Abstract
Acer mono is known to contain bioactive substances that exhibit beneficial effects in osteoporosis, gastric ulcers, hepatic damage, and pathologic angiogenesis. The current study aimed to investigate the effects of Acer mono extract on the invasive activities and cell-cycle progression of human fibrosarcoma cells. Cytotoxicity of Acer mono extract was assessed by MTT assay, in-vitro invasiveness of HT1080 fibrosarcoma cells was measured using matrigel assay, expression of invasion- and cell-cycle-related proteins was analyzed by western blot analysis, and that of E2F target genes was quantified using qRT-PCR. Acer mono extract did not show distinct cytotoxicity in the experimental concentrations used. Invasiveness of HT1080 fibrosarcoma cells and expression of cyclin D1 and CDK4 in them were significantly reduced in a dose-dependent manner after treatment with Acer mono extract. Acer mono extract showed inhibitory effects on the G1/S transition during cell-cycle progression; the active phosphorylated Rb protein level was decreased, and expression of E2F target genes was downregulated by the Acer mono extract. Our data collectively demonstrated that Acer mono extract exerts inhibitory effects on the invasiveness and cell-cycle progression of HT1080 human fibrosarcoma cells.
Keywords: Acer mono, Matrix Metalloproteinase 2, Plant Extracts, Cell-Cycle
INTRODUCTION
Acer mono Max. is a wild plant distributed across East Asia, especially Korea, Japan, China, Mongolia, and the Russian Far East. A species of Acer mono is a deciduous tree with five to seven lobular leaves and growing up to 15–20 m in height. It has been reported to have beneficial effects on osteoporosis.1 The leaves, roots, and sap of Acer mono have been used in Korean folk medicine for the treatment of hemostasis, arthralgia, cataclasis, difficulty in urination, constipation, other gastrointestinal disorders, and neuralgia. Several studies have demonstrated its effects on angiogenesis, hepatic protection, and stress-induced gastric ulceration.2,3,4,5 Acer species are known to contain various secondary metabolites, such as coumarinolignans, diarylheptanoids, flavonoids, sterols, and triterpenoids.6,7 However, there has been no report on the bioactive characteristics of Acer mono extract in relation to cell invasion and proliferation of tumor cells.
Cancer cells differ from normal cells in many cellular characteristics, including loss of contact inhibition, increased invasiveness and metastasis, continuous cell proliferation, loss of differentiation, and decreased drug sensitivity. The differences arise from increased proteolytic activities in the extracellular matrix and uncontrolled cellular proliferation of tumor cells. Tumor cell migration and metastasis have been known to be closely related to the expression of matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinases (TIMP).8,9,10,11,12,13,14 In our previous pilot study, Acer mono duramen extract had been found to function as a matrix metalloproteinase inhibitor (MMPI). The present study aimed to investigate the effects of Acer mono on the invasive activities and expression of MMPs and TIMPs in HT1080 fibrosarcoma cells.
The cell-cycle is a conserved proliferative signaling cascade consisting of a series of events occurring in a dividing cell, specifically the G1, S, G2, and M phases.15 When cells are stimulated by mitogens to divide, active G1-CDK (cyclin D1-CDK4) accumulates and phosphorylates Rb proteins, reducing their binding to E2F. The liberated E2F proteins then activate the transcription of G1/S genes, including cyclin E and cyclin A. Therefore, dysregulation of cell-cycle progression leads to changes in cell proliferation characteristics. Overexpression of cyclin D1 could be a reason behind tumor progression and metastasis.16,17 Although the relationship between tumor cell invasion and proliferation still remains unclear, we analyzed the effects of Acer mono on cell-cycle progression to elucidate the mechanism of anticancer activity of Acer mono extract in tumor cells and explore its potential as a chemotherapeutic agent in anticancer therapy.
MATERIALS AND METHODS
1. Materials
An extract of the medicinal plant Acer mono was purchased from Plant Extract Bank, Daejeon, Korea. The extract was dissolved in methanol and stored at 4℃. EMEM medium, trypsin-EDTA, and fetal bovine serum (FBS) were obtained from Gibco BRL (Life Technologies, USA). HT1080 fibrosarcoma cells were obtained from the American Type Culture Collection (USA). Antibodies were purchased from R&D (USA), and matrigel was purchased from BD Biosciences (USA).
2. Cell culture
HT1080 cells were grown as monolayers in tissue culture flasks (Nunc, Denmark) in EMEM medium supplemented with 10% fetal bovine at 5% CO2 and 37℃ in a humidified atmosphere. Cells were passaged thrice a week by treating with trypsin-EDTA, and then used for subsequent experiments. Once the cells adhered to the culture plate, the medium was replaced with EMEM medium containing 1% FBS, and the extract was added at various concentrations. The conditioned medium was collected after 24 h of incubation for subsequent analysis.
3. MTT assay
Cytotoxic levels of extracts in HT-1080 cells were measured using the 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Cells were grown in 96-well plates at a density of 5×103 cells/well. After 24 h, they were washed with fresh medium and treated with different concentrations of extracts. After 24-h incubation, cells were re-washed, 20 µL of MTT (5 mg/mL) was added, and incubated again for 4 h. Finally, dimethyl sulfoxide (DMSO, 100 µL) was added to solubilize the formazan salt formed, and the amount of formazan salt was determined by measuring the OD at 490/540 nm using a microplate reader (Molecular Devices, USA). Data are expressed as the mean of three independent experiments.
4. Invasion assay
For the invasion assay, 5×104 HT-1080 cells in 250 µL EMEM were seeded in the upper chamber and serum-free EMEM was placed in the lower chamber. Matrigel matrix-coated membranes were inserted between the two chambers. After incubation at 37℃ in a 5% CO2 incubator overnight, the medium in upper chamber was replaced by EMEM containing 1% FBS (250 µL) and treated with various concentrations of the extract (0–200 µg/mL). The chamber was then incubated at 37℃ in a 5% CO2 incubator for 24 h. At the end of the incubation period, the membrane was fixed and stained according to the manufacturer's instructions (Becton-Dickinson, USA).
5. Western blot analysis
The treated cells were suspended in lysis buffer (1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 mM Na-phosphate buffer (pH 7.4), 100 % aprotinin (100 µL), 1 mg/mL leupeptin (10 µL), and 250 mM Na3VO4 (20 µL)) for 10 min on ice. The nuclei were pelleted by centrifugation at 6,000 rpm at 4℃ for 5 min, and the supernatants containing cytosolic proteins were collected. Equal amounts of protein and an equal volume of 2× sample buffer were mixed and electrophoresed on 8% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). The proteins were then electro-transferred to a nitrocellulose membrane (Amersham, USA). The membrane was blocked with 5% skimmed milk in 1× PBS (pH 7.6) for 2 h at room temperature with shaking, and incubated with the primary antibody, followed by secondary anti-mouse/goat IgG. The immunoreactive protein bands were visualized with enhanced chemiluminescence (ECL) reagents (Amersham, USA).
6. qRT-PCR
RNA extraction was performed using TRI reagent according to the manufacturer's instructions (Molecular Research Center, USA). Total RNA (3 µg) was reverse-transcribed using an M-MLV cDNA synthesis system (Invitrogen, USA), and the reverse-transcribed cDNA was subjected to PCR. The amplification reaction was performed under the following conditions: 40 cycles of denaturation at 94℃, annealing at 60℃, and extension at 72℃. Disassociation curves were generated after each PCR run to ensure that a single product of the appropriate length was amplified. The mean threshold cycle were calculated from individual Ct values obtained from triplicates per stage. In each reaction, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primers of E2F target genes, such as cyclin A (CCNA), cyclin E (CCNE), CDC6 (cell division cycle 6), dehydrofolate reductase (DHFR), and thymidine kinase (TK), were purchased from Quiagen.
7. Statistical analysis
The p value was determined by the one-tailed Student's t-test. The level of statistical significance was set at p<0.05.
RESULTS
1. Cytotoxicity of the Acer mono extract in HT1080 cells
Cytotoxicity of the Acer mono extract in HT-1080 cells was evaluated by the MTT assay. Cells (5×103) were treated with different concentrations of the Acer mono extract (0, 50, 100, 150, and 200 µg/mL) and incubated for 24 h. The Acer mono extract had no effect on the viability of HT1080 cells under the experimental concentrations (Fig. 1).
FIG. 1. Effect of Acer mono extract on the viability of HT1080 cells. HT1080 cells (5×103) were treated with the Acer mono extract in a dose-dependent manner (0, 50, 100, 150, and 200 µg/mL) for 24 h. Cell viability was determined using the standard MTT assay. Cell viability is represented as the percentage of relative absorbance compared to that in the control. Results are presented as the mean±SD of three independent experiments.
2. Acer mono extract inhibited invasive behavior of HT1080 cells
The invasive activities of HT1080 cells, treated with different concentrations of the Acer mono extract, were evaluated using a matrigel assay. Results showed the Acer mono extract to significantly inhibit the invasive activity of HT1080 cells in a dose-dependent manner (Fig. 2A).
FIG. 2. Inhibitory effect of Acer mono extract on the invasiveness of HT1080 cells. Effect of Acer mono extract on matrigel invasion of HT1080 cells is shown (A). HT1080 cells were treated with indicated concentrations of the Acer mono extract. Photograph of cells penetrating the matrigel is shown. The conditioned medium was collected from each well and treated with different concentrations of Acer mono extract (50, 100, and 200 µg/mL). MMP2 and MMP9 activity was analyzed by zymography (B). TIMP2, MMP2, MMP9, and MMP14 protein expression in cells treated with different concentrations of the Acer mono extract was analyzed by western blotting (C). The scale bar represents 20 µm (*p<0.05, **p<0.01).
3. Acer mono extract inhibited the expression of MMP2 and MMP9
Metastasis is a multistep and complex process that includes cell proliferation, ECM degradation and invasion. The MMP2 (72 kD gelatinase A) and MMP9 are ECM degrading enzymes, associated with the invasive metastatic potential of various tumor and cancer cells. So, zymography was performed to examine the activity of MMP-2 in HT1080 cells. MMP-2 activity was significantly decreased in a dose-dependent manner (Fig. 2B). In addition, TIMP2 expression was significantly reduced (Fig. 2C). This result indicated that the inhibitory effect on cell invasion in the matrigel assay was mediated by MMP-TIMP imbalance.
4. Acer mono extract inhibited the expression of cyclin D1, CDK4, and pRb in HT1080 cells
HT1080 cells were treated with DMSO or Acer mono extract (50, 100, and 200 µg/mL) for 24 h, and the expression of pRb (Ser795), pRb (Ser807/811), cyclin D1, and CDK4 was examined by western blot analysis. Cyclin D1 and CDK4 protein expressions were decreased in a dose-dependent manner, with maximum inhibition occurring at 200 µg/mL Acer mono extract (Fig. 3B ,C).
FIG. 3. Effect of Acer mono extract on the expression of pRb, cyclin D1, and CDK4. HT1080 cells were treated with Acer mono extract (50, 100, and 200 µg/mL) for 24 h. Cell lysates were subjected to SDS-PAGE and analyzed by western blotting. Rb phosphorylated on Ser795 or Ser807/811 was measured by immunoblotting with a specific anti-Ser795-phospho-Rb or Ser807/811-phospho-Rb antibody. Representative blots are shown (A, B). Total Rb expression was measured by immunoblotting using a rabbit polyclonal antibody, which recognizes total Rb protein. Densitometric analysis of the effect of Acer mono extract on the expression of p-Rb, cyclin D1, and CDK4 (C) (*p<0.05).
5. Cyclin/CDK-Rb signaling pathway was involved in the inhibition of E2F function in G1/S transition
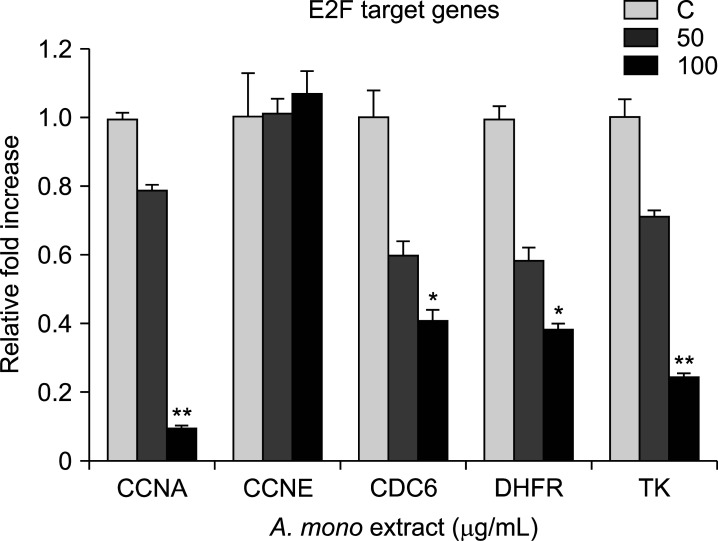
Acer mono extract blocked the G1/S transition by interfering with the Rb-E2F signaling pathway via regulation of the expression of cyclin D1, CDK4, and E2F. Furthermore, the mRNA levels of downstream targets of E2F, including CCNA, CDC6, DHFR, and TK genes, were significantly lower in Acer mono extract-treated cells than in DMSO-treated cells (Fig. 4). The data suggested that Acer mono extract may inhibit the G1/S transition by suppressing multiple targets, including cyclin D1, CDK4, and Rb-E2F, which in turn attenuate the transcription of S-phase genes.
FIG. 4. Real-time qRT-PCR analysis of E2F target gene mRNAs. Cells were incubated with Acer mono extract (50 and 100 µg/mL). Total RNA was isolated from cells after 48 h of treatment and used for qRT-PCR analysis with the indicated E2F target gene primers (CCNA, CCNE, CDC6, DHFR, and TK). GAPDH was used as a control gene for normalization. The mean and standard error for the three independent datasets are shown (*p<0.05, **p<0.01).
DISCUSSION
Metastasis is the major cause of death in patients with cancer. Extracellular secretion of MMPs to destroy extracellular matrix is an important key step in tumor cell invasion and metastasis. HT1080 human fibrosarcoma cells secret abundant MMPs and exhibit strong metastatic characteristics. For this reason, we selected HT1080 cells, which are widely used to measure the invasive activities of tumor cells to evaluate the effect of Acer mono extract in matrigel assay. As shown in Fig. 2A, Acer mono extract inhibited the invasive activities of HT1080 cells in a dose-dependent manner, and the inhibitory effect on tumor cell invasion was considered to be a result of the downregulation of MMP activities, in addition to increased expression of TIMP2 (Fig. 2C). However, whether Acer mono extract also exhibits similar effects on invasive activities of other tumor cell types is necessary to be studied in the future. Although there is no report elucidating the mechanisms of invasiveness and cell proliferation, Cheu and Pan had demonstrated that PD-0332991 inhibits cellular growth and suppresses migration, invasion, and MMP-2 expression of esophageal squamous cell carcinoma (ESCC) cells.18 While the mechanism underlying the invasion-proliferation relationship has not yet been clearly elucidated, the current study indicated that there is a link between cellular invasion and proliferation.
The cell-cycle control system plays a pivotal role in regulating cell proliferation in the body tissues. The central components of this system includes members of a family of protein kinases known as cyclin-dependent kinases (CDKs). Activities of the kinases change as the cell progresses through the cycle, leading to cyclical changes in the phosphorylation of intracellular proteins that initiate or regulate the major events of cell-cycle. Cyclins are the most important CDK regulators. When cyclin forms a complex with, the protein kinase is activated to trigger specific cell-cycle progression. G1/S-cyclins activate CDKs in late G1 phase and promote cell-cycle progression. The G1/S transition is a crucial event in cell-cycle progression, which requires cyclin proteins and their associated kinases, especially cyclin D1, in the early G1 phase.19,20 Deregulation of the cyclin/CDK-Rb-E2F pathway, such as amplification, mutation, and overexpression of cyclin D, is crucial for the development of human cancer. Moreover, cancers displaying activation of specific oncogenic pathways may also be particularly sensitive to CDK4/ CDK6 inhibition.17 Our present study revealed that entry of HT1080 cells into G1/S phase transition is inhibited after treatment with Acer mono extract (Fig. 3). To investigate the changes in proteins that are important for the progression of cell-cycle, the expression levels of cyclin D1 and CDK4 were measured by western blot analysis. As shown in Fig. 3 cyclin D1 and CDK4 were downregulated in HT1080 cells treated with Acer mono extract in a dose-dependent manner. We also measured the activities and expressions of other cell-cycle and proliferation related proteins including cyclinD3, Cdk6, Chk1, Chk2, p53, p27, p15 and p16 after treatment of Acer mono extract, however any significant changes were not found in our experiment. (data not shown). The results suggested that Acer mono extract inhibits cell-cycle progression by suppressing G1/CDK action, which is required for the G1/S transition. Cyclin D1 overexpression has been observed in various tumor cells.21,22,23 Similar to our data, other molecules inhibiting cell-cycle progression in the G0/G1 phase (for example, luteolin and silibinin) have also been reported to show anti-proliferative activities.24,25 In this study, the potent inhibitory effect on cyclin D1 and CDK4 provides molecular evidence of an anti-proliferative effect directed at cell-cycle arrest.
G1/CDK complexes activate Rb protein and subsequently liberate gene regulatory factors known as E2F proteins, which promote the expression of genes that encode proteins required for S-phase entry.
In this study, we found that phosphorylated Rb proteins are decreased in HT1080 cells treated with Acer mono extract (Fig. 3A). As a result, expression of E2F target genes, including CCNA (cyclin A), CDC6 (cell division cycle 6), DHFR (dehydrofolate reductase), and TK (thymidine kinase) genes, were downregulated (Fig. 4).
Taken together, the results indicated that Acer mono extract inhibits cell-cycle progression by suppressing the G1/CDK-Rb-E2F pathway in HT1080 cells.
Our data further suggested that Acer mono extract has anti-tumor activity and can be applied in the development of therapeutic resource for cancer therapy.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI16019-1) from Chonnam National University Hospital Biomedical Research Institute.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Lee GS, Byun HS, Kim MH, Lee BM, Ko SH, Jung EM, et al. The beneficial effect of the sap of Acer mono in an animal with low-calcium diet-induced osteoporosis-like symptoms. Br J Nutr. 2008;100:1011–1018. doi: 10.1017/S0007114508959195. [DOI] [PubMed] [Google Scholar]
- 2.Seo EJ, Kuete V, Kadioglu O, Krusche B, Schröder S, Greten HJ, et al. Antiangiogenic activity and pharmacogenomics of medicinal plants from traditional Korean medicine. Evid Based Complement Alternat Med. 2013;2013:131306. doi: 10.1155/2013/131306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Sung SH, Kim YC. Two new hepatoprotective stilbene glycosides from Acer mono leaves. J Nat Prod. 2005;68:101–103. doi: 10.1021/np0497907. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Lee MK, Kim YC. Protective activities of stilbene glycosides from Acer mono leaves against H2O2-induced oxidative damage in primary cultured rat hepatocytes. J Agric Food Chem. 2005;53:4182–4186. doi: 10.1021/jf050093+. [DOI] [PubMed] [Google Scholar]
- 5.Park CH, Son HU, Son M, Lee SH. Protective effect of Acer mono Max. sap on water immersion restraint stress-induced gastric ulceration. Exp Ther Med. 2011;2:843–848. doi: 10.3892/etm.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morikawa T, Tao J, Toguchida I, Matsuda H, Yoshikawa M. Structures of new cyclic diarylheptanoids and inhibitors of nitric oxide production from Japanese folk medicine Acer nikoense. J Nat Prod. 2003;66:86–91. doi: 10.1021/np020351m. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Zaid MM, Helson BV, Nozzolillo C, Arnason JT. Ethyl m-digallate from red maple, Acer rubrum L., as the major resistance factor to forest tent caterpillar, Malacosoma disstria Hbn. J Chem Ecol. 2001;27:2517–2527. doi: 10.1023/a:1013683600211. [DOI] [PubMed] [Google Scholar]
- 8.Huang LL, Wang Z, Cao CJ, Ke ZF, Wang F, Wang R, et al. AEG-1 associates with metastasis in papillary thyroid cancer through upregulation of MMP2/9. Int J Oncol. 2017;51:812–822. doi: 10.3892/ijo.2017.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swayampakula M, McDonald PC, Vallejo M, Coyaud E, Chafe SC, Westerback A, et al. The interactome of metabolic enzyme carbonic anhydrase IX reveals novel roles in tumor cell migration and invadopodia/MMP14-mediated invasion. Oncogene. 2017;36:6244–6261. doi: 10.1038/onc.2017.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao Y, Hu K, Wang X, Wang L. MicroRNA-552 promotes migration and invasion of osteosarcoma through targeting TIMP2. Biochem Biophys Res Commun. 2019;511:63–68. doi: 10.1016/j.bbrc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen MK, Liu YT, Lin JT, Lin CC, Chuang YC, Lo YS, et al. Pinosylvin reduced migration and invasion of oral cancer carcinoma by regulating matrix metalloproteinase-2 expression and extracellular signal-regulated kinase pathway. Biomed Pharmacother. 2019;117:109160. doi: 10.1016/j.biopha.2019.109160. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin SL, Ice RJ, Rajulapati A, Kozyulina PY, Livengood RH, Kozyreva VK, et al. NEDD9 depletion leads to MMP14 inactivation by TIMP2 and prevents invasion and metastasis. Mol Cancer Res. 2014;12:69–81. doi: 10.1158/1541-7786.MCR-13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Yang B, She Y, Ye Y. The lncRNA TP73-AS1 promotes ovarian cancer cell proliferation and metastasis via modulation of MMP2 and MMP9. J Cell Biochem. 2018;119:7790–7799. doi: 10.1002/jcb.27158. [DOI] [PubMed] [Google Scholar]
- 14.Chen SW, Zhang Q, Xu ZF, Wang HP, Shi Y, Xu F, et al. HOXC6 promotes gastric cancer cell invasion by upregulating the expression of MMP9. Mol Med Rep. 2016;14:3261–3268. doi: 10.3892/mmr.2016.5640. [DOI] [PubMed] [Google Scholar]
- 15.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 16.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Pan J. Dual cyclin-dependent kinase 4/6 inhibition by PD-0332991 induces apoptosis and senescence in oesophageal squamous cell carcinoma cells. Br J Pharmacol. 2017;174:2427–2443. doi: 10.1111/bph.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ. Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians. 1995;107:181–186. [PubMed] [Google Scholar]
- 21.Sun S, Zimmet JM, Toselli P, Thompson A, Jackson CW, Ravid K. Overexpression of cyclin D1 moderately increases ploidy in megakaryocytes. Haematologica. 2001;86:17–23. [PubMed] [Google Scholar]
- 22.Dworakowska D, Jassem E, Jassem J, Boltze C, Wiedorn KH, Dworakowski R, et al. Prognostic value of cyclin D1 overexpression in correlation with pRb and p53 status in non-small cell lung cancer (NSCLC) J Cancer Res Clin Oncol. 2005;131:479–485. doi: 10.1007/s00432-004-0661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii Y, Pirkmaier A, Alvarez JV, Frank DA, Keselman I, Logothetis D, et al. Cyclin D1 overexpression and response to bortezomib treatment in a breast cancer model. J Natl Cancer Inst. 2006;98:1238–1247. doi: 10.1093/jnci/djj334. [DOI] [PubMed] [Google Scholar]
- 24.Ong CS, Zhou J, Ong CN, Shen HM. Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt-GSK-3β-Cyclin D1 pathway. Cancer Lett. 2010;298:167–175. doi: 10.1016/j.canlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143:58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]