Key Points
Question
Do patients with cancer develop antibodies after SARS-CoV-2 vaccination, and how do their antibody levels compare with those of health care workers?
Findings
In this cohort study of 901 samples from 595 patients with hematooncological diseases and a control group of health care workers, anti–SARS-CoV-2 spike antibodies after full immunization could be detected, although antibody levels were lower in patients than in health care workers. However, specific subgroups, such as patients who received B-cell–targeting therapy, showed impaired seroconversion.
Meaning
The study findings suggest that lower SARS-CoV-2 antibody levels in patients with cancer after vaccination compared with vaccinated health care workers, and particularly weak seroconversion in specific subgroups, highlight the need for dedicated vaccination trials in patients with cancer.
Abstract
Importance
To our knowledge, little is known about antibody development after SARS-CoV-2 vaccination in immunocompromised individuals, such as patients with cancer.
Objective
To determine whether hematooncological patients develop anti–SARS-CoV-2 antibodies after vaccination.
Design, Setting, and Participants
This retrospective cohort study included 2 independent cohorts of patients who were treated for hematological and solid malignant tumors between October 2020 and May 2021, comprising 901 samples from 595 patients and 58 health care workers (HCWs). Serum samples were collected from patients who were treated at an academic center and a community hospital in a rural area and a control group of HCWs, all of whom received SARS-CoV-2 vaccination.
Main Outcomes and Measures
Total anti–SARS-CoV-2 nucleocapsid (anti-NC) and antispike protein (anti-S) antibodies were measured retrospectively.
Results
In total, 595 patients (320 women [53.8%] and 275 men [46.2%]; median [range] age, 67 [19-96] years) and 58 HCWs (40 women [69.0%] and 18 men [31.0%]; median [range] age, 42 [24-60] years) were included. Previous SARS-CoV-2 infection was documented in 43 of 595 (7.2%), while anti-NC antibodies that suggested previous infections were observed in 49 of 573 evaluable patients (8.6%). In both cohorts, anti-S antibody levels were higher in fully vaccinated patients compared with patients who received 1 dose. After the first vaccination, patients with hematological cancer who received B cell–targeting agents had lower anti-S levels (median, 1.6 AU/mL; range: 0-17 244 AU/mL) than patients who received other therapies (median, 191.6 AU/mL; range, 0-40 000; P < .001) or patients with solid tumors (median, 246.4 AU/mL; range, 0-40 000 AU/mL; P < .001). Anti-S levels after the first vaccination differed according to ongoing antineoplastic treatment modalities, with the lowest median levels in patients who received chemotherapy alone (157.7 AU/mL; range, 0-40 000 AU/mL) or in combination with immunotherapy (118.7 AU/mL; range, 14.1-38 727 AU/mL) and the highest levels in patients with no ongoing antineoplastic treatment (median, 634.3 AU/mL; range, 0-40 000 AU/mL; P = .01). Antibody levels after full immunization were higher in HCWs (median, 2500 U/mL; range, 485-2500 U/mL) than in patients with cancer (median, 117.0 U/mL; range, 0-2500 U/mL; P < .001).
Conclusions and Relevance
In this cohort study of patients with hematooncological diseases and a control group of HCWs, anti-SARS-CoV-2 antibodies after vaccination could be detected in patients with cancer. Lower antibody levels compared with HCWs and differences in seroconversion in specific subgroups underscore the need for further studies on SARS-CoV-2 vaccination in patients with hematooncological disease.
This cohort study examines the development of anti–SARS-CoV-2 antibodies in patients with hematooncological cancer after COVID-19 vaccination.
Introduction
Patients with cancer are at high risk of mortality when infected with SARS-CoV-2, with up to 30% mortality in inpatient populations.1 Although regular hospital visits seem safe if strict safety measures are used, antineoplastic therapy might increase the risk for adverse outcomes.2,3,4 Therefore, COVID-19 vaccination is recommended for all patients with cancer.5
As immunocompromised patients were not included in the studies on messenger RNA (mRNA) or viral vector vaccines, COVID-19 vaccine efficacy data are limited in this vulnerable subgroup.6,7,8 The immunogenicity of mRNA vaccines in solid organ transplant recipients was lower than in immunocompetent study populations.9,10 The first data showed antibody responses in approximately 80% of patients with tumors who were receiving active therapy after the second mRNA vaccine dose.11,12 However, patients with hematooncological disease showed lower seroconversion rates and lower antibody titers than healthy individuals.12,13,14,15,16 Antibody responses were substantially reduced in patients with chronic lymphocytic leukemia who were receiving active or previous anti-CD20 antibody treatment.15,16
Still, large-scale real-life studies that assess the response of patients with hematooncological disease to SARS-CoV-2 vaccines are rare. In this article, we aim to identify factors associated with antibody responses in patients with solid tumors and hematological cancer and compare them with health care workers (HCWs).
Methods
Patient Cohorts
Samples from 2 cohorts with patients with hematooncological disease and 1 control group with HCWs were included. Ongoing treatments included cytotoxic chemotherapy, immune checkpoint inhibitors (ICIs), targeted therapies (eg, tyrosine kinase inhibitors, monoclonal antibodies except ICI), and combinations thereof. Patients who were undergoing treatment and HCWs were routinely tested by nasopharyngeal swabs, and control measures were established, as described previously.2,17,18 In all cohorts, individuals with verified SARS-CoV-2 infections were defined as individuals for whom SARS-CoV-2 RNA could be detected by reverse transcriptase polymerase chain reaction (rt-PCR) in respiratory specimens.
All included patients and HCWs provided written informed consent, and study procedures were performed according to the Declaration of Helsinki and its amendments and according to local and institutional guidelines. The study was approved by the ethics committee of the Medical University of Vienna and the Südtiroler Sanitätsbetrieb. This article follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies as appropriate.
Vienna Patient Cohort
Patients with cancer treated at the Department of Medicine I, Division of Oncology (Medical University of Vienna; Vienna, Austria) and included in the biobanking program after providing written informed consent were identified and included if documentation of SARS-CoV-2 vaccination was available in patient records. BioNTech/Pfizer (BNT162b2), Moderna (mRNA-1273), or AstraZeneca (AZD1222) COVID-19 vaccines were used according to approval status and Austrian federal regulations. Blood samples were processed and stored by the MedUni Wien Biobank facility according to standard operating procedures in an ISO 9001:2015–certified environment19 as part of the biobanking program of the Division of Oncology. Antinucleocapsid (anti-NC) antibodies were measured in serum samples using the Elecsys Anti–SARS-CoV-2 N immunoassay (Roche Diagnostics). A cutoff index of greater than 1.0 as determined by electrochemiluminescence was regarded as positive. Antispike protein (anti-S) antibodies were quantified using the Elecsys Anti-SARS-CoV-2 S (Roche) electrochemiluminescence sandwich immunoassay (quantification range, 0.4-2500.0 U/mL). Anti-NC and anti-S assays detected total antibodies (immunoglobulin [Ig] A, IgG, and IgM) directed against the respective epitopes and were measured on Cobas e801 modular analyzers (Roche).
Meran Patient Cohort
All patients treated for hematological or oncological cancers between October 15, 2020, and May 1, 2021, at the day hospital unit of the Franz Tappeiner Hospital (Meran, Italy) were included and received the BNT162b2 vaccine. Blood samples for anti-S and anti-NC antibodies were taken on the day of the administration of the second vaccination dose. Repeated sampling 21 days after the second dose was performed in patients for whom no anti-S seroconversion (<50 AU/mL) could be observed at the first point. The SARS-CoV-2 IgG II Quant-test (Abbott Laboratories) was used to quantify anti-S antibodies (measurement range, 0-40000 AU/mL). Anti-NC antibodies were detected using the Abbott SARS-CoV-2 IgG assay (chemiluminescent microparticle assay) on the Abbott Alinity platform, in which chemiluminescence was measured as relative light units that were associated with the amount of IgG antibodies. A calibrated index of 1.4 or greater was regarded as positive.
HCW Cohort
The HCW cohort comprised physicians, nurses, physical therapists, environmental service workers, administrative staff, and dietitians working at the Division of Oncology (Medical University of Vienna). Blood biobanking throughout the COVID-19 pandemic was continued as described previously.20 Data on demographic parameters, known SARS-CoV-2–infected contact persons, quarantines, SARS-CoV-2 rt-PCR test results, and information on SARS-CoV-2 vaccination were collected using a structured questionnaire. Plasma of HCWs was retrieved from the biobank, and measurements of anti-NC/anti-S levels were performed as described previously for the Vienna patient cohort.
Statistical Analysis
The independence of categorical variables was assessed using the χ2 test. Means of continuous variables were assessed with the Mann-Whitney U, Wilcoxon signed-rank, and Kruskal-Wallis tests. Correlations between metric variables were evaluated using the Spearman correlation coefficient. Results were considered significant at P ≤ .05. Because of the hypothesis-generating scope of the study, no correction for multiple testing was applied.21 Statistical analysis was performed using GraphPad Prism, version 9.1.2 and R, version 4.0.2 (The R Foundation for Statistical Computing), with RStudio, version 1.3.1056 (RStudio Inc).
Results
Patient Characteristics
In the Vienna patient cohort, 111 patients (18.7%) with solid tumors were included. The median age was 64 years (range, 19-87 years). Most patients received a diagnosis of lung cancer (35 of 111 [31.5%]) and underwent chemotherapy (42 of 111 [37.8%]).
In the Meran patient cohort, 484 patients (81.3%) with a median age of 69 years (range, 24-96 years) were enrolled. Of these, 271 patients (56.0%) had solid tumors, and 213 (44.0%) received a diagnosis of hematological cancer. Most patients received chemotherapy (156 of 484 [32.2%]). Thirty-seven patients (7.6%) received a B cell–targeting agent (eg, rituximab, obinutuzumab, or ibrutinib). Further baseline characteristics are described in Table 1.
Table 1. Baseline Characteristics of the Patient Cohorts.
| Characteristic | Cohort, No. (%) | |
|---|---|---|
| Vienna | Meran | |
| No. | 111 | 484 |
| Age, median (range) | 64 (19-87) | 69 (24-96) |
| Sex | ||
| Men | 55 (49.5) | 220 (45.5) |
| Women | 56 (50.5) | 264 (54.5) |
| Solid tumors | 111 (100) | 271 (56.0) |
| Cancer | ||
| Lung | 35 (31.5) | 26 (9.6) |
| Breast | 14 (12.6) | 69 (40.4) |
| Head and neck | 14 (12.6) | 2 (0.7) |
| Pancreatic | 12 (10.8) | 8 (3.0) |
| Colorectal | 4 (3.6) | 34 (12.5) |
| Upper gastrointestinal | 4 (3.6) | 11 (4.0) |
| Kidney | 1 (0.9) | 14 (5.2) |
| Ovarian | NA | 24 (8.6) |
| Prostate | NA | 28 (10.3) |
| Other | 27 (24.3) | 55 (20.3) |
| Hematological cancer | 0 | 213 (44.0) |
| Essential thrombocythemia | NA | 42 (19.7) |
| Chronic lymphocytic leukemia | 35 (16.4) | |
| Multiple myeloma | 27 (12.7) | |
| Chronic myeloid leukemia | 16 (7.5) | |
| Polycythemia vera | 16 (7.5) | |
| Follicular lymphoma | 15 (7.0) | |
| Myelodysplastic syndrome | 15 (7.0) | |
| Diffuse large B-cell lymphoma | 10 (4.7) | |
| Other | 37 (17.4) | |
| Ongoing treatment | ||
| Chemotherapy | 42 (37.8) | 156 (32.2) |
| Targeted therapy | 1 (0.9) | 127 (26.2) |
| Immune checkpoint inhibition | 30 (27.0) | 29 (6.0) |
| Chemotherapy + targeted therapy | 15 (13.5) | 42 (8.7) |
| Chemotherapy + ICI | 16 (14.4) | 5 (1.0) |
| Targeted therapy + ICI | 4 (3.6) | 0 |
| No ongoing antineoplastic treatment | 3 (2.7) | 95 (19.6) |
| Othera | NA | 30 (6.2) |
| B cell–targeting agent (rituximab, obinutuzumab, ibrutinib) | NA | 37 (7.6) |
Abbreviations: ICI, immune checkpoint inhibitors; NA, not applicable.
Other included hormonal therapy, intravenous immunoglobulins, radiotherapy, and bisphosphonates.
SARS-CoV-2 Seroprevalence and COVID-19 Infections in Patients With Hematooncological Disease
SARS-CoV-2 infections could be verified in 5 of 111 patients (4.5%) in Vienna and 38 of 484 patients (7.9%) in Meran (P = .22). Anti-NC antibodies could be detected in 7 of 111 patients (6.3%) in Vienna and 42 of 462 patients (9.1%) in Meran (P = .35). Three of 111 patients (2.7%) in Vienna and 11 of 462 (2.4%) in Meran showed anti-NC antibodies in the absence of prior positive SARS-CoV-2 rt-PCR results, suggesting unnoticed past infection. Conversely, in 1 of 111 patients (0.9%) in Vienna and 7 of 462 patients (1.5%) in Meran with verified SARS-CoV-2 infections, no anti-NC antibodies could be detected (eTable 1 in the Supplement).
Seroconversion After SARS-CoV-2 Vaccination in Patients With Hematooncological Disease
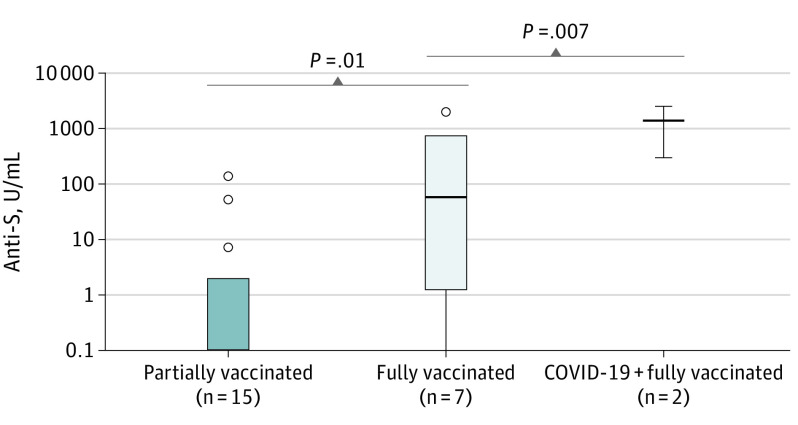
Patient characteristics for vaccinated patients are described in Table 2. In the Vienna cohort, sampling after vaccination was available in 24 of 111 patients (21.6%). Applied vaccines included BNT162b2 in 14 of 24 (58.3%), mRNA-1273 in 6 of 24 (25.0%), and AZD1222 in 4 of 24 (16.7%). Sampling was available in 15 of 24 (62.5%) partially vaccinated patients (1 dose of the vaccination schedule) and 9 of 24 (37.5%) fully vaccinated (2 doses) patients, of whom 2 had documented prior SARS-CoV-2 infection. In partially vaccinated patients, the median time from vaccination to sampling was 14 days (range, 2-35 days), while in fully vaccinated patients, sampling was performed a median of 13 days after the second dose (range, 2-25 days). Median anti-S levels were 0.0 U/mL (range, 0.0-135 U/mL; less than detection range [<0.4 U/mL], 11 of 15 [73.3%]) in partially vaccinated patients, 57.7 U/mL (range, 0.0-2007 U/mL; <0.4 U/mL, 1 of 7 [14.3%]) in fully vaccinated patients, and 295 as well as 2500 U/mL in fully vaccinated patients who had prior SARS-CoV-2 infection (Figure 1). Observed differences were significant between partially and fully vaccinated patients (P = .01) as well as between partially and fully vaccinated patients with prior SARS-CoV-2 infection (P = .007). In partially vaccinated patients, no difference in seroconversion according to the used vaccine could be detected (antibody levels [median (range)]: AZD1222, 0.0 U/mL [0.0-7.28]; <0.4 U/mL, 3 of 4 [75.0%]; BNT162b2, 0.0 U/mL [0.0-135.0]; <0.4 U/mL, 5 of 7 [71.4%]; mRNA-1273, 0.0 U/mL [0.0-2.02]; <0.4 U/mL, 3 of 4 [75.0%]; P = .80). Statistical testing according to the administered vaccination in fully vaccinated patients was not performed because of small sample sizes. Further correction for tumor entities and applied anticancer treatments in the Vienna cohort was not feasible because of small sample sizes in subgroups. There were no documented SARS-CoV-2 infections after the first or second vaccination until data cutoff in the Vienna cohort.
Table 2. SARS-CoV-2 Infection and Vaccination Characteristics in Vaccinated Patients.
| Characteristic | Cohort, No. (%) | |
|---|---|---|
| Vienna | Meran | |
| No. | 24 | 484 |
| Verified SARS-CoV-2 infections | 2 (4.5) | 38 (7.9) |
| Positive anti-NC antibodiesa | 2 (4.5) | 42 (9.1) |
| Used vaccine | ||
| BNT162b2 | 14 (58.3) | 484 (100) |
| mRNA-1273 | 6 (25.0) | NA |
| AZD1222 | 4 (16.7) | NA |
| No. of received doses at time of sampling | ||
| 1 (partially vaccinated) | 15 (62.5) | 484 (100)b |
| 2 (fully vaccinated) | 9 (37.5) | 125 (25.8)b |
Abbreviations: anti-NC, anti–SARS-CoV-2 nucleocapsid; AZD1222, AstraZeneca; BNT162b2, BioNTech/Pfizer; mRNA-1273, Moderna; NA, not applicable.
Available in 462 of 484 patients (95.5%) in the Meran cohort.
Sampling after 1 dose was available in all patients in the Meran cohort, whereas sampling after 2 doses was performed in patients with anti-S levels less than 50 AU/mL after the first vaccination.
Figure 1. Antispike (Anti-S) Antibody Levels in Partially and Fully Vaccinated Patients in the Vienna Cohort and Fully Vaccinated Patients With Prior SARS-CoV-2 Infection.
P values as determined by Mann-Whitney U test.
In the Meran cohort, all patients were vaccinated with BNT162b2 and underwent blood sampling after the first dose. No serious adverse events after vaccination were observed. The median anti-S level after the first dose was 201.2 AU/mL (range, 0-40 000 AU/mL). In 125 of 484 patients (25.8%) with anti-S levels of less than 50 AU/mL, a repeated measurement after the second vaccination dose was performed. Anti-S levels increased in those patients after the second dose (median [range] first vaccination, 1.7 AU/mL [0-49.4 AU/mL] vs second vaccination: 50 AU/mL [0-40 000 AU/mL]; P < .001; Figure 2A). Still, 62 of 125 patients (49.6%) with impaired seroconversion after the first dose had low anti-S levels after the second vaccination. Patients with hematological cancer had lower anti-S levels (median, 139.3 AU/mL; range, 0-40 000 AU/mL; below detection range [<50 AU/mL], 69 of 213 [32.4%]) than patients with solid tumors (median, 246.4 AU/mL; range, 0-40 000 AU/mL; <50 AU/mL, 58 of 271 [21.4%]; P = .01). This difference was attributable to patients with hematological cancer receiving B cell–targeting therapies who had lower anti-S levels than those without B cell–targeting therapy in hematological cancer and solid tumors after the first (patients receiving B cell–targeting agents, median 1.6 U/mL; range, 0-17244 AU/mL; <50 AU/mL, 26 of 37 [70.3%]; hematological cancer without B cell–targeting agents, 191.6 U/mL; range, 0-40 000 AU/mL; <50 AU/mL, 43 of 176 [24.4%]; solid tumors, 246.4 U/mL; range, 0-40 000 AU/mL; <50 AU/mL, 58 of 271 [21.4%]; Figure 2B) and second vaccination dose (patients receiving B cell–targeting agents: median, 0 U/mL; range: 0-140.6 AU/mL; <50 AU/mL, 24 of 26 [92.3%]; hematological malignancies without B cell–targeting agents, 172.8 U/mL; range, 0.0-13346 AU/mL; <50 AU/mL, 18 of 41 [43.9%]; solid tumors, 124.9 U/mL; range, 0-40 000 AU/mL; <50 AU/mL, 20 of 58 [34.5%]; Figure 2C). After the first dose, seroconversion differed according to the ongoing antineoplastic treatment in solid tumors, with the lowest median levels observed in patients who were receiving chemotherapy (157.7 AU/mL; range, 0-40 000 AU/mL; <50 U/mL, 33 of 101 [32.7%]) or chemotherapy plus ICI (118.7 AU/mL; range, 14.1-38 727 AU/mL; <50 U/mL, 1 of 4 [25.0%]) and the highest median levels in patients with no ongoing antineoplastic treatment (634.3 AU/mL; range, 0-40 000 AU/mL; <50 U/mL, 4 of 26 [15.4%]; Figure 2D). In patients with solid tumors for whom a second sample was obtained, no differences were observed (eFigure in the Supplement). There were no correlations between age and anti-S levels after the first (Spearman r = −0.11; P = .02) and second (r = 0.005; P = .96) vaccination. Similarly, anti-S levels did not differ between men and women after the first dose (median [range] for men, 185.0 AU/mL [0-40 000 AU/mL]; <50 AU/mL, 59 of 220 [26.8%]; women: 237.7 AU/mL [0-40 000 AU/mL]; <50 AU/mL, 68 of 264 [25.8%]; P = .38) and dose 2 (men: 58.4 AU/mL [0-13 346 AU/mL]; <50 AU/mL, 26 of 59 [44.1%]; women: 36.75 AU/mL [0-40 000 AU/mL]; <50 AU/mL, 36 of 66 [54.5%]; P = .35). Two patients were infected between the first and second dose of vaccination; no further SARS-CoV-2 infections after vaccinations were recorded until end of the study.
Figure 2. Antispike (Anti-S) Levels in the Meran Cohort.
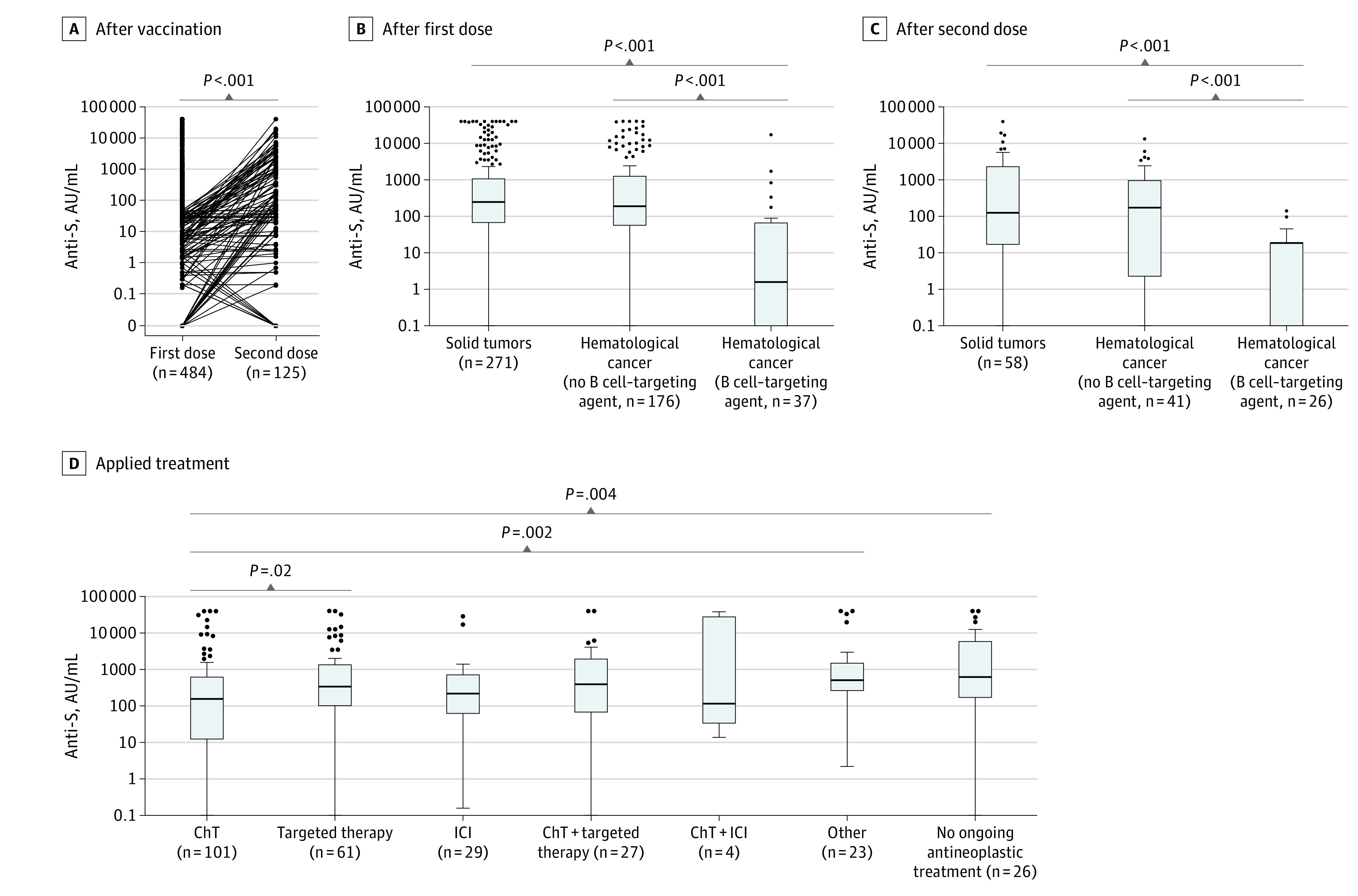
A, After the first and second dose of BioNTech/Pfizer (BNT) 162b2 vaccination. P value as determined by Wilcoxon signed rank test. Anti-S levels after the first (B) and second (C) dose of BNT162b2 vaccination in patients with solid tumors and hematological cancer with/without B cell–targeted treatment. P values as determined by Mann-Whitney U test. D, Anti-S levels after the first dose according to applied treatment in patients with solid tumors (other including hormonal therapy, radiotherapy, and bisphosphonates). P values as determined by Mann-Whitney U test. ChT indicates chemotherapy; ICI, immune checkpoint inhibitor.
HCW Cohort
Samples from 58 HCWs were available, including 19 physicians/medical oncologists (32.8%), 24 nurses (41.4%), 6 administrative staff members (10.3%), and 9 other professionals (15.5%). In total, 37 participants (63.8%) worked primarily at the inpatient ward and clinic, followed by 13 (22.4%) at the outpatient department and 8 (13.8%) who worked in administrative/laboratory environments and had no direct contact with patients. Baseline characteristics of the HCW cohort are described in eTable 2 in the Supplement.
In our previous article based on blood samples taken between April and June 2020,20 2 individuals with verified SARS-CoV-2 infection had detectable anti-NC antibodies that were still measurable in November 2020. Additionally, 1 HCW had anti-NC antibodies, although no prior SARS-CoV-2 infection was documented. However, that individual underwent quarantine because of a SARS-CoV-2 infection of a household member. In all 3 HCWs who previously had COVID-19, anti-S antibodies were detected before vaccination (25.8 U/mL, 26.2 U/mL, and 2152 U/mL, respectively).
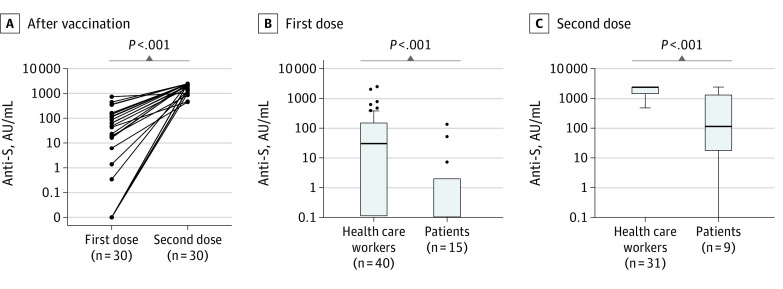
Longitudinal sampling could be performed in 49 HCWs (84.5%). Until the second follow-up, 2 additional HCWs were infected with SARS-CoV-2 and developed anti-NC antibodies. In total, 33 of 49 individuals (67.3%) were vaccinated with BNT162b2, followed by 10 HCWs (20.4%) who received AZD1222. At the first follow-up, 31 of 48 evaluable HCWs (64.6%) had received 1 dose (median days from dose 1 to sampling, 17; range, 3-26 days), while 17 (35.4%) were unvaccinated. At the second follow-up, 31 of 48 evaluable HCWs (64.6%) had undergone the full vaccination schedule (median days from dose 2 to sampling, 29; range, 19-48 days), while 9 (18.8%) were vaccinated with 1 dose (median days from vaccination to sampling, 18; range, 5-31 days) and 8 (16.7%) were still unvaccinated. Anti-S levels after 1 dose showed a median of 49.6 U/mL (range, 0-780 AU/mL; <0.4 U/mL, 8 of 31 [25.8%]) in those who received BNT162b2 and a median of 21.1 U/mL (range, 0-2500 AU/mL;<0.4 U/mL, 2 of 9 [22.2%]) in those who received AZD1222 (P = .99). Full follow-up after the first and second doses was available for 30 HCWs (51.7%) (Figure 3A) who were vaccinated with BNT162b2. All individuals developed anti-S levels of greater than 100 U/mL after the second dose.
Figure 3. Antispike (Anti-S) Antibody Levels in Health Care Workers and Patients With Cancer.
A, In health care workers after the first and second dose of vaccination. P values as determined by Wilcoxon signed rank test. Anti-S levels in healthy controls/health care workers and patients with cancer after the first (B) and second (C) dose. P values as determined by the Mann-Whitney U test.
Differences in Seroconversion After Vaccination in Patients vs HCWs
As different assays were used, a direct comparison of anti-S levels between patients and HCWs was only feasible with the Vienna patient cohort. After the first dose, HCWs had median anti-S levels of 30.60 U/mL (range, 0-2500 AU/mL; <0.4 U/mL, 10 of 40 [25.0%]) compared with 0 U/mL in patients (range, 0.0-135 AU/mL; <0.4 U/mL, 11 of 15 [73.3%]; P < .001; Figure 3B). Similarly, anti-S levels after full immunization were higher in HCWs (median, 2500 U/mL; range, 485-2500 AU/mL; <0.4 U/mL, 0 of 31 [0%]) than in patients (median, 117 U/mL; range, 0-2500 AU/mL; <0.4 U/mL, 1 of 9 [11.1%]; P < .001; Figure 3C).
Discussion
Recent studies reported that 1 dose yields low seroconversion in patients with cancer, whereas antibody levels were considerably higher after the second dose,11,12,22,23 highlighting the importance of following the full vaccination schedule in patients with cancer. Therefore, policies to defer the second dose to reach broad single-dose coverage in the population, such as those adopted in the UK, may not be appropriate in patients with hematooncological disease.24 The results from this study suggest that patients with hematooncological disease have lower seroconversion levels than HCWs after the first dose, with substantially increasing antibody levels after the booster dose. In addition, our data suggest that fully vaccinated patients who have had previous COVID-19 infection (with consequently triple antigen exposure) have the numerically highest antibody levels. Previously published data showed that patients with cancer who recovered from COVID-19 had high antibody levels even after 1 dose of the BNT162b2 vaccine.25 Conversely, it was observed that cellular and humoral immune responses were not consistently more accentuated in the 17 patients with prior SARS-CoV-2 infections compared with naïve patients.22 These data highlight the importance of repeated antigenic exposure by full vaccination to mount effective immune responses in patients with cancer.
Patients who were receiving anti–B-cell therapies exhibited impaired serological immune responses, confirming previous findings in patients with hematological cancer.15,16 Similar results have been reported in patients with chronic inflammatory diseases who were receiving B cell–depleting therapies,26 suggesting that impaired vaccination-induced humoral responses are associated with ongoing anti–B-cell therapies rather than the underlying conditions. Indeed, overall anti-S antibody levels of patients with hematological disease who were not treated with B cell–targeting agents were comparable with patients with solid cancer in this study cohort. Patients with myeloma were found to yield IgG seroconversion rates of 56% after the first vaccination,27 which is similar to the percentage seen in solid tumors.13
Adequate immune activity after vaccinations is orchestrated by a complex interplay between cellular and humoral immunity. Still, data on cellular immune responses after vaccinations in patients during rituximab therapy are conflicting.28,29,30 Concerning SARS-CoV-2 vaccination, T cell–mediated immune responses after BNT162b2 vaccination could be observed in patients with rheumatic diseases, suggesting that BNT162b2 vaccination may nevertheless exert activity in B cell–depleted patients.31 Recently, it was similarly shown in patients with cancer that CD4+ and CD8+ T cell responses could be observed after mRNA vaccination.32 However, it remains unclear whether these in vitro results translate to clinical efficacy and how stable these immune responses are given the evolution of virus variants.
In the study’s patient cohorts, 4.5% and 7.9% had prior SARS-CoV-2 infection, with anti-NC antibodies in 6.3% and 9.1% in Vienna and Meran, respectively. In contrast, approximately 7% and 14% of the general population in the respective areas recovered from COVID-19 according to official numbers (effective June 18, 2021). Strict safety measures in hematooncological departments and the self-protecting behavior of patients may have contributed to prevent uncontrolled viral spread throughout the pandemic.33 However, with impaired vaccination-induced immune responses, continued measures and patient education are still necessary to ensure the safety of patients with hematooncological disease. This is particularly relevant, as valid antibody thresholds or biomarkers reflecting sufficient immunity from SARS-CoV-2 infection are elusive,34 especially as SARS-CoV-2 variants are emerging that may hamper the efficacy of the currently available vaccines.
Limitations
This study has limitations. First, immunoassays from different manufacturers were used in the Vienna and the Meran cohorts, precluding pooled analyses of the included cohorts. Although the World Health Organization issued an international standard on SARS-CoV-2 antibody assays and antibody levels can be converted to universal binding antibody units, it was recently shown that those values are not interchangeable, underscoring the need for further standardization.34 Second, the retrospective study design of the included cohorts is inherently associated with heterogeneous cohorts and small numbers in specific subgroups. Lastly, times between vaccination and sampling varied in the Vienna patient and HCW cohorts. Thus, seroconversion may not have been measurable at the time of sampling, considering the kinetics of humoral immune responses.
Conclusions
This cohort study of patients with hematooncological diseases and a control group of HCWs suggests that patients with cancer are able to develop SARS-CoV-2-specific antibodies. Based on 2 independent cohorts from an academic center and a communal hospital in a rural area located in 2 European countries, we observed that antibody levels were lower in patients than in a HCW control group, and specific subgroups, such as patients who were receiving chemotherapy and B cell–targeting agents, showed a particularly impaired serological response. To our knowledge, this is the largest study to measure anti-SARS-CoV-2 antibody levels after vaccination in this vulnerable population. The included patients represent a wide spectrum of solid tumors and blood cancer as well as antineoplastic treatments, providing real-life insights. However, dedicated trials that evaluate the effectiveness of SARS-CoV-2 vaccination in patients with cancer are needed to ensure optimal cancer care during the pandemic and beyond.
eFigure. Anti-S levels after the second dose according to applied treatment in patients with solid tumors (other including hormonal therapy, radiotherapy and bisphosphonates
eTable 1. Confirmed SARS-CoV-2 infections and anti-NC detection rates in hemato-oncological patients
eTable 2. Health care workers’ cohort
References
- 1.Desai A, Gupta R, Advani S, et al. Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta-analysis of cohort studies. Cancer. 2021;127(9):1459-1468. doi: 10.1002/cncr.33386 [DOI] [PubMed] [Google Scholar]
- 2.Berghoff AS, Gansterer M, Bathke AC, et al. SARS-CoV-2 testing in patients with cancer treated at a tertiary care hospital during the COVID-19 pandemic. J Clin Oncol. 2020;38(30):3547-3554. doi: 10.1200/JCO.20.01442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lièvre A, Turpin A, Ray-Coquard I, et al. ; GCO-002 CACOVID-19 Collaborators/Investigators . Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19). Eur J Cancer. 2020;141:62-81. doi: 10.1016/j.ejca.2020.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218-1223. doi: 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Society for Medical Oncology . Covid-19 vaccinations and patients with cancer—an ESMO call to action. Accessed June 17, 2021. https://www.esmo.org/policy/esmo-call-to-action-on-covid-19-vaccinations-and-patients-with-cancer-vaccinate-monitor-educate-endorsements [DOI] [PMC free article] [PubMed]
- 6.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. doi: 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784-1786. doi: 10.1001/jama.2021.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204-2206. doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shroff RT, Chalasani P, Wei R, et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. medRxiv. Posted May 14, 2021. doi: 10.1101/2021.05.13.21257129 [DOI]
- 12.Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;(July):1-8. doi: 10.1001/jamaoncol.2021.2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palich R, Veyri M, Marot S, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32(8):1051-1053. doi: 10.1016/j.annonc.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in elderly myeloma patients after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674-3676. doi: 10.1182/blood.2021011904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-3173. doi: 10.1182/blood.2021011568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;(May):1-3. doi: 10.1038/s41375-021-01270-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong D, Rauch S, Petter C, Haspinger E, Alber M, Mitterer M. Infection rate and clinical management of cancer patients during the COVID-19 pandemic: experience from a tertiary care hospital in northern Italy. ESMO Open. 2020;5(3):e000810. doi: 10.1136/esmoopen-2020-000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong D, San Nicolò KO, Alber M, Mitterer M. Evaluating the longitudinal effectiveness of preventive measures against COVID-19 and seroprevalence of IgG antibodies to SARS-CoV-2 in cancer outpatients and healthcare workers. Wien Klin Wochenschr. 2021;133(7-8):359-363. doi: 10.1007/s00508-020-01807-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslacher H, Gerner M, Hofer P, et al. Usage data and scientific impact of the prospectively established fluid bioresources at the hospital-based MedUni Wien Biobank. Biopreserv Biobank. 2018;16(6):477-482. doi: 10.1089/bio.2018.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuereder T, Berghoff AS, Heller G, et al. SARS-CoV-2 seroprevalence in oncology healthcare professionals and patients with cancer at a tertiary care centre during the COVID-19 pandemic. ESMO Open. 2020;5(5):e000889. doi: 10.1136/esmoopen-2020-000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54(4):343-349. doi: 10.1016/S0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- 22.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-778. doi: 10.1016/S1470-2045(21)00213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133-1140. doi: 10.1001/jamaoncol.2021.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero-Brufau S, Chopra A, Ryu AJ, et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ. 2021;373(May):n1087. doi: 10.1136/bmj.n1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong D, Mair MJ, Mitterer M. High levels of anti-SARS-CoV-2 IgG antibodies in previously infected patients with cancer after a single dose of BNT 162b2 vaccine. Eur J Cancer. 2021;154:4-6. doi: 10.1016/j.ejca.2021.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv. Posted April 9, 2021. doi: 10.1101/2021.04.05.21254656 [DOI]
- 27.Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389-e392. doi: 10.1016/S2352-3026(21)00110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv. 2021;5(12):2624-2643. doi: 10.1182/bloodadvances.2021004629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643-1648. doi: 10.1016/j.vaccine.2010.12.072 [DOI] [PubMed] [Google Scholar]
- 30.Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946-1953. doi: 10.1182/blood-2013-04-494096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;annrheumdis-2021-220781. doi: 10.1136/annrheumdis-2021-220781 [DOI] [PubMed] [Google Scholar]
- 32.Mairhofer M, Kausche L, Kaltenbrunner S, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;(August):S1535-6108(21)00441-4. doi: 10.1016/j.ccell.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curigliano G, Banerjee S, Cervantes A, et al. ; Panel members . Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31(10):1320-1335. doi: 10.1016/j.annonc.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkmann T, Perkmann-Nagele N, Koller T, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;e0024721. doi: 10.1128/spectrum.00247-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Anti-S levels after the second dose according to applied treatment in patients with solid tumors (other including hormonal therapy, radiotherapy and bisphosphonates
eTable 1. Confirmed SARS-CoV-2 infections and anti-NC detection rates in hemato-oncological patients
eTable 2. Health care workers’ cohort