Abstract
Background and purpose
Depression is common after stroke and is often treated with antidepressant medications (AD). ADs have also been hypothesised to improve stroke recovery, although recent randomised trials were neutral. We investigated the patterns of in-hospital AD initiation after ischaemic stroke and association with clinical and readmission outcomes.
Methods
All Medicare fee-for-service beneficiaries aged 65 or older hospitalised for ischaemic stroke in participating Get With The Guidelines-Stroke hospitals between April and December 2014 were eligible for this analysis. Outcome measures included days alive and not in a healthcare institution (home time), all-cause mortality and readmission within 1-year postdischarge. Propensity score (PS)-adjusted logistic regression models were used to evaluate the associations between AD use and each outcome measure. We also compared outcomes in patients prescribed selective serotonin reuptake inhibitors (SSRIs) AD versus those prescribed non-SSRI ADs.
Results
Of 21 805 AD naïve patients included in this analysis, 1835 (8.4%) were started on an AD at discharge. Patients started on an AD had higher rates of depression and prior ischaemic stroke, presented with higher admission National Institutes of Health Stroke Scale score and were less likely to be discharged home. Similarly, patients started on an SSRI had lower rates of discharge to home. Adjusting for stroke severity, patients started on an AD had worse all-cause mortality, all-cause readmission, major adverse cardiac events, readmission for depression and decreased home-time. However, AD use was also associated with an increased risk for the sepsis, a falsification endpoint, suggesting the presence of residual confounding.
Conclusions
Patients with ischaemic stroke initiated on AD therapy are at increased risk of poor clinical outcomes and readmission even after PS adjustment, suggesting that poststroke depression requiring medication is a poor prognostic sign. Further research is needed to explore the reasons why depression is associated with worse outcome, and whether AD treatment modifies this risk or not.
Keywords: stroke
Introduction
Depression is a common complication of stroke that is associated with poor functional outcomes and increased mortality.1–4 The use of antidepressant medications (ADs) to treat poststroke depression, therefore, holds potential to improve neuropsychiatric outcomes.5 6 Beyond the treatment of poststroke depression, ADs have also been hypothesised to improve stroke functional recovery, but without definitive evidence so far from clinical trials. In animal models of stroke, ADs attenuate infarct growth, promote neurogenesis and have neuroprotective effects.7–11 Early-stage clinical investigations of acute and chronic stroke patients treated with selective serotonin reuptake inhibitors (SSRIs) have also shown favourable associations with neuroplasticity and motor recovery.12–16 Larger scale clinical trials investigating SSRIs for improving poststroke functional outcomes, including three phase 3 randomised controlled trials of fluoxetine after ischaemic or haemorrhagic stroke (FOCUS, EFFECTS and AFFINITY), however, showed fluoxetine treatment was associated with increased risk of fractures and no improvement of functional outcomes.17–19 The results of these large, randomised controlled trials suggest that SSRI treatment does not significantly improve functional outcomes poststroke. If ADs improve neuropsychiatric outcomes after ischaemic stroke, however, then they may be associated with better discharge and clinical outcomes including lower risk of hospital readmission.
We have previously shown using a local Get With The Guidelines-Stroke (GWTG-Stroke) Registry that AD or SSRI use prior to hospitalisation for ischaemic stroke was associated with lower rates of discharge to home despite no difference in admission stroke severity.20 In this study, we investigated national patterns of AD prescription on discharge and the association between ADs with long-term outcomes at 1-year poststroke.
Methods
Study population
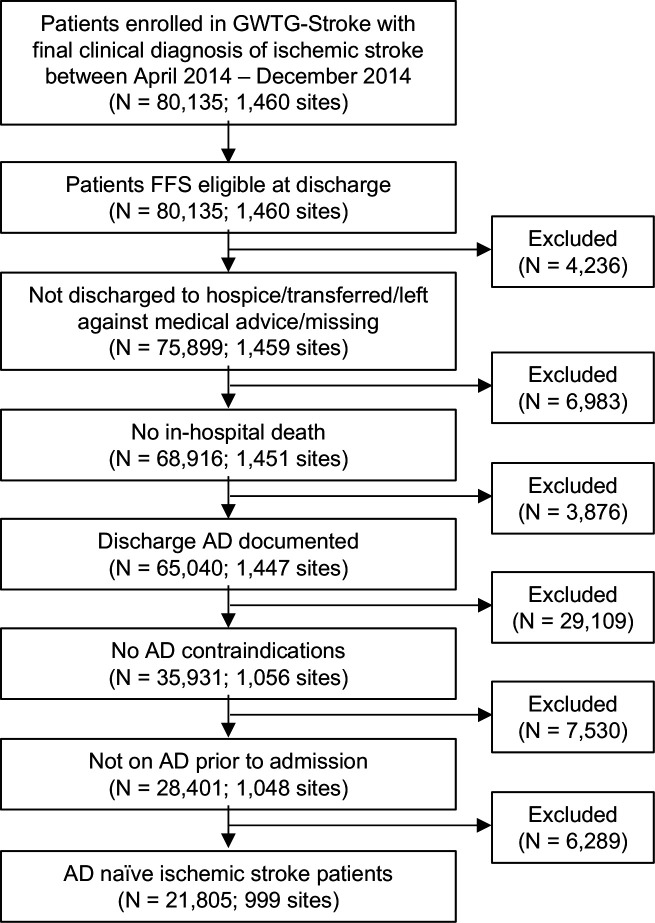
All data and materials used for this analysis are available from the GWTG-Stroke Registry. Data collection and case ascertainment from the national GWTG-Stroke Registry have been described previously.21 22 In brief, all patients aged 65 or older enrolled in a fully participating GWTG-Stroke hospital with a final clinical diagnosis of ischaemic stroke, discharged between April and December 2014, and linked to Centers for Medicare & Medicaid Services inpatient data were eligible for inclusion in this analysis. Patients were excluded from this analysis if they met any of the following criteria: (1) fee for service ineligible at discharge; (2) discharged to hospice or left against medical advice; (3) in-hospital death; (4) discharge AD not documented and (5) any contraindications to AD use (figure 1).
Figure 1.
Inclusion/exclusion criteria. AD, antidepressant; FFS, fee for service; GWTG, get with the guidelines.
Variables of interest
AD and SSRI use were classified from medication reconciliation data review of preadmission and hospital discharge medications. Patients were assigned to the AD group if they were taking any ADs regardless of the indication. SSRI use was defined if any of following medications were noted: citalopram, escitalopram, fluoxetine, paroxetine and sertraline. All other AD therapy was defined as non-SSRI ADs. AD and SSRI naïve patients were defined as those with no AD or SSRI on preadmission medication list and new AD or SSRI on hospital discharge.
Patient demographics and socioeconomic measures, including age, sex, race and insurance status, were recorded (see online supplemental materials for details). Medical history was obtained from medical records including depression, prior ischaemic stroke or transient ischaemic attack (TIA), diabetes, coronary artery disease, atrial fibrillation, heart failure, hypertension, tobacco use and peripheral vascular disease. On presentation, details of stroke severity (National Institutes of Health (NIH) Stroke Scale, National Institutes of Health Stroke Scale (NIHSS), ambulatory status, emergency medical services transport, time of arrival, time from ischaemic stroke onset to arrival and prior AD use were recorded. The variables recorded on discharge included ambulatory status, discharge disposition and discharge medications. Most variables were missing in less than 3% of patients, except for the following: NIHSS (14.8%), ambulatory status at admission (25.5%) and ambulatory status at discharge (3.4%). See online supplemental table S1 for details of how missing data were handled for each variable.
svn-2020-000691supp001.pdf (1.3MB, pdf)
Outcomes/endpoints
The primary outcome was the total number of days spent alive and outside of a hospital, skilled nursing facility or inpatient rehabilitation facility within 1 year since discharge (home time). Dates of hospital discharge and readmission were ascertained from the inpatient institutional claims. Secondary outcomes included all-cause mortality, major adverse cardiac events (MACE, a composite endpoint of all-cause mortality, cardiovascular (CV) and stroke readmissions) and readmission for: all-cause, ischaemic stroke/TIA, CV, non-CV, and depression 1-year postdischarge (see online supplemental materials for details).
Statistical analysis
The respective distributions of baseline characteristics among AD/SSRI naïve patients receiving (1) any AD versus no AD, and (2) SSRI vs non-SSRI were compared. Categorical variables were represented as proportions and continuous variables as mean±SD. Differences between groups were evaluated using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Because patients who receive AD may differ from those who do not on important baseline and clinical characteristics that may affect the likelihood of adverse events, an overlap weighting method was used to create comparable groups. First, a logistic regression model was used to assign a probability of treatment selection to each patient based on the distribution of a defined set of variables, including age, race, sex, insurance status, medical history (depression, ischaemic stroke, TIA, diabetes mellitus, coronary artery disease, atrial fibrillation, heart failure, hypertension, peripheral vascular disease, carotid stenosis, dyslipidaemia and smoking), EMS transport, on-hour arrival and ambulatory status prior to ischaemic stroke admission. The binary outcome for the selection model was whether the patient received AD treatment (yes/no). Next, each subject was weighted by the overlap weights, calculated as the probability of being assigned to the opposite treatment group, resulting in a pseudorandomisation of patients to each treatment. Following the weighting step, the primary and secondary endpoints were compared between AD and SSRI treatment groups. The unadjusted associations of AD or SSRI use with all-cause mortality, all-cause readmission, CV readmission, depression-related admission and stroke/TIA readmission were examined using a Cox proportional hazards model. Adjusted event rates were estimated using the same model with overlap weights. Differences in home time were examined using unadjusted negative binomial model with hospital-specific random intercepts, and then with overlap weights adjustment. Modelling was subsequently repeated for the following clinically relevant subgroups: age (<80 vs ≥80), sex (male vs female), race (white vs non-white), NIHSS (≤4 vs >4) and medical history of depression. To evaluate the potential for residual confounding after multivariable adjustment, we investigated the association between AD use at discharge and a falsification endpoint, readmission for sepsis.
Kaplan-Meier curves were then constructed for all-cause mortality at 1 year following the index hospitalisation for patients prescribed an AD at hospital discharge. Log-rank tests were used to examine differences in mortality between AD treatment groups. For the readmission endpoints, the incidence at 1 year based on estimates from the cumulative incidence function was calculated and the Gray test was used to test for differences between groups for these outcomes.
Results
After excluding patients without prior AD use documented or those on any AD prior to admission, 21 805 AD naïve patients from 999 hospitals were included in this analysis. Patients prescribed an AD at discharge (N=1835) were younger, more likely to be female and white, have higher rates of medical history of depression and prior ischaemic stroke, and present at later time from onset with higher admission NIHSS and reduced ability to ambulate compared with the no AD group (N=19 970, table 1).
Table 1.
Baseline characteristics by discharge AD use in AD naïve stroke patients (N=21 805)
| Variable | Overall | AD yes | AD no | P value |
| N=21 805 | N=1835 | N=19 970 | ||
| Demographics | ||||
| Age | 79 (72–86) | 78 (71–85) | 79 (72–86) | <0.001 |
| Gender | <0.001 | |||
| Female | 54.43 | 61.36 | 53.80 | |
| Male | 45.57 | 38.64 | 46.20 | |
| Race/ethnicity | 0.002 | |||
| White | 79.60 | 83.26 | 79.26 | |
| Black | 11.80 | 9.71 | 12.00 | |
| Hispanic (any race) | 3.84 | 3.22 | 3.90 | |
| Asian | 1.92 | 1.31 | 1.97 | |
| Other (includes UTD) | 2.84 | 2.51 | 2.87 | |
| Insurance status | 0.728 | |||
| Self pay/no insurance | 0.37 | 0.28 | 0.38 | |
| Medicare | 53.32 | 52.29 | 53.42 | |
| Medicaid | 6.72 | 6.95 | 6.70 | |
| Private/VA/champus/other | 39.59 | 40.49 | 39.50 | |
| Medical history | ||||
| Depression | 4.74 | 17.71 | 3.55 | <0.001 |
| Atrial fibrillation/flutter | 23.64 | 23.00 | 23.70 | 0.497 |
| Stroke | 24.25 | 26.65 | 24.03 | 0.012 |
| TIA | 10.28 | 10.63 | 10.25 | 0.612 |
| Coronary artery disease/prior MI | 29.97 | 29.81 | 29.99 | 0.874 |
| Carotid stenosis | 4.51 | 4.85 | 4.48 | 0.467 |
| Diabetes mellitus | 33.07 | 34.99 | 32.90 | 0.069 |
| Peripheral vascular disease | 5.60 | 6.16 | 5.54 | 0.273 |
| Hypertension | 81.01 | 82.40 | 80.89 | 0.114 |
| Smoker | 10.13 | 11.34 | 10.02 | 0.074 |
| Dyslipidaemia | 51.06 | 51.72 | 51.00 | 0.559 |
| Heart failure | 10.11 | 10.35 | 10.09 | 0.715 |
| Renal insufficiency | 8.81 | 8.23 | 8.86 | 0.362 |
| Medications prior to admission | ||||
| Antiplatelets | 57.39 | 59.34 | 57.21 | 0.09 |
| Anticoagulants | 23.84 | 23.76 | 23.84 | 0.951 |
| Antihypertensives | 76.11 | 76.13 | 76.10 | 0.980 |
| Cholesterol reducers | 48.84 | 50.52 | 48.69 | 0.134 |
| Diabetic medications | 26.61 | 27.55 | 26.52 | 0.341 |
| Presentation | ||||
| EMS arrival | 51.24 | 53.55 | 51.03 | 0.042 |
| On time arrival (non-holiday, M-F 7a-6p) | 50.03 | 48.03 | 50.21 | 0.074 |
| Onset to arrival times (min) | 225 (79–606) | 243.5 (98–600.5) | 224 (77–606) | 0.006 |
| Onset to arrival times (min) | 0.001 | |||
| ≥241 | 48.15 | 50.27 | 47.95 | |
| ≥120 and <241 | 17.03 | 19.60 | 16.79 | |
| <120 | 34.82 | 30.13 | 35.26 | |
| Initial NIHSS | 3 (1–8) | 5 (2–11) | 3 (1–8) | <0.001 |
| Initial NIHSS | <0.001 | |||
| ≥15 | 11.79 | 15.83 | 11.41 | |
| ≥6 and <15 | 23.95 | 30.41 | 23.35 | |
| <6 | 64.26 | 53.75 | 65.24 | |
| Recorded initial NIHSS score | 85.64 | 87.08 | 85.50 | 0.065 |
| Ambulatory status at admission | <0.001 | |||
| Able to ambulate independently | 38.94 | 28.09 | 39.90 | |
| With assistance from person | 31.80 | 32.76 | 31.72 | |
| Unable to ambulate | 29.26 | 39.16 | 28.38 | |
| Stroke symptoms resolved at time of presentation | 9.32 | 7.32 | 9.51 | 0.01 |
| Discharge | ||||
| Ambulatory status at discharge | <0.001 | |||
| Able to ambulate independently | 49.55 | 35.63 | 50.83 | |
| With assistance from person | 37.63 | 45.89 | 36.87 | |
| Unable to ambulate | 12.82 | 18.49 | 12.30 | |
| Discharge destination | <0.001 | |||
| Home | 45.54 | 30.63 | 46.91 | |
| Inpatient rehabilitation facility | 28.08 | 34.01 | 27.54 | |
| Skilled nursing facility | 24.68 | 33.46 | 23.88 | |
| Other healthcare facility | 1.70 | 1.91 | 1.68 | |
| Antihypertensives | 88.61 | 91.63 | 88.34 | <0.001 |
| Antithrombotics | 96.23 | 96.29 | 96.23 | 0.247 |
| Antiplatelets | 87.46 | 87.44 | 87.47 | 0.971 |
| Aspirin | 72.15 | 72.55 | 72.12 | 0.697 |
| Anticoagulants | 30.53 | 32.20 | 30.38 | 0.111 |
Bold denotes statistically significant p value < 0.05.
AD, antidepressant; EMS, emergency medical services; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischaemic attack; UTD, unable to determine; VA, veterans affairs.
On discharge, the group started on an AD were less likely to be ambulatory or discharged home. Of those patients started on a SSRI (N=377), the SSRI group was also younger but had no difference in admission NIHSS or ambulatory status compared with the no SSRI group (online supplemental table S2). Patients started on a SSRI were more likely to ambulate independently at discharge and also had lower rates of discharge to home.
To create comparable AD treatment groups, we employed an overlap-weight-adjusted propensity model for the probability of being started on an AD. After overlap weighting, the propensity score distributions of being on an AD in the two treatment groups showed sufficient overlap, and all model adjustment variables were balanced as indicated by the small postweighting standardised differences (online supplemental figure S1 and S2).
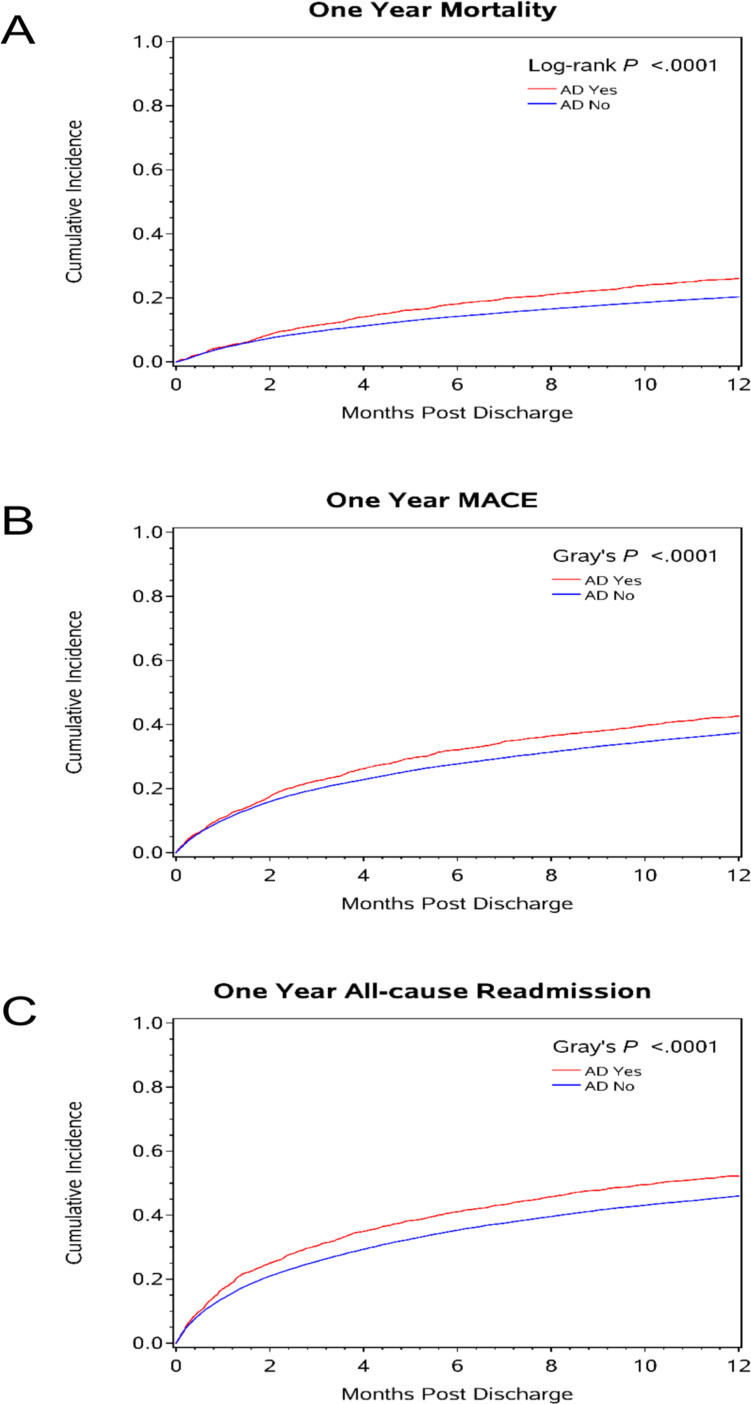
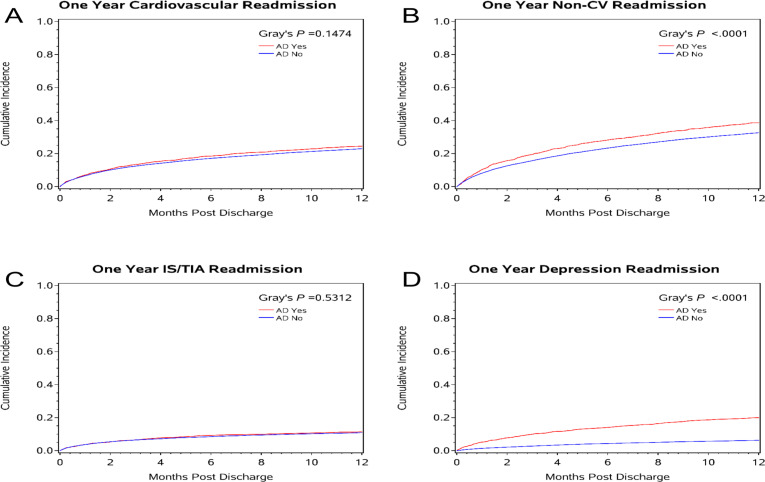
After overlap weight adjustment, AD initiation among AD naïve patients was associated with shorter home time and worse clinical outcomes at 1 year, including: all-cause mortality, all-cause readmission, MACE and readmission for depression or non-CV reasons (table 2, figure 2, online supplemental figure S3). Notably, no difference was observed in stroke/TIA or CV readmissions between the AD and no AD groups (table 2, figure 3).
Table 2.
AD use and clinical outcomes at 1-year post-IS discharge
| Outcomes at 1 year | Cumulative incidence |
Unadjusted | Weight adjusted | NIHSS weight adjusted | |||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| All-cause mortality | |||||||
| AD yes | 478 (26.05) | 1.32 (1.21 to 1.45) | <0.001 | 1.29 (1.17 to 1.41) | <0.001 | 1.21 (1.09 to 1.34) | <0.001 |
| AD no | 4050 (20.28) | Reference | Reference | Reference | |||
| All-cause readmission | |||||||
| AD yes | 940 (52.19) | 1.22 (1.14 to 1.30) | <0.001 | 1.19 (1.11 to 1.27) | <0.001 | 1.16 (1.08 to 1.24) | <0.001 |
| AD no | 9008 (46.01) | Reference | Reference | Reference | |||
| Composite of mortality and all-cause readmission | |||||||
| AD yes | 1079 (59.90) | 1.23 (1.15 to 1.31) | <0.001 | 1.19 (1.12 to 1.27) | <0.001 | 1.16 (1.08 to 1.24) | <0.001 |
| AD no | 10 274 (52.46) | Reference | Reference | Reference | |||
| CV readmission | |||||||
| AD yes | 440 (24.46) | 1.10 (1.00 to 1.22) | 0.056 | 1.11 (1.00 to 1.22) | 0.05 | 1.08 (0.96 to 1.20) | 0.19 |
| AD no | 4496 (22.99) | Reference | Reference | Reference | |||
| MACE | |||||||
| AD yes | 766 (42.61) | 1.18 (1.10 to 1.27) | <0.001 | 1.17 (1.08 to 1.26) | <0.001 | 1.11 (1.03 to 1.20) | 0.009 |
| AD no | 7313 (37.40) | Reference | Reference | Reference | |||
| IS/TIA readmission | |||||||
| AD yes | 205 (11.38) | 1.07 (0.92 to 1.24) | 0.389 | 1.07 (0.91 to 1.25) | 0.41 | 1.02 (0.86 to 1.21) | 0.803 |
| AD No | 2135 (10.89) | Reference | Reference | Reference | |||
| ICH readmission | |||||||
| AD yes | 19 (1.05) | 1.19 (0.75 to 1.89) | 0.466 | 1.07 (0.66 to 1.72) | 0.794 | 0.99 (0.59 to 1.67) | 0.975 |
| AD no | 178 (0.91) | Reference | Reference | Reference | |||
| Non-CV readmission | |||||||
| AD yes | 694 (38.61) | 1.27 (1.18 to 1.36) | <0.001 | 1.20 (1.12 to 1.30) | <0.001 | 1.18 (1.08 to 1.28) | <0.001 |
| AD no | 6370 (32.62) | Reference | Reference | Reference | |||
| Readmission with depression | |||||||
| AD yes | 352 (19.93) | 3.53 (3.13 to 3.98) | <0.001 | 2.37 (2.09 to 2.70) | <0.001 | 2.31 (2.02 to 2.65) | <0.001 |
| AD no | 1191 (6.27) | Reference | Reference | Reference | |||
| Readmission with sepsis | |||||||
| AD yes | 218 (12.13) | 1.46 (1.27 to 1.67) | <0.001 | 1.37 (1.19 to 1.58) | <0.001 | 1.36 (1.16 to 1.59) | <0.001 |
| AD no | 1703 (8.73) | Reference | Reference | Reference | |||
| Mean (SD) | Difference (95% CI) | P value | Difference (95% CI) | P value | Difference (95% CI) | P value | |
| Home-time, days | |||||||
| AD yes | 262.1 (125.8) | −24.2 (−29.6 to −18.8) | <0.001 | −27.6 (−33.0 to −22.1) | <0.001 | −25.3 (−31.1 to −19.4) | <0.001 |
| AD no | 284.3 (120.8) | Reference | Reference | Reference | |||
Bold denotes statistically significant p value < 0.05.
AD, antidepressant; CV, cardiovascular; ICH, intracranial haemorrhage; IS, ischaemic stroke; MACE, major adverse cardiac events; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischaemic attack.
Figure 2.
Cumulative incidence plots for 1-year follow-up outcomes in AD naïve patients for (A) 1-year mortality; (B) 1-year MACE and (C) 1-year all-cause readmission. AD, antidepressant; MACE, major adverse cardiac events.
Figure 3.
Cumulative incidence plots for 1-year follow-up readmission outcomes in AD naïve patients for (A) cardiovascular readmission; (B) non-cardiovascular readmission; (C) IS/TIA readmission and (D) depression readmission. AD, antidepressant; CV, cardiovascular; IS, ischaemic attack; TIA, transient ischaemic attack.
In contrast, in the SSRI subgroup, rates of readmission for stroke/TIA were reduced in the population started on an SSRI as compared with the no SSRI group, whereas no difference was observed in rates of all-cause mortality or other readmissions (online supplemental table S3). However, AD use was also associated with the falsification endpoint, readmission for sepsis, suggesting the presence of residual confounding after adjustment.
Lastly, we examined the heterogeneity of the AD treatment effect across prespecified subgroups including age, sex, race, mild stroke and patients with medical history of depression (table 3, online supplemental table S4).
Table 3.
AD use and clinical outcomes at 1-year post-IS discharge by subgroups
| Outcomes at one year | Unadjusted | Weight adjusted | NIHSS weight adjusted | |||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| All-cause mortality | ||||||
| 0.3 | 0.615 | 0.661 | ||||
| Age ≥80 years | 1.32 (1.18 to 1.48) | <0.001 | 1.28 (1.14 to 1.43) | <0.001 | 1.20 (1.06 to 1.36) | 0.005 |
| Age <80 years | 1.46 (1.25 to 1.71) | <0.001 | 1.34 (1.14 to 1.58) | <0.001 | 1.26 (1.06 to 1.50) | 0.01 |
| 0.826 | 0.674 | 0.697 | ||||
| Female | 1.32 (1.17 to 1.49) | <0.001 | 1.31 (1.15 to 1.48) | <0.001 | 1.23 (1.08 to 1.40) | 0.002 |
| Male | 1.29 (1.11 to 1.50) | <0.001 | 1.25 (1.08 to 1.46) | 0.004 | 1.18 (1.00 to 1.39) | 0.057 |
| 0.266 | 0.2934 | 0.348 | ||||
| White | 1.29 (1.16 to 1.43) | <0.001 | 1.25 (1.13 to 1.39) | <0.001 | 1.18 (1.06 to 1.32) | 0.003 |
| Non-white | 1.48 (1.19 to 1.85) | <0.001 | 1.44 (1.15 to 1.80) | 0.002 | 1.34 (1.06 to 1.71) | 0.016 |
| 0.004 | 0.007 | 0.006 | ||||
| NIHSS >4 | 1.08 (0.95 to 1.22) | 0.237 | 1.08 (0.95 to 1.22) | 0.258 | 1.08 (0.95 to 1.23) | 0.221 |
| NIHSS ≤4 | 1.47 (1.24 to 1.73) | <0.001 | 1.44 (1.21 to 1.71) | <0.001 | 1.46 (1.23 to 1.73) | <0.001 |
| 0.021 | 0.016 | 0.008 | ||||
| History of depression Yes | 0.96 (0.73 to 1.27) | 0.794 | 0.94 (0.72 to 1.24) | 0.685 | 0.84 (0.63 to 1.13) | 0.254 |
| History of depression No | 1.36 (1.23 to 1.49) | <0.001 | 1.35 (1.22 to 1.49) | <0.001 | 1.28 (1.15 to 1.43) | <0.001 |
| All-cause readmission | ||||||
| 0.482 | 0.685 | 0.763 | ||||
| Age ≥80 years | 1.20 (1.09 to 1.32) | <0.001 | 1.17 (1.06 to 1.29) | 0.002 | 1.14 (1.03 to 1.27) | 0.011 |
| Age <80 years | 1.27 (1.14 to 1.40) | <0.001 | 1.21 (1.09 to 1.34) | <0.001 | 1.17 (1.05 to 1.31) | 0.005 |
| 0.682 | 0.613 | 0.864 | ||||
| Female | 1.20 (1.10 to 1.31) | <0.001 | 1.17 (1.07 to 1.28) | <0.001 | 1.15 (1.05 to 1.26) | 0.004 |
| Male | 1.24 (1.12 to 1.37) | <0.001 | 1.21 (1.09 to 1.35) | <0.001 | 1.16 (1.04 to 1.30) | 0.007 |
| 0.439 | 0.526 | 0.396 | ||||
| White | 1.21 (1.13 to 1.30) | <0.001 | 1.17 (1.09 to 1.26) | <0.001 | 1.14 (1.06 to 1.23) | <0.001 |
| Non-white | 1.30 (1.11 to 1.52) | 0.001 | 1.24 (1.06 to 1.46) | 0.009 | 1.24 (1.04 to 1.47) | 0.016 |
| 0.104 | 0.234 | 0.226 | ||||
| NIHSS >4 | 1.11 (1.01 to 1.22) | 0.033 | 1.10 (1.00 to 1.21) | 0.058 | 1.10 (1.00 to 1.21) | 0.045 |
| NIHSS ≤4 | 1.25 (1.12 to 1.39) | <0.001 | 1.20 (1.08 to 1.34) | 0.001 | 1.21 (1.08 to 1.35) | <0.001 |
| 0.011 | 0.008 | 0.016 | ||||
| History of depression yes | 0.96 (0.80 to 1.16) | 0.692 | 0.94 (0.79 to 1.13) | 0.542 | 0.92 (0.76 to 1.12) | 0.412 |
| History of depression no | 1.24 (1.15 to 1.33) | <0.001 | 1.23 (1.14 to 1.32) | <0.001 | 1.20 (1.11 to 1.29) | <0.001 |
| MACE | ||||||
| 0.522 | 0.722 | 0.829 | ||||
| Age ≥80 years | 1.17 (1.06 to 1.29) | 0.002 | 1.16 (1.05 to 1.28) | 0.004 | 1.10 (0.99 to 1.23) | 0.074 |
| Age <80years | 1.23 (1.09 to 1.39) | <0.001 | 1.19 (1.05 to 1.34) | 0.005 | 1.12 (0.99 to 1.28) | 0.076 |
| 0.969 | 0.945 | 0.887 | ||||
| Female | 1.17 (1.06 to 1.30) | 0.001 | 1.17 (1.06 to 1.29) | 0.003 | 1.12 (1.00 to 1.24) | 0.043 |
| Male | 1.18 (1.05 to 1.32) | 0.005 | 1.16 (1.03 to 1.31) | 0.011 | 1.10 (0.97 to 1.25) | 0.139 |
| 0.557 | 0.568 | 0.684 | ||||
| White | 1.17 (1.08 to 1.27) | <0.001 | 1.15 (1.06 to 1.25) | <0.001 | 1.10 (1.01 to 1.20) | 0.026 |
| Non-white | 1.24 (1.04 to 1.48) | 0.018 | 1.22 (1.02 to 1.47) | 0.031 | 1.15 (0.95 to 1.39) | 0.146 |
| 0.008 | 0.022 | 0.021 | ||||
| NIHSS >4 | 1.00 (0.90 to 1.11) | 0.955 | 1.01 (0.91 to 1.12) | 0.871 | 1.01 (0.91 to 1.13) | 0.805 |
| NIHSS ≤4 | 1.25 (1.11 to 1.41) | <0.001 | 1.23 (1.08 to 1.39) | 0.001 | 1.24 (1.09 to 1.40) | <0.001 |
| 0.038 | 0.027 | 0.037 | ||||
| History of depression yes | 0.96 (0.78 to 1.18) | 0.689 | 0.94 (0.76 to 1.16) | 0.552 | 0.89 (0.71 to 1.11) | 0.302 |
| History of depression no | 1.21 (1.12 to 1.31) | <0.001 | 1.21 (1.12 to 1.31) | <0.001 | 1.15 (1.06 to 1.25) | 0.001 |
Italics denotes p value for the interaction of AD use and outcomes between subgroups.
Bold denotes statistically significant p value < 0.05.
AD, antidepressant; CV, cardiovascular; IS, ischaemic stroke; MACE, major adverse cardiac events; NIHSS, National Institutes of Health Stroke Scale.
Adjusting for stroke severity, patients with mild stroke started on an AD were at increased risk of all-cause mortality, readmission, MACE and non-CV readmission compared with the no AD group. Among patients without a prestroke history of depression, starting an AD was also associated with worse clinical outcomes at 1 year (online supplemental table S4).
Discussion
In this analysis from a national registry of inpatient ischaemic stroke patients, we observe that approximately 1 in 12 patients with ischaemic stroke were newly initiated on an AD and these patients are a clinically vulnerable population at higher risk for poor clinical outcomes and readmissions. Despite adjusting for stroke severity and other comorbidities, patients started on an AD during their index ischaemic stroke hospitalisation had decreased home time and increased all-cause mortality and non-stroke readmissions, but no difference in stroke or TIA readmission up to 1-year postdischarge. Additionally, the association between AD use and a falsification endpoint, readmission for sepsis, persisted even after adjustment, suggesting the presence of residual confounding.
Our findings suggest that patients with ischaemic stroke initiated on an AD during their index hospitalisation are at increased risk for poor clinical outcomes, in particular increased mortality and non-CV readmission but not CV or ischaemic stroke/TIA readmission. In part, this observation may be due to the increased admission NIHSS score and higher rates of premorbid depression in the AD group. The subsequent subgroup analysis, however, provides additional insight into these findings as we observed that patients with mild ischaemic stroke severity and those without a prestroke diagnosis of depression started on an AD were also at increased risk of all-cause mortality and readmission. These observations in patients with minor stroke or no history of depression, suggest the possibility of unmeasured confounders that potentially influence the clinical decision making for initiation of an AD. We pursued propensity model analysis to account for preselected clinically relevant factors for AD treatment, including NIHSS. Unfortunately, the model still suggested that both the non-treated and AD treated groups had low overall chances of being prescribed an AD. The positive association with a falsification endpoint also suggests the presence of unmeasured confounders for AD initiation in this patient cohort. Overall these findings underscore the importance of recognising that patients started on an AD during their ischaemic stroke hospitalisation are high risk for early mortality and non-CV readmission and, as a result, increased attention should be paid to transitions of care and preventative medicine in this patient population.
The underlying explanation for why patients initiated on an AD during their index ischaemic stroke hospitalisation would have worse clinical outcomes and readmissions is less clear, and should be the subject of future research. The large, randomised controlled trials, FOCUS, EFFECTS and AFFINITY, demonstrated that fluoxetine after ischaemic or haemorrhagic stroke did not significantly improve functional outcomes but was associated with excess bone fracture risk (1%), which could possibly contribute to the increased risk of non-CV readmissions.18 19 A propensity score match analysis of a Danish Stroke Registry observed reduced 30-day mortality in patients started on an AD during the acute hospitalisation, however, this was a shorter time period of follow-up without additional data on readmission rates.23 The observation that patients without a diagnosis of depression or with mild stroke that were started on an AD had worse clinical outcomes suggests unmeasured factors that may predispose to poor outcomes. Further emphasising that patients with ischaemic stroke started on an AD during their hospitalisation are high risk, we observed this group to have higher rates of readmission for sepsis. One possible explanation for the observed worse clinical outcomes in this cohort may be clinical suspicion for poststroke depression as the indication for AD initiation. Poststroke depression occurs in up to one in three of patients with stroke and is associated with poor functional outcomes and increased mortality.2 3 24–26 Compounding this issue, poststroke depression has also been shown to influence participation in rehabilitation activities27 28 and increase inpatient utilisation times.29 Along these lines, patients started on an AD in our analysis had higher likelihood of a readmission for depression.
There are several strengths of our study. First, this was a large-scale analysis of national AD/SSRI initiation restricted to patients with ischaemic stroke with detailed clinical outcomes and readmission endpoints. Second, as compared with clinical trial databases, analysis of the GWTG-Stroke Registry provides insight into the real-world practice patterns for AD/SSRI treatment of patients with ischaemic stroke. Third, unlike other studies which were restricted to individual AD/SSRI agents or antidepressant classes in combined haemorrhagic and ischaemic populations, our analysis explored all ADs and SSRIs exclusively in patients with ischaemic stroke who have a more homogeneous distribution of outcomes as opposed to a cohort of both ischaemic and haemorrhagic stroke subtypes.
There are several important considerations regarding the results of this study. First, this was a retrospective analysis of a national registry during a finite time period, which may limit the overall generalisability. However, the large patient population and diverse clinical settings mitigates these concerns somewhat as they highlight the broad patient population included in this analysis. Second, information on the indication, dose, duration or adherence to AD/SSRI treatment is lacking, which holds potential to provide further insight into the underlying nature of our observations. Future studies further exploring the indication for AD initiation and the relationship of AD dose and duration in relation to poststroke outcomes are necessary. Lastly, direct information on rates of poststroke depression is not available in this analysis. As a surrogate, however, the information on readmission for depression indirectly addresses this limitation.
Conclusions
Among patients hospitalised with acute ischaemic stroke, 8.4% were newly started on an AD during their stroke hospitalisation and these patients were at risk for higher mortality and readmission for non-stroke related causes. These findings suggest that the decision to start an ischaemic stroke patient on an AD identifies patients at risk for poor clinical outcomes, even after controlling for differences in stroke severity.
Footnotes
Twitter: @Shree_Stroke
Contributors: ME analysed and interpreted the data and drafted the manuscript. SS, LM, DH, BLi, BLy, LT, EES, GCF, LHS, DLB and AH provided critical revisions to the manuscript for intelllectual content. XH, YX and ECO analysed the data and provided critical revisions to the manuscript for intellectual content. All authors reviewed and gave final approval to the submitted manuscript.
Funding: ME, SS, XH, YX, LM, DH, BLi, BLy, LT and EES report no relevant funding. GCF and LHS report funding from PCORI and being a member of the GWTG-Stroke Systems of Care Advisory Group. The GWTG-Stroke program is provided by the American Heart Association/American Stroke Association. GWTG-Stroke is sponsored, in part, by Novartis, Boehringer Ingelheim Lilly, Novo Nordisk, Sanofi, AstraZeneca and Bayer. PROSPER study was supported by an award (CE-1304-7073) from the Patient-Centered Outcomes Research Institute (PCORI).
Competing interests: DLB discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Coinvestigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. All data and materials used for this analysis are available from the GWTG-Stroke Registry.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke depression: a scientific statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2017;48:e30–43. 10.1161/STR.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 2.Bartoli F, Lillia N, Lax A, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat 2013;2013:1–11. 10.1155/2013/862978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke 2014;9:1026–36. 10.1111/ijs.12356 [DOI] [PubMed] [Google Scholar]
- 4.El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16. 10.1161/STROKEAHA.111.643130 [DOI] [PubMed] [Google Scholar]
- 5.Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother 2008;9:1291–8. 10.1517/14656566.9.8.1291 [DOI] [PubMed] [Google Scholar]
- 6.Hackett ML, Anderson CS, House AO. Management of depression after stroke: a systematic review of pharmacological therapies. Stroke 2005;36:1098–103. 10.1161/01.STR.0000162391.27991.9d [DOI] [PubMed] [Google Scholar]
- 7.McCann SK, Irvine C, Mead GE, et al. Efficacy of antidepressants in animal models of ischemic stroke: a systematic review and meta-analysis. Stroke 2014;45:3055–63. 10.1161/STROKEAHA.114.006304 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 2007;18:391–418. 10.1097/FBP.0b013e3282ee2aa8 [DOI] [PubMed] [Google Scholar]
- 9.Lim C-M, Kim S-W, Park J-Y, et al. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res 2009;87:1037–45. 10.1002/jnr.21899 [DOI] [PubMed] [Google Scholar]
- 10.Shin TK, Kang MS, Lee HY, et al. Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol 2009;13:257–63. 10.4196/kjpp.2009.13.3.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schäbitz WR, Schwab S, Spranger M, et al. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1997;17:500–6. 10.1097/00004647-199705000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Chollet F, Tardy J, Albucher J-F, et al. Fluoxetine for motor recovery after acute ischaemic stroke (flame): a randomised placebo-controlled trial. Lancet Neurol 2011;10:123–30. 10.1016/S1474-4422(10)70314-8 [DOI] [PubMed] [Google Scholar]
- 13.Siepmann T, Kepplinger J, Zerna C, et al. The effects of pretreatment versus de novo treatment with selective serotonin reuptake inhibitors on short-term outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis 2015;24:1886–92. 10.1016/j.jstrokecerebrovasdis.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 14.Zittel S, Weiller C, Liepert J. Citalopram improves dexterity in chronic stroke patients. Neurorehabil Neural Repair 2008;22:311–4. 10.1177/1545968307312173 [DOI] [PubMed] [Google Scholar]
- 15.Acler M, Robol E, Fiaschi A, et al. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol 2009;256:1152–8. 10.1007/s00415-009-5093-7 [DOI] [PubMed] [Google Scholar]
- 16.Siepmann T, Penzlin AI, Kepplinger J, et al. Selective serotonin reuptake inhibitors to improve outcome in acute ischemic stroke: possible mechanisms and clinical evidence. Brain Behav 2015;5:e00373. 10.1002/brb3.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legg LA, Tilney R, Hsieh C-F, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev 2019;2019. doi: 10.1002/14651858.CD009286.pub3. [Epub ahead of print: 26 Nov 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FOCUS Trial Collaboration . Effects of fluoxetine on functional outcomes after acute stroke (focus): a pragmatic, double-blind, randomised, controlled trial. Lancet 2019;393:265–74. 10.1016/S0140-6736(18)32823-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham C, Lewis S, Forbes J, et al. The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: statistical and health economic analysis plan for the trials and for the individual patient data meta-analysis. Trials 2017;18:627. 10.1186/s13063-017-2385-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etherton MR, Siddiqui KA, Schwamm LH. Prestroke selective serotonin reuptake inhibitor use and functional outcomes after ischaemic stroke. Stroke Vasc Neurol 2018;3:9–16. 10.1136/svn-2017-000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonarow GC, Reeves MJ, Smith EE, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes 2010;3:291–302. 10.1161/CIRCOUTCOMES.109.921858 [DOI] [PubMed] [Google Scholar]
- 22.Schwamm LH, Fonarow GC, Reeves MJ, et al. Get with the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009;119:107–15. 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 23.Mortensen JK, Johnsen SP, Larsson H, et al. And all-cause 30-day mortality in patients with ischemic stroke. Cerebrovasc Dis 2015;40:81–90. [DOI] [PubMed] [Google Scholar]
- 24.Whyte EM, Mulsant BH, Rovner BW, et al. Preventing depression after stroke. Int Rev Psychiatry 2006;18:471–81. 10.1080/09540260600935470 [DOI] [PubMed] [Google Scholar]
- 25.Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002;52:253–64. 10.1016/S0006-3223(02)01424-5 [DOI] [PubMed] [Google Scholar]
- 26.El Husseini N, Goldstein LB, Peterson ED, et al. Depression status is associated with functional decline over 1-year following acute stroke. J Stroke Cerebrovasc Dis 2017;26:1393–9. 10.1016/j.jstrokecerebrovasdis.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 27.Skidmore ER, Whyte EM, Holm MB, et al. Cognitive and affective predictors of rehabilitation participation after stroke. Arch Phys Med Rehabil 2010;91:203–7. 10.1016/j.apmr.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillen R, Tennen H, McKee TE, et al. Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch Phys Med Rehabil 2001;82:1645–9. 10.1053/apmr.2001.26249 [DOI] [PubMed] [Google Scholar]
- 29.Ghose SS, Williams LS, Swindle RW. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med Care 2005;43:1259–64. 10.1097/01.mlr.0000185711.50480.13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000691supp001.pdf (1.3MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. All data and materials used for this analysis are available from the GWTG-Stroke Registry.