Abstract
Background and purpose
To evaluate relationship between fluid-attenuated inversion recovery vascular hyperintensity (FVH) after intravenous thrombolysis and outcomes in different lesion patterns on diffusion-weighted imaging (DWI).
Methods
Patients with severe internal carotid or intracranial artery stenosis who received intravenous thrombolysis from March 2012 to April 2019 were analysed. They were divided into four groups by DWI lesion patterns: border-zone infarct (BZ group), multiple lesions infarct (ML group), large territory infarct (LT group), and single cortical or subcortical lesion infarct (SL group). Logistic regression was performed to identify risk factors for outcome (unfavourable outcome, modified Rankin Scale (mRS) ≥2; poor outcome, mRS ≥3).
Results
Finally, 203 participants (63.3±10.2 years old; BZ group, n=72; ML group, n=64; LT group, n=37; SL group, n=30) from 1190 patient cohorts were analysed. After adjusting for confounding factors, FVH (+) was associated with unfavourable outcome in total group (OR 3.02; 95% CI 1.49 to 6.13; p=0.002), BZ group (OR 4.22; 95% CI 1.25 to 14.25; p=0.021) and ML group (OR 5.44; 95% CI 1.41 to 20.92; p=0.014) patients. FVH (+) was associated with poor outcome in total group (OR 2.25; 95% CI 1.01 to 4.97; p=0.046), BZ group (OR 5.52; 95% CI 0.98 to 31.07; p=0.053) and ML group (OR 4.09; 95% CI 1.04 to 16.16; p=0.045) patients, which was marginal significance. FVH (+) was not associated with unfavourable or poor outcome in LT and SL groups.
Conclusion
This study suggests that association between FVH and outcome varies with different lesion patterns on DWI. The presence of FVH after intravenous thrombolysis may help to identify patients who require close observations in the hospitalisation in patients with border-zone and multiple lesion infarcts.
Keywords: stroke, stenosis, MRI, artery
Introduction
Stroke is one of the leading causes of disability and death worldwide,1 and there are million new stroke cases each year in China.2 Previous studies indicated that there are several mechanisms of ischaemic stroke including thrombosis leading to complete artery occlusion, artery-to-artery embolism, haemodynamic compromise and local branch occlusion. Different mechanisms of stroke always resulted in different lesion patterns and different outcomes.3 Lesion patterns have been widely analysed to explore the mechanism of stroke.4
Fluid-attenuated inversion recovery (FLAIR) vascular hyperintensity (FVH) was first reported by Cosnard et al in 1999,5 which was frequently encountered in patients with acute ischaemic stroke with significant intracranial artery stenosis or occlusion.6 Pathologically, FVHs are considered as slow flow, flow-related enhancement (slow, but not static flow) and clot-signal intensity (oxyhaemoglobin),7 and the slow flow is regarded as slow retrograde flow in leptomeningeal collaterals8 or antegrade flow representing the impaired haemodynamics.9 There are many studies focusing on the association between FVH and outcomes in patients with ischaemic stroke. However, some studies demonstrated that FVHs were reflected poor collaterals and poor outcomes,9–11 while other studies indicated that FVHs were related to good collaterals and favourable outcomes.12–14 The exact reason for the discrepancy is still unknown. But these literatures did not consider the effect of stroke mechanisms, and did not classify the ischaemic stroke based on lesion patterns. Different lesion patterns related to different mechanisms of stroke may also be related to different causes of formation of FVH. In this study, we try to focus on the relationship between FVH and outcome according to different lesion patterns on diffusion-weighted imaging (DWI) in large artery atherosclerosis stroke.
Methods
Patients
Patients with ischaemic stroke within 4.5 hours of symptom onset who received recombinant tissue plasminogen activator (r-tPA) therapy and brain MRI in Xuanwu Hospital, Capital Medical University, from April 2012 to March 2019 were retrospectively enrolled. The inclusion criteria are as follows: (1) >18 years old; (2) had acute infarct lesions within the middle cerebral artery (MCA) distribution territory determined by DWI; (3) had ipsilateral severe stenosis (>50% stenosis) or occlusion in MCA, internal carotid artery or common carotid artery identified by CT angiography, magnetic resonance angiography (MRA) or digital subtraction angiography. Patients with the following conditions were excluded from this study: (1) no FLAIR images or MRI with poor image quality; (2) no DWI hyperintensity; (3) no medical history; (4) r-tPA not finished or received mechanical thrombectomy; (5) not MCA territory stroke; (6) non-atherosclerotic vasculopathy that may predispose to stroke such as dissection, moyamoya disease, vasculitis, evidence of cardioembolism or undetermined aetiology; (7) artery stenosis degree <50%. The demographics and clinical characteristics of patients were collected from the medical record, including age, sex, vascular risk factors such as hypertension, diabetes mellitus, coronary artery disease, hyperlipidaemia, smoking, drinking, history of previous stroke, the National Institute of Healthy Stroke Scale (NIHSS) score before r-tPA therapy and hospitalisation days. Hypertension15 and diabetes mellitus16 were defined as published standards.
Magnetic resonance imaging
Brain MRI was performed for all included patients on 3T MR scanners (Magnetom Verio and Magnetom Trio; Siemens, Germany) with 12-channel head coils. In the routine brain imaging protocol, the sequences of FLAIR and DWI were acquired using the following parameters: T2-FLAIR: turbo spin-echo, TR/TE 10 000 ms/119 ms, FOV 23.0×23.0 cm2, matrix 308×308 and slice thickness 5 mm; DWI: echo‐planar imaging, TR/TE 2824 ms/93 ms, FOV 23.0×23.0 cm2, matrix 164×122 and slice thickness 5 mm. Time-of-flight-MRA: fast low-angle shot, TR/TE 19 ms/3.5 ms, FOV 200×200 mm2, matrix 400×268 and slice thickness 1.1 mm. All MRIs were performed after thrombolytic therapy, and the time from finishing thrombolysis to MRI was within 7 days.
Neuroimaging analysis
All DWI images were evaluated for the determination of an acute ischaemic infarction.17 White matter hyperintensity (WMH) was assessed on FLAIR images using the Fazekas score.18 Lacunar infarct was defined as small infarct lesion (sized ≤15 mm in diameter, hyperintensity on FLAIR images and iso-intensity on T1W images) located in the subcortical white matter, thalamus or basal ganglia. Artery stenosis degree was measured as the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis trial.19
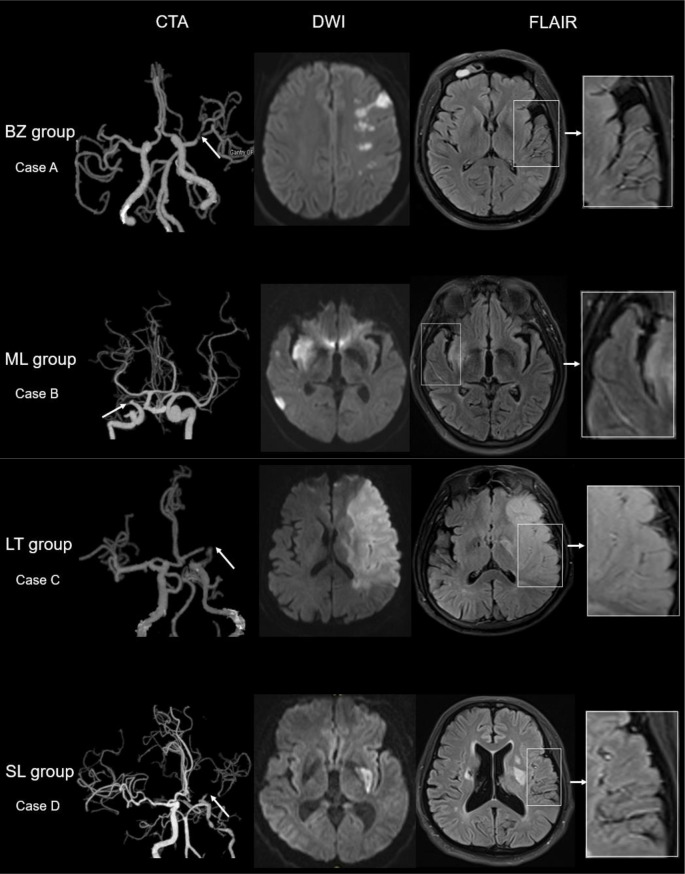
The topography of infarct lesions was defined using published templates,4 and the templates have been modified. Accordingly, all patients were categorised into four groups: border-zone infarct group (BZ group), multiple lesion infarcts group (ML group), large territory infarct group (LT group) and single cortical or subcortical lesion group (SL group). Border-zone infarcts (BZ group) were defined as infarcts at anterior or posterior cortical border-zone or internal border-zone. Multiple infarcts (ML group) referred to multiple infarct lesions in MCA territory but not at the border-zone. When cases with most of the infarcts are located at the border-zone it will be classified into BZ group, and when cases with most of the infarcts are located at non-border-zone regions it will be classified into ML group. BZ group has the priority when it comes to mixture lesion pattern cases. Large territory infarct (LT group) was defined as an infarct occurring in the vascular territories supplied by the stem of MCA and more than one-third of MCA territory. Single cortical or subcortical lesion (SL group) was defined as a single infarct occurring in the cortical or subcortical territory of the MCA and less than one-third of MCA territory (figure 1).
Figure 1.
Illustrative cases according to the DWI lesion patterns. Case A (BZ group) shows severe stenosis of left MCA on CTA, a border-zone infarct lesion on DWI and with FVH positive in FLAIR; FVHs located beyond the lesions. Case B (ML group) shows occlusion of the right MCA, two lesions in different artery territories on DWI and with FVH positive on FLAIR; FVHs located beyond the lesions. Case C (LT group) shows occlusion of the left MCA on CTA, a large territory infarct lesion on DWI and with FVH positive on FLAIR; FVHs located within or very closed to the lesions. Case D (SL group) shows near occlusion of the left MCA on CTA, a lesion in the basal ganglia on DWI and with FVH positive on FLAIR; FVHs located beyond the lesions. BZ group, border-zone infarct group; CTA, CT angiography; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; FVH, FLAIR vascular hyperintensity; LT group, large territory infarct group; MCA, middle cerebral artery; ML group, multiple lesion infarcts group; SL group, single cortical or subcortical lesion group.
The FVHs after intravenous thrombolysis are defined as focal, tubular or serpentine hyperintensities in the subarachnoid space against the relative hypointensity of cerebrospinal fluid and corresponding to the typical arterial course on FLAIR images20 at the ipsilateral side of acute ischaemic lesions on FLAIR images.
All brain images were reviewed by two experienced radiologists who had, respectively, 19 years and 6 years of experience in neuroimaging blinded to the clinical data. Disagreements were resolved by consensus.
Clinical outcome
Modified Rankin Scale (mRS) was used to assess the clinical outcome of all the patients at discharge after thrombolysis treatment.21 The mRS ≥2 was defined as unfavourable outcome; mRS score ≥3 was defined as poor outcome.
Intraobserver and interobserver agreement
Thirty patients were randomly selected for testing the intraobserver and interobserver reliability in identifying presence of FVH, lesion pattern of infarct, and WMH (subcortical) and WMH (periventricular). A time interval of 2 months was set for testing the intraobserver reliability to minimise the bias of memory.
Statistical analysis
The continuous variables were described as mean±SD or median and IQR. The binary variables were expressed as count and percentage. The clinical characteristics were compared between FVH (+) and FVH (−) groups using independent t-test, Mann-Whitney U test, Χ2 test or Fisher’s exact test according to the type of variable. The κ coefficient was used to assess interobserver agreements for the lesion patterns and FVH. Multivariate logistic regression analysis was performed to identify independent predictors of unfavourable (mRS ≥2, model 1) and poor outcome (mRS ≥3, model 2) at discharge. FVH (+), artery occlusion, NIHSS at admission (NIHSS-ad), age and sex were put into multivariate logistic regression model. A value of p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS V.20.0.
Results
Basic clinical characteristic
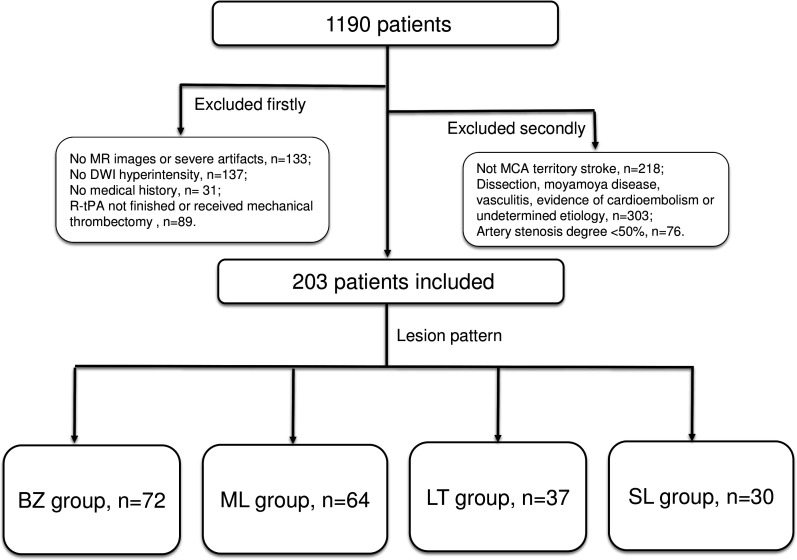
Finally, 203 participants (63.3±10.2 years old; 169 men (83.3%)) who met the inclusion criteria among 1190 consecutive patients with ischaemic stroke (BZ group, n=72; ML group, n=64; LT group, n=37; SL group, n=30) were included, while 987 patients were excluded (no MRI or severe artefacts, n=133; no DWI hyperintensity, n=137; no medical history, n=31; r-tPA intravenous thrombolysis therapy not finished or received mechanical thrombectomy, n=89; not MCA territory stroke, n=218; dissection, moyamoya disease, vasculitis or evidence of cardioembolism, n=303; artery stenosis degree <50%, n=76;) (figure 2). Among the remaining 203 patients, the presence of FVH was observed in 100 patients (49.3%). The time from finishing thrombolysis to MRI was within 7 (median (IQR), 2 (1–3)) days.
Figure 2.
The flow chart of patients included. BZ group, border-zone infarct group; DWI, diffusion-weighted imaging; LT group, large territory infarct group; MCA, middle cerebral artery; ML group, multiple lesion infarcts group; r-tPA, recombinant tissue plasminogen activator; SL group, single cortical or subcortical lesion group.
Intraobserver and interobserver agreement
For the intraobserver agreement in assessing the presence of FVH, lesion pattern of infarct, and WMH (subcortical) and WMH (periventricular), the κ values were 1.00, 0.95, 0.94 and 0.95 (all p<0.001), respectively. For the interobserver agreement in assessing the presence of FVH, lesion pattern of infarct, and WMH (subcortical) and WMH (periventricular), the κ values were 0.92, 0.90, 0.87 and 0.90 (all p<0.001), respectively.
The differences between FVH (+) and FVH (−) group
Cases with FVH (+) tended to have higher prevalence of artery occlusion (58.0% vs 10.7%, p<0.001), higher NIHSS-ad score (7.5 (4–12) vs 5 (3–9), p=0.002), higher mRS score at discharge (2 (1–4) vs 1 (1–2), p<0.001) and longer hospitalisation (8 (7–10) vs 7 (6–9), p=0.009) compared with patients with FVH (−). This was similar in BZ and ML groups. In LT group, patients with FVH (+) were found to have a significantly higher prevalence of artery occlusion (65.4% vs 27.3%, p=0.033). In SL group, patients with FVH tended to have higher prevalence of previous stroke (83.3% vs 20.8%, p=0.015) and artery occlusion (66.7% vs 4.2%, p=0.002). Neither NIHSS-ad score (7.2±2.3 vs 5.0±2.4, p=0.064) nor mRS score at discharge (1 (1–1.25) vs 1.5 (1–3), p=0.210) was significantly different between FVH (+) and FVH (−) patients. All demographic and clinical characteristics were presented in table 1.
Table 1.
Clinical and demographic characteristics of FVH (+) and FVH (−) patients in all patients and four subgroups
| All patients | BZ group | ML group | LT group | SL group | |||||||||||
| FVH− | FVH+ | P value | FVH− | FVH+ | P value | FVH− | FVH+ | P value | FVH− | FVH+ | P value | FVH− | FVH+ | P value | |
| N=103 (%) | N=100 (%) | N=43 (%) | N=29 (%) | N=25 (%) | N=39 (%) | N=11 (%) | N=26 (%) | N=24 (%) | N=6 (%) | ||||||
| Sex (men) | 82 (79.6) | 87 (87.0) | 0.159 | 35 (81.4) | 25 (86.2) | 0.830 | 21 (84.0) | 33 (84.6) | 0.999 | 9 (81.8) | 23 (88.5) | 0.989 | 17 (70.8) | 6 (100) | 0.331 |
| Age | 63.2±10.1 | 63.4±10.4 | 0.870 | 64.7±9.4 | 63.6±10.1 | 0.634 | 62.7±9.5 | 63.3±10.2 | 0.826 | 66.6±12.7 | 62.9±11.6 | 0.395 | 59.3±10.1 | 65.7±9.8 | 0.174 |
| Hypertension | 81 (78.6) | 72 (72) | 0.272 | 34 (79.1) | 19 (65.5) | 0.201 | 20 (80.0) | 28 (71.8) | 0.460 | 7 (63.6) | 21 (80.8) | 0.490 | 20 (83.3) | 4 (66.7) | 0.732 |
| Diabetes mellitus | 34 (33.0) | 36 (36.0) | 0.654 | 17 (39.5) | 12 (41.4) | 0.876 | 6 (24.0) | 11 (28.2) | 0.710 | 2 (18.2) | 10 (38.5) | 0.412 | 9 (37.5) | 3 (50.0) | 0.926 |
| Coronary heart disease | 14 (13.6) | 19 (19.0) | 0.296 | 6 (14.0) | 6 (20.7) | 0.667 | 2 (8.0) | 7 (17.9) | 0.454 | 3 (27.3) | 6 (23.1) | 0.999 | 3 (12.5) | 0 (0) | 0.999 |
| Hyperlipidaemia | 55 (53.4) | 42 (42.0) | 0.104 | 25 (58.1) | 15 (51.7) | 0.591 | 14 (56.0) | 17 (43.6) | 0.332 | 4 (36.4) | 9 (34.6) | 0.999 | 12 (50.0) | 1 (16.7) | 0.311 |
| Previous stroke | 29 (28.2) | 25 (25.0) | 0.611 | 11 (25.6) | 8 (27.6) | 0.850 | 10 (40.0) | 7 (17.9) | 0.051 | 3 (27.3) | 5 (19.2) | 0.915 | 5 (20.8) | 5 (83.3) | 0.015* |
| Smoking | 58 (56.3) | 62 (62.0) | 0.410 | 24 (55.8) | 17 (58.6) | 0.814 | 15 (60.0) | 23 (59.0) | 0.935 | 8 (72.7) | 19 (73.1) | 0.999 | 11 (45.8) | 3 (50.0) | 0.999 |
| Drinking | 57 (55.3) | 54 (54.0) | 0.848 | 24 (55.8) | 15 (51.7) | 0.733 | 13 (52.0) | 25 (64.1) | 0.336 | 7 (63.6) | 13 (50.0) | 0.447 | 13 (54.2) | 1 (16.7) | 0.234 |
| Lacunar infarct | 56 (54.4) | 62 (62.0) | 0.271 | 23 (53.5) | 18 (62.1) | 0.471 | 16 (64.0) | 26 (66.7) | 0.827 | 4 (36.4) | 15 (57.7) | 0.235 | 13 (54.2) | 3 (50.0) | 0.999 |
| Occlusion | 11 (10.7) | 58 (58.0) | <0.001* | 2 (4.7) | 13 (44.8) | <0.001* | 5 (20.0) | 24 (61.5) | 0.001* | 3 (27.3) | 17 (65.4) | 0.033* | 1 (4.2) | 4 (66.7) | 0.002* |
| WMH (subcortical) | 0 (0–1) | 1 (0–1) | 0.366 | 1 (0–1) | 0 (0–1) | 0.643 | 0 (0–1) | 1 (0–2) | 0.265 | 0 (0–1) | 0 (0–1) | 0.750 | 0 (0–1) | 1 (0–2.25) | 0.209 |
| WMH (periventricular) | 1 (0–2) | 1 (0–2) | 0.992 | 1 (1–2) | 1 (0–1.5) | 0.471 | 1 (0.5–1.5) | 1 (1–3) | 0.295 | 1 (0–2) | 1 (0–1) | 0.559 | 1 (0.25–2) | 1 (1–1.25) | 0.732 |
| NIHSS-ad | 5 (3–9) | 7.5 (4–12) | 0.002* | 5 (3–9) | 6 (4–10.5) | 0.149 | 5 (4–9.5) | 5 (4–11) | 0.830 | 8.7±6.0 | 11.1±5.1 | 0.224 | 5.0±2.4 | 7.2±2.3 | 0.064 |
| mRS ≥2 | 36 (34.3) | 69 (65.7) | <0.001* | 10 (23.3) | 21 (72.4) | <0.001* | 8 (32.0) | 24 (61.5) | 0.021* | 6 (54.5) | 22 (84.6) | 0.126 | 12 (50.0) | 1 (16.7) | 0.311 |
| mRS ≥3 | 18 (17.5) | 47 (47.0) | <0.001* | 3 (7.0) | 14 (48.3) | <0.001* | 4 (16.0) | 15 (38.5) | 0.055 | 4 (36.4) | 18 (69.2) | 0.135 | 7 (29.2) | 0 (0) | 0.331 |
| mRS at discharge | 1 (1–2) | 2 (1–4) | <0.001* | 1 (0–1) | 2 (1–4) | <0.001* | 1 (1–2) | 2 (1–4) | 0.011* | 2 (1–4) | 4 (2–4) | 0.088 | 1.5 (1–3) | 1 (1–1.25) | 0.210 |
| Hospitalisation (days) | 7 (6–9) | 8 (7–10) | 0.009* | 7 (6–8) | 8 (7–10) | 0.014* | 7 (6–8) | 9 (7–10) | 0.007* | 10(9-11) | 9 (6.75–11.25) | 0.277 | 8 (6.25–10) | 7.5 (6.5–8.5) | 0.529 |
| r-tPA to MRI time (days) | 2 (1–3) | 2 (1–2) | 0.624 | 2 (1–3) | 2 (1–3) | 0.915 | 2 (1–3) | 1 (1–3) | 0.057 | 2 (1–4) | 2 (1–2) | 0.180 | 1 (1–2) | 1.5 (1–2.25) | 0.668 |
Data are presented as median (IQR), mean±SD or n (%).
*Significantly different.
BZ group, border-zone infarct group; FVH, fluid-attenuated inversion recovery vascular hyperintensity; LT group, large territory infarct group; ML group, multiple lesion infarcts group; mRS, modified Rankin Scale; NIHSS-ad, National Institute of Healthy Stroke Scale at admission; r-tPA, recombinant tissue plasminogen activator; SL group, single cortical or subcortical lesion group; WMH, white matter hyperintensity.
The association between FVH and outcome in different groups
Multiple analysis of the clinical and radiological factors associated with unfavourable outcome (mRS ≥2, model 1) and poor outcome (mRS ≥3, model 2) according to DWI lesion patterns is reported in table 2. In model 1, FVHs (+) were associated with unfavourable outcome after adjusting for confounding factors in total group (OR 3.02, 95% CI (1.49 to 6.13), p=0.002), BZ group (OR 4.22, 95% CI (1.25 to 14.25), p=0.021) and ML group (OR 5.44, 95% CI (1.41 to 20.92), p=0.014) patients. In LT group, only artery occlusion (OR 20.51; 95% CI 1.69 to 248.68; p=0.018) was associated with unfavourable outcome. In SL group, all of FVHs (+), age, male, occlusion and NIHSS-ad did not show significance. In model 2, after adjusting for confounding factors, FVH (+) was also associated with poor outcome in total group (OR 2.25; 95% CI 1.01 to 4.97; p=0.046), BZ group (OR 5.52; 95% CI 0.98 to 31.07; p=0.053) and ML group (OR 4.09; 95% CI 1.04 to 16.16; p=0.045) patients, which was marginal significance. In addition, NIHSS-ad was also associated with poor outcome in these three groups. In LT group, age (OR 0.88, 95% CI (0.77 to 1.00), p=0.051, which was marginal significance) and NIHSS-ad (OR 1.69, 95% CI (1.18 to 2.44), p=0.004) were associated with poor outcome. In SL group, none of these factors showed significance.
Table 2.
Results of multiple logistic regression analysis for unfavourable (mRS ≥2) and poor (mRS ≥3) outcome in all patients and four subgroups
| All patients | BZ group | ML group | LT group | SL group | ||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Model 1, mRS ≥2 | ||||||||||
| FVH (+) | 3.02 (1.49 to 6.13) | 0.002 | 4.22 (1.25 to 14.25) | 0.021 | 5.44 (1.41 to 20.92) | 0.014 | 1.95 (0.24 to 15.90) | 0.531 | 0.24 (0.01 to 7.36) | 0.410 |
| Male | 2.00 (0.86 to 4.66) | 0.110 | 0.20 (0.02 to 2.12) | 0.180 | 0.91 (0.17 to 4.99) | 0.911 | 8.74 (0.31 to 246.91) | 0.203 | 3.55 (0.49 to 25.78) | 0.211 |
| Age | 1.00 (0.97 to 1.03) | 0.870 | 1.01 (0.96 to 1.08) | 0.654 | 1.02 (0.96 to 1.09) | 0.448 | 0.97 (0.89 to 1.06) | 0.499 | 1.01 (0.93 to 1.11) | 0.761 |
| Occlusion | 1.67 (0.77 to 3.59) | 0.192 | 1.14 (0.97 to 1.33) | 0.103 | 0.58 (0.15 to 2.18) | 0.420 | 20.51 (1.69 to 248.68) | 0.018 | 1.08 (0.03 to 33.89) | 0.965 |
| NIHSS-ad | 1.18 (1.09 to 1.28) | <0.001 | 14.19 (1.40 to 143.85) | 0.025 | 1.24 (1.06 to 1.46) | 0.007 | 1.24 (0.96 to 1.60) | 0.093 | 1.03 (0.72 to 1.46) | 0.882 |
| Model 2, mRS ≥3 | ||||||||||
| FVH (+) | 2.25 (1.01 to 4.97) | 0.046 | 5.52 (0.98 to 31.07) | 0.053 | 4.09 (1.04 to 16.16) | 0.045 | 1.53 (0.16 to 14.40) | 0.709 | NA | NA |
| Male | 1.40 (0.54 to 3.62) | 0.488 | 0.30 (0.02 to 3.94) | 0.363 | 0.56 (0.09 to 3.58) | 0.537 | 8.48 (0.33 to 217.26) | 0.197 | 0.70 (0.08 to 6.26) | 0.754 |
| Age | 1.00 (0.97 to 1.03) | 0.937 | 1.05 (0.96 to 1.14) | 0.338 | 1.03 (0.97 to 1.09) | 0.318 | 0.88 (0.77 to 1.00) | 0.051 | 1.04 (0.93 to 1.16) | 0.511 |
| Occlusion | 2.90 (1.32 to 6.37) | 0.008 | 32.43 (3.21 to 327.27) | 0.003 | 2.10 (0.57 to 7.82) | 0.268 | 5.39 (0.54 to 54.04) | 0.152 | 0.01 (0.01 to 999.99) | 0.999 |
| NIHSS-ad | 1.21 (1.11 to 1.31) | <0.001 | 1.34 (1.07 to 1.68) | 0.010 | 1.14 (1.02 to 1.27) | 0.019 | 1.69 (1.18 to 2.44) | 0.004 | 1.30 (0.83 to 2.02) | 0.253 |
Model 1 used mRS ≥2 as dependent variable; model 2 used mRS ≥3 as dependent variable.
The bolded entries mean significance.
BZ group, border-zone infarct group; FVH, fluid-attenuated inversion recovery vascular hyperintensity; LT group, large territory infarct group; ML group, multiple lesion infarcts group; mRS, modified Rankin Scale; NA, not available; NIHSS-ad, National Institute of Healthy Stroke Scale at admission; SL group, single cortical or subcortical lesion group.
The location of FVHs
In total patients, FVHs of 14% patients were located within DWI lesions; FVHs of 73% patients were located beyond DWI lesions; FVHs of 13% patients were located both within and beyond DWI lesion FVHs. In BZ and ML groups, FVHs were most frequently located beyond DWI lesions; in LT group, most FVHs were located within, both within and beyond DWI lesions; in SL group, all FVHs were located beyond DWI lesions (table 3). We also explored the relationship between FVH location and outcome in all FVH (+) patients. We found that there was no association between different FVH locations and outcomes. After adjusting for confounding factors, only occlusion and NIHSS-ad were associated with poor outcome (mRS ≥3) (online supplemental tables 4 and 5).
Table 3.
The distribution of FVHs in four subgroups
| FVH within DWI lesion | FVH beyond DWI lesion | FVH (both within and beyond) | |
| Total patients, n=100, (%) | 14 (14.0) | 73 (73.0) | 13 (13.0) |
| BZ group, n=29, (%) | 1 (3.4) | 26 (89.7) | 2 (6.9) |
| ML group, n=39, (%) | 0 (0) | 38 (97.4) | 1 (2.6) |
| LT group, n=26, (%) | 13 (50.0) | 3 (11.5) | 10 (38.5) |
| SL group, n=6, (%) | 0 (0) | 6 (100) | 0 (0) |
BZ group, border-zone infarct group; DWI, diffusion-weighted imaging; FVH, fluid-attenuated inversion recovery vascular hyperintensity; LT group, large territory infarct group; ML group, multiple lesion infarcts group; SL group, single cortical or subcortical lesion group.
svn-2020-000641supp001.pdf (46.7KB, pdf)
Discussion
This study investigated the relationship between FVH and clinical outcomes in different lesion types on DWI. We found that the prognostic value of FVH is different depending on the lesion patterns. FVH is an independent risk factor for unfavourable (mRS ≥2) and poor outcome (mRS ≥3) in patients with border-zone (BZ group) infarct and multiple lesion (ML group) infarct, while this association disappears when it comes to patients with large territory (LT group) stroke and single lesion (SL group) stroke.
In the present study, FVH was associated with poor outcome in total patients, which was in keeping with several previous studies.9 10 22 23 The prognostic value of FVH in ischaemic stroke has been widely investigated, with greatly divergent results. But these studies just investigated the associations between FVH and clinical outcome, not classifying stroke according to the mechanism. Besides populations, sample size, end points and FVH classifications, we suspected that the mechanism of stroke may also play an important role in the discrepancies.
According to the Chinese ischaemic stroke subclassification, the underlying mechanism of territorial infarct could be a sudden obstruction from an embolus and have little time to generate collaterals.24 Previous studies have indicated that the pathophysiology of FVH was slow flow, flow-related enhancement (slow, but not static flow) and clot-signal intensity (oxyhaemoglobin). There are two underlying aetiologies of slow flow: one opinion is that it is retrograde artery flow of the leptomeningeal collateral circulation, the other is that it is antegrade flow representing the impaired haemodynamics.10 14 In BZ and ML groups, patients of FVH (+) had a higher incidence of artery occlusion and a higher mRS score at discharge than those of FVH (−). No significant differences were found in NIHSS-ad score between patients of FVH (+) and FVH (−) group. This means that patients with FVH are more frequent to suffer from a deterioration of stroke in these two groups. The pathophysiology of border-zone area infarcts was thought to be dynamic disturbance and microembolisation, and the aetiology of multiple lesion infarcts was multiple emboli from the upstream artery.24 25 In the multivariate logistic regression analysis, FVH is found to be an independent risk factor for poor outcome after adjusting for confounding factors in these two groups. FVHs in these two groups are speculated as antegrade flow representing impaired haemodynamics, and patients with FVH (+) suffered hypoperfusion for a much longer time than those without FVH. This assumption was strengthened by the view that most FVHs are located beyond DWI lesions in BZ group and ML group. In this study, FVH is found to be significantly associated with artery occlusion, which also supports this opinion. In addition, there may be also some retrograde artery flow of leptomeningeal collaterals participating in the formation of FVHs, but not able to counteract the consumption of artery occlusion and impaired haemodynamics.
In LT group, patients with FVH tended to have higher prevalence of artery occlusion, and FVH is not an independent risk factor for poor outcome after adjusting for confounding factors. This points to that the majority of FVHs in LT group do not represent impaired haemodynamics, which may be clot-signal intensities (oxyhaemoglobin) and the ‘flow effect’ disappeared. This view is supported by the phenomenon that FVHs are always located within or very closed to the DWI lesions in LT group (figure 1). In addition, age is associated with outcome, as a protect factor, this may be explained that a few old patients suffered from cerebral haemorrhage and these patients did not undergo MRI, were not included in the present study, which would have led to few bias.
In SL group, the incidence of FVH is 20%, which is much lower than the other three groups. As similar constitution of artery stenosis or occlusion, the main reason to have a mild symptom in this group is that there are good collaterals. Patients with FVH tend to have a higher prevalence of previous stroke and artery occlusion (table 1) compared with patients without FVH, but the NIHSS-ad score and mRS at discharge were not significantly different. A higher prevalence of previous stroke meant that patients were in a long stay in artery stenosis and had enough time to generate collaterals. FVHs in SL group are considered as combination of retrograde artery flow of leptomeningeal collaterals and antegrade flow of new vessels, like moyamoya vessels near the occlusion MCA.26 It is interesting that the prevalence of poor outcome in FVH (–) group was higher than FVH (+) group, but the difference did not reach statistical significance. Further study with large samples is warranted.
A recent meta-analysis27 reported that FVHs beyond DWI lesions were associated with better outcome. We found that FVHs beyond or both beyond and within DWI lesion were not associated with outcome in patients with FVH (+). This difference maybe because of the fact that FVH was obtained after intravenous thrombolysis in our study. In addition, the intravenous thrombolysis treatment would have changed vascular occlusion status and the appearance of infarct lesion on MRI. Therefore, we should take into consideration the time of MRI performed.
As far as we know, this is the first study that investigates the associations between FVH and clinical outcomes according to different lesion patterns on DWI. This study explains the associations in the stroke mechanism way, offering a new method for the investigations in the FVH fields. This study demonstrates that we should observe different details in different stroke lesion patterns in clinical activities. Physicians should pay close attention to artery occlusion and NIHSS score rather than FVH in patients with large territory infarct. However, in patients with border-zone infarcts and multiple lesion infarcts, FVH should be considered as important as artery occlusion and NIHSS-ad score. In addition, we only enrolled patients who received intravenous thrombolysis, which could minimise the impact of therapy to a certain degree. Otherwise, the associations between FVH and clinical outcome in different lesion patterns should be further studied in patients without intravenous thrombolysis.
This study has some limitations. First, there are no follow-up MRIs in these patients, and future studies need to be performed to find out the duration of FVH, which would help to explain the mechanism more accurately. Second, we have not divided FVHs into proximal or distal subtype, because most patients both have these two subtypes, and we evaluate the general tendency of FVH in this study. Third, patients who had severe clinical complications such as large volume of cerebral haemorrhage were not able to perform MRI scan, therefore these patients were not included in this study, which may lead to some bias. Finally, there was a marginal significant difference in the total group, and the sample sizes of LT and SL groups were small when compared with that of the BZ or ML group. In generalising the results to the broader r-tPA patient population with carotid/intracranial artery stenosis, a study with more cases of patients with large territory and single lesion infarct is warranted.
In conclusion, this study suggests that the association between FVH and clinical outcome varies with different lesion patterns on DWI. FVH is an independent risk factor for unfavourable and poor outcome in patients with border-zone infarct and multiple lesion infarcts, but not in patients with large territory infarct or patients with single lesion infarct. The presence of FVHs after thrombolysis may help to identify patients with border-zone infarct and multiple lesion infarcts who require close observations in the hospitalisation.
Acknowledgments
The authors thank all the patients who participated in this study and they also thank the doctors in the Department of Neurology in Xuanwu Hospital.
Footnotes
EW and CW contributed equally.
Contributors: EW—study concept design, statistical analysis, gathering the clinical information and writing the manuscript. CW—study concept design, statistical analysis, gathering the clinical information and writing the manuscript. DY—MRI analysis and critical revision of the manuscript. XZ—critical revision of the manuscript for important intellectual content. JZ—gathering the clinical information and critical revision of the manuscript. HC—gathering the clinical information and critical revision of the manuscript. QY—study concept and design, study supervision, MRI analysis and critical revision of the manuscript for important intellectual content.
Funding: This study was funded by the National Natural Science Foundation of China (8191101305).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Anonymised individual participant data will be shared with qualified parties on request to the corresponding author.
Ethics statements
Patient consent for publication
Not required.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394–405. 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lu Z, Sun S, et al. Risk factors, topographic patterns and mechanism analysis of intracranial atherosclerotic stenosis ischemic stroke. Int J Neurosci 2017;127:267–75. 10.1080/00207454.2016.1188298 [DOI] [PubMed] [Google Scholar]
- 4.Lee DK, Kim JS, Kwon SU, et al. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke 2005;36:2583–8. 10.1161/01.STR.0000189999.19948.14 [DOI] [PubMed] [Google Scholar]
- 5.Cosnard G, Duprez T, Grandin C, et al. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with Mr angiography. Neuroradiology 1999;41:342–6. 10.1007/s002340050761 [DOI] [PubMed] [Google Scholar]
- 6.Cheng B, Ebinger M, Kufner A, et al. Hyperintense vessels on acute stroke fluid-attenuated inversion recovery imaging: associations with clinical and other MRI findings. Stroke 2012;43:2957–61. 10.1161/STROKEAHA.112.658906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Xu G, Yue X, et al. Hyperintense vessels on FLAIR: a useful non-invasive method for assessing intracerebral collaterals. Eur J Radiol 2011;80:786–91. 10.1016/j.ejrad.2010.09.043 [DOI] [PubMed] [Google Scholar]
- 8.Sanossian N, Saver JL, Alger JR, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. Am J Neuroradiol 2009;30:564–8. 10.3174/ajnr.A1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam K-W, Kim CK, Kim TJ, et al. Flair vascular hyperintensities predict early ischemic recurrence in TIA. Neurology 2018;90:e738–44. 10.1212/WNL.0000000000005034 [DOI] [PubMed] [Google Scholar]
- 10.Kufner A, Galinovic I, Ambrosi V, et al. Hyperintense vessels on FLAIR: hemodynamic correlates and response to thrombolysis. Am J Neuroradiol 2015;36:1426–30. 10.3174/ajnr.A4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nave AH, Kufner A, Bücke P, et al. Hyperintense vessels, collateralization, and functional outcome in patients with stroke receiving endovascular treatment. Stroke 2018;49:675–81. 10.1161/STROKEAHA.117.019588 [DOI] [PubMed] [Google Scholar]
- 12.Mahdjoub E, Turc G, Legrand L, et al. Do fluid-attenuated inversion recovery vascular hyperintensities represent good collaterals before reperfusion therapy? Am J Neuroradiol 2018;39:77–83. 10.3174/ajnr.A5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Scalzo F, Rao NM, et al. Fluid-Attenuated inversion recovery vascular hyperintensity topography, novel imaging marker for revascularization in middle cerebral artery occlusion. Stroke 2016;47:2763–9. 10.1161/STROKEAHA.116.013953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KY, Latour LL, Luby M, et al. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology 2009;72:1134–9. 10.1212/01.wnl.0000345360.80382.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2199–269. 10.1016/j.jacc.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 16.Petersmann A, Nauck M, Müller-Wieland D, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2018;126:406–10. 10.1055/a-0584-6223 [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazekas F, Chawluk JB, Alavi A, et al. Mr signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6. 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 19.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. 10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Seo KD, Kim JH, et al. Correlation between hyperintense vessels on FLAIR imaging and arterial circulation time on cerebral angiography. Magn Reson Med Sci 2016;15:105–10. 10.2463/mrms.2015-0006 [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 22.Nam K-W, Kwon H-M, Park S-W, et al. Distal hyperintense vessel sign is associated with neurological deterioration in acute ischaemic stroke. Eur J Neurol 2017;24:617–23. 10.1111/ene.13259 [DOI] [PubMed] [Google Scholar]
- 23.Dong X, Bai C, Nao J. Influential factors and clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in transient ischemic attacks of carotid arterial system. Neuroradiology 2017;59:1093–9. 10.1007/s00234-017-1906-z [DOI] [PubMed] [Google Scholar]
- 24.Gao S, Wang YJ, Xu AD, et al. Chinese ischemic stroke subclassification. Front Neurol 2011;2:6. 10.3389/fneur.2011.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moustafa RR, Momjian-Mayor I, Jones PS, et al. Microembolism versus hemodynamic impairment in rosary-like deep watershed infarcts: a combined positron emission tomography and transcranial Doppler study. Stroke 2011;42:3138–43. 10.1161/STROKEAHA.111.616334 [DOI] [PubMed] [Google Scholar]
- 26.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226–37. 10.1056/NEJMra0804622 [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Malavera A, Yoshimura S, et al. Clinical prognosis of FLAIR hyperintense arteries in ischaemic stroke patients: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2020;91:475–82. 10.1136/jnnp-2019-322625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000641supp001.pdf (46.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Anonymised individual participant data will be shared with qualified parties on request to the corresponding author.