Abstract
Respiratory syncytial virus (RSV) is a common cause of acute lower respiratory tract infections and hospitalisations among young children and is globally responsible for many deaths in young children, especially in infants aged <6 months. Furthermore, RSV is a common cause of severe respiratory disease and hospitalisation among older adults. The development of new candidate vaccines and monoclonal antibodies highlights the need for reliable surveillance of RSV. In the European Union (EU), no up-to-date general recommendations on RSV surveillance are currently available. Based on outcomes of a workshop with 29 European experts in the field of RSV virology, epidemiology and public health, we provide recommendations for developing a feasible and sustainable national surveillance strategy for RSV that will enable harmonisation and data comparison at the European level. We discuss three surveillance components: active sentinel community surveillance, active sentinel hospital surveillance and passive laboratory surveillance, using the EU acute respiratory infection and World Health Organization (WHO) extended severe acute respiratory infection case definitions. Furthermore, we recommend the use of quantitative reverse transcriptase PCR-based assays as the standard detection method for RSV and virus genetic characterisation, if possible, to monitor genetic evolution. These guidelines provide a basis for good quality, feasible and affordable surveillance of RSV. Harmonisation of surveillance standards at the European and global level will contribute to the wider availability of national level RSV surveillance data for regional and global analysis, and for estimation of RSV burden and the impact of future immunisation programmes.
Short abstract
Recommendations for developing a feasible and sustainable national surveillance strategy for respiratory syncytial virus that will enable harmonisation and data comparison at the European level. https://bit.ly/3rWUOOI
Introduction
Human respiratory syncytial virus (RSV), also known as human orthopneumovirus, is an important global respiratory pathogen, affecting mostly the upper airways. Particularly in children aged <5 years, RSV can also cause infection of the lower airways, e.g. bronchiolitis or bronchopneumonia, which can lead to respiratory failure. It is the most common cause of hospitalisation among young children admitted for an acute lower respiratory infection (ALRI) worldwide, and is estimated to cause about 120 000 deaths in children aged <5 years globally per year [1]. Almost half of RSV-ALRI-associated hospitalisations (45%) and in-hospital deaths (46%) in these children occur in infants aged <6 months. By the age of 1 year, 60–70% of children have been infected with RSV [2]. Furthermore, RSV infection in early life has been associated with the development of recurrent wheezing and asthma in later infancy and childhood [3]. RSV can cause severe disease in premature infants, infants with comorbidities (such as congenital heart disease, bronchopulmonary dysplasia and Down syndrome) [4], older adults (≥65 years) [5] and adults with comorbidities such as chronic obstructive pulmonary disease [6]. In addition to severe respiratory disease, RSV infections also lead to high utilisation of outpatient services such as visits to emergency rooms, general practitioners (GPs) and/or paediatricians, although this impact has not yet been well defined. As a result of widespread acute RSV infections and the long-term chronic consequences, most countries face high RSV-associated healthcare expenditure. RSV causes seasonal epidemics worldwide [7], and in Europe RSV has demonstrated seasonality with a moderate correlation between timing of the epidemic and higher latitude of the country [8]. In general, RSV activity peaks consistently during winter months in temperate countries but shows greater variability in seasonal pattern in the tropics [9].
Several candidate RSV vaccines are currently in the pipeline, with a variety of different working mechanisms and target groups, including pregnant women through maternal vaccinations [10]. The first of these current candidate vaccines reported results from a phase 3 trial in 2019 [11]. In addition to the monoclonal antibody palivizumab, recommended as immunoprophylaxis for RSV for high-risk infants on a monthly basis before and during the RSV season [12], a new monoclonal antibody is being developed with an enhanced neutralising effect and longer half-life [13]. Therefore, it is possible that new monoclonal antibodies, if they indeed show higher efficacy and sufficient half-life, and are cost-effective, will become more broadly available for the general population and not only for at-risk groups. These developments support the prospect that severe RSV infections may be preventable in the coming years. As novel RSV vaccines and monoclonal antibodies reach the final stages of development, the need to develop systems for monitoring population-level impact and vaccine effectiveness is becoming more urgent. National and supranational surveillance systems offer an efficient infrastructure through which to obtain baseline data and monitor the future impact of RSV immunisation programmes and the effectiveness of RSV vaccines and monoclonal antibodies. The World Health Organization (WHO) has started a global effort to develop standards for RSV surveillance, based on the Global Influenza Surveillance and Response System. A pilot study was started in 2017 [14], followed by a 3-year extension phase from 2018 to 2021 [15] in over 20 countries.
In the EU, no up-to-date general recommendations on RSV surveillance are currently available for Member States who want to establish or improve RSV surveillance. From 1996 to 2008, RSV data were collected and shared through the European Influenza Surveillance Scheme [16], which was a disease surveillance network funded (mainly) by the European Commission and based on agreed surveillance recommendations [17]. The main purpose was to estimate the incidence of influenza-like illness (ILI) during the early part of the influenza season. In September 2008, after moving to the European Centre for Disease Prevention and Control (ECDC), the network was given the name European Influenza Surveillance Network (EISN). Together with the WHO Regional Office for Europe, collection of data on national RSV laboratory test results has continued, but without updating the existing surveillance recommendations. This is because RSV is not yet in the list of notifiable diseases at the EU level (see below). Therefore, RSV surveillance in the EU is currently based on a variety of surveillance platforms [18] that are informative for describing trends and seasonality on the national level, but have poor comparability across countries [8]. These data are also not very useful for estimating healthcare burden or impact of future immunisation programmes.
In addition to collecting data on laboratory results, nearly all EU/European Economic Area countries have a primary care (e.g. GPs and community-based paediatricians) sentinel surveillance system providing data on consultation rates for ILI and/or acute respiratory infection (ARI) and respiratory sampling of patients across all age groups [18]. Testing for RSV for severe acute respiratory infection (SARI) or hospitalised ARI cases is also primarily conducted in hospitals as part of the influenza surveillance programme, and hospital RSV-testing practices are highly variable within and between most European countries [18]. A substantial proportion of young children hospitalised with lower respiratory tract infections like bronchiolitis and pneumonia are tested for RSV, but the reported differences in RSV detection [8] most likely reflect differences in clinical diagnostic guidelines and protocols rather than differences in disease prevalence.
Many countries in Europe have established national electronic healthcare databases and registries that are currently mainly used to inform policies for immunoprophylaxis [19]. These registries include routinely collected data such as data from laboratory testing, hospital admissions, outpatient and GP attendance, medical prescriptions and mortality [20]. Healthcare registries usually have complete population coverage and are designed to support direct patient healthcare delivery [21]. Secondary uses of these data can include surveillance of diseases, research, public health guidance, resource planning and management, and service evaluation and improvement [22]. National laboratory registries for infectious diseases, to which all positive results from any diagnostic laboratory in the country are reported, provide the opportunity for real-time pathogen surveillance.
In order to further enhance and harmonise European collaboration in the field of RSV surveillance, a workshop was organised by ECDC, Statens Serum Institut (SSI) and the National Institute for Public Health and the Environment (RIVM) to develop recommendations for RSV surveillance in Europe. In total, 30 experts working in the fields of RSV-associated epidemiology, virology, public health and paediatrics from 17 different European countries and two representatives from ECDC and WHO participated in this workshop. The recommendations described here will help in the development of a feasible and sustainable surveillance strategy at the national level and enable harmonisation and data comparison at the European level. The recommendations can be used by public health institutes to set up new or enhance existing RSV surveillance strategies.
Linking with the WHO RSV surveillance initiative and EU surveillance infrastructure
Surveillance of RSV in the European region will eventually form a core arm of the global surveillance of RSV by the WHO. It is therefore essential that we collaborate closely with and contribute to global RSV surveillance with both clinical and virological data collection. Globally determined RSV surveillance standards and European approaches should be fully aligned to avoid conflicting guidance at the national level. Structures such as European RSV reference laboratories (one of the global reference laboratories for the WHO RSV surveillance phase 2 pilot [15] is located in the UK) would be instrumental in this. The harmonisation of surveillance standards at the European and global level will ultimately contribute to the delivery of national-level RSV surveillance data to regional analyses at the ECDC and WHO Regional Office for Europe and further for global analysis at the WHO headquarters, similar to the current routine practice in influenza surveillance [23]. The EU Decision on serious cross-border threats to health (no. 1082/2013/EU) [24] mandates that the European Commission establishes and updates the list of communicable diseases and related special health issues and provides case definitions concerning each communicable disease, as well as updates the procedures for operating the epidemiological surveillance network. Currently, RSV is not included in the list of diseases to be covered by epidemiological surveillance in the EU. For this reason, the ECDC currently has a very limited mandate to develop EU-level surveillance for RSV. While in some European countries RSV is a notifiable disease, in most countries reporting is voluntary [18]. However, because the WHO has proceeded with a global RSV surveillance pilot in more than 20 countries and the ECDC has a history of collecting RSV data as part of influenza surveillance and already publishes visual summaries of RSV in its Surveillance Atlas (www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases), there is a need to standardise surveillance systems and data collection across countries and to advocate for the inclusion of RSV on the list of notifiable diseases.
Recommendations for national RSV surveillance
We here provide recommendations for three components of RSV surveillance: 1) active community surveillance, 2) active hospital surveillance (where intensive care unit (ICU) surveillance can either be a stand-alone surveillance solution or nested within hospital surveillance) and 3) passive surveillance using national healthcare registries. With respect to the implementation of active sentinel RSV surveillance, recommendations for optimal diagnostic and virus characterisation are provided.
The preferred RSV surveillance system will depend on the national objectives of the surveillance (box 1 and table 1) and available resources. Active sentinel surveillance systems are used to systematically obtain high-quality data. We recommend 1) using existing RSV surveillance platforms and upgrading these where relevant to accommodate relevant standards, 2) leveraging existing surveillance systems for influenza surveillance as cost-effective RSV surveillance platforms [14] or 3) setting up active sentinel surveillance systems (community and/or hospital) for fast and efficient extraction of systematically collected high-quality data. Whereas most sentinel surveillance platforms in Europe are based on community cases [18], targeting both community and hospital surveillance within one country would provide insight into the full spectrum of RSV disease. Because the primary aim of a future immunisation programme is likely to be to prevent severe illness in infants, the optimal surveillance platform includes RSV hospital admission data in infants. In addition to collecting data from RSV cases, assessing all-cause ARI GP consultations and all-cause SARI admissions may be of value when assessing RSV vaccine effectiveness, given that RSV vaccination may affect the risk of subsequent (RSV and non-RSV) ALRI and complications [11]. Furthermore, this ARI-based surveillance could facilitate the introduction of a more flexible surveillance system into which other respiratory pathogens, e.g. severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can be integrated. Surveillance systems based on electronic health registry and/or laboratory data have the advantage of covering a larger part (often comprehensively all) of the population and are less expensive to maintain than sentinel surveillance. Linking to or using laboratory data is crucial for passive RSV surveillance, given the non-specific clinical symptoms of RSV.
BOX 1. Objectives for respiratory syncytial virus surveillance
Following an expert consultation in November 2015 [25], the European Centre for Disease Prevention and Control's Advisory Forum considered the potential objectives for respiratory syncytial virus (RSV) surveillance noted below to be appropriate and proportional (adjusted from [25]):
1) Describe seasonality and monitor regional, national or European trends for RSV infection • to describe RSV circulation and identify the start and end of RSV seasons • to inform prevention and treatment strategies.
2) Measure positivity rates of RSV across different age groups.
3) Measure incidence of RSV infection and support the estimation of healthcare burden of RSV in different age and target groups.
4) Contribute to the overall understanding of the role (e.g. the attributable fraction) of RSV in respiratory disease and define RSV risk groups.
5) Monitor genetic and antigenic characteristics and changes of RSV: • collect samples to monitor the circulation of the two RSV subtypes, genetic diversity among circulating strains, and the stability of antigenic epitopes targeted by existing and in-the-pipeline monoclonal antibodies and vaccines.
6) Provide a platform and baseline data to estimate the impact of immunisation programmes, when available on the market.
TABLE 1.
Potential objectives of RSV surveillance and corresponding surveillance data indicators from sentinel and registry based surveillance
| Objective | Sentinel surveillance (community and hospital) | Passive surveillance using RSV laboratory surveillance database |
| 1) Describe seasonality and trends for RSV | ARI/extended SARI incidence ARI/extended SARI RSV incidence |
RSV laboratory-confirmed cases |
| 2) Measure positivity rates of RSV across different age groups | % of RSV among ARI/extended SARI cases | % of RSV among tested patients |
| 3) Support the estimation of healthcare burden of RSV | Proportion of hospitalisations associated with RSV ARI/extended SARI incidence ARI/extended SARI RSV incidence |
RSV laboratory-confirmed cases Duration of hospitalisation, etc. |
| 4) Contribute to the overall understanding of the role of RSV in respiratory disease | % of RSV among ARI/extended SARI cases Ratios of RSV positivity compared with other respiratory pathogens |
Ratios of RSV detections/cases compared to detections/cases of other pathogens |
| 5) RSV types and genetic diversity | Genotypic characterisation Phenotypic characterisation |
Sequence data stored in an RSV dedicated or general (GenBank) sequence database Existing laboratory databases containing detailed genetic information |
| 6) Platform and baseline to access impact of immunisation programmes | VE of RSV ARI/extended SARI VE of RSV bronchiolitis (hospital only) RSV incidence before and after implementation (focus on primary target group for vaccination) |
If immunisation status is available: VE among different risk groups RSV incidence before and after implementation (focus on primary target group for vaccination) |
RSV: respiratory syncytial virus; ARI: acute respiratory infection; SARI: severe acute respiratory infection; VE: vaccine effectiveness (this term includes the effectiveness of monoclonal antibodies).
Recommendations for active community and hospital surveillance
The recommendations in this section apply to active surveillance, both in the community and in hospitals.
Case definitions
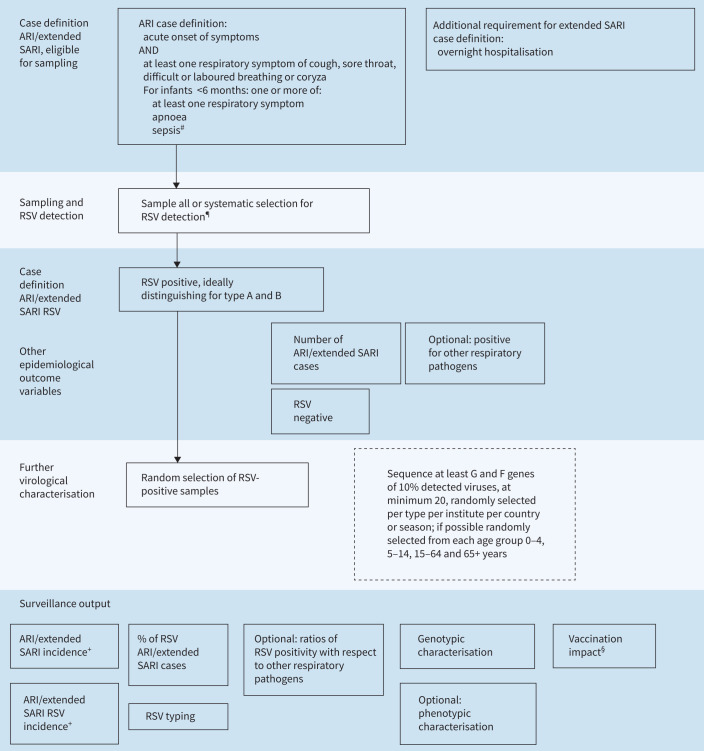
Because the definition of ARI without the necessity of fever is more sensitive than that of ILI (which requires fever) [26, 27] in capturing RSV infection, the use of the ECDC ARI case definition [28] should be considered as the preferred option for RSV surveillance. This case definition is also recommended for the WHO phase 2 pilot [29]. This case definition encompasses acute onset of infectious symptoms with at least one respiratory symptom of cough, sore throat, difficult or laboured breathing or coryza. For children aged <6 months, apnoea and sepsis should also be included to cover the wider clinical presentation in this age group.
The SARI case definition for hospital RSV surveillance can exclude up to 50% of RSV cases in young children and older adults because of the requirement for fever [30]. In line with the WHO recommendations for RSV surveillance [14, 29], it is more appropriate to adopt the ECDC ARI case definition with the addition that for hospitalised cases overnight admission is required. This definition is similar to the extended SARI definition of the WHO [29] that furthermore includes sepsis and apnoea for infants aged <6 months (figure 1).
FIGURE 1.
Testing and diagnostic algorithm for respiratory syncytial virus (RSV) surveillance: active community surveillance and active hospital surveillance. ARI: acute respiratory infection; SARI: severe acute respiratory infection. #: sepsis defined as fever >37.5°C or hypothermia, shock or seriously ill without apparent cause; ¶: using nasopharyngeal swab, within 10 days after onset of disease but ideally within 4 days after onset, by quantitative reverse transcriptase PCR (qRT-PCR) or molecular point-of-care tests (mPOCT), ideally distinguishing by type A and B; +: note that (background) denominator data are needed; §: note that additional variables (e.g. vaccination coverage) are needed.
Age groups
RSV infects all age groups, not only infants and frail older adults. Furthermore, the RSV transmission pattern between young and older children or adults, the role of other age groups (including healthcare workers) in transmitting RSV and the burden of RSV infection in adults is not well understood and may have important economic consequences. Therefore, specimen collection needs to cover all ages [31]. For feasibility reasons and in keeping with the WHO RSV surveillance phase 2 pilot [15], children aged <2 years (who have a high burden of RSV) may be prioritised for specimen collection [1]. Because of the high incidence of RSV and high proportion of severe RSV cases in the first years of life, in particular in the first 6 months [1], adoption of the following age groups is recommended if specific age in months and years cannot be collected: <3 months, 3–5 months, 6–11 months, 12–23 months, 2–4 years, 5–14 years, 15–64 years and 65+ years. This would allow direct comparison of RSV and influenza age data from EISN and the WHO RSV surveillance initiative [14, 29]. If detailed age groups in infants are not possible then sub-groups directly aligned with the above proposed age groups should be adopted (e.g. <2 years and 2–4 years or 0–4 years).
Start and end of each season
The great majority of RSV cases across Europe is captured between week 40 and week 20 [7], but the onset of RSV circulation is often close to week 40 [8]. To ensure that unexpected early epidemics are identified, to assess regularity of RSV seasons and to be able to document sporadic RSV cases, RSV surveillance should in principle be conducted year-round, at least for the first few years of surveillance. When this is not possible, the focus could be on the season defined through existing multi-year data, typically weeks 40–20 [8], but this is only possible in countries with a well-characterised RSV season.
Defining the start and end of the RSV season enables the surveillance system to inform healthcare providers and health authorities so that measures can be implemented as needed. Currently, there is no generally accepted method to define the start and end of the RSV season in Europe based on data from a community-based sentinel system. For this reason, each country needs to apply the best calculation method according to data availability and local circumstances, until standard methods are agreed upon and widely adopted. Several methods exist (box 2). We recommend the use of either the WHO method or the Moving Epidemic Method (MEM), because these can be used prospectively. The MEM is commonly used for defining the influenza season and additionally assesses intensity levels [33].
BOX 2. Methods for defining start and end of respiratory syncytial virus season
Recommended real-time methods:
1) Average epidemic curve method: this method, recommended by the World Health Organization for influenza surveillance, determines average epidemic curves (appendix 8 of [32]). A specific example of this is the moving epidemic method (MEM) [33, 34], which estimates a pre- and post-epidemic threshold, and additional intensity levels of an epidemic, based on data from previous seasons.
Other methods:
2) Annual mean percentage [35]: comparing the weekly proportion of positive tests to the annual mean percentage.
3) 3% threshold method [36]: threshold of a weekly percentage of 3% tests positive by PCR testing. This could also be used real-time.
4) 1.2% threshold method [8]: >1.2% of total respiratory syncytial virus (RSV)-positive specimens per country with surveillance system and season RSV detections also exceeding threshold continuously during the season (with one gap week allowed). This method can only be used retrospectively and can be used when no denominator data on number of tested specimens are available.
Denominators
Two different denominators are important in RSV community and hospital surveillance: first, the (age-stratified) population denominator to establish the ARI and extended SARI incidence; and second, the number of samples to estimate the percentage of RSV positivity. Methods to calculate incidence rates for SARI and outpatient ILI surveillance described by the WHO [32, 37, 38] can also be applied for the incidence of extended SARI and ARI. In some countries, a population denominator will relate to the population served by the sentinel GPs and community paediatricians (e.g. via patient lists). Also, some countries may have clear catchment areas defined for hospitals. In other countries, these would need to be estimated using methods described by the WHO, e.g. by mapping addresses of patients of a certain sentinel site while also taking into account other health facilities in that area. If these data are not available, additional surveys on healthcare utilisation might be necessary [38]. Alternative methods may be used if the catchment population per sentinel site is unknown (e.g. calculate the percentage of sentinel physicians compared to the total number of physicians and apply the percentage to the population pyramid) [39]. These numbers need to be updated every season. To estimate denominators for RSV positivity, it is important to maintain a weekly record of all tested patients, including those whose sample tested negative for RSV. In circumstances in which the national reference laboratory receives all sentinel samples, a simple (aggregated) denominator of number of all tested specimens can be obtained at the national level to calculate the percentage of positive RSV samples. For this to be reliable, testing should be performed on either all eligible cases, or on a systematic basis, specified a priori. To extrapolate data to the national level for accurate healthcare burden estimates, more detailed population data may be necessary, e.g. on prevalence of risk factors and on healthcare-seeking behaviour [40].
Data reporting
Countries may consider requesting community and hospital sentinel sites to collect and report individual case-based data (for a limited set of variables (table 2)) by period (week, month) of specimen collection at the national level. Data reporting to the supranational level can also be in case-based format as is already done in the WHO pilot [14]. Reports of case-based data will assist in data validation and linking with laboratory results. Additionally, these data are useful for future vaccine effectiveness calculations. When case-based data are not available for sharing at the national level, data reporting will need to be aggregated by predefined age groups as described above. Weekly data collection, as conducted for influenza surveillance, is likely feasible in many countries. The advantages of weekly data reporting are that it allows the start of the RSV season to be identified and facilitates healthcare planning, such as additional bed capacity in hospitals. At a minimum, data should be reported weekly during the respiratory season (weeks 40–20) and on a monthly basis thereafter.
TABLE 2.
Recommended set of core and other optional variables in case-based reporting of community and hospital surveillance
| Community surveillance | Hospital surveillance | |
| CORE SET variables | ||
| Patient variables | Date of consultation | Date of admission |
| Age in years# | Age in years# | |
| Age in months (children aged <24 months)¶ | Age in months (children aged <24 months)¶ | |
| Sex | Sex | |
| Clinical variables | Date of onset | Date of onset |
| Measured temperature >38°C, cough, sore throat, coryza, difficult or laboured breathing, (for infants aged <6 months) apnoea, sepsis+ | Measured temperature >38°C, cough, sore throat, coryza, difficult or laboured breathing, respiratory rate frequency above WHO threshold for pneumonia,§ (for infants aged <6 months) apnoea, sepsis+ | |
| Virological variables | Date of sampling | Date of sampling |
| Type of specimen | Type of specimen | |
| RSV detection result positive/negative | RSV detection result positive/negative | |
| RSV type | RSV type | |
| For subset: genotyping and analysis of antigenic sites | For subset: genotyping and analysis of antigenic sites | |
| Other optional variables | ||
| Clinical variablesƒ | Length of stay (days) | |
| Supplemental oxygen use (yes/no) | ||
| ICU admission yes/no | ||
| Ventilatory support (yes/no OR subdivided in invasive and non-invasive) | ||
| Died during hospitalisation (yes/no) | ||
| RSV vaccination status of patientƒ | RSV vaccination status of patientƒ | |
| RSV vaccination status of mother (for children aged <1 year)## | RSV vaccination status of mother (for children aged <1 year)## | |
| Monoclonal Ab use | Monoclonal Ab use | |
| If yes, date of most recent monoclonal Ab use | If yes, date of most recent monoclonal Ab use | |
| Risk groups | Preterm birth (<37 weeks of gestation) | Preterm birth (<37 weeks of gestation) |
| Underlying conditions | Underlying conditions |
WHO: World Health Organization; RSV: respiratory syncytial virus; ICU: intensive care unit; Ab: antibody. #: for the oldest age groups, a category such as 90+ years may be required depending on the size of demographic strata for reported data to be anonymised; ¶: if strata are too small, age groups (<3 months, 3–5 months, 6–11 months, 12–23 months) could be used; +: all variables should be recorded as yes/no/unknown; §: WHO respiratory rate threshold for pneumonia [96]: a) age <2 months ≥60 breaths·min−1, b) age 2–11 months ≥50 breaths·min−1, c) age 12–59 months ≥40 breaths·min−1, d) age ≥60 months ≥20 breaths·min−1; ƒ: some optional outcomes would require patient follow-up during hospitalisation, which will not be feasible in all surveillance settings; ##: vaccination status is depending on availability of vaccine and the type of vaccination (maternal, paediatric, etc.).
In community surveillance systems that are currently reporting and sampling patients who present with ARI in a systematic manner, we recommend leveraging the system to include virological testing of specimens for RSV (including from infants) if these components are not currently in place. Most commercial PCR panels for respiratory testing already include RSV. Recommendations on additional information to collect are presented in table 2. For those systems that currently report and sample patients with ILI symptoms, the following changes are recommended: 1) expand reporting and sampling to the broader ARI case definition and 2) collect additional data on symptoms if not already done. Patients who are sampled on the broader ARI case definition need to have individual symptoms recorded so that ILI cases can still be extracted from this national case-based dataset. When possible, both ILI and ARI incidence in the community should be reported. This sampling strategy meets the WHO RSV community surveillance guidelines and does not change influenza surveillance according to the present ILI definition. Another advantage of this approach is that influenza cases without typical ILI symptoms, although a minority [26, 41], will also be identified. A practical recommendation when resources do not allow enhanced sampling of ARI patients is to continue sampling patients presenting with ILI but expand to the broader ARI case definition in the age groups with the highest RSV burden, i.e. the youngest (<2 years) and oldest (≥65 years) age groups, and to record individual symptoms so that ILI cases can be derived from these data.
Passive surveillance using RSV laboratory surveillance databases
Reporting cases through a passive surveillance structure means that there is no active case finding and systematic sampling involved, but that cases are recorded through laboratory and/or clinical coding systems. These cases have generally been tested for RSV for clinical reasons, or have been coded as RSV cases based on clinical diagnosis.
For passive RSV surveillance, laboratory registry data on RSV testing is the recommended surveillance system. A sustainable, feasible model of an RSV laboratory surveillance database includes the following minimum data elements: an accurate record of the date of sample, patient information (patient identification, date of birth and/or age, sex) and testing information (test type and result, RSV type and the healthcare setting from where the sample was taken) (table 3). As a minimum, these reports should include weekly aggregated data on total number of RSV tests and RSV-positive laboratory tests, stratified by age group (table 3). Negative laboratory results provide an exact denominator of number of tested together with number of positive specimens and this allows for a more accurate interpretation of trends in RSV positivity than recording the number of RSV-positive tests alone [42]. Similar to active surveillance, we recommend adopting the age groups specified above if individual month/year of age cannot be collected. Although other types of registries could be used to identify RSV-related healthcare episodes, such as hospital admission or GP registries [43], we currently do not recommend these as stand-alone sources for RSV surveillance owing to the high variation in quality of diagnostic coding within these types of administrative data and the potential for misclassification bias [20, 44, 45]. The use of International Classification of Diseases 10th revision (ICD10) codes for capturing RSV cases is being assessed [43] and exploring the use of ICD10 codes for RSV surveillance is also one of the goals of the WHO RSV surveillance phase 2 pilot [15].
TABLE 3.
Optimal data elements to be collected on all RSV laboratory tests in an RSV laboratory surveillance dataset and core data on RSV-positive laboratory tests to be reported as a minimum
| Core data elements to be collected | Minimum reported data |
| Patient ID and/or personal identifier | |
| Date of birth and/or age at sampling | Minimum: age group#,¶ Preferably:
|
| Date of sample | ISO calendar week and year of sample |
| Sex | Female/male/other/unknown |
| Reporting laboratory/site | Data source+ or laboratory ID |
| Test type | PCR/antigen/rapid test/etc. |
| Test result | Positive/negative |
| RSV type | A/B/untyped |
| Healthcare setting | Hospital/ICU/GP/unknown |
RSV: respiratory syncytial virus; ID: identity; ISO: International Organization for Standardization; ICU: intensive care unit; GP: general practitioner. #: for the oldest age groups, a category such as 90+ years may be required depending on the size of the demographic strata for reported data to be anonymised; ¶: if strata are too small, age groups (<3 months, 3–5 months, 6–11 months, 12–23 months) could be used; +: data source is a more comprehensive description of surveillance system where multiple variables (e.g. geographical coverage, population, active/passive, sentinel/comprehensive) within data source need to be defined; this is reported only when specific surveillance type is started or if there are changes to the system.
Virological considerations and recommendations for RSV detection and characterisation
Laboratory confirmation of clinically suspected RSV cases is essential for the accuracy and validity of any surveillance system. Two critical factors influence the sensitivity of virus detection: sufficient and appropriate specimen sampling, and timing of sampling as compared to the onset of disease (box 3). Four modalities of tests are being used for RSV detection: molecular detection using nucleic acid amplification (PCR) techniques, direct or indirect immunofluorescence assay (DFA/IFA), rapid antigen detection tests (RADT) and virus culture [46, 47]. While virus culture is still required for studies of phenotypic properties of the virus, it is no longer used as a primary diagnostic tool because of its complexity and long assay duration. Instead of virus culture, quantitative reverse transcription PCR (qRT-PCR)-based assays are currently the gold standard and are in widespread use. Despite being less specific and sensitive than qRT-PCR-based assays, RADTs are still used because of lower costs and less requirement in terms of time, expertise and maintenance compared with qRT-PCR. Serology as a diagnostic tool for use in surveillance is not mentioned here because it is only useful for sero-epidemiological studies and research purposes and not for diagnosis of an acute RSV infection [47]. Genetic characterisation of RSV by direct sequencing of sub-genomic regions or full genomic sequencing will be an important part of RSV surveillance to monitor potential antigenic changes in the circulating viruses that might affect the efficacy of future immunisation strategies.
BOX 3. Summary of recommendations on specimen collection, transport and storage
-
1)
Specimen collection: all eligible patients or systematic selection
-
2)
Timing of specimen collection: • routine diagnostics: specimen should be collected preferably in the first 4 days following onset of disease • hospital setting: specimens should be collected as soon as possible after admission
-
3)
Site of sampling: • nasopharyngeal specimens give the best sensitivity • routine diagnostics: follow guidelines of World Health Organization respiratory syncytial virus surveillance initiative including upper and lower respiratory tract sampling • surveillance purpose: only less invasive upper respiratory tract sampling should be considered to encourage patient participation
-
4)
Sampling and transport: • use flocked swab with a plastic shaft • send specimens to the laboratory as soon as possible after sampling, preferably the same day • store specimens at ∼4°C until transport • transport at ambient temperature if temperature <25°C; if ambient temperature is higher, transport at ∼4°C
Specimen collection, transport and storage
For virological surveillance, specimens could either be collected from all eligible patients or a subset of these patients. The number of specimens needed will depend on the surveillance objectives and can be calculated using e.g. the ARI incidence, the total population size and the expected RSV positivity [48, 49]. If it is not feasible or necessary to test all eligible patients, patients should be selected on a systematic basis defined a priori (figure 1), e.g. the first predefined number of patients per week, or every second patient. For further sequencing, we recommend a minimum of 10% of the detected viruses with a minimum of 20 randomly selected per RSV type per country or institute per season; if possible, these should be randomly selected from each age group <3 months, 3–5 months, 6–11 months, 12–23 months, 2–4 years, 5–14 years, 15–64 years and 65+ years.
Timing of sampling in relation to day of onset of disease greatly impacts the chances of a correct laboratory diagnosis. The duration of RSV shedding in an outpatient setting is an average of 9.8±4.8 days for adults [50] and can be even longer (up to 30 days) in children (especially of very young age) [51] and immunocompromised patients [52]. Therefore, patient age and condition as well as time of sampling from onset of disease should be taken into account when interpreting diagnostic results. The effect of shedding patterns on confirmation of the presence of RSV in a clinical specimen depends on the technique used. The number of positive samples drops more rapidly with time since onset of disease using antigen detection compared to qRT-PCR, indicating that the sensitivity of antigen detection is only high during the first few days after onset of disease [53]. For highest sensitivity in any test, we recommend specimen collection preferably in the first 4 days following onset of disease for routine diagnostics. However, collection can reliably be done up to 10 days following onset of disease or even longer (taking into account assay-type sensitivity as well as patient age and condition-specific limitations that influence the shedding period) [50–53]. In the hospital setting, specimens should be collected as soon as possible after admission.
The anatomical site from which specimens are collected is also important for the sensitivity of diagnostic laboratory tests. Nasopharyngeal swabs are more sensitive than oropharyngeal swabs owing to a higher viral load in the nasopharynx than the oropharynx [54]. Also, nasopharyngeal specimens seem to have greater sensitivity for RSV than mid-turbinate specimens owing to the higher number of cells collected [55, 56]. Therefore, we recommend only using nasopharyngeal swab for surveillance purposes. Although the WHO RSV surveillance initiative recommends upper respiratory tract and the more invasive lower respiratory tract for sampling (supplementary table S1 [57, 58]), using the less invasive nasopharyngeal swab only for surveillance purposes [54, 59] may be beneficial and lead to higher acceptance among participants. For the youngest children, mid-turbinate sampling rather than nasopharyngeal sampling might be less challenging. For routine diagnostics, we recommend following the guidelines for age group-specific optimal sampling of anatomical sites and type of clinical specimens provided by the WHO RSV surveillance initiative [57].
Flocked swabs are slightly preferable to rayon swabs because they are more efficient in collecting infected epithelial cells [55, 60]. This is of benefit for molecular techniques [60] and antigen detection, but especially for DFA/IFA [55]. Swabs with a cotton tip, calcium alginate-aluminium swabs and swabs with a wooden shaft should not be used because of inhibition of PCR and/or virus isolation [61, 62]. Therefore, we recommend flocked swabs with a plastic shaft. The transport medium should enhance the preservation of virus infectivity and RNA integrity and prevent the overgrowth of bacteria during transport [63]. Transport and storage of specimens should also take into account the subsequent analysis type because conditions for molecular and antigen detection are less critical than for virus isolation [63–65]. Regarding viral transport medium, we recommend following WHO guidelines, which include guidance for commercial and in-house laboratory developed tests [66]. We recommend sending specimens to the laboratory as soon as possible after sampling and preferably the same day or the next day, at the latest. Specimens should be stored at 4°C until transport to prevent viral RNA degradation. For virus isolation, the specimen should ideally be transferred immediately to the laboratory and inoculated on cells, where applicable. If the specimen needs to be transported, it should be kept at 4°C at all times and the time between specimen collection and transport should ensure same-day arrival at the laboratory and testing and/or inoculation on cells [64, 67]. Transport of specimens for routine testing can be done at ambient temperature in regions with a temperate climate. When the ambient temperature exceeds 25°C, transport should ideally be done at 4°C. Upon arrival at the laboratory, the specimen should be aliquoted, with one aliquot kept at 4°C for testing within 1–3 days and the other aliquots stored at −70°C or lower for future testing. As a guideline, at least the RSV-positive specimens should be stored in the freezer until genetic characterisation for a season is completed. A subset of sequenced clinical specimens should be stored for a longer time as reference material, with the duration dependent on available freezer capacity. If specimens need to be stored for future virus isolation, an infectivity preservative should be added first and freezing avoided [65]. Cultured viruses should be stored in a biobank for antigenic characterisation and as reference material, with the duration dependent on available freezer capacity.
Detection methods
Molecular detection
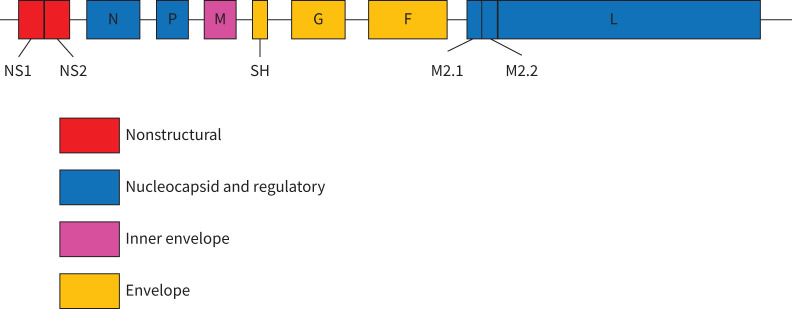
The landscape of molecular detection assays in use for RSV diagnostics is illustrated by the results of three RSV external quality assessment (EQA) programmes of Quality Control for Molecular Diagnostics (QCMD) carried out in 2018 (results used with permission from QCMD). For the three programmes, 118, 89 and 86 datasets were reported by 101, 72 and 72 laboratories, respectively, and this exercise described the current practice of specialist and general clinical laboratories worldwide. Of these laboratories, 63–72% used commercial molecular detection assays and 28–37% used in-house-developed molecular assays. Commercial assays included RSV-specific assays and multiplex respiratory panel assays including RSV. Some of these assays are considered molecular point-of-care tests (mPOCT), which are increasingly being used, especially for emergency room testing. Only 21–29% of laboratories reported typing information, indicating that the majority of tests did not differentiate between RSV-A and RSV-B. The majority of assays used (47–72%) targeted the nucleoprotein (N) gene (figure 2 for the RSV genomic overall structure for genes coding the indicated proteins). Other genes targeted were the matrix (M) protein gene (10–22%) and the fusion glycoprotein (F) gene (6–12%) or genes coding for the large polymerase subunit (L), nonstructural protein-1 (NS1), nonstructural protein-2 (NS2), the M2-2 transcription factor and phosphoprotein (P) (each <3%). Despite this diversity in the targeted genes, there were no differences in the ability to detect and differentiate RSV-A and RSV-B and no obvious differences in sensitivity. However, at least an annual review of primers and probes against available sequence data is needed because ongoing evolution may lead to mutations in primer and probe target sites and subsequently to reduced sensitivity and under-recognition [68, 69]. As for other viral RNA detection assays, virus controls should be updated frequently to include new emerging variants that may affect assay performance.
FIGURE 2.
Respiratory syncytial virus genomic overall structure of genes coding for proteins. NS: nonstructual protein; SH: small hydrophobic protein.
Antigen detection
RSV antigen detection by RADT through antigen capture and by DFA/IFA through antigen detection in infected cells by monoclonal antibodies are both less sensitive than qRT-PCR [70]. They suffer from higher false positive results due to cross-reactivity with similar proteins of related viruses, and higher false negative results mostly due to antigenic variation among viruses [71]. The protein most often targeted is the F-protein, but the N-protein and G-protein are also used. Mutations in the genes coding for these proteins may result in changes in antigenic epitopes used by the detecting antibodies. A recent study concluded that for optimal development of monoclonal antibodies, only selected regions of F and N should be used and combined with selected regions of G. This is because F and N of RSV and of human metapneumovirus are highly related, which can cause false positivity [71]. Indeed, this type of targeted development of monoclonal antibodies, although against other proteins, resulted in higher sensitivity and specificity in the ELISA format [72]. Nevertheless, the key advantage of RADT (its faster turnaround time) has been challenged by mPOCTs, which are increasingly used in clinical laboratories and which provide results in a turnaround time comparable to RADT, but with the performance of qRT-PCR [73].
Virus isolation
RSV is a virus that rapidly loses infectivity if not appropriately treated after a specimen has been collected. Increased temperature, freeze–thaw cycles and changes in pH have a detrimental effect on viral infectivity [64]. Immediate inoculation of cells, appropriate specimen collection and addition of phosphate sucrose to preserve infectivity in storage medium improve the success of virus isolation [65, 67]. The most commonly used cell line for RSV isolation from clinical specimens is HEp-2, although A549 cells are also widely used [46]. Although virus isolation as a diagnostic test has been largely replaced by molecular and antigenic tests [46], cultivation is still needed to obtain viruses for phenotypic analysis (e.g. for analysing susceptibility to vaccine-induced neutralising antibodies, antiviral susceptibility and antigenic likeness with vaccine strains) and as controls for other assay types.
Based on these data, we have three recommendations for RSV detection, listed in box 4.
BOX 4. Summary of detection recommendations
Given the strengths and weaknesses of the methods for respiratory syncytial virus (RSV) detection in clinical specimens described above, we recommend:
-
1)
quantitative reverse transcriptase PCR (either in-house, commercial or in molecular point-of-care test format) as the standard detection method: a) able to detect both RSV types A and B and optimally also distinguishes between types A and B; b) ideally, targets at least two of the highly conserved genes as N, P, M or L;
-
2)
pre-seasonal review of primers and probes against sequences of recent circulating strains;
-
3)
annual evaluation of assay performance by external quality assessment.
Genotyping
Sequencing of the RSV genome or sub-genomic regions serves different purposes: describing the genetic evolution and global spread of RSV [74, 75], examining the association of genotypes with severity of disease [76, 77] and monitoring the evolution of proteins that are targets for antigen detection and vaccines (active and passive) and antivirals under development [78, 79]. In particular, any possible changes in the virus that may be accelerated by implementing an immunisation programme should be carefully identified and followed up.
Recent studies with full RSV genomes show a complexity of RSV evolution that has not been captured previously with sequencing of sub-genomic regions [74, 80]. Comparison of different studies is complicated by the lack of standardisation of the nomenclature for RSV strains and genomic clades and of criteria for assigning genomic clades. Indeed, combined analysis of sequences (G-protein gene as well as full genome) from different studies assigned viruses with different country-specific clade nomenclature to the same clade [74, 81]. Whole genomes show that RSV circulates on a global scale, with the same predominant clades of viruses found in countries around the world [74]. This global analysis also showed that complete G-protein gene sequences, but no other genes or the widely used partial G-protein gene sequences, generated similar phylogenetic topology compared to whole genomes [74]. Therefore, consensus over sequencing sub-genomic regions and criteria and nomenclature for genomic clades is needed to maximise the ability to share sequence data for merged analyses. Furthermore, sequence sharing should be facilitated by the development of a global curated database dedicated to RSV, similar to the GISAID database for influenza. Whole genome sequencing should be performed, preferably for at least a representative subset, and if that is not feasible, for full G-protein gene for phylogenetic analysis. In addition, we consider sequencing of the F-protein gene to be highly relevant because the F-protein demonstrates significant variability [82] and is targeted by several promising vaccines under development and by the therapeutic monoclonal antibodies either existing or under development, as well as by antivirals [10]. Recommendations for genotypic characterisation are summarised in box 5.
BOX 5. Summary of genotyping
As guidance for representative sequencing, we recommend:
-
1)
sequencing of the whole G-protein gene as a minimum or if possible full genomes for molecular epidemiology and analysis of potential impact of amino acid changes on epidemiology and severity of disease
-
2)
sequencing of the F-protein gene, at a minimum covering antigenic sites Ø and II for analysing the potential impact of amino acid changes on antigenicity
-
3)
sequencing of 10% of detected viruses at minimum with a minimum of 20 randomly selected per respiratory syncytial virus type per country or institute per season; if possible, samples should be randomly selected from each age group <3 months, 3–5 months, 6–11 months, 12–23 months, 2–4 years, 5–14 years, 15–64 years and 65+ years.
Phenotypic characterisation
The study of phenotypic properties is necessary to understand the impact of genetic diversification on e.g. virus replication [75] and proposed effectiveness of immunisation [79] and antivirals [78]. Pre- and post-vaccination serum specimens are needed to analyse the protective antibody response following vaccination in addition to analysing the availability of recently circulating strains [83]. Antigenic characterisation can be performed using neutralisation assays in cell culture systems.
External quality assessment
EQA is an important mechanism for assessing the quality of a laboratory's performance in detecting RSV, even when these laboratories use a wide variety of molecular techniques [45]. However, strains used in EQA panels are often outdated or not characterised [84]. Recent changes in RSV that may affect the sensitivity of tests or even their ability to detect new strains [69] may not be covered by EQA schemes, providing false confidence in the performance of used tests. One of the objectives of the second phase of the WHO RSV pilot is to perform an RSV detection and typing EQA using molecular diagnostics with a panel composition that takes these considerations into account [85]. EQA schemes for RSV isolation, DFA/IFA or RADT are not widely available. However, several national schemes offer such specialised EQAs (e.g. [86]). Increased use of sequence analysis including next-generation sequencing (NGS) techniques necessitates the establishment of an EQA scheme for NGS-based and full genome analysis [87]. With immunisation strategies on the horizon, EQA for characterisation of RSV antigenic drift may become relevant in the near future.
Ethical and governance considerations
When setting up or altering RSV surveillance systems, public health institutes and national governments need to be aware of the legal and ethical considerations of surveillance systems. WHO recently published guidelines on the obligations that (public health) institutes, countries and global communities have to ensure that surveillance will be well conducted in terms of privacy, autonomy, equity and the common good [88]. At the European level, the new EU General Data Protection Regulation (GDPR) regulates the processing of personal data relating to individuals by an individual, a company or an organisation [89]. Personal data that are identifiable or pseudonymised and therefore theoretically traceable are all within the scope of the GDPR. Only data that are irreversibly anonymised are not considered as personal data [89]. This is of importance both at the national level and when sharing data at the European or global level. One of the crucial factors is whether RSV surveillance falls under the umbrella of lawful purposes, which depends, among other factors, on the decision of the European Commission to add RSV to the list of reportable diseases at EU level. At the national level, practical interpretation of the GDPR will be slightly different across countries, depending on national legislation.
Discussion
In this article we provide suggested guidelines to prioritise and shape new and enhanced RSV surveillance systems, building on the recommendations developed in 2006 by the European Influenza Surveillance System [17] and considering the findings of the WHO RSV surveillance pilot [14, 29, 40]. Minimum dataset requirements are outlined to allow comparison of a core dataset at the European level. We also propose recommendations for optimal requirements, where feasible, for data collection and reporting on a national level and/or EU level. Furthermore, we propose recommendations for optimal diagnostics to support sensitive surveillance of RSV. These include the best respiratory tract sampling site and procedure, optimum time period after onset of diseases for specimen collection, optimal specimen transport conditions, most sensitive techniques for virus detection and external quality assessment procedures. Because resources for surveillance are limited, assessing trends and seasonality (objective 1) are the minimum requirements for sustainable and feasible surveillance on a European scale. Depending on available resources and the healthcare system within each country, either active sentinel surveillance or passive laboratory register surveillance could be applied to achieve this. Second, setting up a platform to assess the impact of immunisation (objective 6) is highly relevant in countries that may be introducing immunisation strategies into national programmes, given the current developments regarding candidate RSV vaccines and monoclonal antibodies [90]. However, a surveillance system in which the impact of the programme and immunisation/vaccine effectiveness can be assessed will require more extensive development, in terms of both patient numbers and the information required per patient. A surveillance system that is set up to assess the impact of immunisation programmes would be more beneficial if it additionally covers other RSV surveillance objectives, as described in this manuscript. It will be important to harmonise data collection for impact assessment in different countries, so that data can theoretically be pooled as is done for influenza vaccine effectiveness by the I-MOVE project [91, 92]. Adding sequence data will be important to interpret vaccine effectiveness outcomes correctly or even stratify vaccine effectiveness according to emerging clades with altered antigenic sites.
We suggest using the extended SARI case definition, instead of the SARI case definition as used in influenza surveillance. Although it will be less informative to compare extended SARI RSV and SARI influenza incidences in hospital, this extended SARI definition will be more sensitive for capturing RSV cases.
Integration of RSV surveillance into other respiratory surveillance systems, as recommended by the ECDC advisory forum (personal communication, E. Broberg) and the WHO [14], should make RSV surveillance more feasible. The use of the ECDC ARI and extended SARI case definitions, as suggested here, should allow future extension of surveillance with other pathogens if necessary, by assessing those pathogens in the same specimens.
Which of the surveillance components discussed here can be best applied in a country will also depend on the national healthcare system and the healthcare-seeking behaviour of different population strata. In some countries, parents will be more likely to visit emergency departments of hospitals with symptomatic children than primary care. In others, working-age adults will seek primary care for insurance purposes. Implementation should, therefore, be seen in the context of other existing or future surveillance activities, such as laboratory or hospital-based surveillance. Hospital-based surveillance for RSV is currently not implemented in many countries in Europe [18], and could first be piloted at a limited number of sentinel sites in a few countries to identify challenges and barriers to implementation before being scaled up to national level throughout Europe.
In general, the preferred RSV surveillance system will be active sentinel surveillance, with both primary care and hospital patients being systematically sampled and tested for RSV. One important limitation of this surveillance system is that the use of the ARI case definition may increase the burden and will be a major change for physicians, who often have participated in the existing surveillance networks of ILI for a long time. These two components may compromise influenza surveillance and this should be monitored carefully. However, according to the first results of the WHO pilot, combining RSV and influenza surveillance into one system actually appears beneficial for both systems [14]. Furthermore, the cost and effort to add RSV as a component to this surveillance were reported to be marginal and incremental [14]. Coordinated planning should also consider the need for severe acute respiratory syndrome coronavirus 2019 (COVID-19) surveillance, which has been included in sentinel influenza surveillance schemes in many countries during 2020. To assess the total burden of RSV, monitoring and sampling of community patients with otitis media, which poses a substantial socioeconomic as well as healthcare burden, could be additionally considered. Because this is a sequela of RSV-associated ARI, this is better captured outwith an ARI- or SARI-based surveillance through well-designed prospective clinical studies.
Surveillance in ICUs could be considered, as part of total hospital surveillance or stand alone, using the same extended SARI case definition. Specific surveillance on neonatal or paediatric ICUs would, however, be needed to cover the lowest age group.
The benefits of using passive surveillance of RSV, via laboratory database surveillance, are that it is nationally representative and a relatively inexpensive strategy compared to active surveillance, once set up [28, 93]. Furthermore, inclusion of a personal identifier within the laboratory surveillance dataset, where feasible, allows linkage to other national databases such as clinical data or vaccination or immunisation registries. This will likely not facilitate real-time surveillance, but would allow secondary research where appropriate [93]. Measures to ensure data privacy would be necessary to allow data linkage. Introducing RSV to the list of notifiable diseases (e.g. by laboratories) could be an alternative method of providing the number of positive RSV cases per age group to cover the minimum reported data.
However, laboratory database-based surveillance also has limitations. First, minimal or no clinical data are available and variation or changes in policy, health service capacity, healthcare-seeking behaviour and testing practices cannot be controlled for unless negative tests and clinical data are also recorded. Second, while many countries in the Northern, Central and Western European regions have established national electronic healthcare databases, many countries in Eastern Europe have not [19]. A lack of resources to set up such a national registry is likely to limit the capacity to set up an RSV laboratory surveillance database [94]. Similarly, it is difficult to capture clinical information within the surveillance database without requiring additional resources from the reporting laboratories. Furthermore, the increasing use of mPOCT in hospitals, often without involvement of the laboratory [95], may greatly affect the number of cases reported to national public health institutes, especially if the levels of recording in clinical records, reporting to laboratories and the registration of negative test results are unknown. Finally, if patient identifiers (or patient identifiable information) are not included in the database, it is not possible to carry out de-duplication and individual-level analysis, or linkage to other existing, structured datasets containing clinical information. This linking of clinical data with laboratory information is important to support research on the burden of RSV and cost-effectiveness analysis of future RSV immunisation strategies.
Critical for the ascertainment of a laboratory-confirmed case of RSV infection is optimal sampling and transport of specimens as outlined in box 3. For surveillance purposes we recommend using a nasopharyngeal swab only, whereas the WHO initiative recommends collection of upper respiratory and lower respiratory specimens as well (supplementary table S1 [57, 58]). In our opinion a slightly lower sensitivity when using upper respiratory tract nasopharyngeal swabs is only acceptable if this significantly reduces the rate of refusals of, in particular, parents to have their sick children sampled. We recommend the use of qRT-PCR or its mPOCT equivalent for the most sensitive detection of RSV. Harmonising this approach by the use of one type and brand of test by all surveillance sites is not recommended because it is not practical, and may lead to delays in recognising when there are issues with assay sensitivity/specificity or other test failures for whatever reason [69]. Therefore, the use of a diverse palette of clinically well-validated and well-performing tests (despite being variable in design) is preferable. However, quality should be assessed annually by EQA and primers and probes checked for fit with recent circulating strains. For commercially available tests, the manufacturer is responsible for the latter if the manufacturer does not release primer and probe information.
Conclusions
To facilitate countries establishing or upgrading existing RSV surveillance, we propose three different types of surveillance: active sentinel community surveillance, active sentinel hospital surveillance and passive laboratory surveillance, considering ethical and policy-related issues. Based on current diagnostics, we propose the use of qRT-PCR-based assays as the standard detection method for RSV and virus genetic characterisation, if possible, to monitor genetic evolution. These guidelines should provide the basis for a feasible, affordable and robust RSV surveillance system for RSV in Europe and beyond: it offers a unique platform for comparison of RSV activity, virological features and disease burden locally, nationally and across country borders. This represents a possible solution to the unmet need for estimating RSV healthcare burden and provides the basis for an approach for assessing the impact of future immunisation programmes.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03766-2020.SUPPLEMENT (41.8KB, pdf)
Shareable PDF
Acknowledgements
We thank QCMD for the opportunity to get insight into the RSV EQA 2018 supplementary report and for providing additional data. We thank Maria Zambon and Joanna Ellis (WHO reference laboratory for RSV at Public Health England, Collindale, UK) for their input to the standardised virology component of RSV surveillance in the European region. We thank Pasi Penttinen (ECDC, Stockholm, Sweden) for valuable comments during the process of writing the manuscript and for critically reviewing the pre-final version.
The content of the workshop on RSV surveillance was organised by SSI, RIVM and ECDC. All participants, except E.K. Broberg and S.S. Hirve, were refunded for travel and hotel costs by the Respiratory Syncytial Virus Consortium in Europe (RESCEU). RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement 116019. This Joint Undertaking receives support from the EU's Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations. This workshop was only attended by publicly funded participants of academic and public health bodies. No industrial partners participated in the meeting or were involved in writing this manuscript. S.S. Hirve was funded through WHO, E.K. Broberg through ECDC and A. Stuwitz Berg through the Norwegian Institute of Public Health.
The institutions of the following co-authors are partners in RESCEU: National Institute for Public Health and the Environment, RIVM, the Netherlands (A.C. Teirlinck, A. Meijer); Statens Serum Institute, SSI, Denmark (T.K. Fischer, R. Trebbien and H-D. Emborg); University of Edinburgh (H. Nair, H. Campbell and R.M. Reeves).
The institutions of the following co-authors are affiliated partners in RESCEU: Norwegian Institute of Public Health (A. Stuwitz Berg and H. Bøås); Nivel, the Netherlands (J. Paget), Finnish Institute for Health and Welfare THL (H. Nohynek).
Footnotes
This article has supplementary material available from erj.ersjournals.com
Disclaimer: The views and opinions expressed herein are the authors’ own and do not necessarily state or reflect those of ECDC. ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn.
Author contributions: A.C. Teirlinck, T.K. Fischer and E.K. Broberg organised the meeting and had the lead in organising the manuscript and preparing the first draft. A. Stuwitz Berg, H. Campbell, A. Meijer and R.M. Reeves wrote, based on the working group discussions, sections of the manuscript on RSV surveillance in hospitals, RSV surveillance in the community, virology aspects of RSV surveillance and RSV surveillance using national registries, respectively. All authors critically reviewed the final manuscript and agreed on publication.
Conflict of Interest: A.C. Teirlinck reports grants from Innovative Medicines Initiative, during the conduct of the study.
Conflict of interest: E.K. Broberg has nothing to disclose.
Conflict of interest: A. Stuwitz Berg has nothing to disclose.
Conflict of interest: H. Campbell reports grants from Innovative Medicines Initiative, and grants and personal fees (paid via the university) from WHO, Bill and Melinda Gates Foundation and Sanofi, during the conduct of the study.
Conflict of interest: R.M. Reeves reports grants from Innovative Medicines Initiative, during the conduct of the study.
Conflict of interest: A. Carnahan has nothing to disclose.
Conflict of interest: B. Lina has nothing to disclose.
Conflict of interest: G. Pakarna has nothing to disclose.
Conflict of interest: H. Bøås has nothing to disclose.
Conflict of interest: H. Nohynek reports grants from GSK, SanofiPasteur and Pfizer (to their institute THL, not their unit), outside the submitted work; and membership of the ESWI Scientific Committee.
Conflict of interest: H-D. Emborg reports grants from Innovative Medicines Initiative (grant agreement 116019), during the conduct of the study.
Conflict of interest: H. Nair reports grants from Innovative Medicines Initiative, during the conduct of the study; grants and personal fees from Bill and Melinda Gates Foundation, World Health Organization and Sanofi, and personal fees from Janssen and Abbvie, outside the submitted work.
Conflict of interest: J. Reiche has nothing to disclose.
Conflict of interest: J.A. Oliva has nothing to disclose.
Conflict of interest: J.O. Gorman has nothing to disclose.
Conflict of interest: J. Paget reports grants from Sanofi Pasteur, WHO, and Foundation for Influenza Epidemiology, outside the submitted work.
Conflict of interest: K. Szymański has nothing to disclose.
Conflict of interest: K. Danis has nothing to disclose.
Conflict of interest: M. Socan has nothing to disclose.
Conflict of interest: M. Gijon has nothing to disclose.
Conflict of interest: M. Rapp has nothing to disclose.
Conflict of interest: R. Trebbien reports grants from Innovative Medicines Initiative, during the conduct of the study.
Conflict of interest: R. Guiomar has nothing to disclose.
Conflict of interest: S.S. Hirve has nothing to disclose.
Conflict of interest: S. Buda has nothing to disclose.
Conflict of interest: S. van der Werf reports non-financial support from RESCEU (travel and hotel costs to attend the workshop on RSV surveillance), during the conduct of the study and is a Board member of ISIRV.
Conflict of interest: A. Meijer reports grants from Innovative Medicines Initiative, during the conduct of the study.
Conflict of interest: T.K. Fischer has nothing to disclose.
Support statement: This work was supported by the Innovative Medicines Initiative (grant agreement 116019). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. doi: 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 3.Shi T, Ooi Y, Zaw EM, et al. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis 2020; 222: Suppl. 7, S628–S633. [DOI] [PubMed] [Google Scholar]
- 4.Sommer C, Resch B, Simoes EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J 2011; 5: 144–154. doi: 10.2174/1874285801105010144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222: Suppl. 7, S577–S583. [DOI] [PubMed] [Google Scholar]
- 6.Zwaans WA, Mallia P, van Winden ME, et al. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease – a systematic review. J Clin Virol 2014; 61: 181–188. doi: 10.1016/j.jcv.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7: e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5 [DOI] [PubMed] [Google Scholar]
- 8.Broberg EK, Waris M, Johansen K, et al. Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Euro Surveill 2018; 23: 17-00284. doi: 10.2807/1560-7917.ES.2018.23.5.17-00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom-Feshbach K, Alonso WJ, Charu V, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One 2013; 8: e54445. doi: 10.1371/journal.pone.0054445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18: e295–e311. doi: 10.1016/S1473-3099(18)30292-5 [DOI] [PubMed] [Google Scholar]
- 11.Novavax . Novavax Announces Topline Results From Phase 3 PrepareTM Trial of ResVax™ for Prevention of RSV Disease in Infants via Maternal Immunisation. 2019. http://ir.novavax.com/news-releases/news-release-details/novavax-announces-topline-results-phase-3-preparetm-trial
- 12.Groothuis JR, Hoopes JM, Hemming VG. Prevention of serious respiratory syncytial virus-related illness. II: immunoprophylaxis. Adv Ther 2011; 28: 110–125. doi: 10.1007/s12325-010-0101-y [DOI] [PubMed] [Google Scholar]
- 13.Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9: eaaj1928. [DOI] [PubMed] [Google Scholar]
- 14.Broor S, Campbell H, Hirve S, et al. Leveraging the Global Influenza Surveillance and Response System for global respiratory syncytial virus surveillance – opportunities and challenges. Influenza Other Respir Viruses 2020; 14: 622–629. doi: 10.1111/irv.12672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . WHO RSV Surveillance Phase-2 (2019–2021). 2019. www.who.int/influenza/rsv/RSV_surveillance_phase2/en/Date last accessed: September 16, 2020.
- 16.Meerhoff TJ, Mosnier A, Schellevis F, et al. Progress in the surveillance of respiratory syncytial virus (RSV) in Europe: 2001–2008. Euro Surveill 2009; 14: 19346. [PubMed] [Google Scholar]
- 17.Meerhoff TJ, Fleming D, Smith A, et al. Surveillance recommendations based on an exploratory analysis of respiratory syncytial virus reports derived from the European Influenza Surveillance System. BMC Infect Dis 2006; 6: 128. doi: 10.1186/1471-2334-6-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollers M, Barnadas C, Broberg EK, et al. Current practices for respiratory syncytial virus surveillance across the EU/EEA Member States, 2017. Euro Surveill 2019; 24: 1900157. doi: 10.2807/1560-7917.ES.2019.24.40.1900157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacurariu A, Plueschke K, McGettigan P, et al. Electronic healthcare databases in Europe: descriptive analysis of characteristics and potential for use in medicines regulation. BMJ Open 2018; 8: e023090. doi: 10.1136/bmjopen-2018-023090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholls SG, Langan SM, Benchimol EI. Routinely collected data: the importance of high-quality diagnostic coding to research. CMAJ 2017; 189: E1054–E1055. doi: 10.1503/cmaj.170807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iezzoni LI. Assessing quality using administrative data. Ann Intern Med 1997; 127: 666–674. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00048 [DOI] [PubMed] [Google Scholar]
- 22.Safran C, Bloomrosen M, Hammond WE, et al. Toward a national framework for the secondary use of health data: an American Medical Informatics Association White Paper. J Am Med Inform Assoc 2007; 14: 1–9. doi: 10.1197/jamia.M2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Centre for Disease Control and World Health Organization-Europe . Flu News Europe. https://flunewseurope.org/Date last accessed: September 16, 2020.
- 24.Eur-Lex . Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on Serious Cross-border Threats to Health and Repealing Decision No 2119/98/EC Text with EEA Relevance. 2013. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013D1082Date last accessed: September 16, 2020.
- 25.European Centre for Disease Control . Workshop on Burden of RSV Disease in Europe: ECDC Expert Consultation Meeting Stockholm, November 23–24, 2015. https://ecdc.europa.eu/sites/portal/files/media/en/press/events/Documents/Meeting%20report%20ECDC%20RSV%20surv%20and%20burden%20of%20disease%20workshop%2023-24%20Nov.pdfDate last accessed: September 16, 2020.
- 26.Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis 2014; 209: 1873–1881. doi: 10.1093/infdis/jit839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha S, Pandey BG, Choudekar A, et al. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalisations among children in a rural community of northern India. J Glob Health 2015; 5: 010419. doi: 10.7189/jogh.05.020419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EU Commission . Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the Communicable Diseases and Related Special Health Issues To Be Covered by Epidemiological Surveillance as well as Relevant Case Definitions. 2018. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018D0945&qid=1618995914874Date last accessed: September 16, 2020.
- 29.Hirve S, Crawford N, Palekar R, et al. Clinical characteristics, predictors, and performance of case definition – interim results from the WHO global respiratory syncytial virus surveillance pilot. Influenza Other Respir Viruses 2020; 14: 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rha B, Dahl RM, Moyes J, et al. Performance of surveillance case definitions in detecting respiratory syncytial virus infection among young children hospitalized with severe respiratory illness—South Africa, 2009–2014. J Pediatric Infect Dis Soc 2019; 8: 325–333. [DOI] [PubMed] [Google Scholar]
- 31.Fleming DM, Elliot AJ, Cross KW. Morbidity profiles of patients consulting during influenza and respiratory syncytial virus active periods. Epidemiol Infect 2007; 135: 1099–1108. doi: 10.1017/S0950268807007881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . Global Epidemiological Surveillance Standards for Influenza. Geneva, World Health Organization, 2013. [Google Scholar]
- 33.Vega T, Lozano JE, Meerhoff T, et al. Influenza surveillance in Europe: establishing epidemic thresholds by the moving epidemic method. Influenza Other Respir Viruses 2013; 7: 546–558. doi: 10.1111/j.1750-2659.2012.00422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos LM, Teirlinck AC, Lozano JE, et al. Use of the moving epidemic method (MEM) to assess national surveillance data for respiratory syncytial virus (RSV) in the Netherlands, 2005 to 2017. Euro Surveill 2019; 24: 1800469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumeister E, Duque J, Varela T, et al. Timing of respiratory syncytial virus and influenza epidemic activity in five regions of Argentina, 2007–2016. Influenza Other Respir Viruses 2019; 13: 10–17. doi: 10.1111/irv.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Midgley CM, Haynes AK, Baumgardner JL, et al. Determining the seasonality of respiratory syncytial virus in the United States: the impact of increased molecular testing. J Infect Dis 2017; 216: 345–355. doi: 10.1093/infdis/jix275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . WHO Regional Office for Europe Guidance for Sentinel Influenza Surveillance in Humans. Copenhagen, World Health Organization Regional Office for Europe, 2011. [Google Scholar]
- 38.World Health Organization . A manual for estimating disease burden associated with seasonal influenza. Geneva, World Health Organization, 2015. [Google Scholar]
- 39.der Heiden MA, Köpke K, Buda S, et al. Estimates of excess medically attended acute respiratory infections in periods of seasonal and pandemic influenza in Germany from 2001/02 to 2010/11. PLoS One 2013; 8: e64593. doi: 10.1371/journal.pone.0064593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pebody R, Moyes. J, Hirve S,, et al. Approaches to use the WHO respiratory syncytial virus surveillance platform to estimate disease burden. Influenza Other Respir Viruses 2020; 14: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta V, Dawood FS, Rai SK, et al. Validity of clinical case definitions for influenza surveillance among hospitalized patients: results from a rural community in North India. Influenza Other Respir Viruses 2013; 7: 321–329. doi: 10.1111/j.1750-2659.2012.00401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reeves RM, Hardelid P, Gilbert R, et al. Epidemiology of laboratory-confirmed respiratory syncytial virus infection in young children in England, 2010–2014: the importance of birth month. Epidemiol Infect 2016; 144: 2049–2056. doi: 10.1017/S0950268816000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai W, Tolksdorf K, Hirve S, et al. Evaluation of using ICD-10 code data for respiratory syncytial virus surveillance. Influenza Other Respir Viruses 2020; 14: 630–637. doi: 10.1111/irv.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang KL, Lucyk K, Quan H. Coder perspectives on physician-related barriers to producing high-quality administrative data: a qualitative study. CMAJ Open 2017; 5: E617–E622. doi: 10.9778/cmajo.20170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benchimol EI, Manuel DG, To T, et al. Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol 2011; 64: 821–829. doi: 10.1016/j.jclinepi.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 46.Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev 2017; 30: 277–319. doi: 10.1128/CMR.00010-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popow-Kraupp T, Aberle JH. Diagnosis of respiratory syncytial virus infection. Open Microbiol J 2011; 5: 128–134. doi: 10.2174/1874285801105010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Association of Public Health Laboratories . Influenza Virologic Surveillance Right Size Sample Size Calculators. October 16, 2019. www.aphl.org/programs/infectious_disease/influenza/Influenza-Virologic-Surveillance-Right-Size-Roadmap/Pages/Calculator-A.aspxDate last accessed: September 16, 2020.
- 49.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open source epidemiologic statistics for public health. 2013. www.OpenEpi.comDate last accessed: November 5, 2019. Date last updated: April 6, 2013.
- 50.Walsh EE, Peterson DR, Kalkanoglu AE, et al. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 2013; 207: 1424–1432. doi: 10.1093/infdis/jit038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wathuo M, Medley GF, Nokes DJ, et al. Quantification and determinants of the amount of respiratory syncytial virus (RSV) shed using real time PCR data from a longitudinal household study. Wellcome Open Res 2016; 1: 27. doi: 10.12688/wellcomeopenres.10284.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson L, Brite J, Del Castillo M, et al. Comparison of respiratory virus shedding by conventional and molecular testing methods in patients with haematological malignancy. Clin Microbiol Infect 2016; 22: 380. doi: 10.1016/j.cmi.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shafik CF, Mohareb EW, Youssef FG. Comparison of direct fluorescence assay and real-time RT-PCR as diagnostics for respiratory syncytial virus in young children. J Trop Med 2011; 2011: 781919. doi: 10.1155/2011/781919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackenzie GA, Vilane A, Salaudeen R, et al. Respiratory syncytial, parainfluenza and influenza virus infection in young children with acute lower respiratory infection in rural Gambia. Sci Rep 2019; 9: 17965. doi: 10.1038/s41598-019-54059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daley P, Castriciano S, Chernesky M, et al. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J Clin Microbiol 2006; 44: 2265–2267. doi: 10.1128/JCM.02055-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wouters Y, Keyaerts E, Rector A, et al. Comparison of the Idylla Respiratory (IFV-RSV) panel with the GeneXpert Xpert(R) Flu/RSV assay: a retrospective study with nasopharyngeal and midturbinate samples. Diagn Microbiol Infect Dis 2019; 94: 33–37. doi: 10.1016/j.diagmicrobio.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization . Collection, Transport and Storage of Clinical Samples for RSV Surveillance Pilot. 2017. www.who.int/influenza/rsv/rsv_collection_transport_storage_samples/en/Date last updated: May 16, 2020. Date last accessed: September 16, 2020.
- 58.World Health Organization . WHO strategy to pilot global respiratory syncytial virus surveillance based on the global influenza surveillance and response system (GISRS). 2017. www.who.int/influenza/rsv/WHO_RSV_pilot_strategy_21112017.pdf?ua=1. Date last accessed: May 31, 2019.
- 59.Spencer S, Thompson MG, Flannery B, et al. Comparison of respiratory specimen collection methods for detection of influenza virus infection by reverse transcription-PCR: a literature review. J Clin Microbiol 2019; 57: e00027-19. doi: 10.1128/JCM.00027-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernes SS, Quarsten H, Hagen E, et al. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis 2011; 30: 159–165. doi: 10.1007/s10096-010-1064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nat Protoc 2014; 9: 2663–2681. doi: 10.1038/nprot.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wadowsky RM, Laus S, Libert T, et al. Inhibition of PCR-based assay for Bordetella pertussis by using calcium alginate fiber and aluminum shaft components of a nasopharyngeal swab. J Clin Microbiol 1994; 32: 1054–1057. doi: 10.1128/JCM.32.4.1054-1057.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson FB. Transport of viral specimens. Clin Microbiol Rev 1990; 3: 120–131. doi: 10.1128/CMR.3.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hambling MH. Survival of the respiratory syncytial virus during storage under various conditions. Br J Exp Pathol 1964; 45: 647–655. [PMC free article] [PubMed] [Google Scholar]
- 65.Howell CL, Miller MJ. Effect of sucrose phosphate and sorbitol on infectivity of enveloped viruses during storage. J Clin Microbiol 1983; 18: 658–662. doi: 10.1128/JCM.18.3.658-662.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization . Collecting, Preserving and Shipping Specimens for the Diagnosis of Avian Influenza A(H5N1) Virus Infection. Guide For Field Operations. October 2006. Annex 8. Viral Transport Media (VTM) 2006.www.who.int/ihr/publications/Annex8.pdf Date last accessed: May 31, 2019.
- 67.Bromberg K, Daidone B, Clarke L, et al. Comparison of immediate and delayed inoculation of HEp-2 cells for isolation of respiratory syncytial virus. J Clin Microbiol 1984; 20: 123–124. doi: 10.1128/JCM.20.1.123-124.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunson RN, Collins TC, Carman WF. Real-time RT-PCR detection of 12 respiratory viral infections in four triplex reactions. J Clin Virol 2005; 33: 341–344. doi: 10.1016/j.jcv.2004.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamau E, Agoti CN, Lewa CS, et al. Recent sequence variation in probe binding site affected detection of respiratory syncytial virus group B by real-time RT-PCR. J Clin Virol 2017; 88: 21–25. doi: 10.1016/j.jcv.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chartrand C, Tremblay N, Renaud C, et al. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol 2015; 53: 3738–3749. doi: 10.1128/JCM.01816-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Souza C, Zanchin NI, Krieger MA, et al. In silico analysis of amino acid variation in human respiratory syncytial virus: insights into immunodiagnostics. Mem Inst Oswaldo Cruz 2017; 112: 655–663. doi: 10.1590/0074-02760170013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.González LA, Vázquez Y, Mora JE, et al. Evaluation of monoclonal antibodies that detect conserved proteins from respiratory syncytial virus, metapneumovirus and adenovirus in human samples. J Virol Methods 2018; 254: 51–64. doi: 10.1016/j.jviromet.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 73.Banerjee D, Kanwar N, Hassan F, et al. Comparison of six sample-to-answer influenza A/B and respiratory syncytial virus nucleic acid amplification assays using respiratory specimens from children. J Clin Microbiol 2018; 56: e00930-18. doi: 10.1128/JCM.00930-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bose ME, He J, Shrivastava S, et al. Sequencing and analysis of globally obtained human respiratory syncytial virus A and B genomes. PLoS One 2015; 10: e0120098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elawar F, Griffiths CD, Zhu D, et al. A virological and phylogenetic analysis of the emergence of new clades of respiratory syncytial virus. Sci Rep 2017; 7: 12232. doi: 10.1038/s41598-017-12001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinello RA, Chen MD, Weibel C, et al. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis 2002; 186: 839–842. doi: 10.1086/342414 [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez-Fernandez R, Tapia LI, Yang CF, et al. Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J Infect Dis 2017; 217: 24–34. doi: 10.1093/infdis/jix543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heylen E, Neyts J, Jochmans D. Drug candidates and model systems in respiratory syncytial virus antiviral drug discovery. Biochem Pharmacol 2017; 127: 1–12. doi: 10.1016/j.bcp.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 79.Melero JA, Mas V, McLellan JS. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 2017; 35: 461–468. doi: 10.1016/j.vaccine.2016.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schobel SA, Stucker KM, Moore ML, et al. Respiratory syncytial virus whole-genome sequencing identifies convergent evolution of sequence duplication in the C-terminus of the G gene. Sci Rep 2016; 6: 26311. doi: 10.1038/srep26311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tapia LI, Shaw CA, Aideyan LO, et al. Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLoS One 2014; 9: e90786. doi: 10.1371/journal.pone.0090786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mas V, Nair H, Campbell H, et al. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine 2018; 36: 6660–6673. doi: 10.1016/j.vaccine.2018.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev 2000; 13: 1–15. doi: 10.1128/CMR.13.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meerhoff TJ, MacKay WG, Meijer A, et al. The impact of laboratory characteristics on molecular detection of respiratory syncytial virus in a European multicentre quality control study. Clin Microbiol Infect 2008; 14: 1173–1176. doi: 10.1111/j.1469-0691.2008.02100.x [DOI] [PubMed] [Google Scholar]
- 85.World Health Organization . WHO Meeting to Launch Phase-2 of the RSV Surveillance Pilot Based on the Global Influenza Surveillance and Response System. 2019. www.who.int/influenza/rsv/who_rsv_surveillance_2nd_phase/en/Date last updated: May 14, 2020. Date last accessed: September 16, 2020.
- 86.Labquality . Annual EQA Programme. www.labquality.fi/en/external-quality-assessment/annual-eqa-programme/ Date last accessed: October 8, 2019.
- 87.Gargis AS, Kalman L, Lubin IM. Assuring the quality of next-generation sequencing in clinical microbiology and public health laboratories. J Clin Microbiol 2016; 54: 2857–2865. doi: 10.1128/JCM.00949-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Health Organization . WHO guidelines on ethical issues in public health surveillance. Geneva, World Health Organization, 2017. [Google Scholar]
- 89.EU Commission . 2018 Reform of EU Data Protection Rules. 2018. https://ec.europa.eu/info/law/law-topic/data-protection/eu-data-protection-rules_en Date last accessed: April 21, 2021.
- 90.PATH . RSV Vaccine and mAb Snapshot. www.path.org/resources/rsv-vaccine-and-mab-snapshot/ Date last accessed: July 17, 2019. Date last updated: April 2019.
- 91.Kissling E, Valenciano M, Pozo F, et al. 2015/16 I-MOVE/I-MOVE+ multicentre case-control study in Europe: moderate vaccine effectiveness estimates against influenza A(H1N1)pdm09 and low estimates against lineage-mismatched influenza B among children. Influenza Other Respir Viruses 2018; 12: 423–437. doi: 10.1111/irv.12520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rondy M, Launay O, Castilla J, et al. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35: 4298–4306. doi: 10.1016/j.vaccine.2017.06.088 [DOI] [PubMed] [Google Scholar]
- 93.Jutte DP, Roos LL, Brownell MD. Administrative record linkage as a tool for public health research. Annu Rev Public Health 2011; 32: 91–108. doi: 10.1146/annurev-publhealth-031210-100700 [DOI] [PubMed] [Google Scholar]
- 94.Onyebujoh PC, Thirumala AK, Ndihokubwayo JB. Integrating laboratory networks, surveillance systems and public health institutes in Africa. Afr J Lab Med 2016; 5: 431. doi: 10.4102/ajlm.v5i3.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rogan DT, Kochar MS, Yang S, et al. Impact of rapid molecular respiratory virus testing on real-time decision making in a pediatric emergency department. J Mol Diagn 2017; 19: 460–467. doi: 10.1016/j.jmoldx.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.World Health Organization.IMCI Chart Booklet Geneva, World Health Organization, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03766-2020.SUPPLEMENT (41.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03766-2020.Shareable (385.1KB, pdf)