To the Editor—In the global coronavirus disease 2019 (COVID-19) pandemic, up to 80% of the patients in intensive-care units (ICUs) have required invasive mechanical ventilation (IMV).1 Inpatients receiving endotracheal intubation and IMV have increased risk of ventilator-associated pneumonia (VAP).1,2
Oral hygiene with chlorhexidine-based mouthwash is an important prevention measure for VAP3; however, outbreaks of Burkholderia cepacia complex associated with these products have been reported.4,5 To our knowledge, this is the first report of a VAP outbreak caused by B. cepacia complex in COVID-19 patients admitted in ICUs involving 2 hospitals.
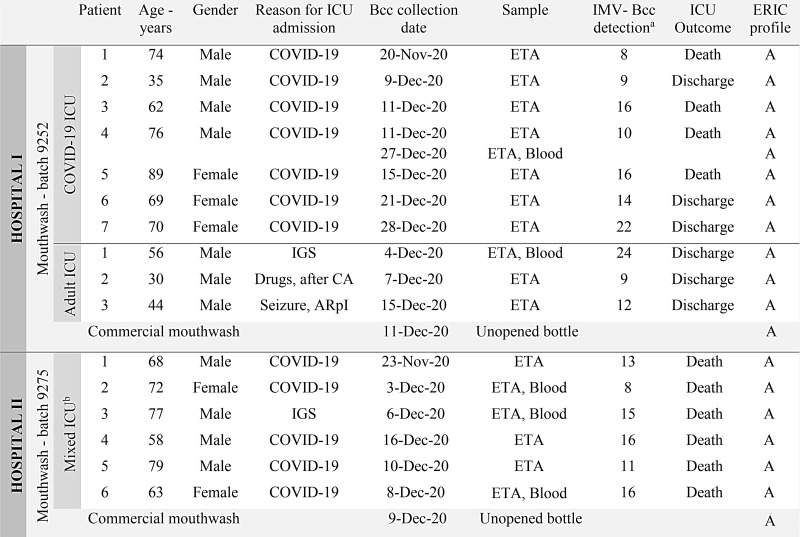
In November and December 2020, in a tertiary-care university hospital (hospital 1) in southern Brazil, 7 patients in a COVID-19 ICU and 3 patients in an adult ICU had positive cultures for B. cepacia complex (>106 CFU/mL) from endotracheal aspirate (ETA). During this period, 6 other patients in a mixed ICU in a private hospital (hospital 2) in the same region showed B. cepacia complex–positive cultures (Fig. 1).
Fig. 1.
Schematic description of B. cepacia complex isolates recovered from mechanically ventilated patients and unopened mouthwash bottles in an intra- and interhospital outbreak. (a) Time (in days) between the beginning of invasive mechanical ventilation (IMV) and B. cepacia complex detection (collection of clinical sample). (b) Patients with and without COVID-19 are admitted to the mixed ICU. Note. ICU, intensive care unit; IGS, Instability after gastrointestinal surgery; CA, cardiac arrest; ARpI, acute respiratory insufficiency; ETA, endotracheal aspirate; ERIC, Enterobacterial repetitive intergenic consensus polymerase chain reaction.
As part of the intervention, contact-isolation precautions were implemented for all patients with B. cepacia complex–positive cultures. Microbiological data were reviewed to track the source of this contamination, and as reported previously, hospital 1 had experienced consecutive outbreaks of B. cepacia complex as a result of the use of intrinsically contaminated mouthwash, so this source was investigated first.6
Burkholderia cepacia complex isolates recovered from ETA and mouthwashes at hospital 1 were characterized phenotypically using the BD-Phoenix automated system (Becton-Dickinson, Franklin Lakes, NJ). Hospital 2 used the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) system (Bruker Daltonics GmbH, Leipzig, Germany). All isolates (hospitals 1 and 2) were typed using the enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) technique.7 BioNumerics 6.5 software (Applied Maths, Sint-Martens-Latem, Belgium) was used to analyze band patterns.
In total, 16 patients had B. cepacia complex–positive cultures recovered from ETA; 12 (75%) these patients were hospitalized with COVID-19 (positive RT-PCR for severe acute respiratory coronavirus virus 2 [SARS-CoV-2]) (Fig. 1). The mean age of these patients was 66 years, and 69% were male. All patients received IMV from the first day of ICU admission. The median time between the beginning of IMV and the first isolation of B. cepacia complex was 14 days (interquartile range [IQR], 9–16).
Burkholderia cepacia complex was recovered (>2.7×105 CFU/mL) in unopened mouthwash bottles containing 0.12% chlorhexidine used in both hospital 1 (batch C9252, 250 mL) and hospital 2 (batch C9275, 1000 mL), all from the same company. This company’s mouthwashes had been used at hospital 1 since January 2020 without isolation of B. cepacia complex in infections.
All isolates evaluated showed 100% genetic similarity, characterizing a monoclonal outbreak involving 3 ICUs and 2 hospitals caused by B. cepacia (confirmed by MALDI-TOF MS).
The manufacturer of these contaminated batches was implicated in a previous B. cepacia complex outbreak at hospital 1, 4 years prior (data reported by our research group).6 In the current outbreak, the hospitals notified again the National Health Surveillance Agency (ANVISA) and the manufacturer. More effectively, a voluntary national recall on December 16, 2020, by the manufacturer resulted in removal of all affected batches. According to the FDA, a likely source of B. cepacia complex contamination in aqueous products appears to be contaminated water used in manufacturing.4 The presence of B. cepacia complex in unopened bottles from different batches of mouthwash strongly suggests contamination during the manufacturing process, and as with B. lata in a study conducted by Leong et al8, our findings also suggest contamination during manufacturing.
Nosocomial cross transmission between patients with B. cepacia complex appears unlikely in this case. In hospital 1, the facilities and staff are not shared between the ICUs, and the adult ICU has single-bed rooms and the COVID-19 ICU 2-bed rooms. In hospital 2, inpatients with COVID-19 are single-bed rooms.
Of the total of 12 patients with VAP by B. cepacia complex and with COVID-19, 9 (75%) died. Of the 4 patients with VAP by B. cepacia complex and without COVID-19, only 1 (25%) died (Fig. 1). The time of IVM of these patients (without COVID-19) was 54.8% shorter than the patients with B. cepacia complex and SARS-CoV-2 coinfection. The median times of IVM were 31 for patients with COVID-19 and 17 days for patients without COVID-19. These results suggest that coinfection with SARS-CoV-2 and B. cepacia complex may increase the time of IMV, similarly to the case reported by Osman and Nguyen.9
Another observation here was the high number of deaths, although attributable mortality was not calculated. Although data on coinfection between SARS-CoV-2, fungi or bacteria, including B. cepacia complex, were reported,10 data on the time of IMV and mortality attributed to these patients are still little explored and require further investigation.
Outbreaks of B. cepacia complex PAV caused by intrinsically contaminated chlorhexidine-based mouthwashes have been well reported.4-6 The ability of B. cepacia complex to remain viable in chlorhexidine appears to result from a combination of efflux pump activity, biofilm formation, and cell-wall impermeability.8 These factors in themselves are extremely important because these products are used for critically ill patients. However, in the context of the COVID-19 pandemic, an outbreak appears to have even more serious consequences. The few cases reported in hospital 2 showed that VAP occurred in a short period, with a high incidence (50%) of bacteremia secondary to VAP and 100% mortality of affected patients.
In conclusion, effective surveillance with practical monitoring by a multidisciplinary team and rapid implementation of outbreak control are even more necessary in mixed ICUs and COVID-19 ICUs. We strongly suggest that national regulatory authorities establish protocols for the detection of B. cepacia complex in chlorhexidine-based products, ensuring microbiological quality of the finished product in addition to patient safety, so that similar outbreaks can be prevented.
Acknowledgments
The authors thank Dr Janet W. Reid for the English text review.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001. These government funds covered only the cost of laboratory materials and had no role in the study design or the decision to submit the work for publication.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1.Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care Lond Engl 2021;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blonz G, Kouatchet A, Chudeau N, et al. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: a multicenter retrospective study in 188 patients in an un-inundated French region. Crit Care 2021;25:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hocková B, Riad A, Valky J, et al. Oral complications of ICU patients with COVID-19: case-series and review of two hundred ten cases. J Clini Med 2021;10:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares M, Kozak M, Balola A, Sá-Correia I.Burkholderia cepacia complex bacteria: a feared contamination risk in water-based pharmaceutical products. Clin Microbiol Rev 2020;33:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaban R, Sotomayor-Castillo C, Nahidi S, et al. Global burden, point sources, and outbreak management of healthcare-associated Burkholderia cepacia infections: an integrative review. Infect Control Hosp Epidemiol 2020;41:777–783. [DOI] [PubMed] [Google Scholar]
- 6.Saalfeld SMS, Shinohara DR, Szczerepa MMA, et al. Consecutive outbreaks of Burkholderia cepacia complex caused by intrinsically contaminated chlorhexidine mouthwashes. Am J Infect Control 2020;48:1348–1353. [DOI] [PubMed] [Google Scholar]
- 7.Silbert S, Pfaller MA, Hollis RJ, et al. Evaluation of three molecular typing techniques for nonfermentative gram-negative bacilli. Infect Control Hosp Epidemiol 2004;25:847–851. [DOI] [PubMed] [Google Scholar]
- 8.Leong LEX, Lagana D, Carter GP, et al. Burkholderia lata infections from intrinsically contaminated chlorhexidine mouthwash, Australia, 2016. Emerg Infect Dis 2018;24:2109–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osman H, Nguyen P.First case of COVID-19 complicated with Burkolderia cepacia pneumonia and bacteremia. Chest 2020;158:A544. [Google Scholar]
- 10.Yang S, Hua M, Liu X, et al. Bacterial and fungal coinfections among COVID-19 patients in intensive care unit. Microbes Infect 2021;104806. [DOI] [PMC free article] [PubMed] [Google Scholar]