Abstract
Inflammation drives the pathogenesis of nonalcoholic steatohepatitis (NASH). The current study examined changes in intestinal inflammation during NASH. In male C57BL/6J mice, feeding a methionine- and choline-deficient diet (MCD) resulted in severe hepatic steatosis and inflammation relative to feeding a chow diet (CD). MCD-fed mice exhibited characteristics of mucosal and submucosal inflammatory responses compared with CD-fed mice. Moreover, intestinal phosphorylation states of c-Jun N-terminal protein kinase p46 and mRNA levels of IL-1B, IL-6, tumor necrosis factor alpha, and monocyte chemoattractant protein-1 were significantly higher and intestinal mRNA levels of IL-4 and IL-13 were significantly lower in MCD-fed mice compared with those in CD mice. Surprisingly, upon treatment with MCD-mimicking media, the proinflammatory responses in cultured intestinal epithelial CMT-93 cells did not differ significantly from those in CMT-93 cells treated with control media. In contrast, in RAW264.7 macrophages, MCD-mimicking media significantly increased the phosphorylation states of c-Jun N-terminal protein kinase p46 and mitogen-activated protein kinases p38 and mRNA levels of IL-1B, IL-6, IL-10, and tumor necrosis factor alpha under either basal or lipopolysaccharide-stimulated conditions. Collectively, these results suggest that increased intestinal inflammation is associated with NASH phenotype. Thus, elevated proinflammatory responses in macrophages likely contribute to, in large part, increased intestinal inflammation in NASH.
Nonalcoholic steatohepatitis (NASH) is the advanced form of nonalcoholic fatty liver disease (NAFLD) characterized by overt inflammatory liver damage.1,2 Growing evidence indicates that NASH is becoming the most common cause of terminal liver diseases, including liver cirrhosis and hepatocellular carcinoma.3, 4, 5, 6 To date, there is a lack of established treatment for NASH. It is therefore necessary to better understand the pathogenesis of NASH-related inflammation leading to the development of new therapeutic strategies for NASH.
Because inflammation promotes the progression of simple steatosis to NASH, numerous studies have focused on how liver inflammation is triggered or exacerbated. For instance, how dysregulated fat metabolism elicits hepatocyte proinflammatory responses is an extensively studied research area of NAFLD/NASH. Using a hepatocyte cell line, Nakamura et al7 showed that accelerated oxidation of palmitate, the most abundant fatty acid in most forms of fats stored in hepatocytes,8,9 acts by causing excessive electron flux in the mitochondrial respiratory chain and generating reactive oxygen species to activate c-Jun N-terminal protein kinase (JNK) signaling. In addition to triggering hepatocyte proinflammatory responses, fat deposition–driven hepatocyte apoptosis or death is further shown to release mitochondrial DNA. The latter functions as a trigger to activate the stimulator of interferon genes in liver Kupffer cells, which in turn critically contributes to the development of liver inflammation.10 Interestingly, nutrition stress also can directly activate macrophages, thereby triggering or exacerbating liver inflammation and contributing to the development of NAFLD or NASH phenotypes.11, 12, 13 Because the incidence of NAFLD in obese populations is increased by seven to tenfold relative to that in lean subjects,14,15 obesity-associated inflammation, in particular inflammation in white adipose tissue (WAT), is considered a critical factor that triggers or aggravates NAFLD. Notably, increasing evidence shows the existence of NAFLD, NASH, or even liver fibrosis in lean subjects,16,17 as well as HIV-positive subjects with normal body mass index or lipodystrophy.18,19 This indicates that tissues additional to WAT are also involved in the pathogenesis of NASH.
Along with nutrition stress–induced WAT inflammation, intestinal inflammation is increased in mice fed a high-fat diet (HFD; 60% fat calories), accompanied by altered composition of gut microbiota involved in the development of NAFLD.13 It is noteworthy that all these inflammatory events occur in the presence of obesity. This finding raised a critical question regarding the extent to which, in the absence of obesity, increased inflammation occurs in tissues other than WAT (eg, the intestine) and is associated with NAFLD/NASH phenotypes. To address this question, the current study examined intestinal inflammation in mice upon feeding a methionine- and choline-deficient diet (MCD) and showed that increased intestinal inflammation is associated with diet-induced hepatic steatosis and inflammation. Moreover, increased proinflammatory responses in macrophages may account for MCD-induced intestinal inflammation.
Materials and Methods
Animal Experiments
C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME), maintained on a 12:12-hour light–dark cycle (lights on at 6:00 am), and fed ad libitum except those that were used for dietary feeding studies. To induce NASH, 11- to 12-week–old male C57BL/6J mice were fed an MCD (Table 1) (Research Diets, Inc., New Brunswick, NJ) for 5 weeks, as previously described.12 Sex- and age-matched mice were fed a regulator chow diet (CD) and used as control. During the feeding period, body weights of all mice were recorded weekly. Also, food consumed during the feeding period was recorded, and food intake per cage was calculated. At 1 week before the end of the feeding period, mice were subjected to glucose tolerance tests. At the end of the feeding period, all mice were euthanized and tissue samples were collected. Epididymal, mesenteric, and perinephric fat depots were weighed,20 and intestine and liver tissues were collected. After weighing, parts of each liver were fixed and embedded for histologic and immunohistochemical analyses. Additional samples from the liver and small intestine were frozen in liquid nitrogen, and samples were stored at −80°C for further analyses. All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Table 1.
Diet Composition
| Macronutrients | Chow diet |
MCD |
||
|---|---|---|---|---|
| g% | kCal% | g% | kCal% | |
| Protein | 24.1 | 28.7 | 17 | 16 |
| Carbohydrates | 57.6 | 57.9 | 66 | 63 |
| Fat | 11.4 | 13.4 | 10 | 21 |
| kCal/g | 3.36 | 4.21 | ||
| Ingredients | g% | kCal% | g% | kCal% |
|---|---|---|---|---|
| Casein | 20.00 | 20.71 | 17.0 | 16% |
| l-methionine | 0.30 | 0.31 | 0 | 0 |
| Choline bitartrate | 2250 ppm∗ | 0 | 0 | 0 |
| Cornstarch | 15 | 15.54 | 15 | 14 |
| Sucrose | 50.00 | 51.79 | 45.53 | 42.48 |
| Maltodextrin | 0 | 0 | 5 | 5 |
| Cellulose | 5 | 0 | 3 | 0 |
| Corn oil | 5 | 11.65 | 10 | 21.19 |
Research Diets, Inc. Brunswick, NJ
Glucose Tolerance Tests
Glucose tolerance tests were performed as described elsewhere.13 After the feeding period, mice were fasted for 4 hours and received an intraperitoneal injection of d-glucose (2 g/kg of body weight). For glucose tolerance tests, blood samples (5 μL) were collected from the tail vein before and at 30, 60, 90, and 120 minutes after the glucose bolus injection. A Glucose (HK) Assay kit (MilliporeSigma, Burlington, MA) was used to measure the concentrations of glucose in the samples collected.
Analysis of Body Composition
At 1 day before harvest, the mice in Study 1 were subjected to body composition analysis using the EchoMRI-100H (EchoMRI LLC, Houston, TX), as previously described.12
Cell Culture and Treatment
To gain insights into MCD-induced intestinal inflammation, CMT-93 cells, which are intestinal epithelial cells (IECs) from a rectal carcinoma cell line, and RAW264.7 cells, which are a macrophage cell line, were subjected to treatment with the MCD-mimicking media. In brief, the cells were originally grown in Iscove's Modified Dulbecco's Medium (IMDM) glucose medium in petri dishes and then transferred into 6-well plates and placed in a humidified incubator with 5% carbon dioxide at 37°C, as previously described.13,21 At approximately 80% confluence, the original IMDM was removed and replaced with either complete 1:1 Dulbecco’s modified Eagle’s medium/Ham’s F-12 media (Ctrl) or 1:1 Dulbecco’s modified Eagle’s medium/Ham’s F-12 media without methionine and choline (MCD-mimicking media, MCD) for an additional 24 hour incubation. At 30 minutes before harvest, the cells were treated with lipopolysaccharide (LPS; 100 ng/mL) or phosphate-buffered saline. Cell lysates were prepared and subjected to Western blot analysis described in Western Blot Analysis. Additional CMT-93 cells and RAW264.7 cells were incubated with MCD-mimicking media for 24 hours and supplemented with or without LPS (20 ng/mL) for the last 6 hours. RNA samples were harvested and subjected to analyses of the mRNA levels of cytokines described in Analysis of Gene Expression.
Histologic and Immunohistochemical Analysis
Mouse liver and small intestine (SI) blocks embedded in paraffin were cut into 5 μm thick sections and stained with hematoxylin and eosin, as previously described.11, 12, 13 To analyze liver and SI inflammation, additional liver sections were stained for F4/80 expression with rabbit anti–F4/80 antibodies (1:1000) (AbD Serotec, Raleigh, NC), whereas additional SI sections were stained for CD68 expression with mouse monoclonal anti-CD68 antibodies (sc-20060, 1:1000; Santa Cruz Biotechnology, Dallas, TX).11, 12, 13
Western Blot Analysis
To determine intestine and cell proinflammatory signaling, lysates of frozen intestines or cultured cells were evaluated by using Western blot analysis as described elsewhere.21, 22, 23 All primary antibodies were purchased from Cell Signaling Technology (Danvers, MA). ImageJ software version 1.52a (NIH, Bethesda, MD; https://imagej.nih.gov/ij) was used to quantify the maximum intensity of each band. The signal strength of phosphorylated NF-κB, phosphorylated JNK p46, phosphorylated mitogen-activated protein kinase p38, and their corresponding total proteins were determined. Glyceraldehyde-3-phosphate dehydrogenase was used as a control for total protein.
Analysis of Gene Expression
Frozen liver and intestine tissues, as well cell samples were used to isolate total RNA using an RNA extraction kit. Reverse transcription was performed by using the GoScript Reverse Transcription System (Promega, Madison, WI), and real-time PCR analysis was performed by using SYBR Green (LightCycler 480 system; Roche, Basel, Switzerland).22,24 The mRNA levels were analyzed for IL-1B, IL-6, IL-10, tumor necrosis factor alpha (TNF), and monocyte chemoattractant protein-1, as well as the expression of genes for cell surface markers and for macrophage activation status such as CD11c, CD163, CD206, arginase 1 (ARG1), IL-4, and IL-13. Each reaction included a total of 0.1 μg RNA. After PCR, the data were normalized to 18s ribosomal RNA and graphed as relative amount to the average of mice fed a CD or Ctrl-treated cells, which was set as 1. Primer sequences are listed in Table 2.
Table 2.
Primer Sequences Used for Real-Time RT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ARG1 | 5′-TGGCTTGCGAGACGTAGAC-3′ | 5′-GCTCAGGTGAATCGGCCTTTT-3′ |
| CD11c | 5′-CTGGATAGCCTTTCTTCTGCTG-3′ | 5′-GCACACTGTGTCCGAACTC-3′ |
| CD163 | 5′-TCCACACGTCCAGAACAGTC-3′ | 5′-CCTTGGAAACAGAGACAGGC-3′ |
| CD206 | 5′-CAGGTGTGGGCTCAGGTAGT-3′ | 5′-TGTGGTGAGCTGAAAGGTGA-3′ |
| IL1B | 5′-TGTCTTGGCCGAGGACTAAGG-3′ | 5′-TGGGCTGGACTGTTTCTAATGC-3′ |
| IL4 | 5′-AGATGGATGTGCCAAACGTCCTCA-3′ | 5′-AATATGCGAAGCACCTTGGAAGCC-3′ |
| IL6 | 5′-ACAACCACGGCCTTCCCTACTT-3′ | 5′-CACGATTTCCCAGAGAACATGTG-3′ |
| IL10 | 5′-ACCTGGTAGAAGTGATGCCCCAGGCA-3′ | 5′-CTATGCAGTTGATGAAGATGTCAAA-3′ |
| IL13 | 5′-TGAGGAGCTGAGCAACATCACACA-3′ | 5′-TGCGGTTACAGAGGCCATGCAATA-3′ |
| MCP1 | 5′-CCACTCACCTGCTGCTACTCAT-3′ | 5′-TGGTGATCCTCTTGTAGCTCTCC-3′ |
| TNF | 5′-ACGGCATGGATCTCAAAGAC-3′ | 5′-AGATAGCAAATCGGCTGACG-3′ |
Statistical Methods
Numeric data are presented as means ± SEM. Unpaired, two-tailed analysis of variance or t-tests were used to determine statistical significance, which was considered significant at the two-tailed P < 0.05.
Results
MCD Feeding Induces Significant Weight Loss and Lipoatrophy in C57BL/6J Mice
Unlike mouse models of HFD-induced NAFLD, in which body weight and visceral fat mass are significantly increased, MCD-induced mouse models of NASH reveal marked decreases in body weight and adiposity. In the current study C57BL/6J mice exhibited significantly decreased body weight with MCD feeding for 5 weeks compared with mice maintained on a CD (Figure 1A). MCD-fed mice also displayed significant decreases in food intake starting at 3 weeks of MCD feeding compared with mice fed a CD (Figure 1B). Considering that weight loss of the MCD-fed mice started at 1 week after initiation of feeding, it is not likely that decreased food intake is a major factor contributing to the weight loss effect of MCD, at least during the first 1 to 2 weeks of MCD feeding. Consistent with a marked decrease in body weight, MCD-fed mice exhibited altered body composition that was characterized by a dramatic decrease in fat mass compared with mice fed a CD (Figure 1C). This decrease accounted for the increased ratio of lean mass to body weight in MCD-fed mice, whereas the absolute lean mass in MCD-fed mice was also decreased significantly compared with that in the CD-fed mice. In addition, the mass of both epididymal and mesenteric fats in MCD-fed mice was significantly smaller than that in the mice fed a CD (Figure 1D); perinephric fat was undetectable in most mice. These results confirm that MCD-fed mice display lipoatrophy.
Figure 1.
Methionine- and choline-deficient diet (MCD) feeding causes marked weight loss and lipoatrophy in mice. A: Body weight. B: Food intake. C: Body composition. D: Abdominal fat mass. A–D: Male C57BL/6J mice, at 11 to 12 weeks of age, were fed an MCD or kept on a chow diet (CD) for 5 weeks. A and B: Body weight was recorded during and after the feeding period. Also, food consumed during the feeding period was recorded and calculated for food intake. C: Body composition was analyzed at 1 day before the end of the feeding period. D: Abdominal fat mass was recorded immediately after the harvest tissue samples. Data are expressed as means ± SEM. n = 10 to 13 (A, C, and D); n = 5 (B). ∗P < 0.05, ∗∗P < 0.01 versus MCD at the same time point; †††P < 0.001 versus MCD for the same type of mass.
MCD Feeding Improves Glucose Tolerance
Obesity-associated NAFLD is commonly accompanied by glucose intolerance. Glucose tolerance in the MCD-fed mice was significantly improved compared with that in the CD-fed mice (Figure 2A). In addition, area under the curve, calculated based on glucose tolerance test data, in MCD-fed mice, was much lower than that in CD-fed mice (Figure 2B). Therefore, MCD feeding improved systemic glucose metabolism, which is likely due to marked weight loss and lipoatrophy.
Figure 2.
Methionine- and choline-deficient diet (MCD) feeding improves systemic glucose tolerance. A: Glucose tolerance tests (GTTs). B: Area under the curve. For A and B, male C57BL/6J mice, at 11 to 12 weeks of age, were fed an MCD or kept on a chow diet (CD) for 5 weeks. Before GTT, mice were fasted for 4 hours and given an intraperitoneal injection of glucose (2 g/kg body weight). Glucose levels were assayed before and at the indicated time points after intraperitoneal glucose injection. For B, area under the curve was calculated based on the data for GTT. Data are expressed as means ± SEM. n = 10. ∗P < 0.05, ∗∗∗P < 0.001 versus MCD at the same time point; ††P < 0.01 versus CD.
MCD-Fed Mice Display Severe Phenotype of NASH
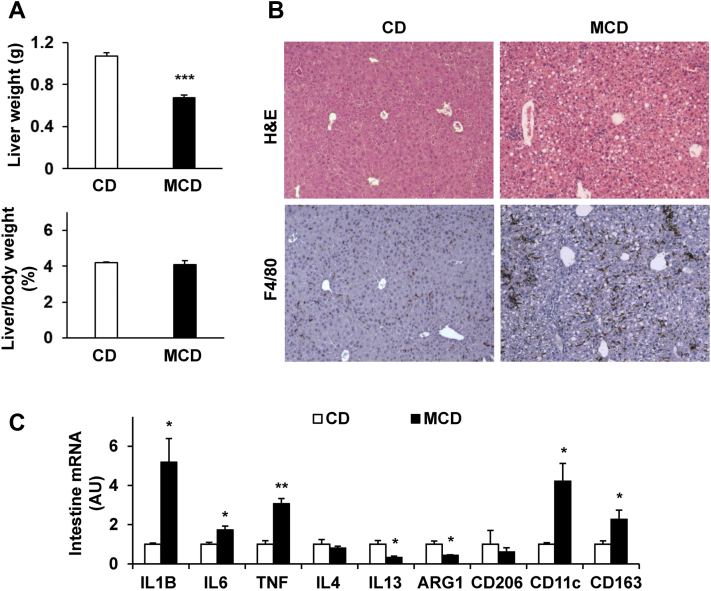
While inducing weight loss and markedly decreasing adiposity (abdominal fat mass), MCD feeding also caused a significant decrease in liver weight (Figure 3A). This decrease in liver weight was proportionally associated with weight loss as the ratios of liver weight to body weight in MCD-fed mice were comparable to those in mice fed a CD (Figure 3A). Consistent with NASH phenotype, the severity of hepatic steatosis and inflammation was significantly increased in MCD-fed mice compared with mice fed a CD, as indicated by histologic staining in liver sections (Figure 3B). Specifically, the livers of MCD-fed mice displayed marked increases in macrophage infiltration, most of which were aggregated (Figure 3B), relative to those in CD–fed mice. mRNA levels of CD11c and CD163 (markers for macrophage proinflammatory activation) in MCD-fed mice were significantly higher than their respective levels in CD-fed mice (Figure 3C). These increases were accompanied by significantly increased mRNA levels of proinflammatory cytokines such as IL-1B, IL-6, and TNF (Figure 3C). In contrast, hepatic mRNA levels of the genes related to anti-inflammatory cytokines (eg, IL-4, IL-13) and macrophage anti-inflammatory activation (eg, ARG1) in MCD-fed mice were significantly decreased compared with their respective levels in mice fed a CD (Figure 3C). However, the mRNA levels of CD206, a maker for macrophage anti-inflammatory activation, revealed no significant differences between the two groups. These results suggest that MCD feeding induced massive accumulation of macrophages that exhibited increased proinflammatory activation and/or decreased anti-inflammatory activation.
Figure 3.
Methionine- and choline-deficient diet (MCD) feeding induces nonalcoholic steatohepatitis phenotype in mice. A: Liver weight (top panel) and ratios of liver weight to body weight (bottom panel). B: Liver histology and immunohistochemistry. Liver sections were stained with hematoxylin and eosin (H&E) or for F4/80 expression. C: Liver expression of surface markers for macrophage activation status. A–C: Male C57BL/6J mice, at 11 to 12 weeks of age, were fed an MCD or kept on a chow diet (CD) for 5 weeks. C: The mRNA levels were quantified by using real-time RT-PCR. Data are expressed as means ± SEM. n = 10 (A) or n = 6 (C). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus CD. Original magnification, ×10 (B). ARG1, arginase 1; TNF, tumor necrosis factor alpha.
MCD Feeding Induces Proinflammatory Changes in SI
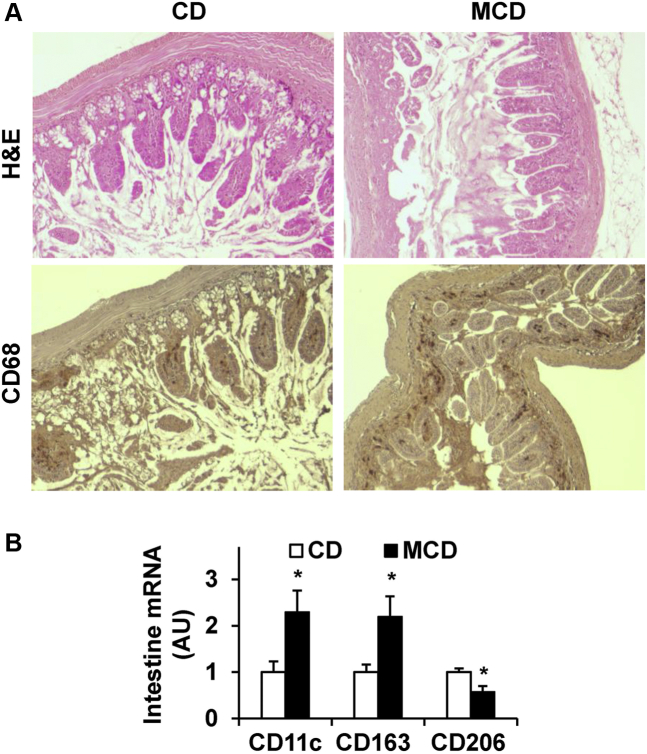
In the MCD-induced mouse model of NASH, the severity of liver inflammation is reportedly greater than that in the HFD-fed mouse model of NAFLD.12,25 Considering that MCD-fed mice displayed extremely low or undetectable amount of epididymal fat mass, we proposed that tissues other than WAT (eg, the intestine) likely revealed increased inflammation and are associated with NASH. As such, morphologic changes were examined in key features related to SI inflammation. Upon staining with hematoxylin and eosin, SI sections of MCD-fed mice displayed mucosal changes characterized by a loss of normal crypt compared with that of mice fed a CD (Figure 4A). Moreover, SI sections of MCD-fed mice revealed significantly increased infiltration of immune cells (macrophages) relative to that of CD-fed mice (Figure 4A), indicated by the results upon staining SI sections for the expression of CD68. Further analysis for the expression of the markers for macrophage activation status indicated significantly higher levels of CD11c and CD163 mRNA. On the other hand, the mRNA levels of CD206 were significantly lower in the SI of MCD-fed mice than in the SI of CD-fed mice (Figure 4B). These results suggest that MCD feeding induces intestinal inflammation characterized by increased mucosal and submucosal accumulation of proinflammatory macrophages.
Figure 4.
Methionine- and choline-deficient diet (MCD) feeding induces morphologic changes in the small intestine. A: Small intestine histology and immunohistochemistry. Sections of small intestine were stained with hematoxylin and eosin (H&E) (top row) or for the expression of CD68 (bottom row). B: Intestinal expression of surface markers for macrophage activation status. The mRNA levels were quantified by using real-time RT-PCR. Data are expressed as means ± SEM. n = 6. ∗P < 0.05 versus CD. Original magnification, ×10 (A). ARG1, arginase 1; AU, arbitrary unit; CD, chow diet.
To further validate intestinal inflammation, proinflammatory signaling was examined in the intestines of mice. Compared with those in CD-fed mice, the phosphorylation states of NF-κB p65 in livers of MCD-fed mice were not significantly altered (Figure 5, A and B). However, the phosphorylation states of JNK p46 in livers of MCD-fed mice were significantly increased compared with those in CD-fed mice (Figure 5, A and B). mRNA levels of key proinflammatory cytokines or chemokine (eg, IL-1B, IL-6, TNF and monocyte chemoattractant protein-1) in intestines of MCD-fed mice were significantly higher than their respective levels in CD-fed mice (Figure 5C). In contrast, intestinal mRNA levels of anti-inflammatory cytokines and genes (eg, IL-4, IL-13, ARG1) in MCD-fed mice were significantly lower than their respective levels in CD-fed mice (Figure 3C). These results, along with those described above, suggest that the MCD-induced increase in intestinal inflammation is associated with the NASH phenotype.
Figure 5.
Methionine- and choline-deficient diet (MCD) feeding increases intestinal proinflammatory responses. A: Intestinal proinflammatory signaling. Representative blots from five individual samples per group. B: Quantification of phosphorylation. C: Intestinal expression of inflammatory mediators. A–C: Male C57BL/6J mice, at 11 to 12 weeks of age, were fed an MCD or kept on a chow diet (CD) for 5 weeks. Data are expressed as means ± SEM (B and C). n = 5 (B); n = 6 (C). ∗P < 0.05 and ∗∗P < 0.01 versus CD. AU, arbitrary unit; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCP1, monocyte chemoattractant protein-1; TNF, tumor necrosis factor alpha.
MCD-Mimicking Media Do Not Significantly Alter the Proinflammatory Responses of IECs
Considering that MCD feeding increased intestinal inflammation, the direct effect of MCD-mimicking media was examined on IEC proinflammatory responses, because IECs are a major cell type of the intestine. In the absence of LPS, the phosphorylation states of NF-κB p65 in MCD-mimicking medium-treated IECs did not differ from those in Control (Ctrl) medium-treated IECs after a 4-hour incubation period (Figure 6, A and B). Upon incubation for 24-hours, however, the phosphorylation states of NF-κB p65 in MCD-mimicking medium-treated IECs were, surprisingly, lower than those in Ctrl-treated IECs. In the presence of LPS, the phosphorylation states of NF-κB p65 in MCD-mimicking medium-treated IECs were comparable to those in Ctrl-treated IECs, although the increase in LPS-stimulated phosphorylation states of NF-κB p65 in MCD-mimicking medium-treated IECs was greater than that in Ctrl-treated IECs (Figure 6, A and B). mRNA levels of proinflammatory cytokines IL-1B, IL-6, and IL-10 in MCD-mimicking medium-treated IECs were comparable to their respective levels in control IECs under either basal or LPS-stimulated conditions; however, LPS-stimulated TNF mRNAs in MCD-mimicking medium-treated IECs were higher than those in control IECs (Figure 6C). Taken together, these results suggest that MCD-mimicking media have a limited effect on increasing IEC proinflammatory responses.
Figure 6.
Methionine- and choline-deficient diet (MCD)-mimicking media do not significantly alter the proinflammatory responses in intestinal epithelial cells (IECs). A: IEC proinflammatory signaling. Representative blots from 2 to 3 individual samples per group. B: Quantification of phosphorylation. C: IEC expression of proinflammatory cytokines. A–C: CMT-93 cells, an IEC line, were treated with MCD-mimicking media or maintained on control (Ctrl) media for the indicated time period (4 or 24 hours in A, and 24 hours in B and C). Before harvest, IECs were treated with lipopolysaccharide (LPS) (100 ng/mL for 30 minutes in A and B or 20 ng/mL for 6 hours in C) or phosphate-buffered saline (PBS). A: cell lysates were evaluated by using Western blot analysis. C: RNAs were subjected to reverse transcription, followed by real-time PCR. Data are expressed as means ± SEM (B and C). n = 2 to 3 (A and B); n = 4 to 6 (C). ∗P < 0.05 and ∗∗P < 0.01 versus Ctrl; †P < 0.05 and ††P < 0.01 versus PBS within the same group. AU, arbitrary unit; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCP1, monocyte chemoattractant protein-1; TNF, tumor necrosis factor alpha.
MCD-Mimicking Media Enhance Macrophage Proinflammatory Responses
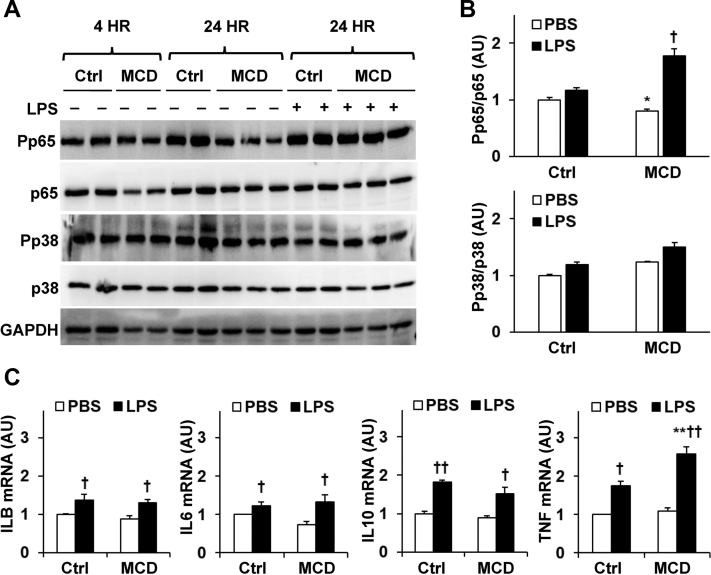
Macrophages critically regulate intestinal inflammation.26 The direct effect of MCD-mimicking media on the proinflammatory responses was examined in RAW264.7 cells. Under either basal or LPS-stimulated conditions, the phosphorylation states of NF-κB p65 in MCD-mimicking media-treated RAW234.7 cells were comparable to those in Ctrl cells (Figure 7, A and B). However, the phosphorylation states of JNK p46 and mitogen-activated protein kinase p38 in MCD-mimicking media-treated RAW264.7 cells were significantly higher than their respective levels in Ctrl cells under either basal or LPS-stimulated conditions (Figure 7, A and B). mRNA levels of the proinflammatory cytokines IL-1B, IL-6, IL-10, and TNF in MCD-mimicking medium-treated RAW264.7 cells were significantly higher than their respective levels in control RAW264.7 cells under either basal or LPS-stimulated conditions (Figure 7C). Taken together, these results suggest that incubation with MCD-mimicking media enhances macrophage proinflammatory responses.
Figure 7.
Methionine- and choline-deficient diet (MCD)-mimicking media enhance macrophage proinflammatory responses. A: Macrophage proinflammatory signaling. Representative blots from two individual samples per group. B: Quantification of phosphorylation. C: Macrophage expression of proinflammatory cytokines. A–C: RAW264.7 cells were treated with MCD-mimicking media or maintained on control (Ctrl) media for 24 hours. Before harvest, RAW264.7 cells were treated with lipopolysaccharide (LPS) [100 ng/mL for 30 minutes in A and B or 20 ng/mL for 6 hours in (C) or phosphate-buffered saline (PBS)]. A: Cell lysates were subjected to Western blot analysis. C: RNAs were subjected to reverse transcription, followed by real-time PCR. Data are expressed as means ± SEM (B and C). n = 2 (A and B); n = 4 to 6 (C). ∗P < 0.05 and ∗∗P < 0.01 versus Ctrl with the same treatment; †P < 0.05 and ††P < 0.01 versus PBS within the same group.
Discussion
The involvement of the intestine in the development and progression of NAFLD/NASH has been largely illustrated by the leaky gut theory. In this theory, nutrition stress causes dysregulation of the composition of gut microbiota.27 The latter, in turn, results in increased intestine permeability to enhance the absorption or draining of nutrients and bacterial product (eg, LPS) into the liver to trigger or exacerbate fat deposition and inflammation. In addition, alterations in the production of microbiota metabolites also contribute to the pathogenesis of NAFLD, as evidenced by the gut production of indole, an anti-inflammatory microbiota metabolite, being decreased in its circulating and hepatic levels in a mouse model of NAFLD.13 Increasing evidence also suggests that nutrition stress (eg, feeding a HFD to mice) increases intestinal inflammation, which is indicated by the elevated proinflammatory signaling through the NF-κB and JNK pathways in intestines.21,26 Moreover, the status of intestinal inflammation is closely associated with the outcomes of distal effects (eg, inflammation in liver and adipose tissues) and systemic insulin sensitivity in mice.22,28 These observations led us to postulate a link between intestinal inflammation and the pathophysiology of NASH in the current study. As suggested by increased mucosal and submucosal infiltration of proinflammatory macrophages and elevated intestinal proinflammatory signaling through JNK pathways and cytokine expression in mice fed an MCD relative to their respective levels in mice fed a CD, intestinal proinflammatory responses were increased in MCD-fed mice, and this increase was associated with NASH phenotype. Of note, this association between increased intestinal inflammation and NASH is accompanied by lipoatrophy. Indeed, in the current study, most mice in the MCD-fed group had zero or undetectable WAT mass, suggesting that WAT inflammation does not play a role in the pathogenesis of NASH phenotype as it does in obesity-associated NAFLD. Therefore, MCD-induced intestinal inflammation may significantly contribute to liver inflammation of MCD-induced NASH. This view, however, warrants future investigations to determine the extent to which altering intestinal inflammation specifically influences NASH phenotype in MCD-fed mice.
Nutrition stress–associated intestinal inflammation is attributable to, to a large extent, increased proinflammatory responses in IECs. As supporting evidence, HFD feeding causes activation of NF-κB, largely in IECs.26 Likewise, palmitate, a major nutrient of a HFD, acts to enhance the proinflammatory responses of IECs.21 Based on this finding, we postulated that increased intestinal inflammation in MCD-fed mice is attributable to increases in IEC proinflammatory responses. However, this was not the case. MCD-mimicking media did not significantly increase the phosphorylation states of NF-κB p65 in the treated IECs compared with control media. However, it enhanced the effect of LPS on stimulating NF-κB p65 phosphorylation. Additionally, it did not alter the phosphorylation states of mitogen-activated protein kinase p38 compared with control media. These findings suggest that nutrients in MCD seem to function differently from nutrients in HFD in terms of regulating the proinflammatory responses of intestines, in particular IECs. Alternatively, nutrients not presented in MCD, but presented in HFD, function to suppress IEC proinflammatory responses. In either case, in vivo, the differences in diets or dietary components seem to determine the proinflammatory responses of IECs, as suggested by the current study and by other published studies.21,26 Nevertheless, it remains interesting but unsolved as to how methionine and/or choline deficiency does not significantly alter IEC proinflammatory responses, in vivo, while increasing macrophage proinflammatory responses (see below).
In mice with MCD-induced NASH, liver inflammation occurs largely due to increased macrophage proinflammatory responses.25 In particular, treatment of macrophages, but not primary hepatocytes, with MCD-mimicking media reveal significantly increased proinflammatory responses under either basal or LPS-stimulated conditions. In the current study, a primary line of evidence indicates that the SI of MCD-fed mice accumulates significantly more macrophages relative to that of CD-fed mice. Moreover, intestinal macrophages exhibited increased status of proinflammatory activation and decreased anti-inflammatory activation, as supported by increased intestinal expression of CD11c and CD163 and decreased expression of CD206 and ARG1. Based on this finding, we also postulated that MCD-induced intestinal inflammation is attributable, in a large part, to increased macrophage proinflammatory activation and decreased anti-inflammatory activation. Thus, macrophages exert a more critical role than IECs in initiating or exacerbating MCD-induced intestinal inflammation. Examining the direct effects of MCD-mimicking media on macrophages corroborated the observation that in the presence of MCD-mimicking media, macrophage proinflammatory responses were significantly enhanced, as indicated by the increased phosphorylation states of JNK p46 and mitogen-activated protein kinase p38 and the expression of proinflammatory cytokines under either basal (in the absence of LPS) or LPS-stimulated conditions. These findings were consistent with those reported previously concerning the effect of MCD on increasing liver inflammation.25 As such, we argue that macrophages account for increased intestinal inflammation associated with NASH phenotype in MCD-fed mice. As mentioned above, IECs respond differentially to different nutrients (ie, nutrients from HFD or MCD). This, however, may not be the case for macrophages, considering that both saturated fatty acids (palmitate and/or stearate) and MCD-mimicking media act to enhance macrophage proinflammatory responses.25,29
Various mechanisms have been proposed to explain how nutrition stress causes or exacerbates macrophage proinflammatory activation.13,30, 31, 32, 33, 34, 35 From the perspective of metabolic reprograming, impaired tricarboxylic acid cycle and aspartate-argininosuccinate shunt and increased pentose phosphate pathway, along with disrupted glycolysis, are associated with macrophage proinflammatory activation.30, 31, 32,36 However, no published data address precisely how MCD feeding induces macrophage proinflammatory activation, although several studies have repeatedly confirmed the proinflammatory effects of MCD on macrophage activation. Considering that MCD feeding alters the expression of genes related to macrophage activation, as well as hepatic lipogenesis, in ways different from those upon HFD feeding,37,38 deficiency of choline and methionine should account for the effect of MCD feeding on inducing macrophage gene expression. Indeed, compared with methionine deficiency, methionine supplementation to diets suppresses hepatic expression of IL-1 and IL-18 in broilers.39 However, whether this effect of methionine is specific to macrophages remains unclear, and warrants further investigation.
In summary, the current study provides evidence to support an association between increased intestinal inflammation and NASH phenotype in MCD-fed mice. The significant finding is that intestinal proinflammatory signaling and cytokine expression in mice fed a MCD were significantly higher than those in mice fed a CD. In addition, the results from cultured cells indicate that macrophages, but not IECs, have increased proinflammatory responses upon treatment with MCD-mimicking media. This suggests that macrophages play an important role in triggering or exacerbating intestinal inflammation than IECs. As such, dietary approaches for alleviating intestinal inflammation may be valuable for managing liver inflammation, and thereby NASH.
Acknowledgments
D.R.M. and C.W. thank Wenya Huang, Linqiang Ma, Xianjian Luo, and Ya Pei for their scientific and intellectual contributions to the current study.
Footnotes
Supported in part by NIH grants DK095862 (C.W.), DK124854 (C.W.) DK108959 (H.F.), DK119421 (H.F.), DK054811 (G.A., S.G.), DK115184 (G.A., S.G.), DK110035 (G.A., S.G.), and AA028711 (G.A., S.G.); the Hatch Program of the National Institutes of Food and Agriculture (C.W.); the Hickam Endowed Chair, Gastroenterology, Medicine (G.A.); Indiana University; and the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative (G.A., H.F.).
D.R.M. and H.L. contributed equally to this work.
Disclosures: None declared.
The views expressed in this article are those of the authors. They do not necessarily represent the views or policies of the Department of Veterans Affairs or the United States Government.
Author Contributions
D.R.M., H.L, and J.Z. performed the majority of the mice experiments; D.R.M. and H.L. performed the majority of the in vitro experiments; D.R.M. and H.L. collected tissue and cell samples and performed molecular and biochemical assays; H.L. performed histologic assays; Q.L., S.G., H.F., and G.A. contributed to scientific discussion; C.W. conceived the study, supervised all experiments, and edited the manuscript.
References
- 1.Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E., Leone N., Vanni E., Marchesini G., Brunello F., Carucci P., Musso A., De Paolis P., Capussotti L., Salizzoni M., Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Marrero J.A., Fontana R.J., Su G.L., Conjeevaram S.H., Emick D.M., Lok A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 5.Powell E.E., Jonsson J.R., Clouston A.D. Steatosis: co-factor in other liver diseases. Hepatology. 2005;42:5–13. doi: 10.1002/hep.20750. [DOI] [PubMed] [Google Scholar]
- 6.Starley B.Q., Calcagno C.J., Harrison S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S., Takamura T., Matsuzawa-Nagata N., Takayama H., Misu H., Noda H., Nabemoto S., Kurita S., Ota T., Ando H., Miyamoto K.-I., Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H., Li H., Woo S.-L., Kim S.-M., Shende V.R., Neuendorff N., Guo X., Guo T., Qi T., Pei Y., Zhao Y., Hu X., Zhao J., Chen L., Chen L., Ji J.-Y., Alaniz R.C., Earnest D.J., Wu C. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J Biol Chem. 2014;289:16374–16388. doi: 10.1074/jbc.M113.539601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Zhang J., Sun H., Liu X., Zheng Y., Xu D., Wang J., Jia D., Han X., Liu F., Nie J., Shi Y. Defective phosphatidylglycerol remodeling causes hepatopathy, linking mitochondrial dysfunction to hepatosteatosis. Cell Mol Gastroenterol Hepatol. 2019;7:763–781. doi: 10.1016/j.jcmgh.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y., Liu Y., An W., Song J., Zhang Y., Zhao X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J Clin Invest. 2019;129:546–555. doi: 10.1172/JCI121842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y., Li H., Liu M., Pei Y., Zheng J., Zhou J., Luo X., Huang W., Ma L., Yang Q., Guo S., Xiao X., Li Q., Zeng T., Meng F., Francis H., Glaser S., Chen L., Huo Y., Alpini G., Wu C. Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology. 2018;68:48–61. doi: 10.1002/hep.29777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X., Li H., Ma L., Zhou J., Guo X., Woo S.-L., Pei Y., Knight L.R., Deveau M., Chen Y., Qian X., Xiao X., Li Q., Chen X., Huo Y., McDaniel K., Francis H., Glaser S., Meng F., Alpini G., Wu C. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology. 2018;155:1971–1984.e4. doi: 10.1053/j.gastro.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L., Li H., Hu J., Zheng J., Zhou J., Botchlett R., Matthews D., Zeng T., Chen L., Xiao X., Athrey G., Threadgill D.W., Li Q., Glaser S., Francis H., Meng F., Li Q., Alpini G., Wu C. Indole alleviates diet-induced hepatic steatosis and inflammation in a manner involving myeloid cell 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3. Hepatology. 2020;72:1191–1203. doi: 10.1002/hep.31115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 15.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman A., Eder S.K., Felder T.K., Kedenko L., Paulweber B., Stadlmayr A., Huber-Schönauer U., Niederseer D., Stickel F., Auer S., Haschke-Becher E., Patsch W., Datz C., Aigner E. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol. 2017;112:102–110. doi: 10.1038/ajg.2016.318. [DOI] [PubMed] [Google Scholar]
- 17.Kim D., Kim W., Joo S.K., Kim J.H., Harrison S.A., Younossi Z.M., Ahmed A. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non-obese nonalcoholic fatty liver disease. Liver Int. 2019;39:332–341. doi: 10.1111/liv.13983. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Matute P., Pérez-Martínez L., Blanco J.R., Oteo J.A. Role of mitochondria in HIV infection and associated metabolic disorders: focus on nonalcoholic fatty liver disease and lipodystrophy syndrome. Oxid Med Cell Longev. 2013;2013:493413. doi: 10.1155/2013/493413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Welzen B.J., Mudrikova T., El Idrissi A., Hoepelman A.I.M., Arends J.E. A review of non-alcoholic fatty liver disease in HIV-infected patients: the next big thing? Infect Dis Ther. 2019;8:33–50. doi: 10.1007/s40121-018-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo Y., Guo X., Li H., Wang H., Zhang W., Wang Y., Zhou H., Gao Z., Telang S., Chesney J., Chen Y.E., Ye J., Chapkin R.S., Wu C. Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. J Biol Chem. 2010;285:3713–3721. doi: 10.1074/jbc.M109.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botchlett R., Li H., Guo X., Qi T., Zhao J., Zheng J., Woo S.-L., Pei Y., Liu M., Hu X., Chen G., Guo T., Yang S., Li Q., Xiao X., Huo Y., Wu C. Glucose and palmitate differentially regulate PFKFB3/iPFK2 and inflammatory responses in mouse intestinal epithelial cells. Sci Rep. 2016;6:28963. doi: 10.1038/srep28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo X., Li H., Xu H., Halim V., Thomas L.N., Woo S.-L., Huo Y., Chen Y.E., Sturino J.M., Wu C. Disruption of inducible 6-phosphofructo-2-kinase impairs the suppressive effect of PPAR[gamma] activation on diet-induced intestine inflammatory response. J Nutr Biochem. 2013;24:770–775. doi: 10.1016/j.jnutbio.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo S.-L., Xu H., Li H., Zhao Y., Hu X., Zhao J., Guo X., Guo T., Botchlett R., Qi T., Pei Y., Zheng J., Xu Y., An X., Chen L., Chen L., Li Q., Xiao X., Huo Y., Wu C. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9:e91111. doi: 10.1371/journal.pone.0091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X., Li H., Xu H., Halim V., Zhang W., Wang H., Ong K.T., Woo S.-L., Walzem R.L., Mashek D.G., Dong H., Lu F., Wei L., Huo Y., Wu C. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One. 2012;7:e39286. doi: 10.1371/journal.pone.0039286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J., Li H., Cai Y., Ma L., Matthews D., Lu B., Zhu B., Chen Y., Qian X., Xiao X., Li Q., Guo S., Huo Y., Zhao L., Tian Y., Li Q., Wu C. Mice lacking adenosine 2A receptor reveal increased severity of MCD-induced NASH. J Endocrinol. 2019;243:199–209. doi: 10.1530/JOE-19-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding S., Chi M.M., Scull B.P., Rigby R., Schwerbrock N.M.J., Magness S., Jobin C., Lund P.K. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang X., Zheng J., Zhang S., Wang B., Wu C., Guo X. Advances in the involvement of gut microbiota in pathophysiology of NAFLD. Front Med (Lausanne) 2020;7:361. doi: 10.3389/fmed.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X., Xu K., Zhang J., Li H., Zhang W., Wang H., Lange A.J., Chen Y.E., Huo Y., Wu C. Involvement of inducible 6-phosphofructo-2-kinase in the anti-diabetic effect of peroxisome proliferator-activated receptor gamma activation in mice. J Biol Chem. 2010;285:23711–23720. doi: 10.1074/jbc.M110.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z., Kahn B.B., Shi H., Xue B.-Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haschemi A., Kosma P., Gille L., Evans R., Burant F., Starkl P., Knapp B., Haas R., Schmid A., Jandl C., Amir S., Lubec G., Park J., Esterbauer H., Bilban M., Brizuela L., Pospisilik J.A., Otterbein L E., Wagner O. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., Zheng L., Gardet A., Tong Z., Jany S.S., Corr S.C., Haneklaus M., Caffrey B.E., Pierce K., Walmsley S., Beasley F.C., Cummins E., Nizet V., Whyte M., Taylor C.T., Lin H., Masters S.L., Gottlieb E., Kelly V.P., Clish C., Auron P.E., Xavier R.J., O'Neill L.A.J. Succinate is an inflammatory signal that induces IL-1[beta] through HIF-1[alpha] Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanman L.E., Qian Y., Eisele N.A., Ng T.M., van der Linden W.A., Monack D.M., Weerapana E., Bogyo M. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;5:e13663. doi: 10.7554/eLife.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Cao Q., Yu L., Shi H., Xue B., Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. 2016;1:e87748. doi: 10.1172/jci.insight.87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijayan V., Pradhan P., Braud L., Fuchs H.R., Gueler F., Motterlini R., Foresti R., Immenschuh S. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide—a divergent role for glycolysis. Redox Biol. 2019;22:101147. doi: 10.1016/j.redox.2019.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang S., Ma H.-Y., Zhong Z., Dhar D., Liu X., Xu J., Koyama Y., Nishio T., Karin D., Karin G., McCubbin R., Zhang C., Hu R., Yang G., Chen L., Ganguly S., Lan T., Karin M., Kisseleva T., Brenner D.A. NADPH oxidase 1 in liver macrophages promotes inflammation and tumor development in mice. Gastroenterology. 2019;156:1156–1172.e6. doi: 10.1053/j.gastro.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jha K., Huang C.-C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., Chmielewski K., Stewart K.M., Ashall J., Everts B., Pearce E.J., Driggers E.M., Artyomov M.N. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Rizki G., Arnaboldi L., Gabrielli B., Yan J., Lee G.S., Ng R.K., Turner S.M., Badger T.M., Pitas R.E., Maher J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280–2290. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Crespo M., Gonzalez-Teran B., Nikolic I., Mora A., Folgueira C., Rodríguez E., Leiva-Vega L., Pintor-Chocano A., Fernández-Chacón M., Ruiz-Garrido I., Cicuéndez B., Tomás-Loba A., A-Gonzalez N, Caballero-Molano A., Beiroa D., Hernández-Cosido L., Torres J.L., Kennedy N.J., Davis R.J., Benedito R., Marcos M., Nogueiras R., Hidalgo A., Matesanz N., Leiva M., Sabio G. Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. Elife. 2020;9:e59258. doi: 10.7554/eLife.59258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng J.L., Bai S.P., Wang J.P., Ding X.M., Zeng Q.F., Zhang K.Y. Methionine deficiency decreases hepatic lipid exportation and induces liver lipid accumulation in broilers. Poult Sci. 2018;97:4315–4323. doi: 10.3382/ps/pey317. [DOI] [PubMed] [Google Scholar]