Abstract
Colonization and development of the gut microbiome is a crucial consideration for optimizing the health and performance of livestock animals. This is mainly attributed to the fact that dietary and management practices greatly influence the gut microbiota, subsequently leading to changes in nutrient utilization and immune response. A favorable microbiome can be implanted through dietary or management interventions of livestock animals, especially during early life. In this review, we explore all the possible factors (for example gestation, colostrum, and milk feeding, drinking water, starter feed, inoculation from healthy animals, prebiotics/probiotics, weaning time, essential oil and transgenesis), which can influence rumen microbiome colonization and development. We discuss the advantages and disadvantages of potential strategies used to manipulate gut development and microbial colonization to improve the production and health of newborn calves at an early age when they are most susceptible to enteric disease. Moreover, we provide insights into possible interventions and their potential effects on rumen development and microbiota establishment. Prospects of latest techniques like transgenesis and host genetics have also been discussed regarding their potential role in modulation of rumen microbiome and subsequent effects on gut development and performance in neonatal ruminants.
Keywords: Gut development, Microbial colonization, Rumen microbiota, Health, Performance
1. Introduction
The gut microbiota is essential for crucial functional activities, such as gut development, feed digestion and utilization, and immune response in livestock animals. Changes in gut microbial colonization in livestock animals during early life often result in permanent effects on the establishment of rumen microbiota and resultant effects on host phenotype (Furman et al., 2020; Guo et al., 2020). Ruminal microbes are fundamentally anaerobic and produce various compounds during rumen fermentation, which are directly used by the host or other microbes. Methane is produced by methanogens through utilizing metabolic hydrogen during rumen fermentation (Hassan et al., 2020). Overall, enteric methane emission from ruminants constitutes about 18% of total methane emissions from all anthropogenic sources (Mizrahi et al., 2021). Methane is the second most potent greenhouse gas after CO2 that contributes to global warming. Moreover, methane emanation is a loss of dietary energy, which would otherwise be used by the host to produce meat and milk. Therefore, manipulating the rumen microbiome of livestock animals is considered as an approach to reduce their environmental impacts while increasing production efficiency (O'hara, 2019). During later stages of life, it is more challenging to change rumen microbial ecology over a long time, however, in early life, a favorable microbiome can be implanted via dietary or management interventions with potentially a long-lasting effect (Dill-Mcfarland et al., 2017; Furman et al., 2020; Malmuthuge et al., 2019; Palma-Hidalgo et al., 2020). Gastrointestinal tracts (GIT) of ruminants are colonized by different micro-organisms (bacteria, archaea, virus, protozoa, and fungi) in their adult life, and are considered sterile at birth. However, within 20 min of birth, fibrolytic bacteria and methanogens appear in the rumen of newborn animals (Guzman et al., 2015). One day post-birth the microbial density of rumen microbiota reaches up to 109 cells per milliliter (Li et al., 2012; Yáñez-Ruiz et al., 2015), with cellulolytic bacteria, Ruminococcus flavefaciens, Ruminococcus albus and members of the Prevotella genus often being detected at that time. These microbes are involved in various critical energy-harvesting functions (McLoughlin et al., 2020; Shabat et al., 2016), such as cellulose and hemicellulose degradation (Jami et al., 2013).
The ruminant digestive system switches from that of a monogastric to that of a fully functional foregut rumen fermenter post-weaning, with an ability to digest fibrous feed. During the suckling period of calves, milk bypasses the rumen due to the esophageal groove. The developed rumen comprises 60% to 80% of the total digestive system volume as compared with the monogastric stomach in early life. Besides this, during early life, rumen villi are not yet developed which are necessary for the absorption of nutrients (Krishnamoorthy and Moran, 2012; Van Soest, 2018). Rumen microbial populations have a considerable impact on rumen structure and physiological development. Initial microbial GIT colonizers constitute both aerobic and facultative anaerobic microbial taxa, which later on mostly are replaced by anaerobic taxa (Minato et al., 1992). Consequently, one-day-old calves have a very different bacterial population compared to three-day-old calves (Jami et al., 2013). The oxidative condition within the rumen is a primary regulator of change within the rumen ecosystem and redox in newborns, with an inert impact on the advancement of methanogenic species (Friedman et al., 2017). The primary changes which occur as rumen development ensues include modification in density configuration within the Bacteroidetes phylum. In the developed rumen, this phylum is dominated by the genus Prevotella across several species (Henderson et al., 2015). Nevertheless, during primary stages of development, Bacteroides is the main genus within phylum Bacteroidetes and is immediately replaced by the Prevotella during the first 2 months of life (Rey et al., 2014).
The period from birth to weaning is important for rumen microbial colonization and adaptation. Regarding this, transmission is one of the most important factors that affects the development of microbiota in the GIT (Dominguez-Bello et al., 2010; Francino, 2014). The composition of this complex microbial community is shaped by the highly dynamic physical, chemical, and predatory conditions within the rumen, and potentially by genetic factors of the host (Sasson et al., 2017). Interaction of the host and microbes is essentially responsible for the development of microbial colonization known as co-evolution (Malmuthuge et al., 2015; O'hara et al., 2020a). The microbial population is established by successive waves where convergence of microbial populations is seen reaching a more stable population structure (Furman et al., 2020).
Once the development and maturation of rumen and the microbiome are complete, it is difficult to permanently manipulate or change the rumen ecosystem due to microbial adaptation and resilience to external mediators (Clemmons et al., 2019) and the control that the host genome has been shown to have on the microbiome (Ribeiro et al., 2017; Abbas et al., 2020; Weimer et al., 2017). That is why developing a rumen ecosystem during weaning age is key for getting higher growth rates and better health at a later stage of life (De Barbieri et al., 2015; O'hara et al., 2020b). Another possible strategy for improving feed efficiency is the fortification of rumen microorganisms in calves during early life. The main objective of such strategies is to overcome the risk of undesirable health consequences associated with an altered gut microbiome in neonatal animals and restoration of the gut microbial community following dysbiosis. A complete understanding of early gut colonization is necessary to design different effective strategies to manipulate the GIT microbiome.
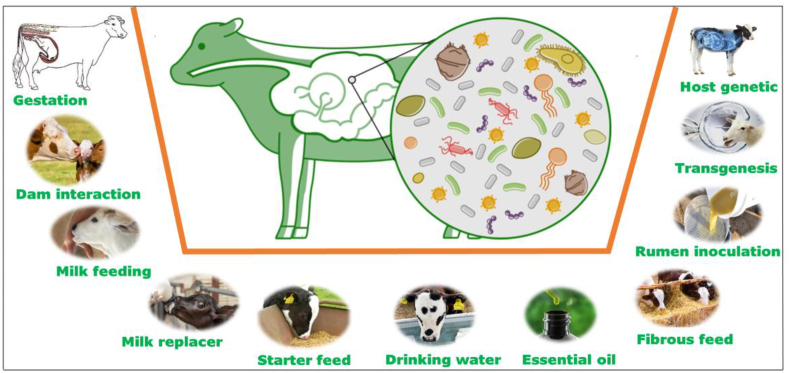
Although a wealth of literature is available on different aspects of the rumen microbiome in adult animals and early colonization of gut microbiota, information regarding the role of host genetics and microbial interactions in the early development of the gut microbiota is limited. This review focuses on providing insights into the initial colonization of rumen microbiota, while focusing on challenges in our understanding of the complex interaction between different factors, especially between the host and the microbiome. We also present some potential strategies to manipulate the rumen microbiome in early life (Fig. 1), to desirably enhance gut development and microbial colonization for improved health and production in ruminants.
Fig. 1.
Possible factors involved in rumen microbial colonization during early life.
2. Relationship of gut microbiota with host immunity
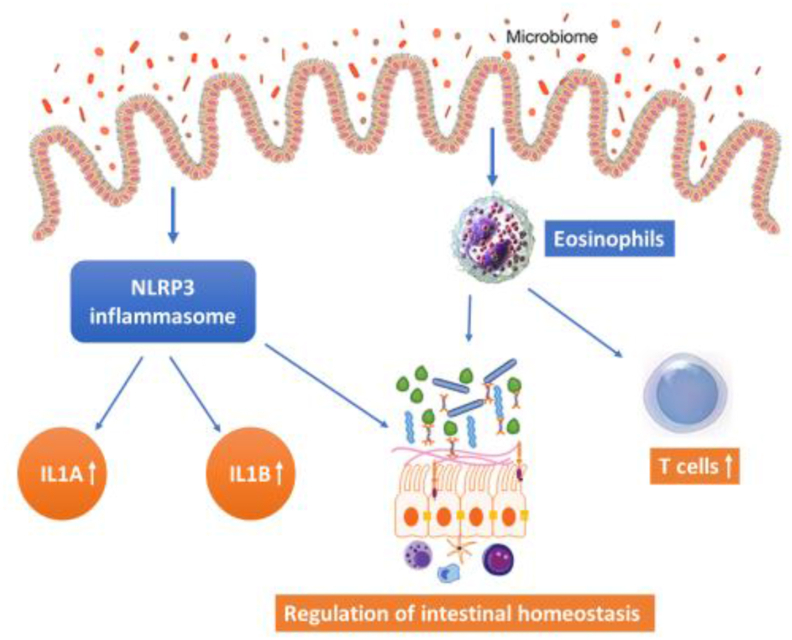
Developing better immunity is crucial for the prevention and elimination of pathogens, and to maintain the health status of young ones. Newborn ruminants have no immunity at the time of birth, which can be induced through colostrum feeding from the mother. Immunoglobulins (IgG, IgA, and IgM) in colostrum provide immunity and protection against inhaled and ingested pathogens (Woof and Kerr, 2004). The presence of IgA reduces intestinal pro-inflammatory signaling to mediate tolerance in the gut, and regulate gut bacterial composition while maintaining intestinal homeostasis between the host and GIT microbes (Bauer et al., 2006a). Microbial colonization has a critical role in the development of host innate immunity (Janeway Jr and Medzhitov, 2002). Tracheal antimicrobial peptide (TAP) and β-defensin genes are front-line protectants against pathogens (Caverly et al., 2003). TAP also provides a link between innate and adaptive immune responses against microbial invasions (Yang et al., 2002). Notably, the β-defensin 1 and β-defensin 2 were detected in the first 6 to 8 weeks of life, with substantial expression in pre-natal lambs in the digestive tract (Huttner et al., 1998; Meyerholz et al., 2006). Toll-like receptors (TLR) are another class of host proteins with important roles in inducing an immune response in the GIT with the help of gut microbes in calves (Malmuthuge et al., 2012). Exogenous bacterial colonization in calves triggers immune and defense responses causing induction of expression of different genes involved in the adaptive and innate immune system (Li et al., 2019) and studies indicate that the genes related to host innate immunity have an important role in developing a composite interface between the colonized microbial community of fresh rumen and host immune surveillance. The majority of studies have reported developmental ontogeny of the digestive tract in the neonatal calf (usually on the rumen), however, the richest site of immune cell deposition along the intestine is in the ileum (small intestine) of cattle. The submucosa of the ileum has lymphoid nodules called Peyer's patches, also known as “immune sensor” of the intestine (Jung et al., 2010). Recently, Lyons et al. (2020) revealed an important portal of host–microbe interaction with the presence of large number of innate immune cells (eosinophils ad macrophages) in the ileum. A strong relationship between the relative abundance of Bacteroidetes and T cells has been observed in the calf's ileum. Webb et al. (2016) demonstrated that genus Bacteroidetes could stimulate regulatory T cells which promote epithelial repair, promote tolerance to microbes and initiate suppression of immune responses to self and bacterial antigens. Appropriate activation of the inflammasome sensors (such as NOD-like receptor pyrin domain-containing protein 3 [NLRP3]) and the expansion of eosinophils in ileal tissue are likely to be important in terms of regulating ileal homeostasis and maladaptive inflammation immediately after birth and warrants further investigation (Fig. 2).
Fig. 2.
Activation of the NLRP3 inflammasome and the expansion of eosinophils in ileal tissue. NLRP3 inflammasome (member of multi-protein innate immune complex) regulates intestinal homeostasis (Hirota et al., 2011). Interleukin-1 alpha (IL1A) and interleukin-1 beta (IL1β) proteins are mediated through NLRP3 inflammasome. IL1A, known as “pro-inflammatory”, stimulates the activity of genes involved in inflammation and immunity (Lee et al., 2008). IL1β is involved in T-cell activation, antibody production, and promotes Th17 differentiation of T-cells (Tominaga et al., 2000). NLRP3 = NOD-like receptor pyrin domain-containing protein 3.
3. Factors affecting the development of gut microbiota in neonatal ruminants
The colonization and establishment of microbiota play a key role in the development and function of the GIT, which is subsequently associated with higher body weight and feed efficiency of young ruminants (Wickramasinghe et al., 2020). There are numerous factors that directly or indirectly affect microbial colonization and gut development in ruminants. Some crucial considerations regarding these are discussed below.
3.1. Gestation
Maternal nutrition in pregnancy has an important role in the health of the offspring later in life through modulating in utero developmental processes. In this regard, gut microbiota provides a potential mediating factor (Chu et al., 2016). Transmission of bacteria from the mother to her offspring is required for developing a healthy nascent microbiome, which may impact growth, immune system maturation, and even neurodevelopment of human infants (Foster and Neufeld, 2013; Hansen et al., 2012; Schwarzer et al., 2016). Similarly, microbial colonization in the bovine gut in early life is important for efficient metabolic functions, immune system, and future health (De Agüero et al., 2016). However, microbes were observed in newborn calf meconium (Alipour et al., 2018), suggesting that microbial colonization of the bovine hindgut might begin before birth, and that prenatal dam-to-fetus efflux of commensal bacteria occurs in utero via placental barrier transmission (Funkhouser and Bordenstein, 2013). However, Malmuthuge and Griebel (2018) reported that the fetal intestine and fetal environment are sterile during the third trimester of pregnancy and after the rupture of the amniotic membrane, colonization of the GIT only begins during the birth process. However, another study has also supported the idea of dam-to-fetus efflux of bacteria by detecting hindgut bacteria in neonatal calves immediately after birth (Elolimy et al., 2019). Therefore, there is currently no consensus on whether or not colonization occurs pre-birth. It remains unclear whether small numbers of microbial cells also translocate to the fetus through the placenta (Seferovic et al., 2019; Theis et al., 2019).
During pregnancy, antimicrobial treatments may increase the risk of immunological and metabolic disorders in offspring (Korpela et al., 2016). Maternal microbiota plays a key role in the development of the intestinal immune system during the fetal period through circulating microbial metabolites (De Agüero et al., 2016). Dietary supplementation of methionine to maternal diet during late pregnancy in dairy cows has exhibited a positive impact on hindgut functionality and health in their offspring via modifications in the fecal microbiota and metabolome although feed intake was similar (Elolimy et al., 2019). Furthermore, these modifications may also provide essential nutrients to the neonate and prevent pathogen colonization of the hindgut (Elolimy et al., 2020). Other beneficial effects of the maternal supply of methionine to neonatal calves, including faster maturation of hepatic metabolic pathways and better innate immune function have also been observed (Jacometo et al., 2017; Wang et al., 2019a).
3.2. The microbiota of newborn calves and their dams
Bacterial colonization in the GIT of newborns depends upon microbes possessed by the mother and the environment, during fetal development and after birth (Maynard et al., 2012; Stinson et al., 2017), but the observations are controversial due to challenges in reliable sampling and analysis of low-abundance microbial taxa (Alipour et al., 2018). Irrespective, on the whole, exposure of an offspring to the mother's microbial environment appears to be beneficial in terms of life-long immune homeostasis (Torow and Hornef, 2017). The fecal microbiota of newborn calves was derived from the inoculation of the birth canal during birth, because the cow vaginal and calf fecal microbiota are quite similar (Klein-Jöbstl et al., 2019). However, other sources were not examined, which is why the environment and the uterus ante natum might be a source of colonization. The newborn rectal microbiota in farm animals usually resembles the dam's oral rather than fecal or vaginal vestibular microbiota; this is mainly composed of Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes. Following birth in lambs, before colostrum feeding, the most abundant genera included: Actinobacillus (6.1%), Halomonas (5.7%), Mannheimia (3.8%), Sphingomonas (3.8%), and Lactobacillus (1.3%) (Wang et al., 2019b). The possible sources of these bacteria are the mother's vagina, skin, and the environment (Dominguez-Bello et al., 2010; Pannaraj et al., 2017).
Recently, metagenomic analysis of fecal samples of pre-weaned calves and lactating cows (from 17 dairy farms) revealed an abundance of Bacteroidetes and Verrumicrobia in calves, while Firmicutes, Spirochaetes, Deinococcusthermus, Lentisphaerae, Planctomycetes, and Chlorofexi dominated in cows (Haley et al., 2020). This indicates that composite feces from pre-weaned calves (up to 8 weeks of age) harbor different bacterial communities than lactating cows. Recently, Schwaiger et al. (2020) reported a significant increase in the abundance of Enterococcus in the intestine of newborn healthy calves between 6 and 24 h of birth. The relative abundance of Lactobacillus was also increased in the intestinal tract of calves during the first 7 d after birth (Takino et al., 2017).
There is a perception that vertical transmission of maternal microbiota has a role in microbial colonization of neonatal GIT (Gritz and Bhandari, 2015). Recently, Guzman et al. (2020) demonstrated that fetal GIT was spatially colonized before birth by a pioneer microbiome. They showed that relative abundance of bacterial and archaeal communities in calf fetal fluids and tissues changed between 5 and 7 months of gestation. These findings indicate that initial colonization of the GIT can occur before 5 months gestation, which suggests that microbial colonization of the fetus is a developmental process. However, there is a need to evaluate the path of initial colonization of these microbiomes, their involvement in metabolic interactions with the fetus, and in priming fetal immunity.
The early hindgut microbiome and metabolome at birth before colostrum feeding partly determine feed efficiency (Elolimy et al., 2020). Lambs reared in close contact with their dam exhibited earlier establishment of cellulolytic bacteria during early life compared to lambs with restricted dam exposure (Fonty et al., 1989). Thus, a metabolically active microbiome could be established through the transmission of microbes from the dam to the newborn calf and the colonization of a species-specific microbiome (Belanche et al., 2019). The lambs reared without their dam had no rumen protozoa and exhibited lower microbial diversity, whereas natural rearing accelerated the rumen microbial development and facilitated the transition to a solid diet. Colonization of the alimentary canal probably depends upon the source of connection between newborn calves and their environment. In most cases, feces act as the main source of colonization for the alimentary tract. Piglets that were kept in an environment heavily contaminated with their dam's feces were active at birth and started walking sooner. This favored the rapid entry of considerable numbers of bacteria into the alimentary tract, leading to a massive proliferation of Escherichia coli, Clostridium welchii and Streptococci in the stomach and flooding of the small intestine with these organisms during the short period (Smith, 1965). The incidence of dysbiosis during the neonatal period necessitates potential strategies to restore gut microbiota to avoid adverse health effects associated with the altered gut microbiome. Fecal matter transplantation (FMT) also known as bacteriotherapy, is an intervention that involves the transfer of whole gut microbiome from healthy individuals to microbial dysbiosis individuals (Choi and Cho, 2016). Recently, the use of super-donors to completely restore microbial dysbiosis has been proposed as an option to avoid unreliable and short-term FMT restoration (Wilson et al., 2019). Malmuthuge and Griebel (2019) showed that microbial dysbiosis can be recovered, through reconnecting subsections of the surgically isolated intestinal segment to the adjacent intact intestine during early life in lambs. This model provides insights to explore the effect of early microbial dysbiosis throughout the prolonged neonatal period in large mammals. The intestinal microbiome in vaginally born neonatal calves had a low number of bacterial phyla (Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes), suggesting a shared microbiota between calf meconium and the maternal vaginal vestibulum (Alipour et al., 2018; Yeoman et al., 2018; Klein-Jöbstl et al., 2019). A recent study showed that meconium of full-term calves delivered by elective caesarean section in most cases harbored a small amount of diverse bacterial DNA and possibly rare culturable bacteria. In the amniotic fluid, bacteria were not observed by 16S qPCR or culturing, but a microbial DNA profile was distinguishable from controls by amplicon sequencing. Based on these findings, it is suggested that calves encounter utero maternal–fetal transmission of bacterial components, but the prenatal acquisition of live bacteria is likely not physiologically significant (Husso et al., 2020).
3.3. Colostrum and milk feeding
Diet after birth essentially mediates the colonization of gut microbiota in farm animals. Colostrum feeding is a recommended practice in ruminants to ensure proper early nutrition and development of mucosal immune response. It can effectively prevent the colonization of pathogenic microorganisms in the GIT while decreasing inflammatory reactions (Fischer, 2017; Hang, 2019). Milk can potentially prevent the binding of potential pathogens with gut epithelium as it contains 40 oligosaccharides and lactoferrin which serve as antimicrobial, anti-inflammatory, and iron-chelating agents (Pacheco et al., 2015). After colostrum intake on day 2 of age, an increased Lactococcus abundance was observed with low rumen pH (less than 4) in neonatal lambs (Wang et al., 2019a). Feeding colostrum can resist colonization of Mycobacterium avium subsp. Paratuberculosis in newborn calves infected with these bacterial species (Stabel et al., 2019). Increased milk intake (20% of body weight) after birth has also been shown to result in improved health status (greater blood tumor necrosis factor-α and lower blood urea nitrogen) of Holstein female calves, when compared with low milk intake (10% body weight) during the first 3 weeks of life (Alimirzaei et al., 2020). Increased Lactobacillus abundance was observed in the feces of calves fed more milk, which was associated with improved gut environment and reduced inflammation. Recently, Van Keulen et al. (2020) reported that increased milk allowance (20% of body weight) improved villus height, width and surface area of the small intestine in newborn calves. This improvement may be crucial for intestinal integrity and barrier function with increased cell growth and proliferation in newborn calves.
Early colonization by Gram-negative bacteria in the GIT might adversely affect calf health and growth performance of dairy calves (Bach et al., 2017). Colostrum feeding has been shown to stimulate the colonization of Clostridium and Bifidobacterium, while inhibiting E. coli (Song et al., 2019). Time of colostrum feeding after birth also affects the immune status of newborn calves, as delaying colostrum feeding up to 12 h after birth decreased the passive transfer of immunoglobulin G (IgG) in calves (Fischer et al., 2018a). Recently, Ma et al. (2019) reported that delayed feeding of first colostrum to neonatal calves did not affect the relative abundance of ileum mucosa-associated bacteria at the phylum level. Colostrum replacer can be fed successfully as an alternative to maternal colostrum or as a supplement to colostrum with low IgG (Lopez et al., 2020). However, studies have shown that maternal colostrum feeding to neonatal lambs enhanced volatile fatty acids (VFA) production at weaning compared with the colostrum alternative group (Belanche et al., 2019). However, later in life when all lambs were grouped on the same pasture, they developed similar rumen prokaryotic communities. Based on these findings, it has been suggested that dietary interventions in early life can alter the colonization of gut microbiota with desirable consequences (De Barbieri et al., 2015; Zhong et al., 2014). Although, some effects of early life interventions are long-lived, some of the acquired changes are temporary. However, post-weaning programming exhibits substantial effects on rumen microbiota and performance indices (Dill-Mcfarland et al., 2019). Consequently, it is suggested that effective manipulation of the GIT microbiome should focus on the weaning transition period. This is mainly because dietary interventions aimed at or immediately after weaning are likely most effective with long term effects on rumen microbial ecology and functions (Belanche et al., 2019; Dill-Mcfarland et al., 2017). Very limited data is available regarding the genetics and dynamics of microbial communities of milk-fed newborns before the functional development of the rumen. Therefore, further studies are required to better understand the mechanism of gut colonization and the precise role of different factors affecting microbial establishment, especially host genetics and microbial interactions.
Although feeding colostrum promotes weight gain and colonization of beneficial bacteria in the small intestine of newborn calves (Hammon et al., 2002; Malmuthuge et al., 2015) during colostrum handling, poor hygiene practices can cause microbial contamination, ultimately leading to the exposure of young ruminants to pathogens (Morrill et al., 2012). To prevent such contamination, heat treatment of colostrum is usually suggested, which not only inhibits potential pathogens (Mycoplasma bovis, Listeria monocytogenes, E. coli, and Salmonella enteritidis) in the colostrum but also helps in transferring passive immunity to young ruminants (Godden et al., 2006; Johnson et al., 2007; Rafiei et al., 2019). Intestinal absorption of IgG can be increased through feeding heat-treated colostrum (60 °C for 30 min) owing to a lower bacterial concentration in this colostrum (Gelsinger et al., 2015; Salazar-Acosta and Elizondo-Salazar, 2019; Saldana et al., 2019). Bovine colostrum contains various bioactive proteins such as immunoglobulins, lactoferrin, and lactoperoxidase. Chatterton et al. (2020) subjected bovine colostrum to different processing techniques including low-temperature, long-time pasteurization (63 °C, 30 min) or high-temperature, short-time pasteurization (72 °C, 15 s) and spray-drying (with or without γ-irradiation) to remove microbial contamination. They reported that the high-temperature, short-time pasteurization technique damages several bioactive proteins which are highly sensitive. A recent study showed that heated colostrum improves the abundance of Bifidobacterium and reduces Enterobacteriaceae and E. coli abundance in neonatal calves at 12 h of life (Song et al., 2019). Bovine colostrum contains oligosaccharides, which play a key role in the stimulation of growth of Bifidobacterium (Ward et al., 2007). Heat treatment can denature the oligosaccharide-protein bond resulting in free oligosaccharide contents (Neeser et al., 1991). Studies have shown that heated colostrum contains substantially higher oligosaccharides (3,511.6 μg/g) as compared to non-heated colostrum (1,329.9 μg/g) of Holstein cows (Fischer et al., 2018b). Additionally, calves fed heated colostrum have shown increased fold changes of mucosa-associated Bifidobacterium and Clostridium cluster XIVa in the colon (Song et al., 2019). These data show that heat-treated colostrum can protect the intestine against pathogens, as well as stimulating the development of the immune function. Additionally, chemical additives are also commonly used for preserving colostrum as they also may reduce microbial counts. In a recent study by Morales-Delanuez et al. (2020), glycerol and propylene glycol were used to reduce microbial counts and preserve immune properties in heat-treated goat milk. They reported that glycerol addition to goat colostrum before heat treatment showed no effect on immunogenic properties of colostrum and reduction of microbial (bacterial) counts.
3.4. Drinking water
Offering drinking water to newborn dairy calves is restricted up to 17 d of age. Dairy producers generally assume that water contents of milk or milk replacer could fulfill the water requirement of the calves (Usda, 2016). Recently, Wickramasinghe et al. (2019) reported that dairy calves receiving drinking water from the first day post-birth had a higher body weight, fiber digestibility and feed efficiency compared with those that received drinking water at 17 d of age. Early colonization of bacteria in the gut of newborn calves has been observed as a result of offering drinking water from d 1 post birth with increased abundances of Faecalibacterium prausnitzii and Bifidobacterium breve seen (Wickramasinghe et al., 2020). Furthermore, higher digestibility of acid detergent fiber correlated with Faecalibacterium, suggesting that offering drinking water from birth has a positive impact on the ability of the calf to digest a solid diet efficiently even after weaning.
3.5. Starter feed
Diet is the main factor that influences the composition of the gut microbiota of neonatal calves (Henderson et al., 2015; Rey et al., 2014). Studies have demonstrated that feeding a solid diet during the pre-weaning period of calves improves performance, microbial establishment rumen development and facilitates weaning transition (Malmuthuge et al., 2019; Yuste et al., 2020). The introduction of a solid diet establishes rumen microbiota as milk bypasses the rumen to enter the abomasum (Heinrichs, 2005). Therefore, managing pre-weaning feeding is important for microbial establishment in the rumen during the weaning period. Fermentable carbohydrates have shown most promising effects regarding the composition and activity of the indigenous microbiota of the GIT (Bauer et al., 2006a, 2006b). Supplementing fermentable carbohydrates is essential for the rapid stabilization of the microbial community and bacterial diversity in newly weaned animals (Konstantinov et al., 2003). Therefore, the time of intestinal colonization after birth or during weaning due to rapid change or stress is most crucial for the young animal to consider for optimization for gut development and sustained microbial colonization.
Supplementing starter feed with milk in pre-weaning lambs also promotes rumen development more efficiently and also provides a greater amount of fermentable carbohydrates and ultimately more VFA and acetate proportions to the cecum (Jiao et al., 2015). Increased rumen weight and papillae size, ultimately associated with better health and growth, have been observed in pre-weaning calves with starter feeding (Berends et al., 2012; Pazoki et al., 2017; Sun et al., 2018). Sun et al. (2018) found that starter feeding influenced cecal bacterial communities and decreased inflammatory expression, which has been shown to have a beneficial effect by alleviating the weaning stress in lambs. The relative abundance of the bacterium Alistipes was increased in the cecum with increasing age of the lamb fed starter feed and milk. Alistipes is associated with fiber degradation in the rumen and utilizes degraded soluble sugars as substrates (Zhang et al., 2017b).
The composition of starter feed is a crucial consideration for effective manipulation of the establishment of gut microbiota and rumen development. This is mainly attributed to the fact that different dominant phyla require specific fermentation substrates in the gut to derive energy required for proliferation and colonization (Li et al., 2012). The starter feed and milk-fed lambs have shown similar phyla prevalence in the cecal mucosa of adult goats (Liu et al., 2017). The dietary supplementation of calves with milk replacer, alfalfa hay, and starter feed has been shown to improve cecal VFA abundance and growth performance compared with maternal grazing and nursing (Wu et al., 2019). Also increased abundance of genera, Clostridium sensu stricto, Escherichia/Shigella, and Prevotella was observed in the cecum of calves. These are involved in the utilization of fibrous and non-fibrous carbohydrates and propionate and butyrate production (Zhang et al., 2017a). Early starter feeding has also been shown to influence gene expression in the rumen epithelium as high-grain diets significantly up-regulated the genes involved in VFA absorption and cell proliferation in the rumen (Jing et al., 2018; Yan et al., 2014). Recently, Zhuang et al. (2020) provided new insights into the molecular mechanisms of rumen development in goat kids by revealing the potential role of starter feeding to promote rumen epithelial cell growth, rumen immune function, and activation of lipid metabolism.
3.6. Weaning regimen and time
Usually, the term weaning means the reduction of milk allowance and an increasing supply of solid feed to young calves at different ages of life (6 to10 wk). Different feeding programs or time of weaning could affect solid feed intake, rumen development, and digestibility of calves in different ways. Early weaning (4 to 6 weeks) coupled with offering optimum milk allowance (10% of body weight) to calves, encourages early solid feed consumption and early rumen development (Bhatti et al., 2012). Delaying the age of weaning increases body weight gain in calves fed an elevated plane of nutrition before weaning and decreases the transient reduction of weight gain at weaning (Abbas et al., 2017; Meale et al., 2015). An increased solid feed intake has been observed during the transition period when calves were weaned late compared with early-weaned calves (Eckert et al., 2015). Feeding milk to Sahiwal calves (up to 15% of their body weight), coupled with early weaning at 8 weeks of age, saves milk and labor compared to weaning at 12 weeks (Cheema et al., 2016). Similarly, calves weaned at 6 weeks of age have shown greater feed efficiency, weight gain, and higher dry matter and organic matter digestibility compared to late weaned counterparts (Tao et al., 2018). In addition to weaning time, a strategy known as the step-down method has been suggested, in which calves initially receive a high amount of milk, then by increasing water content, milk supply is gradually reduced (Khan et al., 2007a, 2007b). Recently, studies have also followed a step-down method to feed milk replacer up to 7 weeks of age, and reported that providing increased milk quantity during early life encourages solid feed intake, with positive effects on GIT development and growth, as well as calf health (Schäff et al., 2016, 2018).
Weaning is considered as the most critical phase in early life due to the significant dietary changes, which can lead to stress and ultimately disease susceptibility and attenuation of the immune system and growth (Hickey et al., 2003; Schichowski et al., 2010). Improved morphology of the small intestine, organ development, and immune function has been observed in artificially reared lambs weaned at 4 or 6 weeks of age (Mccoard et al., 2020). Furthermore, pre-weaning diet and feeding have shown pronounced and long-lasting impacts on rumen microbial composition due to host-specificity of the rumen microbiome (Abecia et al., 2013, 2014). Calves weaned at 3 weeks of age with the introduction of solid feed intake showed greater microbial abundance in the rumen as compared to the calves weaned at 6 weeks of age (Anderson et al., 1987). Rumen development can be affected by different weaning practices such as milk quantity, weaning age, weaning scheme, and time of introduction of solid feeds to the young ones (Khan et al., 2016). Morphological and physiological development of the rumen can be improved by opting for a suitable weaning strategy such as a step-down weaning method, which has shown a successful weaning of calves at 4 weeks of age (Carballo et al., 2019). Although studies are available regarding the short-term effects of weaning strategies on growth performance and rumen development, further investigations are required for a better understanding of the effects on gastrointestinal function, the rumen microbiome, and calf health.
4. Potential strategies to manipulate microbial colonization and gut development
We present potential strategies that can be used to desirably affect the process of initial microbial colonization and gut development in neonatal ruminants.
4.1. Rumen transfaunation inoculation from healthy animals
Rumen transfaunation is a process of transferring rumen fluid from healthy animals to others with diverse microorganisms. This transplanted rumen fluid also supplies nutrients and energy to the rumen microbial population (Depeters and George, 2014). Generally, rumen fluid contains different nutrients such as microbial proteins, amino acids, VFA, vitamins, minerals, and diverse enzymes (Elfaki and Abdelatti, 2018; Sarteshnizi et al., 2018). Indigestion of feed in cows could also be eliminated through the transfaunation of small volumes (1 L) of rumen fluid, which also improves rumen function and feed intake of cows (Steiner et al., 2020). Microbial intervention can also be induced artificially in young calves which can potentially affect calf health and establishment of rumen microbiota. Studies have shown that inoculation of fresh or lyophilized or autoclaved rumen fluid from adult animals improved feed efficiency and weight gain of newborn animals (Muscato et al., 2002; Zhong et al., 2014). A recent study in sheep also reported that the inoculation of newborn lambs with mature lyophilized rumen fluid significantly improved starter feed digestibility and growth performance during and after weaning (Yu et al., 2020). Inoculation also improved ruminal propionate concentration, rumen amylase activities, and decreased acetate-to-propionate ratio. The Streptococcus ruminantium population was increased with the inoculation of mature lyophilized rumen fluid as it is associated with the utilization of starch (Wang et al., 2019b). Similarly, the relative abundance of 8 bacterial genera (Acidiphilium, Jeotgalibaca, Polaribacter, Pseudodesulfovibrio, Bdellovibrio, Microbacterium, Eubacterium and Sporosarcina) belonging to 4 phyla (Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria) were significantly higher in young calves fed with exogenous rumen fluid obtained from an adult cow (Li et al., 2019). A recent study related to goat kids also revealed that inoculation of fresh rumen fluid from adult goat promotes early rumen colonization through the abundant protozoal community, with higher feed intake, greater rumen VFA production and absorption during the preweaning period (Belanche et al., 2020). Inclusion of spray-dried rumen fluid with 1% maltodextrin in suckling dairy calves stimulated the calves’ immune system as revealed by lower serum concentrations of interleukin-6 (Sarteshnizi et al., 2020). These data suggest that providing exogenous rumen fluid to young ruminants not only improves weight gain but also desirably influences diet digestibility, immunity, and health.
4.2. Supplementation of prebiotics and probiotics during early life
It is well established that many pathogenic strains, including E. coli, cause diarrhea in calves (Moxley and Francis, 1986). These pathogens can be potentially controlled through different feeding strategies. Prebiotics and probiotics have shown desirable effects regarding calf health improvement. Supplementation of prebiotics (galacto oligosaccharides) to 2-week-old calves increased the relative abundance of Lactobacillus and Bifidobacterium in the colon, which was less pronounced in 4-week old calves (Castro Marquez, 2014). Mannan oligosaccharides (MOS) from yeast cell walls can bind the thread-like fimbriae on pathogenic bacteria and can prevent their intestinal colonization leading to improved gut health (Hooge, 2006; Spring et al., 2015). It has been observed that oligosaccharides as a prebiotic can stimulate beneficial microbiota (Swennen et al., 2006). Slow fermentable polysaccharides might have more potential compared to rapidly fermented oligosaccharides, through the production of short-chain fatty acid and suppression of protein metabolism more distally in the colon. However, supplementing probiotics is a comparatively easier way to manipulate the microbiome during early life, when gut microbiota is being established, because in later stages it is less effective as the rumen is large and highly anaerobic.
Administration of Bifidobacterium and Lactobacillus to young calves during early life has caused an increase in weight gain and a decrease in the occurrence of diarrhea (Abe et al., 1995; Shehta et al., 2019). Conversely, Vazquez-Mendoza et al. (2019) reported that supplementation of active Bacillus amyloliquefaciens in non-medicated milk replacer to calves resulted in similar performance and economic efficiency, compared with ruminants fed pasteurized waste milk. Therefore, replacement of pasteurized waste milk can be possible with non-medicated milk replacers fortified with probiotics without having a negative impact on calf growth rate and health status of calves. For instance, Brown et al. (2005) and Kmicikewycz et al. (2013) demonstrated that feeding non-medicated milk replacer to calves produced higher growth rates than feeding waste milk.
An increase in total serum IgG concentration has been observed following supplementation of Lactobacillus in young calves (Al-Saiady, 2010). Similarly, the administration of probiotic Bifidobacteria to calves decreased E. coli and total coliforms while increasing the relative abundance of lactobacilli (Geigerová et al., 2016). Furthermore, they reported that the freeze-dried form of the probiotic is more suitable and convenient for feeding to calves from a practical viewpoint. Supplementation of a fungal probiotic has also been shown to increase dietary intake and live weight gain in weaning calves (Theodorou et al., 1990). Recently, Villot et al. (2019) revealed that yeast (Saccharomyces cerevisiae boulardii CNCM I-1079) supplemented to veal calves reduced episodes of diarrhea and maintained a healthy microbial community with Fecalibacterium as a predominant genus. Furthermore, fermentation products of S. cerevisiae can stimulate the colonization of Lachnospiraceae and Ruminococcaceae in the rumen and large intestine, respectively (Xiao et al., 2019). The lower relative abundance of Prevotellaceae as compared to highly abundant Lachnospiraceae has suggested that S. cerevisiae might change the fermentation and stimulate the degradation of recalcitrant fiber substrates in the pre-mature rumen of the calves. However, further investigations are required to fully understand the precise role of probiotics and prebiotics in gut microbial colonization and microbiota composition during early life.
4.3. Supplementation of plant essential oils during early life
Plant essential oils can change ruminal fermentation and resultant VFA concentrations through altering the ruminal microbial communities (Zhou et al., 2020). Essential oils possess different properties such as antibacterial, antiviral, antifungal, insecticidal, and herbicidal activities, and can induce conformational changes in the cell membrane rendering it less impermeable (Benchaar et al., 2008; Calsamiglia et al., 2007; Miguel, 2010). Different phytochemicals presented in essential oils have different properties; thymol and carvacrol mainly act as potent antimicrobials against pathogens such as E. coli, Salmonella typhimurium, Staphylococcus aureus, epidermidis and L. monocytogenes (Benchaar et al., 2008). Similarly, oregano essential oil having second-highest oxygen radical scavenging ability after cloves, followed by cinnamon, ginger, and rosemary (Bentayeb et al., 2014). Dietary supplementation of a liquid blend of essential oils and a prebiotic have been shown to improve the immune status of young calves by increasing IgA titers (Swedzinski et al., 2020).
Dietary supplementation of essential oils can also potentially mitigate ammonia nitrogen and methane through deamination and methanogenesis, respectively (Mcintosh et al., 2003). Essential oils have also been shown to improve the intake of calf starter, feed efficiencies, and body weight gain while increasing beneficial bacteria and inhibiting the growth of E. coli in the gut (Hill et al., 2007; Santos et al., 2015). Dietary supplementation of an essential oil blend (2.5 g/d) improved the average daily gains and increased immunity of calves compared to calves fed the control and higher inclusion rates (Froehlich et al., 2017). Supplementation of essential oil and phenol improved feed intake and reduced the weaning age of calves (Seifzadeh et al., 2017). Inclusion of oregano essential oil increased the relative abundance of Prevotella and Dialister bacteria, which indicate its potential to manipulate ruminal fermentation and reduce methane emissions (Zhou et al., 2020).
Similarly, supplementation of ruminant diets with a blend of essential oil and a prebiotic has shown promising effects on growth, feed efficiency, nutrient digestibility, and immunity of calves during a period of 70 d from birth (Liu et al., 2020). Supplementation of essential oil in neonatal calves during the pe-weaning period showed that essential oil can improve the production of short-chain fatty acid and growth of specific rumen bacterial groups. Increased propionate concentrations and ruminal bacterial communities (Bacteriodetes) were observed in calves fed essential oil, which was primarily attributed to the higher abundance of Prevotellaceae (Poudel et al., 2019). Furthermore, a blend of essential oils (carvacrol, caryophyllene, p-cymene, cineole, terpinene, and thymol) has shown excellent potential to increase propionate concentration and abundance of Prevotella ruminicola (Poudel et al., 2018). The above-mentioned findings clearly indicate that essential oils possess the substantial ability to modulate the ruminal ecosystem and microbiota, to improve nutrient utilization, performance, and health during the early stages of the rumen development.
4.4. Feeding of fibrous plant material (forage) to stimulate rumen development and microbial colonization
Different feeding regimes may lead to different establishment of microbial populations in the rumen of young ruminants. A meta-analysis of literature spanning from 1998 to 2016 showed that feeding forage in starter feed has the potential to improve feed intake and performance of calves (Imani et al., 2017). Many inconsistent findings regarding dietary intake, weight gain, and growth performance have been reported regarding the provision of forage during the pre-weaning period of the calf (Xiao et al., 2020). However, forage provision has consistently been shown to stabilize rumen pH and enhance the muscular development of the rumen through positively affecting rumen microbiota and fermentation characteristics (Castells et al., 2012; Lin et al., 2018). A recent study showed an increased relative abundance of Bacteroidales, Ruminobacter, and Selenomonas in neonatal lambs after 14 d when they were allowed to graze alongside ewes (Wang et al., 2019a). Forage or pasture feeding in this regard can be a potential source to achieve good muscle or fat deposition, and growth performance of young calves during early life (Khan et al., 2020). On the contrary, feeding a high level of forage to lambs has resulted in low dry matter intake, lower feed efficiency, higher nitrogen excretion, and greater urinary urea-nitrogen loss compared with a low level of forage or solid feed (Yuste et al., 2020). Another study of Xia et al. (2018) reported that feeding a high concentration of forage in mixed diet improved Lactobacillus abundance and decreased the abundance of pathogenic microbes, which indicated that a high forage diet for weaned calves improves growth and health of male dairy calves. Furthermore, increased acetate proportion and cellulolytic microbial growth (R. albus) in the rumen has been observed as a result of forage provision (Castells et al., 2013; Suárez et al., 2006). Similarly, a higher abundance of Prevotella and cellulolytic bacteria (R. flavefaciens and R. albus) was observed in calves supplemented with forages (Kim et al., 2016). Moreover, Prevotella was the predominant genus in animals fed high-fiber diets rather than high-caloric diets (Jami et al., 2013). A higher abundance of Bacteroidetes has been observed in the hay supplemented group, with increased rumen growth and volume (Lin et al., 2018). Recently, feeding of a mixture of calf starter and corn silage showed similar growth, weight gain, and health of calves compared to the calf starter (Kehoe et al., 2019). These findings indicate that provision of forage during early life may has a potential impact on the development of predominant microbiota in the rumen.
4.5. Transgenesis
Dietary and inoculation techniques are crucial considerations for improving gut development and microbiota colonization in livestock animals to enhance feed efficiency and meet increasing demands for milk and meat while reducing climatic impacts. In this regard, recent biological techniques, such as transgenesis are getting attention for improving the efficiency of animal production and developing alternatives to antibiotics in the future (Wu and Bazer, 2019). Generally, the production performance of livestock depends upon their genome, environmental factors (nutrition and disease etc.), and their interactions (Bazer et al., 2011). Usually, in a transgenic animal, a foreign gene of interest is inserted into its genome. The foreign gene is constructed in vitro using recombinant DNA technology. Generally, transgenic technologies are categorized into two approaches; embryo-mediated and cell-mediated (Laible, 2018). Transgenic animal technology allows the animal to establish a desirable trait. This technology has been used for the successful production of different transgenic animals, including pigs, mice, rats, cattle, rabbits, sheep, and chickens (Prather et al., 2013; Tiley, 2016). Furthermore, Wang et al. (2016), evaluated the effect of a neomycin phosphotransferase transgene by studying changes in the gut microbiota of piglets using high throughput sequencing. Neo-transgenic expression in transgenic pigs not only changed the relative abundance of some bacteria (Firmicutes, Bacteroidetes, and Proteobacteria) in the intestine but also decreased the levels of potentially harmful bacteria such as Escherichia, Shigella, and Hafnia. From these studies, it is speculated that animal transgenesis is a promising technology in the future to overcome the limitation of conventional methods for improving feed efficiency and improving gut microbiota especially in the early age of life. However, to be used globally, regulations regarding genetically modified organisms would need to be revised.
5. Redundancy, resilience, and host specificity of gut microbiota
As mentioned in previous sections, microbial colonization starts at birth, although it has been proposed that it may even start pre-birth, and dynamically proceeds exhibiting substantial changes induced by many factors like birth type, colostrum/milk feeding, diet and antimicrobial treatment (Bokulich et al., 2016). Once microbiota colonizes and occupies specific niches, the relative abundance of the microbial community consists of a core microbiome that is representative of that particular host species which shows inherent resilience to different perturbations like an established ecosystem (Dethlefsen and Relman, 2011; Martínez et al., 2013; Schloissnig et al., 2013). The development of rumen with age significantly affects microbial diversity and colonization (Furman et al., 2020). Rumen bacteria are the most abundant and diverse group of microbes that constitute more than 95% of the total rumen microbiome (Jami EMizrahi, 2012; Petri et al., 2013). The core microbiome in bovines mainly includes Bacteroidetes, Firmicutes and Proteobacteria which constitute more than 90% of total rumen bacteria (Petri et al., 2013). Studies have shown that this core microbiome fluctuates within these phyla right after birth but becomes stable after rumen development completes and starts proper functioning. This shows the redundancy and resilience of the core microbiome in bovines and other farm animals. Remarkable similarity observed among functional classes of the microbiome at different early stages of life (age groups) suggests that rumen microbiota possess the effective potential to maintain a sustainable physiological and metabolic potential in ruminants (Jami et al., 2013). Redundancy of rumen microbiome stems from overlapping physiological abilities across multiple microbial taxa (Weimer, 2015).
Studies have shown that relative abundance of core phyla varies distinctly with age, for example, composition at 14 d was different from 42 d in Holstein calves. Although overall 170 genera were observed in calves, the core microbiome just consisted of 45 genera in pre-ruminant calves (Li et al., 2012). The process of rumen development significantly affects bacterial diversity as revealed by a considerable compositional heterogeneity of rumen microbiota observed in pre-ruminant calves during early development. It is mainly attributed to substantial changes in the anatomy of the rumen wall and subsequent alterations in shifts in physiological function and metabolism (Li et al., 2012). The intimate cross-talk between rumen microbiome, its metabolites, diet, and the host is responsible for successive changes that occur during rumen development. For example, VFA produced by microbes ultimately determine the size and shape of rumen ruminal papilla. These ruminal papillary structures affect microbial colonization as they provide niche environments for certain rumen microbes (Rieu et al., 1990). Cellulolytic bacteria, sulfur-reducing bacteria, and hydrogenotrophic species (methanogens) constitute major functional groups of rumen microflora that become established during the first few days of life (Anderson et al., 1987; Morvan et al., 1996).
Despite the presence of a core microbiome, there are also a large number of microbial taxa, which varies among hosts explaining host specificity of rumen microbiota. Such host specificity of microbes for a specific host does not appear to be restricted to rumen bacteria only but also has been seen with other microbial communities including archaea and the protozoa (Zhou et al., 2012). Rumen microbiomes have an impact on host metabolism and production performance owing to a positive association of rumen microbiome with the host metabolism (Xue et al., 2020). Recent studies on the bovine rumen demonstrated that the rumen microbiome is influenced by host genetic factors (Wallace et al., 2019). That is why selective breeding can be the best alternative to unlock a host's genetic potential and to induce desired changes in the rumen microbiota compared to rumen transplantation, and probiotics (Difford et al., 2018). Recently, Abbas et al. (2020) revealed that there is a direct relationship between rumen microbiota and host genotype that leads to selective absorption of volatile fatty acids, which can increase energy availability to the host animal. The interaction of the host genome with rumen bacterial composition opens up a new horizon for using genomic selection to sustainably improve animal health and productivity. This needs further investigation to devise suitable strategies for targeted manipulation of the rumen microbiome.
Very little information is available regarding host-specific microbial taxa and their physiological and metabolic effects. Moreover, mechanistic insights regarding the role of host genetics to establish a specific niche for particular microbial taxa are lacking. Although, recent high throughput sequencing and analytical advances have piled up a huge dataset regarding microbiota composition and diversity which has surpassed our capacity to elucidate the physiological and ecological roles of individual microbial taxa, especially those that substantially affect the performance of ruminants. That is why the most challenging task is to dissect the physiological roles of microbes to exploit them for manipulation of gut development and microbial colonization to subsequently enhance the productivity of ruminants.
6. Future implications
The gut microbiota is a crucial consideration for optimizing better health and the performance of neonatal ruminants. This is mainly because dietary and management practices greatly influence gut microbiota subsequently leading to an alteration in the efficiency of nutrient utilization and the immune response. Studies have clearly demonstrated early life as a window of opportunity in which t manipulation of gut microbiota to mediate the immune response and metabolism of young ones. Dietary interventions in the early days of life have shown substantial effects on gut development and microbial colonization as colostrum feeding and supplementation of pro- and prebiotics has shown desirable effects on calf health and growth. The weaning transition period is also very crucial for the long term and persistent effects on rumen microbiota establishment. Therefore, suitable dietary strategies (like starter feed, supplementation of essential oils and other additives) are required to desirably affect successful rumen development and colonization of beneficial microbes to sustainably improve the performance of neonatal ruminants. However, further studies are required to better understand the mechanism of action of dietary interventions on gut development and microbial colonization. Moreover, a role of host genetics shaping microbial populations and a better understanding of molecular mechanisms through which microbes influence the host physiology during the first days of life is crucial for the identification of pathways and key factors associated with host–microbe interaction.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the Southwest Medical University (SWMU) grant No. 42-00040149 that was awarded to Dr. Ahmad Ud Din. We acknowledge the kind inputs of Dr. Tao Ma, Assistant professor, Key Laboratory of Feed Biotechnology of the Ministry of Agriculture and Rural Affairs, Feed Research Institute, Chinese Academy of Agricultural Sciences during revision of the manuscript.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abbas W., Bhatti S.A., Khan M.S., Saeed N., Warriach H.M., Wynn P., Mcgill D. Effect of weaning age and milk feeding volume on growth performance of Nili-Ravi buffalo calves. Ital J Anim Sci. 2017;16:490–499. [Google Scholar]
- Abbas W., Howard J.T., Paz H.A., Hales K.E., Wells J.E., Kuehn L.A., Erickson G.E., Spangler M.L., Fernando S.C. Influence of host genetics in shaping the rumen bacterial community in beef cattle. Sci Rep. 2020;10:1–4. doi: 10.1038/s41598-020-72011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe F., Ishibashi N., Shimamura S. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. J Dairy Sci. 1995;78:2838–2846. doi: 10.3168/jds.S0022-0302(95)76914-4. [DOI] [PubMed] [Google Scholar]
- Abecia L., Martín-García A., Martínez G., Newbold C., Yáñez-Ruiz D.R. Nutritional intervention in early life to manipulate rumen microbial colonization and methane output by kid goats postweaning. J Anim Sci. 2013;91:4832–4840. doi: 10.2527/jas.2012-6142. [DOI] [PubMed] [Google Scholar]
- Abecia L., Waddams K.E., Martínez-Fernandez G., Martín-García A.I., Ramos-Morales E., Newbold C.J., Yáñez-Ruiz D.R. An antimethanogenic nutritional intervention in early life of ruminants modifies ruminal colonization by Archaea. Archaea. 2014;2014 doi: 10.1155/2014/841463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saiady M. Effect of probiotic bacteria on immunoglobulin G concentration and other blood components of newborn calves. J Anim Vet Adv. 2010;9:604–609. [Google Scholar]
- Alimirzaei M., Alijoo Y., Dehghan-Banadaky M., Eslamizad M. The effects of feeding high or low milk levels in early life on growth performance, fecal microbial count and metabolic and inflammatory status of Holstein female calves. Animal. 2020;14:303–311. doi: 10.1017/S1751731119001691. [DOI] [PubMed] [Google Scholar]
- Alipour M.J., Jalanka J., Pessa-Morikawa T., Kokkonen T., Satokari R., Hynönen U., Iivanainen A., Niku M. The composition of the perinatal intestinal microbiota in cattle. Sci Rep. 2018;8:10437. doi: 10.1038/s41598-018-28733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K., Nagaraja T., Morrill J., Avery T., Galitzer S., Boyer J. Ruminal microbial development in conventionally or early-weaned calves. J Anim Sci. 1987;64:1215–1226. doi: 10.2527/jas1987.6441215x. [DOI] [PubMed] [Google Scholar]
- Bach A., Aris A., Vidal M., Fàbregas F., Terré M. Influence of milk processing temperature on growth performance, nitrogen retention, and hindgut's inflammatory status and bacterial populations in a calf model. J Dairy Res. 2017;84:355–359. doi: 10.1017/S0022029917000401. [DOI] [PubMed] [Google Scholar]
- Bauer E., Williams B.A., Smidt H., Verstegen M.W., Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7:35–52. [PubMed] [Google Scholar]
- Bauer E., Williams B.A., Smidt H., Mosenthin R., Verstegen M.W. Influence of dietary components on development of the microbiota in single-stomached species. Nutr Res Rev. 2006;19:63–78. doi: 10.1079/NRR2006123. [DOI] [PubMed] [Google Scholar]
- Bazer F.W., Kraemer D.C., Mchughen A. 2011. Welfare, health, and biological efficiency of animals through genetics and biotechnology, animal welfare in animal agriculture: husbandry, stewardship, and sustainability in animal production; p. 275. [Google Scholar]
- Belanche A., Yáñez-Ruiz D.R., Detheridge A.P., Griffith G.W., Kingston-Smith A.H., Newbold C.J. Maternal versus artificial rearing shapes the rumen microbiome having minor long-term physiological implications. Environ Microbiol. 2019;21:4360–4377. doi: 10.1111/1462-2920.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanche A., Palma-Hidalgo J., Nejjam I., Jiménez E., Martín-García A., Yáñez-Ruiz D. Inoculation with rumen fluid in early life as a strategy to optimize the weaning process in intensive dairy goat systems. J Dairy Sci. 2020 doi: 10.3168/jds.2019-18002. [DOI] [PubMed] [Google Scholar]
- Benchaar C., Calsamiglia S., Chaves A., Fraser G., Colombatto D., Mcallister T., Beauchemin K. A review of plant-derived essential oils in ruminant nutrition and production. Anim Feed Sci Technol. 2008;145:209–228. [Google Scholar]
- Bentayeb K., Vera P., Rubio C., Nerín C. The additive properties of Oxygen Radical Absorbance Capacity (ORAC) assay: the case of essential oils. Food Chem. 2014;148:204–208. doi: 10.1016/j.foodchem.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Berends H., Van Reenen C., Stockhofe-Zurwieden N., Gerrits W. Effects of early rumen development and solid feed composition on growth performance and abomasal health in veal calves. J Dairy Sci. 2012;95:3190–3199. doi: 10.3168/jds.2011-4643. [DOI] [PubMed] [Google Scholar]
- Bhatti S.A., Ahmed M.F., Wynn P.C., Mcgill D., Sarwar M., Afzal M., Ullah E., Khan M.A., Khan M.S., Bush R. Effect of diet on preweaning performance of Sahiwal calves. Trop Anim Health Prod. 2012;44:819–826. doi: 10.1007/s11250-011-9973-3. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M., Li H., Lieber A.D., Wu F., Perez-Perez G.I., Chen Y. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aad7121. 343ra82-343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Vandehaar M., Daniels K., Liesman J., Chapin L., Keisler D., Nielsen M.W. Effect of increasing energy and protein intake on body growth and carcass composition of heifer calves. J Dairy Sci. 2005;88:585–594. doi: 10.3168/jds.S0022-0302(05)72722-3. [DOI] [PubMed] [Google Scholar]
- Calsamiglia S., Busquet M., Cardozo P., Castillejos L., Ferret A. Invited review: essential oils as modifiers of rumen microbial fermentation. J Dairy Sci. 2007;90:2580–2595. doi: 10.3168/jds.2006-644. [DOI] [PubMed] [Google Scholar]
- Carballo O.C., Khan M.A., Knol F.W., Lewis S.J., Stevens D.R., Laven R.A., Mccoard S.A. Impact of weaning age on rumen development in artificially reared lambs. J Anim Sci. 2019;97:3498–3510. doi: 10.1093/jas/skz148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells L., Bach A., Araujo G., Montoro C., Terré M. Effect of different forage sources on performance and feeding behavior of Holstein calves. J Dairy Sci. 2012;95:286–293. doi: 10.3168/jds.2011-4405. [DOI] [PubMed] [Google Scholar]
- Castells L., Bach A., Aris A., Terré M. Effects of forage provision to young calves on rumen fermentation and development of the gastrointestinal tract. J Dairy Sci. 2013;96:5226–5236. doi: 10.3168/jds.2012-6419. [DOI] [PubMed] [Google Scholar]
- Castro Marquez J. Doctoral dissertation, University of Illinois at Urbana-Champaign; 2014. Calf intestinal health: assessment and dietary interventions for its improvement. [Google Scholar]
- Caverly J.M., Diamond G., Gallup J.M., Brogden K.A., Dixon R.A., Ackermann M.R. Coordinated expression of tracheal antimicrobial peptide and inflammatory-response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect Immun. 2003;71:2950–2955. doi: 10.1128/IAI.71.5.2950-2955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton D.E., Aagaard S., Hansen T.H., Nguyen D.N., De Gobba C., Lametsch R., Sangild P.T. Bioactive proteins in bovine colostrum and effects of heating, drying and irradiation. Food Funct. 2020;11:2309–2327. doi: 10.1039/c9fo02998b. [DOI] [PubMed] [Google Scholar]
- Cheema A.T., Bhatti S.A., Akbar G., Wynn P.C., Muhammad G., Warriach H.M., Mcgill D. Effect of weaning age and milk feeding level on pre-and post-weaning growth performance of Sahiwal calves. Anim Prod Sci. 2016 doi: 10.1071/AN15719. [DOI] [Google Scholar]
- Choi H.H., Cho Y.-S. Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin Endosc. 2016;49:257. doi: 10.5946/ce.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.M., Meyer K.M., Prince A.L., Aagaard K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microb. 2016;7:459–470. doi: 10.1080/19490976.2016.1241357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons B.A., Voy B.H., Myer P.R. Altering the gut microbiome of cattle: considerations of host-microbiome interactions for persistent microbiome manipulation. Microb Ecol. 2019;77:523–536. doi: 10.1007/s00248-018-1234-9. [DOI] [PubMed] [Google Scholar]
- De Agüero M.G., Ganal-Vonarburg S.C., Fuhrer T., Rupp S., Uchimura Y., Li H., Steinert A., Heikenwalder M., Hapfelmeier S., Sauer U. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- De Barbieri I., Hegarty R., Silveira C., Gulino L., Oddy V., Gilbert R., Klieve A., Ouwerkerk D. Programming rumen bacterial communities in newborn Merino lambs. Small Rumin Res. 2015;129:48–59. [Google Scholar]
- Depeters E., George L. Rumen transfaunation. Immunol Lett. 2014;162:69–76. doi: 10.1016/j.imlet.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci Unit States Am. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difford G.F., Plichta D.R., Løvendahl P., Lassen J., Noel S.J., Højberg O., Wright A.D., Zhu Z., Kristensen L., Nielsen H.B., Guldbrandtsen B. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill-Mcfarland K.A., Breaker J.D., Suen G. Microbial succession in the gastrointestinal tract of dairy cows from 2 weeks to first lactation. Sci Rep. 2017;7:1–12. doi: 10.1038/srep40864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill-Mcfarland K.A., Weimer P.J., Breaker J.D., Suen G. Diet influences early microbiota development in dairy calves without long-term impacts on milk production. Appl Environ Microbiol. 2019;85 doi: 10.1128/AEM.02141-18. e02141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci Unit States Am. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert E., Brown H., Leslie K., Devries T., Steele M. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. J Dairy Sci. 2015;98:6315–6326. doi: 10.3168/jds.2014-9062. [DOI] [PubMed] [Google Scholar]
- Elfaki M.O., Abdelatti K.A. Rumen content as animal feed: a review. J Vet Med Anim Prod. 2018;7 [Google Scholar]
- Elolimy A., Alharthi A., Zeineldin M.M., Parys C., Helmbrecht A., Loor J.J. Supply of methionine during late-pregnancy alters fecal microbiota and metabolome in neonatal dairy calves without changes in daily feed intake. Front Microbiol. 2019;10:2159. doi: 10.3389/fmicb.2019.02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elolimy A., Alharthi A., Zeineldin M., Parys C., Loor J.J. Residual feed intake divergence during the preweaning period is associated with unique hindgut microbiome and metabolome profiles in neonatal Holstein heifer calves. J Anim Sci Biotechnol. 2020;11:13. doi: 10.1186/s40104-019-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.J. University of Alberta; 2017. Effects of colostrum management practices on the neonatal dairy calf. Master thesis. [Google Scholar]
- Fischer A., Song Y., He Z., Haines D., Guan L., Steele M. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J Dairy Sci. 2018;101:3099–3109. doi: 10.3168/jds.2017-13397. [DOI] [PubMed] [Google Scholar]
- Fischer A.J., Malmuthuge N., Steele M.A. The effect of heat treatment of bovine colostrum on the concentration of oligosaccharides in colostrum and in the intestine of neonatal male Holstein calves. J Dairy Sci. 2018;101:401–407. doi: 10.3168/jds.2017-13533. [DOI] [PubMed] [Google Scholar]
- Fonty G., Gouet P., Nebout J. Development of the cellulolytic microflora in the rumen of lambs transferred into sterile isolators a few days after birth. Can J Microbiol. 1989;35:416–422. doi: 10.1139/m89-064. [DOI] [PubMed] [Google Scholar]
- Foster J.A., Neufeld K-aM. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Francino M.P. Early development of the gut microbiota and immune health. Pathogens. 2014;3:769–790. doi: 10.3390/pathogens3030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N., Shriker E., Gold B., Durman T., Zarecki R., Ruppin E., Mizrahi I. Diet-induced changes of redox potential underlie compositional shifts in the rumen archaeal community. Environ Microbiol. 2017;19:174–184. doi: 10.1111/1462-2920.13551. [DOI] [PubMed] [Google Scholar]
- Froehlich K., Abdelsalam K., Chase C., Koppien-Fox J., Casper D. Evaluation of essential oils and prebiotics for newborn dairy calves. J Anim Sci. 2017;95:3772–3782. doi: 10.2527/jas.2017.1601. [DOI] [PubMed] [Google Scholar]
- Funkhouser L.J., Bordenstein S.R. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman O., Shenhav L., Sasson G., Kokou F., Honig H., Jacoby S., Hertz T., Cordero O.X., Halperin E., Mizrahi I. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat Commun. 2020;11:1–3. doi: 10.1038/s41467-020-15652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigerová M., Vlková E., Bunešová V., Rada V. Persistence of bifidobacteria in the intestines of calves after administration in freeze-dried form or in fermented milk. Czech J Anim Sci. 2016;61:49–56. [Google Scholar]
- Gelsinger S., Jones C., Heinrichs A.J. Effect of colostrum heat treatment and bacterial population on immunoglobulin G absorption and health of neonatal calves. J Dairy Sci. 2015;98:4640–4645. doi: 10.3168/jds.2014-8790. [DOI] [PubMed] [Google Scholar]
- Godden S., Mcmartin S., Feirtag J., Stabel J., Bey R., Goyal S., Metzger L., Fetrow J., Wells S., Chester-Jones H. Heat-treatment of bovine colostrum. II: effects of heating duration on pathogen viability and immunoglobulin G. J Dairy Sci. 2006;89:3476–3483. doi: 10.3168/jds.S0022-0302(06)72386-4. [DOI] [PubMed] [Google Scholar]
- Gritz E.C., Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr. 2015;3:17. doi: 10.3389/fped.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.Y., Ji S.K., Yan H., Wang Y.J., Liu J.J., Cao Z.J., Yang H.J., Zhang W.J., Li S.L. Dynamic change of the gastrointestinal bacterial ecology in cows from birth to adulthood. Microbiol Open. 2020;9:e1119. doi: 10.1002/mbo3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman C.E., Bereza-Malcolm L.T., De Groef B., Franks A.E. Presence of selected methanogens, fibrolytic bacteria, and proteobacteria in the gastrointestinal tract of neonatal dairy calves from birth to 72 hours. PloS One. 2015;10 doi: 10.1371/journal.pone.0133048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman C.E., Wood J.L., Egidi E., White-Monsant A.C., Semenec L., Grommen S.V., Hill-Yardin E.L., De Groef B., Franks A.E. A pioneer calf foetus microbiome. Sci Rep. 2020;10:1–3. doi: 10.1038/s41598-020-74677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B.J., Kim S.-W., Salaheen S., Hovingh E., Van Kessel JaS. Differences in the microbial community and resistome structures of feces from preweaned calves and lactating dairy cows in commercial dairy herds. Foodb Pathog Dis. 2020;17:494–503. doi: 10.1089/fpd.2019.2768. [DOI] [PubMed] [Google Scholar]
- Hammon H., Schiessler G., Nussbaum A., Blum J. Feed intake patterns, growth performance, and metabolic and endocrine traits in calves fed unlimited amounts of colostrum and milk by automate, starting in the neonatal period. J Dairy Sci. 2002;85:3352–3362. doi: 10.3168/jds.S0022-0302(02)74423-8. [DOI] [PubMed] [Google Scholar]
- Hang B.P.T. Swedish University of Agricultural Sciences; 2019. Colostrum quality, intestinal microbiota and implications for health in young dairy calves. Doctoral thesis. [Google Scholar]
- Hansen C.H.F., Nielsen D.S., Kverka M., Zakostelska Z., Klimesova K., Hudcovic T., Tlaskalova-Hogenova H., Hansen A.K. Patterns of early gut colonization shape future immune responses of the host. PloS One. 2012;7 doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F.U., Arshad M.A., Ebeid H.M., Rehman M.S., Khan M.S., Shahid S., Yang C. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet–microbe interaction. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.575801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs A. Rumen development in the dairy calf. Calf Heifer Rearing. 2005:53–65. [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Abecia L., Angarita E., Aravena P., Arenas G., NAriza C. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M.-C., Drennan M., Earley B. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J Anim Sci. 2003;81:2847–2855. doi: 10.2527/2003.81112847x. [DOI] [PubMed] [Google Scholar]
- Hill T., Aldrich J., Schlotterbeck R., Bateman H., Ii Apex plant botanicals for neonatal calf milk replacers and starters. Prof Anim Sci. 2007;23:521–526. [Google Scholar]
- Hirota S.A., Ng J., Lueng A., Khajah M., Parhar K., Li Y., Lam V., Potentier M.S., Ng K., Bawa M., McCafferty D.M. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooge D.M. MOS may boost calf gain. Feedstuffs. 2006;79:19. [Google Scholar]
- Husso A., Lietaer L., Pessa-Morikawa T., Grönthal T., Govaere J., Van Soom A., Iivanainen A., Opsomer G., Niku M. The composition of the microbiota in the full-term fetal gut and amniotic fluid: a bovine caesarean section study. bioRxiv. 2020 doi: 10.1101/2020.09.28.309476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner K.M., Brezinski-Caliguri D.J., Mahoney M.M., Diamond G. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J Nutr. 1998;128:297S–299S. doi: 10.1093/jn/128.2.297S. [DOI] [PubMed] [Google Scholar]
- Imani M., Mirzaei M., Baghbanzadeh-Nobari B., Ghaffari M. Effects of forage provision to dairy calves on growth performance and rumen fermentation: a meta-analysis and meta-regression. J Dairy Sci. 2017;100:1136–1150. doi: 10.3168/jds.2016-11561. [DOI] [PubMed] [Google Scholar]
- Jacometo C., Zhou Z., Luchini D., Correa M., Loor J.J. Maternal supplementation with rumen-protected methionine increases prepartal plasma methionine concentration and alters hepatic mRNA abundance of 1-carbon, methionine, and transsulfuration pathways in neonatal Holstein calves. J Dairy Sci. 2017;100:3209–3219. doi: 10.3168/jds.2016-11656. [DOI] [PubMed] [Google Scholar]
- Jami EMizrahi I. Composition and similarity of bovine rumen microbiota across individual animals. PloS One. 2012;7 doi: 10.1371/journal.pone.0033306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami E., Israel A., Kotser AMizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013;7:1069–1079. doi: 10.1038/ismej.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway Jr C.A., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jiao J., Li X., Beauchemin K.A., Tan Z., Tang S., Zhou C. Rumen development process in goats as affected by supplemental feeding v. grazing: age-related anatomic development, functional achievement and microbial colonisation. Br J Nutr. 2015;113:888–900. doi: 10.1017/S0007114514004413. [DOI] [PubMed] [Google Scholar]
- Jing X.P., Peng Q.H., Hu R., Zou H.W., Wang H.Z., Yu X.Q., Zhou J.W., Degen A., Wang Z.S. Dietary supplements during the cold season increase rumen microbial abundance and improve rumen epithelium development in Tibetan sheep. J Anim Sci. 2018;96:293–305. doi: 10.1093/jas/skx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.L., Godden S.M., Molitor T., Ames T., Hagman D. Effects of feeding heat-treated colostrum on passive transfer of immune and nutritional parameters in neonatal dairy calves. J Dairy Sci. 2007;90:5189–5198. doi: 10.3168/jds.2007-0219. [DOI] [PubMed] [Google Scholar]
- Jung C., Hugot J.P., Barreau F. Peyer's patches: the immune sensors of the intestine. Int J Inflamm. 2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe S., Dill-Mcfarland K., Breaker J.D., Suen G. Effects of corn silage inclusion in preweaning calf diets. J Dairy Sci. 2019;102:4131–4137. doi: 10.3168/jds.2018-15799. [DOI] [PubMed] [Google Scholar]
- Khan M., Lee H., Lee W., Kim H., Ki K., Hur T., Suh G., Kang S., Choi Y. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J Dairy Sci. 2007;90:3376–3387. doi: 10.3168/jds.2007-0104. [DOI] [PubMed] [Google Scholar]
- Khan M., Lee H., Lee W., Kim H., Kim S., Ki K., Ha J., Lee H., Choi Y. Pre-and postweaning performance of Holstein female calves fed milk through step-down and conventional methods. J Dairy Sci. 2007;90:876–885. doi: 10.3168/jds.S0022-0302(07)71571-0. [DOI] [PubMed] [Google Scholar]
- Khan M., Bach A., Weary D., Von Keyserlingk M. Invited review: transitioning from milk to solid feed in dairy heifers. J Dairy Sci. 2016;99:885–902. doi: 10.3168/jds.2015-9975. [DOI] [PubMed] [Google Scholar]
- Khan M., Burggraaf V., Thomson B., Muir P., Lowe K., Koolaard J., Heiser A., Leath S., Mccoard S. Feeding forage or concentrates early in life influences rumen fermentation, metabolic response, immune function and growth of Wagyu× Friesian calves. Anim Prod Sci. 2020 doi: 10.1071/AN18636. [DOI] [Google Scholar]
- Kim Y.-H., Nagata R., Ohtani N., Ichijo T., Ikuta K., Sato S. Effects of dietary forage and calf starter diet on ruminal pH and bacteria in Holstein calves during weaning transition. Front Microbiol. 2016;7:1575. doi: 10.3389/fmicb.2016.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Jöbstl D., Quijada N.M., Dzieciol M., Feldbacher B., Wagner M., Drillich M., Schmitz-Esser S., Mann E. Microbiota of newborn calves and their mothers reveals possible transfer routes for newborn calves' gastrointestinal microbiota. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmicikewycz A., Da Silva D., Linn J., Litherland N. Effects of milk replacer program fed 2 or 4 times daily on nutrient intake and calf growth. J Dairy Sci. 2013;96:1125–1134. doi: 10.3168/jds.2012-5738. [DOI] [PubMed] [Google Scholar]
- Konstantinov S.R., Zhu W.-Y., Williams B.A., Tamminga S., De Vos W.M., Akkermans A.D. Effect of fermentable carbohydrates on piglet faecal bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. FEMS Microbiol Ecol. 2003;43:225–235. doi: 10.1111/j.1574-6941.2003.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Korpela K., Salonen A., Virta L.J., Kekkonen R.A., Forslund K., Bork P., De Vos W.M. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy U., Moran J. Food and Agriculture Organization of the United Nations (FAO); 2012. Rearing young ruminants on milk replacers and starter feeds. [Google Scholar]
- Laible G. vol. 2. Springer; 2018. pp. 95–121. (Production of transgenic livestock: overview of transgenic technologies, Animal Biotechnology). [Google Scholar]
- Lee S., Temple S., Roberts S., Price P. Complex effects of IL1A polymorphism and calpain inhibitors on interleukin 1α (IL-1α) mRNA levels and secretion of IL-1α protein. Tissue Antigens. 2008;72:67–71. doi: 10.1111/j.1399-0039.2008.01052.x. [DOI] [PubMed] [Google Scholar]
- Li R.W., Connor E.E., Li C., Baldwin V., Ransom L., Sparks M.E. Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ Microbiol. 2012;14:129–139. doi: 10.1111/j.1462-2920.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- Li W., Edwards A., Riehle C., Cox M.S., Raabis S., Skarlupka J.H., Steinberger A.J., Walling J., Bickhart D., Suen G. Transcriptomics analysis of host liver and meta-transcriptome analysis of rumen epimural microbial community in young calves treated with artificial dosing of rumen content from adult donor cow. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Wang J., Hou Q., Wang Y., Hu Z., Shi K., Yan Z., Wang Z. Effect of hay supplementation timing on rumen microbiota in suckling calves. Microbiol Open. 2018;7 doi: 10.1002/mbo3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Bian G., Sun D., Zhu W., Mao S. Starter feeding supplementation alters colonic mucosal bacterial communities and modulates mucosal immune homeostasis in newborn lambs. Front Microbiol. 2017;8:429. doi: 10.3389/fmicb.2017.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Chen H., Bai Y., Wu J., Cheng S., He B., Casper D.P. Calf starter containing a blend of essential oils and prebiotics affects the growth performance of Holstein calves. J Dairy Sci. 2020;103:2315–2323. doi: 10.3168/jds.2019-16647. [DOI] [PubMed] [Google Scholar]
- Lopez A., Jones C., Geiger A., Heinrichs A. Comparison of immunoglobulin G absorption in calves fed maternal colostrum, a commercial whey-based colostrum replacer, or supplemented maternal colostrum. J Dairy Sci. 2020;103:4838–4845. doi: 10.3168/jds.2019-17949. [DOI] [PubMed] [Google Scholar]
- Lyons T., Jahns H., Brady J., O'Hara E., Waters S.M., Kenny D., Doyle E., Meade K.G. Integrated analyses of the microbiological, immunological and ontological transitions in the calf ileum during early life. Sci Rep. 2020;10:1–4. doi: 10.1038/s41598-020-77907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., O'hara E., Song Y., Fischer A., He Z., Steele M., Guan L. Altered mucosa-associated microbiota in the ileum and colon of neonatal calves in response to delayed first colostrum feeding. J Dairy Sci. 2019;102:7073–7086. doi: 10.3168/jds.2018-16130. [DOI] [PubMed] [Google Scholar]
- Malmuthuge N., Griebel P.J. Fetal environment and fetal intestine are sterile during the third trimester of pregnancy. Vet Immunol Immunopathol. 2018;204:59–64. doi: 10.1016/j.vetimm.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Malmuthuge N., Griebel P.J. A novel animal model for regional microbial dysbiosis of the pioneer microbial community. Front Microbiol. 2019;10:1706. doi: 10.3389/fmicb.2019.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmuthuge N., Li M., Fries P., Griebel P.J. Regional and age dependent changes in gene expression of Toll-like receptors and key antimicrobial defence molecules throughout the gastrointestinal tract of dairy calves. Vet Immunol Immunopathol. 2012;146:18–26. doi: 10.1016/j.vetimm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Malmuthuge N., Griebel P.J., Guan L.L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front Vet Sci. 2015;2:36. doi: 10.3389/fvets.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmuthuge N., Liang G., Guan L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019;20:172. doi: 10.1186/s13059-019-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I., Muller C.E., Walter J. Long-term temporal analysis of the human fecal microbiota revealed a stable core of dominant bacterial species. PloS One. 2013;8 doi: 10.1371/journal.pone.0069621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard C.L., Elson C.O., Hatton R.D., Weaver C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccoard S.A., Cristobal-Carballo O., Knol F.W., Heiser A., Khan M.A., Hennes N., Johnstone P., Lewis S., Stevens D.R. Impact of early weaning on small intestine, metabolic, immune and endocrine system development, growth and body composition in artificially reared lambs. J Anim Sci. 2020;98 doi: 10.1093/jas/skz356. skz356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcintosh F., Williams P., Losa R., Wallace R., Beever D., Newbold C. Effects of essential oils on ruminal microorganisms and their protein metabolism. Appl Environ Microbiol. 2003;69:5011–5014. doi: 10.1128/AEM.69.8.5011-5014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin S., Spillane C., Claffey N., Smith P.E., O'Rourke T., Diskin M.G., Waters S.M. Rumen microbiome composition is altered in sheep divergent in feed efficiency. Front Microbiol. 2020;11:1981. doi: 10.3389/fmicb.2020.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meale S., Leal L., Martín-Tereso J., Steele M. Delayed weaning of Holstein bull calves fed an elevated plane of nutrition impacts feed intake, growth and potential markers of gastrointestinal development. Anim Feed Sci Technol. 2015;209:268–273. [Google Scholar]
- Meyerholz D.K., Kawashima K., Gallup J.M., Grubor B., Ackermann M.R. Expression of select immune genes (surfactant proteins A and D, sheep beta defensin 1, and toll-like receptor 4) by respiratory epithelia is developmentally regulated in the preterm neonatal lamb. Dev Immunol. 2006;30:1060–1069. doi: 10.1016/j.dci.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato H., Otsuka M., Shirasaka S., Itabashi H., Mitsumori M. Colonization of microorganisms in the rumen of young calves. J Gen Appl Microbiol. 1992;38:447–456. [Google Scholar]
- Mizrahi I., Wallace R.J., Moraïs S. The rumen microbiome: balancing food security and environmental impacts. Nat Rev Microbiol. 2021 doi: 10.1038/s41579-021-00543-6. [DOI] [PubMed] [Google Scholar]
- Morales-Delanuez A., Hernández-Castellano L.E., Moreno-Indias I., Sánchez-Macías D., Argüello A., Castro N. Use of glycerol and propylene glycol as additives in heat-treated goat colostrum. J Dairy Sci. 2020;103:2756–2761. doi: 10.3168/jds.2019-17535. [DOI] [PubMed] [Google Scholar]
- Morrill K., Conrad E., Lago A., Campbell J., Quigley J., Tyler H. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J Dairy Sci. 2012;95:3997–4005. doi: 10.3168/jds.2011-5174. [DOI] [PubMed] [Google Scholar]
- Morvan B., Bonnemoy F., Fonty G., Gouet P. Quantitative determination of H 2-utilizing acetogenic and sulfate-reducing bacteria and methanogenic archaea from digestive tract of different mammals. Curr Microbiol. 1996;32:129–133. doi: 10.1007/s002849900023. [DOI] [PubMed] [Google Scholar]
- Moxley R.A., Francis D.H. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect Immun. 1986;53:339–346. doi: 10.1128/iai.53.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscato T., Tedeschi L., Russell J. The effect of ruminal fluid preparations on the growth and health of newborn, milk-fed dairy calves. J Dairy Sci. 2002;85:648–656. doi: 10.3168/jds.S0022-0302(02)74119-2. [DOI] [PubMed] [Google Scholar]
- Neeser J.-R., Golliard M., Del Vedovo S. Quantitative determination of complex carbohydrates in bovine milk and in milk-based infant formulas. J Dairy Sci. 1991;74:2860–2871. doi: 10.3168/jds.S0022-0302(91)78467-1. [DOI] [PubMed] [Google Scholar]
- O'hara E. University of Alberta; 2019. Investigating early life microbial and host transcriptomic dynamics in the bovine gastrointestinal tract. Doctor of Philosophy. [Google Scholar]
- O'hara E., Neves A.L., Song Y., Guan L.L. The role of the gut microbiome in cattle production and health: driver or passenger? Annu Rev Anim Biosci. 2020;8:199–220. doi: 10.1146/annurev-animal-021419-083952. [DOI] [PubMed] [Google Scholar]
- O'hara E., Kenny D., Mcgovern E., Byrne C.J., Mccabe M.S., Guan L., Waters S.M. Investigating temporal microbial dynamics in the rumen of beef calves raised on two farms during early life. FEMS Microbiol Ecol. 2020;96 doi: 10.1093/femsec/fiz203. [DOI] [PubMed] [Google Scholar]
- Pacheco A.R., Barile D., Underwood M.A., Mills D.A. The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci. 2015;3:419–445. doi: 10.1146/annurev-animal-022114-111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Hidalgo J.M., Jiménez E., Popova M., Morgavi D.P., Martín-García A.I., Yáñez-Ruiz D.R., Belanche A. Inoculation with rumen fluid in early life accelerates the rumen microbial development and favours the weaning process in goats. Anim Microb. 2020 doi: 10.21203/rs.3.rs-108249/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannaraj P.S., Li F., Cerini C., Bender J.M., Yang S., Rollie A., Adisetiyo H., Zabih S., Lincez P.J., Bittinger K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazoki A., Ghorbani G., Kargar S., Sadeghi-Sefidmazgi A., Drackley J.K., Ghaffari M. Growth performance, nutrient digestibility, ruminal fermentation, and rumen development of calves during transition from liquid to solid feed: effects of physical form of starter feed and forage provision. Anim Feed Sci Technol. 2017;234:173–185. [Google Scholar]
- Petri R.M., Schwaiger T., Penner G.B., Beauchemin K.A., Forster R.J., Mckinnon J.J., Mcallister T.A. Characterization of the core rumen microbiome in cattle during transition from forage to concentrate as well as during and after an acidotic challenge. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel P., Froehlich K., Casper D., St-Pierre B. 438 feeding an essential oils blend to neonatal Holstein dairy calves increased rumen propionate concentration and resulted in higher representation of a previously uncharacterized strain of Prevotella ruminicola. J Anim Sci. 2018;96:235–236. [Google Scholar]
- Poudel P., Froehlich K., Casper D.P., St-Pierre B. Feeding essential oils to neonatal Holstein dairy calves results in increased ruminal Prevotellaceae abundance and propionate concentrations. Microorganisms. 2019;7:120. doi: 10.3390/microorganisms7050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather R.S., Lorson M., Ross J.W., Whyte J.J., Walters E. Genetically engineered pig models for human diseases. Annu Rev Anim Biosci. 2013;1:203–219. doi: 10.1146/annurev-animal-031412-103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiei M., Ghoorchi T., Toghdory A., Moazeni M., Khalili M. Effect of feeding heat-treated and unheated colostrum on immunoglobulin G absorption, health and performance of neonatal Holstein dairy calves. Acta Sci Anim Sci. 2019;41 [Google Scholar]
- Rey M., Enjalbert F., Combes S., Cauquil L., Bouchez O., Monteils V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J Appl Microbiol. 2014;116:245–257. doi: 10.1111/jam.12405. [DOI] [PubMed] [Google Scholar]
- Ribeiro G.O., Oss D.B., He Z., Gruninger R.J., Elekwachi C., Forster R.J., Yang W., Beauchemin K.A., Mcallister T.A. Repeated inoculation of cattle rumen with bison rumen contents alters the rumen microbiome and improves nitrogen digestibility in cattle. Sci Rep. 2017;7:1–16. doi: 10.1038/s41598-017-01269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu F., Fonty G., Gaillard B., Gouet P. Electron microscopy study of the bacteria adherent to the rumen wall in young conventional lambs. Can J Microbiol. 1990;36:140–144. doi: 10.1139/m90-025. [DOI] [PubMed] [Google Scholar]
- Salazar-Acosta E., Elizondo-Salazar J.A. Heat treatment of colostrum increases immunoglobulin absorption in Holstein heifer calves. Agron Mesoam. 2019;30:229–238. [Google Scholar]
- Saldana D., Gelsinger S., Jones C., Heinrichs A.J. Effect of different heating times of high-, medium-, and low-quality colostrum on immunoglobulin G absorption in dairy calves. J Dairy Sci. 2019;102:2068–2074. doi: 10.3168/jds.2018-15542. [DOI] [PubMed] [Google Scholar]
- Santos F., De Paula M., Lezier D., Silva J., Santos G., Bittar C.M.M. Essential oils for dairy calves: effects on performance, scours, rumen fermentation and intestinal fauna. Animal. 2015;9:958–965. doi: 10.1017/S175173111500018X. [DOI] [PubMed] [Google Scholar]
- Sarteshnizi F.R., Benemar H.A., Seifdavati J., Greiner R., Salem A.Z., Behroozyar H.K. Production of an environmentally friendly enzymatic feed additive for agriculture animals by spray drying abattoir's rumen fluid in the presence of different hydrocolloids. J Clean Prod. 2018;197:870–874. [Google Scholar]
- Sarteshnizi F.R., Abdi-Benemar H., Seifdavati J., Khalilvandi-Behroozyar H., Seyedsharifi R., Salem A. Influence of spray-dried rumen fluid supplementation on performance, blood metabolites and cytokines in suckling Holstein calves. Animal. 2020:1–8. doi: 10.1017/S1751731120000518. [DOI] [PubMed] [Google Scholar]
- Sasson G., Ben-Shabat S.K., Seroussi E., Doron-Faigenboim A., Shterzer N., Yaacoby S., Miller M.E., White B.A., Halperin E., Mizrahi I. Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow's capacity to harvest energy from its feed. mBio. 2017;8 doi: 10.1128/mBio.00703-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäff C.T., Gruse J., Maciej J., Mielenz M., Wirthgen E., Hoeflich A., Schmicke M., Pfuhl R., Jawor P., Stefaniak T. Effects of feeding milk replacer ad libitum or in restricted amounts for the first five weeks of life on the growth, metabolic adaptation, and immune status of newborn calves. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäff C., Gruse J., Maciej J., Pfuhl R., Zitnan R., Rajsky M., Hammon H. Effects of feeding unlimited amounts of milk replacer for the first 5 weeks of age on rumen and small intestinal growth and development in dairy calves. J Dairy Sci. 2018;101:783–793. doi: 10.3168/jds.2017-13247. [DOI] [PubMed] [Google Scholar]
- Schichowski C., Moors E., Gauly M. Influence of weaning age and an experimental Haemonchus contortus infection on behaviour and growth rates of lambs. Appl Anim Behav Sci. 2010;125:103–108. [Google Scholar]
- Schloissnig S., Arumugam M., Sunagawa S., Mitreva M., Tap J., Zhu A., Waller A., Mende D.R., Kultima J.R., Martin J. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger K., Storch J., Bauer C., Bauer J. Development of selected bacterial groups of the rectal microbiota of healthy calves during the first week postpartum. J Appl Microbiol. 2020;128:366–375. doi: 10.1111/jam.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer M., Makki K., Storelli G., Machuca-Gayet I., Srutkova D., Hermanova P., Martino M.E., Balmand S., Hudcovic T., Heddi A. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- Seferovic M.D., Pace R.M., Carroll M., Belfort B., Major A.M., Chu D.M., Racusin D.A., Castro E.C., Muldrew K.L., Versalovic J. Visualization of microbes by 16S in situ hybridization in term and preterm placentas without intraamniotic infection. Am J Obstet Gynecol. 2019;221:146.e1–146.e23. doi: 10.1016/j.ajog.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifzadeh S., Mirzaei Aghjehgheshlagh F., Abdibenemar H., Seifdavati J., Navidshad B. The effects of a medical plant mix and probiotic on performance and health status of suckling Holstein calves. Ital J Anim Sci. 2017;16:44–51. [Google Scholar]
- Shabat S.K., Sasson G., Doron-Faigenboim A., Durman T., Yaacoby S., Miller M.E., White B.A., Shterzer N., Mizrahi I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016;10:2958–2972. doi: 10.1038/ismej.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehta A., Omran H., Kiroloss F., Azmi M. Effect of probiotic on growth performance and frequency of diarrhea in neonatal buffalo calves. Adv Anim Vet Sci. 2019;7:876–881. [Google Scholar]
- Smith H.W. The development of the flora of the alimentary tract in young animals. J Pathol Bacteriol. 1965;90:495–513. [PubMed] [Google Scholar]
- Song Y., Malmuthuge N., Li F., Guan L.L. Colostrum feeding shapes the hindgut microbiota of dairy calves during the first 12 h of life. FEMS Microbiol Ecol. 2019;95:fiy203. doi: 10.1093/femsec/fiy203. [DOI] [PubMed] [Google Scholar]
- Spring P., Wenk C., Connolly A., Kiers A. A review of 733 published trials on Bio-Mos®, a mannan oligosaccharide, and Actigen®, a second generation mannose rich fraction, on farm and companion animals. J Appl Anim Nutr. 2015;3 [Google Scholar]
- Stabel J., Krueger L., Jenvey C., Wherry T., Hostetter J., Beitz D. Influence of colostrum and vitamins A, D3, and E on early intestinal colonization of neonatal Holstein calves infected with Mycobacterium avium subsp. paratuberculosis. Vet Sci. 2019;6:93. doi: 10.3390/vetsci6040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S., Linhart N., Neidl A., Baumgartner W., Tichy A., Wittek T. Evaluation of the therapeutic efficacy of rumen transfaunation. J Anim Physiol Anim Nutr. 2020;104:56–63. doi: 10.1111/jpn.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson L.F., Payne M.S., Keelan J.A. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43:352–369. doi: 10.1080/1040841X.2016.1211088. [DOI] [PubMed] [Google Scholar]
- Suárez B., Van Reenen C., Beldman G., Van Delen J., Dijkstra J., Gerrits W. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: I. Animal performance and rumen fermentation characteristics. J Dairy Sci. 2006;89:4365–4375. doi: 10.3168/jds.S0022-0302(06)72483-3. [DOI] [PubMed] [Google Scholar]
- Sun D., Mao S., Zhu W., Liu J. Effect of starter diet supplementation on rumen epithelial morphology and expression of genes involved in cell proliferation and metabolism in pre-weaned lambs. Animal. 2018;12:2274–2283. doi: 10.1017/S1751731118000290. [DOI] [PubMed] [Google Scholar]
- Swedzinski C., Froehlich K.A., Abdelsalam K.W., Chase C., Greenfield T.J., Koppien-Fox J., Casper D.P. Evaluation of essential oils and a prebiotic for newborn dairy calves. Transl Anim Sci. 2020;4:txz150. doi: 10.1093/tas/txz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swennen K., Courtin C.M., Delcour J.A. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr. 2006;46:459–471. doi: 10.1080/10408390500215746. [DOI] [PubMed] [Google Scholar]
- Takino T., Kato-Mori Y., Motooka D., Nakamura S., Iida T., Hagiwara K. Postnatal changes in the relative abundance of intestinal Lactobacillus spp. in newborn calves. J Vet Med Sci. 2017:16–406. doi: 10.1292/jvms.16-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Guo F., Tu Y., Si B.-W., Xing Y.-C., Huang D.-J., Diao Q.-Y. Effect of weaning age on growth performance, feed efficiency, nutrient digestibility and blood-biochemical parameters in Droughtmaster crossbred beef calves. Asian-Australas J Anim Sci. 2018;31:864. doi: 10.5713/ajas.17.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis K.R., Romero R., Winters A.D., Greenberg J.M., Gomez-Lopez N., Alhousseini A., Bieda J., Maymon E., Pacora P., Fettweis J.M. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol. 2019;220:267.e1–267.e39. doi: 10.1016/j.ajog.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou M., Beever D., Haines M., Brooks A. The effect of a fungal probiotic on intake and performance of early weaned calves. Anim Prod. 1990;50:577. [Google Scholar]
- Tiley L. Transgenic animals resistant to infectious diseases. Rev Sci Tech (Int Off Epizoot) 2016;35:121–132. doi: 10.20506/rst.35.1.2422. [DOI] [PubMed] [Google Scholar]
- Tominaga K., Yoshimoto T., Torigoe K., Kurimoto M., Matsui K., Hada T., Okamura H., Nakanishi K. IL-12 synergizes with IL-18 or IL-1β for IFN-γ production from human T Cells. Int Immunol. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- Torow N., Hornef M.W. The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol. 2017;198:557–563. doi: 10.4049/jimmunol.1601253. [DOI] [PubMed] [Google Scholar]
- Usda . USDA-APHIS:VS-CEAH-NAHMS; Fort Collins, CO: 2016. Dairy 2014: dairy cattle management practices in the United States, 2014. [Google Scholar]
- Van Keulen P., Khan M., Dijkstra J., Knol F., Mccoard S. Effect of arginine or glutamine supplementation and milk feeding allowance on small intestine development in calves. J Dairy Sci. 2020;103:4754–4764. doi: 10.3168/jds.2019-17529. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J. Cornell university press; 2018. Nutritional ecology of the ruminant. [Google Scholar]
- Vazquez-Mendoza O., Elghandour M.M., Salem A.Z., Cheng L., Sun X., Lisete Garcia-Flor V., Barbabosa Pilego A., Vazquez-Mendoza PAnele U. Effects of sodium butyrate and active Bacillus amyloliquefaciens supplemented to pasteurized waste milk on growth performance and health condition of Holstein dairy calves. Anim Biotechnol. 2019:1–8. doi: 10.1080/10495398.2019.1578785. [DOI] [PubMed] [Google Scholar]
- Villot C., Ma T., Renaud D., Ghaffari M., Gibson D., Skidmore A., Chevaux E., Guan L., Steele M. Saccharomyces cerevisiae boulardii CNCM I-1079 affects health, growth, and fecal microbiota in milk-fed veal calves. J Dairy Sci. 2019;102:7011–7025. doi: 10.3168/jds.2018-16149. [DOI] [PubMed] [Google Scholar]
- Wallace R.J., Sasson G., Garnsworthy P.C., Tapio I., Gregson E., Bani P., Huhtanen P., Bayat A.R., Strozzi F., Biscarini F., Snelling T.J. A heritable subset of the core rumen microbiome dictates dairy cow productivity and emissions. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Qian L., Jiang S., Cai C., Ma D., Gao P., Li H., Jiang K., Tang M., Hou J. Safety evaluation of neo transgenic pigs by studying changes in gut microbiota using high-throughput sequencing technology. PloS One. 2016;11 doi: 10.1371/journal.pone.0150937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang K., Zhang C., Feng Y., Zhang X., Wang X., Wu G. Dynamics and stabilization of the rumen microbiome in yearling Tibetan sheep. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-56206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Wang R., Liu M., Beauchemin K., Sun X., Tang S., Jiao J., Tan Z., He Z. Dietary starch and rhubarb supplement increase ruminal dissolved hydrogen without altering rumen fermentation and methane emissions in goats. Animal. 2019;13:975–982. doi: 10.1017/S1751731118002410. [DOI] [PubMed] [Google Scholar]
- Ward R.E., Niñonuevo M., Mills D.A., Lebrilla C.B., German J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res. 2007;51:1398–1405. doi: 10.1002/mnfr.200700150. [DOI] [PubMed] [Google Scholar]
- Webb C.R., Koboziev I., Furr K.L., Grisham M.B. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology. 2016;23:67–80. doi: 10.1016/j.pathophys.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front Microbiol. 2015;6:296. doi: 10.3389/fmicb.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P.J., Cox M.S., De Paula T.V., Lin M., Hall M.B., Suen G. Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high-and low-efficiency Holstein cows. J Dairy Sci. 2017;100:7165–7182. doi: 10.3168/jds.2017-12746. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe H., Kramer A., Appuhamy J. Drinking water intake of newborn dairy calves and its effects on feed intake, growth performance, health status, and nutrient digestibility. J Dairy Sci. 2019;102:377–387. doi: 10.3168/jds.2018-15579. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe H., Anast J., Schmitz-Esser S., Serão N., Appuhamy J. Beginning to offer drinking water at birth increases the species richness and the abundance of Faecalibacterium and Bifidobacterium in the gut of preweaned dairy calves. J Dairy Sci. 2020 doi: 10.3168/jds.2019-17258. [DOI] [PubMed] [Google Scholar]
- Wilson B.C., Vatanen T., Cutfield W.S., O'sullivan J.M. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019;9:2. doi: 10.3389/fcimb.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woof J.M., Kerr M.A. IgA function–variations on a theme. Immunol. 2004;113:175. doi: 10.1111/j.1365-2567.2004.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bazer F.W. Application of new biotechnologies for improvements in swine nutrition and pork production. J Anim Sci Biotechnol. 2019;10:28. doi: 10.1186/s40104-019-0337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Cui Z., Chen X., Wang P., Yao J. Changed caecal microbiota and fermentation contribute to the beneficial effects of early weaning with alfalfa hay, starter feed, and milk replacer on the growth and organ development of yak calves. Animal. 2019;9:921. doi: 10.3390/ani9110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.-Q., Niu W.-J., Shao T.-Q., Qiu Q.-H., Huawei S., Cao B.-H. Effects of dietary forage to concentrate ratio and wildrye length on nutrient intake, digestibility, plasma metabolites, ruminal fermentation and fecal microflora of male Chinese Holstein calves. J Integr Agric. 2018;17:415–427. [Google Scholar]
- Xiao J., Alugongo G.M., Ji S., Wu Z., Dong S., Li S., Yoon I., Chung R., Cao Z. Effects of Saccharomyces cerevisiae fermentation products on the microbial community throughout the gastrointestinal tract of calves. Animal. 2019;9:4. doi: 10.3390/ani9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Alugongo G.M., Li J., Wang Y., Li S., Cao Z. How forage feeding early in life influences the growth rate, ruminal environment, and the establishment of feeding behavior in pre-weaned calves. Animal. 2020;10:188. doi: 10.3390/ani10020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M.Y., Sun H.Z., Wu X.H., Liu J.X., Guan L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome. 2020;8:1–9. doi: 10.1186/s40168-020-00819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Zhang B., Shen Z. Dietary modulation of the expression of genes involved in short-chain fatty acid absorption in the rumen epithelium is related to short-chain fatty acid concentration and pH in the rumen of goats. J Dairy Sci. 2014;97:5668–5675. doi: 10.3168/jds.2013-7807. [DOI] [PubMed] [Google Scholar]
- Yáñez-Ruiz D.R., Abecia L., Newbold C.J. Manipulating rumen microbiome and fermentation through interventions during early life: a review. Front Microbiol. 2015;6:1133. doi: 10.3389/fmicb.2015.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Biragyn A., Kwak LWOppenheim J.J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- Yeoman C.J., Ishaq S.L., Bichi E., Olivo S.K., Lowe J., Aldridge B.M. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci Rep. 2018;8:1–4. doi: 10.1038/s41598-018-21440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Zhang G., Liu Z., Wu P., Yu Z., Wang J. Repeated inoculation with fresh rumen fluid before or during weaning modulates the microbiota composition and co-occurrence of the rumen and colon of lambs. BMC Microbiol. 2020;20:29. doi: 10.1186/s12866-020-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste S., Amanzougarene Z., De Vega A., Fondevila M., Blanco M., Casasús I. Effect of preweaning diet on performance, blood metabolites and rumen fermentation around weaning in calves of two beef breeds. Anim Prod Sci. 2020 doi: 10.1071/AN19152. [DOI] [Google Scholar]
- Zhang J., Shi H., Wang Y., Li S., Cao Z., Ji S., He Y., Zhang H. Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in Holstein heifers. Front Microbiol. 2017;8:2206. doi: 10.3389/fmicb.2017.02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Xu C., Huo D., Hu Q., Peng Q. Comparative study of the gut microbiome potentially related to milk protein in Murrah buffaloes (Bubalus bubalis) and Chinese Holstein cattle. Sci Rep. 2017;7:1–11. doi: 10.1038/srep42189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R., Sun H., Li G., Liu H., Zhou D. Effects of inoculation with rumen fluid on nutrient digestibility, growth performance and rumen fermentation of early weaned lambs. Livest Sci. 2014;162:154–158. [Google Scholar]
- Zhou M., Hünerberg M., Beauchemin K., Mcallister T., Okine E., Guan L. Individuality of ruminal methanogen/protozoa populations in beef cattle fed diets containing dried distillers' grain with solubles. Acta Agric Scand, Sec A–Anim Sci. 2012;62:273–288. [Google Scholar]
- Zhou R., Wu J., Lang X., Liu L., Casper D.P., Wang C., Zhang L., Wei S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J Dairy Sci. 2020;103:2303–2314. doi: 10.3168/jds.2019-16611. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Cui K., Huang W., Han Y., Diao Q., Zhang N. Early solid diet supplementation influences proteomic of rumen epithelium in goat kids. Res Square. 2020 doi: 10.21203/rs.3.rs-17915/v1. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]