Abstract
Knock-in homozygote VCPR155H/R155H mutant mice are a lethal model of valosin-containing protein (VCP)-associated inclusion body myopathy associated with Paget disease of bone, frontotemporal dementia and amyotrophic lateral sclerosis. Ceramide (d18:1/16:0) levels are elevated in skeletal muscle of the mutant mice, compared to wild-type controls. Moreover, exposure to a lipid-enriched diet reverses lethality, improves myopathy and normalizes ceramide levels in these mutant mice, suggesting that dysfunctions in lipid-derived signaling are critical to disease pathogenesis. Here, we investigated the potential role of ceramide in VCP disease using pharmacological agents that manipulate the ceramide levels in myoblast cultures from VCP mutant mice and VCP patients. Myoblasts from wild-type, VCPR155H/+ and VCPR155H/R155H mice, as well as patient-induced pluripotent stem cells (iPSCs), were treated with an inhibitor of ceramide degradation to increase ceramide via acid ceramidase (ARN082) for proof of principle. Three chemically distinct inhibitors of ceramide biosynthesis via serine palmitoyl-CoA transferase (L-cycloserine, myriocin or ARN14494) were used as a therapeutic strategy to reduce ceramide in myoblasts. Acid ceramidase inhibitor, ARN082, elevated cellular ceramide levels and concomitantly enhanced pathology. Conversely, inhibitors of ceramide biosynthesis L-cycloserine, myriocin and ARN14494 reduced ceramide production. The results point to ceramide-mediated signaling as a key contributor to pathogenesis in VCP disease and suggest that manipulating this pathway by blocking ceramide biosynthesis might exert beneficial effects in patients with this condition. The ceramide pathway appears to be critical in VCP pathogenesis, and small-molecule inhibitors of ceramide biosynthesis might provide therapeutic benefits in VCP and related neurodegenerative diseases.
Introduction
Inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (IBMPFD) also known as multisystem proteinopathy (MSP)—was first characterized by Dr. Virginia Kimonis in 2000 and the causative gene encoding valosin-containing protein (VCP) was identified in 2004 (1–3). VCP controls a growing number of cellular processes ranging from cellular proteostasis to genome stability and cellular metabolism (4,5), and plays a critical role in muscular and neuronal degenerative disorders. VCP disease is associated with progressive limb girdle muscle weakness and early demise from respiratory insufficiency. Muscle pathology is significant for vacuoles, TAR DNA binding protein-43 (TDP-43), and ubiquitin-positive inclusions. TDP-43 cytoplasm mislocalization and translocation from the nucleus and deposition of ubiquitinated and hyper-phosphorylated TDP-43 into inclusion bodies is the key pathological feature in related disorders such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (6). There is great interest in understanding and improving the cellular and molecular pathophysiological mechanisms underlying VCP disease in view of the overlap with other common neurodegenerative diseases such as ALS and frontotemporal dementia.
VCP mouse models carrying the common R155H mutation display typical features of the human disease. Homozygous knock-in VCPR155H/R155H mice exhibit rapid progressive weakness and accelerated pathology, including disrupted autophagosome formation and mitochondrial dysfunction, and typically die by 21 days of age (7). In vivo studies demonstrated that tissue levels of ceramide (d18:1/16:0) and non-esterified palmitic acid, two lipotoxic mediators, were elevated in skeletal muscle and liver from homozygous VCPR155H/R155H mice, compared with their wild-type (WT) or heterozygote counterparts (7). Of note, ceramides have been implicated in the binding and recruiting of LC3B-labeled autophagosomes to damaged mitochondria for degradation, a process known as ‘lethal mitophagy’ (8–10). Moreover, these compounds may also contribute to other neurodegenerative disorders—such as Alzheimer’s disease (AD) and ALS—as well as to lysosomal storage diseases such as Fabry, Farber disease (FD) and Neimann-Pick C1 disease (9,11–16).
Feeding pregnant VCPR155H/+ heterozygous dams with a 9% lipid-enriched diet (LED) resulted in a striking reversal of the lethal phenotype in homozygous offspring, with survival extended for up to 2 years. This dietary intervention also improved motor activity, muscle pathology and autophagy/mitophagy signaling cascades, while concomitantly normalizing the levels of ceramide and palmitic acid in skeletal muscle of VCPR155H/R155H mice. In this study, we investigated the possible role played by ceramide in VCP-related neurodegenerative diseases using pharmacological interventions that either increase or decrease ceramide levels in cells. As shown in Figure 1A, ceramide biosynthesis starts with the condensation of palmitate with serine. This reaction, which is catalyzed by the enzyme serine palmitoyl-Co A synthase/serine palmitoyltransferase (SPT), is selectively inhibited by various pharmacological agents—including antibiotics such as L-cycloserine and myriocin (17–19), and synthetic small molecules such as the indolin-2-one derivative ARN14494 (20) (Fig. 1B)—leading to a decrease in cellular ceramide content (17,19,21). Ceramide-mediated signaling is terminated by acid ceramidase (also known as N-acylsphingosine amidohydrolase-1, ASAH-1) (22), whose inhibition by N-hexyl-2,4-dioxo-pyrimidine-1-carboxamides such as ARN082 elevates ceramide levels in cells (23). In addition to providing useful experimental tools to dissect the role of ceramide in VCP disease, these agents may also offer much-needed treatments for this devastating condition.
Figure 1 .
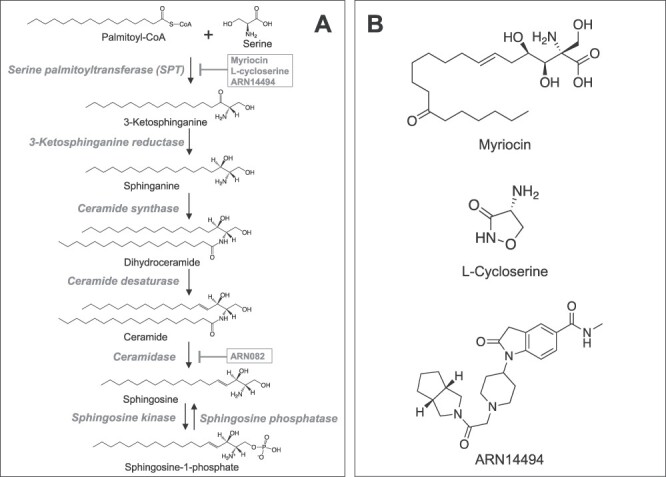
De novo synthesis of ceramide and the degradation pathway. (A) The de novo pathway produce ceramide from the condensation of palmitate and serine, which is catalyzed by the serine palmitoyl-Co A synthase/serine palmitoyltransferase (SPT). This reaction is known to be the rate-limiting step of the de novo pathway, and selective inhibitors for the SPT, Myriocin, L-cycloserine and ARN14494, reduce the biosynthesis of ceramide. The product of SPT, 3-keto-dihydrosphingosine/3-keto-sphinganine, is reduced to dihydrosphingosine/sphinganine, which is followed by an acylation reaction to produce dihydroceramide. Finally, ceramide is produced by the catalysis of dihydroceramide desaturase. Ceramide is inactivated by the enzyme ceramidase, an enzyme that is selectively inhibited by the compound ARN082. (B) Chemical structure of the myriocin, L-cycloserine and ARN14494.
Currently, there are no treatments for this progressive neuromuscular disorder. The results from these studies provide the rationale for in vivo treatment using these ceramide inhibitors in the VCP disease mouse model. Successful reversal of the disease phenotype in the mouse model will improve the chance of these inhibitors being approved for trials in patients with VCP disease.
Results
Ceramide levels in primary myoblasts from WT versus VCP155H/R155H mice and patient with VCP disease
Confirming our previous results in skeletal muscles from VCPR155H mutant mice (7), LC–MS/MS analyses revealed that levels of all ceramide species, including ceramide (d18:1/16:0), were substantially elevated in primary myoblasts from homozygous VCP mice, compared to WT controls (Fig. 2A, Table 1). In a separate experiment, we found that the levels of ceramide (d18:1/16:0) and total ceramide in primary myoblasts cultures from heterozygous (HET) VCP mice did not display statistically significant differences when compared to those of WT mice, although slight trends of elevations were observed (ceramide (d18:1/16:0), 130.8 ± 11.2%, P = 0.105; total ceramide, 124.9 ± 10.9%, P= 0.163; n = 6 per group). Comparable changes in cellular ceramide levels were also observed in primary cultures of human VCP patient-derived myoblasts, when compared with the control myoblast from normal volunteers (n = 2–3) (Fig. 2B).
Figure 2 .
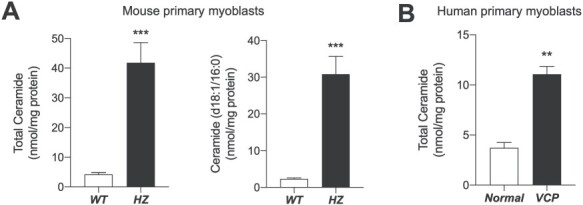
Lipid analyses of cultured primary myoblasts from wild-type and VCPR155H/R155H mice. Levels of cellular ceramide were quantified from primary myoblasts in culture. (A) Cells from the WT (at passage 8 in vitro) and VCPR155H/R155H (HZ, at passage 7) mice were cultured and scraped into methanol. The lipids were extracted with chloroform and the levels of total ceramides and the major ceramide species in the myoblast, ceramide (d18:1/16:0), were determined using LC–MS/MS (n = 5–6). (B) Comparable changes in cellular ceramide levels were also observed in primary cultures of human VCP patient-derived myoblasts, when compared with the control myoblast from normal volunteers (n = 2–3). Values are normalized by protein quantity. Data are expressed as mean ± SEM. ***P < 0.001, **P < 0.01, and *P < 0.05 by two-tailed t-test.
Table 1.
Levels of ceramide species in cultured primary myoblasts from wild-type and VCPR155H/R155H mice
| Ceramide | Mean ± SEM (nmol/mg protein) | |
|---|---|---|
| WT | HZ | |
| (d18:1/16:0) | 2.34 ± 0.30 | 30.82 ± 4.86*** |
| (d18:1/18:0) | 0.10 ± 0.01 | 0.92 ± 0.16*** |
| (d18:1/24:1) | 0.96 ± 0.11 | 6.36 ± 1.24*** |
| (d18:1/24:0) | 0.87 ± 0.13 | 3.69 ± 0.59*** |
Levels of cellular ceramide were quantified from primary myoblasts in culture. Cells from the WT (at passage 8 in vitro) and VCPR155H/R155H (HZ, at passage 7) mice were cultured and scraped into methanol. The lipids were extracted with chloroform and the levels of various ceramide species were determined by using LC–MS/MS (n = 5–6). Data are expressed as mean ± SEM. ***P < 0.001 by two-tailed t-test.
Exogenous ceramide stimulates autophagy, a key feature of VCP disease pathology
To determine whether ceramide-mediated signaling might affect autophagy in VCP disease, we treated myoblasts prepared from WT, heterozygous and homozygous mice with 25 μM of C8 ceramide, a synthetic cell-permeable ceramide analog. Baseline expression of the autophagosomal marker the autophagy receptor p62/SQSTM1 and LC3B was substantially higher in myoblasts from homozygous myoblasts, relative to WT controls (Fig. 3A). Moreover, C8 ceramide increased, in a concentration-dependent manner, the expression of LC3B and p62/SQSTM1 in all three myoblast lines (Fig. 3B). The ability of exogenous C8 ceramide to stimulate autophagy in myoblasts is consistent with previous findings in other cellular systems (24), and compatible with the hypothesis that endogenous ceramides are involved in this process, which is a hallmark of VCP pathology.
Figure 3 .
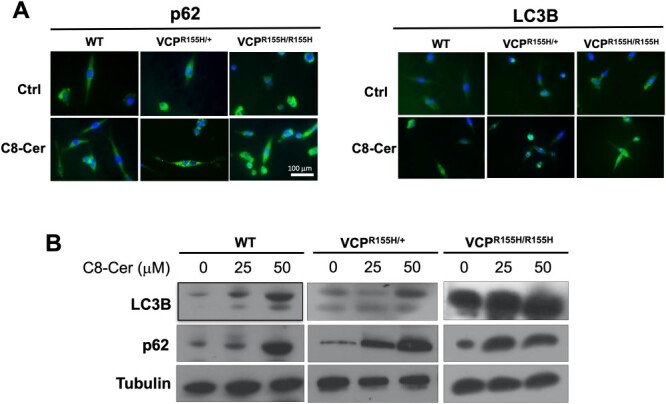
Increased expression levels of LC3B and p62/SQSTM1 in ceramide treated mouse myoblasts. Myoblasts obtained from the wild-type (WT), VCPR155H/+, and VCPR155H/R155H mice were treated with 0, 25 and 50 μM of C8 ceramide, respectively, a cell-permeable analog of naturally occurring ceramides for 24 hours (n = 5–6). The expression of LC3B and p62 was assessed by immunocytochemistry (A) and western blotting (B) and representative immunocytochemistry images (A) are shown with the 25 μM ceramide dose.
Ceramide degradation can be prevented by agents that inhibit the intracellular activity of acid ceramidase (AC), a lysosomal hydrolase that catalyzes the conversion of ceramide to sphingosine. To block ceramide degradation, we used the potent AC inhibitor ARN082 (22). Previous work has shown that this agent inhibits mouse AC activity in vitro with a median effective concentration (IC50) of 67.0 ± 5.0 nM, acting through an irreversible mechanism (22). However, treatment with ARN082 produced no changes in the expression of TDP-43 and LC3B as compared with untreated VCP cells (Fig. 4A-D). Additionally, ARN082 did not affect the abnormal localization of TDP-43 in patient myoblasts (Fig. 5). We interpret these results as indicating that AC-dependent ceramide hydrolysis does not contribute to ceramide-mediated stimulation of autophagy in myoblasts.
Figure 4 .
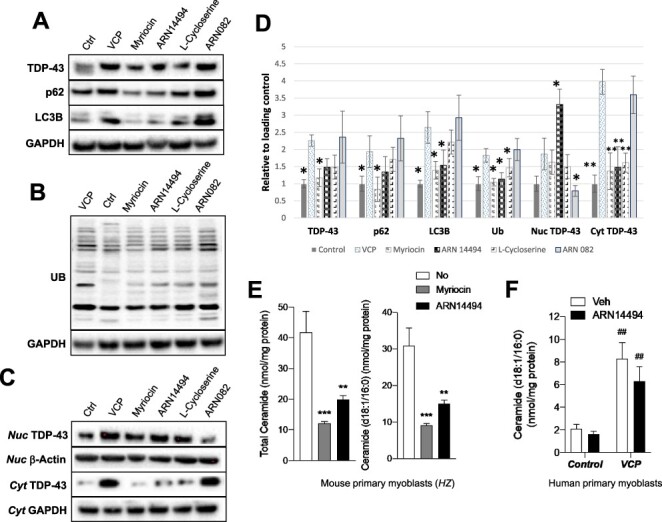
Effect of ceramide biosynthesis inhibitors on mouse and human myoblasts. Expression levels of ubiquitin, TDP-43, p62, and LC3B autophagy markers were lower when treated with ceramide biosynthesis inhibitors (Myriocin at 0.25 μM, ARN14494 at 2 μM, L-cycloserine at 30 μM) in comparison to untreated VCP myoblasts (A, B). Treatment with ceramide degradation inhibitor (ARN082) resulted in similar TDP-43 and LC3B protein expression levels as untreated VCP myoblasts. Nuclear TDP-43 and cytoplasmic TDP-43 (C) including the densitometry results (D). (E) Myoblasts from VCPR155H/R155H homozygous mouse treated with the ceramide biosynthesis inhibitors, Myriocin (0.25 μM at 16 hours) or ARN14494 (2 μM at 16 hours), significantly decreased cellular levels of total ceramide and ceramide (d18:1/16:0). (F) Similar trends were also observed in the VCP patient-derived myoblasts treated with ARN14494, when compared with vehicle controls.***P < 0.001, **P < 0.01 and *P < 0.05 by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison post-test. ##P<0.01 compared with control group by two-way ANOVA with Turkey’s multiple comparison.
Figure 5 .
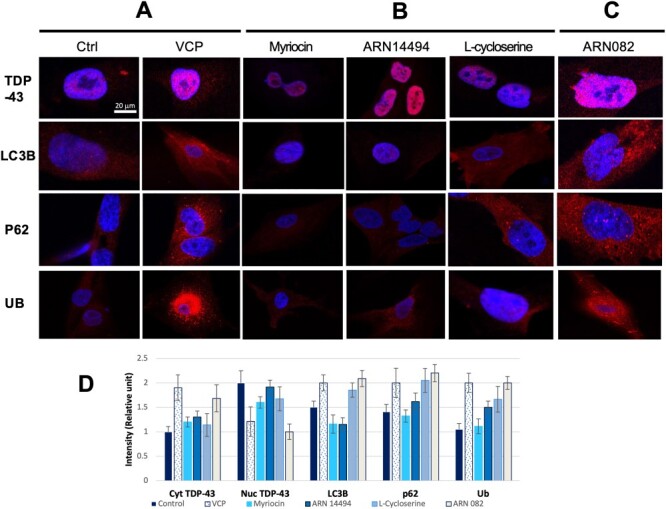
Immunocytochemistry of VCP patient iPSC-derived myoblasts treated with ceramide biosynthesis and degradation inhibitors. The localization of TDP-43 outside the nucleus in the cytoplasm is indicative of VCP pathology. VCP myoblasts treated with ceramide biosynthesis inhibitors (myriocin, ARN14494, L-Cycloserine) had localization of TDP-43 in the nucleus (B) similar to control myoblasts (A), while myoblasts treated with ceramide degradation inhibitors (ARN082) had localization of TDP-43 in the cytoplasm similar to untreated patient myoblasts (C and A). Protein density was showed from three representative images (D). This suggests that an overall decrease in ceramide concentration in the cell can help alleviate VCP pathology based on the localization of certain autophagy markers. Data are representative of three independent experiments.
Inhibition of ceramide biosynthesis reduces ceramide in mouse and human myoblasts
The condensation of palmitate with serine, catalyzed by SPT, is the rate-limiting step in ceramide biosynthesis. Pharmacological inhibition of SPT activity has been shown to decrease ceramide biosynthesis and ceramide levels in various model systems (17,19,21). To investigate the role of ceramide in the pathogenesis of VCP disease and to explore the therapeutic potential of modulating ceramide signaling in this and related neurodegenerative conditions, we treated mice and human myoblasts in culture with two chemically different SPT inhibitors, the antibiotic myriocin and the synthetic reversible inhibitor ARN14494 (Fig. 1B) (17,19,21). The compound ARN14494 inhibits mouse SPT activity in vitro with an IC50 of 21.8 ± 0.9 nM, acting through a fully reversible mechanism. In vivo, ARN14494 inhibits mouse SPT activity with a median effective dose (ID50) of approximately 20 mg per kg intraperitoneally.
Mouse myoblasts in cultures were incubated in the presence of myriocin (0.25 μM) and ARN14494 (2 μM), and ceramide content was measured by LC–MS/MS. Both compounds significantly reduced the levels of ceramide (d18:1/16:0) in myoblasts obtained from WT and homozygous mice (Fig. 4E). Notably, we also found that treatment with ARN14494 reduced levels of ceramide (d18:1/16:0), which were significantly elevated in VCP patient-derived myoblasts compared with normal controls (Fig. 4F).
Inhibition of ceramide biosynthesis mitigates VCP-associated autophagy and TDP-43 pathology
We also tested whether treatment with three structurally distinct SPT inhibitors—myriocin, ARN14494 and L-cycloserine, affects the expression of ubiquitin and autophagy markers, p62 and LC3B. As expected, VCP myoblasts expressed significantly higher levels of autophagy marker proteins under baseline conditions, and displayed abnormal localization of TDP-43 in the cytoplasm, rather than within the nucleus, a pathological hallmark of VCP and related diseases. Treatment with myriocin at different doses (0.25, 2, 5,10 μM) or ARN14494 (2, 5, 10 μM) or L-cycloserine (30, 60 and 90 μM) effectively reduced the levels of p62 and LC3B proteins in iPSC-derived human myoblasts and resulted in correct localization of TDP-43 into nuclei in comparison with untreated myoblasts. We did not observe any additional improvements in the effects on the autophagy markers and TDP-43 compared with the lowest doses tested (Figs 4 and 5). In addition to western blotting analysis, we performed immunocytochemical staining to visualize the protein expression levels. From 10x magnification, we noted that approximately 90% of VCP myoblasts treated with myriocin, 70% with ARN14494 and 60% with L-cycloserine showed TDP-43 relocalization compared with none in untreated VCP patient myoblasts. These results suggest that an overall decrease in ceramide signaling by targeting the ceramide pathway may correct the VCP-associated impairment in localization and expression of autophagy markers and TDP-43 localization and might thus help to alleviate pathology.
Discussion
Multisystem proteinopathy caused by mutations in the VCP gene, includes combinations of phenotypically heterogeneous disorders such as hereditary IBMPFD and amyotrophic lateral sclerosis. An effective treatment with bisphosphonates for VCP disease only exists for Paget’s disease component (1). Therefore, understanding the mechanism with the hope of developing targeted novel therapies is an unmet need for this important disorder, which shares a similar TDP-43 and autophagy pathogenesis with more common diseases.
We have previously found that VCPR155H/R155H homozygous mice display progressive weakness and accelerated pathology prior to their early demise by postnatal day 21 and accumulate multiple ceramides including ceramide (d18:1/16:0) (7). Feeding pregnant dams prenatally and the pups postnatally with an LED dramatically rescues the lethal phenotype and mice showed improved survival, and markers of muscle pathogenesis, in addition to amelioration of ceramide accumulation in muscle and liver. Other studies have also shown that LED improves neurological deficit caused by defective astrocyte lipid metabolism in mitochondrial myopathy,27 regulation of autophagy flux and skeletal muscle homeostasis in HDAC1/2 mutant mice (25,26), and improves myelination and alleviation of peripheral nerve pathology in neuropathic mice (27).
In this study, we report that myoblasts derived from VCP mice or from a patient with VCP disease also show elevated ceramide levels. Ceramides are a class of bioactive lipids that mediate many critical cellular processes—including cell growth, cell adhesion, cell migration, apoptosis and autophagy (28,29)—and are increasingly recognized as interconnected and compartmentalized signaling molecules within the cell (28,30–32). They have been implicated in neurodegenerative disorders such as lysosomal storage disorders but also Alzheimer’s disease and ALS (9,11–14). These lipid signals can be produced in cells via three primary pathways: cleavage of membrane sphingomyelin by sphingomyelinase, recycling of sphingosine via the salvage pathway or de novo biosynthesis starting with the condensation of palmitate with serine by SPT (Fig. 1). Pharmacological inhibition of intracellular SPT activity decreases ceramide concentrations in several model systems (17,19,21). Our results show that selective SPT inhibition is an effective strategy to reduce ceramide levels in tissues with VCP-associated mutation (Fig. 4).
Autophagy and ceramide
The regulatory role of ceramides in the pathogenesis of VCP is supported by lipidomic analysis data, which showed a significant elevation of ceramides in VCPR155H/R155H mouse as well as in VCP patient myoblasts. We carried out further investigation clarifying the crosstalk between ceramides and the autophagy pathways in VCP disease in order to provide the rationale for follow-on translational studies. Ceramide treatment of myoblasts resulted in an elevation in the autophagy markers p62 and LC3B. Our study also found that treatment with several inhibitors of ceramide biosynthesis corrected the mislocalization of TDP-43 and expression of autophagy markers in mouse and human myoblasts. All three SPT inhibitors studied showed benefits, the most effective, being myriocin and ARN14494 in reducing cytoplasmic TDP-43 and autophagic markers. Autophagy is a highly regulated self-digestive process that targets misfolded proteins or damaged organelles to lysosomal degradation (33–36), maintaining homeostasis during stress, but also contributes to cell death under specific contexts. Dysfunction in autophagy has been involved in aging as well as in numerous pathologies, including neurodegenerative diseases, metabolic syndrome and cancer (8).
Although ceramides are well-established inducers of apoptosis, studies have also implicated them in the induction of autophagy through activation of c-Jun N-terminal kinase (JNK), upregulation of Beclin-1 or BNIP3 and downregulation of nutrient transporter proteins (37). Thus, these lipids have emerged as important effectors in the regulation of the autophagic pathway, mediating the crosstalk between apoptosis and autophagy. This regulation depends on the subcellular localization of ceramides (mitochondria or endoplasmic reticulum), fatty acid chain length (C18-ceramide or C16-ceramide) and the presence of downstream targets of ceramides (Drp1, LC3B-II) for induction of lethal or survival autophagy. Ceramide is one of the lipids critical for exosome formation. The previous finding has shown that exosomes from ALS brain caused cytoplasmic relocation of TDP-43 in neuro2a cells, suggesting that exosomes might have important roles in the propagation of TDP-43 proteinopathy (38). Sentelle et al. reported that ceramide synthase 1 (CerS1) directly interacts with LC3BII and targets autophagosomes to the mitochondrial outer membrane leading to mitophagy (9). CerS1-derived ceramide (d18:1–18:0) has also been implicated in human aging (39). In a high-fat diet rat model of nonalcoholic fatty liver disease with impaired autophagy function, Yang et al. recently investigated the impact of myriocin to block ceramide synthesis and demonstrated the role of ceramide in down-regulating autophagy (40).
In sum, our results support the hypothesis that ceramides may be key effectors of inclusion body pathogenesis, and that decreasing the production of these cytotoxic lipid mediators might ameliorate the pathophysiologic manifestations in VCP myopathy. The ability of three structurally distinct small-molecule inhibitors of intracellular SPT activity—L-cycloserine, myriocin and ARN14494, to reduce ceramide biosynthesis and ameliorate key cellular signs of VCP myopathy brings the promise of new pharmacologic avenues for interventions and treatments for VCP and related neurodegenerative diseases.
Materials and Methods
Ethics statement
Human cell studies were approved by the University of California, Irvine Institutional Review Board protocol # 2007–5832 and hSCRO protocol 2009–1005. Animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of University of California Irvine, under protocols IACUC 2007–1195 and AUP-19-075.
Generation of mouse myoblasts for these studies
Quadriceps muscles from 19-month-old WT, VCPR155H/+ [HET] and VCPR155H/R155H [HZ], mice were used for generation of primary myoblasts using a published protocol (41).
Cell cultures and treatments
Mouse myoblasts (WT, HET and HZ) and human myoblasts (patient and control) were cultured in humidified 5% CO2 at 37°C incubator in DMEM supplemented with 10% fetal bovine serum. Cells were seeded onto 6-well plates (for western blotting) or chamber slides (Ibidi 80 826 for immunostaining). At 60–80% confluency, cells were treated with C8 ceramide (Cayman Chemical, Ann Arbor and MI at 0, 25 and 50 μM, respectively, for 24 h), ARN082 (3 μM for 3 h) or L-cycloserine (30, 60 and 90 μM for 16 h), myriocin (0.25, 2, 5,10 μM for 16 h) and ARN14494 (2, 5, 10 μM for 16 h) (https://patentscope.wipo.int/search/en/detail.jsf?docId=WO20080843000) (8,9,21,40,42).
Targeted lipid analyses
Lipid analyses were conducted as previously described (43). Primary myoblasts from WT (at passage 8 in vitro), or HZ (at passage 7) mice were maintained in primary culture as previously described (44). After drug treatment, the cells were washed with ice-cold phosphate-buffered saline (PBS), and scraped into 1 mL of methanol containing internal standard [ceramide (d18:1/12:0), 250 pmol/mL final concentration) for quantitation. The cells (n = 6 individual cultures) were homogenized, lipids were extracted with chloroform and the levels of ceramide were measured by LC–MS/MS (43). Values were normalized by protein concentration, which was measured using the Pierce BCA protein assay kit (Thermo Fisher Scientific). Ceramide analyses were carried out using an Agilent 1260 series LC coupled to an Agilent 6460C Triple Quadrupole MSD (45). The compounds were separated on a Zorbax Eclipse XDB C18, 1.8 μm, 2.1 id x 50 mm L (Agilent Technologies, Wilmington, DE) with a guard column (Zorbax Eclipse XDB C18 (2.1 x 5.0 mm, 1.8 μm), and the column operating temperature was maintained at 50°C. The injection volume was 2 μL, and the flow rate was 0.5 mL/min. Mobile phase consisted of A—80:20 water:acetonitrile, and B—80:20 isopropanol:acetonitrile. A step gradient starting with 78% B for 3 min followed by 95% B at 3.01 min continuing to 4 min, to elute any strongly bound compounds from the column, then at 4.01 min to 78% B continuing to 7.0 min, as a re-equilibration step. Detection was in the positive mode using the following fragmentation transitions:ceramide (d18:1/16:0) [M-H2O+H]+ (m/z = 538.52>264.2), ceramide (d18:1/18:0) [M-H2O+H]+ (m/z = 566.55>264.2), ceramide (d18:1/24:0) [M-H2O+H]+ (m/z = 650.65>264.2) and ceramide (d18:1/24:1) [M-H2O+H]+ (m/z = 648.63>264.2). Ceramide (d18:1/12:0) [M-H2O+H]+ (m/z = 482.46>264.2) (Avanti Polar Lipids, Alabaster, AL) was included as an internal standard.
Antibodies and reagents
All primary antibodies were from Abcam (Cambridge, MA): β-actin, ab8227; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), ab9485; microtubule-associated protein 1A/1B-light chain 3 (LC3B), ab192890; sequestosome 1 (SQSTM1)/p62, ab56416; TAR DNA-binding protein (TDP-43), ab190963; beta-tubulin, ab6046; and ubiquitin, ab7780.
Western blot analyses
Protein lysates from myoblasts were prepared for western blotting as previously described (46). We analyzed autophagy signaling intermediates including TDP-43, LC3B, p62/SQSTM1 in mouse myoblasts (47). Cytoplasmic and nuclear protein fractions were extracted using NE-PER kit (Thermo Scientific). Densitometry was performed to quantitate the signals from western blot images from 3 to 4 independent experiments using Image J Program (National Institutes of Health, Bethesda, MD).
Immunofluorescence imaging
Myoblasts were subjected to immunohistochemistry as described previously (47). The cells were fixed in 4% paraformaldehyde (PFA) for 15 min. They were washed 3 times with PBS and permeabilized for immunocytochemistry (ICC) using Triton X-100. After blocking in donkey serum (Sigma-Aldrich, D9663) for 1 h, cells were incubated overnight at 4°C in the presence of primary antibodies for VCP, ubiquitin, LC3B, p62/SQSTM1 and TDP-43. The cells were then incubated with fluorescein-conjugated secondary antibodies (Thermal Fisher) for 1 h and mounted with 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting media (Vector Laboratories) (47). The myoblast sections were analyzed by fluorescence microscopy (LSM 700) using a Zen image capture system (Carl Zeiss, Thornwood, NY). Densitometry using Image J Program was performed to quantitate the signals from immunocytochemical staining images.
Statistical analyses
Results were expressed as means ± SEM, and significance was determined using a two-tailed Student’s t-test, one-way or two-way analysis of variance with Turkey’s multiple comparisons post-test. P ≤ 0.05 was considered statistically significant.
Acknowledgements
This work was supported by the National Institute of Health (AR AR050236 R01 and R56 to V.K.), and the Institute of Clinical and Translational Science, University of California Irvine.
Contributor Information
Lan Weiss, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA.
Kwang-Mook Jung, Department of Anatomy & Neurobiology, University of California-Irvine, Irvine, CA, USA.
Angele Nalbandian, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA; Department of Ophthalmology, University of California-Irvine, Irvine, CA, USA.
Katrina Llewellyn, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA.
Howard Yu, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA.
Lac Ta, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA.
Isabela Chang, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA.
Marco Migliore, Drug Discovery and Development, Istituto Italiano di Tecnologia, Genoa, 16162, Italy; Aptuit (Verona) Srl, Verona, 37135 Italy.
Erica Squire, Department of Anatomy & Neurobiology, University of California-Irvine, Irvine, CA, USA.
Faizy Ahmed, Department of Anatomy & Neurobiology, University of California-Irvine, Irvine, CA, USA.
Daniele Piomelli, Department of Anatomy & Neurobiology, University of California-Irvine, Irvine, CA, USA; Pharmaceutical Sciences, University of California-Irvine, Irvine, CA, USA; Biological Chemistry, University of California-Irvine, Irvine, CA, USA.
Virginia Kimonis, Division of Genetic and Genomic Medicine, Department of Pediatrics, University of California-Irvine, Irvine, CA, USA; Department of Neurology, University of California-Irvine, Irvine, CA, USA; Department of Pathology, University of California-Irvine, Irvine, CA, USA; Division of Occupational and Environmental Medicine, Department of Medicine, University of California-Irvine, Irvine, CA, USA.
References
- 1.Kimonis, V. (1993) Inclusion Body Myopathy with Paget Disease of Bone and/or Frontotemporal Dementia. 2007 May 25 [Updated 2019 Sep 12]. In Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K. and Amemiya, A. (eds), GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1476/ [Google Scholar]
- 2.Kimonis, V., Donkervoort, S. and Watts, G. (1993) In Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Ledbetter, N., Mefford, H.C., Smith, R.J.H. and Stephens, K. (eds), Gene Reviews®, Seattle (WA); University of Washington, 1993–2020. [Google Scholar]
- 3.Watts, G.D., Wymer, J., Kovach, M.J., Mehta, S.G., Mumm, S., Darvish, D., Pestronk, A., Whyte, M.P. and Kimonis, V.E. (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet., 36, 377–381. [DOI] [PubMed] [Google Scholar]
- 4.Meyer, H. and Weihl, C.C. (2014) The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J. Cell Sci., 127, 3877–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parzych, K., Saavedra-Garcia, P., Valbuena, G.N., Al-Sadah, H.A., Robinson, M.E., Penfold, L., Kuzeva, D.M., Ruiz-Tellez, A., Loaiza, S., Holzmann, V. et al. (2019) The coordinated action of VCP/p97 and GCN2 regulates cancer cell metabolism and proteostasis during nutrient limitation. Oncogene, 38, 3216–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad, A., Bharathi, V., Sivalingam, V., Girdhar, A. and Patel, B.K. (2019) Molecular mechanisms of TDP-43 Misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci., 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llewellyn, K.J., Nalbandian, A., Jung, K.M., Nguyen, C., Avanesian, A., Mozaffar, T., Piomelli, D. and Kimonis, V.E. (2014) Lipid-enriched diet rescues lethality and slows down progression in a murine model of VCP-associated disease. Hum. Mol. Genet., 23, 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang, W. and Ogretmen, B. (2013) Ceramide stress in survival versus lethal autophagy paradox: ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Autophagy, 9, 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sentelle, R.D., Senkal, C.E., Jiang, W., Ponnusamy, S., Gencer, S., Selvam, S.P., Ramshesh, V.K., Peterson, Y.K., Lemasters, J.J., Szulc, Z.M. et al. (2012) Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol., 8, 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, W. and Ogretmen, B. (2014) Autophagy paradox and ceramide. Biochim. Biophys. Acta, 1841, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippov, V., Song, M.A., Zhang, K., Vinters, H.V., Tung, S., Kirsch, W.M., Yang, J. and Duerksen-Hughes, P.J. (2012) Increased ceramide in brains with Alzheimer's and other neurodegenerative diseases. J. Alzheimers Dis., 29, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gertow, J., Kjellqvist, S., Stahlman, M., Cheung, L., Gottfries, J., Werngren, O., Boren, J., Franco-Cereceda, A., Eriksson, P. and Fisher, R.M. (2014) Ceramides are associated with inflammatory processes in human mediastinal adipose tissue. Nutr. Metab. Cardiovasc. Dis., 24:124–31. [DOI] [PubMed] [Google Scholar]
- 13.Holland, W.L., Brozinick, J.T., Wang, L.P., Hawkins, E.D., Sargent, K.M., Liu, Y., Narra, K., Hoehn, K.L., Knotts, T.A., Siesky, A. et al. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab., 5, 167–179. [DOI] [PubMed] [Google Scholar]
- 14.Schonemann, W., Gallienne, E., Ikeda-Obatake, K., Asano, N., Nakagawa, S., Kato, A., Adachi, I., Gorecki, M., Frelek, J. and Martin, O.R. (2013) Glucosylceramide mimics: highly potent GCase inhibitors and selective pharmacological chaperones for mutations associated with types 1 and 2 Gaucher disease. Chem Med Chem, 8:1805–17. [DOI] [PubMed] [Google Scholar]
- 15.Desnick, R.J., Schuchman, E.H., Astrin, K.H. and Cheng, S.H. (2013) Emery and Rimoin's Principles and Practice of Medical Genetics (Sixth Edition). Academic Press, Oxford, pp. 1–30. [Google Scholar]
- 16.Desnick, R.J., Astrin, K.H. and Bishop, D.F. (1989) Fabry disease: molecular genetics of the inherited nephropathy. Adv. Nephrol. Necker Hosp., 18, 113–127. [PubMed] [Google Scholar]
- 17.Castro, E.V., Yoneyama, K.G., Haapalainen, E.F., Toledo, M.S., Takahashi, H.K. and Straus, A.H. (2013) Myriocin, a serine palmitoyltransferase inhibitor, blocks cytokinesis in Leishmania (Viannia) braziliensis promastigotes. J. Eukaryot. Microbiol., 60, 377–387. [DOI] [PubMed] [Google Scholar]
- 18.Lizarazo, D., Zabala, V., Tong, M., Longato, L. and de la Monte, S.M. (2013) Ceramide inhibitor myriocin restores insulin/insulin growth factor signaling for liver remodeling in experimental alcohol-related steatohepatitis. J. Gastroenterol. Hepatol., 28, 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadsworth, J.M., Clarke, D.J., McMahon, S.A., Lowther, J.P., Beattie, A.E., Langridge-Smith, P.R., Broughton, H.B., Dunn, T.M., Naismith, J.H. and Campopiano, D.J. (2013) The chemical basis of serine palmitoyltransferase inhibition by myriocin. J. Am. Chem. Soc., 135, 14276–14285. [DOI] [PubMed] [Google Scholar]
- 20.Bolton, G.L., Hutchings, R.H., Kohrt, J.T., Park, W.K.C. and Van Huis, C.A. (2006) Inhibitors of Serine Palmitoyl Transferase. In Fuller, G., Jr. (ed), International Patent Classification: C07D 401/04 (2006.01); C07D 401/14 (2006.01); A61K 31/454 (2006.01). C/o George, Nancy, McGraw Pfizer Inc., MS8260–1615 Eastern Point Road Groton. CT 06340 (US). [Google Scholar]
- 21.Kurek, K., Piotrowska, D.M., Wiesiolek-Kurek, P., Lukaszuk, B., Chabowski, A., Gorski, J. and Zendzian-Piotrowska, M. (2013) Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int., in press. [DOI] [PubMed] [Google Scholar]
- 22.Realini, N., Solorzano, C., Pagliuca, C., Pizzirani, D., Armirotti, A., Luciani, R., Costi, M.P., Bandiera, T. and Piomelli, D. (2013) Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Sci. Rep., 3, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzirani, D., Pagliuca, C., Realini, N., Branduardi, D., Bottegoni, G., Mor, M., Bertozzi, F., Scarpelli, R., Piomelli, D. and Bandiera, T. (2013) Discovery of a new class of highly potent inhibitors of acid ceramidase: synthesis and structure-activity relationship (SAR). J. Med. Chem., 56, 3518–3530. [DOI] [PubMed] [Google Scholar]
- 24.Lavieu, G., Scarlatti, F., Sala, G., Carpentier, S., Levade, T., Ghidoni, R., Botti, J. and Codogno, P. (2008) Sphingolipids in macroautophagy. Methods Mol. Biol., 445, 159–173. [DOI] [PubMed] [Google Scholar]
- 25.Ahola-Erkkila, S., Carroll, C.J., Peltola-Mjosund, K., Tulkki, V., Mattila, I., Seppanen-Laakso, T., Oresic, M., Tyynismaa, H. and Suomalainen, A. (2010) Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet., 19, 1974–1984. [DOI] [PubMed] [Google Scholar]
- 26.Camargo, N., Brouwers, J.F., Loos, M., Gutmann, D.H., Smit, A.B. and Verheijen, M.H. (2012) High-fat diet ameliorates neurological deficits caused by defective astrocyte lipid metabolism. FASEB J., in press. [DOI] [PubMed] [Google Scholar]
- 27.Zhou, Y., Bazick, H., Miles, J.R., Fethiere, A.I., Salihi, M.O.A., Fazio, S., Tavori, H. and Notterpek, L. (2019) A neutral lipid-enriched diet improves myelination and alleviates peripheral nerve pathology in neuropathic mice. Exp. Neurol., 321, 113031. [DOI] [PubMed] [Google Scholar]
- 28.Hannun, Y.A. and Obeid, L.M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol., 9, 139–150. [DOI] [PubMed] [Google Scholar]
- 29.Giansanti, V., Torriglia, A. and Scovassi, A.I. (2011) Conversation between apoptosis and autophagy: "is it your turn or mine?". Apoptosis, 16, 321–333. [DOI] [PubMed] [Google Scholar]
- 30.Bartke, N. and Hannun, Y.A. (2009) Bioactive sphingolipids: metabolism and function. J. Lipid Res., 50, S91–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gault, C.R., Obeid, L.M. and Hannun, Y.A. (2010) An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol., 688, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill, A.H., Jr. (2011) Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev., 111, 6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klionsky, D.J. (2018) Why do we need to regulate autophagy (and how can we do it)? A cartoon depiction. Autophagy, 14, 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klionsky, D.J. (2018) Why do we need autophagy? A cartoon depiction. Autophagy, 14, 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klionsky, D.J. (2020) Autophagy participates in, well, just about everything. Cell Death Differ., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klionsky, D.J., Abdelmohsen, K., Abe, A., Abedin, M.J., Abeliovich, H., Acevedo Arozena, A., Adachi, H., Adams, C.M., Adams, P.D., Adeli, K. et al. (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy, 12, 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarlatti, F., Bauvy, C., Ventruti, A., Sala, G., Cluzeaud, F., Vandewalle, A., Ghidoni, R. and Codogno, P. (2004) Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J. Biol. Chem., 279, 18384–18391. [DOI] [PubMed] [Google Scholar]
- 38.Iguchi, Y., Eid, L., Parent, M., Soucy, G., Bareil, C., Riku, Y., Kawai, K., Takagi, S., Yoshida, M., Katsuno, M. et al. (2016) Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain, 139, 3187–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stith, J.L., Velazquez, F.N. and Obeid, L.M. (2019) Advances in determining signaling mechanisms of ceramide and role in disease. J. Lipid Res., 60, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, R.X., Pan, Q., Liu, X.L., Zhou, D., Xin, F.Z., Zhao, Z.H., Zhang, R.N., Zeng, J., Qiao, L., Hu, C.X. et al. (2019) Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids Health Dis., 18, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahini, A., Vydiam, K., Choudhury, D., Rajabian, N., Nguyen, T., Lei, P. and Andreadis, S.T. (2018) Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res., 30, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Vita, T., Albani, C., Realini, N., Migliore, M., Basit, A., Ottonello, G. and Cavalli, A. (2019) Inhibition of serine Palmitoyltransferase by a small organic molecule promotes neuronal survival after astrocyte amyloid Beta 1-42 injury. ACS Chem. Neurosci., 10, 1627–1635. [DOI] [PubMed] [Google Scholar]
- 43.Astarita, G., Ahmed, F. and Piomelli, D. (2009) Lipidomic analysis of biological samples by liquid chromatography coupled to mass spectrometry. Methods Mol. Biol., 579, 201–219. [DOI] [PubMed] [Google Scholar]
- 44.Hindi, L., McMillan, J.D., Afroze, D., Hindi, S.M. and Kumar, A. (2017) Isolation, culturing, and differentiation of primary myoblasts from skeletal muscle of adult mice. Bio-protocol, 7, e2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astarita, G., Avanesian, A., Grimaldi, B., Realini, N., Justinova, Z., Panlilio, L.V., Basit, A., Goldberg, S.R. and Piomelli, D. (2015) Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PLoS One, 10, e0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nalbandian, A., Llewellyn, K.J., Badadani, M., Yin, H.Z., Nguyen, C., Katheria, V., Watts, G., Mukherjee, J., Vesa, J., Caiozzo, V. et al. (2013) A progressive translational mouse model of human valosin-containing protein disease: the VCP(R155H/+) mouse. Muscle Nerve, 47, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llewellyn, K.J., Nalbandian, A., Weiss, L.N., Chang, I., Yu, H., Khatib, B., Tan, B., Scarfone, V. and Kimonis, V.E. (2017) Myogenic differentiation of VCP disease-induced pluripotent stem cells: a novel platform for drug discovery. PLoS One, 12, e0176919. [DOI] [PMC free article] [PubMed] [Google Scholar]


