Abstract
One of the key drivers of antibiotic resistance (ABR) and drug-resistant bacterial infections is the misuse and overuse of antibiotics in human populations. Infection management and antibiotic decision-making are multifactorial, complex processes influenced by context and involving many actors. Social constructs including race, ethnicity, gender identity and cultural and religious practices as well as migration status and geography influence health. Infection and ABR are also affected by these external drivers in individuals and populations leading to stratified health outcomes. These drivers compromise the capacity and resources of healthcare services already over-burdened with drug-resistant infections. In this review we consider the current evidence and call for a need to broaden the study of culture and power dynamics in healthcare through investigation of relative power, hierarchies and sociocultural constructs including structures, race, caste, social class and gender identity as predictors of health-providing and health-seeking behaviours. This approach will facilitate a more sustainable means of addressing the threat of ABR and identify vulnerable groups ensuring greater inclusivity in decision-making. At an individual level, investigating how social constructs and gender hierarchies impact clinical team interactions, communication and decision-making in infection management and the role of the patient and carers will support better engagement to optimize behaviours. How people of different race, class and gender identity seek, experience and provide healthcare for bacterial infections and use antibiotics needs to be better understood in order to facilitate inclusivity of marginalized groups in decision-making and policy.
The persisting challenge of antibiotic resistance
Antibiotic resistance (ABR) is an intractable problem that remains a persistent challenge to global health security. It is driven by antibiotic use in human and animal populations, both of which are consequences of a complex mix of social, economic and contextual factors.1,2
Existing efforts to address ABR focus primarily on interventions that are not easily scalable, as they fail to consider the complex inter-relationships between infection, antibiotic use and the emergence and spread of resistance through a sociocultural lens.3 A sociocultural perspective can help make sense of the wider set of cultural and societal factors which not only influence ABR but also the availability and allocation of resources and the capacity dedicated to efforts to respond to this public health threat. Gaps in capacity and resources significantly affect those populations most vulnerable to the consequences of ABR.4 Moreover, by failing to consider different contexts, existing efforts may be ignoring or contributing to the societal inequities that drive ABR.
Reflecting these concerns, in 2015 the World Health Assembly endorsed a global action plan on antimicrobial resistance calling for the development and implementation of national action plans (NAPs).5 Progress towards implementing effective strategies is, however, impeded by the unequal risks and opportunities within and across countries. If we are to tackle ABR effectively we need to understand and address these inequalities and their consequences.6 To do so, we need to consider the diverse sociocultural and political contexts in which ABR emerges and spreads. We must identify those communities and individuals, many already disadvantaged and in low- and middle-income countries (LMICs), who are at greatest risk. Disadvantaged populations may have greater risks associated with ABR for several reasons. First, their burden of infection is highest,7,8 placing them at greatest risk of what may become effectively untreatable infections.9 Second, they may struggle to obtain access to vaccination programmes that reduce the risk of disease.10 Third, they are often exposed to conditions that promote the emergence and spread of ABR, including interrupted, inadequate or inappropriate treatment, dependent on antibiotics supplied via informal outlets without the need for a prescription.11
While the impact of socioeconomic inequalities on health has received some attention in high-income countries(HICs), such as the USA and the UK,12–14 there is very little evidence from elsewhere. A study investigating the association between educational attainment of NHS patients and their use of health services found that higher education was positively associated with greater use of outpatient care.12 This indicates that better educated patients are better able to navigate the health system and access services more effectively. The concept of critical allyship has been applied to conceptualize health inequalities and help everyone who works in health to take collective action on systems of inequality that impact health.14 In the field of infection and ABR, research gaps in understanding the impact of socioeconomic inequalities on outcomes hinder the progress of critical allyship. This is despite the inclusion—in the 2018 WHO guidance on gender and equity in antimicrobial resistance—of recommendations for stakeholders developing NAPs, which acknowledge the persisting gaps in research on how inequalities impact on ABR.6 It is also despite the commitments made by the world’s governments to tackling inequalities in health contained in the United Nations sustainable development goals (SDGs).15 Goal 3 focuses on good health and well-being, while many other goals also contribute to or are connected to health. The other SDG goals include quality education, gender equality, no poverty, and clean water and sanitation.
The SDGs incorporate the principle that we should ‘leave no one behind’. While many countries were making progress towards a number of the SDG targets before the COVID-19 pandemic,16 persisting imbalances of power and resources continue to pose seemingly intractable challenges and there are fears that the financial damage caused by the COVID-19 pandemic may lead to a reversal of the progress so far.17 There is growing recognition that a new approach is needed, based on people- and patient-centred policies that engage with communities, recognizing the need to better understand the social construct of health inequities.18
In this review we explore the potential impact of socioeconomic inequalities (uneven distribution of resources) and cultural inequities (avoidable imbalances due to cultural exclusion and poor governance and policy-making) on ABR. We frame ABR as a social issue influenced by intersecting sociocultural and economic factors, which not only drive its emergence and spread but also create barriers to tackling it. While we acknowledge the lack of comparable data on the socioeconomic disparities and their association with ABR, we consider the data that do exist so as to identify the research and policy gaps that need to be addressed if we are to fully understand the diverse impacts of ABR on different populations and provide solutions that are widely implementable and accessible.
Socioeconomic disparities and ABR from global to local
The limited research that exists on the relationship between sociocultural imbalances and inequities and ABR offers some stark findings. An investigation of the social gradient in health in Europe describes how structural factors, such as crowding, homelessness and displacement, increase the risk of infections and ABR.19 These conditions constrain the opportunities available for some people to take measures that will reduce their risk of infectious diseases, as well as limiting their timely access to appropriate healthcare when needed. Affordable health services (including vaccination programmes) are critical to the prevention and spread of infectious diseases, as are access to water, sanitation and hygiene (WASH). The distribution of each of these is driven by upstream political, social and environmental factors.20 These basic resources remain out of reach of millions of people worldwide. Living and working conditions, combined with vulnerabilities shaped by individual characteristics, such as ethnicity or migration status, influence the risks of infection through poor living conditions and lack of access to WASH, vaccination programmes and effective diagnostics and antibiotics to treat infections.21
The interactions that lead to health inequalities relevant to infectious diseases are complex and multifaceted. To take a few examples, low vaccination coverage persists in marginalized communities in many settings.22 Socioeconomic deprivation is a risk factor for many infectious diseases, including TB and meningococcal disease.19 In LMICs, the impact of poverty (encompassing many sociocultural factors) on specific pathogens has been reported, e.g. increased resistance amongst Streptococcus pneumoniae and Acinetobacter baumannii isolates and a 7-fold higher infection rate.23 The consequences of the inter-relationship between poverty and ABR are dire, with one estimate suggesting that ABR could push an additional 24 million people into extreme poverty by 2030. Of the projected 10 million cases from drug-resistant infections annually by 2050, the vast majority are estimated to occur in LMICs of Africa and Asia.24
Sociocultural drivers of ABR
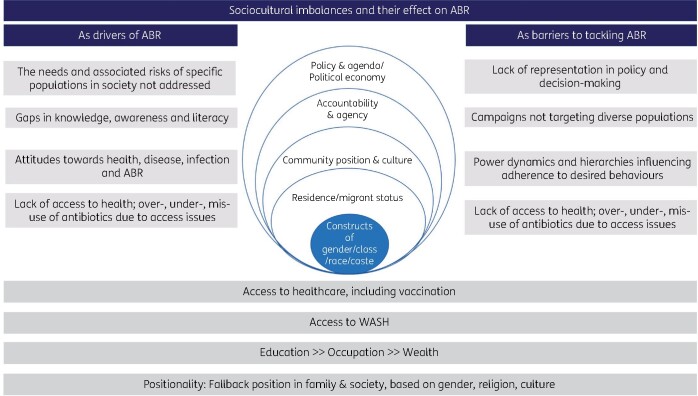
Much of health is decided outside of healthcare.25 Understanding the experience of ABR from individuals differentially affected by the social determinants of health is essential if we are to build programmes that are sustainable and meet the needs of entire populations. Power dynamics and hierarchies embedded and translated within cultures impact health equity. Positionality of individuals within society and culture is influenced by their gender identity, class, religion and culture (Figure 1). These in turn influence individuals’ access to education, wealth and health, including to WASH, and public health interventions such as vaccination programmes. Inequities in the representation within wider society can act as both drivers for ABR and as barriers to efforts to tackle ABR. For example, the division of power and hierarchies (including by gender, socioeconomic status, race, ethnicity, class) across structures within society can be predictors of health-providing and health-seeking behaviours in the context of infection and ABR (Figure 1). Understanding and addressing these dynamics will help sustain efforts to meet the SDGs and develop contextually fit, culturally sensitive and responsive interventions to tackle ABR across different settings.
Figure 1.
Intersectional view of social constructs and ABR.
Sociocultural inequalities also act as barriers to the interventions required to mitigate ABR, including influencing health-providing behaviours. Though understudied, this is an important consideration. Existing research has highlighted the hierarchies within medical and surgical teams in hospitals that determine antibiotic decision-making.26–28 Such hierarchies can get in the way of practices that optimize antibiotic treatment, for example, by making it more difficult to challenge senior doctors who are not adhering to evidence-based guidelines and recommendations. In the health workspace, historical exclusion and hierarchical structures can exclude key groups such as pharmacists and nurses in favour of doctors.29 Pharmacists receive training that allows them to optimize antibiotic therapies while nurses make critical contributions to reducing risks of infection developing and spreading. The exclusion of these groups from the decision-making process is likely to lead to suboptimal patient care. The roles and responsibilities of the allied healthcare professional groups are not fully understood or integrated into the relevant policy and guidelines.30
The roles of patients and carers and the knowledge that they bring to their care are also overlooked. Emerging evidence from India has emphasized the critical yet overlooked role that carers play in infection-related inpatient care (S. Surendran, E. Castro-Sánchez, V. Nampoothiri, S. Joseph, S. Singh, C. Tarrant, A. Holmes and E. Charani, unpublished data). The existing global infection prevention and control (IPC) narrative considers patients as sole decision-makers of their own care needs (S. Surendran, E. Castro-Sánchez, V. Nampoothiri, S. Joseph, S. Singh, C. Tarrant, A. Holmes and E. Charani, unpublished data). This overlooks an opportunity to view the wider sociocultural context and the constellation of persons who play a role in care and by extension decisions about IPC and ABR.26 Furthermore, the ABR narrative is explained as a battle between bugs and drugs, which overlooks human agency,6 leading to a partial and confusing narrative.31 For example, the discourse should perhaps be focused on antibiotic use and misuse, which remains the key issue, rather than antimicrobial use and misuse. Culture-sensitive IPC and ABR policies, which recognize the communication needs of different populations and embrace the roles that informal carers play, are urgently needed. We must generate knowledge and evidence to address the gaps in existing culture and practice and develop contextually fit solutions that will facilitate change in organizations and society to impact healthcare provider and user behaviours.
Gender and ABR
Gender inequalities also have consequences for ABR. Women often face reduced access to financial resources, creating barriers to healthcare, including screening for infection.32,33 Women also suffer more from inadequate access to WASH,34 for example during pregnancy and childbirth, when they are at particular risk of waterborne pathogens leading to miscarriage, while lack of access to clean water during childbirth increases the risk of maternal and neonatal sepsis, cord infection and surgical site infections.34 Being female is an independent risk factor for sepsis while, in community settings, women receive significantly higher numbers of antibiotic prescriptions than men.35–37 Recent literature indicates that the gender of both healthcare provider and recipient may influence not only the communication style but also antibiotic-prescribing behaviours.38 A study from the Netherlands investigating antibiotic prescribing for sore throat reported that female doctors were more likely to adopt a ‘wait and see’ policy with female patients, whilst male doctors were more likely to prescribe antibiotics.38 Inequalities that impact the infection-related healthcare needs of individuals who do not identify as male or female and those who have transitioned to a different gender remain largely unaddressed. Recent studies have, however, reported a higher than normal rate of surgical site infection and urethral complications in individuals undergoing gender reassignment surgery.39,40
Behaviours, roles and opportunities are based on different levels of power.41 The majority of the workforce driving IPC are female (nurses) and in stewardship teams, too, the pharmacy workforce responsible for reviewing antibiotic prescriptions have a higher female-to-male ratio. In LMICs community healthcare workers, who are predominantly female, are critical to making health services accessible to dispersed and diverse populations. In India, Accredited Social Health Activists (ASHAs) and auxiliary nurse midwives have major roles in infection prevention through delivering vaccination programmes, early detection of infections in the community—including sepsis and pneumonia—and promoting safe childbirth.42 Yet these community health workers must navigate many challenges, including religious, cultural and gender norms, which limit the work they can do.43 Furthermore, their training and safety needs are rarely met while prevailing gender and social hierarchies mean that their advice and recommendations are not consistently adhered to.43
Education, communication and ABR
Socioeconomic status and ethnicity intersect with education.44 A lack of formal education and awareness about infections amongst the public has also been linked to ABR.23,45 Similarly, gaps in ABR-related health literacy have been described together with the risk these pose to behaviours that may lead to emergence of ABR and infections, including hand hygiene practices and inappropriate antibiotic use.46,47 Current ABR-related public health promotion and education efforts do not address the inequalities in literacy and education in their target populations, failing to reach those who may be most vulnerable to the consequences of ABR. Furthermore, the success of such campaigns in reaching the target audience are rarely evaluated meaning there is no capacity for learning from any potential impact they may have had.48
Health promotion amongst healthcare workers also needs to recognize that individuals operate within social networks and are influenced by sociocultural perspectives that need to be better understood. The inconsistencies in education and training and engagement on public health interventions impact healthcare worker infection-related behaviours. Inadequate training can lead to lapses in IPC practices and inappropriate antibiotic prescribing, administration and monitoring, which may all drive drug-resistant infections. The impact of healthcare worker racial and ethnic disparities on vaccine uptake has been reported not only for the influenza vaccine, but more recently for vaccines developed for the COVID-19 pandemic.49,50 Being able to address legitimate concerns requires understanding the sociocultural beliefs that influence attitudes and behaviours. Only then can we develop culturally sensitive, effective communication and build trust in order to increase vaccination coverage and reduce the burden of infection in healthcare worker populations.50
The COVID-19 pandemic and the widening inequalities that impact ABR
The COVID-19 pandemic has exacerbated the existing inequalities and will have long-lasting social and economic consequences impacting health and well-being of populations for years to come.51 This pandemic has shed a light on how sociocultural constructs and inequalities between different populations interact resulting in significant disparities in health outcomes.52–54 Socioeconomic status, including income and living conditions, impacts on the ability to adhere to public health interventions seeking to prevent SARS-CoV-2 transmission. This is enhanced by all the same factors that drive ABR, including lack of sustainable access to health, education and social services, often in the setting of weak health systems. All have negatively impacted socioeconomically vulnerable populations during the COVID-19 pandemic.
The long-lasting legacies of health inequality on other pandemics, such as HIV/AIDS for example, continue to this day.33 A national cohort study of patients with COVID-19 admitted to ICUs in the UK has identified higher rates of intensive care admission and death in areas with greatest socioeconomic deprivation.55 In the USA, a recent study across three major cities has described the correlation between high social vulnerability—measures include socioeconomic status, household composition, minority status—with high clusters of COVID-19 positivity, incidence and mortality.56 Longitudinally, the impact of the pandemic on women is predicted to be significantly worse due to the disproportionate burden of unpaid domestic and care work as well as less job security and pay.57
Whilst the global response is rightly focused on SARS-CoV-2, secondary bacterial infections and both inappropriate and appropriate antibiotic use during this pandemic place additional burden on already severely ill individuals and overstretched health systems.58 In LMICs, national programmes such as the directly observed therapy (DOT) for TB treatment have been severely affected due to disruption in essential services, with the countries worst hit being Indonesia, South Africa, India and the Philippines, all of which reported at least 25% reduction in TB treatment since the beginning of the pandemic.59 Additionally, migrant labourers and individuals from lower socioeconomic strata (the majority of patients on DOT) returning to their villages and home cities during the lockdown have also lost out on essential TB treatment.60
The reported use of azithromycin for treatment of COVID-19 will influence the resistance patterns of bacteria that are usually treated with these agents. Another threat to the global efforts to address ABR will be the increased use of community drug vendors being used as the first point of care for the public in LMICs, as they try to self-medicate and avoid high costs of consultation fees/user fees, to procure however much of a course of antibiotics that can based on the money they have. This is in part driven by the convenience of avoiding long queues in public hospitals but also the lack of confidence in health systems due to perpetual stockouts.5,61 Reframing ABR as a social as well as a medical problem would facilitate the efforts needed to address its underlying socioeconomic drivers and facilitate context-specific interventions to mitigate them.
An intersectional framework for addressing ABR
Intersectional enquiry62,63 has been applied to many topics, including in health, to better understand the complex interplay of social hierarchies and constructs in intersecting and interactive ways.64 Intersectionality hinges on understanding human beings as shaped by the interaction of these different social constructs and conditions (e.g. race, caste, gender identity, sexual identity, class, geography, religion, migration status and cultural trauma) that interact within connected systems and structures of power e.g. laws, policies and media.65 Recognizing these complex interactions and dynamics allows for a better understanding of social inequalities as well as their drivers allowing researchers, public health policy-makers and governments to respond to them.18 To date, most health systems research on intersectionality has been from high-income settings. This is despite the fact that many of the greatest challenges arising from inequalities in health are in LMICs. In this context, it is deeply concerning that the UK government has decided to cut its Official Development Assistance (ODA) funds, threatening many international collaborative research projects focused on ABR.
Effective and sustainable responses to ABR require an understanding of how people of different races, classes and genders seek, experience and provide infection-related healthcare and use antibiotics. The questions that remain unanswered are: Is the incidence and perceived or measurable impact of ABR the same for everyone? Do any groups in society face greater or different risks of exposure to ABR or more challenges in accessing, using and benefiting from the information, services and solutions to tackle ABR? These questions were posed in the WHO report on tackling ABR, with a focus on gender identity and equity.6 To answer these questions, we need to apply an intersectional lens to research across HICs and LMICs. To address ABR through an inclusive policy approach and transformative implementation lens, there needs to be a proactive engagement with the prevailing inequities. At the structural and policy level (Figure 2) there need to be greater efforts to promote inclusivity in policy-agenda setting and decision-making for ABR. This must include greater equity in global representation across stakeholders, researchers and clinicians from LMICs as well as from different populations within society. The equity in representation and in division of funding for ABR research must be supported by equitable healthcare systems, which include vaccination programmes. The reach of structural and policy strategies in ABR will also be influenced by language, communication and advocacy efforts, which must recognize the culture- and context-specific needs of populations. In order for them to be used as a meaningful measure, qualitative and quantitative data related to ABR must be disaggregated by gender, ethnicity and socioeconomic vulnerability index.66
Figure 2.
A framework to address ABR through an inclusive policy approach and transformative implementation lens that considers the impact of the prevailing socioeconomic disparities.
Interventions targeting ABR need to address transformative implementation questions (Figure 2) to better understand the intersection of social constructs with ABR and infection. Accounting for power dynamics in the context of ABR and infection will promote inclusivity, enable greater participation in health and facilitate capacity. This will help sustain efforts to meet the SDG goals and develop contextually fit, culture-sensitive and responsive interventions to tackle ABR across different settings. Building on the WHO focus report on gender equity and ABR, we call for a broader and inclusive approach to the research and implementation strategy to address this public health threat across HICs and LMICs. This approach will give a platform to the narratives and experiences of different groups of people within society as well as different healthcare professional groups in relation to ABR and infection management. This will be a step in the right direction to assuring equitable access to optimized infection-related care.
Funding
This work was supported by funding from the following. (i) the Economic and Social Research Council (ESRC) and the National Institute for Health Research ASPIRES project (Antibiotic use across Surgical Pathways: Investigating, Redesigning and Evaluating Systems) (https://www.imperial.ac.uk/arc/aspires/). The support of ESRC as part of the Antimicrobial Cross Council initiative supported by the seven UK research councils, and also the support of the Global Challenges Research Fund, is gratefully acknowledged. (ii) The National Institute for Health Research, UK Department of Health [HPRU-2012-10047] in partnership with Public Health England. The funders had no role in the design and conduct of the study; collection, management, analysis, review or approval of the manuscript; and decision to submit the manuscript for publication.
Transparency declarations
None to declare.
References
- 1.Holmes AH, Moore LSP, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 2.Chandler CIR.Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun 2019; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey P, Marwick CA, Scott CL. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; issue 2: CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colzani E.Beyond morbidity and mortality: the burden of infectious diseases on healthcare services. Epidemiol Infect 2019; 147: e251. [Google Scholar]
- 5.Mpundu M. Moving from Paper to Action—The Status of National AMR Action Plans in African Countries. 2020. https://revive.gardp.org/moving-from-paper-to-action-the-status-of-national-amr-action-plans-in-african-countries/.
- 6.WHO. Tackling Antimicrobial Resistance Together. Working Paper 5.0: Enhancing the focus on gender and equity. 2018. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-working-papers/tackling-amr-together-working-paper-5-genderandequity-sept2018-en.pdf?sfvrsn=8b53f887_1.
- 7.Charani E, Cunnington AJ, Yousif AEHA. et al. In transition: current health challenges and priorities in Sudan. BMJ Glob Health 2019; 4: e001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitty CJM, MacEwen C, Goddard A. et al. Rising to the challenge of multimorbidity. BMJ 2020; 368: l6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker MG, Barnard LT, Kvalsvig A. et al. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 2012; 379: 1112–9. [DOI] [PubMed] [Google Scholar]
- 10.Jones KE, Patel NG, Levy MA. et al. Global trends in emerging infectious diseases. Nature 2008; 451: 990–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein EY, Milkowska-Shibata M, Tseng KK. et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis 2021; 21: 107–15. [DOI] [PubMed] [Google Scholar]
- 12.Stoye G, Zaranko B, Shipley M. et al. Educational inequalities in hospital use among older adults in England, 2004-2015. Milbank Q 2020; 98: 1134–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resnick A, Galea S, Sivashanker K. The Painful Price of Ignoring Health Inequities. The BMJ Opinion, 2020. https://blogs.bmj.com/bmj/2020/03/18/covid-19-the-painful-price-of-ignoring-health-inequities/.
- 14.Nixon SA.The coin model of privilege and critical allyship: implications for health. BMC Public Health 2019; 19: 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Fact Sheet on the SDGs: Health Targets and Antimicrobial Resistance (2017). https://www.euro.who.int/en/health-topics/health-policy/sustainable-development-goals/publications/2017/fact-sheets-on-the-sustainable-development-goals-sdgs-health-targets/fact-sheet-on-the-sdgs-antimicrobial-resistance-2017.
- 16.Lozano R, Fullman N, Abate D. et al. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 2091–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekwebelem OC, Ofielu ES, Nnorom-Dike OV. et al. Threats of COVID-19 to achieving United Nations sustainable development goals in Africa. Am J Trop Med Hyg 2020; 104: 457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson E, George A, Morgan R. et al. 10 Best resources on… intersectionality with an emphasis on low- and middle-income countries. Health Policy Plan 2016; 31: 964–9. [DOI] [PubMed] [Google Scholar]
- 19.ECDC. Health Inequalities, the Financial Crisis, and Infectious Disease in Europe. 2013. https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Health_inequalities_financial_crisis.pdf.
- 20.UNICEF & WHO. Progress on Household Drinking Water, Sanitation and Hygiene 2000-2017. https://washdata.org/sites/default/files/documents/reports/2019-07/jmp-2019-wash-households.pdf.
- 21.Nellums LB, Thompson H, Holmes A. et al. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18: 796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournet N, Mollema L, Ruijs WL. et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Public Health 2018; 18: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alividza V, Mariano V, Ahmad R. et al. Investigating the impact of poverty on colonization and infection with drug-resistant organisms in humans: a systematic review. Infect Dis Poverty 2018; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O ’Neill J. Tackling Drug-Resistant Infections Globally: an Overview of Our Work. The Review on Antimicrobial Resistance, 2016. https://wellcomecollection.org/works/e8njjeed/items.
- 25.Horton R.Offline: the pretensions of global health elites. Lancet 2020; 395: 672. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Mendelson M, Surendran S. et al. Investigating infection management and antimicrobial stewardship in surgery: a qualitative study from India and South Africa. Clin Microbiol Infect 2021; doi: 10.1016/j.cmi.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Charani E, Castro-Sanchez E, Sevdalis N. et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of ‘prescribing etiquette’. Clin Infect Dis 2013; 57: 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charani E, Ahmad R, Rawson T. et al. The differences in antibiotic decision-making between acute surgical and acute medical teams: an ethnographic study of culture and team dynamics. Clin Infect Dis 2019; 69: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charani E, Castro-Sanchéz E, Bradley S. et al. Implementation of antibiotic stewardship in different settings—results of an international survey. Antimicrob Resist Infect Control 2019; 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mostaghim M, Snelling T, McMullan B. et al. Nurses are underutilised in antimicrobial stewardship—results of a multisite survey in paediatric and adult hospitals. Infect Dis Health 2017; 22: 57–64. [Google Scholar]
- 31.Mendelson M, Balasegaram M, Jinks T. et al. Antibiotic resistance has a language problem. Nature 2017; 545: 23–5. [DOI] [PubMed] [Google Scholar]
- 32.Bingham AL, Kavanagh AM, Fairley CK. et al. Income inequality and Neisseria gonorrhoeae notifications in females: a country-level analysis. Sex Health 2014; 11: 556–60. [DOI] [PubMed] [Google Scholar]
- 33.Cummings B, Mengistu M, Negash W. et al. Barriers to and facilitators for female participation in an HIV prevention project in rural Ethiopia: findings from a qualitative evaluation. Cult Health Sex 2006; 8: 251–66. [DOI] [PubMed] [Google Scholar]
- 34.WaterAid. WASH and Poverty. 2013. https://www.wateraid.org/uk/sites/g/files/jkxoof211/files/wateraid-annual-report-2013-14.pdf.
- 35.Schröder W, Sommer H, Gladstone BP. et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J Antimicrob Chemother 2016; 71: 1800–6. [DOI] [PubMed] [Google Scholar]
- 36.Leibovici L, Paul M, Weinberger M. et al. Excess mortality in women with hospital-acquired bloodstream infection. Am J Med 2001; 111: 120–5. [DOI] [PubMed] [Google Scholar]
- 37.Pietropaoli A, Glance L, Oakes D. et al. Gender differences in mortality in patients with severe sepsis and septic shock. Gend Med 2010; 7: 422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggermont D, Smit MAM, Kwestroo GA. et al. The influence of gender concordance between general practitioner and patient on antibiotic prescribing for sore throat symptoms: a retrospective study. BMC Fam Pract 2018; 19: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao JJ, Marchaim D, Palla MB. et al. Surgical site infections in genital reconstruction surgery for gender reassignment, Detroit: 1984–2008. Surg Infect 2014; 15: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nassiri N, Maas M, Basin M. et al. Urethral complications after gender reassignment surgery: a systematic review. Int J Impot Res 2020; doi: 10.1038/s41443-020-0304-y. [DOI] [PubMed] [Google Scholar]
- 41.Manandhar M, Hawkes S, Buse K. et al. Gender, health and the 2030 agenda for sustainable development. Bull World Health Organ 2018; 96: 644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanchard A, Ansari S, Rajput R. et al. Free facilities or false promises? The effects of Accredited Social Health Activists’ home visits on maternal and newborn health equity in Uttar Pradesh, India: a mixed methods study. Lancet Glob Heal 2019; 7: S3. [Google Scholar]
- 43.Sarin E, Lunsford SS.. How female community health workers navigate work challenges and why there are still gaps in their performance: a look at female community health workers in maternal and child health in two Indian districts through a reciprocal determinism framework. Hum Resour Health 2017; 15: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabbani F, Perveen S, Aftab W. et al. Health workers’ perspectives, knowledge and skills regarding community case management of childhood diarrhoea and pneumonia: a qualitative inquiry for an implementation research project ‘Nigraan’ in District Badin, Sindh, Pakistan. BMC Health Serv Res 2016; 16: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Bank Group. Drug-Resistant Infections: A Threat to Our Economic Future. 2017. http://documents.worldbank.org/curated/en/323311493396993758/final-report.
- 46.Castro-Sánchez E, Chang PWS, Vila-Candel R. et al. Health literacy and infectious diseases: why does it matter? Int J Infect Dis 2016; 43: 103–10. [DOI] [PubMed] [Google Scholar]
- 47.Hermsen ED, MacGeorge EL, Andresen ML. et al. Decreasing the peril of antimicrobial resistance through enhanced health literacy in outpatient settings: an underrecognized approach to advance antimicrobial stewardship. Adv Ther 2020; 37: 918–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redfern J, Bowater L, Coulthwaite L. et al. Raising awareness of antimicrobial resistance among the general public in the UK: the role of public engagement activities. JAC Antimicrob Resist 2020; 2: dlaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ojha RP, Stallings-Smith S, Flynn PM. et al. The impact of vaccine concerns on racial/ethnic disparities in influenza vaccine uptake among health care workers. Am J Public Health 2015; 105: e35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razai MS, Osama T, McKechnie DGJ. et al. Covid-19 vaccine hesitancy among ethnic minority groups. BMJ 2021; 372: n513. [DOI] [PubMed] [Google Scholar]
- 51.The COVID Decade. Understanding the Long-Term Societal Impacts of COVID-19. The British Academy, 2021. https://www.thebritishacademy.ac.uk/documents/3238/COVID-decade-understanding-long-term-societal-impacts-COVID-19.pdf.
- 52.Pan D, Sze S, Minhas JS. et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine 2020; 23: 100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan NE, El Ayadi AM.. A call for a gender-responsive, intersectional approach to address COVID-19. Glob Public Health 2020; 15: 1404–12. [DOI] [PubMed] [Google Scholar]
- 54.Wenham C, Smith J, Morgan R.. COVID-19: the gendered impacts of the outbreak. Lancet 2020; 395: 846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lone N, McPeake J, Stewart NI. et al. Influence of socioeconomic deprivation on interventions and outcomes for patients admitted with COVID-19 to critical care units in Scotland: a national cohort study. Lancet Reg Health Eur 2021; 1: 100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilal U, Tabb LP, Barber S. et al. Spatial inequities in COVID-19 testing, positivity, confirmed cases, and mortality in 3 U.S. cities: an ecological study. Ann Intern Med 2021; 174: 936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cela B. Expert’s take: The Gendered Impact of COVID-19 Requires Transformative Changes in Economic Policies. 2020. https://www.preventionweb.net/news/view/74074.
- 58.Li J, Wang J, Yang Y. et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control 2020; 9: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.1.4 Million with Tuberculosis, Lost Out on Treatment During First Year of COVID-19. United Nations News, 2021. https://news.un.org/en/story/2021/03/1087962.
- 60.Wingfield T, Karmadwala F, MacPherson P. et al. Challenges and opportunities to end tuberculosis in the COVID-19 era. Lancet Respir Med 2021; 9: 556–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pai M. Can Pharmacies Improve Global Health Delivery by Taking Tests Closer to People? https://www.forbes.com/sites/madhukarpai/2019/05/25/can-pharmacies-improve-global-health-delivery-by-taking-tests-closer-to-people/?sh=7aa67f5f3185.
- 62.Eddo-Lodge R.Why I'm No Longer Talking to White People About Race, 2nd edn. Bloomsbury Publishing, 2018. [Google Scholar]
- 63.Crenshaw K.Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev 1991; 43: 1241–99. [Google Scholar]
- 64.Kapilashrami A, Hankivsky O.. Intersectionality and why it matters to global health. Lancet 2018; 391: 2589–91. [DOI] [PubMed] [Google Scholar]
- 65.Hankivsky O.Women’s health, men’s health, and gender and health: implications of intersectionality. Soc Sci Med 2012; 74: 1712–20. [DOI] [PubMed] [Google Scholar]
- 66.Nadimpalli M, Chan C, Doron S.. Antibiotic resistance: a call to action to prevent the next epidemic of inequality. Nat Med 2021; 27: 187–8. [DOI] [PMC free article] [PubMed] [Google Scholar]