.8–1.0, as opposed to the high-end of 1.0–1.2). Trial participants for drugs indicated for acne vulgaris and atopic dermatitis demonstrated fully adequate female representation because their 95% CIs were completely within the range of 0.8 and 1.2. Participants for drugs indicated for acute bacterial skin and skin structure infections and metastatic melanoma were in a similar situation; however, the lower band of their 95% CIs missed the range slightly (0.77 and 0.78, respectively). Although the female representation for clinical trials of many dermatological drugs is acceptable, these findings should not dismiss the importance of continued advocacy for improved equity in representation.
Sex and racial disparity are known to exist in academic dermatology (Karol et al., 2021, Lu et al., 2020, Shah et al., 2018); however, to our knowledge, this study is the first to offer a quantitative breakdown of demographics in pivotal clinical trial participation for a variety of dermatological drugs. An interesting finding in Table 3 was that the distributions of Caucasian (p = .228), Black (p = .339), Asian (p = .511), and Hispanic/Latino (p = .577) participants were not significantly different across study location (North America, Europe, or multicontinental). Despite the varying population demographics of different countries and continents, where a study was conducted seemed to have minimal influence on the central tendency to predominantly enroll Caucasian participants. However, significant differences in the distributions of Caucasian (p = .001), Black (p = .002), and Hispanic/Latino (p = .041) participants were noted between U.S. and non-U.S. pharmaceutical sponsor companies. Non-U.S. pharmaceutical sponsors tended to enroll more Caucasian participants than their U.S. counterparts, and conversely, U.S. pharmaceutical sponsors generally recruited more Black and Hispanic/Latino participants compared with their non-U.S. counterparts. Exactly how trial enrollment practices continually enforce the overrepresentation of Caucasian participants is unclear. Although participant factors unrelated to sex, race, and ethnicity are important considerations in trial enrollment, subconscious biases manifested by investigators and researchers may be a propagator of inadequate minority representation (Niranjan et al., 2020).
Caucasian participants consistently outnumbered Black and Asian minorities between 1995 and 2019. According to the 2018 Census Bureau’s American Community Survey, approximately 60% of the U.S. population were Caucasian; meanwhile the proportions of Blacks and Asians were 12% and 6%, respectively (Kaiser Family Foundation, 2018). In addition, 80.4% of overall participants in pivotal clinical trials for drugs with dermatological disease indications were Caucasian (Table 2); therefore, there was likely a systemic issue at play. Fig. 2 compares the Caucasian–Black and Caucasian–Asian participation ratios to illustrate the differences in magnitude of minority underrepresentation throughout the years. Between 1995 and 2019, Asian participants were frequently more underrepresented than their Black counterparts. This was not surprising given that throughout history, there has simply been more Black than Asian individuals living in the United States (FDA, 2018). On the other hand, in 1996, 2007, and 2014, Blacks were more underrepresented than their Asian counterparts, but this was not representative of the true population demographics. Although extremely large ratios are appearing less frequently in the 21st century, consistent and adequate representation of minorities in clinical trials remains a dilemma with no clear solution. It has been suggested that participants from minority backgrounds do not build rapport as easily with study investigators when they perceive a racial barrier (Khan et al., 2020, Weisfeld et al., 2012). A lack of trust due to differences in the socioeconomic background of the participant versus the study investigators may result in a low retention rate (Yancey et al., 2006). Of course, this is only one of many possible explanations for the underrepresentation of minorities. Researchers in the 21st century must continue to tackle this decades-old problem; however, dermatology is not facing the issue alone (McGarry and McColley, 2016, Nazha et al., 2019).
This study was subject to a few limitations. First, data on race and ethnicity were unavailable for several trials. The lack of ethnicity reporting was especially notable because only 36.2% (n = 13,860 of 38,320) of the total population had their ethnicity ascertained. To address this limitation, demographic percentages were adjusted to reflect the proportion among trials that reported data, and trial populations with missing or incomplete demographic data were subtracted from the corresponding total population. Furthermore, some studies reported Hispanic/Latino as a race instead of an ethnicity, which contributed to the lack of data uniformity. A consequence of this was potentially incomplete racial data, but this limitation is inherent to any demographic data from FDA clinical trials. For instance, a multiracial participant (e.g., Caucasian–Black or Asian–Black) can only select the race with which they identify most closely identify. Therefore, participants who fit into multiple race groups are not completely accounted for.
Second, the clinical trials abstracted for analysis were essentially all phase 3 trials and did not offer much insight into the representation of demographics during early clinical studies. Future research focused on evaluating adequate representation of women and minority groups could benefit from investigating the earlier stages of clinical trials. Lastly, the PPR analysis was only conducted for six dermatological diseases. Diseases were included for PPR analysis only if their global prevalence was available in the Global Burden of Diseases database. Prevalence data from independent publications were not accepted. These criteria were established to ensure the PPR was calculated with appropriate control and comparable at the same scale. However, for the disease indication “metastatic melanoma,” the Global Burden of Disease data for “malignant skin melanoma” was selected due to the lack of a more appropriate classification (Appendix Table A1). As a result of this inexact match, the accuracy of the PPR analysis for metastatic melanoma may be lower than for the other five indications.
Conclusion
The PPR analysis suggested adequate female representation for five of six disease indications; nonetheless, efforts should be continued to further improve sex equity in clinical trials. Blacks and Asians were consistently underrepresented while Caucasians were overrepresented between 1995 and 2019. Persistence of demographic disparities in pivotal clinical trials can limit the overall quality of the data; therefore, generalizations of the safety and efficacy of therapies for minorities and women should be made with discretion. Clinical trials are conducted for the benefit of all population demographics, and enrollment practices must address the need for adequate representation. Possible ways to circumvent and mitigate this issue as a dermatologist (both academic and clinical) include advocating for more equitable trial demographics and being cognizant of the quality and generalizability of data produced by trials that fail to address demographic disparities. Future studies may benefit from evaluating the representation of sex, race, and ethnicity in earlier stages of clinical trials.
Conflicts of interest
Dr. Faisal Khosa is the recipient of the American College of Radiology – Global Humanitarian Award (2021) and the Association of Faculties of Medicine of Canada – May Cohen Equity, Diversity and Gender Award (2020). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijwd.2021.02.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anderson G.D. Sex and racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 2005;14:19–29. doi: 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- Chen M.-L. Ethnic or racial differences revisited: Impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45:957–964. doi: 10.2165/00003088-200645100-00001. [DOI] [PubMed] [Google Scholar]
- Chen W., Mempel M., Traidl-Hofmann C., Al Khusaei S., Ring J. Gender aspects in skin diseases. J Eur Acad Dermatol Venereol. 2010;24:1378–1385. doi: 10.1111/j.1468-3083.2010.03668.x. [DOI] [PubMed] [Google Scholar]
- Clark L.T., Watkins L., Piña I.L., Elmer M., Akinboboye O., Gorham M. Increasing diversity in clinical trials: Overcoming critical barriers. Curr Probl Cardiol. 2019;44:148–172. doi: 10.1016/j.cpcardiol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Eshera N., Itana H., Zhang L., Soon G., Fadiran E.O. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am J Ther. 2015;22:435–455. doi: 10.1097/MJT.0000000000000177. [DOI] [PubMed] [Google Scholar]
- Gandhi M., Aweeka F., Greenblatt R.M., Blaschke T.F. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Collaborative Network. Global burden of disease study 2017 results [Internet]. 2018 [cited 2020 July 23]. Available from: https://gbd2017.healthdata.org/gbd-search/.
- Heinrich J. U.S. General Accounting Office; Washington, DC: 2001. Drug safety: most drugs withdrawn in recent years had greater health risks for women. [Google Scholar]
- Kaiser Family Foundation. Population distribution by race/ethnicity [Internet]. 2018 [cited 2020 July 30]. Available from: https://www.kff.org/other/state-indicator/distribution-by-raceethnicity/.
- Karol D.L., Sheriff L., Jalal S., Ding J., Larson A.R., Trister R. Gender disparity in dermatologic society leadership: a global perspective. Int J Womens Dermatol. 2021;7(4):445–450. doi: 10.1016/j.ijwd.2020.10.003. S2352647520301519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S., Shahid I., Siddiqi T.J., Khan S.U., Warraich H.J., Greene S.J. Ten-year trends in enrollment of women and minorities in pivotal trials supporting recent U.S. Food and Drug Administration approval of novel cardiometabolic drugs. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann E., Ovseiko P.V., Kurmeyer C., Gutiérrez-Lobos K., Steinböck S., von Knorring M. Closing the gender leadership gap: a multi-centre cross-country comparison of women in management and leadership in academic health centres in the European Union. Hum Resour Health. 2017;15:2. doi: 10.1186/s12960-016-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.D., Tiwana S., Das P., Siddiqi J., Khosa F. Gender and racial underrepresentation in academic dermatology positions in the United States: a retrospective, cross-sectional study from 2007–2018. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.06.067. S0190962220311658. [DOI] [PubMed] [Google Scholar]
- McGarry M.E., McColley S.A. Minorities are underrepresented in clinical trials of pharmaceutical agents for cystic fibrosis. Ann Am Thorac Soc. 2016;13:1721–1725. doi: 10.1513/AnnalsATS.201603-192BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazha B., Mishra M., Pentz R., Owonikoko T.K. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019:3–10. doi: 10.1200/EDBK_100021. [DOI] [PubMed] [Google Scholar]
- Niranjan S.J., Martin M.Y., Fouad M.N., Vickers S.M., Wenzel J.A., Cook E.D. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126:1958–1968. doi: 10.1002/cncr.32755. [DOI] [PubMed] [Google Scholar]
- Pinn V.W. The role of the NIH’s Office of Research on Women’s Health. Acad Med J Assoc Am Med Coll. 1994;69:698–702. doi: 10.1097/00001888-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Poon R., Khanijow K., Umarjee S., Fadiran E., Yu M., Zhang L. Participation of women and sex analyses in late-phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007–2009. J Womens Health. 2013;22:604–616. doi: 10.1089/jwh.2012.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K., Hsiao J., Shi V. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatol Basel Switz. 2019;237:97–102. doi: 10.1159/000504911. [DOI] [PubMed] [Google Scholar]
- Price K.N., Krase J.M., Loh T.Y., Hsiao J.L., Shi V.Y. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378–380. doi: 10.1111/bjd.18938. [DOI] [PubMed] [Google Scholar]
- Rahrovan S., Fanian F., Mehryan P., Humbert P., Firooz A. Male versus female skin: What dermatologists and cosmeticians should know. Int J Womens Dermatol. 2018;4:122–130. doi: 10.1016/j.ijwd.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Jalal S., Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171–176. doi: 10.1111/ijd.13875. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Collection of race and ethnicity data in clinical trials [Internet]. 2016 [cited 2020 September 3]. Available from: https://www.fda.gov/media/75453/download.
- U.S. Food and Drug Administration. Compilation of CDER new molecular entity (NME) drug and new biologic approvals [Internet]. 2020 [cited 2020 August 9]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/compilation-cder-new-molecular-entity-nme-drug-and-new-biologic-approvals.
- U.S. Food and Drug Administration. Enhancing the diversity of clinical trial populations — Eligibility criteria, enrollment practices, and trial designs [Internet]. 2019 [cited 2020 September 4]. Available from: https://www.fda.gov/media/127712/download.
- U.S. Food and Drug Administration. General considerations for the clinical evaluation of drugs [Internet]. 1977 [cited 2020 September 1]. Available from: https://www.fda.gov/media/ 71495/download.
- U.S. Food and Drug Administration. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs [Internet]. 1993 [cited 2020 August 3]. Available from: https://www.fda.gov/media/71107/download.
- U.S. Food and Drug Administration. What we do [Internet]. 2018 [cited 2020 August 2]. Available from: https://www.fda.gov/about-fda/what-we-do.
- Weisfeld V., English R., Claiborne A. National Academies Press; Washington, DC: 2012. Recruitment challenges in clinical trials for different diseases and conditions. [Google Scholar]
- World Health Organization. The global burden of disease concept [Internet]. 2017 [cited 2020 July 20]. Available from: https://www.who.int/quantifying_ehimpacts/publications/en/9241546204chap3.pdf.
- Yancey A.K., Ortega A.N., Kumanyika S.K. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.