Abstract
Introduction:
Primary care physicians (PCPs) are considered the gatekeepers of genetic services, but they often underutilize or inappropriately utilize such services, leading to lack of early treatment, incorrect diagnoses, and unnecessary procedures. This study aims to delineate PCP referral patterns, including the frequency of, motivators for, and barriers to genetic referrals and testing in the present landscape of genomics.
Methods:
A 34-item online survey was distributed to PCPs in the United States (US). PCP demographics, practice characteristics, and referral patterns, motivators, and barriers were analyzed. Six hypothetical clinical scenarios included in the survey also were presented to a cohort of clinical geneticists. We calculated PCPs’ rates of ordering genetic tests and of referral to genetics services in the past year. Rates and responses to clinical scenarios were compared based on respondents’ personal and practice characteristics.
Results:
A total of 95 PCPs and 25 clinical geneticists participated. Among the PCPs, 79% reported referring and 50% reported ordering genetic testing in the last year. PCPs with genetic counselors (GCs) in their clinic referred at significantly higher rates than those without (P = .008). White PCPs referred at significantly higher rates compared to Black or African American PCPs (P = .009). The most commonly reported motivators for referring patients to genetic services were preference for specialist coordination, lack of knowledge, and family’s desire for risk information. The most commonly reported barriers were patient refusal, provider concerns about costs to patients, and uncertainty of when a genetic referral is appropriate. In response to clinical scenarios, clinical geneticists were in agreement about the need for genetic testing or referral for 2 of the scenarios. For these 2 scenarios, only 48% and 71% of PCPs indicated that they would offer genetic testing or referral, respectively.
Conclusions:
Responses to clinical scenarios suggest that it is not clear to PCPs when referrals or testing are needed. Collaboration with GCs is one approach to reducing barriers to and improving PCPs’ utilization of genetic services. Clear guidelines from clinical geneticists may help facilitate appropriate use of genetics services by PCPs. Additional research is needed to further describe barriers that PCPs face in genetic testing/referrals.
Keywords: primary care, genetics, referrals, clinical scenarios, genetic testing, genetic counseling
Introduction
Primary care providers (PCPs) are considered the gatekeepers of genetic services,1 and their roles include initiating appropriate genomic testing and other diagnostic evaluations, and recognizing indications for subspecialty referral.2 Patient benefits from genetic services may include genetics evaluation, risk assessment, genetic counseling, genetic testing, interpretation of genetic testing results, medical management, and psychosocial support. These services help guide precision medicine, provide appropriate treatment, shorten the diagnostic odyssey, and initiate screenings based on a known familial risk. However, many studies indicate that PCPs underutilize such services.1,3-19 In a study presenting clinical scenarios, 71% of physicians’ hypothetical actions would have adhered to genetic counseling and testing recommendations on BRCA1/2 for average risk-women, but only 41% would have adhered to guidelines for high-risk women.4 Another study presenting hypothetical scenarios for global developmental delay (GDD) found that only 21% of pediatricians would order genetic testing for children with isolated GDD,19 whereas the American College of Medical Genetics and Genomics recommends microarray analysis as a first-tier test for unexplained GDD.20 In addition to underutilization of genetic services, there also is evidence of errors in risk calculation.3-7 In 2011, when presented with several hypothetical clinical scenarios, 45% of physicians ordered BRCA1/2 testing for low-risk patients,3 incorrectly assessing them as high-risk for BRCA1/2 mutations.
Underutilization and inappropriate utilization of genetic services can have detrimental repercussions for patients. For example, a case series of adverse events including incorrect genetic test ordering, misinterpretation of genetic testing results, provision of incorrect information to patients, and unnecessary surgeries were reported in at least 30 patients who were not initially provided or referred to genetic counseling prior to these events.5-7 Utilizing genetic services also may reduce psychological distress experienced by patients receiving genetic information. A systematic review of 103 studies on BRCA1/2 testing for the United States (US) Preventive Services Task Force identified 28 studies demonstrating that genetic counseling is associated with reduced breast cancer worry, anxiety, and depression as well as increased understanding of risk.21 These studies support that underutilization of genetic services can adversely affect patient care. An additional study evaluating the test review process at Associate Regional University Pathologists showed that 26% of genetic tests for germline mutations were changed (cancelled, changed to appropriate testing, or added additional testing) after genetic counselor (GC) review, saving referring institutions an average of $48 000 per month.22 Thus, underutilization and inappropriate utilization of genetic services are costly both to patients and institutions.
As genetics gains an increasing relevance across all areas of medicine, PCPs’ ability to understand and utilize genetic services becomes increasingly crucial. A 2019 US genetics workforce study demonstrated that the average new patient caseload for genetic services had increased, but the number of genetics providers had not.23 Overall, it was estimated that there were 2 geneticists for every 1 million individuals in the US. Therefore, PCPs need to assume more responsibility in facilitating genetics care for their patients. However, studies have shown that PCPs lack genetic knowledge and have low comfort levels regarding genetic testing and counseling.10-12 Lack of knowledge is the most commonly self-reported barrier to utilization of genetic services among PCPs.10 Among physicians in a community-based health system in 2013, 61% of PCPs reported “no to minimal knowledge” concerning “when and how to incorporate genomics into practice.”10 Areas of knowledge that represent barriers include basic genetic concepts, awareness of available genetic services and testing, and how to refer to genetic services.10,13 Notably, physicians who felt more prepared to interpret and counsel on genetic testing results were more likely to order genetic testing or refer to genetic services.14
Additional barriers to genetics referrals can be predicted by PCPs’ demographics (such as gender and specialty)4,9 and the availability of educational resources. Physicians who have more recently graduated and those who practice in academic settings have higher genetics knowledge15 and are more likely to refer their patients to genetic services.9,14,16 However, another study found more recent graduates were less comfortable discussing genetic testing with their patients, suggesting that increased levels of genetics knowledge may include acknowledging the complexities of genetics and one’s lack of understanding.10 Given that comfort level can predict genetics services utilization,14 these studies highlight a complex interaction between knowledge, comfort, and utilization.
Patient demographics also can predict genetic services utilization. Interestingly, the greatest predictor of utilization of genetic services is patient interest and inquiry.9,11,16 Therefore, patient education and awareness also seem to be important factors.16 Furthermore, Shields et al14 found that minority-serving physicians were less likely to refer or order genetic testing for their patients, highlighting disparities in genetic services utilization among minority patients.
While many physicians have expressed interest in receiving education and support to increase their genetics knowledge,10,13 studies have shown that educational programs increase physicians’ knowledge about genetics, but do not increase genetics services utilization.24,25 This may be explained by other barriers including cost, lack of time, distance to genetics services, concerns about insurance discrimination, perceived benefit to the patient in terms of meaningful results, or an inability to stay current with genetics.9,13,17 On the other hand, increased knowledge may improve physicians’ referral selection,24 which could explain lack of observed increase in utilization. Overall, findings suggest that physicians’ level of genetics knowledge by itself does not predict utilization of genetics services and underscores the need to better understand factors that influence referral patterns.
These studies describing genetic risk assessment, testing, and referrals were conducted over a decade ago, before newer genetic technologies were integrated into clinical care and before many medical schools comprehensively incorporated genetics in their curriculum. This study aims to generate quantitative data regarding the frequency at which PCPs refer to genetic services and/or order genetic testing in the current landscape of genomics. In addition, we assessed PCPs’ and clinical geneticists’ responses to hypothetical clinical scenarios in order to characterize the appropriateness of PCPs’ referring practices. These data were analyzed to characterize referral patterns and identify motivators, barriers, and potential demographic-specific differences. Elucidation of current referral patterns and barriers to genetic services for PCPs can guide efforts to improve genetic service utilization.
Materials and Methods
This study was approved by the Emory University Institutional Review Board (IRB). Written informed consent was provided, in accordance with the requirement of the IRB. We developed a 34-item online survey based on previous literature3,5-7,10,16 to assess PCP demographics and practice characteristics (18 items), current genetics services referral patterns (4 items), factors that affect decisions to refer (1 item), barriers to referrals (1 item), responses to hypothetical clinical scenarios (6 items; Table 1), and feedback/comments (1 item). For each scenario, response choices were (1) would not see this type of patient due to indication or age range, (2) would not recommend genetics evaluation, as it is not appropriate, (3) would recommend genetics evaluation, genetic counseling, and/or genetic testing, or (4) not sure.
Table 1.
Clinical Scenarios.
| Scenario | Scenario details |
|---|---|
| 1. Developmental delay | A 10-month-old male presents for a well visit. His mother is concerned he is behind on his milestones. He is rolling over, sitting up by support but not independently, not babbling, and not passing objects from hand to hand. He was seen by physical therapy for the past 2 months with no progress. He was born full-term with a normal pregnancy history and is non-dysmorphic. |
| 2. Dyslexia | A 7-year-old male has dyslexia and had to repeat first grade. His father also has dyslexia. The patient has a history of lactose intolerance and constipation. His weight is in the 75th percentile and his height is in the 98th percentile. |
| 3. Elevated homocysteine | A 15-year-old female presents with a history of elevated homocysteine levels (16-18 µmol/L). She has a family history of cardiovascular disease, diabetes, high cholesterol, and high blood pressure. |
| 4. Possible familial hypercholesterolemia | A 41-year-old male has a history of high LDL-C levels. His recent blood work shows total cholesterol of 340 mg/dL, LDL-C of 200 mg/dL, and normal triglycerides. He has a normal BMI, reportedly exercises regularly, and eats a healthy diet. |
| 5. Breast cancer | A 58-year-old female presents with a personal history of breast cancer (unilateral, single episode), which was diagnosed at 56. There is no family history of cancer. |
| 6. Family history of colon and uterine cancer | A 59-year-old male presents at his annual exam. His father had colon cancer at 53, his paternal uncle had colon cancer at 40, and his paternal grandmother had endometrial cancer at 57. |
The study population included licensed PCPs in the US who see at least 10 patients per week. Enrollment occurred from June 2019 to January 2020. Participants were recruited via an email distributed to a stratified random sample of 4591 pediatricians, general practice, family medicine, internal medicine, and adolescent medicine physicians in the US from an American Medical Association listserv and via personal and online networking by contacting healthcare systems primarily in the southeast to directly distribute the survey to their physicians. We also presented the clinical scenarios to clinical geneticists currently practicing in the US, recruited at the 2019 Southeast Regional Genetics Group Annual Meeting, and via Twitter and personal networking.
Descriptive statistics of demographics and referral practices responses were calculated. Due to non-normal distribution of responses, medians are reported and non-parametric tests of association were used to assess the impact of demographics on rates of referrals.
It was not possible to compare median rates of genetic testing ordered due to the low overall median rate of 0 among respondents. Therefore, statistical tests were performed to compare demographics between 2 groups of respondents, those who had and had not ordered genetic testing in the past 12 months.
Results from the clinical scenarios for which clinical geneticists were in agreement (80% or more with the same response) were analyzed. We compared provider and practice characteristics of PCPs who would and who would not recommend genetic testing or referral.
Statistical significance was defined as P <.01 to correct for multiple comparisons and trending associations were defined as P < .05.
Results
We received completed surveys from 95 PCPs, whose specialty included pediatrics (55%), family practice/general practice (36%), adolescent medicine (2%), and other (7%). In addition, 25 clinical geneticists completed the clinical scenarios. Participant demographics are described in Table 2. Practice characteristics of the PCP respondents are presented in Table 3. Physician race had an association approaching significance (P = .01) with percentage of non-Hispanic White patients, where on average, White respondents had a larger percentage of non-Hispanic White patients (mean difference = 20.9%) compared to Black respondents (P = .011).
Table 2.
Demographics of Participating Primary Care Providers (PCPs) and Clinical Geneticists.
| PCPs (n = 95) | Clinical geneticists (n = 25) | |
|---|---|---|
| Gender, n (%) female | 61 (64) | 13 (52) |
| Ethnicity, n (%), non-Hispanic | 90 (95) | 22 (88) |
| Race, n (%) | ||
| White | 61 (64) | 23 (92) |
| Asian | 17 (18) | 1 (4) |
| Black or African American | 15 (16) | 0 (0) |
| Other | 2 (2) | 1 (4) |
| Graduation year, median (range) | 1996 (1967-2015) | 1998 (1979-2016) |
| >2007, No. (%) | 73 (77) | 6 (24) |
| ≤2007, No. (%) | 22 (23) | 19 (76) |
| Age, median (range), years | 50 (30-75) | 47 (26-70) |
Table 3.
Practice Demographics of Participating Primary Care Providers (PCPs).
| n (%) | |
|---|---|
| Practice setting (n = 94) | |
| Physician’s private practice | 33 (35) |
| Health maintenance organization | 24 (26) |
| Private hospital/medical facility | 14 (15) |
| University medical center | 9 (10) |
| Other | 8 (9) |
| Public hospital/medical facility | 6 (6) |
| Practice location (n = 94) | |
| Suburban | 56 (60) |
| Urban | 26 (28) |
| Rural | 11 (12) |
| Other | 1 (1) |
| Distance to genetics facility (miles) (n = 94) | |
| 0-10 | 34 (36) |
| 11-30 | 36 (38) |
| 31-50 | 8 (9) |
| >50 | 13 (14) |
| Not sure | 3 (3) |
| Genetic counselor in clinic (n = 94) | |
| Yes | 10 (11) |
| No | 79 (84) |
| Not sure | 5 (5) |
| Patient population characteristics | Median (range) |
| % Non-Hispanic White (n = 92) | 50 (1-99) |
| % Speak non-English first language (n = 92) | 10 (0-99) |
| Average number seen/month (n = 91) | 300 (35-600) |
Referral Practices
Most respondents (89%) reported that they had ever referred a patient to genetic services, 10% had never referred a patient, and 1% were not sure. Respondents (n = 88) reported referring a median of 3 patients (range of reported patients: 0-20) in the past 12 months, where 21% had not referred any patients during that time. The most common drivers of referrals (respondents could select multiple responses) were diagnostic genetic testing (endorsed by 57% of respondents), geneticist evaluation (55%), and predictive genetic testing (39%).
There were significant differences (P = .009) in median referral rates (MRR) between White (n = 53) and Black or African American respondents (n = 14), whose MRRs were 0.1042% (Q1 = 0.0556%, Q3 = 0.2083%) and 0.0658% (0%, 0.0677%), respectively. There were no significant differences in MRRs between these respondents and Asian respondents (n = 15), whose MRR was 0.0625% (0%, 0.1667%). There were also significant differences in MRRs (P = .008) between respondents who had (n = 9) or did not have (n = 76) a GC in their practice: 0.2083% (0.1019%, 0.4292%) and 0.0694% (0.02437%, 0.1806%), respectively. There were no associations between respondent race and GC in practice (P = 1). There were no significant differences in MRRs between different specialties, graduation year, age, gender, region, practice setting, practice location, distance to a genetic facility, percentage of non-Hispanic White patients, or percentage of non-English as a first language patients.
The top 3 motivators for respondents to refer patients to genetic services were preference for specialist coordination (55% of respondents), lack of specific knowledge base (33%), and the family’s desire for recurrence information or relatives’ risk (30%; Table 4). There were no significant associations between provider demographic groups and these 3 motivators.
Table 4.
Most Common Motivators and Barriers for Referring to Genetic Services.
| Most common reasons to refer (n = 86) | n (%) | Most common reasons not to refer (n = 87) | n (%) |
|---|---|---|---|
| Prefer specialist coordination | 47 (55) | Patient refusal | 40 (46) |
| Lack the specific knowledge base | 29 (33) | Concerns about financial cost of test | 32 (37) |
| Family’s desire for recurrence information/relatives’ risk | 26 (30) | Uncertain when genetic referral is appropriate | 21 (24) |
| Severity of the disorder | 23 (27) | Concerns about privacy/moral/ethical implications | 11 (13) |
| Accuracy of genetic test | 20 (23) | Distance to service too great | 10 (11) |
| Availability of treatment | 18 (22) | Get information from genetics provider | 9 (10) |
| Patient’s interest in the referral | 19 (22) | Order appropriate genetic testing | 9 (10) |
| Lack of time to explain risks/benefits | 1 (1) | Perform appropriate genetic counseling | 3 (3) |
| Concerns about accuracy of available testing | 3 (3) | ||
| Poor access/long wait time | 2 (2) |
The top 3 barriers to referring patients to genetic services were patient refusal (46%), concerns about financial cost of test (37%), and uncertainty of when a genetic referral is appropriate (24%) (Table 4). There were no significant associations between provider demographic groups and these 3 barriers.
Testing Practices
Respondents (n = 84) reported ordering genetic testing for a median of 0 patients (range: 0-15) in the past 12 months. Half of respondents (n = 47) reported they had ordered genetic testing in the past 12 months while half (n = 47) reported they had not. The most common purposes for these genetic tests were diagnostic genetic testing (62%), predictive genetic testing (34%), and pharmacogenetic testing (17%). There were no significant differences between respondents who ordered or who did not order genetic testing in the past 12 months across demographic groups.
Responses to Clinical Scenarios
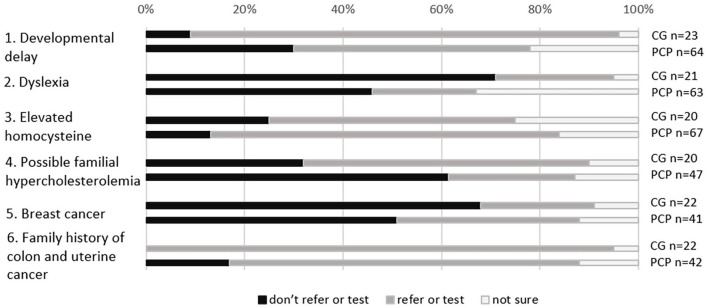
There were no scenarios for which PCPs were in agreement about whether or not to pursue genetic testing or referrals (Figure 1).
Figure 1.
Responses to hypothetical clinical scenarios by primary care providers (PCPs) and clinical geneticists (CGs).
Twenty-five clinical geneticists responded to the hypothetical clinical scenarios (Figure 1). Overall, 87% and 95% of clinical geneticists, respectively, would recommend genetic testing or referral for scenarios 1 (unexplained developmental delay) and 6 (family history of colon and uterine cancer).
For clinical scenario 1, 48% of responding PCPs indicated that they would recommend genetics evaluation, genetic counseling, and/or genetic testing (Figure 1). PCPs who would or would not test/refer did not differ for any personal or practice characteristics.
For clinical scenario 6, 71% of responding PCPs indicated that they would recommend genetics evaluation, genetic counseling, and/or genetic testing (Figure 1). PCPs who worked in Health Maintenance Organizations (n = 10) were less likely to report that they would test or refer than were PCPs who worked in other settings (n = 27) (50% vs 93%, p = 0.0091).
For scenario 3 (elevated homocysteine) and scenario 5 (breast cancer), PCPs were more likely than clinical geneticists to respond that genetic testing or genetic evaluation was warranted.
Preferred Educational Methods
We asked PCPs to select their preferred methods for obtaining information about genetics and genomics in clinical care (Table 5). The top 2 preferred methods were online continuing medical education (CME) activities and online medical references sites. Seventy-eight percent of respondents endorsed one or both of these methods.
Table 5.
Genetics and Genomics Educational Methods Endorsed by PCPs (N = 82).
| Method | n (%) |
|---|---|
| Online CME activities | 52 (63) |
| Online medical reference site (up-to-date, etc.) | 40 (49) |
| Journal articles/reviews | 36 (44) |
| Genetics consult (formal or “curbside”) | 31 (38) |
| In-person regional meetings | 25 (30) |
| Colleagues | 21 (26) |
| Society guidelines | 21 (26) |
| Institutional guidelines (your hospital/university guidelines) | 21 (26) |
| General search engine | 17 (21) |
| In-person national/international meetings (ACMG, ASHG, etc.) | 16 (20) |
| PubMed/OMIM/Ovid search | 10 (12) |
| Media (email alerts, TV, radio, magazines, internet blogs, etc.) | 6 (7) |
| Genetic laboratory websites | 4 (5) |
| Textbooks | 3 (4) |
| None of the above | 3 (4) |
Discussion
Our results indicate that PCPs refer to genetics more often than they order genetic tests, but both occur at very low frequencies. Our respondents reported several barriers to referring their patients to genetic services or ordering genetic testing. A large proportion of responding physicians reported lacking knowledge or uncertainty when referral is appropriate. Lack of genetics knowledge and uncertainty around when a genetics referral is appropriate are consistent with previous studies that highlighted these barriers.9,10,16,26,27
We evaluated additional barriers that exist even when physicians decide to refer. Concerns for financial cost to patients was the most common barrier that PCPs reported in regard to the referral of patients to genetic services. This is consistent with previous studies where PCPs reported cost as a barrier.16,17,28 While distance is often cited as a barrier to accessing genetic services,26,27 our results indicate low referral rates and genetic testing ordering despite the majority of PCPs practicing within 30 miles of a genetics center.
We also found that White PCPs had higher median referral rates compared to Black or African American and Asian PCPs. The reason for this difference is unknown. One study found that PCPs’ gender and race have an impact on the factors that they weigh in selecting a specialist for referral; for example, Black or African American PCPs were more likely to indicate patient convenience as a factor than were White PCPs.29 However, it is not known whether these factors also influence the decision to refer to specialists such as geneticists. The difference could also be related to the concordance between patient and provider race. We found that PCPs who were White were less likely to serve larger proportions of minority patients compared to Black or African American and Asian PCPs. This finding aligns with previous studies that found that minority-serving physicians were less likely to refer or order genetic testing for their patients.11,14 Furthermore, previous studies demonstrated that minority cancer patients are less likely to receive genetic referrals or testing.30,31 Another study demonstrated that while 12% of women from a predominantly Hispanic population met criteria for BRCA1/2 testing, less than 5% reported receiving this testing.32 This suggests additional barriers exist and that there is a need to further evaluate systematic and patient barriers beyond physician education. System-related barriers may include access to resources, trained staff, up-to-date materials, costs for community-based genetic testing, and timely communication from referrals.26 Patient barriers among minority patients may include limited awareness and knowledge about genetic counseling/testing, as well as concerns about cost and insurance coverage, confidentiality, stigma and discrimination, and the impact on the family.33,34 Among Asian Americans, language barriers, interpretation issues, genetic literacy challenges, and cultural expectations of directiveness may also play a role.35 GCs’ main roles include helping patients and providers navigate these barriers and serve as a valuable resource to PCPs.
Recently, PCPs expressed that they expected an increased role for genetics in their practice, but there was a lack of consensus on associated responsibilities, as well as a lack of preparation and support.26 While PCPs need to increase their referral and genetic testing rates, these efforts need to be informed decisions. PCPs and their staff must be able to overcome barriers such as appropriately assessing need, navigating insurance, ordering correct genetic testing, and accurately interpreting results. GCs have clearly defined roles and scopes of practice in regard to providing genetic services to patients compared to PCPs and integration of GC expertise into PCP practices is critical.36 It is crucial to further explore and measure how PCP collaboration with GCs can improve utilization of and access to genetic services in the primary care setting.
Improved patient care in collaboration with GCs does not exclude the need to improve PCPs’ awareness and use of genetic services. With an estimated two clinical geneticists for every 1 million individuals in the US,23 available genetic providers are at capacity. Likewise, Hoskovec et al37 demonstrated a shortage of GCs, with a model predicting patient demand would not be met until 2030. This emphasizes the need for PCPs to be able to provide some level of initial genetics evaluation, counseling, and testing in collaboration with GCs. Consultation of GC expertise is especially needed to address changes in the genetics landscape with the last decade, which can impact provider clinical care.
We collected information on responses to hypothetical clinical scenarios to assess clinical genetics utilization. We found that there was disagreement regarding recommended care among clinical geneticists in response to 4 of the 6 clinical scenarios. The ability to achieve ≥80% concordance within the geneticist population appeared to decrease as the cases became less straightforward, indicating that the genetics community may need to spend some time discussing and developing recommendations. For the 2 scenarios for which clinical geneticists were in agreement, there was discordance among PCPs about whether or not to utilize genetics. Clear guidelines may be helpful to PCPs in determining when genetic testing or referrals are warranted. Guidelines could be based on both evidence and professional consensus, such as those developed for the nutrition management of phenylketonuria.38 These guidelines were based on a modified Delphi method that included a systematic literature review, 2 surveys of professional practice and opinion, an expert panel nominal group, and review of guidelines by national experts.38 Including professional input and consensus in the guideline development process would ensure that the guidelines account for real-world experiences, needs, and barriers, and that they are in alignment with what clinical geneticists need from PCPs.
Once the guidelines are developed, they must be made available to PCPs. Over 3-quarters of the respondents to our survey indicated that they preferred online CME activities or online medical reference sites or both as a means to receive information about genetics and genomics. Clinical genetics professionals, including clinical geneticists and GCs, should work closely with PCPs to develop and disseminate clear advice about genetic testing and referrals in an easily-accessible, online format that is updated as new information becomes available.
There are several important limitations to this study. Because the sample size was small, we cannot rule out the possibility of self-selection bias among our respondents. For example, those who responded may have an interest in genetics and genomics, which would limit extrapolation of results to other PCPs. However, if this bias occurred, we would expect non-respondents to be even less aware of recommendations that affect genetics referrals than our respondents. Furthermore, because almost half of our respondents were from the southeastern region, our respondents may not be representative of all US PCPs. The response rate from PCPs recruited from a national email listserv was lower than expected, which made it difficult to compare results across US regions. While this analysis focused on the rates of genetics referrals and genetic testing, it did not assess PCPs’ knowledge of how to make a referral and their awareness of available resources such as genetic providers. Future studies are needed to further evaluate how this contributes to their reported lack of knowledge and uncertainty, as well as if this barrier contributes to the observed low referral rates.
Acknowledgments
We would like to thank Beth A. Tarini, Hong Li, Reneé H. Moore, Julia Gallini, and Nathaniel H. Robin for their assistance in development of the survey and guidance in statistical analysis plans.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication was supported by the Southeast Regional Genetics Network (SERN), which is funded by the Health Resources and Services (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of an award totaling $600 000 with 0% financed with non-governmental sources (UH7MC30772). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, HRSA, HHS, or the U.S. Government. For more information, please visit HRSA.gov. Partial funding for this study was also obtained from the Georgia Cancer Genetics Network. The funding sources played no role in the conduct of this study.
ORCID iD: Aileen Kenneson  https://orcid.org/0000-0002-5205-5121
https://orcid.org/0000-0002-5205-5121
References
- 1.Lucassen A, Watson E, Harcourt J, Rose P, O’Grady J. Guidelines for referral to a regional genetics service: GPs respond by referring more appropriate cases. Fam Pract. 2001;18:135-140. [DOI] [PubMed] [Google Scholar]
- 2.Hull LE, Gold NB, Armstrong KA. Revisiting the roles of primary care clinicians in genetic medicine. JAMA. 2020;324(16):1607-1608. [DOI] [PubMed] [Google Scholar]
- 3.Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. Primary care physicians. Am J Prev Med. 2011;40(1):61-66. [DOI] [PubMed] [Google Scholar]
- 4.Trivers KF, Baldwin LM, Miller JW, et al. Reported referral for genetic counseling or BRCA 1/2 testing among United States physicians: a vignette-based study. Cancer. 2011;117(23):5334-5343. [DOI] [PubMed] [Google Scholar]
- 5.Bonadies DC, Brierley KL, Barnett RE, et al. Adverse events in cancer genetic testing: the third case series. Cancer J. 2014;20(4):246-253. [DOI] [PubMed] [Google Scholar]
- 6.Farmer MB, Bonadies DC, Mahon SM, et al. Adverse events in genetic testing: the fourth case series. Cancer J. 2019;25(4):231-236. [DOI] [PubMed] [Google Scholar]
- 7.Brierley KL, Blouch E, Cogswell W, et al. Adverse events in cancer genetic testing: medical, ethical, legal, and financial implications. Cancer J. 2012;18(4):303-309. [DOI] [PubMed] [Google Scholar]
- 8.Rutz A, Dent KM, Botto LD, Young PC, Carbone PS. Brief report: pediatrician perspectives regarding genetic evaluations of children with Autism spectrum disorder. J Autism Dev Disord. 2019;49(2):794-808. [DOI] [PubMed] [Google Scholar]
- 9.Hayflick SJ, Eiff MP, Carpenter L, Steinberger J. Primary care physicians’ utilization and perceptions of genetics services. Genet Med. 1998;1:13-21. [DOI] [PubMed] [Google Scholar]
- 10.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomark. 2013;17(3):219-225. [DOI] [PubMed] [Google Scholar]
- 11.Klitzman R, Chung W, Marder K, et al. Attitudes and practices among internists concerning genetic testing. J Genet Couns. 2013;22(1):90-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baars MJ, Henneman L, Ten Kate LP. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: a global problem. Genet Med. 2005;7(9):605-610. [DOI] [PubMed] [Google Scholar]
- 13.Diamonstein C, Stevens B, Shahrukh Hashmi S, Refuerzo J, Sullivan C, Hoskovec J. Physicians’ awareness and utilization of genetic services in Texas. J Genet Couns. 2018;27(4):968-977. [DOI] [PubMed] [Google Scholar]
- 14.Shields AE, Burke W, Levy DE. Differential use of available genetic tests among primary care physicians in the United States: results of a national survey. Genet Med. 2008;10(6):404-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofman KJ, Tambor ES, Chase GA, Geller G, Faden RR, Holtzman NA. Physicians’ knowledge of genetics and genetic tests. Acad Med. 1993;68:625-632. [DOI] [PubMed] [Google Scholar]
- 16.Haga SB, Carrig MM, O’Daniel JM, et al. Genomic risk profiling: attitudes and use in personal and clinical care of primary care physicians who offer risk profiling. J Gen Intern Med. 2011;26(8):834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MJ, Holt CL, Lose EJ, Robin NH. The use by Alabama pediatricians of genetics consultation in the evaluation of developmental delay. Am J Med Genet A. 2008;146A(4):421-425. [DOI] [PubMed] [Google Scholar]
- 18.Hunter A, Wright P, Cappelli M, Kasaboski A, Surh L. Physician knowledge and attitudes towards molecular genetic (DNA) testing of their patients. Clin Genet. 1998;53(6):447-455. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay I, Janvier A, Laberge AM. Paediatricians underuse recommended genetic tests in children with global developmental delay. Paediatr Child Health. 2018;23(8):e156-e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86(5):749-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson HD, Pappas M, Cantor A, Haney E, Holmes R, Stillman L. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322(7):666-685. [DOI] [PubMed] [Google Scholar]
- 22.Miller CE, Krautscheid P, Baldwin EE, et al. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A. 2014;164(5):1094-1101. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins BD, Fischer CG, Polito CA, et al. The 2019 US medical genetics workforce: a focus on clinical genetics. Genet Med. 2021;23:1458-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clyman JC, Nazir F, Tarolli S, Black E, Lombardi RQ, Higgins JJ. The impact of a genetics education program on physicians’ knowledge and genetic counseling referral patterns. Med Teach. 2007;29(6):e143-e150. [DOI] [PubMed] [Google Scholar]
- 25.Carroll JC, Rideout AL, Wilson BJ, et al. Genetic education for primary care providers: improving attitudes, knowledge, and confidence. Can Fam Physician. 2009;55(12):e92-e99. [PMC free article] [PubMed] [Google Scholar]
- 26.Harding B, Webber C, Ruhland L, et al. Primary care providers’ lived experiences of genetics in practice. J Community Genet. 2019;10(1):85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17(3):169-176. [DOI] [PubMed] [Google Scholar]
- 28.Fogleman AJ, Zahnd WE, Lipka AE, et al. Knowledge, attitudes, and perceived barriers towards genetic testing across three rural Illinois communities. J Community Genet. 2019;10(3):417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinchen KS, Cooper LA, Levine D, Wang NY, Powe NR. Referral of patients to specialists: factors affecting choice of specialist by primary care physicians. Ann Fam Med. 2004;2(3):245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-1736. [DOI] [PubMed] [Google Scholar]
- 31.Muller C, Lee SM, Barge W, et al. Low referral rate for genetic testing in racially and ethnically diverse patients despite universal colorectal cancer screening. Clin Gastroenterol Hepatol. 2018;16(12):1911-1918.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuinness JE, Trivedi MS, Silverman T, et al. Uptake of genetic testing for germline BRCA1/2 pathogenic variants in a predominantly Hispanic population. Cancer Genet. 2019;235-236:72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hann KEJ, Freeman M, Fraser L, et al. Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health. 2017;17(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams CD, Bullard AJ, O’Leary M, Thomas R, Redding TS, Goldstein K. Racial/ethnic disparities in BRCA counseling and testing: a narrative review. J Racial Ethn Health Disparities. 2019;6(3):570-583. [DOI] [PubMed] [Google Scholar]
- 35.Young JL, Mak J, Stanley T, Bass M, Cho MK, Tabor HK. Genetic counseling and testing for Asian Americans: a systematic review. Genet Med. 2021;23(8):1424-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Society of Genetic Counselors. Accessed June 2021. https://www.nsgc.org/Policy-Research-and-Publications/State-Licensure-for-Genetic-Counselors/Model-Legislative-Provisions
- 37.Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16-20. [DOI] [PubMed] [Google Scholar]
- 38.Singh RH, Cunningham AC, Mofidi S, et al. Updated, web-based nutrition management guideline for PKU: an evidence and consensus based approach. Mol Genet Metab. 2016;118(2):72-83. [DOI] [PubMed] [Google Scholar]