Abstract
Background and purpose
Both the magnitude and cumulative exposure of atherogenic lipoproteins have an impact on the atherosclerosis risk, and the exploration focus has shifted from one single lipoprotein assessment to the cumulative exposure of lipoproteins. We aim to investigate the relationship between cumulative exposure to different lipid parameters and the risk of newly developed carotid plaque in this study.
Methods
In the Asymptomatic Polyvascular Abnormalities Community study, 2947 participants were included with follow-up every 2 years from 2006 to 2012. Lipid parameters including total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDLC), high-density lipoprotein cholesterol (HDLC) and non-HDLC were measured. Cumulative exposure was calculated by adding the weighted sum of the difference between the measured value and the cut-off value of each parameter. Newly developed carotid plaques were identified by carotid ultrasound performed at the third and fourth follow-ups.
Results
In the univariate analysis, non-HDLC burden had the highest ORs among the five lipid parameters for newly developed carotid plaque in each quartile, as 1.0 (reference), 1.35 (1.09–1.67), 1.68 (1.36–2.08) and 2.74 (2.22–3.38) from the lowest to the highest quartile. In the multivariate analysis and sensitivity analysis, we obtained similar results.
Conclusions
TC burden, TG burden, LDLC burden and non-HDLC burden are all independent risk factors for newly developed carotid plaque, especially for the vulnerable plaques. Among lipid parameters, non-HDLC burden is an optimal predictor. Moreover, the predictive value remained significant for participants under the age of 65 years old or free of hypertension, diabetes mellitus and hyperlipidaemia.
Keywords: atherosclerosis, plaque, ultrasound
Introduction
China bears the largest stroke burden worldwide with a prevalence of 1596 per 100 000 people, and 77.8% is ischaemic stroke.1 It was reported carotid plaque is a crucial cause of thromboembolism with an estimation of 40% among all atherosclerosis.2 The heavy socioeconomic burden urges the need for risk factor controls to slow down the asymptomatic atherosclerosis progression at the early stage.
Hyperlipidaemia is an important risk factor for atherosclerosis and cardiovascular guidelines have specific recommendations for targeted lipid control levels on the one-time measurement. Recently, the Journal of the American College of Cardiology introduced a concept that both the magnitude and cumulative exposure duration to atherogenic lipoproteins have an impact on the cardiovascular disease risk, shifting the focus from one single assessment to the cumulative exposure of lipoproteins.3 This hypothesis has been proved by the Mendelian randomisation studies that the genetic variants maintained an ideal level of apo-B containing lipoproteins all lifelong could effectively slow down the progression of atherosclerotic plaques,4–6 while the clinical evidence is still absent.
Therefore, in this study, we aimed to investigate the predictive association between cumulative exposure to different lipid parameters and newly developed carotid plaque, especially the vulnerable plaque, in the Asymptomatic Polyvascular Abnormalities Community (APAC) prospective study.
Subjects and methods
Study population
The APAC study is a community-based, prospective and long-term observational study which has been described in detail previously.7 As a subpopulation of Kailuan study,8 a total of 5440 participants were enrolled from June 2006 to October 2007 when met the inclusion criteria: (1) older than 40 years old; (2) without previous cardiovascular or cerebrovascular diseases, including transient ischaemic attack, stroke and coronary disease at baseline. For this study, participants were eligible if they attended four consecutive examinations in 2006–2007 (exam 1), 2008–2009 (exam 2), 2010–2011 (exam 3) and 2012–2013 (exam 4). Finally, we included 2947 participants (1272 men and 1675 women) with complete information on questionnaire assessment, clinical evaluation, laboratory test every 2 years together with two times of carotid ultrasound on exam 3, and exam 4 in the current analyses.
Lipid parameters measurement and lipid burden calculation
For each clinical examination, blood samples were drawn under fasting conditions and analysed within 4 hours using an autoanalyser (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan hospital. Total cholesterol (TC) was measured using the end-point test method. Low-density lipoprotein cholesterol (LDLC) and high-density lipoprotein cholesterol (HDLC) were measured using a direct test method and triglycerides (TGs) were measured using the glycerol phosphate oxidase (GPO) method (interassay coefficient of variation <10%; Mind bioengineering Co., Shanghai, China). Non-HDLC level is calculated routinely as total TC minus HDLC. We calculated the cumulative burden of TC, TG, LDLC or HDLC for each subject from baseline to the fourth examination ((mmol/L) * year) based on ideal cut-off levels as follows: 5.2, 1.7, 1.8, 1.0 mmol/L.9 10 The cumulative burden of TC was calculated as the weighted sum of the difference between the measured value and the cut-off value:
TC burden2006–2008=[(TC2006−5.2) + (TC2008−5.2)]/2*time2006–2008
TC burden2006–2012= TC burden2006–2008+ TC burden2008–2010+ TC burden2010–2012
TG burden and LDLC burden were calculated by the same method. For HDLC calculation, the subtraction was opposite as the decrease of HDLC is abnormal: HDLC burden2006–2008=[(1.0−HDLC2006) + (1.0−TC2008)]/2*time2006–2008. For non-HDLC calculation, due to the lack of recommended target levels from any guidelines, we use the equation: non-HDLC burden2006–2008 = (non-HDLC2006 +non-HDLC2008)/2*time2006–2008.
Demographic, behavioural and clinical characteristics assessment
Baseline demographic characteristics were self-reported on a questionnaire at baseline including age, sex, physical activity, income status, medical history (including hypertension, diabetes mellitus (DM), hyperlipidaemia), smoking status, drinking status and current medication status. Blood pressure (BP) was measured by trained nurses on admission and body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). The fasting blood glucose was measured with the hexokinase/glucose-6-phosphate dehydrogenase method and uric acid (UA) was determined with the uricase–peroxidase method.
Newly developed carotid plaque assessment
Newly developed carotid plaque was defined as the first occurrence of carotid plaque in exam 4, either the stable or vulnerable plaque. High-resolution B-mode ultrasounds (Philips iU-22 ultrasound system, Philips Medical Systems, Bothell, Washington, USA) with a 5–12 MHz linear array transducer were used to detect plaques bilaterally on three segments: the common carotid artery, the bifurcation and the internal carotid artery. An atherosclerotic plaque was defined as a focal structure encroaching into the arterial lumen of 0.5 mm or 50% of the surrounding intima–media thickness value, or thinker than 1.5 mm as measured from the intima–lumen interface to the media–adventitia interface.11 The plaque vulnerability is determined based on morphological ultrasound characteristics, including the presence of surface irregularity or ulceration, or anaechogenic plaque, or heterogeneous plaque as previously described.12 13
Statistical analysis
Continuous variables were described by medians and IQR because of skewed distributions, and categorical variables were presented as counts and percentages. The non-parametric Wilcoxon or Kruskal-Wallis test was used to compare group differences for continuous variables, and the χ2 test was used for categorical variables.
The associations of cumulative exposure to different lipid parameters and newly developed carotid plaque, especially the vulnerable plaque, were assessed by logistic regression after adjusting for different cardiovascular risk factors. The TC burden, TG burden, LDLC burden, HDLC burden and non-HDLC burden from 2006 to 2012 were all divided into four groups according to their quartiles, and the lowest quartile was defined as the reference group. Both univariate and multivariate analyses were expressed by ORs and 95% CI. Sensitivity analysis was performed to test whether the results would change after excluding participants aged over 65 years old or with a history of hypertension, DM and hyperlipidaemia.
Overall, a two-sided value of p<0.05 was considered statistically significant. All statistical analyses were performed by SAS software, V.9.1 (SAS Institute, Cary, North Carolina, USA).
Results
Baseline characteristics
Of the 2947 participants enrolled in our study, 55.04% (1622/2947) with pre-existing or non-existing carotid plaque, 44.96% of participants (1325/2947) were diagnosed as newly formed carotid plaque. Among them, 56.53% (749/1325) were stable plaque and 43.47% (576/1325) were vulnerable plaque. Those with a newly developed carotid plaque were more likely to be older and women; had slightly higher BP, BMI, UA, TC, TG, non-HDLC; had higher proportions of the current smoker, history of DM, hypertension, hypercholesterolaemia and current medication in use (p<0.001, table 1).
Table 1.
Baseline characteristics
| Pre-existing or non-existing carotid plaque | Newly developed carotid plaque | P value | ||
| Stable plaque | Vulnerable plaque | |||
| N | 1622 | 749 | 576 | |
| Age, years | 46.72 (43.28–52.94) | 54.60 (48.02–62.96) | 59.97 (51.25–70.90) | <0.001 |
| Male, % | 810 (49.94) | 285 (38.05) | 177 (30.73) | <0.001 |
| SBP, mm Hg | 120.0 (110.0–130.0) | 130.0 (119.3–140.0) | 130.0 (120.0–147.0) | <0.001 |
| DBP, mm Hg | 80.0 (70.7–85.0) | 80.0 (78.7–90.0) | 80.0 (77.7–90.0) | <0.001 |
| BMI, kg/m2 | 24.42 (22.37–26.75) | 24.95 (22.90–27.31) | 24.84 (22.77–27.12) | <0.001 |
| FBG, mg/dL | 5.09 (4.64–5.60) | 5.10 (4.61–5.78) | 5.09 (4.63–5.70) | 0.448 |
| Uric acid, μmol/L | 262.0 (214.0–319.0) | 294.0 (240.0–356.0) | 299.5 (248.0–363.5) | <0.001 |
| TC, mg/dL | 4.82 (4.20–5.41) | 5.01 (4.38–5.62) | 5.09 (4.63–5.70) | <0.001 |
| TG, mg/dL | 1.19 (0.84–1.74) | 1.30 (0.91–2.04) | 1.35 (0.95–1.94) | <0.001 |
| HDLC, mg/dL | 1.53 (1.32–1.77) | 1.49 (1.28–1.76) | 1.49 (1.30–1.73) | 0.137 |
| LDLC, mg/dL | 2.30 (1.85–2.70) | 2.24 (1.81–2.74) | 2.31 (1.83–2.80) | 0.459 |
| non-HDLC, mg/dL | 3.26 (2.70–3.87) | 3.49 (2.82–4.09) | 3.52 (2.90–4.13) | <0.001 |
| Physical activity | <0.001 | |||
| None | 162 (9.99) | 116 (15.49) | 134 (23.26) | |
| Seldom | 1301 (80.21) | 507 (67.69) | 337 (58.51) | |
| Always | 159 (9.80) | 126 (16.82) | 105 (18.23) | |
| Income status | <0.001 | |||
| <600 | 450 (27.74) | 244 (32.58) | 235 (40.80) | |
| 600–800 | 900 (55.49) | 353 (47.13) | 255 (44.27) | |
| 800–1000 | 136 (8.38) | 73 (9.75) | 39 (6.77) | |
| >1000 | 136 (8.38) | 79 (10.55) | 47 (8.16) | |
| Current smoker, n (%) | 395 (24.35) | 228 (30.44) | 170 (29.51) | <0.001 |
| Current drinker, n (%) | 574 (35.39) | 271 (36.18) | 192 (33.33) | 0.281 |
| Medical history | ||||
| Diabetes mellitus | 22 (1.36) | 36 (4.81) | 30 (5.21) | <0.001 |
| Hypertension | 115 (7.09) | 122 (16.29) | 100 (17.36) | <0.001 |
| Hypercholesterolaemia | 80 (4.93) | 85 (11.35) | 60 (10.42) | <0.001 |
| Antihypertensive medication, n (%) | 93 (5.73) | 108 (14.42) | 94 (16.32) | <0.001 |
| Antidiabetic medication, n (%) | 17 (1.05) | 30 (4.01) | 26 (4.51) | <0.001 |
| Lipid-lowering medication, n (%) | 9 (0.55) | 14 (1.87) | 10 (1.74) | <0.001 |
BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Correlation between different lipids burden and newly formed carotid plaque
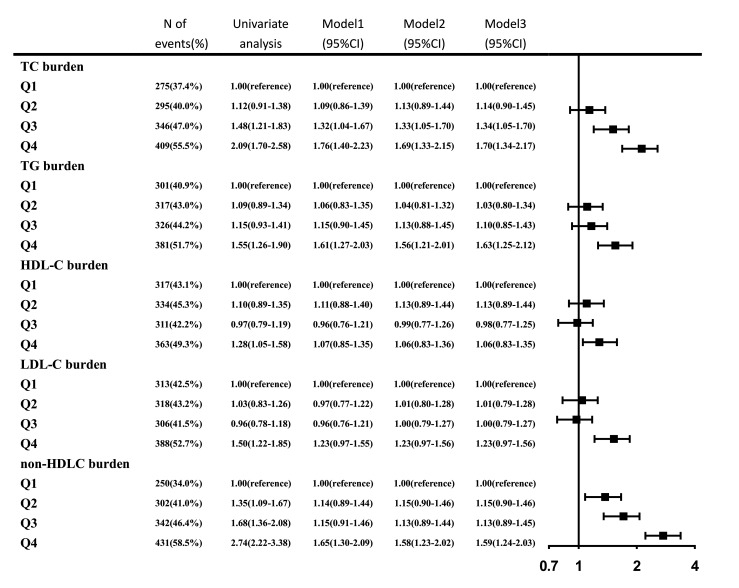
In the univariate analysis, the associations between each lipid burden and newly developed carotid plaque were all significant (p<0.05). Non-HDLC burden had the highest OR value for newly developed carotid plaque in each quartile, as 1.0 (reference), 1.35 (1.09–1.67), 1.68 (1.36–2.08) and 2.74 (2.22–3.38) from the lowest to the highest quartile (p<0.001). In the multivariate analysis, the statistical significance of the HDLC burden disappeared after adjustment for age, sex, smoking status, drinking status, BMI, hypertension, DM, hypercholesterolaemia, physical activity, income status, antihypertensive medication, antidiabetic medication and lipid-lowering medication. The rest four lipid burdens were still positively associated with newly developed carotid plaque after adjusting for potential covariates (figure 1).
Figure 1.
ORs for newly developed carotid plaque for various level of lipid burdens. 95% CI; model 1: adjusted for age, sex; model 2: add smoker, drinker, body mass index, diabetes mellitus, hypertension, hypercholesterolaemia, physical activity, income status to model 1; model 3: add antihypertensive medication, antidiabetic medication, lipid-lowering medication to model 2. HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
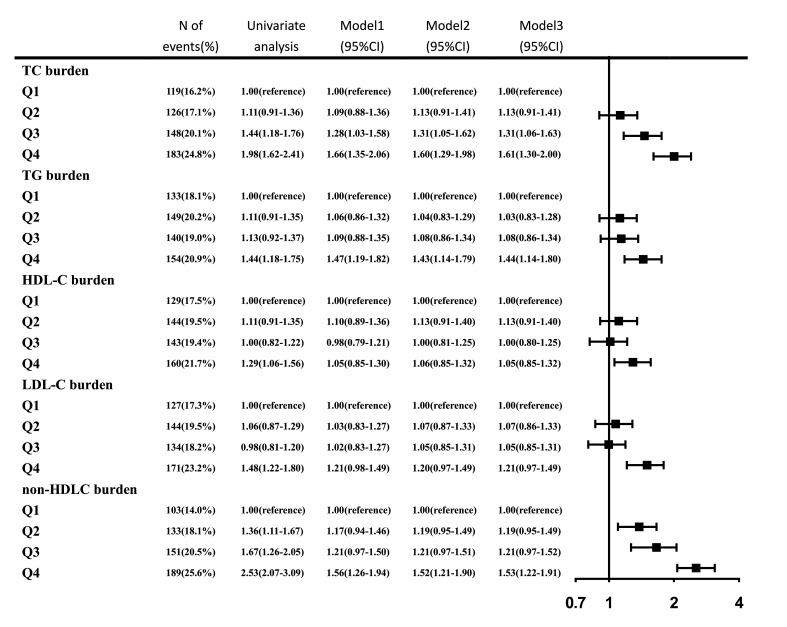
With further examination of the relationship between each lipid burden and newly developed vulnerable carotid plaque, similar results were obtained. Non-HDLC burden was superior to the other four lipid burdens, having the highest OR value as 1.0 (reference), 1.36 (1.11–1.67), 1.67 (1.26–2.05), 2.53 (2.07–3.09) in each quartile (p<0.001). After adjustment for potential covariates, the significance remained except for HDL-C burden (figure 2). Besides, sensitivity analysis coincided with these results among participants under the age of 65 years or free of hypertension, DM and hyperlipidaemia, additional information is given in table 2.
Figure 2.
ORs for newly developed vulnerable carotid plaque for various level of lipid burdens. 95% CI; model 1: adjusted for age, sex; model 2: add smoker, drinker, body mass index, diabetes mellitus, hypertension, hypercholesterolaemia, physical activity, income status to model 1; model 3: add antihypertensive medication, antidiabetic medication, lipid-lowering medication to model 2. HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Table 2.
ORs for newly developed carotid plaque for various level of lipid burdens in sensitivity analysis
| Newly developed carotid plaque | Newly developed vulnerable carotid plaque | |||
| Model 4 (95% CI) | Model 5 (95% CI) | Model 4 (95% CI) | Model 5 (95% CI) | |
| TC burden | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 1.12 (0.87 to 1.44) | 1.09 (0.84 to 1.41) | 1.12 (0.88 to 1.42) | 1.08 (0.85 to 1.37) |
| Q3 | 1.31 (1.01 to 1.68) | 1.30 (1.00 to 1.68) | 1.31 (1.03 to 1.67) | 1.28 (1.01 to 1.63) |
| Q4 | 1.67 (1.29 to 2.15) | 1.54 (1.18 to 2.00) | 1.68 (1.32 to 2.14) | 1.49 (1.17 to 1.89) |
| P for trend | <0.001 | 0.003 | <0.001 | <0.001 |
| Continuous scale | 1.03 (1.01 to 1.05) | 1.03 (1.01 to 1.04) | 1.03 (1.02 to 1.05) | 1.03 (1.01 to 1.04) |
| TG burden | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 1.04 (0.81 to 1.35) | 1.05 (0.81 to 1.37) | 1.05 (0.82 to 1.34) | 1.03 (0.82 to 1.31) |
| Q3 | 1.09 (0.84 to 1.43) | 1.15 (0.88 to 1.49) | 1.09 (0.85 to 1.40) | 1.14 (0.90 to 1.45) |
| Q4 | 1.65 (1.26 to 2.16) | 1.64 (1.24 to 2.16) | 1.56 (1.21 to 2.01) | 1.51 (1.18 to 1.95) |
| P for trend | 0.020 | 0.017 | 0.015 | 0.010 |
| Continuous scale | 1.01 (1.00 to 1.03) | 1.02 (1.00 to 1.03) | 1.01 (1.00 to 1.02) | 1.02 (1.00 to 1.03) |
| HDLC burden | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 1.16 (0.90 to 1.48) | 1.20 (0.92 to 1.56) | 1.21 (0.95 to 1.54) | 1.23 (0.97 to 1.57) |
| Q3 | 0.96 (0.74 to 1.24) | 1.01 (0.77 to 1.32) | 1.03 (0.81 to 1.31) | 1.09 (0.85 to 1.39) |
| Q4 | 1.09 (0.84 to 1.41) | 1.17 (0.89 to 1.53) | 1.12 (0.87 to 1.43) | 1.17 (0.91 to 1.50) |
| P for trend | 0.454 | 0.166 | 0.433 | 0.182 |
| Continuous scale | 1.02 (0.97 to 1.07) | 1.04 (0.99 to 1.09) | 1.02 (0.97 to 1.07) | 1.03 (0.99 to 1.08) |
| LDLC burden | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 0.98 (0.76 to 1.26) | 1.04 (0.80 to 1.35) | 1.03 (0.81 to 1.31) | 1.10 (0.87 to 1.40) |
| Q3 | 0.98 (0.77 to 1.26) | 0.94 (0.73 to 1.23) | 1.02 (0.80 to 1.29) | 1.04 (0.82 to 1.33) |
| Q4 | 1.20 (0.93 to 1.54) | 1.30 (1.00 to 1.69) | 1.19 (0.94 to 1.51) | 1.31 (1.03 to 1.67) |
| P for trend | 0.016 | 0.006 | 0.009 | 0.001 |
| Continuous scale | 1.03 (1.01 to 1.05) | 1.04 (1.01 to 1.06) | 1.03 (1.01 to 1.05) | 1.04 (1.01 to 1.06) |
| Non-HDLC burden | ||||
| Q1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 1.21 (0.94 to 1.56) | 1.12 (0.87 to 1.45) | 1.26 (0.99 to 1.60) | 1.17 (0.92 to 1.48) |
| Q3 | 1.18 (0.91 to 1.52) | 1.15 (0.88 to 1.49) | 1.21 (0.95 to 1.55) | 1.16 (0.91 to 1.47) |
| Q4 | 1.56 (1.20 to 2.03) | 1.53 (1.17 to 2.01) | 1.60 (1.24 to 2.05) | 1.53 (1.20 to 1.96) |
| P for trend | 0.005 | 0.008 | <0.001 | <0.001 |
| Continuous scale | 1.02 (1.01 to 1.04) | 1.02 (1.01 to 1.04) | 1.02 (1.01 to 1.04) | 1.03 (1.01 to 1.04) |
Model 4: model 3 excluding subjects with age ≥65 years.
Model 5: model 3 excluding subjects with history of hypertensive, diabetes mellitus, hyperlipidaemia.
HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; TC, toal cholesterol; TG, triglyceride.
Discussion
In this study, among the five lipid parameters, non-HDLC burden, calculated as the weighted sum of average non-HDLC in the four examinations from 2006 to 2012, was the superior independent predictor for newly developed carotid plaque, especially for the vulnerable plaques. The significance remained in participants under the age of 65 years or free of hypertension, DM and hyperlipidaemia.
Previous studies revealed the association between different lipid parameters and carotid plaque.14–18 The cross-sectional study based on one single time-point measurement could not indicate causality. Atherosclerotic plaques grow over time as additional lipoprotein particles retain, and the size of plaque is determined by both the magnitude and cumulative exposure to the lipids. Major guidelines endorse one single assessment of lipid parameters19 20; it could not be expected to adequately reflect the dynamic change. There were studies focused on cumulative exposure to other atherosclerotic risk factors, such as C-reactive protein and resting heart rate.21 22 Thus, we aim to evaluate the cumulative exposure to lipid over time derived from multiple measurements to predict newly developed carotid plaque, which might be a better method to quantify the relationship between different lipid parameters and asymptomatic carotid plaques.
When TG levels are higher than 2.3 mmol/L, non-HDLC surpass LDLC being the optimal representative for all atherogenic lipoproteins.9 In the series of APAC study, the predictive value of non-HDLC was not better than LDLC on extracranial internal carotid stenosis, which could be partially attributed to the small percentage of participants with hypertriglyceridaemia.23 The data are limited to validate the predictive values of different lipid parameters for early-stage asymptomatic atherosclerosis before developing into ischaemic cardiovascular disease or stroke. Our study suggests the predictive value of non-HDLC burden is superior to LDLC burden on newly developed carotid plaque. Atherosclerosis is a progressive process, from the normal arterial intima, to the fatty streak, fibrous plaque, atherosclerotic plaque and eventually to plaque disruption.24 As newly developed carotid plaque could not quantitively reflect the atherosclerosis degree, our study further investigated the relationship between cumulative exposure to different lipid parameters and newly developed vulnerable carotid plaque, which indicates a more severe degree of atherosclerosis progression. The study highlights the need of taking cumulative exposure into account rather than a single risk factor measurement. When the atherosclerotic lipoproteins accumulate to a certain extent, the plaque rapture probability increases. Individualised lipid-lowering therapy should be administrated to slow down the plaque progression speed. Aggressive treatment is recommended to patients with high lipids burden.
It is noteworthy that the recommended levels of TC, TG, LDLC and HDLC are under 5.2, 1.7, 2.6, 1.0 mmol/L in the Third Report of the National Cholesterol Education Program.9 The cut-off value of LDLC was set at 1.8 mmol/L instead of 2.6 mmol/L, as the 2019 European guideline highlighted a more aggressive control of LDLC to achieve a significant reduction in the risk of stroke.10 25 The cumulative exposure to increased TC, TG, LDLC, HDLC and total non-HDLC over 6 years could not fully reflect the lipid burden of lifelong health. Therefore, we conducted a sensitivity analysis excluding participants over 65 years old to minimise the age-related growth of carotid plaque attributed to lipids deposition.26 Hypertension, DM and hyperlipidaemia are recognised risk factors for atherosclerosis,27 sensitivity analysis was performed and the same results were obtained that the non-HDLC burden was concentration dependently associated with newly developed carotid plaque.
Our study has some limitations. As cumulative exposure is also relevant to non-lipid risk factors, the ideal expression of cardiovascular risk theoretically would be an integration of cumulative exposure to all-risk covariates, such as tobacco pack years, drinking years and environmental pollution factors. Second, both carotid plaque and vulnerable carotid plaque are semiquantitative endpoint events. The size, area or numbers of carotid plaque should also be taken into consideration when seeking for the relationship between cumulative exposure to lipid parameters and early-stage asymptomatic atherosclerosis. Although we collected the information in ultrasonography, further research is needed to provide insight into the relationship.
Conclusions
In conclusion, TC burden, TG burden, LDLC burden and non-HDLC burden are all independent risk factors for newly developed carotid plaque, especially the vulnerable plaque. Among them, non-HDLC burden is the optimal predictor. The predictive value remained in participants under the age of 65 years old or free of hypertension, DM and hyperlipidaemia.
Acknowledgments
The authors gratefully appreciate all the participants and staff for their contributions.
Footnotes
Contributors: JW and YW performed the experiments, interpreted the results of statistical analysis, and drafted the manuscript. AW and JX performed data analysis. KK and JX commented on the drafts. XZ revised the manuscript for intellectual content. All authors read and approved the final manuscript, and agreed to be accountable for all aspects of the work.
Funding: The APAC study was supported by grants from the Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China (No. 2008 BAI52B03) and the National Natural Science Foundation of China (No. 81202279).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available to researchers on request for purpose of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was conducted in accordance with the guidelines from the Helsinki Declaration and approved by the Ethics Committees of the Kailuan General Hospital and Beijing Tiantan Hospital. The Kailuan study, as the original population of APAC study, was registered at the International Clinical Trials Registry Platform with study identifying number ChiCTR-TNRC-11001489. Written informed consent was obtained from all participants.
References
- 1.Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759–71. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 2.Scarborough P, Peto V, Bhatnagar P, et al. Stroke statistics. Oxford: British Heart Foundation & Stroke Association, 2009. [Google Scholar]
- 3.Ference BA, Graham I, Tokgozoglu L, et al. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol 2018;72:1141–56. 10.1016/j.jacc.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 4.Ference BA, Majeed F, Penumetcha R, et al. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial Mendelian randomization study. J Am Coll Cardiol 2015;65:1552–61. 10.1016/j.jacc.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144–53. 10.1056/NEJMoa1604304 [DOI] [PubMed] [Google Scholar]
- 6.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA 2017;318:947–56. 10.1001/jama.2017.11467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Li Y, Xu L, et al. Asymptomatic polyvascular abnormalities in community (APAC) study in China: objectives, design and baseline characteristics. PLoS One 2013;8:e84685. 10.1371/journal.pone.0084685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang A, Wu J, Zhou Y, et al. Measures of adiposity and risk of stroke in China: a result from the Kailuan study. PLoS One 2013;8:e61665. 10.1371/journal.pone.0061665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421. 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 10.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 11.Touboul P-J, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the Advisory Board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European stroke conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012;34:290–6. 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Zhou Y, Liu C, et al. Homocysteine and carotid plaque stability: a cross-sectional study in Chinese adults. PLoS One 2014;9:e94935. 10.1371/journal.pone.0094935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloutier G, Cardinal M-HR, Ju Y, et al. Carotid plaque vulnerability assessment using ultrasound elastography and echogenicity analysis. AJR Am J Roentgenol 2018;211:847–55. 10.2214/AJR.17.19211 [DOI] [PubMed] [Google Scholar]
- 14.Gardener H, Della Morte D, Elkind MSV, et al. Lipids and carotid plaque in the Northern Manhattan study (NOMAS). BMC Cardiovasc Disord 2009;9:55. 10.1186/1471-2261-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang R, Zhang N, Wang C, et al. Relations between plasma ox-LDL and carotid plaque among Chinese Han ethnic group. Neurol Res 2011;33:460–6. 10.1179/016164111X13007856083927 [DOI] [PubMed] [Google Scholar]
- 16.Zambon A, Puato M, Faggin E, et al. Lipoprotein remnants and dense LDL are associated with features of unstable carotid plaque: a flag for non-HDL-C. Atherosclerosis 2013;230:106–9. 10.1016/j.atherosclerosis.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 17.Hou Q, Li S, Gao Y, et al. Relations of lipid parameters, other variables with carotid intima-media thickness and plaque in the general Chinese adults: an observational study. Lipids Health Dis 2018;17:107. 10.1186/s12944-018-0758-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marston NA, Giugliano RP, Im K, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019;140:1308–17. 10.1161/CIRCULATIONAHA.119.041998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of Dyslipidaemias. Eur Heart J 2016;37:2999–3058. 10.1093/eurheartj/ehw272 [DOI] [PubMed] [Google Scholar]
- 20.Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American College of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87. 10.4158/EP171764.APPGL [DOI] [PubMed] [Google Scholar]
- 21.Wang A, Liu J, Li C, et al. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.005610. [Epub ahead of print: 24 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q, Li H, Wang A, et al. Cumulative resting heart rate exposure and risk of all-cause mortality: results from the Kailuan cohort study. Sci Rep 2017;7:40212. 10.1038/srep40212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Wang Y, Wang A, et al. Association between non-high-density lipoprotein cholesterol levels and the prevalence of asymptomatic extracranial internal carotid artery stenosis in a Chinese community-based study. Eur J Neurol 2019;26:740–6. 10.1111/ene.13882 [DOI] [PubMed] [Google Scholar]
- 24.Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–66. 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- 25.Amarenco P, Goldstein LB, Szarek M, et al. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke 2007;38:3198–204. 10.1161/STROKEAHA.107.493106 [DOI] [PubMed] [Google Scholar]
- 26.Pelisek J, Wendorff H, Wendorff C, et al. Age-Associated changes in human carotid atherosclerotic plaques. Ann Med 2016;48:541–51. 10.1080/07853890.2016.1204468 [DOI] [PubMed] [Google Scholar]
- 27.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to researchers on request for purpose of reproducing the results or replicating the procedure by directly contacting the corresponding author.