This cohort study assesses the structural and functional determinants of monocular reading performance to establish its concurrent validity in geographic atrophy, the ability to detect a change in reading performance, and whether patients with geographic atrophy are affected by binocular inhibition of reading performance.
Key Points
Question
What is the association of reading performance in patients with geographic atrophy secondary to age-related macular degeneration with established visual function and structural biomarkers?
Findings
In this cohort study of 150 eyes of 85 participants, reading acuity was most strongly associated with best-corrected visual acuity and geographic atrophy area in the central and inner-right Early Treatment Diabetic Retinopathy Study subfield. Regarding reading speed, the most associated variables were best-corrected visual acuity, low-luminance visual acuity, and geographic atrophy area in the central, the inner-right, and the inner-upper Early Treatment Diabetic Retinopathy Study subfields.
Meaning
The association of reading performance with visual functional and structural biomarkers supports the validity of reading performance as an end point in clinical trials.
Abstract
Importance
As a disabling and frequent disease, geographic atrophy secondary to age-related macular degeneration (AMD) constitutes an important study subject. Emerging clinical trials require suitable end points. The characterization and validation of reading performance as a functional outcome parameter is warranted.
Objective
To prospectively evaluate reading performance in geographic atrophy and to assess its association with established visual function assessments and structural biomarkers.
Design, Setting, and Participants
The noninterventional, prospective natural history Directional Spread in Geographic Atrophy study included patients with geographic atrophy secondary to AMD who were recruited at the University Hospital in Bonn, Germany. Participants were enrolled from June 2013 to June 2016. Analysis began December 2019 and ended January 2021.
Main Outcomes and Measures
Reading acuity and reading speed were assessed using Radner charts. Longitudinal fundus autofluorescence and infrared reflectance images were semiautomatically annotated for geographic atrophy, followed by extraction of shape-descriptive variables. Linear mixed-effects models were applied to investigate the association of those variables with reading performance.
Results
A total of 150 eyes of 85 participants were included in this study (median [IQR] age, 77.9 [72.4-82.1] years; 51 women [60%]; 34 men [40%]). Reading performance was impaired with a median (IQR) monocular reading acuity of 0.9 (0.4-1.3) logarithm of the reading acuity determination and a reading speed of 52.8 (0-123) words per minute. In the multivariable cross-sectional analysis, best-corrected visual acuity, area of geographic atrophy in the central Early Treatment Diabetic Retinopathy Study (ETDRS) subfield, classification of noncenter vs center-involving geographic atrophy, and area of geographic atrophy in the inner-right ETDRS subfield showed strongest associations with reading acuity (cross-validated R2 for reading acuity = 0.69). Regarding reading speed, the most relevant variables were best-corrected visual acuity, low-luminance visual acuity, area of geographic atrophy in the central ETDRS subfield, in the inner-right ETDRS subfield, and in the inner-upper ETDRS subfield (R2 for reading speed = 0.67). In the longitudinal analysis, a similar prediction accuracy for reading performance was determined (R2 for reading acuity = 0.73; R2 for reading speed = 0.70). Prediction accuracy did not improve when follow-up time was added as an independent variable. Binocular reading performance did not differ from reading performance in the better-seeing eye.
Conclusions and Relevance
The association of reading acuity and speed with visual functional and structural biomarkers supports the validity of reading performance as a meaningful end point in clinical trials. These findings suggest that measures in clinical and low-vision care for patients with geographic atrophy should focus primarily on the better-seeing eye.
Introduction
Age-related macular degeneration (AMD) is the most common cause of central vision loss in industrialized countries.1,2,3 Around 11.26 million people are affected by late AMD, and an increase to 18.57 million people by 2040 is expected.4 The high prevalence as well as the expected overall increase of affected patients owing to the demographic trend and the lack of treatment options make the dry late form of AMD a critical study subject.2 Therapeutic trials currently are emerging and require suitable end points.
Geographic atrophy (GA) as the nonexudative late-stage manifestation is hallmarked by atrophy of the retinal pigment epithelium and concurrent atrophy of the outer neuroretina and choriocapillaris.5,6,7,8 Typically, GA foci manifest initially in the parafovea by sparing the fovea itself.5,9,10,11 Over time, these foci gradually expand in size, coalescence, and eventually involve the fovea.5,11 Best-corrected visual acuity (BCVA), the most frequently applied functional trial end point in ophthalmology, may be preserved in patients with foveal sparing, while these patients experience severe impairment in daily visual tasks due to parafoveal atrophy.12,13,14
Overall, BCVA is suboptimal to assess the continuous progression of GA in a considerable fraction of patients. Given the clear preference of regulatory agencies, including the US Food and Drug Administration, toward performance outcome measures over anatomic end points,15 characterization and validation of an alternative performance outcome measures besides BCVA in a disease-specific manner is warranted. Reading performance is an obvious candidate. Hypothetically, it is more sensitive to GA (scotoma) progression in the parafovea than BCVA.
Previously, it has been shown that monocular reading speed is correlated linearly with the square-root GA area cross-sectionally16 and that monocular (and binocular) reading speed declines over time.17,18 While the association between retinal structure and reading acuity has recently been studied and modeled in the context of foveal sparing,19,20,21,22 no detailed structure-function correlation has been reported for the overall cohort of patients with GA, to our knowledge. In addition, reading performance quantified in terms of reading acuity may constitute an alternative to reading speed and warrants investigation. Moreover, the phenomenon of binocular inhibition of reading performance has been reported for other retinal diseases23 but not studied specifically in the context of GA. For clinical and low-vision care, it is warranted to understand the structure-function correlation precisely and adjust visual aids accordingly.
This study aimed to (1) identify the structural and functional determinants of monocular reading performance to establish its concurrent validity in GA, (2) assess the ability to detect a change in reading performance, and (3) examine whether patients with GA are affected by binocular inhibition of reading performance.
Methods
Participants
Participants were recruited in the context of the noninterventional, prospective natural history study Directional Spread in Geographic Atrophy (NCT02051998) at the Department of Ophthalmology at the University Hospital in Bonn, Germany.11,24 Participants with GA in both eyes and who were 55 years or older were included. Exclusion criteria were (1) presence of choroidal neovascular membrane, (2) any ocular disease that may confound retinal assessment, (3) a limited survival prognosis, and (4) difficulties in adhering to the examination schedule.
This study adhered to the tenets of the Declaration of Helsinki25 and was approved by the institutional review board of the University of Bonn. Written informed consent was obtained, and participants received no stipend. Participants were enrolled from June 2013 to June 2016.
Clinical Assessment
Data on age, sex, and medical history were transferred to the case report forms from the health insurance data (electronic medical records) with confirmation by the patient. Data on race and ethnicity were not collected. BCVA, low-luminance visual acuity (LLVA) (2.0-log neutral density filter), and reading performance were assessed using the Early Treatment Diabetic Retinopathy Study (ETDRS) and Radner charts, respectively.26 Radner charts include sentences of standardized grammatical construction, equal in number, position, and length of words, lexical difficulty, and syntactical complexity. Print sizes progress geometrically from 0.25 m to 6.3 m. The logarithmic scale is given in logarithm of the reading acuity determination (logRAD). Reading acuity was defined as the logRAD at the smallest sentence (print size) a patient could read in no more than 30 seconds (range, −0.2 to 1.3 logRAD). The fastest time achieved across the varying print sizes (maximum reading speed) defined our reading speed in words per minute (wpm).
Imaging
Following pupil dilatation with tropicamide, 0.5%, and phenylephrine, 2.5%, participants underwent 30° × 30° fundus autofluorescence (λ excitation, 488 nm; λ emission, 500-700 nm), 30° × 30° infrared reflectance (λ, 815 nm), and 30° × 25° spectral-domain optical coherence tomography imaging (121 B-scans, ART 25) using a Spectralis HRA+OCT2 (Heidelberg Engineering).
Fundus-controlled perimetry was performed with the S-MAIA (CenterVue),27 identifying 2 preferred retinal loci (PRL) of fixation, the initial PRL, the PRL after the first 10 seconds of fixation, and the final PRL, based on all fixation measurements of the test.28
Image Grading
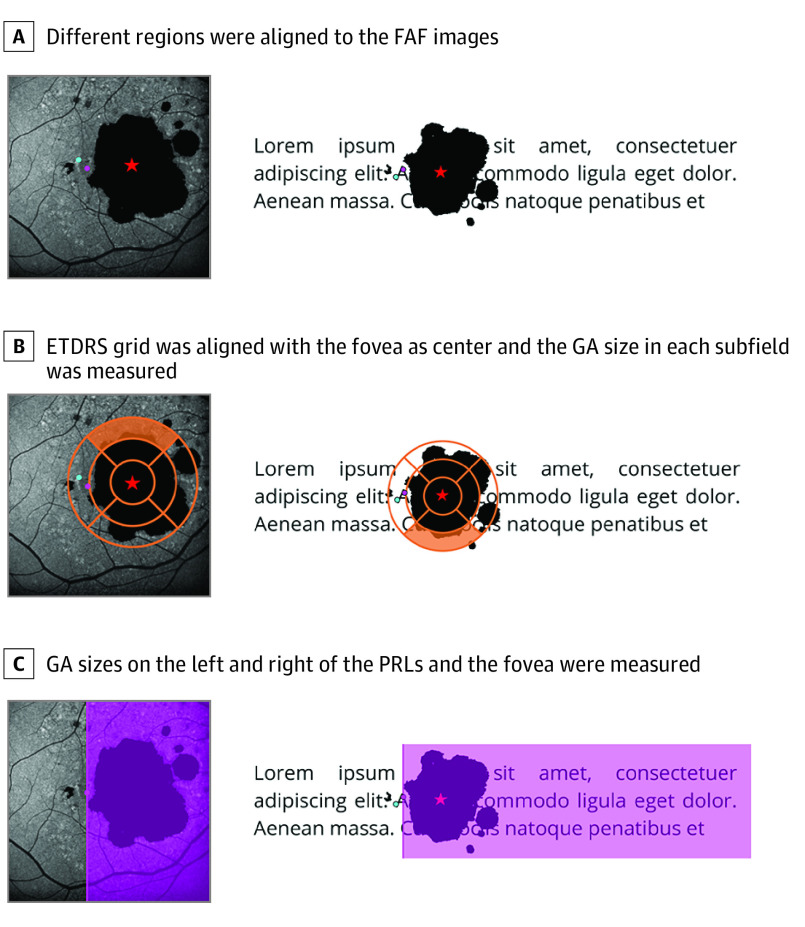
The GA area was semiautomatically annotated using the RegionFinder software in fundus autofluorescence and infrared reflectance images.29,30 The grading task was performed by 2 readers (S.H.K. and L.G.) with arbitration by a third reader (M.P.) if they deviated by more than 0.3 mm2. Subsequently, the coaquired infrared reflectance image with labeled fovea was registered to the fundus autofluorescence images using ImageJ with vascular bifurcations as registration landmarks. We extracted the GA area in the central 4 inner and 4 outer ETDRS subfields (Figure 1B) with an ad hoc–developed plugin for ImageJ. The ETDRS grid was centered to the fovea.
Figure 1. Measurements Extracted From the FAF Images.
Different regions were aligned to the fundus autofluorescence (FAF) images (A). The star indicates the fovea; the pink dot, the initial preferred retinal locus (PRL); and the blue dot, the final PRL. The images on the right show where those regions and the geographic atrophy (GA) are projected on a text while reading. B, The Early Treatment Diabetic Retinopathy Study (ETDRS) grid was aligned with the fovea as center and the GA size in each subfield was measured. The upper outer subfield is marked in orange to highlight its projection on the text. C, The GA sizes on the left and right of the PRLs and the fovea are measured. The GA left of the PRL is in reading direction of a patient (pink area).
In addition, we extracted the overall GA area to the left of the fovea (left in terms of retinal space, ie, in reading direction in visual field terms) and to its right (ie, against reading direction). Similarly, we extracted the GA area left and right to the PRL (Figure 1C), resulting in 15 regions in total. The distances between the initial PRL, the final PRL, the closest GA border, and fovea were determined, resulting in 6 distances (distance from initial to final PRL, distance from initial PRL to GA, distance from initial PRL to fovea, distance from final PRL to GA, distance from final PRL to fovea, and distance from GA to fovea). Also, we explored whether the PRLs were more nasal or temporal than the fovea.
Statistical Analyses
Statistical analyses were performed using the software environment R version 4.0.4 (R Foundation) and the packages lme4, ranger, glmnet, and glmmLasso. BCVA and LLVA were transformed to the base 10 logMAR.31 GA area was square-root transformed. Variables were assessed for normality using normal quantile-quantile plots and the Shapiro-Wilk test. For normally distributed variables, the mean and SD are provided. Otherwise, the median and IQR are presented. All continuous variables were centered scaled to obtain standardized coefficients. The provided P values are 2-sided and not adjusted for multiple comparisons. P values less than .05 were statistically significant.
The better- and worse-seeing eyes were defined by the respective values in each individual determinant. When the values were identical for both eyes, this choice was arbitrary. However, as we only compared the values of the individual determinants, this does not matter because both values will be the same.
First, for the univariable cross-sectional analysis, mixed-effects models were applied to analyze the association between the individual determinants and monocular reading performance (acuity or speed) as a dependent variable. Patients were considered as random effects. The t statistic of the respective fixed factor served as a variable importance measure.
Second, for the cross-sectional multivariable analysis at baseline, we applied least absolute shrinkage and selection operator (LASSO) regression for linear mixed-effects models with the reading performance as a dependent variable and patient as a random-effects term (random intercept model). LASSO regression increases model stability by performing variable selection and shrinking coefficient estimates toward 0. This enhances the prediction accuracy as well as interpretability by providing a parsimonious model (ie, a model with fewer predictors) while taking into consideration of the multicollinearity of the data (eFigure 1 in the Supplement).32 Nested patient-based cross-validation was applied to estimate prediction accuracy (outer leave-1-out cross-validation), while simultaneously optimizing the tuning parameter lambda of the LASSO regression (nested inner leave-1-out cross-validation).33 For comparison, we present the results of a conventional (least-squares) cross-sectional multivariable regression analysis (eFigure 2 in the Supplement). Variables were selected using stepwise backward selection based on the value of the F statistic. Degrees of freedom were calculated using Satterthwaite approximation.
Third, we analyzed the longitudinal multivariable data using LASSO regression for linear mixed-effects models. Here, follow-up time was considered as an independent variable, reading performance as a dependent variable, and eyes nested in patient as a random effect. As before, we additionally present results of regression using backward selection (eFigure 3 in the Supplement). To check the assumptions of the LASSO model (eg, linearity), we further performed a random-forest–based analysis (Figure 2 and eFigure 4 in the Supplement). Here, we did not take random effects into special consideration.
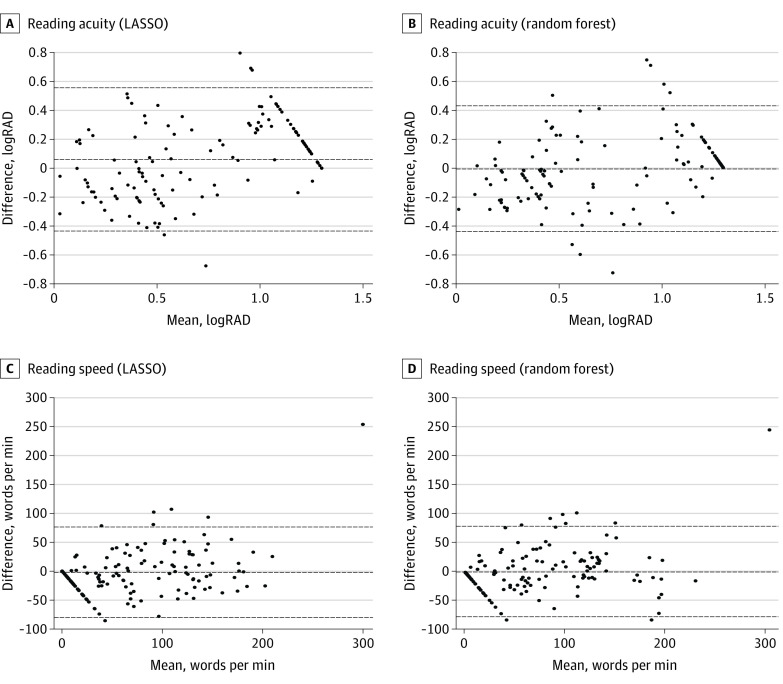
Figure 2. Bland-Altman Plot for Model Comparison.
We compare the predictions of the linear mixed-effect model fitted using least absolute shrinkage and selection operator (LASSO) regression to those of a random-forest model that was trained on the same data set for reading acuity (A and B) and reading speed (C and D). The plots indicate that the 2 models yield comparable results, and thus it is valid to investigate the linear connections. The estimates for the mean prediction-observation difference (middle line) and the 95% limits of agreement (outer lines) are shown. logRAD indicates logarithm of the reading acuity determination.
Last, we fitted a mixed-effects model with binocular reading performance as the dependent variable and better-seeing eye reading performance as independent variable. Visit nested in patient was considered as random effects (random-intercept model). A likelihood-ratio test was used to explore whether the addition of the worse-seeing eye reading performance as an independent variable would improve the model fit. This would indicate that the worse-seeing eye contributes to binocular reading performance. Analysis began December 2019 and ended January 2021.
Results
Baseline Determinants of Reading Performance
A total of 150 eyes of 85 participants with a median (IQR) age of 77.9 (72.4-82.1) years at baseline were included in this study (eTable 1 in the Supplement). Median (IQR) values of BCVA and LLVA were 0.49 (0.2-1.0) logMAR (20/60 [20/32-20/200] Snellen fraction ft) and 1.00 (0.7-1.3) logMAR (20/200 [20/100-20/400] Snellen fraction ft), respectively. The median (IQR) square root–transformed GA area was 3.14 (2.1-4.1) mm at baseline.
Study participants had a median (IQR) reading acuity of 0.90 (0.4-1.3) logRAD and reading speed of 52.8 (0-123) wpm monocularly. In this study, 57 eyes (38%) had a noncenter-involving GA (ie, foveal sparing).
The distribution of reading acuity revealed a negative skew due to frequent recordings of the worst possible reading acuity of 1.3 logRAD, indicating a marked floor effect of the Radner reading charts in this setting of GA. A Spearman correlation analysis revealed strong multicollinearity between the variables (eFigure 1 in the Supplement).
Univariable Analysis of Reading Performance at Baseline
Most of our characteristics exhibited an association with reading speed and reading acuity in a univariable analysis at baseline (eTable 2 in the Supplement). Only age, distance from initial to final PRL, distance from initial PRL to GA, distance from final PRL to GA, GA area left of the final PRL, and GA area left of the initial PRL did not exhibit a considerable association with the reading performance.
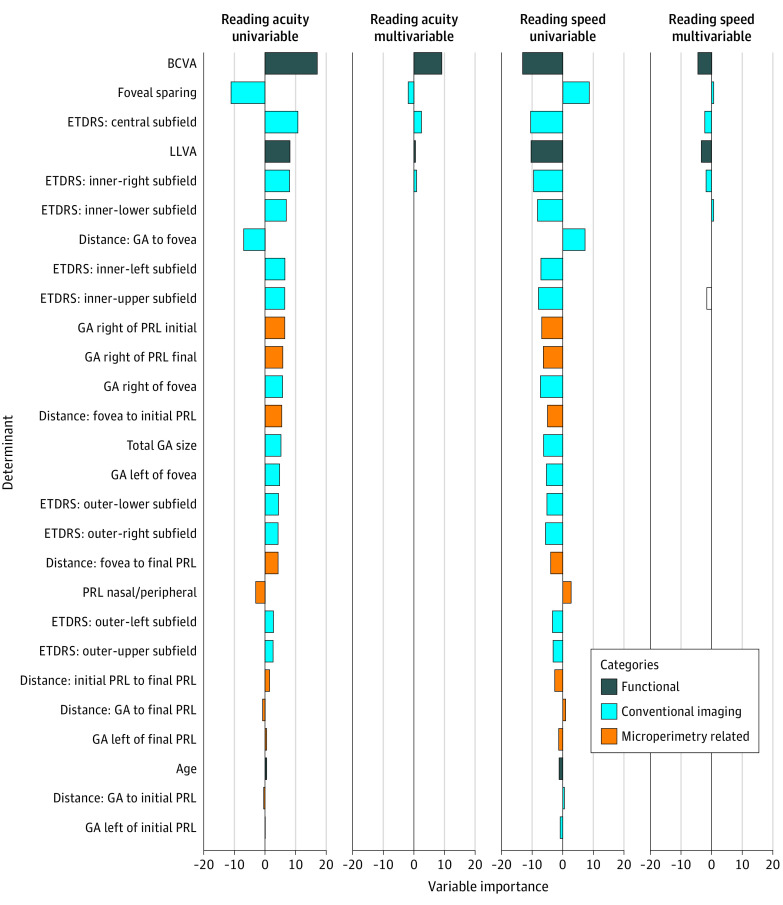
The most important variables of the reading acuity were BCVA, foveal sparing, and the GA in the central ETDRS subfield (Figure 3). In the univariable analysis with reading speed as the dependent variable, the most important variables were BCVA, GA area in the central ETDRS subfield, and LLVA (Figure 3). Interestingly, LLVA was considerably more important for reading speed than for reading acuity (Figure 3).
Figure 3. Variable Importance at Baseline.
The variable importance was measured by the t statistic of the individual univariable linear mixed-effect models (univariable analysis) and respectively of the final multivariable linear mixed-effect model with variables selected via least absolute shrinkage and selection operator regression (multivariable analysis) each for reading speed and reading acuity. BCVA indicates best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; GA, geographic atrophy; LLVA, low-luminance visual acuity; PRL, preferred retinal loci.
Multivariable Analysis at Baseline
The BCVA, the GA in the central subfield, foveal sparing, the GA in the inner-right ETDRS subfield, and the LLVA were selected by the LASSO regression as the most relevant variables (cross-validated R2 = 0.69; Figure 3). For reading speed, BCVA, LLVA, GA area in the central, the inner-right ETDRS subfield, and the inner-upper ETDRS subfield were identified as the most important variables (cross-validated R2 = 0.67; Figure 3). Random-forest analysis, which can also uncover nonlinear associations and interaction associations among the variables, allowed to explain reading acuity (R2 = 0.77) and reading speed (R2 = 0.67) with a similar accuracy (Figure 2 and eFigure 4 in the Supplement).
Longitudinal Determinants of Reading Performance
The longitudinal data contained 75 participants with a median (IQR) of 1 (0-1) follow-up visits spanning a median (IQR) of 0.5 (0-1) years. Participants had a mean (SD) GA progression rate of 0.25 (0.19) mm/year/eye. There was no noticeable change in reading acuity (mean [SD], 0.11 [0.38] logRAD/y) and reading speed (mean [SD], −14.49 [44.29] wpm/y) over time. BCVA (mean [SD], 0.16 [0.38] logMAR/y), and LLVA (mean [SD], 0.17 [0.40] logMAR/y) did not change markedly either (eTable 1 in the Supplement).
Longitudinal Multivariable Analysis
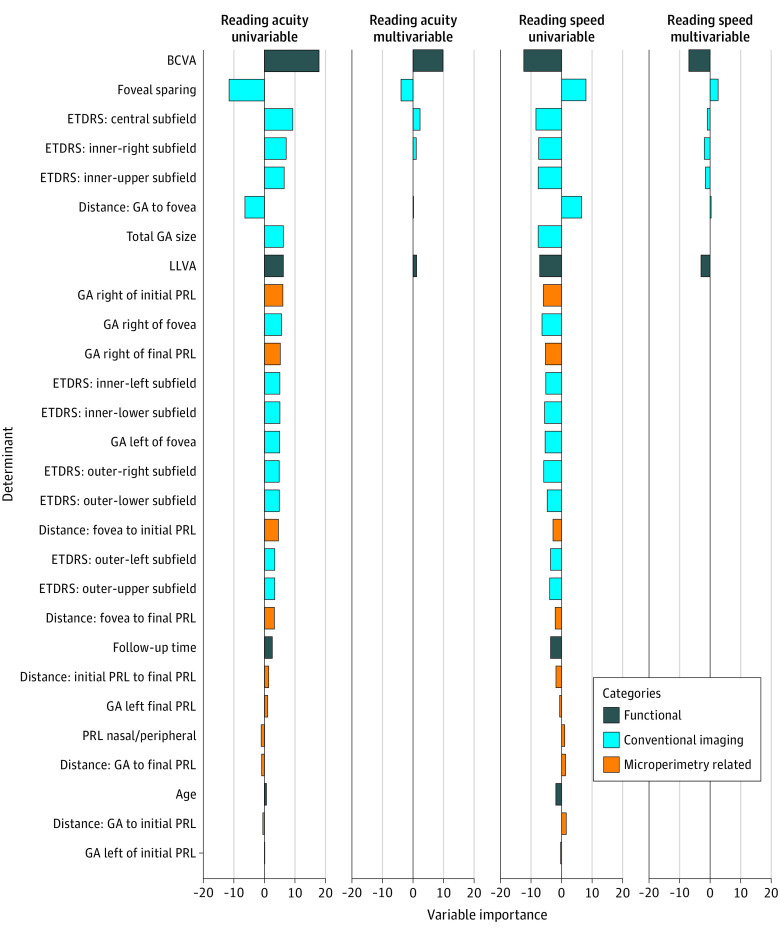
To determine whether longitudinal assessments in reading performance are paralleled in the biomarkers mentioned above or whether a decline in reading performance may be observed independently of these associations, we repeated the cross-sectional multivariable analysis on the longitudinal data, including the follow-up time as a new covariate. The multivariable longitudinal analysis revealed that BCVA, foveal sparing, the GA in the central and in the inner-right ETDRS subfield, and LLVA constituted the most associated variables of the reading acuity (cross-validated R2 = 0.73; Figure 4). Importantly, age and follow-up time were eliminated by LASSO.
Figure 4. Longitudinal Variable Importance.
The variable importance was measured by the t statistic of the individual univariable linear mixed-effect models (univariable analysis) and of the multivariable linear mixed-effect model with variables selected via least absolute shrinkage and selection operator regression (multivariable analysis) each for the reading speed and reading acuity, respectively. BCVA indicates best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; GA, geographic atrophy; LLVA, low-luminance visual acuity; PRL, preferred retinal loci.
For reading speed, BCVA, LLVA, foveal sparing, the GA in the inner-right ETDRS subfield, and in the inner-upper ETDRS subfield were selected as the most relevant variables (cross-validated R2 = 0.70; Figure 4). Again, age and follow-up time were eliminated.
Binocular Reading Performance
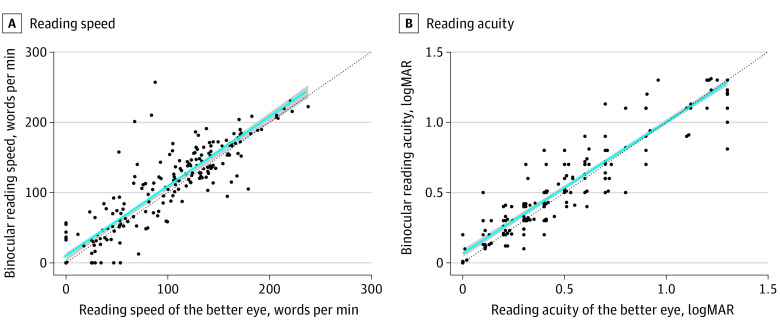
Binocular reading acuity was markedly influenced by both the better-seeing eye (P < .001; effect estimate, 0.94 logRAD/logRAD; 95% CI, 0.91-0.97) and worse-seeing eye (P < .001; effect estimate, 0.74 logRAD/logRAD; 95% CI, 0.63-0.85). The same holds for reading speed (P < .001; effect estimate, 0.54 wpm; 95% CI, 0.47-0.62 and P < .001; effect estimate, 0.81 wpm; 95% CI, 0.68-0.93, respectively). As seen in Figure 5, the binocular reading performance is not noticeably different compared with the reading performance in the better-seeing eye in participants with GA.
Figure 5. Reading Performance: Binocular vs Better-Seeing Eye.
Reading performance was whether recorded from the better-seeing eye (x-axis) or binocularly (y-axis) indifferent. The regression line (blue) is similar to the line of unity (black) highlighting the similarity of the reading performance in the better-seeing eye compared with binocular.
Discussion
This study showed that in patients with GA secondary to AMD, reading performance is strongly correlated with conventional structural imaging biomarkers, microperimetry-based fixation assessment, and functional tests. This is true for both reading speed and acuity. BCVA, GA area in the central ETDRS subfield, and the presence of foveal sparing were found to be associated with both. In contrast, LLVA and GA area in the inner-right and inner-upper ETDRS subfields were associated with reading speed but not acuity. Overall, this analysis revealed a stronger association of lesion-shape determinants with reading speed than reading acuity. The longitudinal analysis further underscored these results, suggesting that reading speed may be superior to reading acuity in terms of the ability to detect change over time.
While BCVA constitutes the most frequently applied functional end point in ophthalmology, patients with GA typically experience primarily an impairment of near activities as highlighted by studies using visual function questionnaires.18,34,35 Multiple studies have previously shown that patients with GA may experience severe reading difficulties, which also includes patients with normal BCVA.16,18,20,35,36 To apply reading speed or acuity as the outcome measure, the concurrent validity and ability to detect changes over time constitute important test characteristics. Regarding the concurrent validity, Sunness et al16 reported that reading speed correlates moderately with the GA area (approximately −24.3 wpm/mm). This is in line with our observation of −25.70 wpm/mm. In contrast with our results, the previous study found no association between BCVA and reading speed.16
Our cross-sectional analyses showed a slightly closer association with structural measures such as the GA area in the inner-right and inner-upper ETDRS subfields. Moreover, LLVA appeared to be more relevant to reading speed compared with reading acuity. In conjunction, these results suggest that reading acuity parallels BCVA at the PRL, whereas reading speed provides additional information regarding retinal function in proximity to the PRL.
Microperimetry-derived biomarkers, such as the position of the PRL, did not exhibit an association with reading acuity and speed in the multivariable analysis, although they carried similar variable importance in the univariable analysis compared with conventional imaging end points. Possibly, PRL measurements as derived by microperimetry are not necessarily identical to the PRL applied for reading.37,38,39
Regarding the ability to detect change over time, we observed a mean (SD) rate of −14.49 (44.29) wpm/y for reading speed and of 0.11 (0.38) logRAD/y for reading acuity, which were not pronounced in terms of statistical effect size but similar to the estimates from the Chroma and Spectri trials (–18.08 wpm per 48 weeks for reading speed).18
Our results are compatible with a larger population-based study of older adults with GA secondary to AMD.40 In terms of binocular reading performance, linear regression analysis highlighted that binocular reading acuity and speed are a function of the better-seeing eye (Figure 5). This underscores the binocular inhibition, as previously observed in the setting of macular telangiectasia type 2,23 is not common in the setting of GA. This highlights that reading parameters, scotoma size, and depth can differ in different macular diseases.
For clinical trial designs, multiple conclusions can be drawn from our study results. First, reading speed appears to be reflective of retinal function beyond BCVA and may therefore be more suitable to acquire less correlated data than reading acuity. Second, in clinical trials, which typically apply treatment to the worse-seeing eye in consideration of patient safety, monocular reading performance must be tested separately.
Given the high prediction accuracy for reading speed based on the multivariable LASSO model (R2 = 0.67), it could be considered to waive reading performance testing and use machine learning–based inferred reading speed as a surrogate outcome measure. Of course, reading performance testing at baseline and the last visit of all participants would be helpful to validate the prediction accuracy. Similar suggestions have been previously made in the context of retinal sensitivity assessment using microperimetry.41,42,43
Limitations
Limitations of this study include the limited sample size (or longitudinal review period), which was underpowered to detect a considerable change in reading acuity and speed over time. Additionally, we investigated the maximum reading speed. For stability, the mean reading speed might be more feasible. However, the current curve-fitting software is not ideal in the setting of the GA because a significant subset of patients show foveal sparing with paradoxical worsening of reading speed for larger letters. Moreover, variations in outer retinal integrity outside of GA area such as incomplete retinal pigment epithelial and outer retinal atrophy were not represented by the current features.44,45
By using nested cross-validation, the estimates for the prediction accuracy are unbiased concerning hyperparameter optimization. Moreover, the additional analysis using random-forest models highlighted that LASSO regression analysis is sufficient to explain the largest fraction of variability in reading performance (Figure 2 and eFigure 4 in the Supplement).
Conclusions
In summary, these data highlight the association of monocular reading acuity and reading speed with established visual function and structural measurements of disease severity. Given that decline in vision-related quality of life is driven by near activities in patients with GA,18,34 these data strongly support the use of reading performance as a functional outcome measure in interventional clinical trials. Particularly in eyes with foveal sparing, assessment of reading speed may provide additional relevant functional information beyond mere BCVA. Extrapolation of these results suggests that clinical and low-vision care should be focused primarily toward the better-seeing eye based on distance visual acuity.
eFigure 1. Correlation among candidate features
eFigure 2. Backward-selected Linear Model: Feature Importance at Baseline
eFigure 3. Backward-selected LM: Longitudinal Feature Importance
eFigure 4. Model accuracy
eTable 1. Cohort characteristics
eTable 2. Determinants reading performance (univariable cross-sectional analysis)
References
- 1.Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy. 2013;33(8):838-855. doi: 10.1002/phar.1264 [DOI] [PubMed] [Google Scholar]
- 2.Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430-1438. doi: 10.1172/JCI71029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen A, Sander B. Long-term longitudinal study of patients treated with ranibizumab for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2014;25(3):158-163. doi: 10.1097/ICU.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 4.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Heal. 2014. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 5.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond). 1988;2(Pt 5):552-577. doi: 10.1038/eye.1988.106 [DOI] [PubMed] [Google Scholar]
- 6.Fleckenstein M, Charbel Issa P, Helb H-MM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137-4144. doi: 10.1167/iovs.08-1967 [DOI] [PubMed] [Google Scholar]
- 7.Zanzottera EC, Ach T, Huisingh C, Messinger JD, Freund KB, Curcio CA. Visualizing retinal pigment epithelium phenotypes in the transition to atrophy in neovascular age-related macular degeneration. Retina. 2016;36(suppl 1):S26-S39. doi: 10.1097/IAE.0000000000001330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677-1691. doi: 10.1016/S0161-6420(97)30079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunness JS, Margalit E, Srikumaran D, et al. ; Enlargement of Atrophy and Implications for Interventional Clinical Trials . The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271-277. doi: 10.1016/j.ophtha.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindner M, Böker A, Mauschitz MM, et al. ; Fundus Autofluorescence in Age-Related Macular Degeneration Study Group . Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122(7):1356-1365. doi: 10.1016/j.ophtha.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 12.Lindner M, Nadal J, Mauschitz MM, et al. Combined fundus autofluorescence and near infrared reflectance as prognostic biomarkers for visual acuity in foveal-sparing geographic atrophy. Invest Ophthalmol Vis Sci. 2017;58(6):BIO61-BIO67. doi: 10.1167/iovs.16-21210 [DOI] [PubMed] [Google Scholar]
- 13.Sayegh RG, Sacu S, Dunavölgyi R, et al. Geographic atrophy and foveal-sparing changes related to visual acuity in patients with dry age-related macular degeneration over time. Am J Ophthalmol. 2017;179:118-128. doi: 10.1016/j.ajo.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 14.Schmitz-Valckenberg S, Nadal J, Fimmers R, et al. ; FAM Study Group . Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica. 2016;235(4):215-224. doi: 10.1159/000445217 [DOI] [PubMed] [Google Scholar]
- 15.Csaky K, Ferris F III, Chew EY, Nair P, Cheetham JK, Duncan JL. Report from the NEI/FDA endpoints workshop on age-related macular degeneration and inherited retinal diseases. Invest Ophthalmol Vis Sci. 2017;58(9):3456-3463. doi: 10.1167/iovs.17-22339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunness JS, Applegate CA, Haselwood D, Rubin GS. Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology. 1996;103(9):1458-1466. doi: 10.1016/S0161-6420(96)30483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunness JS, Applegate CA. Long-term follow-up of fixation patterns in eyes with central scotomas from geographic atrophy that is associated with age-related macular degeneration. Am J Ophthalmol. 2005;140(6):1085-1093. doi: 10.1016/j.ajo.2005.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heier JS, Pieramici D, Chakravarthy U, et al. ; Chroma and Spectri Study Investigators . Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmol Retina. 2020;4(7):673-688. doi: 10.1016/j.oret.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 19.Sunness JS. Reading newsprint but not headlines: pitfalls in measuring visual acuity and color vision in patients with bullseye maculopathy and other macular scotomas. Retin Cases Brief Rep. 2008;2(1):83-84. doi: 10.1097/IAE.0b013e31802fa25d [DOI] [PubMed] [Google Scholar]
- 20.Lindner M, Pfau M, Czauderna J, et al. Determinants of reading performance in eyes with foveal-sparing geographic atrophy. Ophthalmol Retina. 2019;3(3):201-210. doi: 10.1016/j.oret.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008;102(10):600-610. doi: 10.1177/0145482X0810201004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheong AMY, Legge GE, Lawrence MG, Cheung S-H, Ruff MA. Relationship between visual span and reading performance in age-related macular degeneration. Vision Res. 2008;48(4):577-588. doi: 10.1016/j.visres.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzaridis S, Herrmann P, Charbel Issa P, et al. Binocular inhibition of reading in macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2019;60(12):3835-3841. doi: 10.1167/iovs.18-26414 [DOI] [PubMed] [Google Scholar]
- 24.Pfau M, Lindner M, Goerdt L, et al. ; Fundus Autofluorescence in Age-Related Macular Degeneration Study Group . Prognostic value of shape-descriptive factors for the progression of geographic atrophy secondary to age-related macular degeneration. Retina. 2019;39(8):1527-1540. doi: 10.1097/IAE.0000000000002206 [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 26.Radner W. Reading charts in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1465-1482. doi: 10.1007/s00417-017-3659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuchard RA. Preferred retinal loci and macular scotoma characteristics in patients with age-related macular degeneration. Can J Ophthalmol. 2005;40(3):303-312. doi: 10.1016/S0008-4182(05)80073-0 [DOI] [PubMed] [Google Scholar]
- 28.Pfau M, Jolly JK, Wu Z, et al. Fundus-controlled perimetry (microperimetry): application as outcome measure in clinical trials. Prog Retin Eye Res. 2021;82:100907. doi: 10.1016/j.preteyeres.2020.100907 [DOI] [PubMed] [Google Scholar]
- 29.Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al. Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(10):7640-7646. doi: 10.1167/iovs.11-7457 [DOI] [PubMed] [Google Scholar]
- 30.Pfau M, Goerdt L, Schmitz-Valckenberg S, et al. Green-light autofluorescence versus combined blue-light autofluorescence and near-infrared reflectance imaging in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(6):BIO121-BIO130. doi: 10.1167/iovs.17-21764 [DOI] [PubMed] [Google Scholar]
- 31.Pelli DG, Bex P. Measuring contrast sensitivity. Vision Res. 2013;90:10-14. doi: 10.1016/j.visres.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastie T, Tibshirani R, Friedman J.. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; 2009. doi: 10.1007/b94608 [DOI] [Google Scholar]
- 33.Pfau M, Walther G, von der Emde L, et al. [Artificial intelligence in ophthalmology: guidelines for physicians for the critical evaluation of studies]. [Article in German] Ophthalmologe. 2020;117(10):973-988. doi: 10.1007/s00347-020-01209-z [DOI] [PubMed] [Google Scholar]
- 34.Künzel SH, Möller PT, Lindner M, et al. Determinants of quality of life in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61(5):63. doi: 10.1167/iovs.61.5.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimel M, Leidy NK, Tschosik E, et al. Functional Reading Independence (Fri) index: a new patient-reported outcome measure for patients with geographic atrophy. Invest Ophthalmol Vis Sci. 2016;57(14):6298-6304. doi: 10.1167/iovs.16-20361 [DOI] [PubMed] [Google Scholar]
- 36.Varma R, Souied EH, Tufail A, et al. Maximum reading speed in patients with geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59(4):AMD195-AMD201. doi: 10.1167/iovs.18-24238 [DOI] [PubMed] [Google Scholar]
- 37.Crossland MD, Crabb DP, Rubin GS. Task-specific fixation behavior in macular disease. Invest Ophthalmol Vis Sci. 2011;52(1):411-416. doi: 10.1167/iovs.10-5473 [DOI] [PubMed] [Google Scholar]
- 38.Timberlake GT, Sharma MK, Grose SA, Maino JH. Retinal locus for scanning text. J Rehabil Res Dev. 2006;43(6):749-760. doi: 10.1682/JRRD.2005.06.0102 [DOI] [PubMed] [Google Scholar]
- 39.Morales MU, Saker S, Wilde C, Rubinstein M, Limoli P, Amoaku WM. Biofeedback fixation training method for improving eccentric vision in patients with loss of foveal function secondary to different maculopathies. Int Ophthalmol. 2020;40(2):305-312. doi: 10.1007/s10792-019-01180-y [DOI] [PubMed] [Google Scholar]
- 40.Rubin GS, Muñoz B, Bandeen-Roche K, West SK. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Invest Ophthalmol Vis Sci. 2000;41(11):3327-3334. [PubMed] [Google Scholar]
- 41.Kihara Y, Heeren TFC, Lee CS, et al. Estimating retinal sensitivity using optical coherence tomography with deep-learning algorithms in macular telangiectasia type 2. JAMA Netw Open. 2019;2(2):e188029. doi: 10.1001/jamanetworkopen.2018.8029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von der Emde L, Pfau M, Dysli C, et al. Artificial intelligence for morphology-based function prediction in neovascular age-related macular degeneration. Sci Rep. 2019;9(1):11132. doi: 10.1038/s41598-019-47565-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfau M, von der Emde L, Dysli C, et al. Determinants of cone- and rod-function in geographic atrophy: AI-based structure-function correlation. Am J Ophthalmol. 2020;217:162-173. doi: 10.1016/j.ajo.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: classification of atrophy meeting report 4. Ophthalmology. 2020;127(3):394-409. doi: 10.1016/j.ophtha.2019.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfau M, von der Emde L, de Sisternes L, et al. Progression of photoreceptor degeneration in geographic atrophy secondary to age-related macular degeneration. JAMA Ophthalmol. 2020;138(10):1026-1034. doi: 10.1001/jamaophthalmol.2020.2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Correlation among candidate features
eFigure 2. Backward-selected Linear Model: Feature Importance at Baseline
eFigure 3. Backward-selected LM: Longitudinal Feature Importance
eFigure 4. Model accuracy
eTable 1. Cohort characteristics
eTable 2. Determinants reading performance (univariable cross-sectional analysis)