Abstract
The use of herbal medicine to manage chronic conditions including diabetes has become a recent global trend. Diabetes mellitus (DM) is a group of metabolic disorders characterized by hyperglycemia. The present review is aimed to analyze the antidiabetic activity of N. sativa as many type 2 diabetic patients use it as a complementary therapy along with their modern allopathic medications or as an alternative therapy. The literature was reviewed in databases like Medline/PubMed Central/PubMed, Google Scholar, Science Direct, EBSCO, Scopus, Web of science, EMBASE, Directory of open access journals (DOAJ), and reference lists to identify relevant articles supporting the use of N. sativa in diabetes management. Numerous clinical and animal studies have demonstrated the antidiabetic efficacy of black seeds (N. sativa) and its major bioactive constituent thymoquinone. Based on these findings patients with diabetes may use N. sativa as an adjuvant therapy, which may help to reduce the dose and incidence of adverse effects of modern antidiabetic medicines.
Keywords: Diabetes Mellitus, Nigella Sativa, Seeds, Thymoquinone, Hypoglycemic Agents
INTRODUCTION
Diabetes mellitus (DM) is a group of metabolic disorders characterized by hyperglycemia. It is caused by decreased insulin secretion from pancreatic β cells, diminished action of insulin at the periphery or by both.1 Generally, hyperglycemia induces the release of reactive oxygen species (ROS) which stimulate cellular damage leading to complications including peripheral neuropathy, retinopathy and nephropathy.2
In 2019, it was estimated that there were 463 million people living with diabetes across the globe and it has been predicted that the global prevalence of diabetes would reach 578 million cases by 2030 and 700 million by 2045.3 Patients with type 2 diabetes are usually treated with oral antidiabetic drugs such as metformin, sulfonylureas, meglitinides, thiazolidinediones (TZDs), dipeptidyl peptidase 4 (DPP4) inhibitors and SGLT2 inhibitors.4,5
DIABETES AND BLACK SEEDS (NIGELLA SATIVA)
Recently, people around the globe are opting to use herbal medicines to manage chronic conditions such as diabetes, hypertension, cancer, obesity, and others as modern medicines may be associated with harmful and undesirable side effects.6 Perceived failure of allopathic medicines, relatively high cost of allopathic medicines, social cultural practices and/or herbal knowledge, poor accessibility to medical facilities and safety concerns about allopathic medicines are the primary reasons for the patients’ preference of herbal remedies to manage chronic conditions.7
The prevalence of use of herbal medicines is higher among the patients with diabetes.8 A cross sectional survey determined that about 7.3% of 310 Jordanian diabetic patients used N. sativa to manage their diabetes.9
Nigella sativa (Black seeds) is an herb, which belongs to Ranunculacea family. N. sativa has been used to treat various chronic conditions such as diabetes, hypertension, cancer, obesity, and others.10 The most prominent active constituent of N. sativa is thymoquinone (TQ) and it also contains other bioactive constituents including dithymoquinone (DTQ), carvone, limonine, nigellidine, nigellicine, nigellicimine and others.11 The present review is aimed to analyze the antidiabetic activity of N. sativa as many type 2 diabetic patients use it as a complementary therapy along with their modern allopathic medications or as an alternative therapy.
The use of N. sativa is very common in traditional medicines including Unani, Ayurveda, Chinese medicine, and others. Several clinical and pre-clinical studies have already demonstrated the antidiabetic activity of N. sativa and its active constituent Thymoquinone.
The literature reviewed for this article was found in databases like Medline/PubMed Central/PubMed, Google Scholar, Science Direct, EBSCO, Scopus, Web of science, EMBASE, Directory of open access journals (DOAJ), and reference lists to identify relevant articles supporting the use of N. sativa in diabetes management.
CLINICAL STUDIES OF N. SATIVA
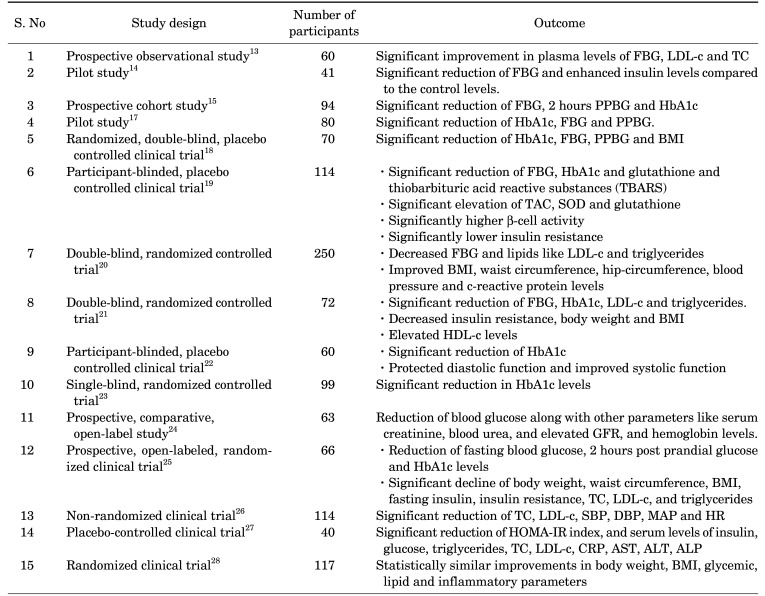
Numerous clinical studies have demonstrated the antidiabetic efficacy of N. sativa (Table 1). The administration of powdered N. sativa seeds for 40 days in 46 patients with type 2 DM produced a significant reduction of fasting blood glucose (FBG), total cholesterol, LDL-cholesterol and triglycerides while increasing the levels of insulin and HDL-cholesterol.12 A prospective observational study found that the administration of 2.5 mL of N. sativa oil 2 times a day in patients taking Atorvastatin 10 mg once daily and Metformin 500 mg twice daily for 6 weeks, resulted in significant improvement in plasma levels of fasting blood glucose, low density lipoprotein (LDL)-cholesterol and total cholesterol.13 Furthermore, a pilot study of 41 patients with type 2 DM revealed that the consumption of N. sativa oil along with their regular antidiabetic medications for 40 days resulted in significant reduction of fasting blood glucose (FBG) and enhanced insulin levels compared to the control levels.14
TABLE 1. Clinical antidiabetic studies of N. sativa.
Moreover, a prospective cohort study of 94 patients with uncontrolled type 2 DM demonstrated that the administration of 2 gm/day of N. sativa for 12 weeks along with their regular antidiabetic medications induced superior diabetes control by reducing FBG, 2 hours post prandial blood glucose (PPBG) and glycosylated hemoglobin A1c (HbA1c) significantly.15 Similarly, the type 2 DM patients who took 5 gm/day of N. sativa tea for 6 months along with their usual antidiabetic drugs, diet and exercise, showed a significant reduction of FBG and HbA1c levels.16 In addition, a study of metabolic syndrome patients with poor glycemic control (HbA1c >7 percentage) demonstrated that the administration of N. sativa for 8 weeks resulted in a significant reduction of HbA1c, FBG and PPBG.17
A randomized, double-blind, placebo controlled clinical trial of 70 type 2 DM patients, demonstrated that the HbA1c, FBG and PPBG were significantly decreased by the administration of 2.5 mL of N. sativa oil 2 times daily for 3 months compared to control group. In addition, the body mass index (BMI) of the patients who received N. sativa oil was also found to be significantly decreased.18
A participant-blinded, placebo controlled clinical trial of 114 type 2 DM patients receiving standard oral antidiabetic drugs demonstrated that the administration of 2 gm of N. sativa daily for 1 year lead to a significant reduction of FBG, HbA1c and glutathione and thiobarbituric acid reactive substances (TBARS) and a significant elevation of total antioxidant capacity (TAC), superoxide dismutase (SOD) and glutathione compared to the control group. In addition, β-cell activity was significantly higher and insulin resistance was significantly lower in patients who received N. sativa along with standard oral antidiabetic drugs.19 Moreover, a double-blind, randomized controlled trial of 250 healthy males with metabolic syndrome found that the supplementation of 1.5 gm of black seeds daily for 8 weeks produced a decrease in FBG and lipids like LDL-cholesterol and triglycerides. Moreover, the administration of black seeds (900 mg/day) in combination with turmeric (1.5 gm/day) for 8 weeks resulted in decreased FBG and LDL-cholesterol along with improved BMI, waist circumference, hip-circumference, blood pressure (BP) and C - reactive protein (CRP) levels.20 In addition, a double-blind, randomized controlled trial of 72 patients with type 2 DM who received 1 gm soft gel capsule of N. sativa oil 3 times daily for 12 weeks, shown a significant reduction of FBG, HbA1c, LDL-cholesterol and triglycerides. Moreover, N. sativa therapy has also decreased insulin resistance, body weight and BMI while increasing HDL-cholesterol levels.21
A participant-blinded, placebo controlled clinical trial of 60 type 2 DM patients taking oral hypoglycemic agents demonstrated that the administration of powdered N. sativa (2 gm/day) for 1 year resulted in significant reduction of HbA1c. In addition, the patients treated with N. sativa for 1 year have shown protected diastolic function and improved systolic function.22 Furthermore, a single-blind, randomized controlled trial of 99 outpatients with metabolic syndrome who received 1.5 mL and 3 mL of oral N. sativa oil daily for 20 days, reported a significant reduction in HbA1c levels.23 In addition, a prospective, comparative, open-label study of patients with chronic kidney disease (stage 3 & 4) due to diabetic nephropathy who received 2.5 mL of oral N. sativa oil daily for 12 weeks along with conservative therapy reported a reduction of blood glucose along with other parameters like serum creatinine, blood urea, and elevated glomerular filtration rate (GFR), and hemoglobin levels.24
A prospective, open-labeled, randomized clinical trial of newly diagnosed type 2 diabetes mellitus patients revealed that the administration of metformin or 1350 mg/day of N. sativa oil capsules for 3 months ensued in reduction of fasting blood glucose, 2 hours post prandial glucose and HbA1c levels caused by N. sativa treatment was found to be inferior to metformin treatment. However, the N. sativa treatment resulted in significant decline of body weight, waist circumference, body mass index, fasting insulin, insulin resistance, total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels, which were comparable to the metformin treatment.25 Moreover, a nonrandomized clinical trial of 114 patients with type 2 diabetes mellitus demonstrated that the supplementation of 2 g/day of N. sativa for 1 year led to a significant reduction of total cholesterol and low-density lipoprotein (LDL) cholesterol along with a significant reduction of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR).26
Similarly, a placebo-controlled clinical trial of 40 patients with type 2 diabetes mellitus demonstrated a significant reduction of Homeostatic model assessment for insulin resistance (HOMA-IR) index, and serum levels of insulin, glucose, triglycerides, total cholesterol and low-density lipoprotein (LDL) cholesterol, c-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), by performing resistance training and consuming N. sativa for 8 weeks.27 In addition, a randomized clinical trial of 117 obese prediabetic subjects determined that the administration of capsules of 450 mg of N. sativa oil 2 times daily or 500 mg of metformin 2 times daily led to statistically similar improvements in body weight, BMI, glycemic, lipid and inflammatory parameters.28
EXPERIMENTAL ANIMAL STUDIES OF N. SATIVA
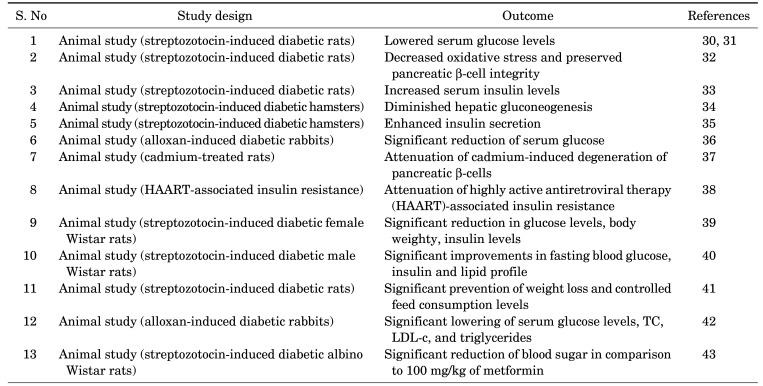
The antidiabetic potential of various extracts of N. sativa has been demonstrated in normal and streptozotocin or alloxan-induced diabetic rats, rabbits or hamsters by various studies (Table 2). Administration of aqueous extract of N. sativa in normal rats resulted in improved glucose tolerance.29 Furthermore, lowered serum glucose levels,30,31 decreased oxidative stress and preserved pancreatic β-cell integrity,32 and increased serum insulin levels33 were observed following the administration of N. sativa in streptozotocin-induced diabetic rats.
TABLE 2. Pre-clinical antidiabetic studies of N. sativa.
In addition, diminished hepatic gluconeogenesis,34 and enhanced insulin secretion35 were observed in streptozotocin-induced diabetic hamsters by the administration of N. sativa oil. Moreover, a significant reduction of serum glucose was noted in alloxan-induced diabetic rabbits by the oral administration of petroleum ether extract of N. sativa oil.36 Similarly, daily intraperitoneal administration of N. sativa attenuated the cadmium-induced degeneration of pancreatic β-cells 37 and highly active antiretroviral therapy (HAART)-associated insulin resistance, in rats.38
An experimental animal study using adult female streptozotocin-induced diabetic Wistar rats demonstrated a significant reduction in glucose levels, body weighty, and insulin levels by the administration of 10 mg/kg of N. sativa extract (Thymoquinone).39 In addition, the administration of 2 mL/kg of N. sativa oil for 30 days produced significant improvements in fasting blood glucose, insulin and lipid profile in streptozotocin-induced diabetic male Wistar rats.40
Moreover, the administration of 24 mg/kg and 48 mg/kg of ethanolic extract of N. sativa for 4 weeks in streptozotocin-induced diabetic rats, resulted in significant prevention of weight loss and controlled feed consumption levels.41 In addition, significant lowering of serum glucose levels, total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides were observed in alloxan-induced diabetic rabbits, following the administration of 2.5 mL/kg of N. sativa oil for 24 days.36
Another experimental animal study using streptozotocin-induced diabetic albino Wistar rats determined that the administration of 1.5 mL of N. sativa oil for 40 days produced a significant reduction of blood sugar in comparison to 100 mg/kg of metformin.42 Similarly, an in-vitro study confirmed the antidiabetic efficacy of N. sativa flavonoids surface coated gold nanoparticles (Au-NPs), which exhibited 78% increase of antidiabetic properties.43
The antidiabetic activity of N. sativa is mainly attributed to thymoquinone, the major bioactive constituent of volatile oil of N. sativa. Daily administration of thymoquinone in streptozotocin-induced diabetic hamsters resulted in decreased hepatic gluconeogenesis via reduction of gluconeogenic precursors such as alanine, glycerol and lactate.44 Similarly, intragastric administration of thymoquinone in streptozotocin nicotinamide-induced diabetic rats ensued in reduction of HbA1c, elevation of insulin and significant, dose dependent hypoglycemic effect.45
An experimental animal study using streptozotocin-induced diabetic rats revealed that the administration of thymoquinone led to significant lowering of plasma glucose levels probably through increased insulin levels and enhanced activities of some cytosolic and mitochondrial enzymes.46 Another experimental animal study using streptozotocin-induced diabetic rats determined a significant reduction of HbA1c, lipid peroxidase and nitric oxide, and higher total antioxidant capacity (TAC) by the supplementation of 50 mg/kg of thymoquinone daily for 4 weeks.47 Moreover, an in-vitro study confirmed that thymoquinone has agonistic activity on peroxisome proliferator activated receptor-γ (PPAR-γ).48
PROPOSED MECHANISMS OF ANTIDIABETIC ACTIVITY OF N. SATIVA
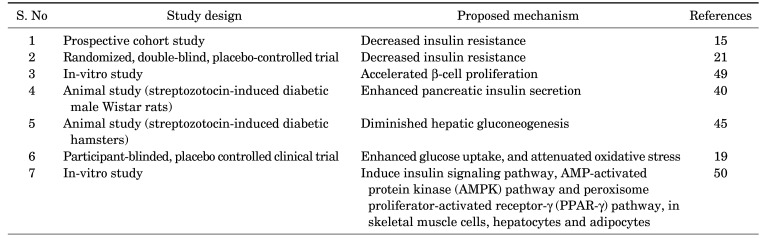
Various mechanisms have been proposed for the antidiabetic activity of N. sativa (Table 3) including decreased insulin resistance,15,21 accelerated β-cell proliferation,49 enhanced pancreatic insulin secretion,40 diminished hepatic gluconeogenesis,45 enhanced glucose uptake, and attenuated oxidative stress.19
TABLE 3. TABLE 3. Proposed mechanisms of antidiabetic effect of N. sativa.
Moreover, the N. sativa seed extract may exert antidiabetic activity through insulin signaling pathways, the AMP-activated protein kinase (AMPK) pathway and peroxisome proliferator-activated receptor-γ (PPAR-γ) pathway, in skeletal muscle cells, hepatocytes and adipocytes.50
DRUG INTERACTIONS POSSIBILITY OF N. SATIVA WITH MODERN ANTIDIABETICS
Interference of effects of one drug by the concomitant administration of other drug(s), food, supplements, herbs, alcohol or tobacco smoke is termed drug interaction.51,52 Drug interactions resulting in enhanced toxicity or diminished therapeutic efficacy are called adverse drug interaction.53,54
The risk of polypharmacy is high among patients with diabetes as they may take many medications to manage comorbidities such as hypertension, dyslipidemia, depression, and others along with their regular antidiabetic medications. Furthermore, black seeds (N. sativa) is recommended as an adjuvant therapy to manage diabetes.55
However, the addition of black seeds (N. sativa) in to the regimen of diabetic patients taking modern antidiabetic medicines may further reduce the blood glucose. Synergistic antidiabetic activity of metformin (1000 mg /day) was observed by the addition of 1 or 2 tablets of Thymoquinone 50 mg daily for 90 days in 60 patients with type 2 diabetes mellitus, through further reduction in the levels of HbA1c and blood glucose.56 Moreover, an experimental animal study using streptozotocin-induced diabetic rats determined that thymoquinone improves the antidiabetic activity of metformin synergistically through its antioxidant properties (decreased malondialdehyde [MDA] and augmented total antioxidant capacity [TAC]).57 Hence, the patients taking this combination should be monitored for the signs and symptoms of hypoglycemia.
CONCLUSION
The use of herbal medicine to manage chronic conditions including diabetes is increasing. Numerous clinical and animal studies have demonstrated the antidiabetic efficacy of black seeds (N. sativa) and its major bioactive constituent thymoquinone. Various mechanisms including decreased insulin resistance, accelerated β-cell proliferation, enhanced pancreatic insulin secretion, diminished hepatic gluconeogenesis, enhanced glucose uptake, and attenuated oxidative stress have been proposed for the antidiabetic activity of N. sativa. The patients with diabetes may use N. sativa as an adjuvant therapy, which may help to reduce the dose of modern antidiabetic medicines and adverse events.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Pinti MV, Fink GK, Hathaway QA, Durr AJ, Kunovac A, Hollander JM. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab. 2019;316:E268–E285. doi: 10.1152/ajpendo.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13:3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Maideen NMP, Balasubramaniam R. Pharmacologically relevant drug interactions of sulfonylurea antidiabetics with common herbs. J Herbmed Pharmacol. 2018;7:200–210. [Google Scholar]
- 5.Pakkir Maideen NM, Manavalan G, Balasubramanian K. Drug interactions of meglitinide antidiabetics involving CYP enzymes and OATP1B1 transporter. Ther Adv Endocrinol Metab. 2018;9:259–268. doi: 10.1177/2042018818767220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James PB, Wardle J, Steel A, Adams J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Glob Health. 2018;3:e000895. doi: 10.1136/bmjgh-2018-000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alqathama A, Alluhiabi G, Baghdadi H, Aljahani L, Khan O, Jabal S, et al. Herbal medicine from the perspective of type II diabetic patients and physicians: what is the relationship? BMC Complement Med Ther. 2020;20:65. doi: 10.1186/s12906-020-2854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otoom SA, Al-Safi SA, Kerem ZK, Alkofahi A. The use of medicinal herbs by diabetic Jordanian patients. J Herb Pharmacother. 2006;6:31–41. [PubMed] [Google Scholar]
- 10.Maideen NMP. Prophetic medicine-Nigella sativa (black cumin seeds) - potential herb for COVID-19? J Pharmacopuncture. 2020;23:62–70. doi: 10.3831/KPI.2020.23.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilal A, Masud T, Uppal AM. BS5-5 Black seed (Nigella sativa) regulates glucose, insulin level and lipid profile in patients with Type 2 diabetes. Diabetes Res Clin Pract. 2008;79 Suppl 1:S19–S20. [Google Scholar]
- 13.Najmi A, Nasiruddin M, Khan RA, Haque SF. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diabetes Dev Ctries. 2008;28:11–14. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilal A, Masud T, Uppal AM, Naveed AK. Effects of Nigella sativa oil on some blood parameters in type 2 diabetes mellitus patients. Asian J Chem. 2009;21:5373–5381. [Google Scholar]
- 15.Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54:344–354. [PubMed] [Google Scholar]
- 16.El-Shamy KA, Mosa MMA, El-Nabarawy SK, El-Qattan GM. Effect of Nigella sativa tea in type 2-diabetic patients as regards glucose homeostasis, liver and kidney functions. J Appl Sci Res. 2011;7:2524–2534. [Google Scholar]
- 17.Najmi A, Nasiruddin M, Khan RA, Haque SF. Therapeutic effect of Nigella sativa in patients of poor glycemic control. Asian J Pharm Clin Res. 2012;5 Suppl 3:224–228. [Google Scholar]
- 18.Hosseini MS, Mirkarimi SA, Amini M, Mohtashami R, Kianbakht S, Fallah Huseini H, et al. Effects of Nigella sativa L. seed oil in type II diabetic patients: a randomized, double-blind, placebo-controlled clinical trial. J Med Plants. 2013;12:93–99. [Google Scholar]
- 19.Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10:e0113486. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin F, Islam N, Anila N, Gilani AH. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome - a double blind randomized controlled trial - TAK-MetS trial. Complement Ther Med. 2015;23:165–174. doi: 10.1016/j.ctim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Heshmati J, Namazi N, Memarzadeh MR, Taghizadeh M, Kolahdooz F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Food Res Int. 2015;70:87–93. [Google Scholar]
- 22.Bamosa A, Kaatabi H, Badar A, Al-Khadra A, Al Elq A, Abou-Hozaifa B, et al. Nigella sativa: a potential natural protective agent against cardiac dysfunction in patients with type 2 diabetes mellitus. J Family Community Med. 2015;22:88–95. doi: 10.4103/2230-8229.155380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rachman PNR, Akrom, Darmawan E. The efficacy of black cumin seed (Nigella sativa) oil and hypoglycemic drug combination to reduce HbA1c level in patients with metabolic syndrome risk. IOP Conf Ser Mater Sci Eng. 2017;259:012018 [Google Scholar]
- 24.Ansari ZM, Nasiruddin M, Khan RA, Haque SF. Protective role of Nigella sativa in diabetic nephropathy: a randomized clinical trial. Saudi J Kidney Dis Transpl. 2017;28:9–14. doi: 10.4103/1319-2442.198093. [DOI] [PubMed] [Google Scholar]
- 25.Moustafa HAM, El Wakeel LM, Halawa MR, Sabri NA, El-Bahy AZ, Singab AN. Effect of Nigella Sativa oil versus metformin on glycemic control and biochemical parameters of newly diagnosed type 2 diabetes mellitus patients. Endocrine. 2019;65:286–294. doi: 10.1007/s12020-019-01963-4. [DOI] [PubMed] [Google Scholar]
- 26.Badar A, Kaatabi H, Bamosa A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: nonrandomized clinical trial. Ann Saudi Med. 2017;37:56–63. doi: 10.5144/0256-4947.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jangjo-Borazjani S, Dastgheib M, Kiyamarsi E, Jamshidi R, Rahmati-Ahmadabad S, Helalizadeh M, et al. Effects of resistance training and nigella sativa on type 2 diabetes: implications for metabolic markers, low-grade inflammation and liver enzyme production. Arch Physiol Biochem. 2021 doi: 10.1080/13813455.2021.1886117. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Mostafa TM, Hegazy SK, Elnaidany SS, Shehabeldin WA, Sawan ES. Nigella sativa as a promising intervention for metabolic and inflammatory disorders in obese prediabetic subjects: a comparative study of Nigella sativa versus both lifestyle modification and metformin. J Diabetes Complications. 2021;35:107947. doi: 10.1016/j.jdiacomp.2021.107947. [DOI] [PubMed] [Google Scholar]
- 29.Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419–424. doi: 10.1016/j.jep.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 30.Kanter M, Meral I, Yener Z, Ozbek H, Demir H. Partial regeneration/ proliferation of the beta-cells in the islets of Langerhans by Nigella sativa L. in streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2003;201:213–219. doi: 10.1620/tjem.201.213. [DOI] [PubMed] [Google Scholar]
- 31.Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol. 2006;44:745–748. [PubMed] [Google Scholar]
- 32.Kanter M, Coskun O, Korkmaz A, Oter S. Effects of Nigella sativa on oxidative stress and beta-cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:685–691. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmeguid NE, Fakhoury R, Kamal SM, Al Wafai RJ. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β-cells of streptozotocin-induced diabetic rats. J Diabetes. 2010;2:256–266. doi: 10.1111/j.1753-0407.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 34.Fararh KM, Atoji Y, Shimizu Y, Shiina T, Nikami H, Takewaki T. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L. oil in streptozotocin-induced diabetic hamsters. Res Vet Sci. 2004;77:123–129. doi: 10.1016/j.rvsc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Fararh KM, Atoji Y, Shimizu Y, Takewaki T. Isulinotropic properties of Nigella sativa oil in Streptozotocin plus Nicotinamide diabetic hamster. Res Vet Sci. 2002;73:279–282. doi: 10.1016/s0034-5288(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 36.Akhtar MT, Qadir R, Bukhari I, Ashraf RA, Malik Z, Zahoor S, et al. Antidiabetic potential of Nigella sativa L seed oil in alloxaninduced diabetic rabbits. Trop J Pharm Res. 2020;19:283–289. [Google Scholar]
- 37.Demir H, Kanter M, Coskun O, Uz YH, Koc A, Yildiz A. Effect of black cumin (Nigella sativa) on heart rate, some hematological values, and pancreatic beta-cell damage in cadmium-treated rats. Biol Trace Elem Res. 2006;110:151–162. doi: 10.1385/BTER:110:2:151. [DOI] [PubMed] [Google Scholar]
- 38.Chandra S, Murthy SN, Mondal D, Agrawal KC. Therapeutic effects of Nigella sativa on chronic HAART-induced hyperinsulinemia in rats. Can J Physiol Pharmacol. 2009;87:300–309. doi: 10.1139/Y09-014. [DOI] [PubMed] [Google Scholar]
- 39.Abduallah AM, Rashed AA, Gamaleldeen AK, Sayed SRM. The effect of Nigella sativa extract (thymoquinone) on glucose insulin levels and body weight of induced diabetic female rats. Am J Life Sci. 2017;5:52–56. [Google Scholar]
- 40.Abdelrazek HMA, Kilany OE, Muhammad MAA, Tag HM, Abdelazim AM. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male Wistar rats. Oxid Med Cell Longev. 2018;2018:8104165. doi: 10.1155/2018/8104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susilowati R, Ghazali A, A'yunin NQ. The potential of black cumin (Nigella sativa L) seeds extract to prevent polyphagia and weight loss in Rattus norvegicus of diabetes mellitus-type 2. El-Haya. 2019;7:119–125. [Google Scholar]
- 42.Sadiq N, Subhani G, Fatima SA, Nadeem M, Zafer S, Mohsin M. Antidiabetic effect of Nigella sativa compared with metformin on blood glucose levels in streptozotocin induced diabetic albino wistar rats. Int J Basic Clin Pharmacol. 2021;10:361–367. [Google Scholar]
- 43.Veeramani S, Narayanan AP, Yuvaraj K, Sivaramakrishnan R, Pugazhendhi A, Rishivarathan I, et al. Nigella sativa flavonoids surface coated gold NPs (Au-NPs) enhancing antioxidant and anti-diabetic activity. Process Biochem. 2021 doi: 10.1016/j.procbio.2021.01.004. [Epub ahead of print] [DOI] [Google Scholar]
- 44.Fararh KM, Shimizu Y, Shiina T, Nikami H, Ghanem MM, Takewaki T. Thymoquinone reduces hepatic glucose production in diabetic hamsters. Res Vet Sci. 2005;79:219–223. doi: 10.1016/j.rvsc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2009;85:830–834. doi: 10.1016/j.lfs.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Fararh KM, Ibrahim AK, Elsonosy YA. Thymoquinone enhances the activities of enzymes related to energy metabolism in peripheral leukocytes of diabetic rats. Res Vet Sci. 2010;88:400–404. doi: 10.1016/j.rvsc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Faisal Lutfi M, Abdel-Moneim AH, Alsharidah AS, Mobark MA, Abdellatif AAH, Saleem IY, et al. Thymoquinone lowers blood glucose and reduces oxidative stress in a rat model of diabetes. Molecules. 2021;26:2348. doi: 10.3390/molecules26082348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Megantara S, Utami D, Puspitasari L, Mustarichie R. Insilico study of thymoquinone as peroxisome proliferator activated receptor gamma agonist in the treatment of type 2 diabetes mellitus. J Pharm Sci Res. 2017;9:1478–1482. [Google Scholar]
- 49.Benhaddou-Andaloussi A, Martineau LC, Spoor D, Vuong T, Leduc C, Joly E, et al. Antidiabetic activity of Nigella sativa. seed extract in cultured pancreatic β-cells, skeletal muscle cells, and adipocytes. Pharm Biol. 2008;46:96–104. [Google Scholar]
- 50.Benhaddou-Andaloussi A, Martineau LC, Vallerand D, Haddad Y, Afshar A, Settaf A, et al. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab. 2010;12:148–157. doi: 10.1111/j.1463-1326.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 51.Maideen NMP, Mohamed N. Pharmacologically relevant drug interactions of nitrovasodilators. Int J Med Rev. 2020;7:30–31. [Google Scholar]
- 52.Maideen NMP. Pharmacodynamic interactions of thiazide diuretics. Int J Med Dev Ctries. 2020;4:1007–1010. [Google Scholar]
- 53.Maideen NMP. Drug interactions of non-dihydropyridine calcium channel blockers involving CYP3A enzymes and P-gp transporter protein. Biointerface Res Appl Chem. 2020;10:6026–6032. [Google Scholar]
- 54.Maideen NMP. Pharmacologically relevant drug interactions of potassium-sparing diuretics. J Pathol Toxicol Res. 2020;1:1–4. [Google Scholar]
- 55.Hamdan A, Haji Idrus R, Mokhtar MH. Effects of Nigella sativa on type-2 diabetes mellitus: a systematic review. Int J Environ Res Public Health. 2019;16:4911. doi: 10.3390/ijerph16244911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali SM, Chen P, Sheikh S, Ahmad A, Ahmad M, Paithankar M, et al. Thymoquinone with metformin decreases fasting, post prandial glucose, and HbA1c in type 2 diabetic patients. Drug Res (Stuttg) 2021;71:302–306. doi: 10.1055/a-1388-5415. [DOI] [PubMed] [Google Scholar]
- 57.El-Aarag B, Hussein W, Ibrahim W, Zahran M. Thymoquinone improves anti-diabetic activity of metformin in streptozotocin-induced diabetic male rats. J Diabetes Metab. 2017;8:780 [Google Scholar]